Abstract
Recent studies demonstrated that human papillomavirus (HPV) specific immunoglobulins (IgG) are present and detectable in non-invasively collected first-void urine (FVU) samples. As IgG levels in urine are low, we evaluated the potential of a highly sensitive HPV16-specific assay based on time-resolved fluorescence, DELFIA, and compared it with three immunoassays, GST-L1-MIA, M4ELISA, and M9ELISA. A total of 225 paired serum and FVU samples from two cohorts of healthy female volunteers were analyzed. Strong Spearman rank correlations between HPV16-specific IgG results measured with DELFIA, M4ELISA, GST-L1-MIA, and M9ELISA were found for both sample types (rs > 0.80). Additionally, total human IgG results, determined in all samples using HTRF human IgG kit and BioPlex Pro™ Human Isotyping Assay, were compared. Moderate correlations between total human IgG concentrations in FVU samples were found for the two total IgG assays (rs ≥ 0.42, p < 0.0001), while correlations for serum were non-significant. In conclusion, the HPV16-DELFIA assay is usable for detecting HPV16-specific antibodies in FVU and serum samples. As total human IgG remains an interesting parameter for the normalization of HPV-specific IgG in FVU, the accuracy of both assays needs to be validated further.
Keywords: First-void urine, Human papillomavirus, HPV, Vaccination, Antibody, Self-sampling
Highlights
-
•
HPV16 DELFIA assay is a suitable immunoassay for the detection of HPV16 antibodies.
-
•
HPV16-specific DELFIA has high sensitivity in first-void urine and serum samples.
-
•
DELFIA sensitivity is similar to existing VLP-based immunoassays M4- and M9ELISA.
-
•
Normalization of antibody concentration with total IgG did not have an added value.
1. Introduction
Human papillomavirus (HPV) vaccination is a crucial step in primary cervical cancer prevention, given the direct association of persistent high-risk HPV infection with nearly all cases of cervical cancer (Walboomers et al., 1999). Currently, assessing the immunogenicity of HPV vaccines predominantly relies on serum samples (Struyf et al., 2015). Interestingly, recent studies have demonstrated that humoral immune responses specific to HPV can be assessed in first-void urine (FVU) (Pattyn et al., 2020a, Teblick et al., 2023, Van Keer et al., 2019). Considering that cervical cancers after HPV infection predominantly develop at the cervical transformation zone, evaluating antibody-mediated immune responses at the cervix, and thus local protective immunity is important (Longet et al., 2011, Stanley et al., 2012).
Vaccine-induced circulating antibodies reach the female genital tract (FGT) through transudation or exudation at microlesion sites. This results in a higher presence of immunoglobulins (IgG) originating from serum, with fewer locally produced IgG and secretory IgA (sIgA) (Mestecky et al., 2005, Scherpenisse et al., 2013). These HPV-related immunological markers, together with discharged mucus and (debris from) exfoliated cells from the female genital organs, accumulate around the urethral opening and the small labia, and are subsequently washed away during urination. The initial part of a urine sample, known as first-void urine contains a higher concentration of FGT secretions compared to the subsequent part. This includes HPV DNA, HPV-type-specific antibodies, and other HPV biomarkers (Bober et al., 2021, Pathak et al., 2014, Van Keer et al., 2022, Van Keer et al., 2021, Vorsters et al., 2014, Zhou et al., 2018). The non-invasive nature of this sample type makes it highly acceptable and allows women to collect it at home, eliminating the need for a blood draw at a medical facility (De Pauw et al., 2021).
As a correlate of protection for HPV infections remains undetermined, the detection of HPV-specific antibodies at the site of infection could emerge as an important immunological monitoring tool (Falcaro et al., 2021, Gillison et al., 2008, Longet et al., 2011, Paavonen et al., 2009, Villa et al., 2006). Recent studies have demonstrated the quantifiability of HPV-specific IgG in FVU samples, revealing a significant difference in HPV-type-specific antibody concentrations between FVU samples from vaccinated and unvaccinated individuals (Pattyn et al., 2020a, Teblick et al., 2023). Given that HPV-type-specific antibody concentrations are approximately three logs higher in serum compared to FVU samples, the concentrations in FVU often reach the detection limit of existing immunoassays, which are exclusively validated for serum. Furthermore, to account for the lower HPV-specific antibody concentrations present in FVU, lower dilutions must be used, potentially leading to more non-specific background compared to serum. To improve HPV-specific antibody detection in FVU a highly sensitive immunoassay is required.
Previous studies were already able to show HPV-specific antibody positivity for HPV16 in more than 80 % of FVU samples from vaccinated women (Pattyn et al., 2020a, Teblick et al., 2023). This was achieved using the bead-based assay (Glutathione S-transferase-L1 multiplex serology assay, GST-L1-MIA) or multi-spot VLP-based ELISAs (M4ELISA, M9ELISA) which are validated and routinely employed for detecting HPV-type specific antibodies in serum samples (Panicker et al., 2015, Panicker et al., 2021, Waterboer et al., 2005). For both assays, results for FVU were close to the detection limit. Consequently, further steps such as sample purification, assay optimization, or the development of a new immunoassay using highly sensitive technology are deemed necessary to enhance sensitivity.
Our research group has developed an HPV16-type-specific Dissociation-Enhanced Lanthanide Fluorescent Immunoassay (DELFIA). This innovative assay combines the principles of time-resolved fluorescence (TRF) with the properties of lanthanide fluorescent compounds, resulting in a potentially more sensitive assay (Hagan and Zuchner, 2011). A major benefit to the use of lanthanides is the delay in measurement between excitation and emission, resulting in a large Stokes shift and a reduction in self-quenching (Hagan and Zuchner, 2011). The technology offers a high sensitivity, making it suitable for the detection of low-abundance analytes, and has a wide dynamic range to cover a broad range of analyte concentrations.
In this study, we assess the efficacy of the TRF-based assay and compare its outcomes for HPV16-specific antibody concentrations with those obtained from the GST-L1-MIA, M4ELISA, and M9ELISA. To achieve this, we will analyse paired FVU and serum samples collected from two distinct cohorts of women.
Because of the heterogeneous nature of a urine sample, both within and between individuals, fluctuations in detectable biomarkers can arise. These fluctuations may stem from differences in time of collection, collection volume, pre-collection washing, concentration of FGT secretions, and potential changes over the menstrual cycle (Nardelli-Haefliger et al., 2003). To ensure accurate comparisons in longitudinal studies, involving samples from the same individual, or between different individuals, normalization and standardization are imperative. A potential normalization parameter for HPV-specific IgG in FVU is the total amount of IgG present in the FVU sample. However, since no validated total human IgG quantification assay exists for FVU, we conducted a comparison between previously obtained results from the bead-based BioPlex Pro™ Human Isotyping Assay (Bio-Rad, USA) and the Homogeneous Time-Resolved Fluorescence (HTRF®) assay (Revvity, Waltham, MA, USA) (Teblick et al., 2023, Van Keer et al., 2019).
2. Methods
2.1. Sample collection and processing
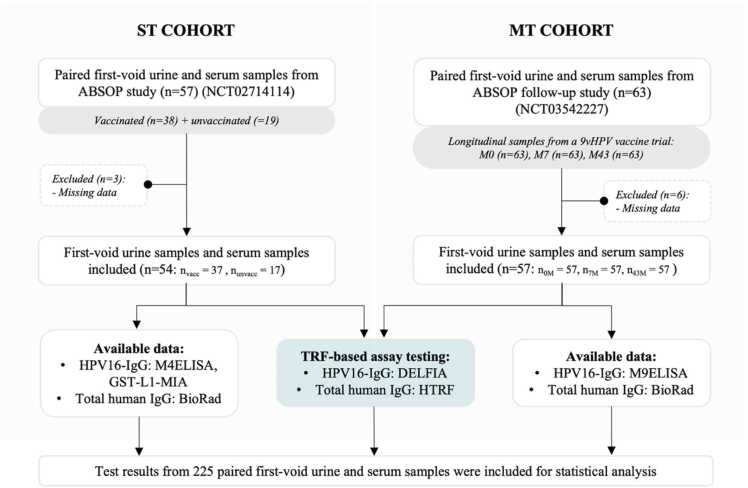
In this study, a total of 225 paired FVU and blood samples were used, originating from two cohorts of female volunteers (Fig. 1). The first cohort, referred to as the single timepoint cohort (ST cohort), comprised 54 female volunteers of which 17 unvaccinated and 37 vaccinated with either a bivalent (2vHPV) or quadrivalent (4vHPV) HPV vaccine (NCT02714114). From each participant paired FVU and serum samples were obtained, resulting in a total of 54 paired samples. The FVU samples were self-collected, while the serum samples were taken by a clinician. The second cohort, referred to as the multiple timepoint cohort (MT cohort), consisted of 57 female volunteers, all of whom collected paired FVU and serum samples at three different time points: before vaccination, 7 months after initial 9vHPV vaccination, and approximately 43 months (3.5 years) after initial 9vHPV vaccination (NCT03542227). This resulted in a total of 171 paired samples. Informed consent was obtained from all volunteers and data was coded to ensure privacy of the participants.
Fig. 1.
Flow diagram of the study. This study included 54 paired FVU and serum samples from the ST cohort, along with 171 samples from the MT cohort. The samples underwent testing for HPV16-IgG utilizing the HPV16 DELFIA assay, alongside assessment for total human IgG using the HTRF assay. Comparative analyses for HPV16-IgG results were done between DELFIA and M4ELISA, as well as GST-L1-MIA results for the ST cohort. For the MT cohort, the comparisons were made between DELFIA and M9ELISA HPV16-IgG results. Additionally, the HTRF-generated total human IgG results were compared to previously generated results with the Bio-Rad.
All FVU samples were self-collected by the volunteers using a 20 ml Colli-Pee™ collection device (Novosanis, Wijnegem, Belgium). Participants were instructed not to thoroughly wash their genitals and not to urinate for at least one hour before FVU sample collection. For MT cohort samples, the device was pre-filled with 6.33 ml urine conservation medium (UCM, Novosanis, Wijnegem, Belgium) whereas for the ST cohort, 20 ml FVU samples were collected without preservative. After collection, all samples were immediately placed on ice or refrigerated at 4 °C before being aliquoted and stored at −80°C until further pre-analytical processing and testing. The median time span between FVU collection and aliquot storage at −80°C was 3 h 50 min (IQR: 0:22–5:19). At all timepoints in each cohort, a paired 10 ml blood sample was collected from each participant using BD Vacutainer serum tubes (Becton-Dickinson, Benelux). The blood samples were allowed to clot for 30–60 minutes and centrifuged at 1000 x g for 10 min at 20 °C. The resulting serum was collected, aliquoted, and stored at −80 °C until further testing. The median time span between blood collection and aliquot storage at −80 °C was 58 min (IQR: 0:49–1:05).
2.2. Pre-analytical processing
FVU samples were concentrated prior to antibody testing. Aliquots of ST cohort samples were thawed and diluted with UCM in a 1:2 ratio (UCM:FVU) before processing. For MT cohort samples, UCM was integrated during the collection process. Subsequently, 4 ml buffered aliquots from each cohort were subjected to centrifugation at 3820 x g for 10 minutes at 20–21 °C using an Amicon Ultra-4 50 K filter. The concentrate on the Amicon filter was diluted with DPBS (Dulbecco’s phosphate buffered saline, Gibco, United Kingdom) to achieve a final volume of 500 µl. This concentrate was stored at −80 °C until testing.
2.3. HPV(16)-specific IgG detection
2.3.1. M4- and M9ELISA
M4- and M9ELISA testing protocols and corresponding results for ST cohort and MT cohort samples, respectively, have been described previously (Pattyn et al., 2020a, Teblick et al., 2023). Antibodies in paired FVU and serum samples were quantified using these multi-spot Virus-Like Particle (VLP)-based assays, as described by Panicker et al., 2015, Panicker et al., 2021. Specifically, ST cohort samples underwent testing with the M4ELISA, assessing HPV type-specific antibody responses to the HPV types included in the 4vHPV vaccine (HPV6, 11, 16, 18). MT cohort samples were subjected to the M9ELISA, which identifies HPV type-specific antibody responses to the HPV types included in the 9vHPV vaccine (4vHPV types plus HPV31, 33, 45, 52, 58). HPV-specific antibody concentrations for all included types were reported in arbitrary units/ml (AU/ml) except HPV16 and 18, which are reported in international units (IU/ml). Arbitrary units were used for all types except HPV16 and 18 because of the absence of an international reference for these types at the time of reporting. The HPV16 (05–134) and HPV18 IU standards (10–140) were obtained from the National Institute for Biological Standards and Controls. Only HPV16-IgG results were used in this comparison study. All concentrations were calculated using the parallel line (PLL) method, described in the World Health Organization (WHO) HPV Labnet Manual 2009 (Ferguson et al., 2009). Notably, for HPV16-IgG concentrations measured by the M9ELISA for the MT cohort, the Relative Light Unit (RLU) signal of a bovine serum albumin (BSA) spot was subtracted before PLL calculation. For all other samples, the raw RLUs were employed. For serum samples, the lower limit of quantification (LLOQ) was employed as described previously (LLOQ = 0.3 IU/ml) (Teblick et al., 2023). For FVU samples, cut-offs were calculated using the mean plus three standard deviations of the unvaccinated samples of the ST cohort for the M4ELISA (cut-off = 0.0048 IU/ml) and of the MT cohort for the M9ELISA (cut-off = 0.0021 IU/ml).
2.3.2. GST-L1-MIA
Results of the GST-L1-MIA were obtained from all ST cohort samples in a previous study (Pattyn et al., 2020a, Van Keer et al., 2019). For this analysis, FVU samples underwent a 1:4 dilution, while serum samples were diluted 1:100 before testing. 1:1 dilution of the FVU samples was not possible due to a large background signal obtained with this dilution. The HPV16-specific antibody results were expressed as Mean Fluorescence Intensity (MFI) values, derived from the analysis of at least 1000 beads per bead set. Cut-offs were determined by calculating the mean plus three standard deviations of the unvaccinated samples. Specifically, for FVU samples, the cut-off was set at 75.8 MFI, while for serum samples, the cut-off was established at 9580.8 MFI.
2.3.3. HPV16-DELFIA
In this study, all paired FVU and serum samples from cohorts 1 and 2 underwent additional testing using the in-house developed HPV16-DELFIA assay. Yellow 96-well DELFIA plates (Revvity, Waltham, MA, USA) were coated with 100 µl/well of 0.5 µg/ml in-house produced HPV16 pseudovirions (PsV), produced following the protocol by Buck et al., 2005. After an overnight incubation at 4°C, the plates were blocked by adding 1X PBS + 1 % DTPA-purified BSA (Revvity, Waltham, MA, USA) to each well and incubated at room temperature (RT) on a plate shaker (300 rpm) for at least one hour. Subsequently, 100 µl of four serial 1:2 dilutions of serum and FVU samples were added to the wells, starting from 1:400 for serum and 1:1 for FVU. Negative control wells contained only assay buffer, while sample control wells contained four serial dilutions of a known HPV16 antibody positive serum sample. After three washes, Europium-anti-human antibodies, specific for the Fc-part of human IgG, were added to bind to the HPV16-specific antibodies bound to the coated PsV. Following a 60-minute incubation at RT and 300 rpm, the plates underwent six washes, and enhancement solution (Revvity, Waltham, MA, USA) was added. The enhancement solution was incubated for at least 30 minutes, and plates were read using the Victor Nivo multimode plate reader with TRF settings, which gives readout of the sample dilutions in fluorescence counts (PerkinElmer, Waltham, MA, USA). A three-point dilution of the HPV16 (05–134) standard was added on each tested plate in duplo to determine a standard curve. Concentrations were determined for each sample using this standard curve reference and the parallel line method (PLL) described in the World Health Organization HPV LabNet Manual 2009 (Ferguson et al., 2009). A sample was given a concentration if it passed all PLL conditions and if the concentration was at or above the lower limit of quantification (LLOQ). A serum or first-void urine sample passed PLL conditions if (1) the correlation between the three selected dilutions was ≥0.9; (2) the absolute value of the slope was ≥0.4; (3) the ratio of the slope of the standard and the test sample was ≥0.5; and (4) if there was not more than one data point out of three points outside the linear range. HPV16-specific antibody concentrations were reported in international units (IU/ml) by using the HPV16 (05–134) standard obtained from the National Institute for Biological Standards and Controls.
LLOQ values for both sample types were generated using a dilution curve. For FVU samples, the LLOQ was defined as the lowest concentration where the coefficient of variation (%CV) of counts was <15 %, and accuracy was between 80 and 120 (LLOQ = 0.0026 IU/ml). For serum, the LLOQ was determined using the formula 10 * SDintercept/slope of the standard calibration curve (LLOQ = 0.0325 IU/ml).
2.4. Total human IgG detection
2.4.1. Bio-Rad BioPlex Luminex assay
Total human IgG results using the BioPlex Pro™ Human Isotyping Assay (Bio-Rad, USA) were obtained previously for both serum and FVU samples (Teblick et al., 2023, Van Keer et al., 2019). Briefly, 50 μl of pre-processed, diluted FVU (1:128; 1:256; 1:512) or serum (1:40,000) was mixed with captured antibodies coupled to fluorescent-labelled, magnetic polystyrene beads, detecting total human IgG using the LX200 platform (Luminex, Austin, Texas, USA). Antibody concentrations (µg/ml) were quantitated from the median fluorescence intensity (MFI) values using a five-parameter logistic regression, within a working range of 0.003–30.27 μg/ml. Total human IgG in FVU was reported as the average of three dilution-corrected concentrations.
2.4.2. HTRF homogenous assay
In this study, total human IgG concentrations were additionally assessed using the Homogeneous Time-Resolved Fluorescence (HTRF) assay (Revvity, Waltham, MA, USA), following the manufacturer's instructions. The FVU samples were diluted 1:300 and the serum samples 1:500,000. Measurements were conducted with the Victor Nivo multimode plate reader (PerkinElmer, Waltham, MA, USA) at wavelengths 665 nm and 620 nm. The analysis was performed using Graphpad Prism version 10.0.3 (Dotmatics, Boston, MA, USA). A four-parameter logistic equation was used to calculate antibody concentrations based on the ratio of 665 nm and 620 nm fluorescence emission values. The reported total IgG concentrations represent the average of two dilution-corrected concentrations.
2.5. Statistical analysis
All analyses were performed using R statistical software version 4.3.1 (packages: tidyverse, readxl, ggbeeswarm, ggpubr, RColorBrewer, ROCR, cutpointr). Data was tested for normal distribution using the Shapiro-Wilk test. Since all data was not normally distributed, a non-parametric Wilcoxon signed-rank test was performed to determine significant differences between antibody titers between assays and between sample types. We used the Spearman rank test to calculate the correlation between the different assays or sample types. Statistical significance was defined as p-adjusted < 0.05 (using Holm-Bonferroni method for p-value adjustment). Figures were made using antibody titers or log10(x) transformed data where zero values were assigned as 0.0001.
3. Results
3.1. Population characteristics
Data from paired FVU and serum samples of 54 participants in the ST cohort and 57 participants in the MT cohort were included for statistical analysis (Fig. 1). The median age at enrollment was 22 years (IQR: 20–24) for the ST cohort and 35 years (IQR: 27–41) for the MT cohort.
In the ST cohort, out of the 54 female volunteers, 37 were vaccinated, and 17 were unvaccinated. All vaccinated women had received three doses of an HPV vaccine, with 32/37 receiving the 4vHPV vaccine, 4/37 the 2vHPV vaccine, and 1/37 a combination of both. The vaccination status of these female volunteers was self-reported. For the ST cohort, paired FVU and serum samples collected at a single timepoint were included for analysis (n = 54). Median time between having received a first vaccine dose and collection of the samples is 7 years (IQR: 5–9).
All participants in the MT cohort received three doses of the 9vHPV vaccine in total, i.e., one dose at timepoints 0 M, 1 M, and 6 M within the HPV V503–004 study (EudraCT NUMBER: 2015-005093-38). For the MT cohort, paired FVU and serum samples collected before vaccination, and at 7 M and 43 M after initial 9vHPV vaccination, were included for analysis (n = 171).
3.2. Evaluation of HPV16-IgG detection using novel TRF assay
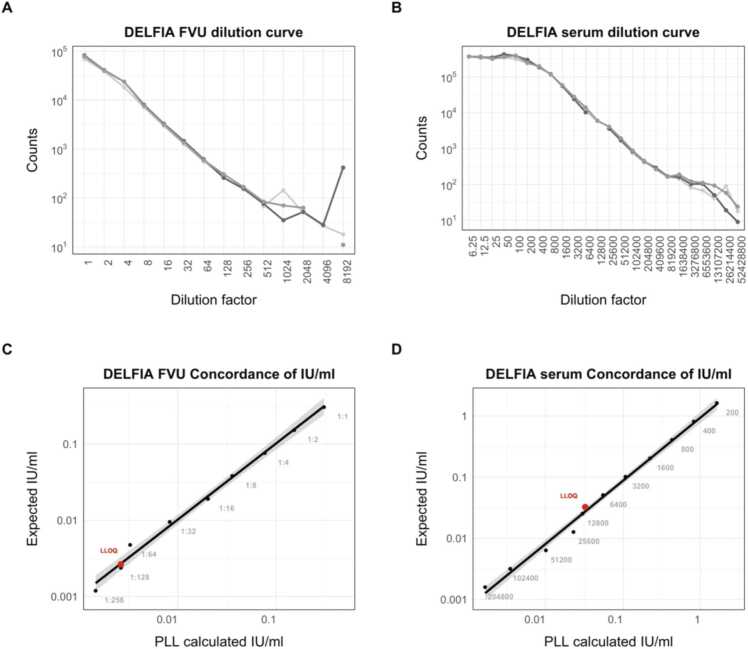
Before testing all samples, we established the LLOQ for the DELFIA assay on Amicon-filtered FVU samples and serum samples through a triplicate dilution series (Appendix Fig. 1). For FVU, the LLOQ was determined by identifying the lowest concentration where the %CV among the three measurements (counts) was below 15 %, and the recovery fell within the range of 80–120 %. For serum, the LLOQ was calculated using the formula 10*SDintercept/slope of the calibration curve. Additionally, the %CV of the negative control wells across the tested plates was 13.38 %, indicating robust assay reproducibility. Furthermore, the %CV of the control samples was 29.06 %, which is considered acceptable.
Appendix_Figure 1.
DELFIA dilution curves of three replicates for A) Amicon filtered FVU and B) serum samples. The concordance between the PLL calculated IU/ml and expected IU/ml concentration (based on known start concentration and dilutions) are presented for C) FVU and D) serum samples. LLOQ values are shown as red dots.
HPV16-specific antibody detection using the TRF-based DELFIA assay was performed on all samples from the ST cohort and the MT cohort (Table 1). For FVU samples, 40/54 (74 %) ST cohort samples and 118/171 (69 %) MT cohort samples had detectable antibody concentrations. Among the vaccinated individuals in the ST cohort, 33/37 (89 %) had detectable antibody concentrations, while in the MT cohort, samples collected at the two time points post-vaccination, at 7 M and 43 M after receiving the first 9vHPV vaccine dose, 107/114 (94 %) tested positive for HPV16-IgG. The median (IQR) HPV16-IgG concentrations for FVU samples from vaccinated volunteers were 0.03 (0.01–0.13) IU/ml for the ST cohort and 0.04 (0.02–0.18) IU/ml for the MT cohort. The geometric mean titer (GMT) for all ST cohort HPV16-IgG positive FVU samples was 0.33 (95 % CI 0.01–0.65) IU/ml, while the GMT for MT cohort FVU samples was 0.28 (95 % CI 0.15–0.41) IU/ml. Among FVU samples from unvaccinated volunteers or collected before vaccination, 7/17 (41 %) ST cohort samples and 11/57 (19 %) MT cohort samples were HPV16-IgG positive. The geometric mean titer (GMT) for all HPV16-IgG positive ST cohort unvaccinated FVU samples was 0.02 (95 % CI 0.00–0.04) IU/ml, while the GMT for MT cohort FVU samples was 0.05 (95 % CI (-0.02)-0.13) IU/ml. In the unvaccinated ST cohort, two participants were exclusively HPV16-IgG positive in FVU using this assay, while the remaining five showed HPV16-IgG positivity with one or more other immunoassays or paired serum (Appendix Table 1).
Table 1.
HPV16-IgG detection (n, %) and concentrations (median, IQR) for all FVU and serum samples from both cohorts using the GST-LA-MIA, M4ELISA and DELFIA for the ST cohort and the M9ELISA and DELFIA for the MT cohort. Concentrations are reported in IU/ml for M4-M9ELISA and DELFIA and in MFI for GST-L1-MIA. FVU/serum ratio are antibody levels in first-void urine (FVU) divided by serum levels, denoted as the median %, plus IQR. P-value (Wilcoxon signed rank test) indicated by an asterisk reports a significant difference between median antibody yield between vaccinated and unvaccinated women for FVU or serum. (*) p<0.05; (**) p<0.01; (***) p<0.001.
|
ALL SAMPLES | ||||||
|---|---|---|---|---|---|---|
| FVU |
Serum |
FVU/Serum % (IQR) ratio | ||||
| Assay | Antibody detection | Median (IQR) | Antibody detection | Median (IQR) | ||
| ST cohort | ||||||
| HPV16-IgG | GST-L1-MIA (MFI) | 33/54 (61 %) | 150 (0−435) |
38/54 (70 %) | 58,575 (0−118,262) |
0.33 (0.17–0.67) |
| M4ELISA (IU/ml) | 41/54 (76 %) | 0.01 (0.00–0.05) |
46/54 (85 %) | 40.65 (6.51–105.50) |
0.03 (0.02–0.07) |
|
| DELFIA (IU/ml) | 40/54 (74 %) | 0.01 (0.00–0.07) |
42/54 (78 %) | 63.56 (27.79–161.45) |
0.03 (0.01–0.07) |
|
| MT cohort | ||||||
| HPV16-IgG | M9ELISA (IU/ml) | 114/171 (67 %) | 0.02 (0.00–0.11) |
124/171 (73 %) | 35.63 (0.00–184.50) |
0.06 (0.03–0.11) |
| DELFIA (IU/ml) | 118/171 (69 %) | 0.02 (0.00–0.09) |
119/171 (70 %) | 33.27 (0.00–259.29) |
0.05 (0.02–0.12) |
|
| FVU SAMPLES | ||||||
| Vaccinated |
Unvaccinated |
P-value | ||||
| Assay | Antibody detection | Median (IQR) | Antibody detection | Median (IQR) | ||
| ST cohort | ||||||
| HPV16-IgG | GST-L1-MIA (MFI) | 32/37 (86 %) | 256 (134−1032) |
1/17 (6 %) | 0 (0−0) |
< 0.001 |
| M4ELISA (IU/ml) | 37/37 (100 %) | 0.03 (0.01–0.13) |
4/17 (24 %) | 0.00 (0.00–0.00) |
< 0.001 | |
| DELFIA (IU/ml) | 33/37 (89 %) | 0.03 (0.01–0.13) |
7/17 (41 %) | 0.00 (0.00–0.01) |
< 0.001 | |
| MT cohort | ||||||
| HPV16-IgG | M9ELISA (IU/ml) | 109/114 (96 %) | 0.05 (0.02–0.21) |
5/57 (9 %) | 0.00 (0.00–0.00) |
< 0.001 |
| DELFIA (IU/ml) | 107/114 (94 %) | 0.04 (0.02–0.18) |
11/57 (19 %) | 0.00 (0.00–0.00) |
< 0.001 | |
| SERUM SAMPLES | ||||||
| Vaccinated |
Unvaccinated |
P-value | ||||
| Assay | Antibody detection | Median (IQR) | Antibody detection | Median (IQR) | ||
| ST cohort | ||||||
| HPV16-IgG | GST-L1-MIA (MFI) | 36/37 (97 %) | 103,375 (55,925−158,675) |
2/17 (12 %) | 0 (0−0) |
< 0.001 |
| M4ELISA (IU/ml) | 37/37 (100 %) | 88.40 (39.40–123.00) |
9/17 (53 %) | 0.92 (0.00–2.83) |
< 0.001 | |
| DELFIA (IU/ml) | 36/37 (97 %) | 130.48 (61.37–202.45) |
6/17 (35 %) | 0.00 (0.00–46.31) |
< 0.001 | |
| MT cohort | ||||||
| HPV16-IgG | M9ELISA (IU/ml) | 114/114 (100 %) | 116.96 (31.79–320.75) |
10/57 (18 %) | 0.00 (0.00–0.00) |
< 0.001 |
| DELFIA (IU/ml) | 113/114 (99 %) | 126.05 (27.41–437.94) |
6/57 (11 %) | 0.00 (0.00–0.00) |
< 0.001 | |
Appendix Table 1.
Unvaccinated FVU or serum samples that have a HPV16-specific IgG titer using DELFIA. (+) indicates HPV16-specific detectable antibodies a; (-) indicates no detectable HPV16-specific antibodies. For cohort 1, two FVU samples (ID: 1.1, 1.9) are exclusively positive using DELFIA. For cohort 2, eight FVU samples (ID: 2.3, 2.6, 2.7, 2.8, 2.9, 2.11, 2.12, 2.15) and one serum sample (ID: 2.13) are exclusively positive using DELFIA.
| HPV16-IgG positivity in unvaccinated samples | |||||||
|---|---|---|---|---|---|---|---|
| ST cohort | |||||||
| FVU | Serum | ||||||
| Sample | GST-L1-MIA | M4ELISA | DELFIA | GST-L1-MIA | M4ELISA | DELFIA | |
| 1.1 | - | - | + | - | - | - | |
| 1.2 | + | + | + | - | + | + | |
| 1.3 | - | + | - | + | + | + | |
| 1.4 | - | - | + | - | - | + | |
| 1.5 | - | - | + | - | + | + | |
| 1.6 | - | - | + | + | + | + | |
| 1.7 | - | + | - | - | + | + | |
| 1.8 | - | + | + | - | - | - | |
| 1.9 | - | - | + | - | - | - | |
| MT cohort | |||||||
| FVU | Serum | ||||||
| Sample | M9ELISA | DELFIA | M9ELISA | DELFIA | |||
| 2.1 | + | + | + | + | |||
| 2.2 | - | + | + | - | |||
| 2.3 | - | + | - | - | |||
| 2.4 | + | + | + | + | |||
| 2.5 | + | - | + | + | |||
| 2.6 | - | + | - | - | |||
| 2.7 | - | + | - | - | |||
| 2.8 | - | + | - | - | |||
| 2.9 | - | + | - | - | |||
| 2.10 | + | - | + | + | |||
| 2.11 | - | + | - | - | |||
| 2.12 | - | + | - | - | |||
| 2.13 | - | - | - | + | |||
| 2.14 | - | - | + | + | |||
| 2.15 | - | + | - | - | |||
For serum samples, 42/54 (78 %) ST cohort samples and 119/171 (70 %) MT cohort samples had detectable antibody concentrations. Among the vaccinated individuals in the ST cohort, 36/37 (97 %) had detectable antibody concentrations while in the MT cohort, samples collected at the two time points post-vaccination showed 113/114 (99 %) testing positive for HPV16-IgG. The total median (IQR) HPV16-IgG concentrations for serum were 130.48 (61.37–202.45) IU/ml for the ST cohort and 126.05 (27.41–437.94) IU/ml for the MT cohort. The geometric mean titer (GMT) for all ST cohort HPV16-IgG positive serum samples was 196.07 (95 % CI 125.78–266.35) IU/ml, while the GMT for MT cohort serum samples was 319.98 (95 % CI 240.56–399.40) IU/ml. Among serum samples from unvaccinated volunteers or collected before vaccination, 6/17 (35 %) ST cohort samples and 6/57 (11 %) MT cohort samples were HPV16-IgG positive. The geometric mean titer (GMT) for all HPV16-IgG positive ST cohort unvaccinated serum samples was 54.84 (95 % CI 42.43–67.25) IU/ml, while the GMT for MT cohort serum samples was 63.66 (95 % CI 31.11–96.20).
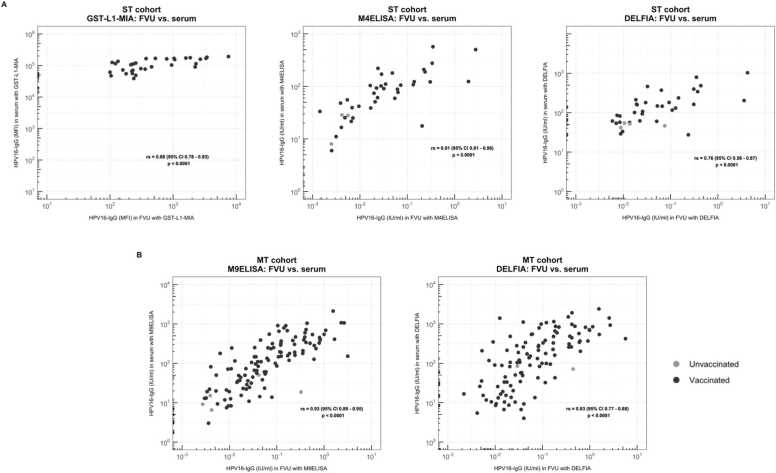
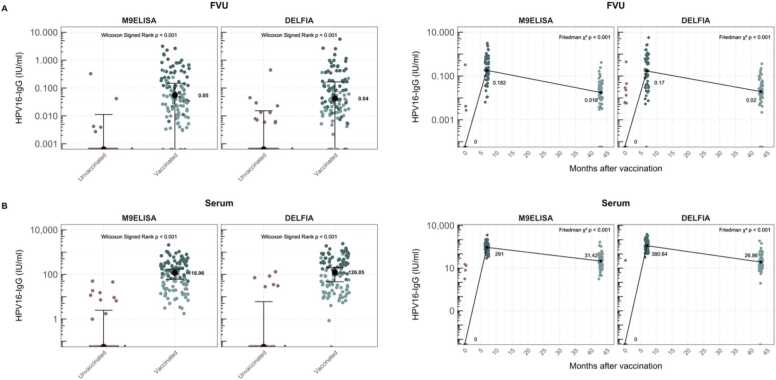
Comparing HPV16-specific median antibody levels in FVU to those in serum measured by DELFIA, the ratios of FVU to serum levels were 0.03 % for the ST cohort and 0.05 % for the MT cohort. Significant differences were found between median antibody levels based on vaccination status in both FVU and serum for both cohorts (p < 0.001). Significant Spearman Rank correlation coefficients were obtained between paired FVU and serum samples for both cohorts (rs ≥ 0.76, p < 0.001) (Fig. 2A).
Fig. 2.
Comparison between FVU and serum antibody results using the (A) GST-L1-MIA, M4ELISA and DELFIA for the ST cohort, and the (B) DELFIA and M9ELISA for the MT cohort. Spearman rank correlation coefficients (95 % CI) are presented in the figure. Black dots represent the vaccinated volunteers, gray dots represent the unvaccinated volunteers.
3.3. Comparison between HPV16-IgG immunoassays
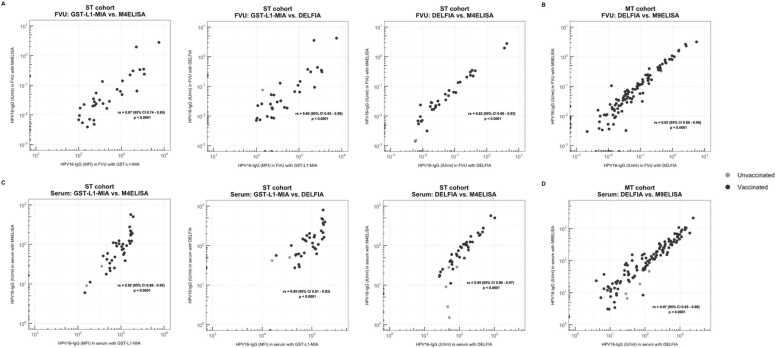
HPV16-IgG results using the DELFIA assay were compared to previously obtained results of the M4ELISA and GST-L1-MIA for the ST cohort and to M9ELISA for the MT cohort. Antibody positivity in samples collected from all vaccinated female volunteers was comparable between all three assays. Specifically, for the vaccinated volunteers, the M4ELISA yielded the most HPV16-IgG positive FVU samples for the ST cohort (37/37, 100 %), while this was the M9ELISA for the MT cohort (109/114, 96 %). GST-L1-MIA detected HPV16-specific antibodies in 32/37 (86 %) and 36/37 (97 %) of vaccinated FVU and serum samples, respectively. For the unvaccinated female volunteers, larger variations in antibody positivity were observed. The DELFIA provided the highest FVU positivity for both cohorts (≥ 19 %), while the M4- or M9ELISA yielded the highest serum positivity (≥ 18 %) for both cohorts (Table 1). GST-L1-MIA detected HPV16-specific IgG in 1/17 (6 %) unvaccinated FVU samples and in 2/17 (12 %) unvaccinated serum samples. Good to excellent correlations between FVU and serum samples were obtained for all assays (rs ≥ 0.76, p < 0.001) (Figs. 2A and 3A). Additionally, the ratio of HPV16-specific antibody concentrations in FVU compared to serum in the ST cohort was 0.03 % for both the M4ELISA and the DELFIA. For the MT cohort, the FVU-serum ratio was 0.06 % for the M9ELISA and 0.05 % for the DELFIA. In the ST cohort, Spearman Rank correlations between the assays ranged from 0.80 to 0.87 for FVU and from 0.89 to 0.95 for serum (Fig. 3A-C). The Spearman rank correlation coefficients between the DELFIA and the M9ELISA for the MT cohort were 0.92 for FVU and 0.97 for serum (Fig. 3B-D).
Fig. 3.
Correlations between assays. Comparison between GST-L1-MIA, M4ELISA and DELFIA HPV16-IgG results for (A) FVU samples and (C) serum samples from the ST cohort. Comparison between M9ELISA and DELFIA HPV16-IgG results for (B) FVU samples and (D) serum samples from the MT cohort. Spearman rank correlation coefficients (95 % CI) are presented in the figure. Black dots represent the vaccinated volunteers, gray dots represent the unvaccinated volunteers. Note differing scales for x- and y-axis in the comparison between GST-L1-MIA and DELFIA/M4ELISA, as assays use different scales for measurement.
For the MT cohort, the antibody concentrations for vaccinated and unvaccinated volunteers were plotted alongside the antibody concentrations at the three different timepoints, using both assays and for both sample types (Fig. 4). The results underscore the usability of both assays in monitoring vaccine induced HPV16 humoral immune response.
Fig. 4.
M9ELISA and DELFIA HPV16-IgG concentrations for unvaccinated and fully 9vHPV vaccinated samples or at all three different timepoints of the MT cohort. Results are presented for (A) FVU and (B) serum samples. The median antibody concentrations are shown in the figure. Wilcoxon Singed Rank test results are reported between median antibody levels in unvaccinated and vaccinated samples. Friedman test results are reported for repeated-measures analysis between all different time points. Pink dots represent the results at 0 M timepoints, dark blue dots at 7 M and light blue dots at 43 M.
3.4. Comparison between Bio-Rad and HTRF total IgG assay
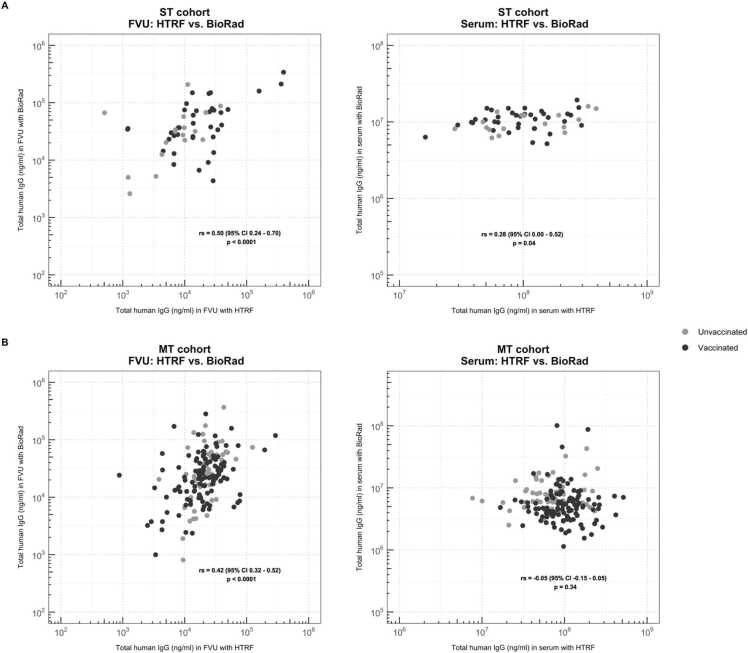
In addition to measuring HPV16-IgG concentrations, we quantified the total human IgG concentrations in all samples. The HTRF results were compared to the Bio-Rad total IgG concentrations for all samples (Fig. 5). In FVU samples, a moderate correlation between total IgG concentrations was observed for both cohorts, being 0.50 (p < 0.0001) and 0.42 (p < 0.0001) for the ST and the MT cohort, respectively. However, for serum samples, only a weak correlation was observed between Bio-Rad and HTRF total IgG concentrations in the ST cohort (rs = 0.28, p = 0.04), and for the MT cohort serum samples, no significant correlation was observed (p = 0.34).
Fig. 5.
Comparison between HTRF and Bio-Rad total human IgG results for (A) all ST cohort samples and (B) all MT cohort samples. Spearman rank correlation coefficients (95 % CI) are presented in the figure. Black dots represent the vaccinated volunteers, gray dots represent the unvaccinated volunteers.
4. Discussion
The use of FVU for the detection of HPV-specific antibodies offers several advantages. Other than being non-invasive, easy-to-collect, and giving the option for at-home self-sampling, this sample is particularly interesting in vaccine- and epidemiological trials where lost-to-follow-up and cost are limiting factors.
Using FVU as a non-invasive sample of the urogenital tract has already provided promising results for follow-up of HPV-related biomarkers (Teblick et al., 2023). Humoral immune responses, specifically HPV-specific antibodies, have demonstrated to be present and detectable in FVU (Pattyn et al., 2020a, Pattyn et al., 2020b, Teblick et al., 2023, Van Keer et al., 2019). As expected, and in line with previous research, antibody concentrations after vaccination were considerably lower in FVU than those in serum, encompassing less than 1 % of HPV16-specific median antibody levels in serum (Pattyn et al., 2020a, Teblick et al., 2023, Van Keer et al., 2019). To be able to adequately define this HPV-specific antibody response in FVU, it is essential to identify assays capable of effectively monitoring these lower-level antibody concentrations.
This study evaluated the performance of a newly developed HPV16 DELFIA assay for the detection and quantification of HPV16-specific antibodies in FVU and serum. A total of 225 paired FVU and serum samples from two different cohorts of female volunteers were tested using the HPV16 DELFIA assay. Results were compared to previously reported M4ELISA and GST-L1-MIA results for the ST cohort (n = 54) or M9ELISA results for the MT cohort (n = 171) (Pattyn et al., 2020a, Teblick et al., 2023, Van Keer et al., 2019).
Very strong correlations between DELFIA and M4ELISA, M9ELISA, or GST-L1-MIA for both sample types (rs > 0.80) were found. In addition, the Spearman rank correlation between FVU and serum for the HPV16 DELFIA was strong (rs = 0.76) to very strong (rs = 0.83) for the ST cohort and the MT cohort, respectively.
Overall, the DELFIA had high sensitivity in both FVU and serum samples, 93 % and 99 %, respectively. The sensitivity was determined using all results from vaccinated female volunteers and based on the vaccination status vs. the observed HPV16-specific antibody concentration. This was done using the formula: true positive/(true positive + false negative). GST-L1-MIA had a sensitivity of 86 % for FVU, while the two VLP-based assays had a sensitivity of 96 % (M9ELISA) and 100 % (M4ELISA). For serum, the sensitivity of the immunoassays ranged from 97 % (GST-L1-MIA) to 100 % (M4ELISA/M9ELISA). Based on these results we can conclude that for detection of vaccine-induced HPV16-specific antibodies present in FVU, DELFIA is more sensitive than GST-L1-MIA (>86 %), and less sensitive than M9ELISA (<96 %) and M4ELISA (<100 %). For vaccine-induced antibodies in serum samples, DELFIA is more sensitive than GST-L1-MIA (>97 %) and less sensitive than both VLP-based ELISAs (<100 %).
For FVU samples, the number of samples with a detectable HPV16-IgG before vaccination was highest using the DELFIA assay in both cohorts. While this might suggest greater sensitivity in identifying naturally induced antibodies, there is only an average agreement with corresponding serum samples or FVU samples testing positive with other immunoassays. In the ST cohort, DELFIA detected HPV16-specific antibodies in 7/17 unvaccinated FVU samples and 6/17 unvaccinated serum samples. Two participants showed exclusive HPV16-IgG positivity in FVU using the DELFIA assay. For the MT cohort, eight samples showed exclusive HPV16-specific antibody positivity with the DELFIA assay (Appendix Table 1). Results reported by Gaudet et al. suggest that humoral immunity in the cervix is comprised of a local and unique spectrum of B-cells (Gaudet et al., 2011). When natural HPV infection occurs, a local immune response is elicited. The local environment could thus have a presence of Ig-expressing B-cells. Those locally produced antibodies might have been detected in the unvaccinated FVU samples by the DELFIA assay. Moreover, a study by Scherer et al. suggests that natural HPV infection elicits a low-avidity and less qualitative serum antibody response than HPV vaccination (Scherer et al., 2016), which might also be the case for locally-produced antibodies after natural infection. Thus, antibodies that are locally-produced after natural infection potentially have a low-avidity, for which DELFIA could prove sufficiently sensitive for detection. These hypotheses may explain the average agreement (rs < 0.52) between sample types in the non-vaccinated cohort. It will be interesting to further explore this in the future.
For serum samples, the overall performance of the DELFIA assay was comparable to that of M4- or M9ELISA, showing average to good agreement between HPV16-IgG positive samples. Notably, the sensitivity for naturally induced antibody detection in serum appeared to be lower when using the GST-L1-MIA. These results showed that the developed DELFIA assay can accurately be used for the detection of HPV16-specific antibodies in both FVU and serum samples.
As mentioned previously, the number of samples with a detectable HPV16-IgG before vaccination was highest using the DELFIA assay in both cohorts for FVU samples. While the limited overlap with corresponding serum samples may indicate presence or detection of locally-produced antibodies after natural infection, it might also be caused by aspecific binding to the DELFIA assay plate. Aspecific binding could be overcome by an extra purification step for the PsV coating antigens, for example by gradient removal, or by adding additional controls to the assay. These steps would benefit the sensitivity as well because lower results would be expected to be detected in negative control wells. Furthermore, there are two FVU and two serum samples that had a concentration near the LLOQ we described for this assay. This non-specific binding seen in the DELFIA assay might be the result of Europium contamination and must be further investigated. In this study, we have used the DELFIA as a singleplex assay. Nevertheless, this technique has the potential to be multiplexed to create a competitive multiplex HPV-specific DELFIA using other lanthanide chelates (Europium, Samarium, Terbium, and Dysprosium). Furthermore, a recent publication by our research group has shown good correlations between HPV16-specific IgG levels and HPV16 neutralizing antibody concentrations in both serum and FVU samples, detected using DELFIA and two orthogonal PBNA readout methods, respectively (Teblick et al., 2024).
The DELFIA principle uses a TRF-based readout from lanthanide fluorescence, while M4/M9ELISA employs electrochemiluminescence technology, and GST-L1-MIA is a bead-based assay that uses suspension array technology (Hagan and Zuchner, 2011, Panicker et al., 2015, Panicker et al., 2021, Waterboer et al., 2005). The performance of the three ELISA assays (DELFIA, M4ELISA, M9ELISA) is expected to be comparable, being direct ELISAs. However, a major benefit to the use of lanthanides is the delay in measurement between excitation and emission, resulting in a reduction in self-quenching (Hagan and Zuchner, 2011). This should make DELFIA suitable for the detection of low-abundance analytes, where for the other assays HPV-type-specific antibodies in FVU were near the limit of the detection (Pattyn et al., 2020a, Van Keer et al., 2019). GST-L1-MIA, which employs the Luminex technology, has an intrinsic problem of direct binding of antibodies from human sera to the beads, resulting in high non-specific background (Waterboer et al., 2006). Furthermore, there is a difference in used antigens between the assays. For DELFIA, plates are coated with pseudovirions (PsV), M4- and M9ELISA use virus-like L1 and L2 particles (VLP), and GST-L1-MIA uses glutathione S-transferase HPV-L1-fusion protein antigens. The difference between PsV and VLP is minor. Both are produced by transfection of Human Embryonic Kidney 293TT cells with plasmid constructs for the desired HPV subtype. PsV, however, incorporate an additional reporter plasmid within its structure. This difference does not affect any of the outcomes of the assay set-ups, as they are based on the binding between the HPV L1 protein and the antibodies present in the tested sample. However, GST-L1-MIA utilizes viral antigens specific for the HPV subtype, bacterially expressed as GST fusion proteins, which do not reflect the HPV capsid in its true three-dimensional conformation. This difference may result in poor or no detection of conformation-dependent neutralizing epitopes by the GST-L1-MIA. In terms of cost and hands-on labour time, the VLP-based assays are comparable to the DELFIA assays, having similar antigen production and assay protocols. The GST-based assay has a lower running cost, as no intact viral particles are used for the antibody detection. Other studies have utilized the DELFIA principle for detection of whole venom-specific and allergenic peptide-specific IgE, sIgG(1) and sIgG(4) or for detection of Norwalk virus-specific IgA and IgG (Van Eeden et al., 2011, Kavanagh et al., 2011). Both papers report the DELFIA as a high-throughput method with a high degree of sensitivity and specificity for detection of the respective antibodies in serum and plasma.
Our DELFIA assay quantifies antibody concentrations in international units, employing the established International Standards for HPV serological methods (Kemp et al., 2022). While these standards have been accessible for HPV16 and HPV18 for over a decade, they are now also available for the remaining 9vHPV vaccine types (HPV6, 11, 31, 33, 45, 52, 58). As we explore potential expansion of this assay into a multiplex format for detecting various HPV vaccine types, we will continue to use these units. The implementation of a uniform unit system among laboratories facilitates the standardization of assays, contributing to the evaluation of antibody levels in both surveillance and vaccine efficacy studies.
This study has some limitations. First, not all assays had a defined LLOQ for both FVU and serum samples. Since the previously used assays were exclusively validated for serum samples, no LLOQ was established for FVU samples. As the matrix of FVU and serum is different, separate LLOQ needs to be defined for each sample type. Where there was no predefined LLOQ, the empirical rule formula was used, which states that 99.7 % of observations fall within the first three standard deviations of the mean. While the use of various cut-off values may impact the results, not using any cut-off values could lead to comparisons with nonspecific outcomes. Although the total sample size is sufficient for this comparative study, the number of samples for the ST cohort comparison is limited. Within this study, we only report on the detection of HPV16-specific antibody responses. Further development of a multiplex platform, detecting additional HPV vaccine-types, of this DELFIA assay is required for HPV vaccine impact or surveillance studies. Moreover, results of the total IgG immunoassays should be interpreted with caution, as no significant correlation between the assays was established. Further studies diving into the possible reasons for these differences must be performed to identify which assay yields the most robust total IgG concentration for both FVU and serum samples. These results also underline why no normalization has been performed in this study. The differences between assays and non-significant correlations between the different total IgG kits should be explained first, for the interpretation of results to be done correctly. Lastly, normalization of the HPV16 specific antibody response quantified in FVU should be further explored. Despite these limitations, we believe that the main outcomes of this study remain unaffected, and a robust assay evaluation and comparison could be made.
In conclusion, we have proven that the HPV16 DELFIA assay is a suitable immunoassay for the detection of HPV16 antibodies. Using this assay, good correlations between HPV16-IgG concentration in FVU and serum were demonstrated. Our developed DELFIA-based assay provided a higher sensitivity for HPV16-IgG detection in vaccinated female volunteers than the GST-L1-MIA and a comparable sensitivity to the M4- and M9ELISA. Interestingly, more HPV16-IgGs were detected before vaccination, potentially elicited by natural infection and produced locally. With this study, we provide data on a novel detection system for HPV-specific antibodies, possibly able to adequately detect low antibody concentrations, as required for the analysis of HPV-related humoral immune response in FVU samples.
CRediT authorship contribution statement
Margo Bell: Writing – review & editing. Anne Van Caesbroeck: Writing – review & editing. Marijana Lipovac: Writing – review & editing, Writing – original draft, Visualization, Methodology, Formal analysis, Data curation. Laura Téblick: Writing – review & editing, Writing – original draft, Visualization, Supervision, Project administration, Methodology, Formal analysis, Data curation, Conceptualization. Peter Delputte: Writing – review & editing, Supervision, Resources. Ilse De Coster: Writing – review & editing, Resources. Annemie De Smet: Writing – review & editing, Methodology, Data curation. Severien Van Keer: Writing – review & editing, Project administration. Wiebren A.A. Tjalma: Writing – review & editing, Resources. Alex Vorsters: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Funding acquisition, Conceptualization.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests. A.V. is a co-founder and former board member of Novosanis (Subsidiary of OraSure Technologies Inc, Wijnegem, Belgium), a spin-off company of the University of Antwerp, and was a minority shareholder until January 2019. The University of Antwerp received grants from Merck, GSK, Hologic, Abbott, Roche, and Cepheid to support the HPV Prevention and Control Board. The University of Antwerp received a project grant and honoraria fee for lectures, presentations, and speaker bureaus from Merck. Other authors declare that they have no conflict of interest.
Acknowledgments
This study was funded by the Industrial Research Fund of the University of Antwerp (IOF-SBO, 44754 and 32387), the Research Foundation Flanders (FWO, Junior postdoctoral fellowship SVK, 1240220 N and Ph.D fellowship MB, 11PJK24N), and the European Union (ERC, URISAMP, 101040588). Views and opinions expressed are, however, those of the author(s) only and do not necessarily reflect those of the European Union or the European Research Council Executive Agency. Neither the European Union nor the granting authority can be held responsible for them. We would like to express our gratitude to Gitika Panicker and Elizabeth R. Unger from the Centers for Disease Control and Prevention (CDC) for testing the samples with the M4ELISA and M9ELISA, as well as Tim Waterboer from the Infections and Cancer Epidemiology Group, German Cancer Research Center (DKFZ) for testing the samples with the GST-L1-MIA. We thank all the volunteers who took part in this study.
Institutional Review Board Statement
The studies were conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of UZA/ University of Antwerp (B300201525585 and B300201525584).
References
- Bober P., Firment P., Sabo J. Diagnostic test accuracy of first-void urine human papillomaviruses for presence cervical HPV in women: systematic review and meta-analysis. Int. J. Environ. Res Public Health. 2021;18 doi: 10.3390/ijerph182413314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pauw H., Donders G., Weyers S., De Sutter P., Doyen J., Tjalma W.A.A., Vanden Broeck D., Peeters E., Van Keer S., Vorsters A., Arbyn M. Cervical cancer screening using HPV tests on self-samples: attitudes and preferences of women participating in the VALHUDES study. Arch. Public Health. 2021;79:155. doi: 10.1186/s13690-021-00667-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcaro M., Castanon A., Ndlela B., Checchi M., Soldan K., Lopez-Bernal J., Elliss-Brookes L., Sasieni P. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. Lancet. 2021;398:2084–2092. doi: 10.1016/S0140-6736(21)02178-4. [DOI] [PubMed] [Google Scholar]
- Ferguson M., Wilkinson D.E., Nardelli-Haefliger D., Sahli R., Eklund C., Hedvall E., Panicker G., Williamson A., Picconi M.A., Ennaifer-Jerbi E., Bharti A.C., Bharadwaj M., Ngamkham J., Sukvirach S., Garland S., Kukimoto I., Kawana K. World Health Organization; 2009. Human Papillomavirus Laboratory Manual; pp. 1–124. [Google Scholar]
- Gaudet R.G., Breden F., Plummer F., Berry J.D. Molecular characterization of the cervical and systemic B-cell repertoire: unique, yet overlapping, immune compartments of an HIV-1 resistant individual. MAbs. 2011;3:181–191. doi: 10.4161/mabs.3.2.14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillison M.L., Chaturvedi A.K., Lowy D.R. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008;113:3036–3046. doi: 10.1002/cncr.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan A.K., Zuchner T. Lanthanide-based time-resolved luminescence immunoassays. Anal. Bioanal. Chem. 2011;400:2847–2864. doi: 10.1007/s00216-011-5047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh O., Estes M.K., Reeck A., Raju R.M., Opekun A.R., Gilger M.A., Graham D.Y., Atmar R.L. Serological responses to experimental Norwalk virus infection measured using a quantitative duplex time-resolved fluorescence immunoassay. Clin. Vaccin. Immunol. 2011;18:1187–1190. doi: 10.1128/CVI.00039-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp T.J., Panicker G., Eklund C., Nie J., Wang Y., Beddows S., Rigsby P., Huang W., Dillner J., Unger E.R., Pinto L.A., Wilkinson D.E. World Health Organization; 2022. Collaborative Study to Evaluate Candidate 1st WHO International Standards for Antibodies to Human Papillomavirus Types 6, 11, 31, 33, 45, 52 and 58; pp. 1–113. [Google Scholar]
- Longet S., Schiller J.T., Bobst M., Jichlinski P., Nardelli-Haefliger D. A murine genital-challenge model is a sensitive measure of protective antibodies against human papillomavirus infection. J. Virol. 2011;85:13253–13259. doi: 10.1128/JVI.06093-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J., Moldoveanu Z., Russell M.W. Immunologic uniqueness of the genital tract: challenge for vaccine development. Am. J. Reprod. Immunol. 2005;53:208–214. doi: 10.1111/j.1600-0897.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- Nardelli-Haefliger D., Wirthner D., Schiller J.T., Lowy D.R., Hildesheim A., Ponci F., De Grandi P. Specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human papillomavirus 16 virus-like particles. J. Natl. Cancer Inst. 2003;95:1128–1137. doi: 10.1093/jnci/djg018. [DOI] [PubMed] [Google Scholar]
- Paavonen J., Naud P., Salmeron J., Wheeler C.M., Chow S.N., Apter D., Kitchener H., Castellsague X., Teixeira J.C., Skinner S.R., Hedrick J., Jaisamrarn U., Limson G., Garland S., Szarewski A., Romanowski B., Aoki F.Y., Schwarz T.F., Poppe W.A., Bosch F.X., Jenkins D., Hardt K., Zahaf T., Descamps D., Struyf F., Lehtinen M., Dubin G., Group H.P.S. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- Panicker G., Rajbhandari I., Gurbaxani B.M., Querec T.D., Unger E.R. Development and evaluation of multiplexed immunoassay for detection of antibodies to HPV vaccine types. J. Immunol. Methods. 2015;417:107–114. doi: 10.1016/j.jim.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicker G., Rajbhandari I., Pathak H.N., Brady A.M., Unger E.R. Multiplex immunoassay to measure antibody response to nine HPV vaccine types. J. Immunol. Methods. 2021;498 doi: 10.1016/j.jim.2021.113136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak N., Dodds J., Zamora J., Khan K. Accuracy of urinary human papillomavirus testing for presence of cervical HPV: systematic review and meta-analysis. BMJ. 2014;349:g5264. doi: 10.1136/bmj.g5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattyn J., Panicker G., Willhauck-Fleckenstein M., Van Keer S., Teblick L., Pieters Z., Tjalma W.A.A., Matheeussen V., Van Damme P., Waterboer T., Unger E.R., Vorsters A. Comparison of a VLP-based and GST-L1-based multiplex immunoassay to detect vaccine-induced HPV-specific antibodies in first-void urine. J. Med. Virol. 2020;92:3774–3783. doi: 10.1002/jmv.25841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattyn J., Van Keer S., Teblick L., Van Damme P., Vorsters A. Non-invasive assessment of vaccine-induced HPV antibodies via first-void urine. Front. Immunol. 2020;11:1657. doi: 10.3389/fimmu.2020.01657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer E.M., Smith R.A., Gallego D.F., Carter J.J., Wipf G.C., Hoyos M., Stern M., Thurston T., Trinklein N.D., Wald A., Galloway D.A. A Single human papillomavirus vaccine dose improves B cell memory in previously infected subjects. EBioMedicine. 2016;10:55–64. doi: 10.1016/j.ebiom.2016.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherpenisse M., Mollers M., Schepp R.M., Meijer C.J., de Melker H.E., Berbers G.A., van der Klis F.R. Detection of systemic and mucosal HPV-specific IgG and IgA antibodies in adolescent girls one and two years after HPV vaccination. Hum. Vaccin Immunother. 2013;9:314–321. doi: 10.4161/hv.22693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley M., Pinto L.A., Trimble C. Human papillomavirus vaccines--immune responses. Vaccine. 2012;30(Suppl 5):F83–F87. doi: 10.1016/j.vaccine.2012.04.106. [DOI] [PubMed] [Google Scholar]
- Struyf F., Colau B., Wheeler C.M., Naud P., Garland S., Quint W., Chow S.N., Salmeron J., Lehtinen M., Del Rosario-Raymundo M.R., Paavonen J., Teixeira J.C., Germar M.J., Peters K., Skinner S.R., Limson G., Castellsague X., Poppe W.A., Ramjattan B., Klein T.D., Schwarz T.F., Chatterjee A., Tjalma W.A., Diaz-Mitoma F., Lewis D.J., Harper D.M., Molijn A., van Doorn L.J., David M.P., Dubin G., Group H.P.S. Post hoc analysis of the PATRICIA randomized trial of the efficacy of human papillomavirus type 16 (HPV-16)/HPV-18 AS04-adjuvanted vaccine against incident and persistent infection with nonvaccine oncogenic HPV types using an alternative multiplex type-specific PCR assay for HPV DNA. Clin. Vaccin. Immunol. 2015;22:235–244. doi: 10.1128/CVI.00457-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teblick L., et al. HPV-specific antibodies in female genital tract secretions captured via first-void urine retain their neutralizing capacity. Hum. Vaccin Immunother. 2024;20(1) doi: 10.1080/21645515.2024.2330168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teblick L., Pattyn J., Van Keer S., De Smet A., De Coster I., Tjalma W.A.A., Rajbhandari I., Panicker G., Unger E.R., Vorsters A. Follow-up of humoral immune response after HPV vaccination using first-void urine: a longitudinal cohort study. J. Med. Virol. 2023;95 doi: 10.1002/jmv.29133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eeden P.E., Wiese M.D., Aulfrey S., Hales B.J., Stone S.F., Brown S.G. Using time-resolved fluorescence to measure serum venom-specific IgE and IgG. PLOS One. 2011;6 doi: 10.1371/journal.pone.0016741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Keer S., Latsuzbaia A., Vanden Broeck D., De Sutter P., Donders G., Doyen J., Tjalma W.A.A., Weyers S., Arbyn M., Vorsters A. Analytical and clinical performance of extended HPV genotyping with BD Onclarity HPV Assay in home-collected first-void urine: a diagnostic test accuracy study. J. Clin. Virol. 2022;155 doi: 10.1016/j.jcv.2022.105271. [DOI] [PubMed] [Google Scholar]
- Van Keer S., Peeters E., Vanden Broeck D., De Sutter P., Donders G., Doyen J., Tjalma W.A.A., Weyers S., Vorsters A., Arbyn M. Clinical and analytical evaluation of the realtime high risk HPV assay in Colli-Pee collected first-void urine using the VALHUDES protocol. Gynecol. Oncol. 2021;162:575–583. doi: 10.1016/j.ygyno.2021.06.010. [DOI] [PubMed] [Google Scholar]
- Van Keer S., Willhauck-Fleckenstein M., Pattyn J., Butt J., Tjalma W.A.A., Van Ostade X., Hens N., Van Damme P., Waterboer T., Vorsters A. First-void urine as a non-invasive liquid biopsy source to detect vaccine-induced human papillomavirus antibodies originating from cervicovaginal secretions. J. Clin. Virol. 2019;117:11–18. doi: 10.1016/j.jcv.2019.05.004. [DOI] [PubMed] [Google Scholar]
- Villa L.L., Costa R.L., Petta C.A., Andrade R.P., Paavonen J., Iversen O.E., Olsson S.E., Hoye J., Steinwall M., Riis-Johannessen G., Andersson-Ellstrom A., Elfgren K., Krogh G., Lehtinen M., Malm C., Tamms G.M., Giacoletti K., Lupinacci L., Railkar R., Taddeo F.J., Bryan J., Esser M.T., Sings H.L., Saah A.J., Barr E. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br. J. Cancer. 2006;95:1459–1466. doi: 10.1038/sj.bjc.6603469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorsters A., Van Damme P., Clifford G. Urine testing for HPV: rationale for using first void. BMJ. 2014;349:g6252. doi: 10.1136/bmj.g6252. [DOI] [PubMed] [Google Scholar]
- Walboomers J.M., Jacobs M.V., Manos M.M., Bosch F.X., Kummer J.A., Shah K.V., Snijders P.J., Peto J., Meijer C.J., Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Waterboer T., Sehr P., Pawlita M. Suppression of non-specific binding in serological Luminex assays. J. Immunol. Methods. 2006;309:200–204. doi: 10.1016/j.jim.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Waterboer T., Sehr P., Michael K.M., Franceschi S., Nieland J.D., Joos T.O., Templin M.F., Pawlita M. Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin. Chem. 2005;51:1845–1853. doi: 10.1373/clinchem.2005.052381. [DOI] [PubMed] [Google Scholar]
- Zhou J.Z., Way S.S., Chen K. Immunology of the uterine and vaginal mucosae. Trends Immunol. 2018;39:302–314. doi: 10.1016/j.it.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]