Abstract
Gadd45a, a p53- and BRCA1-regulated stress protein, has been implicated in the maintenance of genomic fidelity, probably through its roles in the control of cell cycle checkpoint and apoptosis. However, the mechanism(s) by which Gadd45a is involved in the induction of apoptosis remains unclear. We show here that inducible expression of Gadd45a protein causes dissociation of Bim, a Bcl2 family member, from microtubule-associated components and translocation to mitochondria. The Bim accumulation in mitochondria enhances interaction of Bim with Bcl-2, relieves Bax from Bcl-2-bound complexes, and subsequently results in release of cytochrome c into the cytoplasm. Suppression of endogenous Bim greatly inhibits Gadd45a induction of apoptosis. Interestingly, Gadd45a interacts with elongation factor 1α (EF-1α), a microtubule-severing protein that plays an important role in maintaining cytoskeletal stability, and inhibits EF-1α-mediated microtubule bundling, indicating that the interaction of Gadd45a with EF-1α disrupts cytoskeletal stability. A mutant form of Gadd45a harboring a deletion of EF-1α-binding domain fails to inhibit microtubule stability and to induce Bim translocation to mitochondria. Furthermore, coexpression of EF-1α antagonizes Gadd45a's property of suppressing cell growth and inducing apoptosis. These findings identify a novel link that connects stress protein Gadd45a to the apoptotic machinery and address the importance of cytoskeletal stability in apoptotic response to DNA damage.
Mammalian cells exhibit complex cellular responses to genotoxic stress, including cell cycle arrest and apoptosis. Inactivation of these important biological events results in genomic instability and tumorigenesis. The molecular machinery of apoptosis has been intensively studied, and many important regulators and/or components have been identified (17, 24, 29). Two major pathways of apoptosis have been defined: a pathway that involves mitochondria and a pathway that is activated by death receptors (21, 24, 30). In contrast to the death receptor-mediated apoptotic pathway, signaling pathways that regulate mitochondrion-mediated cell death, particularly after genotoxic stress, are poorly understood. Proteins of the Bcl-2 family members have come to be regarded as central players in the control of mitochondrion-mediated apoptotic process. Some proteins of the Bcl-2 family, such as Bcl-2 and Bcl-XL, inhibit apoptosis, whereas others, including Bax, Bad, Bid, and Bim, promote apoptosis (1, 2). Currently, three major Bim isoforms have been characterized: BimL, BimEL, and BimS (18). BimS has been found to only express in 293 human embryonic kidney cells (19), whereas BimL and BimEL are detected in a variety of cell types and tissues (18). It has been demonstrated that BimL and BimEL are normally associated with motor complexes bound to dynein light chain LC8 and sequestered in the cytoskeleton. Bim-induced apoptosis involves the process of BimL/BimEL release from these microtubule-associated complexes and translocation to mitochondria (20). However, the mechanism that regulates the release of Bim from the cytoskeleton remains to be further defined.
There is little understanding of the involvement of the cytoskeleton in apoptotic process. The findings of the release of Bim from microtubule-associated dynein motor complexes and relocation to mitochondria after UV radiation suggest that Bim acts as an important factor in induction of apoptosis induced by genotoxic stress (20). Because most Bim molecules are bound to the LC8 dynein light chain and sequestered to microtubule-associated motor complexes, disruption of cytoskeletal stability might be an initial and critical step for Bim-induced apoptosis (20). Elongation factor 1α (EF-1α) is a highly conserved and ubiquitously expressed protein. In addition to its role in protein translation, EF-1α has been shown to be a microtubule-severing protein since it binds, bundles, and promotes the assembly of microtubules. Therefore, EF-1α plays an important role in microtubule rearrangement and maintains stability of the cytoskeleton (15, 16, 23). EF-1α is also found to associate with cell transformation and malignancy (4, 27). Interestingly, EF-1α has recently been found to regulate stress-induced apoptosis. Overexpression of EF-1α leads to resistance to apoptosis induced by certain genotoxic stress (26). Thus, it appears that the role of EF-1α in stabilizing microtubules might antagonize induction of apoptosis after cell exposure to certain genotoxic stresses.
Gadd45a is a ubiquitously expressed and DNA damage-inducible protein (3, 6). After ionizing radiation (IR), induction of Gadd45a is strictly regulated by tumor suppressor p53 (13, 34). However, induction of Gadd45a by UV radiation or alkylating agents does not require normal cellular p53 function, although p53 may contribute to Gadd45a's response to non-IR stress (35). Gadd45a is also a BRCA1 downstream gene, and the regulation of Gadd45a by BRCA1 is independent of p53 (5, 7, 12). It has been shown that Gadd45a is an important player in cellular response to DNA damage and has been implicated in the maintenance of genomic stability. The mouse embryonic fibroblasts (MEFs), derived from Gadd45a-null mice, exhibit chromosomal aberrations, gene amplification, centrosome amplification, and aneuploidy (9). In addition, Gadd45a-knockout mice exhibit increased carcinogenesis after IR and UVB radiation (8, 9). A number of investigations indicate that Gadd45a plays a negative role in cell proliferation and expression of Gadd45a suppresses cell growth (11, 37). Gadd45a interacts with the Cdc2/cyclin B1 complex, inhibits activity of Cdc2 kinase, and plays a role in the control of cell cycle G2-M checkpoint after DNA damage (32, 33). Previous studies demonstrate that Gadd45-mediated cell cycle G2-M checkpoint, which is dependent on cellular p53 function (11, 32), correlates with Gadd45-induced proliferation suppression in cells containing wild-type p53 (10). In contrast, the mechanism of how Gadd45a inhibits cell growth in cells lacking p53 is unclear.
Gadd45a has also been implicated in the control of apoptotic process. Introduction of a Gadd45a expression vector into tumor cells via transient transfection induces apoptosis (25). UVB radiation-induced apoptosis is deficient in Gadd45a−/− mouse epidermis (8). It has been previously reported that Gadd45a interacts with MEKK4/MTK1 and activates the JNK/p38 signaling pathway that induces apoptosis (25), but this issue is currently controversial as JNK activation remains intact in Gadd45a deficient cells (22, 31). Given the fact that Gadd45a-mediated cell cycle G2-M arrest only correlates with proliferation suppression in cells harboring wild-type p53 (10), the Gadd45a-induced apoptosis may likely account for its suppressive role in cells containing negative p53 status, although the mechanism by which Gadd45a regulates apoptosis remains to be elucidated. We show here that inducible expression of Gadd45a in HeLa cells results in substantial growth suppression and apoptosis. After Gadd45a induction, Bim dissociates from the microtubule-associated dynein motor complex and translocates to mitochondria. Interestingly, Gadd45a interacts with EF-1α and disrupts EF-1α-mediated microtubule bundling. Furthermore, disruption of the EF-1α-binding motif in Gadd45a protein abolishes its ability to induce apoptosis and growth suppression.
MATERIALS AND METHODS
Cell culture and treatment.
The human cervical line HeLa or human colorectal carcinoma line RKO were grown in F-12 medium (Gibco-BRL) supplemented with 10% fetal bovine serum. Gadd45a tet-off inducible HeLa cell line was established and maintained as described previously (11). Briefly, Gadd45a-inducible cells were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine in the presence of tetracycline at a concentration of 2 μg/ml. To induce expression of Gadd45a protein, DMEM containing tetracycline was removed, and the plates were washed four times with phosphate-buffered saline (PBS), and fresh DMEM containing no tetracycline was then added to cells. Cells were collected at the indicated time points for examination of induced Gadd45a protein.
For UV treatment, cells plated in 100-mm dishes were rinsed with PBS and irradiated with UVB at doses between 250 and 400 J m−2. After cell exposure to UVB radiation, fresh medium was added to the plates, and the cells were cultured in the incubator till harvest. For methyl methanesulfonate (MMS) treatment, cells were grown in medium containing MMS at a concentration of 50 μg/ml for 4 h. The medium was replaced, and cells were collected at the indicated times. For colony formation assays, 5 × 105 human cervical carcinoma HeLa cells were seeded onto 100-mm plates and subjected to transfection as described previously (10, 11).
Plasmid clones and antibodies.
Myc-tagged Gadd45a deletion expression vectors were described previously (10, 33). pEGFP-Gadd45a was made by inserting full-length Gadd45a cDNA into BglII/KpnI sites of pEGFP-C1 vector. pEGFP(Δ60-80)Gadd45a was similarly constructed by using mutated Gadd45a cDNA and expresses mutated pEGFP-Gadd45a fusion protein in which amino acids from 60 to 80 were deleted. Myc-tagged-EF-1α was constructed by inserting the open reading frame (ORF) region of EF-1α cDNA clones into pCS2 (Myc tag vector). Both glutathione S-transferase (GST)-Cdc2 and GST-EF-1α were constructed by inserting Cdc2 and EF-1α open reading frames into the EcoR I-XhoI sites of pGEX-5X-1 plasmid. GST-Gadd45a was made by cloning Gadd45a cDNA into the XhoI site of the pGEX-5X-1 vector.
The following antibodies were used in the experiments: Gadd45a, cytochrome c, Bcl-2, and actin were commercially provided from Santa Cruz Biotechnology (Santa Cruz, CA). Caspase-3, caspase-8, caspase-9, Bim, Bax, and EF-1α were purchased from Pharmingen (San Diego, CA).
Cellular protein and GST preparations, immunoprecipitation, and Western blotting assay.
HeLa Gadd45a-inducible cells were exponentially grown in DMEM containing 2 μg of tetracycline/ml. After withdrawal of tetracycline, cells were harvested at the indicated time points for preparation of cell lysates (10, 33). A total of 100 μg of cellular protein was subjected to sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis and transferred to Protran membranes. Membranes were blocked for 1 h at room temperature in 5% milk, washed with PBST (PBS with 0.1% Tween 20), and incubated with the indicated antibodies for 2 h. Membranes were washed four times in PBST, and horseradish peroxidase-conjugated anti-mouse antibody was added at 1:4,000 in 5% milk. After 1 h, membranes were washed, detected by enhanced chemiluminescence (Amersham, Arlington Height, IL), and exposed to X-ray film (Kodak, Rochester, NY).
GST fusion protein expression was induced in Escherichia coli with 0.1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) at room temperature overnight. Bacteria were harvested at 3,000 rpm the following day. Pellets were washed with PBS and resuspended in cold STE buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA, 150 mM NaCl). Freshly prepared lysozyme solution was added in bacteria (100 μg/ml) and tubes were incubated on ice for 20 min. After the addition of DDT (10 mM) and Sarkosyl (0.7%), the bacterial mixtures were subjected to sonication for 1 min. After centrifugation at 16,000 rpm for 20 min, the supernatants were transferred to fresh tubes, and Triton X-100 was included at a final concentration of 2%. The mixtures were incubated at room temperature for 30 min. Next, the glutathione-agarose beads were mixed with supernatant solution of lysed bacteria at 4°C overnight. After several washes with PBS, glutathione-agarose bead-conjugated GST fusion proteins were ready for use in the designed experiments.
For immunoprecipitation, cellular lysates were incubated with 10 μl of antibody and 20 μl of protein A/G agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C for 4 h. Immunocomplexes were washed four times with lysis buffer and examined by immunoblotting analysis.
siRNA transfection.
The Bim small interfering RNA (siRNA) sequence was designed as 5′-(CAAUUGUCUACCUUCUCGG)d(TT)-3′. The nonspecific siRNA (control) sequence was designed as 5′-(GACCACGAGUAAAAGUAGU)d(TT)-3′. These sequences do not match any other sequences in the GenBank. For cell transfection with siRNA, HeLa cells were placed on six-well plates 16 h prior to transfection and grown in RPMI 1640 medium with 10% fetal bovine serum. Then, 5 μl of 20 μM siRNA was added to a tube containing 50 μl of OptiMem. In a separate tube, 1 μl of Lipofectamine was mixed with 50 μl of OptiMem. The two tubes were next mixed and incubated at room temperature for 20 min, and then aliquots were added to each well of the six-well plate that contained 2 ml of DMEM serum-free medium. After 4 h, 2 ml of fresh complete media was added, and transfected cells were incubated for 24 to 72 h until they were ready to be assayed for gene knockdown analysis.
Velocity sedimentation.
HeLa Gadd45a-inducible cells were collected at different time points after the withdrawal of tetracycline. Cells were treated with 5 μM Taxol (Sigma) for 4 h and 0.5 mM AMP-PNP for 30 min before lysis. Cellular lysates were loaded on a 5 to 20% sucrose gradient in 20 mM morpholineethanesulfonic acid, 100 mM NaCl, 30 mM Tris-HCl, and 0.1% Triton X-100 and subjected to centrifugation at 40,000 rpm for 18 h. Fractions were manually collected and analyzed by size separation by gel electrophoresis.
Isolation of mitochondria and mitochondrion-free cytosolic protein.
Cells were washed three times with ice-cold PBS and spun at 1,200 rpm for 5 min. Cell pellets were resuspended with buffer containing 5 mM Tris (pH 7.4), 5 mM KCl, 1.5 mM MgCl2, 0.1 mM EGTA, 1 mM dithiothreitol, 0.2 mM phenylmethylsulfonyl fluoride, 5 μg of leupeptin/ml, and 5 μg of aprotinin/ml, followed by incubation on ice for 30 min. The cell suspension was passed through a 22-gauge needle 15 to 25 times and then spun down at 1,500 rpm for 5 min at 4°C. Supernatants were transferred to new tubes and spun at 11,000 rpm for 30 min. After centrifugation, pellets (mitochondria) and supernatants (mitochondrion-free cytosolic protein) were separated for immunoblotting analysis. In some experiments, cells were subjected to treatment with 1 mM cross-linking agent dithiobis (DSP) at 37°C for 30 min before subcellular fractionation.
In vitro microtubule bundling assay.
This assay was performed according to the manufacturer's instructions for the Fluorescent Microtubule Biochem kit (Cytoskeleton, Inc.) with modifications. Briefly, 10 μg of rhodamine-labeled tubulin was mixed with 40 μg of unlabeled tubulin. The mixture was divided equally into five tubes, each of which contains 10 μl of polymerization buffer. A total of 100 ng (50 ng/μl) of GST-EF-1α, GST-Gadd45a, or GST-(Δ65-80)Gadd45a proteins was added in different tubes. The tubes were incubated at 35°C for 15 min, and 100 μl of Taxol/microtubule buffer was added to each tube to stabilize the microtubule assembly. Then, 20 ml of the reaction from each tube was put onto a glass slide and examined by fluorescence microscopy (Olympus, Ltd.).
Immunofluorescent staining.
HeLa cells were placed in 100-mm dishes and transfected with pEGFP, pEGFP-Gadd45a (1 to 165 amino acids, full length), or pEGFP(Δ65-85)Gadd45a (Gadd45a with deletion from 65 to 85 amino acids). After 48 h, cells were fixed with 2% paraformaldehyde in PBS for 10 min at room temperature and washed with PBS. Next, cells were subjected to fixation with cold methanol at −20°C, followed by treatment with 0.5% Triton X-100 and 0.5% bovine serum albumin in PBS for 30 min. After incubation with antibody to β-tubulin, plates were washed and incubated with Cy3-conjugated goat anti-mouse immunoglobulin G. After being washed with PBS, cells were stained with DAPI (4′,6′-diamidino-2-phenylindole) and examined under a fluorescence microscope.
RESULTS
Inducible expression of Gadd45 results in apoptosis in HeLa cells.
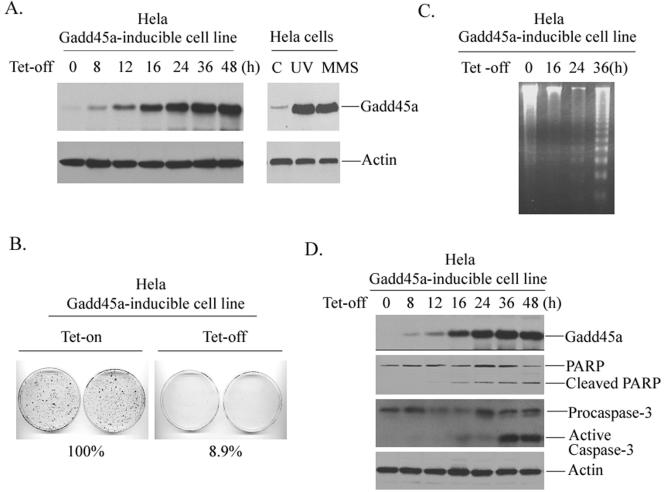
To investigate the role of Gadd45a in the control of cell growth and apoptosis, we established a tetracycline-regulated (tet-off) Gadd45a-inducible cell line in human cervical carcinoma HeLa cells, where p53 is disrupted due to the presence of HPVE6 protein, and examined growth suppression after inducible expression of Gadd45a proteins. As shown in Fig. 1A, the basal level of Gadd45a protein in HeLa cells exhibited a low amount of protein expression. After withdrawal of tetracycline, Gadd45a protein was highly induced. It should be noted here that the Gadd45a induction via the tet-off system is controlled at a similar level to that observed in response to DNA-damaging agents such as MMS or UV. This would be able to make any effect of induced Gadd45a protein on cells similar to the physiological situation. In agreement with our previous observations that Gadd45a negatively regulates cell cycle progression (10, 11, 32), the induction of Gadd45a substantially inhibited cell proliferation by using a colony formation assay (Fig. 1B). In the presence of inducible Gadd45a protein, more than 90% of cell proliferation was suppressed.
FIG. 1.
Inducible expression of Gadd45a results in apoptosis in HeLa cells. (A) a HeLa Gadd45a-inducible cell line was established as described in Materials and Methods. Cells were placed in 100-mm dishes at a density of 4 × 105 and grown in DMEM containing tetracycline at a concentration of 2 μg/ml. After withdrawal of the tetracycline, the cells were collected at the indicated time points for preparation of cellular protein. A total of 100 μg of whole-cell protein was used for immunoblotting analysis with anti-Gadd45a antibody. In addition, Gadd45a protein levels in HeLa cells treated with UV at a dose of 10 J/m2 or MMS at a concentration of 100 μg/ml were examined. As a loading control, anti-actin antibody was included. (B) HeLa Gadd45a-inducible cells were seeded at a density of 1,000 cells per 100-mm dish and grown in medium containing 2 μg of tetracycline/ml. After 16 h, the medium was removed, the plates were washed three times with PBS, and then fresh medium containing no tetracycline was added to the plates. The cells were fixed and stained at 14 days and scored for colonies containing at least 50 cells. The experiments were performed four times, and only representative results are shown here. (C and D) After inducible expression of Gadd45a protein, cells were collected at the indicated time points for the assay of cellular DNA fragmentation (C) or for measurements of cleavages of PARP and caspase-3 (D).
Previous reports from our group and others demonstrate that Gadd45a-induced cell cycle G2-M arrest requires normal cellular p53 function and that inducible expression of Gadd45a fails to induce cell cycle G2-M arrest in HeLa cells. It is speculated that growth inhibition by Gadd45a in cells harboring negative status may correlate with Gadd45a-induced apoptosis. Therefore, we used a DNA fragmentation assay to detect apoptosis in the Gadd45a-inducible HeLa cell line. In Fig. 1C, marked DNA degradation with nucleosome size laddering was detected in cells at 36 h after removal of tetracycline, indicating that inducible expression of Gadd45a protein resulted in apoptosis. In agreement with this observation, the induction of Gadd45a caused protein cleavages for PARP and caspase-3 in HeLa cells (Fig. 1D). In addition, we performed a TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) assay in Gadd45a-inducible HeLa cells and found that ca. 74% cells underwent apoptosis after Gadd45a induction (data not shown). Thus, Gadd45a-induced growth suppression in HeLa cells may mainly correlate with Gadd45a-activated cell death.
Gadd45a induction results in Bim accumulation at the mitochondria.
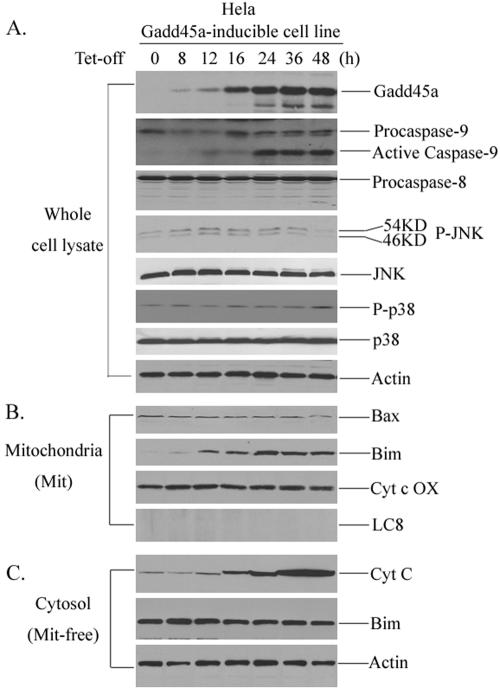
To determine which signaling pathway mainly mediates Gadd45a-activated cell death, the activation of caspase-9 (mitochondria pathway) and caspase-8 (death receptor pathway) after Gadd45a induction was examined. After withdrawal of tetracycline, whole-cell lysates were prepared at different time points and assayed for cleavages of caspase-9 and caspase-8. In Fig. 2A, caspase-9, but not caspase-8, was cleaved at 24, 36, and 48 h, suggesting that the mitochondrion-mediated pathway (cytochrome c/apaf-1/caspase-9) is activated by Gadd45a.
FIG. 2.
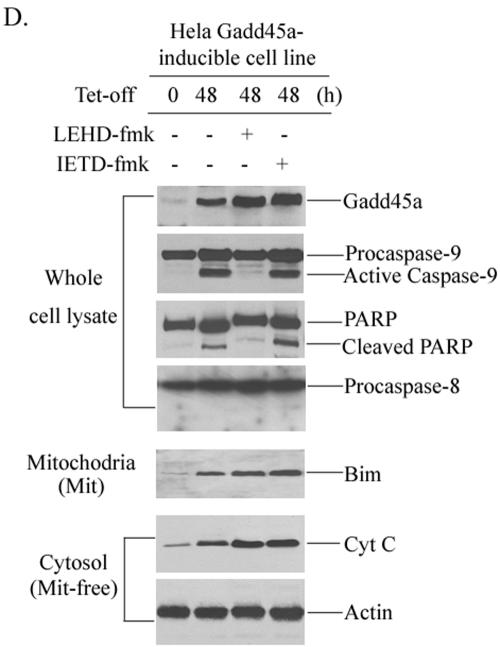
Bim induction in mitochondria by Gadd45a protein. (A) The HeLa Gadd45a-inducible cell line was grown in DMEM in the presence of tetracycline at a concentration of 2 μg/ml. After removal of the tetracycline, the cells were collected at the indicated time points for the preparation of cellular protein. A total of 100 μg of whole-cell protein was examined for activation of caspase-9, caspase-8, JNK, and p38 kinases. In addition, total JNK and p38 protein were also examined. (B) Mitochondrial protein was isolated from HeLa Gadd45a-inducible cells (see Materials and Methods) at different time points and used for the detection of Bim and Bax. (C) Cytochrome c release was measured in mitochondrion (Mit)-free cytosol protein after inducible expression of Gadd45a in Hthe removal of tetracycline, caspase-9 inhibitor (LEHD-fmk) or caspase-8 inhibitor (IETD-fmk) was added to the cell cultures. After 48 h, the cells were collected for the preparation of cellular protein. A total of 100 μg of whole-cell protein was examined for the activation of caspase-9, caspase-8, and PARP cleavage. Bim induction of mitochondria and cytochrome c release were examined as in panels B and C.
Gadd45a has been previously reported to physically associate with and activate MTK1/MEKK4, an upstream activator of JNK/p38 kinase pathways, which may be involved in induction of apoptosis (25). However, this issue is currently under debate since disruption of Gadd45a in Gadd45a-deficient cells does not affect activation of JNK after DNA damage (22, 31). Therefore, we analyzed the activation of both JNK and p38 kinases after inducible expression of Gadd45a in HeLa cells. To carry out this assay, two mitogen-activated protein kinase phosphorylation site-specific antibodies, anti-JNK1/2[pTpY183, 185] and anti-p38, were used. As illustrated in Fig. 2A, no appreciable JNK and p38 activation was observed after Gadd45a induction, and the total amounts of JNK and p38 proteins also remained unchanged. In addition, we included specific inhibitors for both JNK kinase (SP600125) and p38 (SB203580) in the culture of Gadd45a-inducible cells and found that these inhibitors had no effect on Gadd45a-activated apoptosis (result not shown), suggesting that apoptotic process activated by induction of Gadd45a in HeLa cells does not involve activation of JNK/p38 kinases and might utilize differential pathways.
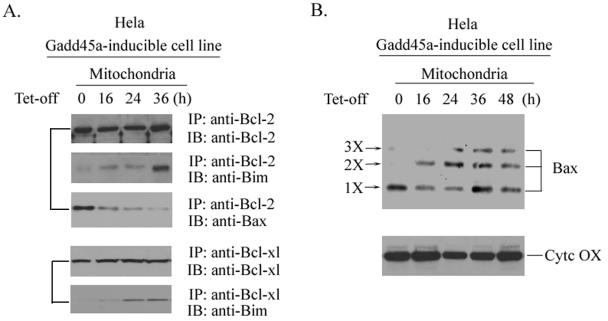
Therefore, the finding that inducible expression of Gadd45a causes caspase-9 cleavage indicates that the mitochondria pathway is likely involved in Gadd45a-activated apoptosis. We then isolated mitochondria from Gadd45a-inducible cells and examined protein levels of Bcl-2 family members, including Bcl-2, Bax, Bim, Bid, Bcl-XL, and Bad. Surprisingly, only Bim protein levels were apparently increased in mitochondria, but others (Bax, Bcl-2, Bid, and Bad) were unchanged (Fig. 2B and results not shown). Since cytochrome c release into the cytoplasm is one of the key steps in the mitochondrion-mediated apoptotic pathway, we extracted mitochondrin-free cytosol proteins from Gadd45a-inducible cells and examined cytochrome c. In Fig. 2C, the levels of cytochrome c were evidently elevated in mitochondrion-free cytosol protein, indicating cytochrome c release into cytoplasm occurred after Gadd45a induction, and this finding goes along with the activation of caspase-9 by Gadd45a. Interestingly, the Bim protein levels in cytoplasm did not exhibit evident changes, suggesting that Gadd45a-caused Bim accumulation in mitochiondria might be due to Bim translocation to mitochondria and not to the overall cellular induction of Bim protein. This concept was further supported by the fact that mRNA or whole cellular protein levels of Bim were not induced after Gadd45a induction (results not shown). To exclude the possibility that Bim translocation to mitochondria by Gadd45a is secondary to the apoptotic event, caspase-9 inhibitor (LEHD-fmk) or caspase-8 inhibitor (IETD-fmk) was added to the cultures of the Gadd45a-inducible cell line. As shown in Fig. 2D, LEHD-fmk was able to block cleavages of both caspase-9 and PARP but did not affect Gadd45a-induced Bim accumulation of mitochondria and cytochrome c release. In contrast, caspase-8 inhibitor (IETD-fmk) did not show any effect on Bim induction and cytochrome c release. These results further confirm that Bim induction of mitochondria by Gadd45a is an upstream event of cytochrome c/caspase-9-mediated apoptosis.
Dissociation of Bim from cytoskeleton after Gadd45a induction.
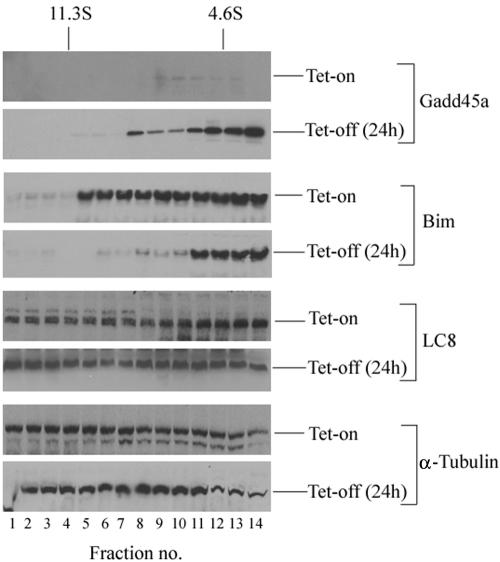
The results presented in Fig. 2B and C suggest a possible correlation of Bim mitochondrial accumulation with Gadd45a-induced apoptosis. A recent report by Puthalakath et al. demonstrate that Bim normally binds to LC8 cytoplasmic dynein light chain and is sequestered in the microtubule-associated motor complex. After certain apoptotic stimuli, such as UV radiation that highly induces Gadd45a expression, Bim is dissociated from the dynein motor complex, translocates to the mitochondria, and exerts its apoptosis-promoting activity (20). Therefore, the subcellular localization of Bim after Gadd45a induction was studied via the approach of velocity sedimentation on sucrose gradients. In Fig. 3, 24 h after tetracycline withdrawal, substantial amounts of Gadd45a were detected in lighter fractions, but the endogenous Gadd45a protein (basal level) was almost undetectable. Interestingly, the sedimentation of Bim on sucrose gradients changed from the heavier fractions (microtubule components) to the lighter fractions. In contrast, the sucrose gradient fractionation assays showed no significant changes in LC8 or α-tubulin subcellular localizations after Gadd45a induction. These results suggest that Gadd45a expression may destabilize the cytoskeleton, dissociate Bim/LC8 from microtubule components, and result in translocation of Bim to the mitochondria.
FIG. 3.
Inducible expression of Gadd45a causes dissociation of Bim from cytoskeleton. HeLa Gadd45a-inducible cells were cultured in DMEM containing tetracycline at a concentration of 2 μg/ml. At 24 h after withdrawal of the tetracycline, cells were treated with 5 μM Taxol (Sigma) for 4 h and 0.5 mM AMP-PNP for 30 min before lysis. Cellular protein extracts were prepared and fractionated by sucrose gradient sedimentation (see Materials and Methods). Proteins in gradient fractions were size separated by gel electrophoresis, transferred to nitrocellulose membranes, and immunoblotted with antibodies to Gadd45a, Bim, LC8, and β-tubulin.
Gadd45a induction enhances interaction of Bim with Bcl-2.
Next, we examined the physical interaction of Bim with Bcl-2 protein. After inducible expression of Gadd45a in HeLa cells, mitochondrial extracts were prepared at 0, 16, 24, or 36 h; incubated with antibody to Bcl-2; and immunoprecipitated with protein A/G-agarose beads. The precipitated complexes were immunoblotted with antibodies to Bim or Bax, and the results are shown in Fig. 4A. In the absence of Gadd45a induction (0-h time point), Bim protein was undetectable in Bcl-2-associated immunocomplexes. However, 16 h after the removal of tetracycline, Bim protein was found to exist in the Bcl-2-precipitated complexes. At 36 h after Gadd45a induction, a great amount of Bim protein was detected in Bcl-2 immunocomplexes. In contrast, Bax was clearly present in Bcl-2 immunocomplexes before Gadd45a was induced, but there was very little detectable Bax in Bcl-2-associated complexes after Gadd45a induction. These results indicate that Bim translocation from cytoskeleton to mitochondria may enhance the association of Bim with Bcl-2 and in turn release Bax from Bcl-2-bound complexes. Consistent with these observations, the oligomeric state of Bax was found to couple with Bim translocation to mitochondria. In Fig. 4B, multimerization of Bax was detected after inducible expression of Gadd45a.
FIG. 4.
Mitochondrion accumulation of Bim after Gadd45a induction increases physical association of Bim with Bcl-2 and enhances Bax multimerization. (A) After inducible expression of Gadd45a protein in the HeLa cell line, cells were collected at the indicated time points. Mitochondrial lysates were prepared (see Materials and Methods) to examine the interactions between Bim and Bcl-2 or Bcl-XL. Lysates were incubated with 10 μl of antibody to Bcl-2 or Bcl-XL and 20 μl of protein A/G agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C for 4 h. Immunocomplexes were washed four times with lysis buffer and examined by immunoblot analysis for the existence of Bim and Bax. (B) After Gadd45a induction, cells were collected at the indicated time points and subjected to treatment with 1 mM DSP (cross-linking agent) at 37°C for 30 min before subcellular fractionation. Mitochondrial proteins were analyzed with antibodies to Bax.
Inhibition of Bim protein levels with siRNA approach disrupts Gadd45a induction of apoptosis.
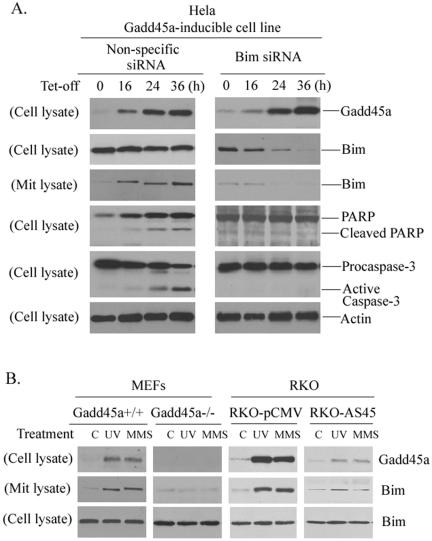
To investigate whether Bim protein mediates Gadd45a-induced cell death, an siRNA approach was used to inhibit endogenous Bim expression and was followed by examination of Gadd45a-induced apoptosis. HeLa Gadd45a-inducible cells were treated with Bim siRNA or nonspecific siRNA and collected at the indicated time points (0, 16, 24, and 36 h) for preparation of whole-cell lysates or mitochondrial proteins. As shown in Fig. 5A, cells treated with Bim siRNA for 16 h exhibited an evident reduced level of cellular Bim protein. At 24 or 36 h, the cellular Bim proteins were substantially suppressed. In agreement with these observations, Bim accumulation of mitochondria after Gadd45a induction was also greatly disrupted. In contrast, nonspecific siRNA had no significant effect on expression of endogenous Bim. Interestingly, induction of Gadd45a proteins failed to induce cleavages for Parp and caspase-3, and the TUNEL assay showed that more than 80% of apoptosis was inhibited (results not shown) in the presence of Bim siRNA, indicating that disruption of endogenous Bim results in inhibition of Gadd45a-activated apoptosis.
FIG. 5.
Suppression of endogenous Bim expression inhibits Gadd45a-induced apoptosis. (A) A total of 5 × 105 HeLa Gadd45a-inducible cells were plated into 100-cm dishes and grown in DMEM containing tetracycline. Upon removal of the tetracycline, the cells were transfected with 21-nucleotide siRNA duplexes that target Bim mRNA transcript via oligofectamine (see Materials and Methods). As a control, nonspecific 21-nucleotide siRNA duplexes were included in the experiments. Cells were collected at the indicated times for preparation of whole-cell lysates or mitochondrial protein. The protein levels of Bim in both whole lysates and mitochondria were analyzed by Western immunoblot assays. In addition, cleavages of PARP and caspase-3 were examined in cells treated with Bim siRNA. (B) Detection of Bim mitochondrial induction in cells with disrupted Gadd45a. MEFs derived from Gadd45a knockouts and RKO-AS45 that expresses antisense Gadd45a were treated with UV radiation at a dose of 10 J/m2 or MMS at a concentration of 50 μg/ml. After 12 h, cells were harvested for the preparation of both whole-cell lysates and mitochondrial protein. Immunoblotting assays were performed to examine the Bim protein levels of mitochondria.
Bim induction of mitochondria was examined in cells with disrupted endogenous Gadd45a. MEFs derived from Gadd45a knockouts or normal animals were exposed to UV radiation (10 J/m2) or MMS (50 μg/ml), and this was followed by analysis of Bim accumulation in mitochondria. After UV or MMS treatments, Bim mitochondrial induction was clearly detected in wild-type MEFs but not in Gadd45a−/− cells. In addition, Bim translocation to mitochondria by UV or MMS was significantly inhibited in RKO-AS45 cells, which are deficient in induction of Gadd45a protein after DNA damage due to the expression of high levels of antisense Gadd45a mRNA. These results suggest that Gadd45a might be required for Bim translocation to mitochondria in response to genotoxic stress.
Gadd45a interacts with EF-1α.
Since there is no evidence that Gadd45a directly interacts with the Bim/LC8 complex (results not shown), the effect of Gadd45a on Bim translocation to mitochondria is more likely mediated through a mechanism that is involved in cytoskeletal stability. In experiments searching for Gadd45a-associated proteins, yeast two-hybrid screening assays demonstrated that Gadd45a interacted with EF-1α, a ubiquitously expressed and microtubule-associated protein. A number of investigations have shown that EF-1 α binds, bundles, stabilizes, and promotes the assembly of microtubules and thus acts as an important regulator of cytoskeletal rearrangements and stability (15, 16, 23). Most recently, the overexpression of EF-1α was found to result in selective resistance to apoptosis induced by certain genotoxic stresses (26), suggesting a role of EF-1α in cellular response to DNA damage. Therefore, EF-1α appears to be a possible target to mediate the effect of Gadd45a on Bim mitochondrial induction that is closely associated with cytoskeletal instability.
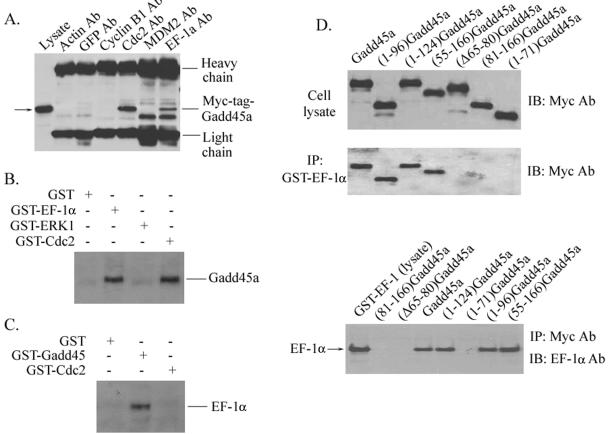
We first performed a series of experiments to confirm the interactions between Gadd45a and EF-1α proteins. In Fig. 6A, cellular lysates from HeLa cells transfected with a Myc-tagged-Gadd45a vector were immunoprecipitated with anti-actin, -GFP, -Cdc2, -MDM2, and -EF-1α antibodies. After immunoblotting analysis, Myc-tagged Gadd45a was detected in the immunoprecipitates by Cdc2 (positive control) and EF-1α antibodies as well but was not detected by antibodies to actin, GFP, and MDM2. An association between cellular Gadd45a and GST-EF-1α proteins was also demonstrated. GST-EF-1α, GST-Cdc2, and GST-ERK1 fusion proteins were incubated with cellular lysates isolated from HeLa Gadd45-inducible cells, which were collected at 24 h after withdrawal of tetracycline and expressed substantial amounts of Gadd45 protein (as shown in Fig. 1A), and were followed by immunoprecipitation. Clearly, both GST-Cdc2 and GST-EF-1α were able to pull down Gadd45, but GST alone or GST-ERK1 did not interact with cellular Gadd45 (Fig. 6B). In addition, GST-Gadd45a was used to immunoprecipitate cellular EF-1α. As shown in Fig. 6C, endogenous EF-1α proteins were detected in GST-Gadd45 immunocomplexes. However, both GST and GST-Cdc2 proteins were unable to pull down EF-1α. Taken together, these results indicate a specific physical association of Gadd45 with EF-1α.
FIG. 6.
Interaction of Gadd45a with EF-1α. (A) Myc-tagged-Gadd45a vector was transiently expressed in HeLa cells via Lipofectamine transfection. At 48 h posttransfection, whole-cell protein extracts were prepared and immunoprecipitated with anti-actin, anti-GFP, anti-Cdc2, anti-MDM2, and anti-EF-1α antibodies. The immunocomplexes were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting with anti-c-Myc antibody. (B) Whole-cell lysates prepared from HeLa cells were incubated with GST-EF-1α, GST-ERK1, GST-Cdc2, or GST alone (conjugated with Sepharose beads) for 6 h at 4°C. Immunocomplexes were then washed four times with lysis buffer and analyzed with antibody to Gadd45a. (C) GST-Gadd45a, GST-Cdc2, and GST alone were incubated with HeLa cellular protein as described in panel B. Immunoblotting assays were used to examine the presence of EF-1α protein in immunocomplexes by using anti-EF-1α antibody. (D) A series of Myc-tagged-Gadd45a deletion mutants were introduced into HeLa cells via transient transfection. Whole-cell lysates were prepared and incubated with GST-EF-1α (conjugated with Sepharose beads). Immunocomplexes were then washed four times with lysis buffer and analyzed with anti-Myc antibody. In addition, cellular lysates isolated from HeLa cells transfected with a series of Myc-tagged Gadd45a deletion mutants were incubated with GST-EF-1α protein and immunoprecipitated with anti-Myc antibody. Immunoprecipitates were analyzed with anti-EF-1α antibody.
Next, we sought to identify the regions of Gadd45a protein that are required for Gadd45a interaction with EF-1α protein by using a series of Myc-tagged Gadd45a deletion protein expression vectors. Cell lysates from HeLa cells transfected with Myc-tagged Gadd45a deletion vectors were immunoprecipitated with antibody to EF-1α and blotted with anti-Myc antibody. As shown in Fig. 6D, there was abundant expression of Myc-tagged Gadd45a deletion proteins in transfected HeLa cells. After incubation with GST-EF-1α (conjugated with Sepharose beads), full-length Myc-tagged-Gadd45a protein was strongly detected. Deletion of the amino terminus of Gadd45a in (55-166)Gadd45a did not interfere with Gadd45a protein binding to EF-1α. The mutants with deletions of the carboxyl terminus of Gadd45a, such as (1-96)Gadd45a and (1-124)Gadd45a, still retained their binding activity for the interaction of Gadd45a with EF-1α. However, (1-70)Gadd45a and (81-166)Gadd45a were deficient for EF-1α binding activity. Collectively, these observations suggest that the central region of the Gadd45a protein (i.e., amino acids between 71 and 80) is needed for EF-1α interaction, and are supported by the evidence that a Gadd45a mutant with truncated central region (i.e., amino acids from 65 to 80) failed to bind to EF-1α (Fig. 6D). To further examine the domain of Gadd45a involved in EF-1α interaction, GST-EF-1α was incubated with cellular protein lysate extracted from HeLa cells expressing Myc-tagged Gadd45a deletion proteins (Fig. 6D). In agreement with the observations in immunoprecipitation by anti-EF-1α, full-length Gadd45a, (1-96)Gadd45a, (1-124)Gadd45a, and (55-166)Gadd45a were pulled down by GST-EF-1α, but (1-70)Gadd45a, (81-166)Gadd45a, and (Δ65-80)Gadd45a did not interact with EF-1α.
Gadd45a affects EF-1α-mediated microtubule bundling and alters cytoskeletal structure.
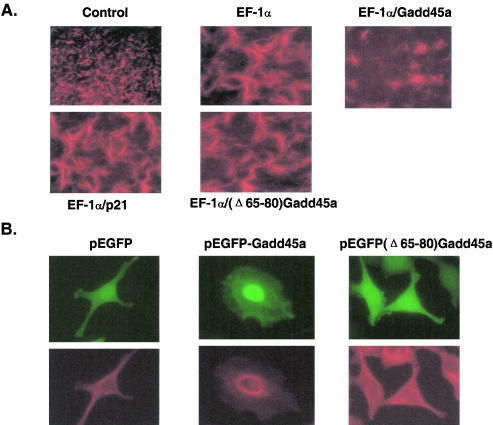
As discussed earlier, EF-1α binds to microtubules and promotes and stabilizes microtubule bundling. Thus, this protein plays an important role in the maintenance of cytoskeletal stability. To examine the effect of Gadd45a on EF-1α-mediated microtubule bundling, an in vitro microtubule assembly assay was conducted. As shown in Fig. 7A, rhodamine-labeled tubulin was seen to form microtubules efficiently (control panel) under an optimal condition for tubulin polymerization (see Materials and Methods). After the addition of GST-EF-1α protein, assembled microtubules presented clear bundles that were manifested by big and/or thick strings, indicating that EF-1α promotes the bundling process of the microtubule. Interestingly, in the presence of GST-Gadd45a protein the bundling of microtubules by EF-1α was substantially disrupted, and the bundled-microtubules exhibited torn or jagged shapes. To exclude nonspecific effects, GST-p21waf1/cip1, which is a p53-regulated stress protein, was included and did not show any influence on EF-1α-mediated microtubule bundles. The (Δ65-80)Gadd45a mutant, in which the EF-1α binding motif had been deleted, also had no effect on EF-1α-mediated microtubule bundles. These results indicate that interaction of Gadd45a with EF-1α is required for Gadd45a inhibition of microtubule bundling. Furthermore, the findings in the in vitro assay were further supported by the in vivo experiments (Fig. 7B), wherein HeLa cells were transfected with pEGFP Gadd45a, pEGFP(Δ65-80) Gadd45a, or pEGFP vectors, and 500 GFP-positive cells from each transfection were subjected to morphological examinations by fluorescence microscopy. At 36 h after transfection, cells expressing either pEGFP or pEGFP(Δ65-80) Gadd45a vectors remained similar in shapes and structures. About 74% cells transfected with pEGFP Gadd45a vector presented obvious morphological alterations, which were manifested by a rounded cell shape, disrupted microtubule structure, and condensed nuclei. Collectively, these data suggest that Gadd45a might be able to disrupt microtubule stability and destabilize cytoskeletal structure through its interaction with EF-1α.
FIG. 7.
(A) Effect of Gadd45a on EF-1α-mediated microtubule bundling. Microtubule assembly and/or bundle assays were carried out by using rhodamine (or fluorescein)-labeled tubulin according to the manufacturer's manual. The assays were performed in the presence of 100 ng of GST-EF-1α, GST-Gadd45a, or GST-p21 proteins and evaluated by using fluorescence microscopy. (B) Effect of Gadd45a expression on cytoskeleton. GFP-Gadd45a and GFP(Δ65-80)Gadd45a expression vectors were transfected into HeLa cells. After 36 h, cells were stained with antibody to β-tubulin and detected by fluorescence microscopy for green fluorescent protein (green fluorescence) or rhodamine staining (red fluorescence).
Interaction of Gadd45a with EF-1α contributes to Gadd45a-induced apoptosis and growth suppression.
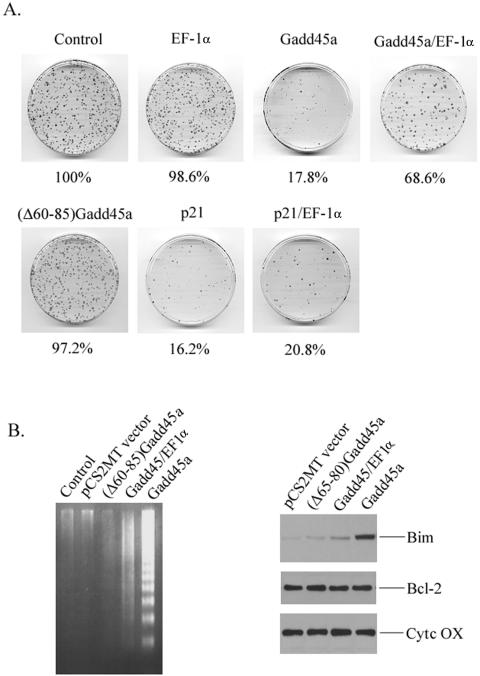
To further demonstrate whether interaction of Gadd45a with EF-1α mediates the role of Gadd45a in induction of apoptosis and growth inhibition, a short-term colony formation analysis was conducted. HeLa cells were cotransfected with pSV2.neo vector and Gadd45a vectors (at a ratio of 1:5) and selected in medium containing G418. After 2 weeks, cell colonies were fixed and stained for cell growth analysis. As shown in Fig. 8A, overexpression of Gadd45a in HeLa cells generated more than 80% growth suppression. In contrast, (Δ60-85)Gadd45a, in which the EF-1α-interacting domain is disrupted, was deficient in cell growth inhibition. Moreover, coexpression of EF-1α substantially antagonized the growth suppressive property of Gadd45a but had no effect on p21waf1/cip-induced growth suppression. Consistent with these observations, (Δ60-85)Gadd45a that harbors a deletion of the EF-1α motif failed to induce apoptosis in HeLa cells (Fig. 8B). Gadd45a-induced cell death was also diminished in the presence of EF-1α protein (Fig. 8B). Furthermore, disruption of the EF-1α domain was shown to abolish Gadd45a-caused Bim translocation to mitochondria (Fig. 8C). Taken together, the interaction of Gadd45a with EF-1α is, to a great extent, required for the role of Gadd45a in cell death and growth suppression.
FIG. 8.
Interaction of Gadd45a with EF-1α contributes to Gadd45a-induced apoptosis and growth suppression. (A) HeLa cells were cotransfected with pCS2MT (Myc-tagged empty vector) or the indicated Myc-tagged expression vectors for Gadd45a, (Δ60-85)Gadd45a, EF-1α, or p21waf1/cip1. After selection with G418 for 2 weeks, cells were fixed, and the colonies that contained at least 50 cells were counted. Quantitative results represent the average of three individual experiments. (B) HeLa cells were transiently transfected with Myc-tagged Gadd45a or Myc-tagged (Δ60-85)Gadd45a. (C) At 48 h posttransfection, cells were harvested for the assay of DNA fragmentation and for analysis of Bim induction in mitochondria.
DISCUSSION
Detailed investigations were undertaken here to define the mechanism by which Gadd45a, a p53- and BRCA1-regulated stress protein, plays a role in the induction of apoptosis. Using Gadd45a tet-off inducible cells, we demonstrated that inducible expression of Gadd45a resulted in apoptosis in HeLa cells and substantially suppressed cell growth. Gadd45a-activated apoptosis was coupled with Bim release from the microtubule-associated dynein motor complex and translocation to the mitochondria. Knockdown of endogenous Bim expression by the siRNA approach greatly inhibited Gadd45a-induced cell death. Interestingly, Gadd45a was shown to interact with EF-1α, a microtubule-severing protein that plays an important role in cytoskeletal rearrangement and in the maintenance of cytoskeletal stability and was able to disrupt EF-1α-mediated microtubule bundling. Disruption of the EF-1α-interacting domain substantially inhibited Gadd45a-activated apoptosis and Gadd45a-induced growth suppression.
Gadd45a is an important player in maintaining genomic fidelity. Disruption of endogenous Gadd45a results in severe genomic instability and increased sensitivity to IR/UV radiation-induced tumorigenesis (8, 9). Gadd45a physically associates with Cdc2 kinase, inhibits its kinase activity, and is able to arrest cells in the G2-M transition following certain DNA damage agents (32, 33). Gadd45a protein suppresses cell proliferation/growth in multiple tumor lines regardless of p53 status (11, 37). Our previous findings demonstrate that Gadd45a-induced cell proliferation inhibition mainly correlates with Gadd45a-mediated cell cycle G2-M arrest in cells that contain functional p53 (10), although weaker apoptosis after inducible expression of Gadd45a is also observed in RKO cells that have wild-type p53 (results not shown). Therefore, Gadd45a-induced apoptosis may primarily account for its growth-suppressive role in cells lacking normal p53. In the present study, inducible expression of Gadd45a in HeLa cells resulted in substantial growth inhibition and apoptosis (Fig. 1). Gadd45a-induced cell death utilized the mitochondrial pathway since Gadd45a induction caused caspase-9, but not caspase-8, activation and cytochrome c release from mitochondria to cytoplasm. Interestingly, Gadd45a induction of apoptosis was likely mediated through Bim accumulation of mitochondria. This elevation of Bim protein in mitochondria was mainly due to Bim release from the cytoskeleton and translocation to mitochondria. Lei and Davis recently reported that JNK phosphorylation of Bim promoted Bim release from microtubule-associated complexes and induced Bax-dependent cell death (14). However, after Gadd45a induction in HeLa cells, only weak activation of JNK was observed (Fig. 2A), and the recruitment of a JNK specific inhibitor did not affect Gadd45a-induced apoptosis (results not shown), indicating that Gadd45a-caused Bim translocation to mitochondria does not require JNK activation in the system of HeLa-Gadd45a tet-off-inducible cells. Indeed, activation of JNK by Gadd45a is currently under debate (22, 31), and it is likely that Gadd45a is required for sustaining JNK activation after DNA damage (8). Thus, this study demonstrated that Gadd45a-induced apoptosis may utilize signaling pathways that differ from JNK activation and may be through Gadd45a destabilization of the cytoskeleton, which is followed by the release of Bim from microtubule-associated dynein motor complexes.
The proapoptotic activity of Bim is found to be regulated by interaction with microtubule-associated complexes. After UV radiation, Bim is dissociated from cytoskeletal compartment and translocates to mitochondria (20). As a consequence of Bim accumulation in mitochondria, increased interactions of Bim with Bcl-2 took place and were coupled with the dissociation of Bcl-2/Bax protein complexes (Fig. 4). This process was finally followed by multimerization of Bax, indicating that Bim mitochondrial translocation is able to remove Bcl-2 inhibition of Bax and promotes its apoptotic property. It appears that Bim release from the cytoskeleton and translocation to mitochondria is a critical step in Gadd45a induction of apoptosis. The use of Bim siRNA, which specifically suppresses the expression of cellular Bim, led to inhibition of Bim accumulation in mitochondria and in turn disrupted Gadd45a-induced apoptosis (Fig. 5A). Similarly, Gadd45a induction may act as an upstream event for Bim mitochondrial translocation. When cells with disrupted endogenous Gadd45a were exposed to treatment with UV or MMS, Bim failed to exhibit mitochondrial induction. These data suggest that Gadd45a might be a regulator in the process of Bim mitochondrial accumulation and further support the role of Gadd45a in cytoskeleton-associated cell death after genotoxic stress.
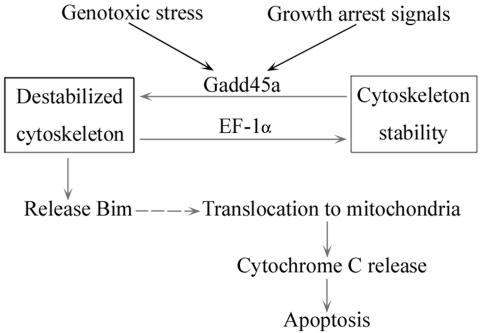
As an important regulator in maintaining microtubule stability (16, 23), EF-1α has also been found to participate in apoptotic response to genotoxic stress (26). Ectopic EF-1α expression conferred protection from cell death induced by growth factor withdrawal and agents that induce endoplasmic reticulum stress (26), indicating that EF-1α may possess antiapoptotic properties. In the present study, the interaction between Gadd45a and EF-1α was shown to disrupt EF-1α-mediated microtubule bundling and alter cytoskeletal structure (Fig. 7). Using Myc-tagged Gadd45a deletion fusion proteins, the EF-1α binding domain was mapped at the central region of Gadd45a, between amino acids 65 and 80. Deletion of this binding motif or coexpression of EF-1α protein abolished the destructive effect of Gadd45a on EF-1α-mediated microtubule bundling. Importantly, the EF-1α binding domain appeared to be required for Gadd45a-induced Bim accumulation in mitochondria and activated apoptosis. Consistent with these observations, disruption of the EF-1α binding domain substantially diminished Gadd45a-induced growth suppression and Bim accumulation of mitochondria. Overexpression of EF-1α in HeLa cells also antagonized the growth-suppressive properties of Gadd45a. As discussed earlier, EF-1α plays a critical role in microtubule assembly, bundling, and maintenance of microtubule stability (16, 23). Disruption of EF-1α function by Gadd45a may destabilize cytoskeletal structure and result in the release of Bim from microtubule-associated motor complexes. Therefore, the findings presented here demonstrate a link between stress protein Gadd45a and microtubule instability after genotoxic stress. A scenario can thus be proposed for this pathway (FIG. 9): after DNA damage or growth arrest signals, Gadd45a protein is induced via either p53-dependent or -independent mechanisms. Increased Gadd45a protein physically interacts with EF-1α and impairs cytoskeletal stability. Cytoskeletal destabilization results in Bim release from microtubule-associated protein complexes and translocation to the mitochondria. Finally, Bim accumulation in mitochondria relieves Bax from Bcl-2- or Bcl-XL-associated complexes and, in turn, induces cell death.
FIG. 9.
Schematic representation of signaling pathway that mediates Gadd45a-induced apoptosis. In cellular response to genotoxic stress and growth arrest signals, Gadd45a is highly induced. Increased Gadd45a protein interacts with EF-1α, a microtubule-severing protein that maintains cytoskeletal stability, and disrupts the stability of the cytoskeleton. Bim protein is in turn released from destabilized microtubule-associated protein complexes and translocates to mitochondria. Induction of Bim in mitochondria relieves Bax from Bcl-2-bound complexes and induces release of cytochrome c. Finally, cells undergo apoptosis.
In addition to regulation by p53 (13), Gadd45a is also characterized as a BRCA1-targeted effector gene (5, 12). The BRCA1 transactivation of Gadd45a is independent of p53 status (12), indicating that Gadd45a may mediate the biological role of BRCA1 in cells lacking p53. A number of investigations have demonstrated that BRCA1 plays an important role in induction of apoptosis, although much effort is required to elucidate the underlying mechanism (7, 28, 36). We recently reported that BRCA1 was cleaved after DNA damage and that the cleavage of BRCA1 could activate the proapoptotic function of the C terminus through removal of the antiapoptotic N terminus. In addition, cleaved BRCA1 was shown to transcriptionally activate Gadd45a that contributes to the apoptotic response to DNA damage (36). Therefore, BRCA1-induced apoptosis may be at least in part mediated through its activation of Gadd45a. The findings in the present study provide evidence that as a BRCA1 downstream effector gene, Gadd45a may also mediate BRCA1's role in the induction of apoptosis and in the maintenance of genomic fidelity. More importantly, the evidence provided here identifies a novel pathway (Gadd45a induction → microtubule instability → Bim release → Bim translocation to mitochondria → cytochrome c release → caspase-9 activation → caspase-3 activation → apoptosis) in the regulation of apoptosis and addresses the importance of cytoskeletal stability in stress-induced apoptosis.
Acknowledgments
This study was supported in part by the National Fundamental Research Program of China (2002CB513101), the National Natural Science Foundation of China (30225018 and 30400074), the Beijing Natural Science Foundation (7043074), and the National Institutes of Health grant R01 CA93640.
We thank Albert J. Fornace and M. Christine Hollander at NIH for providing us with Gadd45a knockout MEFs.
REFERENCES
- 1.Adams, J. M., and S. Cory. 2001. Life-or-death decisions by the Bcl-2 protein family. Trends Biochem. Sci. 26:61-66. [DOI] [PubMed] [Google Scholar]
- 2.Antonsson, B., and J. C. Martinou. 2000. The Bcl-2 protein family. Exp. Cell Res. 256:50-57. [DOI] [PubMed] [Google Scholar]
- 3.Carrier, F., M. L. Smith, I. Bae, K. E. Kilpatrick, T. J. Lansing, C. Y. Chen, M. Engelstein, S. H. Friend, W. D. Henner, T. M. Gilmer, et al. 1994. Characterization of human Gadd45, a p53-regulated protein. J. Biol. Chem. 269:32672-32677. [PubMed] [Google Scholar]
- 4.Edmonds, B. T., J. Wyckoff, Y. G. Yeung, Y. Wang, E. R. Stanley, J. Jones, J. Segall, and J. Condeelis. 1996. Elongation factor-1 alpha is an overexpressed actin binding protein in metastatic rat mammary adenocarcinoma. J. Cell Science 109:2705-2714. [DOI] [PubMed] [Google Scholar]
- 5.Fan, W., S. Jin, T. Tong, H. Zhao, F. Fan, M. J. Antinore, B. Rajasekaran, M. Wu, and Q. Zhan. 2002. BRCA1 regulates GADD45 through its interactions with the OCT-1 and CAAT motifs. J. Biol. Chem. 277:8061-8067. [DOI] [PubMed] [Google Scholar]
- 6.Fornace, A. J., Jr., I. Alamo, Jr., and M. C. Hollander. 1988. DNA damage-inducible transcripts in mammalian cells. Proc. Natl. Acad. Sci. USA 85:8800-8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harkin, D. P., J. M. Bean, D. Miklos, Y. H. Song, V. B. Truong, C. Englert, F. C. Christians, L. W. Ellisen, S. Maheswaran, J. D. Oliner, and D. A. Haber. 1999. Induction of GADD45 and JNK/SAPK-dependent apoptosis following inducible expression of BRCA1. Cell 97:575-586. [DOI] [PubMed] [Google Scholar]
- 8.Hildesheim, J., D. V. Bulavin, M. R. Anver, W. G. Alvord, M. C. Hollander, L. Vardanian, and A. J. Fornace, Jr. 2002. Gadd45a protects against UV irradiation-induced skin tumors, and promotes apoptosis and stress signaling via MAPK and p53. Cancer Res. 62:7305-7315. [PubMed] [Google Scholar]
- 9.Hollander, M. C., M. S. Sheikh, D. V. Bulavin, K. Lundgren, L. Augeri-Henmueller, R. Shehee, T. A. Molinaro, K. E. Kim, E. Tolosa, J. D. Ashwell, M. P. Rosenberg, Q. Zhan, P. M. Fernandez-Salguero, W. F. Morgan, C. X. Deng, and A. J. Fornace, Jr. 1999. Genomic instability in Gadd45a-deficient mice. Nat. Genet. 23:176-184. [DOI] [PubMed] [Google Scholar]
- 10.Jin, S., M. J. Antinore, F. D. Lung, X. Dong, H. Zhao, F. Fan, A. B. Colchagie, P. Blanck, P. P. Roller, A. J. Fornace, Jr., and Q. Zhan. 2000. The GADD45 inhibition of Cdc2 kinase correlates with GADD45-mediated growth suppression. J. Biol. Chem. 275:16602-16608. [DOI] [PubMed] [Google Scholar]
- 11.Jin, S., T. Tong, W. Fan, F. Fan, M. J. Antinore, X. Zhu, L. Mazzacurati, X. Li, K. L. Petrik, B. Rajasekaran, M. Wu, and Q. Zhan. 2002. GADD45-induced cell cycle G2-M arrest associates with altered subcellular distribution of cyclin B1 and is independent of p38 kinase activity. Oncogene 21:8696-8704. [DOI] [PubMed] [Google Scholar]
- 12.Jin, S., H. Zhao, F. Fan, P. Blanck, W. Fan, A. B. Colchagie, A. J. Fornace, Jr., and Q. Zhan. 2000. BRCA1 activation of the GADD45 promoter. Oncogene 19:4050-4057. [DOI] [PubMed] [Google Scholar]
- 13.Kastan, M. B., Q. Zhan, W. S. el-Deiry, F. Carrier, T. Jacks, W. V. Walsh, B. S. Plunkett, B. Vogelstein, and A. J. Fornace, Jr. 1992. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell 71:587-597. [DOI] [PubMed] [Google Scholar]
- 14.Lei, K., and R. J. Davis. 2003. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc. Natl. Acad. Sci. USA 100:2432-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore, R. C., and R. J. Cyr. 2000. Association between elongation factor-1alpha and microtubules in vivo is domain dependent and conditional. Cell Motil. Cytoskeleton 45:279-292. [DOI] [PubMed] [Google Scholar]
- 16.Moore, R. C., N. A. Durso, and R. J. Cyr. 1998. Elongation factor-1alpha stabilizes microtubules in a calcium/calmodulin-dependent manner. Cell Motil. Cytoskeleton 41:168-180. [DOI] [PubMed] [Google Scholar]
- 17.O'Connor, L., D. C. Huang, L. A. O'Reilly, and A. Strasser. 2000. Apoptosis and cell division. Curr. Opin. Cell Biol. 12:257-263. [DOI] [PubMed] [Google Scholar]
- 18.O'Reilly, L. A., L. Cullen, J. Visvader, G. J. Lindeman, C. Print, M. L. Bath, D. C. Huang, and A. Strasser. 2000. The proapoptotic BH3-only protein Bim is expressed in hematopoietic, epithelial, neuronal, and germ cells. Am. J. Pathol. 157:449-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Putcha, G. V., K. L. Moulder, J. P. Golden, P. Bouillet, J. A. Adams, A. Strasser, and E. M. Johnson. 2001. Induction of BIM, a proapoptotic BH3-only BCL-2 family member, is critical for neuronal apoptosis. Neuron 29:615-628. [DOI] [PubMed] [Google Scholar]
- 20.Puthalakath, H., D. C. Huang, L. A. O'Reilly, S. M. King, and A. Strasser. 1999. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Molecular Cell 3:287-296. [DOI] [PubMed] [Google Scholar]
- 21.Roucou, X., B. Antonsson, and J. C. Martinou. 2001. Involvement of mitochondria in apoptosis. Cardiol. Clin. 19:45-55. [DOI] [PubMed] [Google Scholar]
- 22.Shaulian, E., and M. Karin. 1999. Stress-induced JNK activation is independent of Gadd45 induction. J. Biol. Chem. 274:29595-29598. [DOI] [PubMed] [Google Scholar]
- 23.Shiina, N., Y. Gotoh, N. Kubomura, A. Iwamatsu, and E. Nishida. 1994. Microtubule severing by elongation factor 1α. Science 266:282-285. [DOI] [PubMed] [Google Scholar]
- 24.Strasser, A., L. O'Connor, and V. M. Dixit. 2000. Apoptosis signaling. Annu. Rev. Biochem. 69:217-245. [DOI] [PubMed] [Google Scholar]
- 25.Takekawa, M., and H. Saito. 1998. A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell 95:521-530. [DOI] [PubMed] [Google Scholar]
- 26.Talapatra, S., J. D. Wagner, and C. B. Thompson. 2002. Elongation factor-1 alpha is a selective regulator of growth factor withdrawal and ER stress-induced apoptosis. Cell Death Differ. 9:856-861. [DOI] [PubMed] [Google Scholar]
- 27.Tatsuka, M., H. Mitsui, M. Wada, A. Nagata, H. Nojima, and H. Okayama. 1992. Elongation factor-1α gene determines susceptibility to transformation. Nature 359:333-336. [DOI] [PubMed] [Google Scholar]
- 28.Thangaraju, M., S. H. Kaufmann, and F. J. Couch. 2000. BRCA1 facilitates stress-induced apoptosis in breast and ovarian cancer cell lines. J. Biol. Chem. 275:33487-33496. [DOI] [PubMed] [Google Scholar]
- 29.Vaux, D. L., and A. Strasser. 1996. The molecular biology of apoptosis. Proc. Natl. Acad. Sci. USA 93:2239-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang, X. 2001. The expanding role of mitochondria in apoptosis. Genes Dev. 15:2922-2933.11711427 [Google Scholar]
- 31.Wang, X., M. Gorospe, and N. J. Holbrook. 1999. gadd45 is not required for activation of c-Jun N-terminal kinase or p38 during acute stress. J. Biol. Chem. 274:29599-29602. [DOI] [PubMed] [Google Scholar]
- 32.Wang, X. W., Q. Zhan, J. D. Coursen, M. A. Khan, H. U. Kontny, L. Yu, M. C. Hollander, P. M. O'Connor, A. J. Fornace, Jr., and C. C. Harris. 1999. GADD45 induction of a G2/M cell cycle checkpoint. Proc. Natl. Acad. Sci. USA 96:3706-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhan, Q., M. J. Antinore, X. W. Wang, F. Carrier, M. L. Smith, C. C. Harris, and A. J. Fornace, Jr. 1999. Association with Cdc2 and inhibition of Cdc2/cyclin B1 kinase activity by the p53-regulated protein Gadd45. Oncogene 18:2892-2900. [DOI] [PubMed] [Google Scholar]
- 34.Zhan, Q., I. Bae, M. B. Kastan, and A. J. Fornace, Jr. 1994. The p53-dependent gamma-ray response of GADD45. Cancer Res. 54:2755-2760. [PubMed] [Google Scholar]
- 35.Zhan, Q., S. Fan, M. L. Smith, I. Bae, K. Yu, I. Alamo, Jr., P. M. O'Connor, and A. J. Fornace, Jr. 1996. Abrogation of p53 function affects gadd gene responses to DNA base-damaging agents and starvation. DNA Cell Biol. 15:805-815. [DOI] [PubMed] [Google Scholar]
- 36.Zhan, Q., S. Jin, B. Ng, J. Plisket, S. Shangary, A. Rathi, K. D. Brown, and R. Baskaran. 2002. Caspase-3 mediated cleavage of BRCA1 during UV-induced apoptosis. Oncogene 21:5335-5345. [DOI] [PubMed] [Google Scholar]
- 37.Zhan, Q., K. A. Lord, I. Alamo, Jr., M. C. Hollander, F. Carrier, D. Ron, K. W. Kohn, B. Hoffman, D. A. Liebermann, and A. J. Fornace, Jr. 1994. The gadd and MyD genes define a novel set of mammalian genes encoding acidic proteins that synergistically suppress cell growth. Mol. Cell. Biol. 14:2361-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]