Abstract
Activation of the extracellular signal-regulated kinase (ERK) 1/2 cascade by polypeptide growth factors is tightly coupled to adhesion to extracellular matrix in nontransformed cells. Raf-1, the initial kinase in this cascade, is intricately regulated by phosphorylation, localization, and molecular interactions. We investigated the complex interactions between Raf-1, protein kinase A (PKA), and p21-activated kinase (PAK) to determine their roles in the adhesion dependence of signaling from epidermal growth factor (EGF) to ERK. We conclude that Raf-1 phosphorylation on serine 338 (S338) is a critical step that is inhibited in suspended cells. Restoration of phosphorylation at S338, either by expression of highly active PAK or by expression of an S338 phospho-mimetic Raf-1 mutation, led to a partial rescue of ERK activation in suspended cells. Raf-1 inhibition in suspension was not due to excessive negative regulation on inhibitory sites S43 and S259, as these serines were largely dephosphorylated in suspended cells. Finally, strong phosphorylation of Raf-1 S338 provided resistance to PKA-mediated inhibition of ERK activation. Phosphorylation at Raf-1 S43 and S259 by PKA only weakly inhibited EGF activation of Raf-1 and ERK when cells maintained high Raf-1 S338 phosphorylation.
Cell adhesion through integrins to extracellular matrix (ECM) and stimulation by soluble mitogens cooperate in coordinating numerous cellular events, including migration, survival, and proliferation. Integrin engagement results in the recruitment of structural proteins, including talin, vinculin, and paxillin, to focal complexes, specialized adhesive structures that can signal through activation of kinases, such as FAK and Src (1). Entry into the cell cycle normally requires both adhesion to an appropriate ECM, with concurrent organization of the cytoskeleton, and stimulation by growth factors (6). In adherent cells, polypeptide growth factors, such as epidermal growth factor (EGF), activate receptor tyrosine kinases that lead to GTP loading of Ras and activation of the Raf-1/MEK/ERK kinase cascade. Activated extracellular signal-regulated kinase (ERK) can translocate to the nucleus, phosphorylate transcription factors, and induce growth-regulatory genes such as cyclin D1 (29). Loss of adhesion or breakdown of the cytoskeleton disrupts focal complexes and blocks ERK activation (2) and cell proliferation in nontransformed cells (58). Conversely, the uncoupling of growth factor signaling from cell anchorage is a hallmark of malignant transformation (37).
Loss of integrin engagement can influence ERK activation at several points within the signaling cascade. Growth factor receptors have been shown to associate in complexes with integrins in an extracellular matrix-dependent fashion (47), which may concentrate receptors at sites on the plasma membrane to enhance receptor activation (42). Cell detachment from the substratum leads to activation of phosphatases (38) that inhibit ERK activation directly. The organization of the cytoskeleton can also influence kinases, such as protein kinase C (PKC), Src, or p21-activated kinase (PAK) (34), which facilitate Raf-1 or MEK activation. Finally, even with forced activation of ERK in suspended cells, loss of cytoskeletal integrity inhibits ERK translocation to the nucleus (3).
In NIH 3T3 fibroblasts, Ras becomes equally GTP loaded in adherent or suspended cells treated with growth factors, indicating that the signaling pathway is intact to this point (41, 45). We have found that activation of Raf-1 is a key adhesion-dependent step downstream of Ras (41), although others have suggested that abrogation of growth factor signaling in nonadherent cells primarily involves MEK (45). Raf-1 is regulated by functional interactions with many proteins, including kinases (Src, PAK, PKA, PKC, and Akt), phosphatases (PP1 and PP2A), and scaffolding proteins (14-3-3, Hsp90, KSR, and RKIP) (22). Unlike many kinases in which simple phosphorylation of a catalytic loop leads to activation, Raf-1 contains many phospho-regulatory sites, including serines (43, 233, 259, 338, 339, 491, and 621), threonine (494), and tyrosines (340 and 341). The PAKs (10, 49, 51) as well as a rho-dependent kinase (39) seem to play an important role in anchorage-dependent regulation in the ERK cascade. PAK3 phosphorylates Raf-1 on serine 338 (S338), a step that is required for efficient Raf-1 and ERK activation (10). Mutation of Raf-1 S338 to alanine results in a nonactivatable kinase (10, 13). Phosphorylations at this site are not activating for Raf-1 but are thought to relieve an autoinhibitory state to permit activation (17, 51). Importantly, the PAK family kinases are poorly activated in suspended cells due to lack of interaction between PAK and GTP-loaded Rac or cdc42 (19, 20) and because of inhibition by PKA (34).
PKA is a promiscuous kinase also involved in adhesion-dependent signaling to ERK. PKA is transiently activated upon detachment from the substratum (34) and can phosphorylate and inhibit both PAK (34) and Raf-1 (25, 30). PKA activation can have many effects throughout the cell that may impinge on Raf-1 signaling, including cytoskeletal disruption and phosphatase activation. PKA can also directly phosphorylate Raf-1 on at least two critical sites. Phosphorylation of Raf-1 S43 inhibits the Ras-Raf-1 interaction, which is crucial for Raf-1 translocation and activation (4). Phosphorylation of Raf-1 S233 or S259 is thought to inhibit Raf-1 by enhancing 14-3-3 binding to these sites and restricting Raf-1 intra- or intermolecular interactions (26).
The goal of this study was to investigate how cells translate physical adhesion to ECM into critical biochemical events that allow for efficient signaling within the cell. We examined the complex interactions between Raf-1, PKA, and PAK to determine their roles in the adhesion dependence of signaling from EGF to ERK in NIH 3T3 fibroblasts and HEK293 cells. We conclude that the adhesion-dependent regulation of ERK in EGF-treated cells occurs at the level of Raf-1. Unlike adherent cells, suspended cells do not phosphorylate Raf-1 S338 efficiently upon EGF treatment. Raf-1 from suspended cells is generally dephosphorylated on S43 and S259, and so it seems that it is loss of the permissive S338 phosphorylation, rather than excessive inhibitory phosphorylation, that is critical. Restoration of phosphorylation at S338, either by expression of a highly active version of PAK1 (PAK165) or by expression of a phospho-mimetic mutation (Raf-1 S338D) leads to a partial rescue of ERK activation in suspended cells.
PKA activation also plays a critical role in suspended cells. Inhibition of PKA during detachment from the substratum leads to rescue of Raf-1 S338 phosphorylation and partial ERK activation. In adherent cells, PKA activation by forskolin induces Raf-1 phosphorylation on S43 and S259 and inhibits phosphorylation of Raf-1 on S338 and subsequent ERK activation. However, ERK activation can be observed in cells treated with forskolin, provided that S338 is forced to be highly phosphorylated. ERK activation in these cells persists despite phosphorylation on the inhibitory S43 and S259 sites. These observations reveal important insights into how cell adhesion influences positive and negative regulatory phosphorylations in Raf-1 activation with subsequent consequences for signaling in the ERK/MAP kinase pathway.
MATERIALS AND METHODS
Materials.
Forskolin, H89 (Biomol, Plymouth Meeting, PA), and isobutyl-methyl-xanthene (IBMX; Sigma, St. Louis, MO) were maintained as stock solutions in dimethyl sulfoxide (DMSO) at −20°C. Antibodies to Flag (M2; Sigma), hemagglutinin (HA; Covance), ERK (sc-31; Santa Cruz Biotechnology, Santa Cruz, CA), β-14-3-3 (sc-629; Santa Cruz Biotechnology), pERK (Sigma), pS338Raf-1 (05538; Upstate Biotechnology Inc. [UBI], Lake Placid, NY), pS259Raf-1 (Cell Signaling, Beverly, MA), Raf-1 (sc-133; Santa Cruz Biotechnology), CREB and pCREB-133 (Cell Signaling), pS43 Raf-1 (Biosource, Camarillo, CA), pY340/341 (Sigma), 4G10 (UBI), and B-Raf (UBI) were obtained. Trypsin 0.25%-0.5 mM EDTA and soybean trypsin inhibitor (Gibco), bovine serum albumin (Sigma), β-estradiol (Sigma), fibronectin (Fn; BD Biosciences, Bedford, MA), EGF (UBI), myelin basic protein (MBP; UBI), ATP (Cell Signaling), [γ-32P]ATP (New England Nuclear, Boston, MA), and phosphate-buffered saline (PBS) were also used.
Cell culture.
NIH 3T3 cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Sigma) supplemented to 10% newborn calf serum (Sigma) at 37°C and 5% CO2. HEK293 cells were maintained in DMEM supplemented to 10% fetal calf serum (Sigma) at 37°C and 5% CO2.
Adhesion-dependent signaling assay.
Cells were transfected using Superfect (QIAGEN) and were recovered for 12 h. Flag-Raf-1 constructs were transfected at minimum amounts needed to detect effects on signaling: 30% of total transfected DNA for NIH 3T3 cells and 10% of total transfected DNA for HEK293 cells. Cells were grown to near confluence before serum starvation for 12 h in serum-free DMEM. Cells were detached with trypsin-EDTA, blocked with 1 mg/ml soybean trypsin inhibitor, centrifuged at 300 × g, and resuspended in DMEM containing 1% bovine serum albumin. Cells were rocked at 37°C for 30 min, replated to wells coated with 10 μg/ml Fn, or kept in suspension on a rocker at 37°C. After 2 h of plating or rocking, cells were treated with 10 ng/ml EGF for 5 min and then quenched with ice-cold PBS. Cells were centrifuged and washed twice with cold PBS before lysis in modified RIPA buffer (50 mM Tris [pH 7.4], 1% NP-40, 0.5% deoxycholic acid, 2 mM EDTA, 50 mM NaF) supplemented with 500 kallikrein inhibitor units/ml aprotinin, 0.5 mM AEBSF [4-(2-aminoethyl)benzenesulfonyl fluoride], 1 mM 4-nitrophenyl phosphate, 1 mM sodium vanadate, 5 mM benzamidine, and 0.2 μM calyculin A. Lysates were clarified by centrifugation at 14,000 × g, and the supernatant (whole-cell lysate) was used for immunoprecipitation or sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) separation.
Kinase assays.
HA-tagged ERK and Flag-tagged Raf-1 were immunoprecipitated with antibodies and Fast-Flow G-Sepharose beads (Amersham, Uppsala, Sweden). HA-ERK activity was assayed with [32P]ATP and MBP as previously described (20). Flag-Raf-1 activity was assessed using the Raf-1-coupled kinase reaction kit following the manufacturer's protocols (UBI). Incorporation of [32P]ATP into MBP was quantified after SDS-PAGE separation of proteins, exposure of dried gels to phosphorimager cassettes, and analysis on a Storm 840 PhosphorImager (Becton-Dickinson). Kinase activity was adjusted for kinase loading based on densitometric analysis of Western blots (Fluor-S MultiImager; Bio-Rad, Philadelphia, PA). Measurements for adherent cells treated with EGF were set to 100% signal, and other lanes were recalculated accordingly. Graphs with error bars represent means and standard errors of multiple assays.
Constructs.
Flag-tagged Raf-1 constructs (wild type [wt], S338D, and S338A) were generously provided by K. L. Guan (13). Flag-Raf-1CAAX and Flag-Raf-1CAAX338A were obtained from P. Stork (8). PAK165, a highly active N-terminal truncation of Rat PAK1 consisting of residues 165 to 544 (lacking the autoinhibitory and CRIB domains) was obtained from Melanie Cobb. The parent vector pCMVm5 was used as a negative control vector. PKI (8-22) is a small peptide inhibitor of PKA; PKI mut2, a double point mutant of PKI that does not inhibit PKA activation, was used as a negative vector control (34). Raf-1CAAX (from Channing Der), GFP-Raf-1:ER (from Julian Downward), and HA-tagged ERK (from Jacques Pouyssegur) were also used. GFPERK2 (from Kathryn DeFea) was used as a marker for ERK activation in transfected cells and is readily distinguishable from ERK1/2 based on size (65 kDa). Raf-1 small inhibitory RNA (siRNA) was obtained from Ambion (catalog number 51197; Huntingdon, United Kingdom). B-Raf siRNA (AGAAUUGGAUCUGGAUCAU) (33, 53) and our control siRNA (MDR1; GUAUUGACAGCUAUUCGAA) were produced by Dharmacon (Lafayette, CO).
RESULTS
EGF activation of Raf-1 and ERK are tightly coupled to adhesion.
A standard assay was used to examine the factors critical for adhesion-dependent ERK activation downstream of EGF. Both HEK293 cells and NIH 3T3 fibroblasts were serum starved, detached with trypsin, washed, and placed into suspension for 30 min. Cells were then either maintained in suspension or adhered to plates coated with 10 μg/ml fibronectin for 2 h before a 5-min treatment with 10 ng/ml EGF. Cell lysates were subsequently analyzed for phosphorylation events and kinase activity.
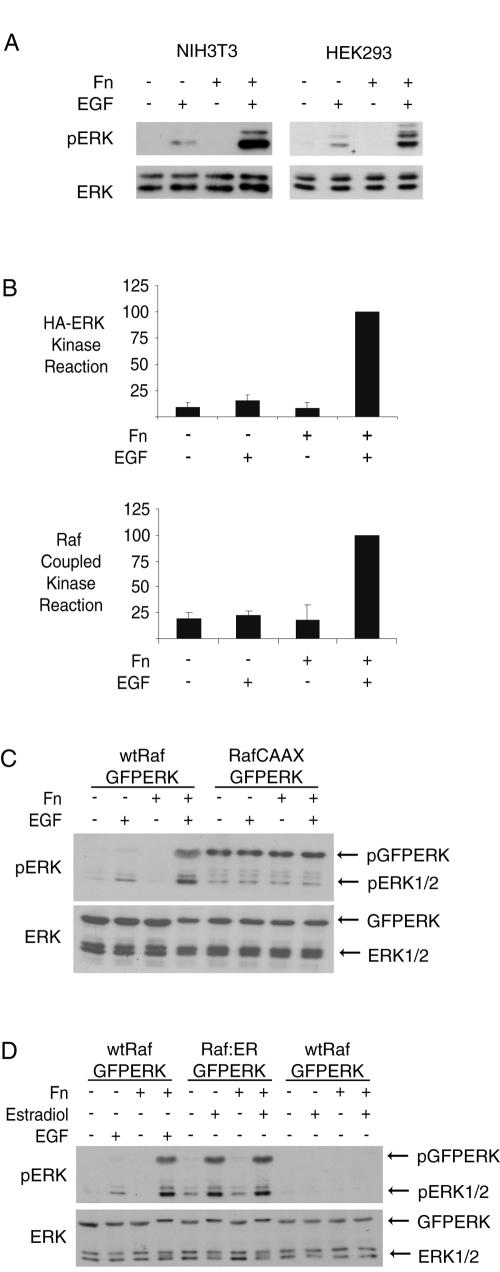
Our laboratory (41) and others (28, 45) have shown that Ras GTP loading in this setting is at least as high in suspended cells as in adherent cells. Thus, we focused on the downstream elements of the ERK signaling cascade. In both HEK293 cells and NIH 3T3 fibroblasts, cells held in suspension showed little ERK activation loop phosphorylation in response to EGF treatment compared to those readhered to fibronectin (Fig. 1A). The lack of ERK phosphorylation in suspension correlated with lack of activation, as quantified by an in vitro ERK kinase assay using MBP (Fig. 1B). To quantify Raf-1 activation, we immunoprecipitated Raf-1 from NIH 3T3 fibroblasts and used a Raf-1-coupled kinase reaction. Figure 1B shows that Raf-1 activation was impaired in nonadherent cells. Suspension did not appear to inhibit signaling downstream of Raf-1, as RafCAAX expression in HEK293 cells led to phosphorylation of coexpressed GFPERK that was independent of both EGF and adhesion to Fn (Fig. 1C). Similarly, the Raf-1-estrogen receptor fusion protein (Raf-1:ER) induced similar phosphorylation of GFPERK after estradiol treatment in both adherent and suspended cells (Fig. 1D). These observations indicate that Raf-1 is a key locus for anchorage regulation of signaling in this context.
FIG. 1.
Adhesion dependence of ERK activation by EGF is regulated at the level of Raf-1. (A) ERK phosphorylation by Western blotting. NIH 3T3 fibroblasts or HEK293 cells were serum starved and plated to fibronectin-coated plates (Fn+) or rocked in suspension(Fn−) for 2 h before treatment with (+) or without (−) 10 ng/ml EGF for 5 min. Whole-cell lysates were resolved by SDS-PAGE and were probed (Western blotted) with antibodies to pERK and ERK. (B) ERK and Raf kinase assays. HA-ERK and Flag-Raf-1 activation was determined from EGF-treated NIH 3T3 cells under adherent and suspended conditions. Immunoprecipitates were quantified for activity using radioactive kinase assays (see Materials and Methods). Adherent, EGF-treated cell activity was set to 100, and other conditions were normalized. Means and standard deviations of three independent experiments are shown. (C and D) Effects of constitutively activated Raf on signaling to ERK. (C) HEK293 cells were cotransfected with GFPERK and Raf-1 wt (500 ng) or Raf-1CAAX (10 ng) and analyzed for adhesion dependence of GFPERK phosphorylation by probing with antibodies to pERK and ERK. (D) HEK293 cells were cotransfected with GFPERK and Raf-1 wt or estradiol-inducible Raf-1:ER. Suspended and adherent cells were treated with or without 10 ng/ml EGF for 5 min or 100 nM β-estradiol for 10 min as indicated and analyzed for adhesion dependence of GFPERK phosphorylation by Western blotting with antibodies to pERK and ERK.
Raf-1 S338 phosphorylation is lost in suspension, but its restoration rescues signaling to ERK.
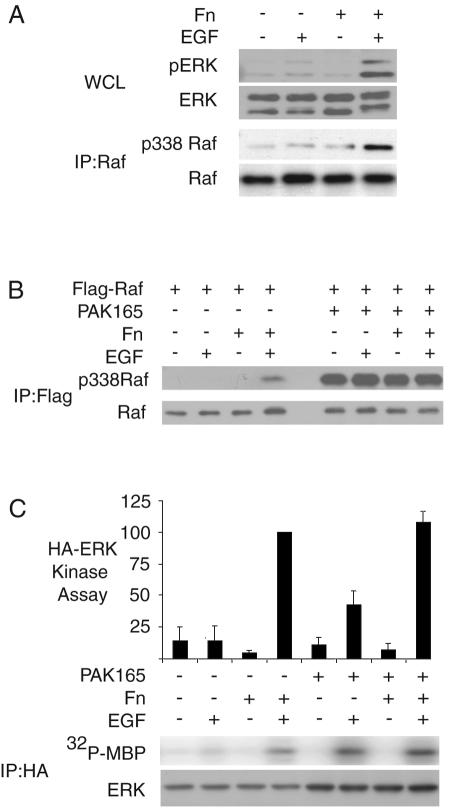
In adherent NIH 3T3 cells in serum-free medium, Raf-1 phosphorylation on S338 was at a low level but could be stimulated by EGF treatment. By contrast, EGF failed to cause phosphorylation of Raf-1 S338 in cells in suspension (Fig. 2A).
FIG. 2.
Raf-1 S338 phosphorylation is a critical adhesion-dependent step in ERK activation. (A) Cell adhesion affects ERK and Raf 338 phosphorylation. Suspended and adherent NIH 3T3 fibroblasts were treated with EGF. Whole-cell lysates were probed for pERK and ERK, and Raf-1 immunoprecipitates were probed for Raf-1 and pS338 Raf-1. (B) Effect of activated PAK on Raf 338 phosphorylation. NIH 3T3 fibroblasts expressing Flag-Raf-1 and vector or constitutively active PAK1 (PAK165) were starved and treated as in panel A. Flag immunoprecipitates were probed with Raf-1 and pS338 antibodies. (C) Effect of activated PAK on ERK kinase activity. NIH 3T3 fibroblasts expressing HA-ERK and vector or PAK165 were assayed for adhesion-dependent HA-ERK activation. A representative blot of immunoprecipitated HA-ERK and an autoradiograph for 32P incorporation into MBP are shown. Bars represent means and standard errors of three experiments.
We could restore Raf-1 S338 phosphorylation in suspended cells through expression of activated PAK (Fig. 2B). PAK165 is an N-terminal truncation of PAK1 that lacks the autoinhibitory and p21 binding domains and is highly active. PAK165 caused a high level of Raf-1 S338 phosphorylation compared to EGF stimulation, and this was not affected by placing cells in suspension. Phosphorylation of S338 was not activating for Raf-1 by itself but led to a partial rescue of EGF-stimulated ERK signaling in suspended cells (Fig. 2C).
The amount of Raf-1 S338 phosphorylation in adherent, EGF-treated NIH 3T3 cells was quite low compared to that which was maximally possible (as in PAK165-treated cells) (Fig. 2B). Thus, it appears that only a small fraction of Raf-1 needs to become S338 phosphorylated and activated in adherent cells in order to permit efficient signaling to ERK. By contrast, in order to partially rescue signaling to ERK in suspended cells, more extensive phosphorylation of S338 by PAK 165 is required.
Raf-1 S338D partially rescues signaling to ERK in suspended cells.
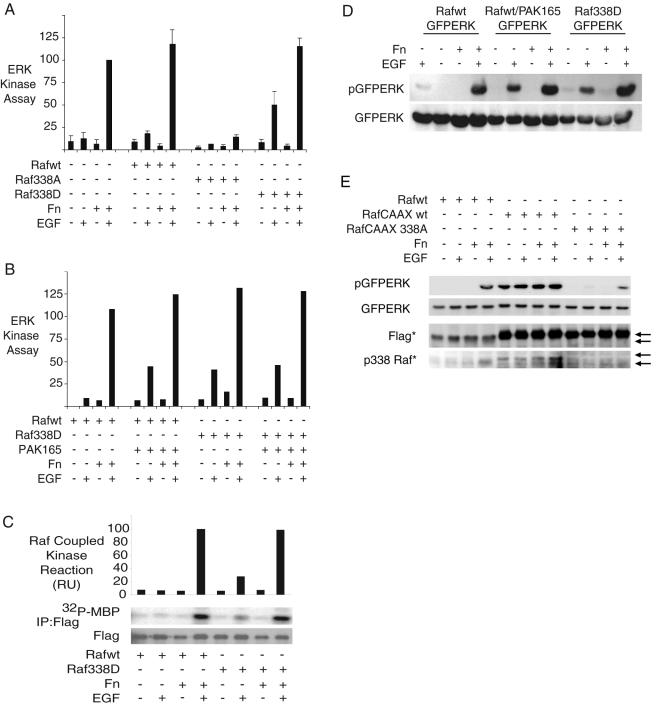
PAK165 may phosphorylate many proteins within a cell, including MEK1 (27), which is directly downstream of Raf-1 in this pathway. PAK phosphorylation of MEK1 is required for adhesion-induced rather than growth factor-dependent ERK activation (49). However, we wanted to more definitively determine the role of S338 in adhesion-dependent signaling through the use of a phospho-mimetic Raf-1 mutant, S338D. We quantitated HA-ERK activation in NIH 3T3 cells coexpressing vector, Raf-1 wt, Raf-1 S338D, or Raf-1 S338A. As seen in Fig. 3A, Raf-1 S338D but not wt Raf-1 caused partial rescue of ERK activation in suspended cells. By contrast, Raf-1 338A ablated signaling in both adherent and suspended cells, reinforcing the observations of others (10, 13) that S338 is critical for Raf-1 activation. The Raf-1 S338D-mediated rescue in suspension cells was nearly identical in strength to that seen with PAK165 (Fig. 3B). Furthermore, it appears that PAK165 works primarily through Raf-1 S338 phosphorylation, as PAK165 and Raf-1 S338D showed no cooperation to further enhance ERK activation in suspended cells. Rescue of ERK activation appears to be at the level of Raf-1, as Raf-1 S338D shows a parallel partial activation in suspension after EGF treatment (Fig. 3C). HEK293 cells behave similarly to NIH 3T3 fibroblasts in terms of adhesion-dependent ERK activation. Thus, expression of PAK165 or Raf-1 S338D also partially rescued ERK activation in suspension in HEK293 cells (Fig. 3D).
FIG. 3.
Raf-1 S338 phosphorylation and phospho-mimetic 338D substitution yield partial rescue of Raf and ERK activation in suspension. (A) Effect of Raf-1 mutants on signaling in suspension. Adhesion dependence of HA-ERK activation by EGF was quantified in NIH 3T3 cells coexpressing vector, Flag-Raf-1 wt, Flag-Raf-1 338A, or Flag-Raf-1 338D. Cells were serum starved and maintained in suspension (Fn−) or adhered (Fn+) for 2 h before 5-min EGF treatment (10 ng/ml) (means and standard errors of at least three experiments). (B) Effects of PAK 165 on signaling in suspension. Adhesion dependence of HA-ERK activation by EGF was quantified in NIH 3T3 cells coexpressing Flag-Raf-1 wt or Flag-Raf-1 338D, with or without PAK165. (C) Raf activity in suspension. Adhesion dependence of Flag-Raf-1 wt and Flag-Raf-1 338D activation by EGF was quantified in NIH 3T3 cells. Raf activation was determined by Raf-coupled kinase reaction of Flag immunoprecipitates. (D) Effects of PAK165 and Raf mutants in HEK293 cells. HEK293 cells expressing GFPERK were cotransfected with Flag-Raf-1 wt, Flag-Raf-1 wt and PAK165, or Flag-Raf-1 338D. Whole-cell lysates were probed with pERK and ERK antibodies. (E) Relative roles of membrane localization and S338 phosphorylation in Raf signaling. HEK293 cells were transfected with GFPERK and Flag-Raf-1 wt, Flag-Raf-1CAAX, or Flag-Raf-1CAAX 338A. Cells were serum starved, and adhesion dependence of GFPERK activation was assessed as previously described. Whole-cell lysates were probed for pERK, ERK, Flag, and pS338 Raf. Note: As indicated with asterisks, Flag-Raf CAAX contains tandem Flag tags and therefore runs slightly higher on a gel and is detected more readily than the singly tagged Flag-Raf-1 wt.
In summary, Raf-1 S338 phosphorylation is not activating by itself, but is a required step for EGF-mediated Raf-1 and subsequent ERK activation that is lost when cells are placed in suspension. In adhered cells a modest level of S338-phosphorylated Raf-1 is sufficient to permit full activation of ERK; this is not significantly enhanced by increasing 338 phosphorylation by PAK165 or by use of the phospho-mimetic 338D mutant (Fig. 2B and 3B). This suggests that the relatively small pool of phosphorylated Raf-1 is efficiently utilized and activated in attached cells. In contrast, restoration of Raf-1 S338 phosphorylation by PAK165 (or use of the phospho-mimetic mutant Raf-1 S338D) allows for partial rescue of ERK activation in suspended cells. However, a larger fraction of the Raf-1 pool needs to be phosphorylated, suggesting that other adhesion-dependent functions influence the efficient activation of Raf-1.
Raf phosphorylation at S338 is critical for membrane-localized Raf.
We wished to explore the relative contributions of membrane localization and S338 phosphorylation in Raf-1 activation and downstream signal transduction. We found that Raf-1 S338 phosphorylation is important even for membrane-localized Raf-1. Flag-tagged versions of Raf-1, Raf-1CAAX, and Raf-1CAAX S338A were transfected into HEK293 cells and assayed for adhesion and EGF dependence of ERK activation (Fig. 3E). Flag-Raf-1 wt-expressing cells showed normal adhesion and EGF dependence of ERK activation. Activation of ERK in Raf-1CAAX-expressing cells was independent of both adhesion and EGF, while expression of Raf-1CAAX S338A inhibited ERK activation. Interestingly, Raf-1CAAX maintained elevated S338 phosphorylation in suspension. These results are similar to published observations that showed that both membrane localization (to specific membrane compartments) and S338 phosphorylation are important for Raf-1 activation (8). Thus, Raf-1 S338 phosphorylation is induced upon membrane localization but is also a separate step required for Raf-1 activation.
Inhibition of PKA in suspended cells rescues ERK signaling through a Raf-1 S338-dependent mechanism.
Activation of PKA has long been known to inhibit growth factor activation of ERK in fibroblasts (7, 22). PKA is transiently activated upon detachment or changes in cell shape (34). PKA activation leads to alterations in the cytoskeleton, loss of tyrosine phosphorylation on crucial structural proteins such as FAK and paxillin, and loss of PAK activation (34), as well as direct phosphorylation and inhibition of Raf-1 (26). As shown previously, expression of a PKA inhibitor peptide (PKI) permits activation of ERK by EGF in suspension cells (34).
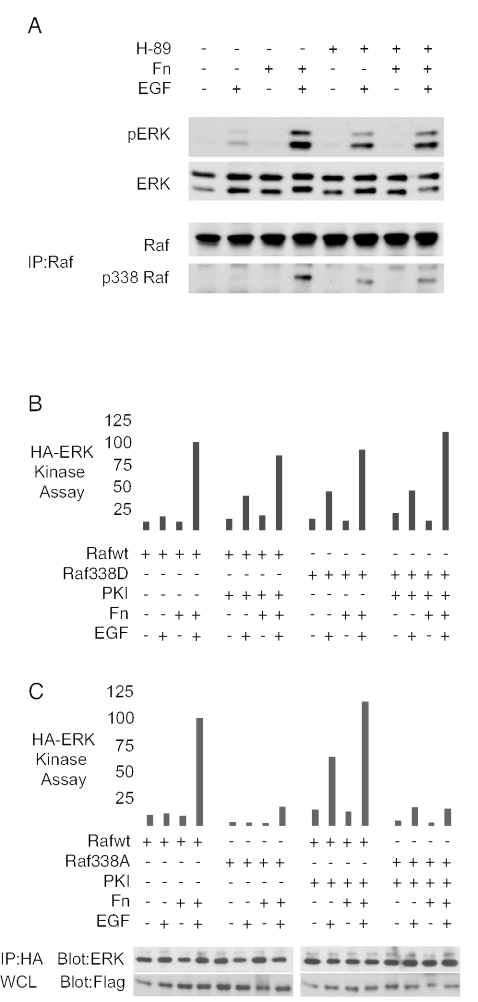
PKA could inhibit Raf-1 either through PAK inhibition or through direct inhibitory phosphorylation of Raf-1. In the latter case, blockade of PKA's direct effects on Raf-1 might supplement the only partial rescue of ERK activation in suspended cells by PAK165 or Raf-1 S338D. Alternatively, if PKA acted through PAK, then the effects of inhibition of PKA should parallel those caused by activation of PAK. Figure 4A shows that treatment of NIH 3T3 fibroblasts with the PKA inhibitor H-89 led to partial rescue of EGF-mediated S338 phosphorylation of endogenous Raf-1 as well as a partial rescue of ERK activation in suspended cells. Similarly, cells expressing PKI, an inhibitor of PKA, also showed partial rescue of signaling to ERK in suspension (Fig. 4B); as well, PKI expression partially rescued S338 phosphorylation in suspension (data not shown). Importantly, cells coexpressing both PKI and Raf-1 338D showed no cooperation or enhancement in terms of rescue of ERK activation in suspension (Fig. 4B). Finally, PKI failed to rescue ERK activation in Raf-1 S338A-expressing cells (Fig. 4C). These data suggest that PKA inhibition rescues ERK activation in suspended cells primarily through a mechanism that involves Raf-1 S338 phosphorylation.
FIG. 4.
Inhibition of PKA leads to partial rescue of ERK activation and Raf-1 S338 phosphorylation in suspension. (A) Effect of the PKA inhibitor H89. NIH 3T3 fibroblasts were pretreated for 1 h with DMSO (−) or H89 (10 μM) and maintained in suspension (Fn−) or adhered (Fn+) for 2 h before a 5-min treatment with 10 ng/ml EGF. Whole-cell lysates were probed for pERK and ERK; endogenous Raf immunoprecipitates were blotted for Raf and pS338. (B) Effect of PKI on ERK activity. Adhesion dependence of HA-ERK activation by EGF was quantified in NIH 3T3 cells coexpressing either Flag-Raf-1 wt or Flag-Raf-1 338D with a plasmid encoding the PKA inhibitor peptide PKI (+) or a control, double point mutation of PKI mut2 (−) peptide (means and standard errors of three experiments). (C) Lack of effect of PKI on Raf 338A. Similar adhesion dependence of HA-ERK activation by EGF was quantified in NIH 3T3 cells coexpressing either Flag-Raf-1 wt or Flag-Raf-1 338A with a plasmid encoding the PKA inhibitor peptide PKI (+) or the PKI mut2 (−) peptide.
PKA blocks ERK activation through inhibition of Raf-1 S338 phosphorylation.
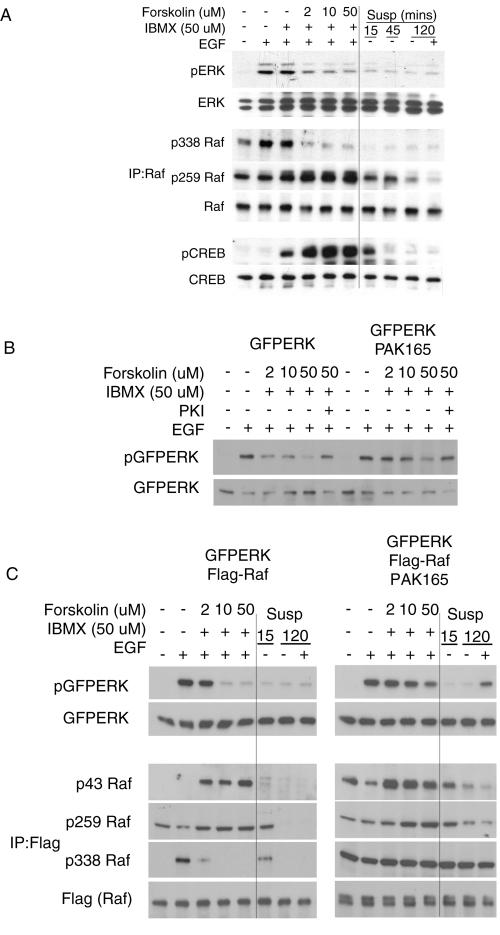
PKA is activated in cells upon a rise in cyclic AMP, which can be produced by treatment with the phosphodiesterase inhibitor IBMX and/or the adenylyl cyclase agonist forskolin, as well as by cell detachment. In adherent NIH 3T3 fibroblasts, IBMX treatment alone caused a moderate increase in phosphorylation of the PKA target CREB but had little effect on EGF-dependent Raf-1 S338 phosphorylation or ERK activation. Forskolin-IBMX treatment inhibited ERK activation by EGF at 2 μM forskolin and to a greater degree at 50 μM forskolin (Fig. 5A); this correlated well with CREB phosphorylation. Examination of Raf-1 phosphorylation showed that S338 phosphorylation was sensitive to Fsk-IBMX treatment and closely correlated with ERK activation. In addition, Raf-1 S259 phosphorylation was elevated in adherent cells treated with increasing forskolin-IBMX concentrations. In contrast, in suspended cells, despite transient activation of PKA (as judged by CREB phosphorylation), Raf-1 S259 phosphorylation was not increased but rather diminished over time in suspension. The reason for this is unclear but may relate to the activation of phosphatases in nonadherent cells (38). In addition, the amount of 14-3-3 protein associated with Raf-1 remains intact in suspended cells despite the low phosphorylation of S259 and S43 (see Fig. S1 in the supplemental material). Thus, loss of ERK activity in suspended cells seems more closely correlated with loss of the permissive S338 phosphorylation rather than increased inhibitory phosphorylation at S259.
FIG. 5.
Loss of ERK activation parallels Raf-1 S338 phosphorylation in cells treated with forskolin and IBMX. (A) Effects of forskolin or cell suspension on phosphorylation of ERK, Raf-1, and CREB. Adherent NIH 3T3 fibroblasts were pretreated for 30 min with 100 μM IBMX and 0, 2, 10, or 50 μM forskolin and compared to cells placed in suspension for 15, 45, or 120 min. Cells were treated with 10 ng/ml EGF for 5 min as indicated. Whole-cell lysates were probed for ERK phosphorylation, and PKA activation was assessed with phospho-specific CREB antibodies. Raf-1 immunoprecipitates were assessed for pS338, pS259, and Raf-1. (B) Active PAK antagonizes the inhibitory effect of forskolin on ERK. NIH 3T3 fibroblasts expressing GFPERK, PAK165, and PKI as indicated were pretreated with 100 μM IBMX and 2, 10, or 50 μM forskolin for 30 min before EGF treatment. Whole-cell lysates were probed for pERK and ERK. (C) Effects of forskolin, active PAK, and cell suspension on phosphorylation of ERK and Raf-1. HEK293 cells were cotransfected with GFPERK, Flag-Raf-1 wt, and PAK165 as indicated. Adherent cells were pretreated for 30 min with DMSO (first two lanes) or 100 μM IBMX and 2, 10, or 50 μM forskolin. Adherent cells were compared to cells placed in suspension for 15 min or 2 h. Whole-cell lysates were probed for pERK and ERK. Flag (Raf-1) immunoprecipitates were probed for pS43, pS259, pS338, and Flag (Raf-1).
Raf-1 phosphorylated on S338 can activate ERK despite high S259 phosphorylation.
Figure 5B shows further evidence that PKA inhibits ERK activation primarily by affecting Raf-1 S338 phosphorylation. PAK 165 (which is not affected by PKA) was able to sustain ERK activation even at high levels of PKA activation. NIH 3T3 cells transfected with GFPERK showed diminished activation loop phosphorylation at or above 2 μM forskolin. However, cells coexpressing GFPERK and PAK165 showed strong ERK activation by EGF up to 10 μM forskolin.
HEK293 cells showed a similar signaling profile with regard to adhesion and PKA activation (Fig. 5C). Cells transfected with GFPERK and Flag-Raf-1 wt and treated with EGF showed dramatic inhibition of GFPERK phosphorylation at both 10 and 50 μM forskolin. However, PAK165-expressing cells showed little inhibition at 10 μM and only moderate inhibition at 50 μM forskolin. In adherent control cells, PKA inhibition of ERK seems to work though PAK, as ERK activation parallels pS338 status. With PAK165 expression and increased S338 phosphorylation, ERK activation diminished only at high forskolin levels. This suggests that low-level PKA activity inhibits the ERK pathway primarily by blocking S338 phosphorylation of Raf-1, while at high levels of PKA activity additional inhibitory mechanisms come into play.
Phosphorylation was readily detected at Raf-1 S43 and S259 sites at high doses of forskolin-IBMX. However, ERK activation was strong in cells with high levels of phosphorylation at S43 and S259, provided that S338 also was highly phosphorylated.
In a similar fashion, placing NIH 3T3 fibroblasts in suspension led to diminished phosphorylation of Raf-1 on S43, S259, and S338 sites in control cells and all but S338 in PAK165-containing cells (data not shown). Once again, these observations suggest that cell adhesion affects Raf-1 primarily by reducing S338 phosphorylation rather than by increasing phosphorylation of negative regulatory sites such as S259.
Raf-1 is required for EGF-dependent ERK activation events.
We wanted to determine the relative contributions of Raf-1 and B-Raf in EGF-dependent ERK activation in the cells studied here. A number of papers have suggested a role for B-Raf in Raf-1 activation (43). Active Ras can induce heterodimerization of B-Raf and Raf-1 (54), and certain B-Raf mutations have been shown to activate ERK through transactivation of Raf-1 (53). In addition, some suggest that B-Raf is the main isoform that couples Ras to MEK (55). This appears to be clearly the case for certain cell types and extracts, particularly from the brain (9, 35). However, in other circumstances Raf-1 seems to make a strong contribution to ERK activation (14, 56). Determination of the role of endogenous B-Raf is difficult, as it has higher activity but lower expression in many tissues (31). Kidney tissue, from which HEK293 cells are derived, has been shown to have low levels of B-Raf mRNA (5). In addition, B-Raf is activated more robustly by serum and Raf-1 is more robustly activated by polypeptide growth factors, while both respond well to phorbol esters (46).
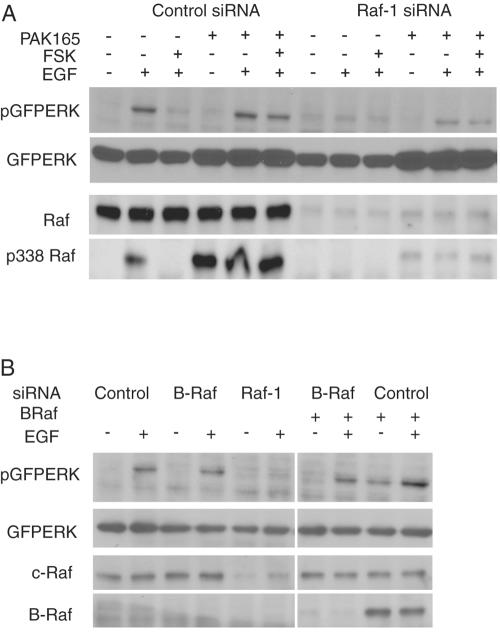
The relative roles of Raf-1 and B-Raf were pursued using RNA interference. As seen in Fig. 6A, in cells treated with a control siRNA that did not affect Raf-1 levels, EGF clearly stimulated Raf-1 S338 phosphorylation as well as GFPERK activation; this was blocked by treatment with forskolin, and the forskolin effect was reversed by coexpression of PAK165, similar to the results described above. In contrast, in cells treated with Raf-1 siRNA the levels of Raf-1 protein were sharply reduced and little activation of GFPERK was detected, even in cells cotransfected with PAK165. This suggests an important role for Raf-1 in the signaling events studied here. In addition, the data imply that PAK165 appears to protect cells against forskolin-induced inhibition of ERK activation through a Raf-1-dependent mechanism.
FIG. 6.
Raf-1 is essential for ERK activation by EGF and for PAK165-dependent rescue of ERK activation in cells treated with forskolin and IBMX. (A) Effects of Raf-1 siRNA. HEK293 cells were transfected with GFPERK, vector (−), or PAK165 (+) and with 50 nM control siRNA (MDR1) or Raf-1 siRNA. After 36 h, cells were serum starved for 12 h, pretreated with DMSO (FSK−) or forskolin (10 μM) and IBMX (50 μM) (FSK +) for 1 h. Wells were dosed with 10 ng/ml EGF for 5 min. Whole-cell lysates were probed for pERK, ERK, Raf, and p338 Raf. (B) Effects of B-Raf siRNA. HEK293 cells were transfected with GFPERK, vector (−), or B-Raf (+), and 50 nM siRNAs to control (MDR1), B-Raf, or Raf-1 and recovered for 36 h. Cells were serum starved for 12 h and dosed with 10 ng/ml EGF for 5 min. Whole-cell lysates were probed for pERK, ERK, Raf-1, and B-Raf.
In contrast, EGF-dependent ERK activation was independent of B-Raf. We could not detect endogenous B-Raf in HEK293 cells; further, these cells activate ERK very poorly in response to 10% FBS, suggesting a lack of this isoform (data not shown). A proven human B-Raf siRNA sequence (33, 36) showed successful knock-down of B-Raf expressed from a cotransfected plasmid (Fig. 6B). Use of this siRNA versus any possible endogenous B-Raf resulted in only slight inhibition of EGF-stimulated GFPERK activation. Thus, the siRNA experiments suggest that Raf-1, rather than B-Raf, is the key intermediary in signaling to ERK in the system studied here. Thus, perturbations of the phosphorylation status of endogenous or transfected Raf-1 will impact on the downstream signaling to ERK.
DISCUSSION
A major goal of our research was to determine if PAK activation and/or Raf-1 S338 phosphorylation is a key adhesion-dependent regulatory step for efficient activation of ERK. Our results do indeed indicate that adhesion-dependent regulation of ERK activation is primarily at the level of S338 phosphorylation of Raf-1. However, restoration of Raf-1 or ERK activation in suspended cells by PAK165 or Raf-1 338D expression is only partial, indicating other levels of anchorage regulation. Although Raf regulation seems to be key, this does not exclude additional loci of anchorage regulation at the level or MEK (27, 49) or even ERK itself (38).
Our data also suggest that, in the adherent state, the signaling that leads to Raf-1 and ERK activation involves a relatively small fraction of the population of Raf-1. Raf-1 S338 phosphorylation following EGF treatment is quite low compared to that seen in cells expressing PAK165, though Raf-1and ERK activation are similar or even more robust. This agrees with reports that show that only a small amount of Raf-1 translocates to the membrane and binds Ras (32).
One interesting set of findings refers to the roles of PAK and PKA in Raf-1 activation in relation to adhesion. As we reported previously (34) and confirm here, signaling in the ERK pathway in suspended cells can be rescued, at least in part, by either expression of an activated PAK or by inhibition of PKA activity. Here we add an important observation that, despite PKA activation upon cell detachment, Raf-1 is largely dephosphorylated on the potential inhibitory PKA sites of S43 and S259, as well as on the permissive S338 site. Thus, rather than excessive phosphorylation of Raf inhibitory sites, it is the PKA-related loss of Raf-1 S338 phosphorylation that appears most critical in reducing signaling in suspended cells. In this way we see the role of PKA activation as being indirect. The low-level transient activation of PKA caused by detachment could contribute to reduced PAK activity through several mechanisms. PKA activation can directly phosphorylate and inhibit PAK, contribute to cytoskeletal disruption (34, 52), or even possibly act through disruption of lipid rafts seen in nonadherent cells (18); however, it is unlikely that adhesion-related PKA activation blocks signaling by direct phosphorylation of inhibitory sites on Raf-1. Although PAK165 clearly phosphorylates S338, we cannot discount the possibility of kinases other than PAK being involved in the phosphorylation of this site (11, 39). However, our observations suggest that other S338 kinases would also be inhibited by PKA.
A second important observation is that cells that maintain high Raf-1 S338 phosphorylation are refractory to PKA activity and high S43 and S259 phosphorylation. This appears contradictory to the notion that strong phosphorylation of these sites leads to an inhibited conformation of Raf-1. Notably, Dhillon et al. described the importance of S259 phosphorylation in forskolin-dependent inhibition of Raf-1 (24). A critical observation is that Raf-1 S259A-expressing cells are resistant to PKA-dependent inhibition of Raf activation. However, there may be experimental differences that contribute to the discordant results. These studies (24) used Cos cells, in which forskolin does not inhibit S338 phosphorylation stimulated by TPA. We find these cells also do not display adhesion-dependent regulation of ERK activation by EGF (unpublished observations). In contrast, forskolin does inhibit PAK activation (34) and S338 phosphorylation in the NIH 3T3 and HEK293 cells used here. An observation consistent with our results is that a Raf-1 S259A/S338A mutation abolishes both basal and stimulated Raf-1 activation (40), implying an intrinsic role for S338 phosphorylation (57).
We do not discount the role of Raf-1 S259 and S43 phosphorylation in Raf-1 inhibition under normal circumstances. Full functional binding of Ras to Raf-1 requires two competent binding sites (15, 21), either of which may be inhibited by phosphorylation at S43 or S233/S259 (26). Both these sites may contribute to a thermodynamic hurdle that Raf-1 must surmount for full activation. A significant consequence of the complete Ras-Raf-1 interaction seems to be to render Raf-1 competent for S338 phosphorylation. Our results fit well with, and add to, models recently proposed by other laboratories (23, 40). Using several mutations, Light et al. showed that mere Ras-Raf-1 interaction was insufficient for Raf-1 activation (40). While not the direct focus of their study, there was a strong correlation between the ability of Ras mutations to displace 14-3-3 from the Raf-1 S259 region (and induce S338 phosphorylation) and their ability to activate Raf-1. Mutation of S259 to alanine produces Raf-1 that is unable to bind 14-3-3 at this site and is more readily phosphorylated on S338 (24), and dephosphorylation of S259 correlates with increased S338 phosphorylation (23). Taken together, these data support the hypothesis that phospho-S259 acts as a negative regulator of a complete Ras-Raf-1 interaction and subsequent S338 phosphorylation. Our data are the first to show kinase activation when Raf-1 is highly phosphorylated on both the positive and negative regulatory sites. We conclude that S259 phosphorylation is not intrinsically inhibitory, but that it prevents essential S338 phosphorylation. Thus, when strong S338 phosphorylation is provided by PAK165, pS43 and pS259 are no longer inhibitory.
A quite different system suggests similar conclusions. When the C-terminal catalytic domain of Raf-1 is expressed in cells, it is highly active but is inhibited by coexpression of the N terminus. Several labs have reported that the role of phosphorylation in the region of S338 or Y341 is to relieve N-terminal autoinhibition of Raf-1 (17, 51). However, Chong and Guan recently proposed an alternate theory about how the N terminus blocks the Raf-1 catalytic domain (12). Their data suggest that the N terminus regulates Raf-1 by inhibiting the necessary positive phosphorylation sites. Mutations, such as S338A, when expressed in the truncated C-terminal catalytic domain are inactive, which implies a positive role for S338 phosphorylation that is intrinsic to the kinase domain and separate from relief of autoinhibition. In addition, C-terminal truncations containing Raf-1 S338D or Y341D mutations are active, even when the N terminus is coexpressed and despite the fact that the N terminus remains bound to the kinase domain. Our results fit well with their hypothesis that the negative inhibition occurs through prevention of the required permissive (S338 or Y341) phosphorylations.
In conclusion, we have shown that phosphorylation of Raf-1 S338 is a critical step in EGF activation of ERK that is lost in nonadherent cells. Lack of Raf-1 activation in suspension is not due to excessive phosphorylation of Raf-1 on negative regulatory sites but primarily due to reduced S338 phosphorylation by PAK (and possibly other kinases). Thus, phosphorylation on the negative regulatory sites S43 and S259 cannot prevent Raf-1 activation if S338 is highly phosphorylated. However, our rescue of ERK activation in suspension with activated PAK or Raf-1 338D expression is only partial. Thus, there are clearly other adhesion-regulated steps required for full activation of ERK. These might involve the altered cytoskeletal organization found in nonadherent cells, as suggested in some studies (2). Alternately, adhesion-dependent regulation of the Raf-1/MEK/ERK cascade may involve scaffolding proteins such as KSR, RKIP, MP-1, and CNK. Few, if any, of these scaffolds have been studied in an adhesion-dependent context, but several are regulated in their activity (16, 44) and could be subject to adhesion-dependent influences.
Supplementary Material
Acknowledgments
We thank Alan Howe and the members of the Juliano lab for their insights and advice on this project.
This work was supported by grants GM26165 and HL4500 to R. L. Juliano.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Aplin, A. E., A. Howe, S. K. Alahari, and R. L. Juliano. 1998. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol. Rev. 50:197-263. [PubMed] [Google Scholar]
- 2.Aplin, A. E., and R. L. Juliano. 1999. Integrin and cytoskeletal regulation of growth factor signaling to the MAP kinase pathway. J. Cell Sci. 112:695-706. [DOI] [PubMed] [Google Scholar]
- 3.Aplin, A. E., S. A. Stewart, R. K. Assoian, and R. L. Juliano. 2001. Integrin-mediated adhesion regulates ERK nuclear translocation and phosphorylation of Elk-1. J. Cell Biol. 153:273-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnard, D., B. Diaz, L. Hettich, E. Chuang, X. F. Zhang, J. Avruch, and M. Marshall. 1995. Identification of the sites of interaction between c-Raf-1 and Ras-GTP. Oncogene 10:1283-1290. [PubMed] [Google Scholar]
- 5.Barnier, J. V., C. Papin, A. Eychene, O. Lecoq, and G. Calothy. 1995. The mouse B-raf gene encodes multiple protein isoforms with tissue-specific expression. J. Biol. Chem. 270:23381-23389. [DOI] [PubMed] [Google Scholar]
- 6.Bohmer, R. M., E. Scharf, and R. K. Assoian. 1996. Cytoskeletal integrity is required throughout the mitogen stimulation phase of the cell cycle and mediates the anchorage-dependent expression of cyclin D1. Mol. Biol. Cell 7:101-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgering, B. M., G. J. Pronk, P. C. van Weeren, P. Chardin, and J. L. Bos. 1993. cAMP antagonizes p21ras-directed activation of extracellular signal-regulated kinase 2 and phosphorylation of mSos nucleotide exchange factor. EMBO J. 12:4211-4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carey, K. D., R. T. Watson, J. E. Pessin, and P. J. Stork. 2003. The requirement of specific membrane domains for Raf-1 phosphorylation and activation. J. Biol. Chem. 278:3185-3196. [DOI] [PubMed] [Google Scholar]
- 9.Catling, A. D., C. W. Reuter, M. E. Cox, S. J. Parsons, and M. J. Weber. 1994. Partial purification of a mitogen-activated protein kinase kinase activator from bovine brain. Identification as B-Raf or a B-Raf-associated activity. J. Biol. Chem. 269:30014-30021. [PubMed] [Google Scholar]
- 10.Chaudhary, A., W. G. King, M. D. Mattaliano, J. A. Frost, B. Diaz, D. K. Morrison, M. H. Cobb, M. S. Marshall, and J. S. Brugge. 2000. Phosphatidylinositol 3-kinase regulates Raf1 through Pak phosphorylation of serine 338. Curr. Biol. 10:551-554. [DOI] [PubMed] [Google Scholar]
- 11.Chiloeches, A., C. S. Mason, and R. Marais. 2001. S338 phosphorylation of Raf-1 is independent of phosphatidylinositol 3-kinase and Pak3. Mol. Cell. Biol. 21:2423-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chong, H., and K. L. Guan. 2003. Regulation of Raf through phosphorylation and N terminus-C terminus interaction. J. Biol. Chem. 278:36269-36276. [DOI] [PubMed] [Google Scholar]
- 13.Chong, H., J. Lee, and K. L. Guan. 2001. Positive and negative regulation of Raf kinase activity and function by phosphorylation. EMBO J. 20:3716-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chong, H., H. G. Vikis, and K. L. Guan. 2003. Mechanisms of regulating the Raf kinase family. Cell Signal. 15:463-469. [DOI] [PubMed] [Google Scholar]
- 15.Chuang, E., D. Barnard, L. Hettich, X. F. Zhang, J. Avruch, and M. S. Marshall. 1994. Critical binding and regulatory interactions between Ras and Raf occur through a small, stable N-terminal domain of Raf and specific Ras effector residues. Mol. Cell. Biol. 14:5318-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corbit, K. C., N. Trakul, E. M. Eves, B. Diaz, M. Marshall, and M. R. Rosner. 2003. Activation of Raf-1 signaling by protein kinase C through a mechanism involving Raf kinase inhibitory protein. J. Biol. Chem. 278:13061-13068. [DOI] [PubMed] [Google Scholar]
- 17.Cutler, R. E., Jr., R. M. Stephens, M. R. Saracino, and D. K. Morrison. 1998. Autoregulation of the Raf-1 serine/threonine kinase. Proc. Natl. Acad. Sci. USA 95:9214-9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.del Pozo, M. A., N. B. Alderson, W. B. Kiosses, H. H. Chiang, R. G. Anderson, and M. A. Schwartz. 2004. Integrins regulate Rac targeting by internalization of membrane domains. Science 303:839-842. [DOI] [PubMed] [Google Scholar]
- 19.del Pozo, M. A., W. B. Kiosses, N. B. Alderson, N. Meller, K. M. Hahn, and M. A. Schwartz. 2002. Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat. Cell Biol. 4:232-239. [DOI] [PubMed] [Google Scholar]
- 20.del Pozo, M. A., L. S. Price, N. B. Alderson, X. D. Ren, and M. A. Schwartz. 2000. Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J. 19:2008-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dent, P., J. Wu, G. Romero, L. A. Vincent, D. Castle, and T. W. Sturgill. 1993. Activation of the mitogen-activated protein kinase pathway in Triton X-100 disrupted NIH-3T3 cells by p21 ras and in vitro by plasma membranes from NIH 3T3 cells. Mol. Biol. Cell 4:483-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhillon, A. S., and W. Kolch. 2002. Untying the regulation of the Raf-1 kinase. Arch. Biochem. Biophys. 404:3-9. [DOI] [PubMed] [Google Scholar]
- 23.Dhillon, A. S., S. Meikle, Z. Yazici, M. Eulitz, and W. Kolch. 2002. Regulation of Raf-1 activation and signalling by dephosphorylation. EMBO J. 21:64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhillon, A. S., C. Pollock, H. Steen, P. E. Shaw, H. Mischak, and W. Kolch. 2002. Cyclic AMP-dependent kinase regulates Raf-1 kinase mainly by phosphorylation of serine 259. Mol. Cell. Biol. 22:3237-3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dumaz, N., Y. Light, and R. Marais. 2002. Cyclic AMP blocks cell growth through Raf-1-dependent and Raf-1-independent mechanisms. Mol. Cell. Biol. 22:3717-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dumaz, N., and R. Marais. 2003. Protein kinase A blocks Raf-1 activity by stimulating 14-3-3 binding and blocking Raf-1 interaction with Ras. J. Biol. Chem. 278:29819-29823. [DOI] [PubMed] [Google Scholar]
- 27.Eblen, S. T., J. K. Slack, M. J. Weber, and A. D. Catling. 2002. Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes. Mol. Cell. Biol. 22:6023-6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fringer, J., and F. Grinnell. 2003. Fibroblast quiescence in floating collagen matrices: decrease in serum activation of MEK and Raf but not Ras. J. Biol. Chem. 278:20612-20617. [DOI] [PubMed] [Google Scholar]
- 29.Giancotti, F. G. 1997. Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr. Opin. Cell Biol. 9:691-700. [DOI] [PubMed] [Google Scholar]
- 30.Hafner, S., H. S. Adler, H. Mischak, P. Janosch, G. Heidecker, A. Wolfman, S. Pippig, M. Lohse, M. Ueffing, and W. Kolch. 1994. Mechanism of inhibition of Raf-1 by protein kinase A. Mol. Cell. Biol. 14:6696-6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagemann, C., and U. R. Rapp. 1999. Isotype-specific functions of Raf kinases. Exp. Cell Res. 253:34-46. [DOI] [PubMed] [Google Scholar]
- 32.Hallberg, B., S. I. Rayter, and J. Downward. 1994. Interaction of Ras and Raf in intact mammalian cells upon extracellular stimulation. J. Biol. Chem. 269:3913-3916. [PubMed] [Google Scholar]
- 33.Hingorani, S. R., M. A. Jacobetz, G. P. Robertson, M. Herlyn, and D. A. Tuveson. 2003. Suppression of BRAF(V599E) in human melanoma abrogates transformation. Cancer Res. 63:5198-5202. [PubMed] [Google Scholar]
- 34.Howe, A. K., and R. L. Juliano. 2000. Regulation of anchorage-dependent signal transduction by protein kinase A and p21-activated kinase. Nat. Cell Biol. 2:593-600. [DOI] [PubMed] [Google Scholar]
- 35.Jaiswal, R. K., S. A. Moodie, A. Wolfman, and G. E. Landreth. 1994. The mitogen-activated protein kinase cascade is activated by B-Raf in response to nerve growth factor through interaction with p21ras. Mol. Cell. Biol. 14:6944-6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karasarides, M., A. Chiloeches, R. Hayward, D. Niculescu-Duvaz, I. Scanlon, F. Friedlos, L. Ogilvie, D. Hedley, J. Martin, C. J. Marshall, C. J. Springer, and R. Marais. 2004. B-RAF is a therapeutic target in melanoma. Oncogene 23:6292-6298. [DOI] [PubMed] [Google Scholar]
- 37.Kerkhoff, E., and U. R. Rapp. 1998. Cell cycle targets of Ras/Raf signalling. Oncogene 17:1457-1462. [DOI] [PubMed] [Google Scholar]
- 38.Laakko, T., and R. L. Juliano. 2003. Adhesion regulation of stromal cell-derived factor-1 activation of ERK in lymphocytes by phosphatases. J. Biol. Chem. 278:31621-31628. [DOI] [PubMed] [Google Scholar]
- 39.Li, W., H. Chong, and K. L. Guan. 2001. Function of the Rho family GTPases in Ras-stimulated Raf activation. J. Biol. Chem. 276:34728-34737. [DOI] [PubMed] [Google Scholar]
- 40.Light, Y., H. Paterson, and R. Marais. 2002. 14-3-3 antagonizes Ras-mediated Raf-1 recruitment to the plasma membrane to maintain signaling fidelity. Mol. Cell. Biol. 22:4984-4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin, T. H., Q. Chen, A. Howe, and R. L. Juliano. 1997. Cell anchorage permits efficient signal transduction between ras and its downstream kinases. J. Biol. Chem. 272:8849-8852. [PubMed] [Google Scholar]
- 42.Miyamoto, S., H. Teramoto, J. S. Gutkind, and K. M. Yamada. 1996. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J. Cell Biol. 135:1633-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mizutani, S., K. Inouye, H. Koide, and Y. Kaziro. 2001. Involvement of B-Raf in Ras-induced Raf-1 activation. FEBS Lett. 507:295-298. [DOI] [PubMed] [Google Scholar]
- 44.Muller, J., S. Ory, T. Copeland, H. Piwnica-Worms, and D. K. Morrison. 2001. C-TAK1 regulates Ras signaling by phosphorylating the MAPK scaffold, KSR1. Mol. Cell 8:983-993. [DOI] [PubMed] [Google Scholar]
- 45.Renshaw, M. W., X. D. Ren, and M. A. Schwartz. 1997. Growth factor activation of MAP kinase requires cell adhesion. EMBO J. 16:5592-5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reuter, C. W., A. D. Catling, T. Jelinek, and M. J. Weber. 1995. Biochemical analysis of MEK activation in NIH 3T3 fibroblasts. Identification of B-Raf and other activators. J. Biol. Chem. 270:7644-7655. [DOI] [PubMed] [Google Scholar]
- 47.Schneller, M., K. Vuori, and E. Ruoslahti. 1997. αvβ3 integrin associates with activated insulin and PDGFβ receptors and potentiates the biological activity of PDGF. EMBO J. 16:5600-5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sendoh, H., C. D. Hu, D. Wu, C. Song, Y. Yamawaki-Kataoka, J. Kotani, T. Okada, F. Shima, K. Kariya, and T. Kataoka. 2000. Role of Raf-1 conserved region 2 in regulation of Ras-dependent Raf-1 activation. Biochem. Biophys. Res. Commun. 271:596-602. [DOI] [PubMed] [Google Scholar]
- 49.Slack-Davis, J. K., S. T. Eblen, M. Zecevic, S. A. Boerner, A. Tarcsafalvi, H. B. Diaz, M. S. Marshall, M. J. Weber, J. T. Parsons, and A. D. Catling. 2003. PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation. J. Cell Biol. 162:281-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang, Y., J. Yu, and J. Field. 1999. Signals from the Ras, Rac, and Rho GTPases converge on the Pak protein kinase in Rat-1 fibroblasts. Mol. Cell. Biol. 19:1881-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tran, N. H., and J. A. Frost. 2003. Phosphorylation of Raf-1 by p21-activated kinase 1 and Src regulates Raf-1 autoinhibition. J. Biol. Chem. 278:11221-11226. [DOI] [PubMed] [Google Scholar]
- 52.Turner, C. E. 2000. Paxillin interactions. J. Cell Sci. 113:4139-4140. [DOI] [PubMed] [Google Scholar]
- 53.Wan, P. T., M. J. Garnett, S. M. Roe, S. Lee, D. Niculescu-Duvaz, V. M. Good, C. M. Jones, C. J. Marshall, C. J. Springer, D. Barford, and R. Marais. 2004. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 116:855-867. [DOI] [PubMed] [Google Scholar]
- 54.Weber, C. K., J. R. Slupsky, H. A. Kalmes, and U. R. Rapp. 2001. Active Ras induces heterodimerization of cRaf and BRaf. Cancer Res. 61:3595-3598. [PubMed] [Google Scholar]
- 55.Wellbrock, C., M. Karasarides, and R. Marais. 2004. The RAF proteins take centre stage. Nat. Rev. Mol. Cell Biol. 5:875-885. [DOI] [PubMed] [Google Scholar]
- 56.Wojnowski, L., L. F. Stancato, A. C. Larner, U. R. Rapp, and A. Zimmer. 2000. Overlapping and specific functions of Braf and Craf-1 proto-oncogenes during mouse embryogenesis. Mech. Dev. 91:97-104. [DOI] [PubMed] [Google Scholar]
- 57.Xiang, X., M. Zang, C. A. Waelde, R. Wen, and Z. Luo. 2002. Phosphorylation of 338SSYY341 regulates specific interaction between Raf-1 and MEK1. J. Biol. Chem. 277:44996-45003. [DOI] [PubMed] [Google Scholar]
- 58.Zhu, X., M. Ohtsubo, R. M. Bohmer, J. M. Roberts, and R. K. Assoian. 1996. Adhesion-dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E-cdk2, and phosphorylation of the retinoblastoma protein. J. Cell Biol. 133:391-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.