Abstract
Chloroplast ATP synthase (CFoCF1) synthesizes ATP by using a proton electrochemical gradient across the thylakoid membrane, termed ΔμH+, as an energy source. This gradient is necessary not only for ATP synthesis but also for reductive activation of CFoCF1 by thioredoxin, using reducing equivalents produced by the photosynthetic electron transport chain. ΔμH+ comprises two thermodynamic components: pH differences across the membrane (ΔpH) and the transmembrane electrical potential (ΔΨ). In chloroplasts, the ratio of these two components in ΔμH+ is crucial for efficient solar energy utilization. However, the specific contribution of each component to the reductive activation of CFoCF1 remains unclear. In this study, an in vitro assay system for evaluating thioredoxin-mediated CFoCF1 reduction is established, allowing manipulation of ΔμH+ components in isolated thylakoid membranes using specific chemicals. Our biochemical analyses revealed that ΔpH formation is essential for thioredoxin-mediated CFoCF1 reduction on the thylakoid membrane, whereas ΔΨ formation is nonessential.
Keywords: chloroplast ATP synthase, proton electrochemical gradient, redox regulation, thioredoxin
In chloroplasts, solar energy is converted to chemical energy via the photosynthetic electron transport chain in the thylakoid membrane. During this process, reducing equivalents are stored as NADPH, and a proton electrochemical gradient (ΔμH+) forms across the membrane. Termed the proton-motive force, ΔμH+ drives chloroplast FoF1-ATP synthase (CFoCF1) to catalyze ATP synthesis (1, 2). Therefore, CFoCF1 is a pivotal enzyme for energy conversion in photosynthesis. Moreover, its activity undergoes precise regulation to maintain efficient chemical energy production under varying light conditions.
ΔμH+ comprises two thermodynamic components: pH differences across the membrane (ΔpH) and the transmembrane electrical potential (ΔΨ). Both components were reported to be kinetically equivalent in FoF1 from thermophilic bacteria (3), and early investigations into CFoCF1 revealed that both contribute to ATP synthesis by this enzyme complex (4, 5, 6). Notably, CFoCF1 exhibits thiol-based redox regulation, distinguishing it among all FoF1 enzymes across species (7, 8), with this regulation system closely linked to ΔμH+. The central axis of CFoCF1, the γ subunit (CF1-γ), harbors a pair of redox-active cysteines (Cys199 and Cys205 in Spinacia oleracea) (9, 10). To activate CFoCF1, chloroplast thioredoxin (Trx) reduces this Cys pair, using reducing equivalents from photosynthetic electron transport reactions (7, 11). In this Trx-dependent activation process, ΔμH+ formation is essential for CF1-γ reduction (12, 13, 14). Conversely, we previously revealed that ΔμH+ dissipation stimulates CF1-γ oxidation (15, 16), facilitated by chloroplast oxidizing factors, such as Trx-like proteins (16, 17, 18). Consequently, CFoCF1 activity is finely tuned to activate only during photosynthetic conditions, promptly deactivating in the dark when ΔμH+ formation ceases. However, the relationship between CFoCF1 redox regulation and the ΔμH+ components, i.e., ΔpH or ΔΨ, remains unexplored.
In chloroplasts, ΔpH plays an important role in regulating photosynthetic performance. Specific ion transporters, such as two-pore potassium channel three and voltage-dependent chloride channel 1, present in the thylakoid membrane contribute to the movement of counter ions for dissipating ΔΨ (19, 20), maintaining a high ΔpH relative to ΔΨ. Acidification of the lumen (i.e., ΔpH formation) regulates the electron transfer activity of the cytochrome b6f complex (21, 22). Additionally, ΔpH serves as a key signal for initiating nonphotochemical quenching (NPQ), which functions as a photoprotection mechanism, especially the qE component (23). Therefore, green plants must maintain a balanced ΔpH-to-ΔΨ ratio within ΔμH+ for safe light utilization.
This study focuses on the thermodynamic components of ΔμH+ required for CF1-γ reduction by Trx. Our investigation involved manipulating ΔμH+ bias in isolated spinach thylakoids using specific chemicals. We also established an in vitro assay system using freshly prepared thylakoid membranes to facilitate CF1-γ reduction by Trx on the membrane.
Results and discussion
Establishing biased ΔμH+ conditions in the thylakoid membrane using ionophores
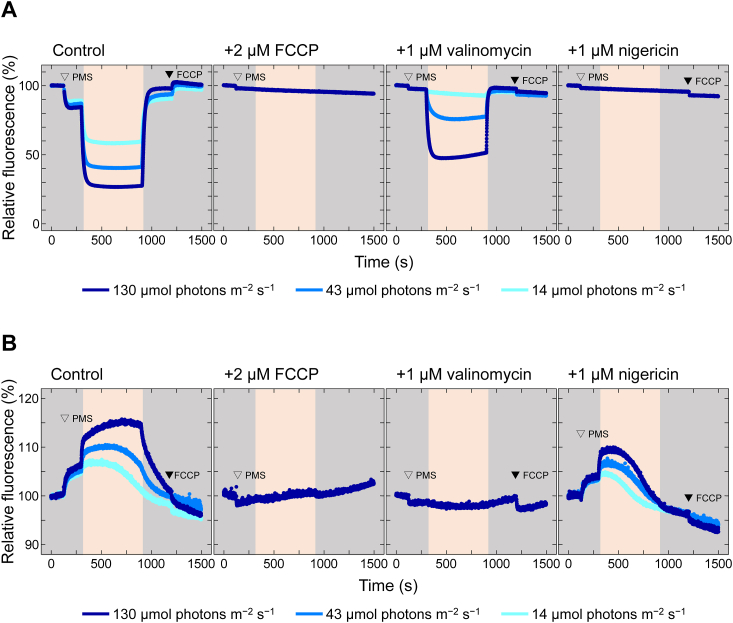
H+ translocation across the membrane concurrently generates ΔpH and ΔΨ. Through the application of ionophores, such as nigericin, valinomycin, or the uncoupler FCCP, we can establish conditions where either ΔpH or ΔΨ forms in the thylakoid membrane or neither form. Using this approach, we aimed to discern differences in the contributions of ΔpH and ΔΨ to CF1-γ redox regulation. Specifically, we used the fluorescent reagents 9-amino-6-chloro-2-methoxyacridine (ACMA) and 8-anilinonaphthalene-1-sulfonic acid (ANS) to monitor ΔpH and ΔΨ formation on the thylakoid membrane, respectively (Fig. 1). ACMA fluorescence intensity decreases upon protonation (24, 25), whereas ANS fluorescence increases upon potentiated membrane binding (26, 27). The addition of the artificial electron mediator 1-methoxy-5-methylphenazinium methylsulfate (PMS) to the thylakoid membrane induces ΔμH+ formation across the membrane under light due to pseudocyclic electron transport around photosystem I (28). We then monitored ΔpH formation by observing the ACMA fluorescence decrease (120 s; Fig. 1A, Control). Subsequently, red light irradiation further enhanced ΔpH formation (300 s; highlighted area of the graph in Fig. 1), dependent on light intensity (14–130 μmol photons m−2 s−1). ΔpH remained constant during red light irradiation and dissipated upon its cessation (900 s), finally returning to initial levels upon FCCP addition (1200 s). Similar temporal events were observed for ΔΨ formation using ANS (Fig. 1B, Control), with ΔΨ gradually declining under low light conditions (14 and 43 μmol photons m−2 s−1).
Figure 1.
Ionophore effects on light-induced ΔμH+in the thylakoid membrane. Observations of ΔpH and ΔΨ across the thylakoid membrane induced by red light irradiation. (A) ΔpH measurement using ACMA. Thylakoid membranes (5 μg chlorophyll (Chl)/ml) were incubated with 0.3 μg/ml ACMA, and ACMA fluorescence intensity (λex = 410 nm, λex = 480 nm) was monitored. (B) ΔΨ measurement using ANS. Thylakoid membranes (5 μg Chl/ml) were incubated with 100 μM ANS, and ANS fluorescence intensity (λex = 330 nm, λex = 455 nm) was monitored. Gray sections indicate dark conditions, whereas highlighted sections indicate red light irradiation periods. Open and closed triangles represent the addition of 2 μM 1-methoxy PMS and 1 μM FCCP, respectively.
The effects of FCCP, valinomycin, and nigericin on ΔpH and ΔΨ formation under the aforementioned light conditions were then examined. FCCP selectively permeates H+ across the membrane, abolishing both ΔpH and ΔΨ (i.e., ΔμH+). Pretreatment of the thylakoid membrane with FCCP almost abolished fluorescence changes in both ACMA and ANS (Fig. 1, A and B, +2 μM FCCP). Nigericin disrupts only ΔpH formation by exchanging lumen H+ for stromal K+, whereas valinomycin disrupts only ΔΨ formation by selectively transporting K+. To use these ionophores, 50 mM KCl was supplemented in the grinding buffer for thylakoid membrane preparation. Pretreatment of the thylakoid membrane with valinomycin led to ACMA but not ANS fluorescence changes in a light-dependent manner (Fig. 1, A and B, +1 μM valinomycin). Conversely, pretreatment of the thylakoid membrane with nigericin caused minimal ACMA fluorescence change (Fig. 1A, +1 μM nigericin). In contrast, changes in ANS fluorescence resembled those of control conditions under any examined light conditions, albeit to a lesser extent (Fig. 1B, +1 μM nigericin). These results highlight the successful identification of varying ionophore actions in the thylakoid membrane as well as the conditions distinguishing both ΔpH and ΔΨ.
Historically, the electrochromic shift in endogenous carotenoid absorbance at 515 nm (ΔA515) has been used to monitor the transmembrane ΔΨ level (29). ΔA515 can be observed by briefly exposing isolated thylakoids to actinic light flashes. However, we employed ACMA and ANS in this study. This is because these fluorescent reagents have a history of being used in combination with ionophores and uncoupler to measure the enzymatic activity of ATP synthase and other ion transporters in in vitro studies, and their effectiveness has been confirmed (16, 30, 31, 32).
Trx-mediated CF1-γ reduction on the thylakoid membrane fails to occur without ΔpH formation
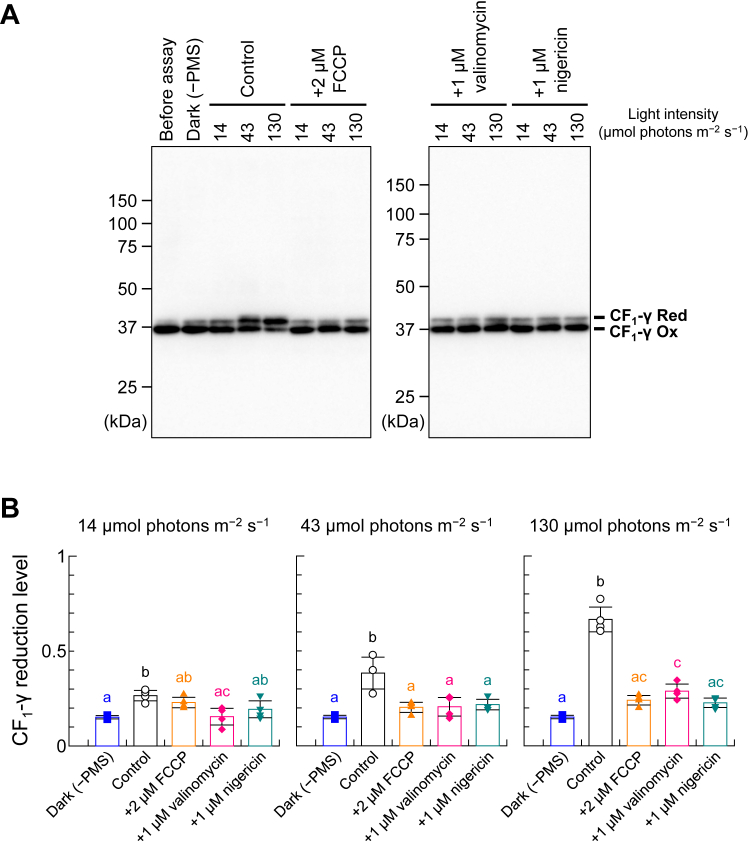
Using the abovementioned conditions to distinguish ΔpH and ΔΨ (Fig. 1), we performed Trx-mediated reduction assays of CF1-γ on the thylakoid membrane (Fig. 2). In vitro reduction experiments involving CF1-γ were performed using the same concentrations of thylakoid membrane [5 μg chlorophyll (Chl)/ml] and 1-methoxy PMS (2 μM) shown in Figure 1. The redox state of CF1-γ was determined using 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid (AMS), as described previously (16). CF1-γ is known to be reduced by Trx-f, a major isoform of chloroplast Trx proteins (33, 34). This reduction process requires prior ΔμH+ formation across the thylakoid membrane (7, 13, 14). As expected, CF1-γ on the thylakoid membrane was not reduced by 1 μM Trx-f in the presence of 100 μM dithiothreitol (DTT) under dark conditions without PMS [Fig. 2B, Dark (−PMS)]. Upon performing the reduction assay under the same light conditions shown in Figure 1 (14–130 μmol photons m−2 s−1), we observed a light intensity-dependent reduction of CF1-γ (Fig. 2B, Control). Thus, the CF1-γ reduction level was controlled by the extent of ΔμH+ across the thylakoid membrane. Next, we investigated the effects of an uncoupler and ionophores under the conditions shown in Figure 1 (2 μM FCCP, 1 μM valinomycin, or 1 μM nigericin), and found that CF1-γ reduction by Trx was significantly inhibited by these chemicals under all tested light conditions.
Figure 2.
Characterization of ΔμH+-dependent thylakoid CF1-γ reduction by Trx.A, determination of the thylakoid CF1-γ redox state. CF1-γ in the thylakoid membrane (5 μg Chl/ml) was reduced by 1 μM Trx-f and 100 μM DTT in the presence of each ionophore under the indicated light conditions for 5 min. After free thiol modification with AMS, proteins underwent nonreducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by western blotting with anti-CF1-γ antibodies. Ox, oxidized form; Red, reduced form. B, quantification of CF1-γ reduction levels for the data shown in (A). Data represent means ± standard deviations (SDs; n = 3–4). Different letters indicate significant differences (p < 0.05; one-way analysis of variance and Tukey’s honest significance differences test).
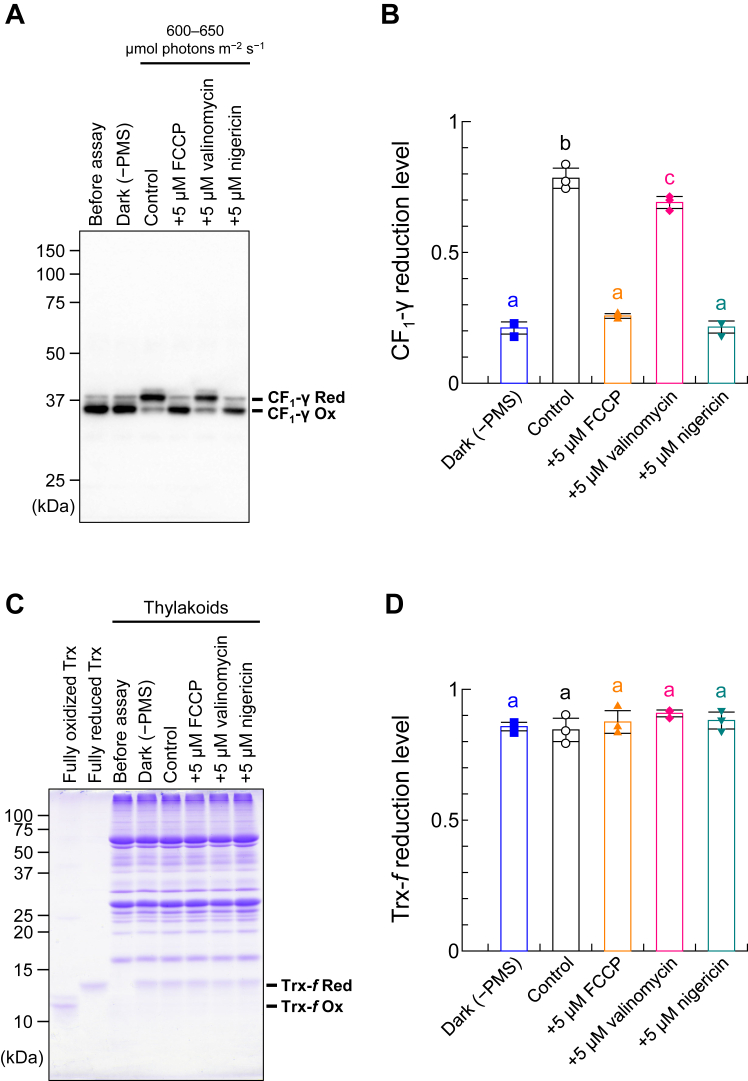
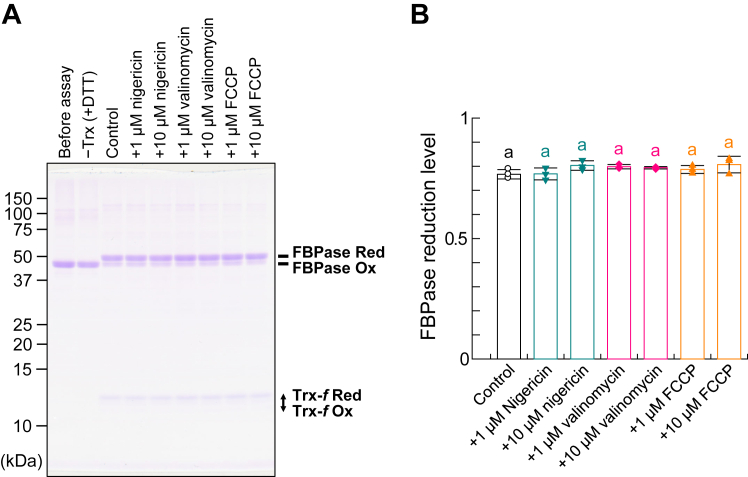
To further explore the relationship between ΔμH+ and CF1-γ reduction, we used higher concentrations of the thylakoid membrane (50 μg Chl/ml) and 1-methoxy PMS (100 μM) under more intense light conditions (600–650 μmol photons m−2 s−1), following our previous study (16, 34) (Fig. 3). Although CF1-γ was not reduced in the dark [Fig. 3, A and B, Dark (−PMS)], it was reduced by approximately 80% when 1 μM Trx and 100 μM DTT were added in the light (Fig. 3, A and B, Control). When FCCP or nigericin was added to the thylakoid membrane beforehand, CF1-γ was not reduced, similar to dark conditions (Fig. 3, A and B, +5 μM FCCP, +5 μM nigericin) as well as the results shown in Figure 2. Both FCCP and nigericin dissipated ΔpH formation across the thylakoid membrane, as confirmed via fluorescence measurements (Fig. 1A, +2 μM FCCP, +1 μM nigericin). However, even in the presence of valinomycin, CF1-γ was reduced by approximately 70% (Fig. 3, A and B, +5 μM valinomycin). Under these experimental conditions, Trx-f was almost completely reduced when sufficient amounts of DTT were added (Fig. 3, C and D). We also examined whether FCCP and ionophores affect the Trx-dependent reduction of other target enzymes by testing FBPase reduction via Trx-f in the presence of FCCP or ionophores. Notably, FBPase is the major target enzyme of Trx-f in chloroplasts (35, 36). As shown in Figure 4, Trx-f reduced FBPase efficiently even in the presence of FCCP or ionophores in the reaction mixture, implying that the inhibited CF1-γ reduction shown in Figures 2 and 3 was due to ΔpH dissipation caused by FCCP or nigericin. Hence, ΔpH but not ΔΨ formation across the thylakoid membrane was required for CF1-γ reduction. The varying results for valinomycin with different thylakoid membrane concentrations may be attributed to differences in membrane stability under the respective experimental conditions. Higher membrane concentrations may maintain stability and reduce H+ leakage.
Figure 3.
In vitro CF1-γ reduction using high-concentration thylakoid membranes. A and C, determination of the thylakoid CF1-γ and Trx-f redox states. CF1-γ in the thylakoid membrane (50 μg Chl/ml) was reduced by 1 μM Trx-f and 100 μM DTT in the presence of each ionophore under 600 to 650 μmol photons m−2 s−1 for 5 min. After free thiol modification with AMS, proteins underwent nonreducing SDS-PAGE, and the redox state was visualized via western blotting with anti-CF1-γ antibodies (A) or Coomassie Brilliant Blue staining (C). Ox, oxidized form; Red, reduced form. B and D, quantification of the CF1-γ and Trx-f reduction levels for the data shown in (A) and (C), respectively. Data represent means ± SDs (n = 3). Different letters indicate significant differences (p < 0.05; one-way ANOVA and Tukey’s HSD test).
Figure 4.
FBPase reduction by Trx-f in the presence of ionophores. A, FBPase (2 μM) was incubated with 1 μM Trx-f and 100 μM DTT for 30 min. After free thiol modification with AMS, proteins underwent nonreducing SDS-PAGE followed by Coomassie Brilliant Blue staining. B, quantification of the FBPase redox state for the data shown in (A). Data represent means ± SDs (n = 3). Different letters indicate significant differences (p < 0.05; one-way ANOVA and Tukey’s HSD test).
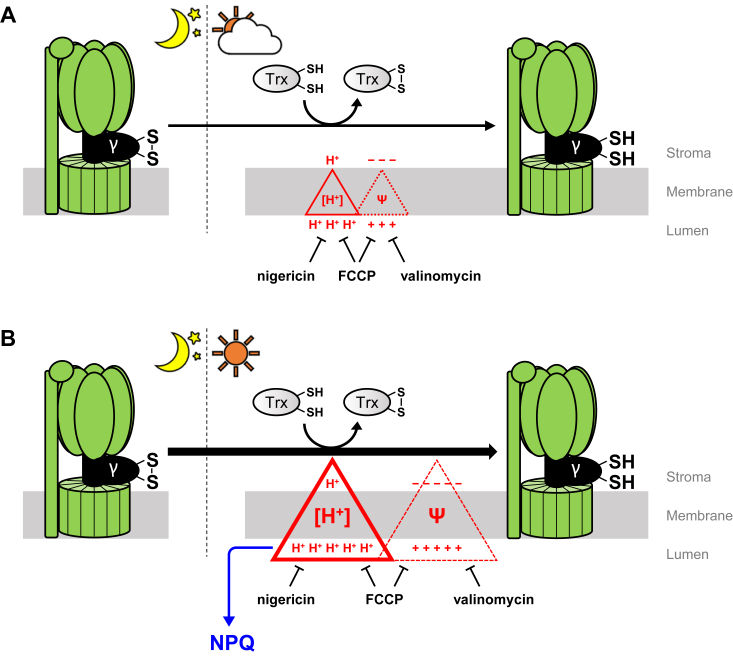
Our results raise an important question: does ΔΨ have any effect on CF1-γ reduction? As shown in Figure 2, CF1-γ reduction did not occur with pretreatment of either nigericin or valinomycin but was observed in the control experiment, especially under 130 μmol photons m−2 s−1. These results imply that ΔΨ supports CF1-γ reduction when ΔpH is low. Notably, the extent of ΔpH with added valinomycin was slightly lower than that under control conditions (Fig. 1A, Control, +1 μM valinomycin). However, CF1-γ was reduced when valinomycin was added under more intense light conditions (Fig. 3, A and B, +5 μM valinomycin). Thus, when sufficient ΔpH forms across the thylakoid membrane, CF1-γ reduction occurs regardless of ΔΨ formation, i.e., the extent of ΔpH governs the CF1-γ reduction process. This reduction process observed in this study under different light conditions was illustrated, along with the effect of ionophores and uncoupler used (Fig. 5). Alkaline conditions near the surface of the thylakoid membrane may be favorable for the dithiol-disulfide exchange reaction between CF1-γ and Trx. However, this detailed molecular mechanism is not yet clear, and further studies are required. As ΔpH across the thylakoid membrane induces NPQ, it is considered crucial in plant physiology, whereas ΔΨ is used exclusively to regulate ΔpH. Overall, the ability to activate CFoCF1 reductively via Trx under fluctuating light conditions without relying on ΔΨ formation is likely advantageous for plants.
Figure 5.
An overview of the relationship between the redox regulation of CF1-γ and ΔpH formation. The formation of ΔμH+ when CF1-γ is reduced by Trx under low light (A) or high light (B) conditions are illustrated. Triangles represent the extent of ΔpH across the thylakoid membrane, and dashed triangles represent the extent of ΔΨ across the thylakoid membrane.
Experimental procedures
Preparation of thylakoid membranes from spinach leaves
Thylakoid membranes were prepared from spinach (S. oleracea) as previously described (34) but with slight modifications. Fresh market spinach was washed thoroughly and left overnight in the dark at 4 °C. Harvested leaves (approximately 10 g fresh weight) were homogenized three times for 3 s in a mixer with 200 ml of grinding buffer [50 mM Tricine-NaOH (pH 7.5), 0.4 M sucrose, 5 mM MgCl2, 10 mM NaCl, and 50 mM KCl]. The homogenate was filtered through four layers of gauze and centrifuged at 3,000g and 4 °C for 10 min. The pellet was then resuspended in the grinding buffer and centrifuged at 300g and 4 °C for 1 min, after which the supernatant was collected and centrifuged at 3,000g and 4 °C for 10 min. After the abovementioned washing step was repeated once, the resulting pellet was resuspended in the grinding buffer to achieve a Chl concentration of 0.5 mg/ml. The preparation was kept in the dark on ice for at least 1 h before the assay.
Monitoring the formation of proton gradients and membrane potential gradients across thylakoid membranes
We measured ΔpH and ΔΨ across the thylakoid membrane using ACMA and ANS, respectively. The grinding buffer for thylakoid preparation was used as the reaction mixture, with the reaction performed at 25 °C. Red light irradiation at 660 nm induced the formation of these thermodynamic gradients across the thylakoid membrane. Before use, ACMA was solubilized at 30 μg/ml in 100% ethanol and stored at −80 °C. ANS was prepared as a 10 mM solution in 10% DMSO and stored at room temperature. The emitted fluorescence of ACMA (λex = 410 nm, λex = 480 nm) and ANS (λex = 330 nm, λex = 455 nm) was measured using a FP-8500 spectrofluorometer (Jasco).
Stored ACMA or ANS solution (20 μl) and 50 μl of 200 μg Chl/ml thylakoid membrane were added to 1910 μl of the grinding buffer in a grass cuvette and left to stand for stabilization of the fluorescence signal. At 120 s after initiating the measurement, 20 μl of 200 μM 1-methoxy PMS was added to the mixture. The final concentrations in the cuvette were 5 μg Chl/ml of thylakoid membranes, 2 μM 1-methoxy PMS, and 0.3 μg/ml ACMA (in 0.1% ethanol) or 100 μM ANS (in 0.01% DMSO). Subsequently, the reaction mixture was irradiated with red light from a direction perpendicular to the cuvette using a light-emitting diode (LED) at 300 s and terminated at 900 s. The photon flux density of the red light is shown in Figure 1. At 1200 s, 2 μl of 2 mM FCCP was added to the mixture to confirm whether ΔμH+ was dissipated compared with the initial condition. The initial fluorescence intensity of each trace was normalized to 100% using the average of the data from approximately 30 to 90 s, i.e., when fluorescence intensity was relatively stable.
Recombinant protein preparation
The recombinant proteins used in this study, spinach Trx-f and Arabidopsis thaliana FBPase, were prepared as described previously (34, 37). Protein concentrations were determined using a BCA protein assay (Pierce).
In vitro assay of Trx-mediated CFoCF1 reduction
For the reduction assay, the grinding buffer for thylakoid membrane preparation was used as the reaction mixture, and the reaction was performed at 25 °C. Prior to the assay, 10 μM Trx-f was incubated with 1 mM DTT in the grinding buffer for 5 min. Subsequently, 200 μl of the Trx-f/DTT mixture and 50 μl of 200 mg Chl/ml thylakoid membrane were added to 1730 μl of the grinding buffer and incubated for 1 min. Next, 20 μl of 200 μM 1-methoxy PMS was added to the mixture. The final concentrations in the mixture were 5 μg Chl/ml thylakoid membranes, 2 μM 1-methoxy PMS, 100 μM DTT, and 1 μM Trx-f. This mixture was irradiated with red light at 660 nm for 5 min using an LED to initiate ΔμH+ formation across the thylakoid membrane. The photon flux density of the red light is shown in Figure 2. Similar experiments were performed using higher thylakoid membrane concentrations (final concentration, 50 μg Chl/ml) and 1-methoxy PMS (final concentration, 100 μM) under more intense light conditions (600–650 μmol photons m−2 s−1). Following the in vitro assay, proteins were precipitated using 10% (w/v) trichloroacetic acid to stop the reduction reaction.
In vitro assay of Trx-mediated FBPase reduction
For FBPase reduction, a medium containing 50 mM Tris-HCl (pH 7.5) and 50 mM NaCl was used, with the reaction performed at 25 °C. Protein and reducing agent concentrations as well as reaction times are described in the Figure 4 legend.
Determination of the protein redox state
The protein redox state was determined by labeling free thiols with AMS and employing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (for Trx-f and FBPase) or immunoblotting (for CF1-γ), as described previously (34, 37). The antibody against CF1-γ were prepared using recombinant Arabidopsis CF1-γ (His-tagged at the C terminus) as an antigen, and its specificity is indicated in our former paper (38). Chemiluminescence was detected using horseradish peroxidase–conjugated secondary antibodies and ECL Prime (Cytiva) and visualized on a LAS 3000 Mini Imaging System (Fuji Film). The resultant band intensities were quantified using ImageJ. The reduction level was calculated as the ratio of the reduced form to the total form. The data in Figure 2, Figure 3, Figure 4 were statistically analyzed using one-way analysis of variance and Tukey’s honest significance differences test (p < 0.05). Statistical analyses were performed using an online calculator at iCalcu.com (https://www.icalcu.com/stat/anova-tukey-hsd-calculator.html).
Data availability
All data are contained within the article.
Conflict of interest
The authors declare that they have no conflicts of interest regarding the content of this article.
Acknowledgments
We acknowledge the Open Facility Center, Tokyo Institute of Technology, for supporting DNA sequencing.
Author contributions
T. S. and T. H. conceptualization; T. S. investigation; K. Y. resources; T. S. writing–original draft; K. Y., K. W., and T. H. writing–review and editing; K. W. and T. H. supervision.
Funding and additional information
This study was supported by Grants-in-Aid for Scientific Research (Grant 21H02502 to T. H.) from the Japan Society for the Promotion of Science, and partly by a Grant-in-Aid for JSPS Research Fellows (Grant 22J13334 to T. S.) as well as the Dynamic Alliance for Open Innovation Bridging Human, Environment and Materials.
Reviewed by members of the JBC Editorial Board. Edited by Joseph Jez
Footnotes
Present Address for Ken-ichi Wakabayashi: Faculty of Life Sciences, Kyoto Sangyo University, Kamigamo-motoyama, Kita-ku, Kyoto 603-8555, Japan.
References
- 1.Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol. Rev. Camb. Philos. Soc. 1966;41:445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- 2.Jagendorf A.T., Uribe E. ATP formation caused by acid-base transition of spinach chloroplasts. Proc. Natl. Acad. Sci. U. S. A. 1966;55:170–177. doi: 10.1073/pnas.55.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soga N., Kinosita K., Jr., Yoshida M., Suzuki T. Kinetic equivalence of transmembrane pH and electrical potential differences in ATP synthesis. J. Biol. Chem. 2012;287:9633–9639. doi: 10.1074/jbc.M111.335356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uribe E.G. ATP synthesis driven by a K+-valinomycin-induced charge imbalance across chloroplast grana membranes. FEBS Lett. 1973;36:143–147. doi: 10.1016/0014-5793(73)80356-4. [DOI] [PubMed] [Google Scholar]
- 5.Junesch U., Graber P. Influence of the redox state and the activation of the chloroplast ATP synthase on proton-transport-coupled ATP synthesis/hydrolysis. Biochim. Biophys. Acta. 1987;893:275–288. [Google Scholar]
- 6.Junesch U., Graber P. The rate of ATP-synthesis as a function of delta pH and delta psi catalyzed by the active, reduced H(+)-ATPase from chloroplasts. FEBS Lett. 1991;294:275–278. doi: 10.1016/0014-5793(91)81447-g. [DOI] [PubMed] [Google Scholar]
- 7.Mills J.D., Mitchell P., Schurmann P. Modulation of coupling factor ATPase activity in intact chloroplasts, the role of the thioredoxin system. FEBS Lett. 1980;112:173–177. [Google Scholar]
- 8.Hisabori T., Sunamura E., Kim Y., Konno H. The chloroplast ATP synthase features the characteristic redox regulation machinery. Antioxid. Redox Signal. 2013;19:1846–1854. doi: 10.1089/ars.2012.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nalin C.M., McCarty R.E. Role of a disulfide bond in the γ subunit in activation of the ATPase of chloroplast coupling factor 1. J. Biol. Chem. 1984;259:7275–7280. [PubMed] [Google Scholar]
- 10.Miki J., Maeda M., Mukohata Y., Futai M. The γ-subunit of ATP synthase from spinach chloroplasts. Primary structure deduced from the cloned cDNA sequence. FEBS Lett. 1988;232:221–226. doi: 10.1016/0014-5793(88)80421-6. [DOI] [PubMed] [Google Scholar]
- 11.Mckinney D.W., Buchanan B.B., Wolosiuk R.A. Activation of chloroplast ATPase by reduced thioredoxin. Phytochemistry. 1978;17:794–795. [Google Scholar]
- 12.Mills J.D., Mitchell P. Modulation of coupling factor ATPase activity in intact chloroplasts. Reversal of thiol modulation in the dark. Biochim. Biophys. Acta. 1982;679:75–82. [Google Scholar]
- 13.Ketcham S.R., Davenport J.W., Warncke K., McCarty R.E. Role of the gamma subunit of chloroplast coupling factor 1 in the light-dependent activation of photophosphorylation and ATPase activity by dithiothreitol. J. Biol. Chem. 1984;259:7286–7293. [PubMed] [Google Scholar]
- 14.Dann M.S., McCarty R.E. Characterization of the activation of membrane-bound and soluble CF(1) by thioredoxin. Plant Physiol. 1992;99:153–160. doi: 10.1104/pp.99.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konno H., Nakane T., Yoshida M., Ueoka-Nakanishi H., Hara S., Hisabori T. Thiol modulation of the chloroplast ATP synthase is dependent on the energization of thylakoid membranes. Plant Cell Physiol. 2012;53:626–634. doi: 10.1093/pcp/pcs018. [DOI] [PubMed] [Google Scholar]
- 16.Sekiguchi T., Yoshida K., Wakabayashi K.I., Hisabori T. Dissipation of the proton electrochemical gradient in chloroplasts promotes the oxidation of ATP synthase by thioredoxin-like proteins. J. Biol. Chem. 2022;298 doi: 10.1016/j.jbc.2022.102541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida K., Hara A., Sugiura K., Fukaya Y., Hisabori T. Thioredoxin-like2/2-Cys peroxiredoxin redox cascade supports oxidative thiol modulation in chloroplasts. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E8296–E8304. doi: 10.1073/pnas.1808284115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yokochi Y., Fukushi Y., Wakabayashi K.I., Yoshida K., Hisabori T. Oxidative regulation of chloroplast enzymes by thioredoxin and thioredoxin-like proteins in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2114952118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carraretto L., Formentin E., Teardo E., Checchetto V., Tomizioli M., Morosinotto T., et al. A thylakoid-located two-pore K+ channel controls photosynthetic light utilization in plants. Science. 2013;342:114–118. doi: 10.1126/science.1242113. [DOI] [PubMed] [Google Scholar]
- 20.Herdean A., Teardo E., Nilsson A.K., Pfeil B.E., Johansson O.N., Unnep R., et al. A voltage-dependent chloride channel fine-tunes photosynthesis in plants. Nat. Commun. 2016;7 doi: 10.1038/ncomms11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jarvi S., Gollan P.J., Aro E.M. Understanding the roles of the thylakoid lumen in photosynthesis regulation. Front. Plant Sci. 2013;4:434. doi: 10.3389/fpls.2013.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malone L.A., Proctor M.S., Hitchcock A., Hunter C.N., Johnson M.P. Cytochrome b(6)f - orchestrator of photosynthetic electron transfer. Biochim. Biophys. Acta Bioenerg. 2021;1862 doi: 10.1016/j.bbabio.2021.148380. [DOI] [PubMed] [Google Scholar]
- 23.Muller P., Li X.P., Niyogi K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001;125:1558–1566. doi: 10.1104/pp.125.4.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuldiner S., Rottenberg H., Avron M. Determination of pH in chloroplasts. 2. Fluorescent amines as a probe for the determination of pH in chloroplasts. Eur. J. Biochem. 1972;25:64–70. doi: 10.1111/j.1432-1033.1972.tb01667.x. [DOI] [PubMed] [Google Scholar]
- 25.Huang C.S., Kopacz S.J., Lee C.P. Mechanistic differences in the energy-linked fluorescence decreases of 9-aminoacridine dyes associated with bovine heart submitochondrial membranes. Biochim. Biophys. Acta. 1983;722:107–115. doi: 10.1016/0005-2728(83)90163-9. [DOI] [PubMed] [Google Scholar]
- 26.Azzi A., Chance B., Radda G.K., Lee C.P. A fluorescence probe of energy-dependent structure changes in fragmented membranes. Proc. Natl. Acad. Sci. U. S. A. 1969;62:612–619. doi: 10.1073/pnas.62.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murakami S., Packer L. The role of cations in the organization of chloroplast membranes. Arch. Biochem. Biophys. 1971;146:337–347. doi: 10.1016/s0003-9861(71)80072-3. [DOI] [PubMed] [Google Scholar]
- 28.Vandermeulen D.L., Govindjee Anthroyl stearate as a fluorescent probe of chloroplast membranes. Biochim. Biophys. Acta. 1976;449:340–356. doi: 10.1016/0005-2728(76)90146-8. [DOI] [PubMed] [Google Scholar]
- 29.Witt H.T. Energy conversion in the functional membrane of photosynthesis. Analysis by light pulse and electric pulse methods. The central role of the electric field. Biochim. Biophys. Acta. 1979;505:355–427. doi: 10.1016/0304-4173(79)90008-9. [DOI] [PubMed] [Google Scholar]
- 30.Azzi A., Baltscheffsky M., Baltscheffsky H., Vainio H. Energy-linked changes of the membrane of Rhodospirillum rubrum chromatophores detected by the fluorescent probe 8-anilinonaphthalene-1-sulfonic acid. FEBS Lett. 1971;17:49–52. doi: 10.1016/0014-5793(71)80561-6. [DOI] [PubMed] [Google Scholar]
- 31.Bashford C.L., Radda G.K., Ritchie G.A. Energy-linked activities of the chromaffin granule membrane. FEBS Lett. 1975;50:21–24. doi: 10.1016/0014-5793(75)81031-3. [DOI] [PubMed] [Google Scholar]
- 32.Dufour J.P., Goffeau A., Tsong T.Y. Active proton uptake in lipid vesicles reconstituted with the purified yeast plasma membrane ATPase. Fluorescence quenching of 9-amino-6-chloro-2-methoxyacridine. J. Biol. Chem. 1982;257:9365–9371. [PubMed] [Google Scholar]
- 33.Schwarz O., Schurmann P., Strotmann H. Kinetics and thioredoxin specificity of thiol modulation of the chloroplast H+-ATPase. J. Biol. Chem. 1997;272:16924–16927. doi: 10.1074/jbc.272.27.16924. [DOI] [PubMed] [Google Scholar]
- 34.Sekiguchi T., Yoshida K., Okegawa Y., Motohashi K., Wakabayashi K.I., Hisabori T. Chloroplast ATP synthase is reduced by both f-type and m-type thioredoxins. Biochim. Biophys. Acta Bioenerg. 2020;1861 doi: 10.1016/j.bbabio.2020.148261. [DOI] [PubMed] [Google Scholar]
- 35.Wolosiuk R.A., Crawford N.A., Yee B.C., Buchanan B.B. Isolation of three thioredoxins from spinach leaves. J. Biol. Chem. 1979;254:1627–1632. [PubMed] [Google Scholar]
- 36.Yoshida K., Hara S., Hisabori T. Thioredoxin selectivity for thiol-based redox regulation of target proteins in chloroplasts. J. Biol. Chem. 2015;290:14278–14288. doi: 10.1074/jbc.M115.647545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yokochi Y., Sugiura K., Takemura K., Yoshida K., Hara S., Wakabayashi K.I., et al. Impact of key residues within chloroplast thioredoxin-f on recognition for reduction and oxidation of target proteins. J. Biol. Chem. 2019;294:17437–17450. doi: 10.1074/jbc.RA119.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshida K., Matsuoka Y., Hara S., Konno H., Hisabori T. Distinct redox behaviors of chloroplast thiol enzymes and their relationships with photosynthetic electron transport in Arabidopsis thaliana. Plant Cell Physiol. 2014;55:1415–1425. doi: 10.1093/pcp/pcu066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are contained within the article.