Version Changes
Revised. Amendments from Version 1
To this revised version of the article, the authors have provided an additional description of the YCharOS antibody characterization platform as well as references to the published standardized protocols and feature article that guides readers on how to interpret the characterization data. This background information has been included to the Introduction. Additionally, the authors have indicated, in the figure legends, which secondary antibodies or detection system was used.
Abstract
SPARC-related modular calcium-binding protein 1, otherwise known as SMOC-1, is a secreted glycoprotein involved in various cell biological processes including cell-matrix interactions, osteoblast differentiation, embryonic development, and homeostasis. SMOC-1 was found to be elevated in asymptomatic Alzheimer’s disease (AD) patient cortex as well as being enriched in amyloid plaques and in AD patientcerebrospinal fluid, arguing for SMOC-1 as a promising biomarker for AD. Having access to high-quality SMOC-1 antibodies is crucial for the scientific community. It can ensure the consistency and reliability of SMOC-1 research, and further the exploration of its potential as both a therapeutic target or diagnostic marker.. In this study, we characterized seven SMOC-1 commercial antibodies for Western blot and immunoprecipitation, using a standardized experimental protocol based on comparing read-outs in knockout cell lines and isogenic parental controls. We identified successful antibodies in the tested applications and encourage readers to use this report as a guide to select the most appropriate antibody for their specific needs.
Keywords: Uniprot ID Q9H4F8, SMOC1, SMOC-1, SPARC-related modular calcium binding protein 1, antibody characterization, antibody validation, western blot, immunoprecipitation
Introduction
The SMOC1 gene encodes the SPARC (secreted protein acidic and rich in cysteine)-related calcium-binding protein 1 (SMOC-1), a secreted glycoprotein involved in numerous extracellular processes. 1 – 3 Expressed in various tissues with localization to the basement membrane and extracellular matrix, SMOC-1 regulates cell-matrix interactions through its ability to bind cell-surface receptors, growth factors, extracellular matrix and cytokines. 2 , 4 Through its binding to receptors on the cells surface, SMOC-1 modulates growth factor signalling involved in osteoblast differentiation. 5 In addition to being a critical regulator of various biological processes, SMOC-1 plays a role in the pathophysiology of diverse diseases, including cancer development and progression. 1
Proteomic studies have uncovered SMOC-1 to be highly enriched in a subpopulation of amyloid plaques, in AD patients and to be elevated in asymptomatic AD cortex. 6 Recently, SMOC-1 was shown to be elevated in cerebrospinal fluid from AD patients. 7 Although it remains unknown why SMOC-1 co-localizes with only some amyloid plaques, it is hypothesized that SMOC-1 may interact with amyloid-beta (Aβ) species that have been subjected to post-translational modifications. 6 More comprehensive research is required to examine the mechanistic role of SMOC-1 in AD.
Mechanistic studies would be greatly facilitated with the availability of high-quality antibodies.
Here we evaluated the performance of seven commercial antibodies for SMOC-1 for use in western blot and immunoprecipitation, enabling biochemical and cellular assessment of SMOC-1 properties and function. The platform for antibody characterization used to carry out this study was endorsed by a committee of industry academic representatives. It consists of identifying human cell lines with adequate target protein expression and the development/contribution of equivalent knockout (KO) cell lines, followed by antibody characterization procedures using most commercially available antibodies against the corresponding protein. The standardized consensus antibody characterization protocols are openly available on Protocol Exchange (DOI: 10.21203/rs.3.pex-2607/v1). 8
The authors do not engage in result analysis or offer explicit antibody recommendations. Our primary aim is to deliver top-tier data to the scientific community, grounded in Open Science principles. This empowers experts to interpret the characterization data independently, enabling them to make informed choices regarding the most suitable antibodies for their specific experimental needs. Guidelines on how to interpret antibody characterization data found in this study are featured on the YCharOS gateway. 9
Results and discussion
Our standard protocol involves comparing readouts from parental and knockout cells. 10 – 14 To identify a cell line that expresses adequate levels of SMOC-1 protein to provide sufficient signal to noise, we examined public proteomics databases, namely PaxDB 15 and DepMap. 16 HeLa was identified as a suitable cell line and thus HeLa was modified with CRISPR/Cas9 to knockout the corresponding SMOC1 gene ( Table 1).
Table 1. Summary of the cell lines used.
SMOC-1 is predicted to be a secreted protein. Accordingly, we collected concentrated culture media from both parental and SMOC1 KO cells and used the conditioned media to probe the performance of the antibodies ( Table 2) side-by-side by Western blot and immunoprecipitation. The profiles of the tested antibodies are shown in Figures 1 and 2.
Table 2. Summary of the SMOC-1 antibodies tested.
| Company | Catalog number | Lot number | RRID (Antibody Registry) | Clonality | Clone ID | Host | Concentration (μg/μL) | Vendors recommended applications |
|---|---|---|---|---|---|---|---|---|
| Abcam | ab313569 ** | 3101091230 | AB_2941846 1 | recombinant-mono | EPR26922-29 | rabbit | 0.50 | Wb, IP, IF |
| Abcam | ab313571 ** | 3101065175 | AB_2941847 1 | recombinant-mono | EPR26922-31 | rabbit | 0.50 | WB, IP |
| Abcam | ab200219 | GR3370372-1 | AB_2833001 | polyclonal | - | rabbit | 0.50 | Wb |
| GeneTex | GTX119208 | 40331 | AB_10618293 | polyclonal | - | rabbit | 0.90 | Wb |
| Thermo Fisher Scientific | PA5-31392 | 130141931 | AB_2548866 | polyclonal | - | rabbit | 0.90 | Wb |
| Thermo Fisher Scientific | PA5-113408 | WL3463969 | AB_2868141 | polyclonal | - | rabbit | 3.50 | Wb |
| ABclonal | A20482 | 125410101 | AB_2909795 | polyclonal | - | rabbit | 2.65 | Wb |
Wb=Western blot; IF= immunofluorescence; IP=immunoprecipitation.
= recombinant antibody.
refers to new antibodies with RRID that have recently been created (August 2023) but will be available on the Antibody Registry in the coming weeks.
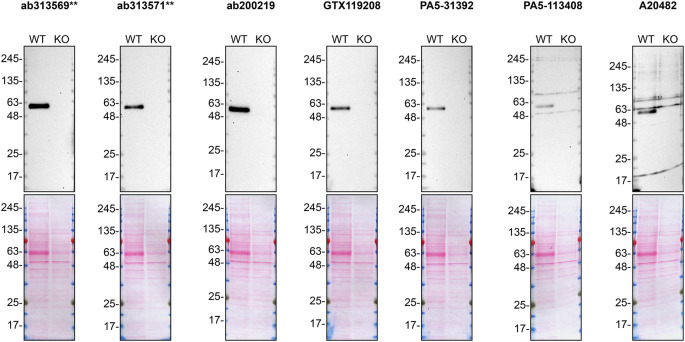
Figure 1. SMOC-1 antibody screening by Western blot on culture media.
HeLa WT and SMOC1 KO were cultured in serum free media, and 30 μg of protein from concentrated culture media were processed for Western blot with the indicated SMOC-1, antibodies. The Ponceau stained transfers of each blot are shown. Peroxidase-conjugated goat anti-rabbit was used as the secondary antibody to detect the signal produced. Antibody dilutions were chosen according to the recommendations of the antibody supplier. All antibodies were tested at 1/2000. Predicted band size: 48 kDa. **= recombinant antibody.
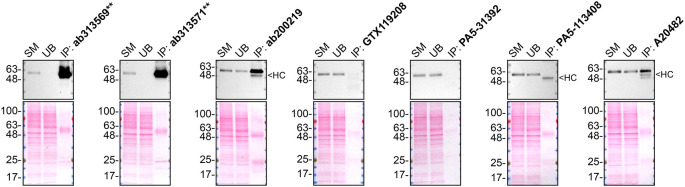
Figure 2. SMOC-1 antibody screening by immunoprecipitation on culture media.
Immunoprecipitation was performed on concentrate culture media from HeLa WT, and using 2.0 μg of the indicated SMOC-1, antibodies pre-coupled to Dynabeads protein A. Samples were washed and processed for Western Blot with the indicated SMOC-1, antibody. For Western blot, ab313569** was used at 1/1000. The Ponceau stained transfers of each blot are shown for similar reasons as in Figure 1. VeriBlot for IP Detection Reagent:HRP was used as a secondary detection system. SM=8% starting material; UB=8% unbound fraction; IP=immunoprecipitated, HC= antibody heavy chain, **= recombinant antibody.
In conclusion, we have screened seven SMOC-1 commercial antibodies by Western blot and immunoprecipitation. Under our standardized experimental conditions, several high-quality antibodies were identified, however, the authors do not engage in result analysis or offer explicit antibody recommendations. A limitation of this study is the use of universal protocols - any conclusions remain relevant within the confines of the experimental setup and cell line used in this study. Our primary aim is to deliver top-tier data to the scientific community, grounded in Open Science principles. This empowers experts to interpret the characterization data independently, enabling them to make informed choices regarding the most suitable antibodies for their specific experimental needs.
The underlying data can be found on Zenodo, an open-access repository. 17 , 18
Methods
Antibodies
All SMOC-1, antibodies are listed in Table 2, together with their corresponding Research Resource Identifiers (RRID), to ensure the antibodies are cited properly. 19 Peroxidase-conjugated goat anti-rabbit is from Thermo Fisher Scientific (cat. number 65-6120).
CRISPR/Cas9 genome editing
HeLa SMOC1 KO clone was generated with low passage cells using an open-access protocol available on Zenodo.org. The guide RNA used to knockout the SMOC1 gene is CUCGUAGGACCUGCCAUCAG.
Cell culture
Both HeLa WT and SMOC1 KO cell lines used are listed in Table 1, together with their corresponding RRID, to ensure the cell lines are cited properly. 20 Cells were cultured in DMEM high-glucose (GE Healthcare cat. number SH30081.01) containing 10% fetal bovine serum (Wisent, cat. number 080450), 2 mM L-glutamate (Wisent cat. number 609065), 100 IU penicillin and 100 μg/mL streptomycin (Wisent cat. number 450201). Cells were starved in DMEM high-glucose containing L-glutamate and penicillin/streptomycin.
Antibody screening by Western blot on culture media
HeLa cells WT and SMOC1 KO were washed three times with PBS 1x and starved for ~18 hrs. Culture media were collected and centrifuged for 10 min at 500 x g to eliminate cells and larger contaminants, then for 10 min at 4500 x g to eliminate smaller contaminants. Culture media were concentrated by centrifuging at 4000 x g for 30 min using Amicon Ultra-15 Centrifugal Filter Units with a membrane NMWL of 10 kDa (MilliporeSigma cat. number UFC901024). Culture media were supplemented with 1x protease inhibitor cocktail mix (MilliporeSigma, cat. number P8340).
Western blots were performed as described in our standard operating procedure. 12 – 14 , 21 Western blots were performed with precast midi 4-20% Tris-Glycine polyacrylamide gels from Thermo Fisher Scientific (cat. number WXP42012BOX) ran with Tris/Glycine/SDS buffer from Bio-Rad (cat. number 1610772), loaded in Laemmli loading sample buffer from Thermo Fisher Scientific (cat. number AAJ61337AD) and transferred on nitrocellulose membranes. BLUelf prestained protein ladder from GeneDireX (cat. number PM008-0500) was used. Proteins on the blots were visualized with Ponceau S staining (Thermo Fisher Scientific, cat. number BP103-10) which is scanned to show together with individual Western blot. Blots were blocked with 5% milk for 1 hr, and antibodies were incubated overnight at 4°C with 5% milk in TBS with 0,1% Tween 20 (TBST) (Cell Signalling Technology, cat. number 9997). Following three washes with TBST, the peroxidase conjugated secondary antibody was incubated at a dilution of ~0.2 μg/ml in TBST with 5% milk for 1 hr at room temperature followed by three washes with TBST. Membranes were incubated with Pierce ECL from Thermo Fisher Scientific (cat. number 32106) prior to detection with the iBright™ CL1500 Imaging System from Thermo Fisher Scientific (cat. number A44240).
Antibody screening by immunoprecipitation on culture media
Immunoprecipitation was performed as described in our standard operating procedure. 12 – 14 , 22 Antibody-bead conjugates were prepared by adding 2 μg of antibody to 500 μL of Pierce IP Lysis Buffer from Thermo Fisher Scientific (cat. number 87788) in a 1.5 mL microcentrifuge tube, together with 30 μL of Dynabeads protein A- (for rabbit antibodies) from Thermo Fisher Scientific (cat. number 10002D). Tubes were rocked for ~1 hr at 4°C followed by two washes to remove unbound antibodies.
Starved HeLa WT culture media were concentrated as described above and supplemented with protease inhibitor. 0.3 mL aliquots at 1.6 mg/mL of protein were incubated with an antibody-bead conjugate for ~1 hr at 4°C. The unbound fractions were collected, and beads were subsequently washed three times with 1.0 mL of IP lysis buffer and processed for SDS-PAGE and Western blot on a precast midi 4-20% Tris-Glycine polyacrylamide gels. VeriBlot for IP Detection Reagent:HRP from Abcam (cat. number ab131366) was used as a secondary detection system at a concentration of 0.3 μg/mL.
Acknowledgment
We would like to thank the NeuroSGC/YCharOS/EDDU collaborative group for their important contributions to the creation of an open scientific ecosystem of antibody manufacturers and knockout cell line suppliers, for the development of community-agreed protocols, and for their shared ideas, resources and collaboration. Members of the group can be found below.
NeuroSGC/YCharOS/EDDU collaborative group: Riham Ayoubi, Thomas M. Durcan, Aled M. Edwards, Carl Laflamme, Peter S. McPherson, Chetan Raina and Kathleen Southern
Thank you to the Structural Genomics Consortium, a registered charity (no. 1097737), for your support on this project. The Structural Genomics Consortium receives funding from Bayer AG, Boehringer Ingelheim, Bristol-Myers Squibb, Genentech, Genome Canada through Ontario Genomics Institute (grant no. OGI-196), the EU and EFPIA through the Innovative Medicines Initiative 2 Joint Undertaking (EUbOPEN grant no. 875510), Janssen, Merck KGaA (also known as EMD in Canada and the United States), Pfizer and Takeda.
An earlier version of this article can be found on Zenodo (doi: 10.5281/zenodo.8277962).
Funding Statement
This work was supported in part by the Accelerating Medicines Partnership Program for Alzheimers disease (AMD-AD) project using a grant from the National Institute on Aging (U54AG065187). It was also supported by a grant from the Canadian Institutes of Health Research Foundation (FDN154305) and by the Government of Canada through Genome Canada, Genome Quebec and Ontario Genomics (OGI-210). RA was supported by a Mitacs fellowship.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
Data availability
Underlying data
Zenodo: Antibody Characterization Report for SMOC-1, https://doi.org/10.5281/zenodo.8277962. 17
Zenodo: Dataset for the SPARC-related modular calcium-binding protein 1 (SMOC-1) antibody screening study, https://doi.org/10.5281/zenodo.8253319. 18
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
References
- 1. Gao Q, Mok HP, Zhuang J: Secreted modular calcium-binding proteins in pathophysiological processes and embryonic development. Chin. Med. J. 2019;132(20):2476–2484. 10.1097/CM9.0000000000000472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vannahme C, Smyth N, Miosge N, et al. : Characterization of SMOC-1, a novel modular calcium-binding protein in basement membranes. J. Biol. Chem. 2002;277(41):37977–37986. 10.1074/jbc.M203830200 [DOI] [PubMed] [Google Scholar]
- 3. Huang XQ, Zhou ZQ, Zhang XF, et al. : Overexpression of SMOC2 Attenuates the Tumorigenicity of Hepatocellular Carcinoma Cells and Is Associated With a Positive Postoperative Prognosis in Human Hepatocellular Carcinoma. J. Cancer. 2017;8(18):3812–3827. 10.7150/jca.20775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bornstein P, Sage EH: Matricellular proteins: extracellular modulators of cell function. Curr. Opin. Cell Biol. 2002;14(5):608–616. 10.1016/S0955-0674(02)00361-7 [DOI] [PubMed] [Google Scholar]
- 5. Choi YA, Lim J, Kim KM, et al. : Secretome analysis of human BMSCs and identification of SMOC1 as an important ECM protein in osteoblast differentiation. J. Proteome Res. 2010;9(6):2946–2956. 10.1021/pr901110q [DOI] [PubMed] [Google Scholar]
- 6. Drummond E, Kavanagh T, Pires G, et al. : The amyloid plaque proteome in early onset Alzheimer's disease and Down syndrome. Acta Neuropathol. Commun. 2022;10(1):53. 10.1186/s40478-022-01356-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnson ECB, Bian S, Haque RU, et al. : Cerebrospinal fluid proteomics define the natural history of autosomal dominant Alzheimer's disease. Nat. Med. 2023;29(8):1979–1988. 10.1038/s41591-023-02476-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ayoubi R, Ryan J, Bolivar SG, et al. : A consensus platform for antibody characterization (Version 1). Protocol Exchange. 2024. [Google Scholar]
- 9. Biddle MS, Virk HS: YCharOS open antibody characterisation data: Lessons learned and progress made. F1000Research. 2023;12:1344. 10.12688/f1000research.141719.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Laflamme C, McKeever PM, Kumar R, et al. : Implementation of an antibody characterization procedure and application to the major ALS/FTD disease gene C9ORF72. elife. 2019;8:8. 10.7554/eLife.48363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alshafie W, Fotouhi M, Shlaifer I, et al. : Identification of highly specific antibodies for Serine/threonine-protein kinase TBK1 for use in immunoblot, immunoprecipitation and immunofluorescence. F1000Res. 2022;11:977. 10.12688/f1000research.124632.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ayoubi R, Southern K, Laflamme C, et al. : The identification of high-performing antibodies for Midkine for use in Western blot and immunoprecipitation [version 1; peer review: awaiting peer review]. F1000Res. 2023;12:148. 10.12688/f1000research.130587.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ayoubi R, Southern K, Laflamme C, et al. : The identification of high-performing antibodies for Secreted frizzled-related protein 1 (sFRP-1) for use in Western Blot and immunoprecipitation [version 1; peer review: awaiting peer review]. F1000Res. 2023;12:291. 10.12688/f1000research.130991.1 [DOI] [Google Scholar]
- 14. Ayoubi R, Southern K, Laflamme C, et al. : The identification of high-performing antibodies for Apolipoprotein E for use in Western Blot and immunoprecipitation [version 2; peer review: 1 approved]. F1000Res. 2023;12:810. 10.12688/f1000research.133899.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang M, Herrmann CJ, Simonovic M, et al. : Version 4.0 of PaxDb: Protein abundance data, integrated across model organisms, tissues, and cell-lines. Proteomics. 2015;15(18):3163–3168. 10.1002/pmic.201400441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DepMap, Broad: DepMap 19Q3 Public ed. 2019.
- 17. Ayoubi R, Bolivar SG, Nicouleau M, et al. : Antibody Characterization Report for SMOC-1. 2023.
- 18. Southern K: Dataset for the SPARC-related modular calcium-binding protein 1(SMOC-1) antibody screening study.[Data set]. Zenodo. 2023.
- 19. Bandrowski A, Pairish M, Eckmann P, et al. : The Antibody Registry: ten years of registering antibodies. Nucleic Acids Res. 2023;51(D1):D358–D367. 10.1093/nar/gkac927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bairoch A: The Cellosaurus, a Cell-Line Knowledge Resource. J. Biomol. Tech. 2018;29(2):25–38. 10.7171/jbt.18-2902-002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ayoubi R, McPherson PS, Laflamme C: Antibody Screening by Immunoblot. 2021.
- 22. Ayoubi R, Fotouhi M, McPherson P, et al. : Antibody screening by Immunoprecitation. 2021.