Abstract
We have demonstrated that Epstein-Barr virus (EBV) confers enhanced growth capability in soft agarose, tumorigenesis in the SCID mouse, and resistance to apoptosis in the Burkitt's lymphoma cell line Akata. Subsequently, we have shown that EBV-encoded small RNAs (EBERs) are responsible for these phenotypes. We constantly observed the upregulation of bcl-2 oncoprotein expression upon EBV infection and expression of EBERs. To test whether these phenotypes were due to the upregulation of bcl-2 expression, we introduced bcl-2 into EBV-negative Akata cells at various levels encompassing the range at which EBV-positive cells expressed it. As cells expressed bcl-2 at higher levels, they became more capable of growing in soft agarose and became resistant to apoptosis. However, clones expressing bcl-2 at a higher level than EBV-positive Akata cells were negative in the tumorigenesis assay in the SCID mouse. On the other hand, introduction of bax into EBV-positive Akata cells reduced the resistance to apoptosis; however, it failed to reduce the growth capability in soft agarose. These data indicate that EBV targets not only bcl-2, but also an unknown pathway(s) to enhance the oncogenic potential of Akata cells.
Previously we established a system to test whether any cellular phenotypes of latency I Burkitt's lymphoma (BL) cells were due to Epstein-Barr virus (EBV), by using a cell line of BL origin, Akata, which has several unique characteristics among BL cell lines (24–27). We have demonstrated that EBV contributes to growth capability in soft agarose, tumorigenesis in immunodeficient mice, and resistance to apoptosis in Akata cells (12, 24). We also reported that EBV-determined nuclear antigen 1 (EBNA1) was not responsible for these phenotypes (12). Similar results were reported by two independent groups (4, 23). We further clarified that EBV-encoded RNAs (EBER-1 and -2) are responsible for these phenotypes (11).
The question that remained to be answered was the mechanism by which EBV contributes to these phenotypes. We constantly observed the upregulation of bcl-2 oncoprotein expression upon EBV infection or expression of EBERs in EBV-negative Akata cell clones (11, 12). A similar finding was also described by Ruf et al. (23). Distinct from other oncogenes, bcl-2 fosters cell survival rather than promoting cell proliferation. Since it is well known for its antiapoptotic function (20), it was assumed that the resistance to apoptosis was due to upregulation of bcl-2 protein. BL cells are predisposed to c-myc-induced apoptosis, since BL cells possess immunoglobulin (Ig)/c-myc translocation, which results in constitutive activation of the c-myc gene (9). Therefore, we hypothesized that upregulation of bcl-2 expression by EBV infection would protect cells from c-myc-induced apoptosis and allow c-myc to exert its oncogenic functions. To test this idea, we employed two approaches: (i) introduction of bcl-2 into EBV-negative Akata cells to test whether any phenotypes were restored and (ii) introduction of bax into EBV-positive Akata cells to antagonize the function of bcl-2 to determine whether any phenotypes were reduced.
Effect of bcl-2 expression on oncogenic potential and resistance to apoptosis in Akata cells.
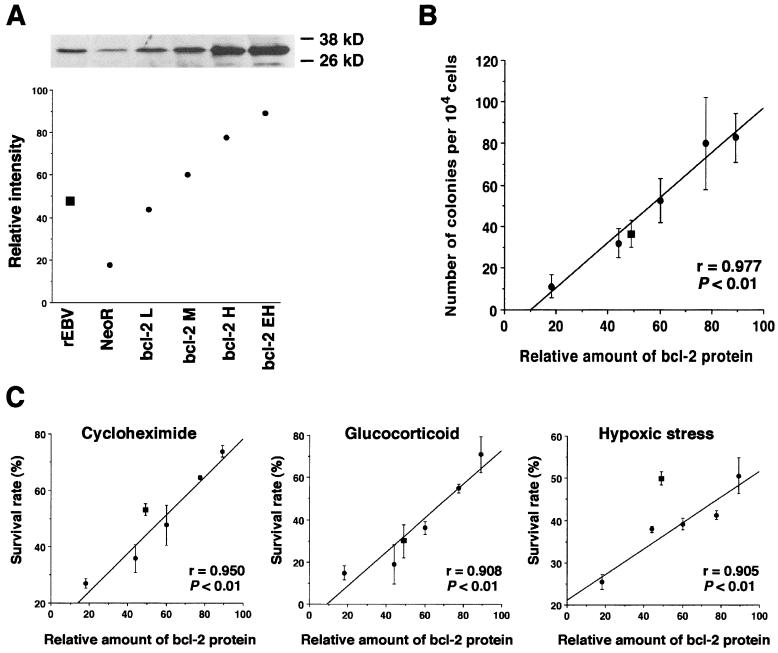
First, we introduced bcl-2 expression vector pBcl-2 into EBV-negative Akata cells. This pcDNA3-based vector carried human bcl-2α under control of a human cytomegalovirus promoter. We successfully isolated clones that expressed low to very high levels of bcl-2 protein (Fig. 1A). The expression of bcl-2 protein was detected by Western blot analysis with antihuman bcl-2 monoclonal antibody bcl-2/100 (Pharmingen). Neomycin resistance gene (neo)-transfected cell clones and EBV-reinfected cell clones were also isolated for use as negative and positive controls, respectively. The average relative signal intensity representing the amount of bcl-2 protein expressed was quantified by densitometric analysis and dot plotted in Fig. 1A. In this experiment, the level of bcl-2 expression detected by Western blot analysis appeared to be within the semiquantitative range. The average level of relative bcl-2 expression of EBV-reinfected cell clones was between those of clones with low and medium levels of bcl-2 expression. The growth rates among these cell clones were almost the same under serum-rich and low-serum conditions, except for clones with high and extra-high levels of bcl-2 expression under low-serum conditions. Using these cell clones, we carried out a soft agarose cloning assay, apoptosis assay, and tumorigenesis assay in SCID mice.
FIG. 1.
Effects of bcl-2 expression on clonability in soft agar and resistance to apoptosis in Akata cells. (A) Western blot analysis detecting bcl-2 protein of bcl-2-transfected cells, which expressed low (L), medium (M), high (H), and extra high (EH) levels. The data presented here are representative of four clones tested. The average relative signal intensity representing the amount of bcl-2 protein expressed in these cells was quantified by densitometric analysis and dot plotted. (B) Clonability in soft agarose. The mean values of the number of colonies that emerged in soft agar were plotted against the relative amounts of bcl-2 protein. Each dot represents the average number of colonies that emerged per 104 cells. (C) Resistance to apoptosis. The mean values of the survival rates against apoptotic stimuli were plotted against the relative amounts of bcl-2 protein. ●, bcl-2-transfected cells; ■, EBV-reinfected cells. Horizontal bars represent the mean values of each group. The bars show the mean values ± standard deviation of four clones.
For the soft agar colony assay, 104 cells were embedded in 0.4% SeaPlaque agarose containing RPMI 1640 and 12% fetal bovine serum as described previously (11). After 2 to 3 weeks of incubation, colonies that contained more than 100 live cells were counted. The mean values of the number of colonies that emerged in soft agarose were plotted against the relative amounts of bcl-2 protein. As a result, the number of colonies in soft agar was found to be in direct proportion to the relative amount of bcl-2 protein (Fig. 1B).
For the apoptosis assay, cells in the log phase were exposed to cycloheximide (20 μg/ml; Wako, Osaka, Japan), glucocorticoid (1 μM; Pharmacia and Upjohn), and a 100% CO2-saturated humidified atmosphere (hypoxic stress) as described previously (11). Viability of cells was quantified by a colorimetric assay (Cell Titer 96; Promega). The percent survival rate (%SR) was calculated by the formula %SR = {[(A570 of the sample) − (A570 of the blank)]/[(A570 of the control) − (A570 of the blank)]} × 100. The mean values of %SRs against all apoptotic stimuli for each clone were plotted against the relative amounts of bcl-2 protein (Fig. 1C). As a result, %SRs were also found to be in direct proportion to the relative amount of bcl-2 protein. It was noted that cells became resistant to hypoxic stress with a minimal increase of bcl-2 expression. This is consistent with the previous finding that upon hypoxic stress, the greatest difference of susceptibility to apoptotic cell death was seen between EBV-positive and -negative clones (11).
The tumorigenic potential of clones expressing higher levels of bcl-2 than EBV-infected Akata cells was tested. A total of 1.5 × 107 cells were inoculated into the thigh subcutis of 4-week-old male SCID mice as described previously (11). Those clones failed to develop tumor masses in the SCID mice (Table 1). Interestingly, the malignant phenotype of bcl-2-expressing Akata cell clones scored differently in the soft agarose colony assay and tumorigenesis assay in the SCID mouse. Historically, these results have been thought to reflect the “tumor cell” phenotype; however, our data suggested that this was not the case.
TABLE 1.
Tumorigenicity of bcl-2-transfected Akata cell clones in SCID micea
| Cell clone | No. of mice with tumors/ no. tested |
|---|---|
| EBV | |
| Positive | 3/3 |
| EBV Negative | 0/3 |
| EBV Reinfected | 8/9 (2/3, 3/3, 3/3) |
| EBER transfected | 7/15 (1/3, 2/3, 2/3, 1/3, 1/3) |
| neor transfected | 0/15 (0/3, 0/3, 0/3, 0/3, 0/3) |
| bcl-2 transfected | 0/15 (0/3, 0/3, 0/3, 0/3, 0/3) |
Five cell clones transfected with neor, EBER, or bcl-2 plasmid and three EBV-reinfected clones derived from an EBV-negative Akata cell clone (1.5 × 107 cells each) were individually inoculated into 4-week-old male SCID mice (Fox Chase C.B-17/Icr-scid Jcl; Clea, Tokyo, Japan). Mice were sacrificed 8 weeks after inoculation, and the developed tumors were measured. The tumors ranged from 0.8 to 4.5 cm in diameter.
Effect of bax expression on oncogenic potential and resistance to apoptosis in Akata cells.
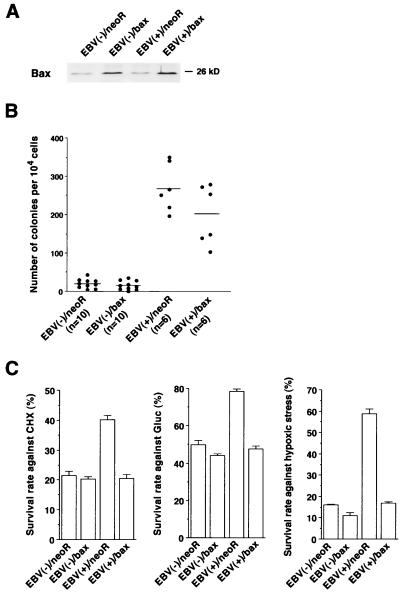
Second, we attempted to antagonize the function of bcl-2 by using bax, a homologue of bcl-2. bax binds to bcl-2 and inhibits its antiapoptotic function (21). We speculated that if the malignant phenotype and resistance to apoptosis depend on bcl-2 protein, expression of bax in EBV-positive Akata cells should lead to a loss of these phenotypes. We transfected bax expression plasmid pBax into both EBV-negative [EBV(−)] and -positive [EBV(+)] Akata cells. The expression vector for bax (pBax) was constructed by inserting the bax-α cDNA downstream of the SRα promoter, which drives transcription of a bicistronic mRNA for bax and neor mediated by an encephalomyocarditis virus internal ribosomal entry site sequence. We isolated G418-resistant cells that were designated EBV(−)/neor, EBV(−)/bax, EBV(+)/neor, and EBV(+)/bax. Expression of bcl-2 and bax protein in these cells was tested by Western blot analysis with antihuman bcl-2 monoclonal antibody bcl-2/100 and a rabbit anti-human bax polyclonal antibody (Pharmingen) (Fig. 2A). A small amount of bax protein was detected in EBV(−)/neoR and EBV(+)/neor cells; in contrast, EBV(−)/bax and EBV(+)/bax cells expressed approximately 2.1- and 2.5-fold more bax protein than EBV(−)/neor and EBV(+)/neor, respectively. The levels of bcl-2 protein expression in these cells were almost the same, except for EBV(−)/bax cells. They expressed 1.9-fold more bcl-2 protein than the others. Since expression of bax might oversensitize EBV(−) cells to apoptosis, cells expressing bcl-2 protein at a higher level seemed to be selected in the cloning process for EBV(−)/bax cells. A slightly reduced growth rate was seen in bax-transfected cells.
FIG. 2.
Effects of bax protein expression on clonability in soft agar and resistance to apoptosis of Akata cells. (A) Expression of bcl-2 and bax proteins in transfected cells. (B) Clonability in soft agarose. Each dot represents the average number of colonies that emerged per 104 cells. Horizontal bars represent the mean values of each group. (C) Resistance to apoptosis against apoptotic stimuli. The bars show the mean values ± standard deviation of three independent experiments. By t test analysis, the differences between mean values from EBV(+)/bax and EBV(+)/neoR cells were significant at P < 0.001 against all apoptotic inducers.
Cells were subjected to a soft agarose colony assay and apoptosis assay (Fig. 2B and C). Both EBV(−)/neor and EBV(−)/bax cells hardly formed colonies in soft agarose. The number of colonies seen for EBV(+)/neor cells was significantly higher than that for EBV(−)/neoR cells, which is consistent with previous findings (11, 12). The number of colonies of EBV(+)/bax cells was not significantly less than that of EBV(+)/neoR cells. In the apoptosis assay, EBV(+)/neoR cells were more resistant to apoptosis than EBV(−)/neoR cells in response to all stimuli. A slight reduction of %SRs was seen in EBV(−)/bax cells compared with EBV(−)/neoR cells. In contrast, a significant reduction of %SRs was found in EBV(+)/bax cells compared with EBV(+)/neoR cells. There is a report that bax protein functions in both bcl-2-dependent and -independent fashions (10, 29). Therefore, it remains a possibility that the phenotype seen in EBV(+)/bax cells might be partly due to the bcl-2-independent function of bax protein.
Using the transfectants derived from an EBV-negative Akata cell clone expressing various levels of bcl-2 proteins encompassing the range of EBV-reinfected Akata cell clones, we demonstrated that: (i) bcl-2 expression conferred resistance to apoptosis, (ii) bcl-2 expression contributed to the growth capability in soft agarose, (iii) the effects of bcl-2 expression in these assays were dose dependent, and (iv) bcl-2 expression was insufficient to support tumorigenesis in the SCID mouse. In the bax study, we demonstrated that the bax expression reduced the resistance to apoptosis, whereas the effect on the growth capability in soft agarose was modest. Those data strongly support the idea that EBV targets not only bcl-2, but also an unknown cellular factor(s) to confer the malignant phenotype and resistance to apoptosis seen in the EBV-positive Akata cells.
The tumorigenic potential of bcl-2 has been clearly demonstrated in rodent systems by transfection of the bcl-2 expression plasmid into NIH 3T3 cells in vitro (22), and in a bcl-2 transgenic mouse study in which follicular lymphoproliferations progressed in the long term to high-grade malignant lymphoma (15, 16). Furthermore, it is widely accepted that bcl-2 synergizes with the c-myc oncogene in tumor progression. This was suggested by clinical investigations indicating that activation of both c-myc and bcl-2 may have conferred an aggressive clinical outcome in lymphoma cases (3, 8, 19). This idea was also demonstrated in a transgenic mouse study, in which bcl-2/c-myc double transgenic mice displayed accelerated lymphomagenesis (6, 14). In mammalian cells, deregulated expression of c-myc has been shown to contribute not only to tumorigenesis (13), but also to induce apoptosis in various cell lines, including BL cell lines (1, 5, 17). The mechanism of bcl-2/c-myc synergy seems to be that bcl-2 protects cells from c-myc-induced apoptosis (2, 28). Like Akata cells (26), all of the BL cells possess a chromosomal translocation involving the c-myc locus, which is believed to result in constitutive activation of the c-myc gene (9). Therefore, BL cells were thought to be predisposed to c-myc-induced apoptosis. Our data imply that EBV infection upregulates expression of bcl-2 protein to protect cells from c-myc-induced apoptosis and to allow c-myc to exert its oncogenic functions. However, other unknown pathways remain to be verified to explain the mechanism by which EBV contributes to the genesis of BL.
The role of bcl-2 in the development of BL has been largely unknown. Although attempts to detect bcl-2 protein expression in tumor biopsy samples failed (7, 18), several lines of evidence supported the hypothesis that BL cell lines with type I latency expressed bcl-2 protein at a low level (18, 23). Since (i) the level of bcl-2 expression in type I BL cell lines is relatively low compared to that in type III BL cell lines and EBV-immortalized lymphoblastoid cell lines (18) and (ii) there is no ideal tissue culture system available to demonstrate the role of bcl-2 in the type I BL cell lines, the significance of bcl-2 expression in the development of BL remains to be validated.
Acknowledgments
We thank S. Takahashi, T. Miyashita, and K. Shimotohno for bcl-2, bax, and internal ribosomal entry site plasmids, respectively. We thank K. Adachi for technical assistance.
This work was supported by grants-in-aid from the Ministry of Education, Science, Sports, and Culture, Japan, and from the Princess Takamatsu Fund.
REFERENCES
- 1.Askew D S, Ashmun R A, Simmons B C, Cleveland J L. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991;6:1915–1922. [PubMed] [Google Scholar]
- 2.Bissonnette R P, Echeverri F, Mahboubi A, Green D R. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature. 1992;359:552–554. doi: 10.1038/359552a0. [DOI] [PubMed] [Google Scholar]
- 3.Brito-Babapulle V, Crawford A, Khokhar T, Laffan M, Matutes E, Fairhead S, Catovsky D. Translocations t(14;18) and t(8;14) with rearranged bcl-2 and c-myc in a case presenting as B-ALL (L3) Leukemia. 1991;5:83–87. [PubMed] [Google Scholar]
- 4.Chodosh J, Holder V P, Gan Y, Belgaumi A, Sample J, Sixbey J W. Eradication of latent Epstein-Barr virus by hydroxyurea alters the growth-transformed cell phenotype. J Infect Dis. 1998;177:1194–1201. doi: 10.1086/515290. [DOI] [PubMed] [Google Scholar]
- 5.Evan G I, Wyllie A H, Gilbert C S, Littlewood T D, Land H, Brooks M, Waters C M, Penn L Z, Hancock D C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 6.Fanidi A, Harrington E A, Evan G I. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature. 1992;359:554–556. doi: 10.1038/359554a0. [DOI] [PubMed] [Google Scholar]
- 7.Henderson S, Rowe M, Gregory C, Croom-Carter D, Wang F, Longnecker R, Kieff E, Rickinson A. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991;65:1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- 8.Karsan A, Gascoyne R D, Coupland R W, Shepherd J D, Phillips G L, Horsman D E. Combination of t(14;18) and a Burkitt's type translocation in B-cell malignancies. Leuk Lymphoma. 1993;10:433–441. doi: 10.3109/10428199309148200. [DOI] [PubMed] [Google Scholar]
- 9.Klein G. The role of gene dosage and genetic transpositions in carcinogenesis. Nature. 1981;294:313–318. doi: 10.1038/294313a0. [DOI] [PubMed] [Google Scholar]
- 10.Knudson C M, Korsmeyer S J. Bcl-2 and Bax function independently to regulate cell death. Nat Genet. 1997;16:358–363. doi: 10.1038/ng0897-358. [DOI] [PubMed] [Google Scholar]
- 11.Komano J, Maruo S, Kurozumi K, Oda T, Takada K. Oncogenic role of Epstein-Barr virus-encoded RNAs in Burkitt's lymphoma cell line Akata. J Virol. 1999;73:9827–9831. doi: 10.1128/jvi.73.12.9827-9831.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Komano J, Sugiura M, Takada K. Epstein-Barr virus contributes to the malignant phenotype and to apoptosis resistance in Burkitt's lymphoma cell line Akata. J Virol. 1998;72:9150–9156. doi: 10.1128/jvi.72.11.9150-9156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Land H, Parada L F, Weinberg R A. Cellular oncogenes and multistep carcinogenesis. Science. 1983;222:771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- 14.Marin M C, Hsu B, Stephens L C, Brisbay S, McDonnell T J. The functional basis of c-myc and bcl-2 complementation during multistep lymphomagenesis in vivo. Exp Cell Res. 1995;217:240–247. doi: 10.1006/excr.1995.1083. [DOI] [PubMed] [Google Scholar]
- 15.McDonnell T J, Deane N, Platt F M, Nunez G, Jaeger U, McKearn J P, Korsmeyer S J. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989;57:79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- 16.McDonnell T J, Korsmeyer S J. Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t(14;18) Nature. 1991;349:254–256. doi: 10.1038/349254a0. [DOI] [PubMed] [Google Scholar]
- 17.Milner A E, Grand R J A, Waters C M, Gregory C D. Apoptosis in Burkitt lymphoma cells is driven by c-myc. Oncogene. 1993;8:3385–3391. [PubMed] [Google Scholar]
- 18.Milner A E, Johnson G D, Gregory C D. Prevention of programmed cell death in Burkitt lymphoma cell lines by bcl-2-dependent and -independent mechanisms. Int J Cancer. 1992;52:636–644. doi: 10.1002/ijc.2910520424. [DOI] [PubMed] [Google Scholar]
- 19.Mohammad R M, Mohamed A N, Smith M R, Jawadi N S, Al-Katib A. A unique EBV-negative low-grade lymphoma line (WSU-FSCCL) exhibiting both t(14;18) and t(8;11) Cancer Genet Cytogenet. 1993;70:62–67. doi: 10.1016/0165-4608(93)90132-6. [DOI] [PubMed] [Google Scholar]
- 20.Nunez G, London L, Hockenbery D, Alexander M, McKearn J P, Korsmeyer S J. Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J Immunol. 1990;144:3602–3610. [PubMed] [Google Scholar]
- 21.Oltvai Z N, Milliman C L, Korsmeyer S J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 22.Reed J C, Cuddy M, Slabiak T, Croce C M, Nowell P C. Oncogenic potential of bcl-2 demonstrated by gene transfer. Nature. 1988;336:259–261. doi: 10.1038/336259a0. [DOI] [PubMed] [Google Scholar]
- 23.Ruf I K, Rhyne P W, Yang H, Borza C M, Hutt-Fletcher L M, Cleveland J L, Sample J T. Epstein-Barr virus regulates c-MYC, apoptosis, and tumorigenicity in Burkitt lymphoma. Mol Cell Biol. 1999;19:1651–1660. doi: 10.1128/mcb.19.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimizu N, Tanabe-Tochikura A, Kuroiwa Y, Takada K. Isolation of Epstein-Barr virus (EBV)-negative cell clones from the EBV-positive Burkitt's lymphoma (BL) line Akata: malignant phenotypes of BL cells are dependent on EBV. J Virol. 1994;68:6069–6073. doi: 10.1128/jvi.68.9.6069-6073.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimizu N, Yoshiyama H, Takada K. Clonal propagation of Epstein-Barr virus (EBV) recombinants in EBV-negative Akata cells. J Virol. 1996;70:7260–7263. doi: 10.1128/jvi.70.10.7260-7263.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takada K, Horinouchi K, Ono Y, Aya T, Osato T, Takahashi M, Hayasaka S. An Epstein-Barr virus-producer line Akata: establishment of the cell line and analysis of viral DNA. Virus Genes. 1991;5:147–156. doi: 10.1007/BF00571929. [DOI] [PubMed] [Google Scholar]
- 27.Takada K, Ono Y. Synchronous and sequential activation of latently infected Epstein-Barr virus genomes. J Virol. 1989;63:445–449. doi: 10.1128/jvi.63.1.445-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaux D L, Cory S, Adams J M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 29.Zha H, Reed J C. Heterodimerization-independent functions of cell death regulatory proteins Bax and Bcl-2 in yeast and mammalian cells. J Biol Chem. 1997;272:31482–31488. doi: 10.1074/jbc.272.50.31482. [DOI] [PubMed] [Google Scholar]