Abstract
Epithelial cells organize into cyst-like structures that contain a spherical monolayer of cells that enclose a central lumen. Using a three-dimensional basement membrane culture model in which mammary epithelial cells form hollow, acinus-like structures, we previously demonstrated that lumen formation is achieved, in part, through apoptosis of centrally localized cells. We demonstrate that the proapoptotic protein Bim may selectively trigger apoptosis of the centrally localized acinar cells, leading to temporally controlled lumen formation. Bim is not detectable during early stages of three-dimensional mammary acinar morphogenesis and is then highly upregulated in all cells of acini, coincident with detection of apoptosis in the centrally localized acinar cells. Inhibition of Bim expression by RNA interference transiently blocks luminal apoptosis and delays lumen formation. Oncogenes that induce acinar luminal filling, such as ErbB2 and v-Src, suppress expression of Bim through a pathway dependent on Erk-mitogen-activated protein kinase; however, HPV 16 E7, an oncogene that stimulates cell proliferation but not luminal filling, is unable to reduce Bim expression. Thus, Bim is a critical regulator of luminal apoptosis during mammary acinar morphogenesis in vitro and may be an important target of oncogenes that disrupt glandular epithelial architecture.
Tissue homeostasis of multicellular organisms is established and maintained by the delicate interplay between cell growth and cell death signals that are often altered in disease states such as cancer. Apoptosis is fundamental in maintaining proper cell number, sculpting of structures, and other cellular process that are necessary for embryonic development and for the maintenance of tissue homeostasis in the adult organism (7). Apoptosis has been implicated in the process of cavitation, or lumen formation in a solid cell mass in several three-dimensional (3D) spheroid models (2, 6, 15, 18). In addition, apoptosis accompanies clearing of the terminal end buds in the developing mammary gland and lumen formation in the salivary gland (19, 20). Many types of early breast cancer lesions such as ductal carcinoma in situ are characterized by loss of acinar organization and filling of the luminal space (14). The molecular mechanisms responsible for creation of the luminal space in epithelial acini are not well defined. In addition, it is not known how oncogenes that induce filling of the luminal space can target these pathways.
We have investigated processes associated with lumen formation in the MCF-10A line of immortalized human mammary epithelial cells. When cultured on a reconstituted basement membrane derived from Engelbreth-Holm-Swarm tumor (Matrigel), immortalized MCF-10A mammary epithelial cells undergo a series of morphogenetic processes resulting in the formation of acinus-like structures containing a single layer of polarized cells surrounded by a hollow lumen (28). Formation of the luminal space in this model follows the development of apico-basal polarity and involves apoptosis and an autophagy-like process in the centrally localized cells (8). Cells that proliferate into the hollow cavity after lumen formation also undergo cell death, indicating that the luminal space is not compatible with cell survival. Oncogenes that induce filling of the luminal space appear to do so, in part, by blocking apoptosis (8).
The evidence that cells which proliferate into the luminal space undergo apoptosis raised the question whether filling of the luminal space by oncogenes that induce hyperproliferation requires antiapoptotic activities. Indeed, we found that filling of the luminal space in MCF-10A acini requires both constitutive stimulation of proliferation and antiapoptotic activities (8). For example, activation of homodimers of the oncogene ErbB2 (HER2/Neu) in preformed acinar structures leads to constitutive proliferation and filling of the luminal space (28), whereas acinar structures that express oncogenes, such as human papillomavirus type 16 (HPV E7) or cyclin D1, proliferate constitutively but contain hollow lumen (8). The ability of ErbB2 to induce luminal filling correlates with its ability to suppress activation of caspase 3 and apoptosis, whereas the proliferating cyclin D1 or HPV E7 expressing acinar cells are unable to promote survival. Moreover, only when HPV E7 or cyclin D1 acinar cells are supplied a prosurvival signal, by coexpressing the antiapoptotic protein Bcl-xL, can they block luminal apoptosis and fill the luminal space. The filled acinar structures induced by ErbB2 or the combination of cyclin D1 and Bcl-xL in this model resemble lesions associated with carcinoma in situ breast tumors. Indeed, amplification or overexpression of ErbB2 is detected in 60 to 85% of comedo-type carcinoma in situ tumors (42); thus, the effects of ErbB2 in this model may mimic events induced by ErbB2 in vivo.
To investigate the molecular mechanisms underlying luminal apoptosis during mammary acinar morphogenesis, we have examined the basis for Bcl-2 and Bcl-xL protection from luminal apoptosis. Pro-survival members of the Bcl-2 family inhibit apoptosis by heterodimerizing with the proapoptotic family members (4). Proapoptotic members fall into two categories; those containing BH domains 1 to 3 (e.g., Bax/Bak/Bok [Bax-like]) and those containing only a BH3 domain (e.g., Bad, Bid, Noxa, and Bim BH3-only]). The multidomain Bax-like members and the BH3-only members can both initiate apoptosis but differ in that the BH3-only members act as damage sensors and antagonists of prosurvival proteins (4), whereas Bax-like members contain innate cytotoxic function (40). The BH3-domain only proteins are well suited for setting thresholds for initiating apoptosis since they are regulated by divergent signaling cascades, and their expression is often tissue specific (17).
We demonstrate here that the ability of Bcl-xL to inhibit luminal apoptosis is dependent on binding to BH3-only proteins and identify the proapoptotic BH3-only protein Bim as a significant contributor to luminal apoptosis. Bim is upregulated during MCF-10A morphogenesis just prior to the induction of apoptosis, is required for early luminal apoptosis, and contributes to luminal clearance in acinar structures. Oncogenes that block luminal cell death inhibit Bim expression through stimulation of Mek/Erk kinase pathway. Thus, the regulation of a BH3-only member, Bim, plays a critical role in luminal apoptosis during acinar morphogenesis in this model system and may be an important target of oncogenes that disrupt glandular epithelial architecture.
MATERIALS AND METHODS
Materials.
MCF-10A cells were obtained from the American Type Culture Collection (Manassas, Virginia) and maintained in DMEM/F12 (Gibco-BRL) supplemented with 5% donor horse serum, 20 ng of epidermal growth factor (EGF)/ml, 10 μg of insulin/ml, 100 ng of cholera toxin/ml, 0.5 μg of hydrocortisone/ml, 50 U of penicillin/ml, and 50 μg of streptomycin/ml. Growth factor-reduced Matrigel was obtained from BD Biosciences. UO126, PD98059, and LY294002 were purchased from Calbiochem. AP1510 was obtained from ARIAD Pharmaceuticals. Antibodies to Bim were obtained from Stressgen (for Western blotting) and Chemicon (for immunofluorescence). Wang et al. (39) have proposed that the Stressgen Bim antibody does not recognize phosphorylated Bim as effectively as its nonphosphorylated counterpart. We have investigated this extensively and found no difference in the detection of phosphoBim (N. Collins, M. J. Reginato, and J. S. Brugge, unpublished data). Similar results were obtained by Marini et al. (26). The following antibodies were obtained as indicated: anti-actin (Santa Cruz); anti-Bcl-xL (BD Pharmingen); anti-phospho Erk, anti-Erk, and anti-activated caspase-3 (Cell Signaling Technologies); and anti-caspase 8 (Upstate). To generate the phospho65-Bim antibody, a phosphopeptide of the sequence CLAPPApSPGPFATR was synthesized and coupled to keyhole limpet hemocyanin by using an Imject Maleimide Activated mcKLH kit (Pierce, Rockford, IL). The antigen was injected into New Zealand White rabbits (Covance Research Products), from which serum was collected approximately every 3 weeks. Serum was affinity purified by subsequent passage on an Immunopure-immobilized protein A column (Pierce) and an agarose-iodoacetyl column (Pierce) to which a synthetic peptide of the sequence CLAPPASPGPFATR was coupled. The final eluate was desalted and concentrated by using Amicon Ultra centrifugal filter devices (Millipore).
Retrovirus vectors and MCF-10A cell lines.
Vesicular stomatitis virus-pseudotyped retroviruses were produced as previously described (32). pMig, pMig-Bcl-xL, pMig-Bcl-xL (M1), and pMig-Bcl-xL (M8) were kindly provided by Stanley Korsmeyer. The construct pBabe-MEK2-DD was kindly provided by Sylvain Meloche. pBabe-vSRC-ER was a generous gift of Martin McMahon. Treatment of vSrc-ER cells with 4-hydroxytamoxifen (OHT) for 48 h caused a marked increase in tyrosine phosphorylation, and treatment with OHT for 48 h in preformed acini led to increased proliferation (data not shown). Vectors encoding the HPV E7 oncoprotein or the empty vector (pLXSN) were obtained as a gift from Denise Galloway and Peter Howley. Stable MCF-10A cells containing p75.B2, a chimeric ErbB2 receptor fused to the bivalent ligand FK-506 binding protein, and its activation via dimerization with the bivalent ligand AP1510 (ARIAD Pharmaceuticals) have been reported previously (28). Full-length BimEL cDNA was cloned by reverse transcription-PCR from RNA isolated from rat cerebellar granule neurons. BimEL cDNA was then subcloned into the polylinker of the mammalian expression vector pCDNA3 (Invitrogen) with an NH2-terminal hemagglutinin tag. Point mutations of Ser-65 were made by using the QuikChange site-directed mutagenesis kit (Stratagene). Similarly, the splicing donor site for BimL was mutated (T126 to G, without an amino acid change) to prevent splicing that generates both BimEL and BimL isoforms as previously been shown (34). Mutations were verified by sequencing. BimEL and BimEL-SA cDNAs were subsequently cloned into the retroviral vector pLPCX. For small interfering RNA (siRNA) experiments, methods for transfecting MCF-10A cells and sequence for control and Bim oligonucleotides have been previously described (32).
Morphogenesis assay.
Assays were performed as previously described (28). Briefly, MCF-10A cells were resuspended in assay medium (DMEM/F12 supplemented with 2% donor horse serum, 10 μg of insulin/ml, 100 ng of cholera toxin/ml, 0.5 μg of hydrocortisone/ml, and 5 ng of EGF/ml). Eight-well RS glass slides (BD Falcon) were coated with 35 μl of Matrigel per well. Then, 5,000 cells were plated per well in assay medium containing a final concentration of 2% Matrigel. Assay medium containing 2% Matrigel was replaced every 4 days. For the stimulation of cells containing ErbB2 chimeras with AP1510, EGF was replaced with 1 μM AP1510 on day 8 or as indicated. For v-Src induction, acini were treated with 1 μM OHT (Sigma) or vehicle control (ethanol,1:2,000). For studies with chemical inhibitors, morphogenesis assays were performed as described until the medium was replaced with assay medium supplemented with dimethyl sulfoxide (1:1,000), UO126 (5 μM), SB202190 (5 μM), or LY294002 (25 μM) for 48 h as indicated.
Quantification of cell death.
Assay media were removed from wells, and acini were washed once with phosphate-buffered saline (PBS). Structures were then incubated for 15 to 30 min at 37°C with 1 μM ethidium bromide (EtBr) in MCF10A growth medium. Cell death was quantified by counting the total number of acini in one well with at least two EtBr-positive cells. At least 100 structures were counted per well. All values are given as percentage of acinar structures exhibiting evidence of cell death in a total population at a given time point. For quantification of apoptosis in cells infected with BimEL or BimEL-SA we used a cell death detection enzyme-linked immunosorbent assay (ELISA) kit (Roche Diagnostics) according to the manufacturer's instructions. Error bars represent the standard error of the mean (SEM) of at least three independent experiments.
Protein extraction and Western analysis from MCF-10A acini.
Acinar structures were washed briefly with 4°C PBS with protease inhibitors (phenylmethylsulfonyl fluoride [10 μg/ml], leupeptin [1 μg/ml], aprotinin [1 μg/ml], pepstatin [1 μg/ml]) and then treated for 15 min at 4°C with radioimmunoprecipitation assay lysis buffer (150 mM NaCl, 20 mM Tris [pH 7.5], 0.1% sodium dodecyl sulfate, 1.0% sodium deoxycholate, 0.1.0% Triton X-100). Matrigel and acini were collected and pulled through a 27-gauge needle three to five times before being placed on ice for 15 min. Lysates were cleared by centrifugation at 16,000 × g for 20 min at 4°C, followed by analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography.
Immunofluorescence and image acquisition.
Structures were prepared as previously described (28). Briefly, acini were fixed in 4% formalin for 25 min at room temperature. Fixed structures were washed with PBS-glycine (130 mM NaCl, 7 mM Na2HPO4, 100 mM glycine) three times for 15 min each time. The structures were then blocked in IF buffer (130 mM NaCl, 7 mM Na2HPO4, 3.5 mM NaH2PO4, 7.7 mM NaN3, 0.1% bovine serum albumin, 0.2% Triton X-100, 0.05% Tween 20) plus 10% goat serum for 1 to 2 h, followed by 20 blocking buffer [i.e., IF buffer containing 10% goat serum and 20 μg of goat anti-mouse F(ab′)2/ml] for 40 min. Primary antibodies were diluted in 20 blocking buffer, followed by incubation overnight 4°C. Structures were washed three times in IF buffer for 15 min each. Anti-mouse or anti-rabbit secondary antibodies coupled with Alexa fluor dyes (Molecular Probes) were diluted in IF buffer containing 10% goat serum, followed by incubation for 60 min. After a wash with IF buffer as described above, structures were incubated with 5 μM Topro-3 Iodide (Molecular Probes) and 0.5 ng of DAPI (4′,6′-diamidino-2-phenylindole; Sigma)/ml before being mounted with the antifade agent Prolong (Molecular Probes). Quantification of Bim staining in ErbB2 structures was performed at ×40 magnification. Only fields including both multiacinar and normal structures were scored. The staining intensity for Bim in multiacinar structures was scored as less than, equal to, or greater than the normal structures visualized in the same field of view. A minimum of 100 multiacinar structures and 100 normal structures were counted per experiment, and each experiment was performed three independent times. Confocal analysis was performed by using the Nikon E800 Bio-Rad Laboratories confocal imaging system (Nikon Imaging Center at Harvard Medical School). Images were generated by using MetaMorph software, converted to Tiff format, and arranged by using Adobe Photoshop 7.0.
Histology.
For Bim immunostaining, acinar structures were fixed and washed with PBS-glycine as described above. Prior to blocking, structures were incubated at 25°C for 15 min each time with 18 and 30% sucrose sequentially. Acini were then mixed with 250 μl of frozen section medium (Stephens Scientific). Samples were then frozen in a dry ice-methanol bath for 10 min and sectioned by using a cryostat. Next, 7 μM sections were prepared, placed on glass slides, and stored at −20°C until use. Immunofluorescence assays were then performed as described above beginning with the addition of in 10 IF blocking buffer. Because of the harsh treatment of cryosectioning, acinus structures were distorted in some sections.
RESULTS
Bcl-xL mutants implicate BH3-only proteins in regulating luminal apoptosis during morphogenesis.
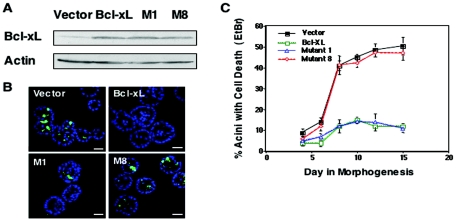
To evaluate the mechanism underlying luminal apoptosis, we investigated the mechanism by which overexpression of Bcl-xL inhibits luminal apoptosis during morphogenesis. We utilized mutant variants of Bcl-xL to examine whether the antiapoptotic function of Bcl-xL in mammary acini is dependent on its heterodimerization with the Bax-like or the BH3-only proapoptotic members (4). MCF-10A cells were infected with retrovirus vectors encoding either wild-type Bcl-xL or one of two Bcl-xL mutant variants: Mt1 (F131V/D133A) lacks functional BH1 and BH2 domains and is unable to interact with Bax or Bak but retains the ability to interact with BH3-only proteins and Mt8 (G138E/R139L/I140N), which lacks a functional BH3 domain and is unable to bind BH3-only proteins or multidomain Bcl-2 family members (4). All proteins were expressed at similar levels (Fig. 1A). Cells undergoing apoptosis in the luminal space begins between day 7 and day 8 and is maximal between day 8 and day 15 as measured by EtBr-positive staining and cleaved, activated caspase-3 staining (Fig. 1B) (8). Acinar structures expressing wild-type Bcl-xL or the Bax-Bak binding mutant (Mt1) displayed significantly less apoptosis, as measured by staining for cleaved, activated caspase-3 (Fig. 1B) or by measuring the percentage of acini in the total culture that contained two or more EtBr-positive cells in the lumen (Fig. 1C). In contrast, cells expressing Mt8, a BH3-domain mutant, did not inhibit luminal apoptosis (Fig. 1B and C). These results suggest that binding to Bcl-xL to BH3-only proteins is critical for its inhibition of luminal apoptosis during morphogenesis and that BH3-only proteins may be involved in regulating apoptosis during lumen formation.
FIG. 1.
Bcl-xL mutants implicate BH3-only proteins in regulating luminal apoptosis during morphogenesis. (A) Lysates from MCF-10A cells infected with retrovirus of empty vector (MIG), Bcl-xL, Bcl-xL (M1), or Bcl-xL (M8) were analyzed for Bcl-xL, and actin levels were analyzed by Western analysis. (B) At day 10 of morphogenesis, MCF-10A cells expressing vector, Bcl-xL, Bcl-xL (M1), or Bcl-xL (M8) were fixed and immunostained with anti-active caspase-3 (green), and nuclei were counterstained with TOPRO-3 (blue). Scale bar, 20 μm. (C) The percentage of acini containing at least two EtBr-positive cells was measured at the indicated time points during morphogenesis. Values represent the mean ± the SEM of three independent experiments. At least 100 acini were counted in each experiment.
The BH3-only protein Bim is induced during morphogenesis and required for luminal apoptosis.
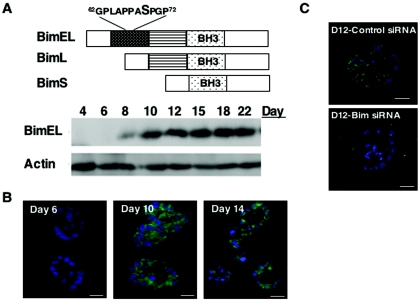
To determine whether expression of BH3-only proteins is regulated during morphogenesis, we analyzed expression of several BH3-only proteins. Although Bad, Bim, and Bid were detected in cells undergoing morphogenesis, only Bim was highly upregulated during morphogenesis (Fig. 2A). Bim mRNA encodes three major isoforms that are generated by alternative splicing: BimEL, BimL, and BimS (30). All three isoforms contain a BH3 domain, but only BimEL contains a consensus Erk site at serine 69 (Fig. 2A). The only form detected in MCF-10A acini comigrates with the protein product of a cDNA form of the largest isoform BimEL (data not shown). BimEL was not detectable during the early stages of morphogenesis and increased in expression at day 8 when apoptosis is initially detected within acini. The levels were maximal at day 10 and then remained high throughout the time period of the experiment (Fig. 2A). BimEL protein levels can fluctuate during morphogenesis, especially after feeding but were consistently higher on day 8 (data not shown).
FIG. 2.
The BH3-only protein BimEL is upregulated during morphogenesis. (A) Schematic of the three most common Bim splice variants: BimEL, BimL, and BimS. All three isoforms contain a BH3 domain, but only BimEL contains consensus Erk site serine 69. Lysates were prepared from acini at the indicated days in morphogenesis and analyzed by Western analysis with antibodies to Bim and actin. (B) Cryosections (7 μm) of acini from days 6, 10, and 14 of morphogenesis were immunostained with antibody to Bim (green) and counterstained with DAPI (blue). Scale bar, 10 μm. (C) At 24 h after transfection with Bim siRNA oligonucleotides or control oligonucleotides, cells were placed in morphogenesis assays and then fixed, cryosectioned, and immunostained with an antibody to Bim (green) and counterstained with DAPI (blue) at day 12. Scale bar, 10 μm.
To determine whether Bim is expressed exclusively in the inner cells that are destined to undergo apoptosis or in all cells of the acini, we immunostained the acini with antiserum to Bim. Although Bim staining was undetectable on day 6, both the inner and outer cells of the acini were strongly positive at day 10 (Fig. 2B). The immunostaining was specific for Bim since cells transfected with siRNA oligonucleotides (siRNA) that target Bim, but not control siRNA, were negative for Bim immunofluorescence (Fig. 2C). Thus, the BH3-only protein Bim is induced in all cells during morphogenesis, coincident with the induction of luminal apoptosis.
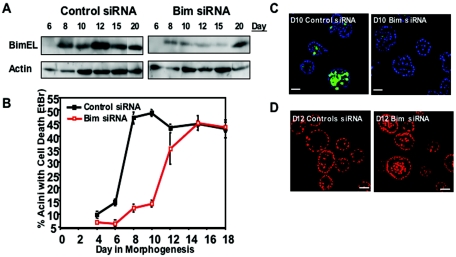
To determine whether Bim expression is required for luminal apoptosis during morphogenesis, we utilized RNA interference to inhibit expression of Bim. Transfection of cells with an siRNA that targeted Bim but not control oligonucleotides significantly reduced expression of Bim through day 15 (Fig. 3A). The expression of other BH3-only proteins such as Bid and Bad was not affected by the Bim siRNA transfection (data not shown). Luminal apoptosis was significantly reduced in cells transfected with the Bim siRNA oligonucleotide but not in control oligonucleotides, as measured by EtBr staining of acini. At day 8 and 10, only 15% of acini derived from cells transfected with Bim siRNA oligonucleotides contained centrally localized, EtBr-positive cells; in contrast, 50% of acini from control transfected cultures exhibited EtBr-positive cells in the luminal space (Fig. 3B). In corroboration with EtBr staining, we also found a dramatic decrease in staining for cleaved, activated caspse-3 in acini transfected with Bim siRNA oligonucleotides compared to control oligonucleotides (Fig. 3C). Day 10 structures transfected with Bim siRNA oligonucleotides contained 16% acini positive for caspase-3 staining compared to 48% acini transfected with control oligonucleotides (data not shown). This level of inhibition of luminal apoptosis was comparable to that seen with cells overexpressing Bcl-xL (Fig. 1B and C). We detected a similar reduction in luminal apoptosis by using an siRNA SMART pool (Dharmacon) targeting Bim (data not shown) and three vectors expressing short hairpin Bim-targeted sequences (T. Schmelzle, E. Lin, and J. S. Brugge, data not shown), supporting the Bim specificity of this siRNA. In addition to blocking apoptosis, Bim siRNA transfection delayed lumen formation since day 12 acini transfected with Bim siRNA oligonucleotides contained significantly greater number of acini with viable cells in the luminal space then control cells (62% filled acini [≥2 cells in the lumen] in Bim siRNA-transfected cells compared to 38% in control acini) (Fig. 3D). The protection from luminal apoptosis in cells transfected with this Bim siRNA oligonucleotides was transient since we observed a complete loss of protection from cell death by day 15 (Fig. 3B). This reduced protection is likely due to loss of Bim siRNA oligonucleotides since the stable hairpin vectors provide constitutive protection from apoptosis. Taken together, these results implicate Bim in early luminal apoptosis and suggest that this proapoptotic protein contributes to luminal clearance.
FIG. 3.
Bim expression involved in luminal apoptosis and luminal clearance during morphogenesis. (A) At 24 h after transfection with Bim siRNA oligonucleotides or matched control oligonucleotides, cells were placed in morphogenesis assays, and cell lysates were collected at the indicated times. Protein levels were analyzed by immunoblotting with Bim or actin antibodies. (B) Cells transfected with Bim or matched control siRNA oligonucleotides were placed in a morphogenesis assay, and the percentage of acini with EtBr-positive cells (two or more cells) was scored after the indicated number of days in culture. Values represent the mean ± the SEM of four independent experiments. (C) Cells transfected with Bim or matched control siRNA oligonucleotides were placed in morphogenesis and were fixed and immunostained at day 10 with anti-active caspase-3 (green), and nuclei were counterstained with TOPRO-3 (blue). Scale bar, 20 μm. (D) Nuclei of representative day 12 structures from cells transfected with Bim or matched control siRNA oligonucleotides were stained with TOPRO-3 (red). Scale bar, 20 μm.
Bim is phosphorylated at Ser-69 during morphogenesis.
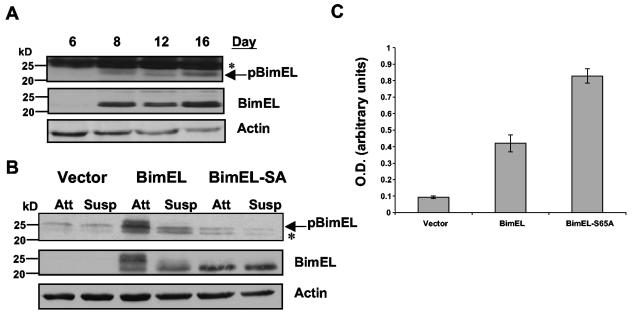
Bim is detected in both the outer cells of acini and the inner cells that eventually undergo apoptosis in a Bim-dependent fashion. This raised the possibility that the activity of Bim might be suppressed in the outer acinar cells. Recent studies have provided evidence that Erk mitogen-activated protein kinase (MAPK) phosphorylation of BimEL at serine 69 (S65 in rodents) decreases its apoptotic activity (1, 22, 25). To investigate whether Bim is phosphorylated in the outer cells, we used affinity-purified antibodies that specifically recognize the serine 69-phosphorylated form of BimEL. Because these antibodies fail to detect Bim in in situ immunofluorescence assays, we examined phosphorylation of serine 69 of Bim (pS69-BimEL) by immunoblotting (Fig. 4A). After days 12 to 14 in culture, the acini are hollow and contain only a single layer of matrix-attached cells; thus, signals detected in lysates of acini from these late stage cultures are derived exclusively from these cells. High levels of pS69-BimEL were detected on all days when Bim was expressed in the acini; even in later days, such as day 16, when the acini are hollow and all inner cells have been cleared. Thus, BimEL is phosphorylated on serine 69 in mature hollow acini, suggesting that outer matrix-attached acinar cells may be phosphorylated on serine 69.
FIG. 4.
Phosphorylation of Bim by matrix signals provides protection from Bim's apoptotic function. (A) Lysates were prepared from acini at indicated days in morphogenesis and analyzed by Western analysis with antibodies to pS69-Bim, Bim, and actin. The nonspecific doublet recognized by the pS69-Bim antibody is indicated by an asterisk. (B) Stable Bcl-2 expressing MCF-10A cells were infected with vector (LPCX), BimEL, or BimEL-SA. At 48 h postinfection, cells were treated with trypsin and either replated or placed in suspension. After 24 h the lysates were collected, the proteins were separated by gel electrophoresis, and the samples were immunoblotted with antibodies for pS69-Bim, Bim, or actin. A nonspecific band recognized by the pS69-Bim antibody is indicated by an asterisk. (C) Cells were infected with an equal titer of vector (LPCX), BimEL, or BimEL-SA retrovirus. Bcl-xL-expressing cells were used to determined the titers of BimEl and BimEl-SA virus. The T126G variant BimEL cDNA that is unable to be spliced to form BimL was used for these studies. At 48 h postinfection cells were collected and then analyzed for apoptosis by DNA fragmentation ELISA. Values represent the mean and the standard deviation of A405 to A490 for at least three independent experiments.
To evaluate the regulation of Bim phosphorylation by matrix, we examined serine 69 phosphorylation of Bim in attached or suspended MCF-10A cells. We expressed ectopic rat BimEL in cells stably transfected with a Bcl-2 expression vector since Bim is barely detectable in adherent cells. Bcl-2 expression prevented the induction of cell death by ectopic Bim. In adherent cells, a strong signal of pS69-BimEL was detected in cells expressing wild-type BimEL but not in cells expressing a mutant form of Bim containing an alanine substitution for serine 65 (BimEL-SA) (Fig. 4B). Cells detached from matrix display minimal pS69-BimEL signal. The lack of the supershifted BimEL band in the S65A mutant indicates that phosphorylation at this site is responsible for the decreased mobility of Bim in the adherent cells (Fig. 4B). Thus, Bim phosphorylation at S69 is maintained in attached cells but lost in cells not in contact with matrix.
Studies in HEK293 cells indicated that phosphorylation of BimEL at this site reduces its apoptotic activity (23). To address whether loss of this phosphorylation site affects Bim's apoptotic activity in MCF-10A cells, we examined the induction of apoptosis in cells 48 h after infection with viruses encoding either wild-type Bim or BimSA. Indeed, we find that exogenous expression of the nonphosphorylatable BimEL-SA mutant induces a twofold increase in apoptosis compared to wild-type BimEL (as measured by DNA fragmentation cell death ELISA) (Fig. 4C) when expressed at equal levels in MCF-10A cells (data not shown). In corroboration, we also see increased caspase-8 cleavage in BimEL-SA-expressing cells compared to wild type (data not shown). These results support previous studies suggesting that Bim phosphorylation at S65 reduces its apoptotic activity. Based on these results, we hypothesize that outer cells in acini that are in direct contact with matrix may be protected from Bim's apoptotic function by reducing the apoptotic activity of Bim via phosphorylation of serine 69. However, reagents that allow us to prove S69 phosphorylation in situ are required to establish definitively whether S69 phosphorylation distinguishes Bim expressed in the inner and outer cells.
Oncogenic regulation of Bim during morphogenesis.
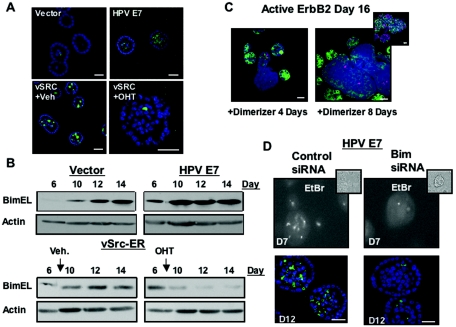
We have previously shown that oncogenes differ in their ability to induce filling of luminal space. For example, whereas both activation of ErbB2 and expression of HPV E7 in MCF-10A cells allow escape from proliferative suppression and constitutive proliferation of acini, activation of the ErbB2 homodimers only leads to production of acini distinguished by a filled luminal space and a marked reduction in apoptosis (8) (Fig. 5C). In contrast, expression of the HPV E7 oncogene does not protect MCF-10A cells from apoptosis in the luminal space and therefore does not block lumen formation (Fig. 5A). We have subsequently found that activation of vSrc in preformed acini can also induce luminal filling. Stable MCF-10A cells expressing the vSrc oncogene fused to estrogen receptor was treated with OHT for 48 h to activate vSrc 8 days after plating cells in Matrigel. Induction of vSrc activation caused inhibition of luminal apoptosis as measured by EtBr staining (data not shown), staining with caspase-3, and filling of the luminal space (Fig. 5A). Treatment of normal acini with OHT during morphogenesis does not alter luminal apoptosis or filling (9), nor does it have any effect on Bim expression during morphogenesis (data not shown). Since Bim expression is critical for luminal apoptosis and lumen formation, we investigated whether the differential regulation of luminal apoptosis by distinct oncogenes is due, in part, to differences in their ability to regulate Bim during morphogenesis. Indeed, activation of v-Src during morphogenesis (day 7) reduced the level of Bim expression significantly (Fig. 5B). In contrast, cells expressing the HPV E7 oncogene did not inhibit Bim expression during morphogenesis (Fig. 5B).
FIG. 5.
Luminal filling of acini by oncogenes involves downregulation of Bim. (A) Acini expressing vector (LXSN) (day 12), HPV E7 (day 12), or v-Src-ER (treated on day 8 with vehicle control [ethanol] or OHT [1 μM] and analyzed after 48 h) were fixed and immunostained with antibodies to activated caspase-3 (green). Nuclei were counterstained with TOPRO-3 (blue). (B) Lysates from acini expressing vector control or HPV E7 and acini expressing v-Src-ER, treated with either vehicle control (ethanol) or OHT (1 μM) at day 7, were collected at the indicated times. Proteins were separated by gel electrophoresis, and samples were immunoblotted with antibodies for Bim or actin. (C) Cells expressing ErbB2 were treated on day 8 with AP1510 for four (left panel) or eight (right panel) days and were immunostained with antibodies to activated caspase-3 (red) or Bim (green). Nuclei were counterstained with TOPRO-3 (blue). The inset image shows multiacinar ErbB2 structures costained with antibodies to Ki67 (red), integrin-α6 (green), or TOPRO-3 (blue) to demonstrate the accessibility of structures to antibodies. (D) Cells expressing HPV E7 were placed in morphogenesis assays 24 h after transfection with Bim or matched control siRNA oligonucleotides and treated at day 7 with EtBr (top panel; with a corresponding phase image [inset]) or immunostained (bottom panel) at day 12 with an antibody to activated caspase-3 (green), and nuclei were counterstained with TOPRO-3 (blue). Scale bar, 20 μm.
We also examined expression of Bim after activation of ErbB2 homodimers in preformed acini by immunostaining since only 25 to 30% of ErbB2-expressing structures form large multiacinar structures after treatment with AP1510 dimerizer. After a minimum of 100 multiacinar structures were counted per experiment, we found that the expression of Bim was decreased in 65% of large multiacinar structures with filled lumens but not in surrounding normal acini that were not affected by ErbB2 activation (Fig. 5C). This observation was reproduced in three independent experiments, thus representing a consistent result in large ErbB2-expressing structures. In contrast, all cells within structures stained with antibody to integrin α6, indicating that antibody accessibility is not altered in large ErbB2-induced structures (Fig. 5C, inset). Thus, two oncogenes, ErbB2 and vSrc, that inhibit luminal apoptosis and fill the luminal space block Bim expression, suggesting that Bim is an important target for oncogenes that are able to fill the luminal space.
Acini expressing the HPV E7 oncogene contained elevated levels of Bim, and Bim was detected at earlier time points than control cells (Fig. 5B), suggesting that the failure of HPV E7-expressing cells to block luminal apoptosis may be due to its inability to inhibit Bim expression. To address this possibility, we used siRNA oligonucleotides to decrease Bim expression in HPV E7 cells. Cells expressing HPV E7 that were transfected with Bim siRNA oligonucleotides but not control oligonucleotides showed a marked reduction of apoptosis during early morphogenesis, as measured by EtBr staining (Fig. 5D). In addition, at a later stage of morphogenesis (day 12), HPV E7 cells transfected with Bim siRNA displayed a significant reduction in activated caspase-3 staining (Fig. 5D). Day 12 structures transfected with Bim siRNA contained a twofold reduction in the number of acini positive for caspase-3 staining (37% of acini) compared to cells transfected with control siRNA (82% acini) (data not shown). Thus, the failure of HPV E7 expressing cells to block Bim expression contributes to their inability to block luminal apoptosis and lumen formation.
Erk MAPK pathway regulates Bim expression and luminal filling during morphogenesis.
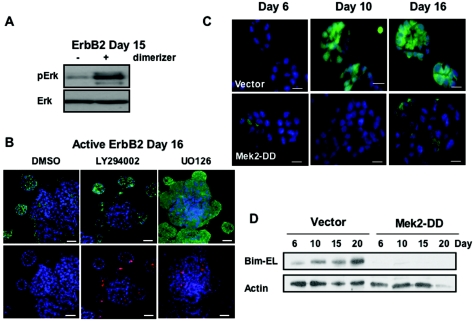
ErbB2 can activate the phosphatidylinositol 3-OH kinase (PI 3-kinase), an activator of PKB/Akt kinase, and the Erk MAPK pathway (16), both of which are known pathways of cell survival. Bim expression can be regulated by either the Akt (11) or the Erk (41) pathway depending on the cell type. We and others have previously shown that in mammary epithelial cells the Erk pathway but not the Akt pathway can negatively regulate Bim levels during anoikis (26, 32). Indeed, we find that activation of ErbB2 in preformed acini leads to high levels of Erk activity compared to control acini (Fig. 6A). To test whether ErbB2 activation of Erk activity is involved in regulating Bim expression during morphogenesis, we examined the effects of blocking activation of Erk and PI 3-kinase. The ability of activated ErbB2 to inhibit Bim expression in large, filled acini was reversed when acini were treated for 48 h with an inhibitor of Mek (UO126) but not when acini were treated for 48 h with the PI 3-kinase inhibitor (LY294002) (Fig. 6B). UO126-treated cultures showed strong staining for Bim throughout the multi-acinar structures, and this inhibitor induced apoptosis in the ErbB2-expressing structures, as demonstrated by staining for activated caspase-3 (Fig. 6B). Similar findings were observed by using the Mek inhibitor PD98069 (data not shown). These results indicate that Bim expression is regulated by the Mek/Erk pathway in ErbB2-activated structures and that signals from Erk may be critical to prevent luminal apoptosis and lumen formation.
FIG. 6.
Involvement of the Mek/Erk pathway in regulating Bim expression. (A) Cells expressing ErbB2 were incubated at day 8 with vehicle control (ethanol) or dimerizer (AP1510), lysates were collected at day 15, and proteins were analyzed by immunoblotting with antibodies to phospho-Erk and Erk. (B) Cells expressing ErbB2 were incubated with AP1510 at day 8 for 8 days and consequently treated with dimethyl sulfoxide, LY294002 (50 μM), or UO126 (40 μM) for 48 h, and D18 acini were immunostained with antibodies to Bim (green; top panel) and activated caspase-3 (red; bottom panel). Nuclei were counterstained with TOPRO-3. Scale bar, 20 μm. (C) Cells expressing vector (Babe) or Mek2-DD were placed in a morphogenesis assay and immunostained at the indicated times with DAPI (blue) and antibody to Bim (green). Scale bar, 10 μm. (D) Cells expressing vector or Mek2-DD were placed in a morphogenesis assay, lysates were collected at the indicated times, and proteins were analyzed by immunoblotting with antibodies to Bim or actin.
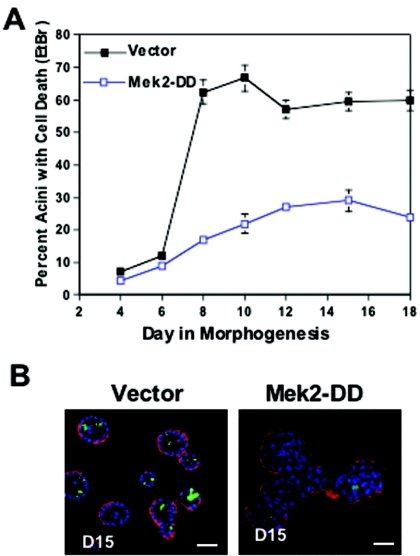
To determine whether activation of the Erk pathway is sufficient to block Bim expression during morphogenesis, stable pools of MCF-10A cells expressing Mek2-DD were generated and cultured in Matrigel. Mek2-DD is an activate variant of Mek2, a kinase that phosphorylates and activates Erk. Cells expressing MEK2-DD contained high levels of activated Erk compared to control acini (data not shown), and the induction of Bim expression during morphogenesis was markedly reduced as seen either by immunostaining (Fig. 6C) or immunoblotting (Fig. 6D) of acinar structures. We also examined whether the inhibition of Bim by the Erk pathway correlated with the loss of luminal apoptosis and lumen formation during morphogenesis. Expression of Mek2-DD blocked luminal apoptosis (as measured by ethidium bromide positivity) (Fig. 7A) and caused luminal filling (Fig. 7B). Thus, activation of the Mek/Erk pathway is sufficient to block Bim expression and correlates with protection from luminal apoptosis and inhibition of lumen formation during morphogenesis.
FIG. 7.
The Mek/Erk pathway regulates luminal apoptosis and luminal filling. (A) Cells expressing vector (Babe) and Mek2-DD were placed in morphogenesis assays, and apoptosis was measured by counting the EtBr-positive acini as described above. Values represent the mean ± the SEM of three independent experiments. (B) Cells expressing vector and Mek2-DD were placed in a morphogenesis assay and immunostained at day 12 with TOPRO-3 (blue), activated caspase-3 (green), and integrin-α6 (red). Scale bar, 20 μm.
Taken together, these results indicate that Bim plays a critical role in regulating luminal apoptosis and lumen formation during mammary morphogenesis in vitro and suggests that oncogenes, such as ErbB2, that are able to induce luminal filling, may do so in part by negatively regulating Bim expression via the Mek/Erk kinase pathway.
DISCUSSION
We have investigated the molecular mechanisms responsible for luminal apoptosis during the morphogenesis of hollow, spheroid epithelial structures in vitro. We identified Bim, a proapoptotic BH3-only Bcl-2 family member, as a critical regulator of luminal apoptosis and lumen formation during morphogenesis of cultured mammary epithelial cells. Bim is one of multiple BH3-only proteins implicated as critical initiators of apoptosis (31). Indeed, we found that expression of Bim, but not Bad or Bid, is induced during morphogenesis and that suppression of Bim significantly delays luminal apoptosis and clearing of the luminal space. Moreover, activation of oncogenes that inhibit luminal apoptosis and fill the luminal space, such as ErbB2 homodimers and vSrc, inhibits Bim expression. In contrast, the oncogene HPV E7, which does not block luminal apoptosis or luminal filling, is unable to block Bim expression. Taken together, our observations establish that Bim plays a critical role in regulating apoptosis in cavitating spheroids in vitro and thus can be considered as a candidate regulator of related processes in vivo. Since Bim can be suppressed by oncogenes, it may serve as a downstream target of oncogenes that promote luminal filling in early stages of carcinogenesis.
These studies are the first to implicate a BH3-only protein in regulating luminal apoptosis and lumen formation of cyst-like epithelial structures. Although epithelial morphogenesis has not been extensively characterized in Bim-deficient mice, it is of interest that loss of Bcl-2 in mice leads to development and eventually death from polycystic kidney disease (29, 35), a disorder linked to alterations in epithelial tube size and morphogenesis (24). Intriguingly, the removal of a single bim allele in these bcl-2−/− mice was sufficient to eliminate polycystic kidney disease (3), suggesting that the interplay between proapototic and antiapoptotic Bcl-2 family members may regulate epithelial morphogenesis in vivo. In mammary epithelial cells, apoptosis has been detected in the presumptive luminal space of the terminal end bud (TEB) in vivo during early development of the mammary gland (19). Bcl-2 transgenic mice showed disruption of cellular organization of the TEB, including reduced levels of apoptosis in the TEBs.
Since we and others have recently shown that Bim expression is upregulated after detachment from matrix in multiple epithelial cells and contributes to anoikis in MCF-10A cells (26, 32) and that centrally localized acinar cells during morphogenesis are not in contact with basement membrane (8), we initially hypothesized that Bim might be induced specifically in the inner, matrix-deprived cells and thus contribute to the selective apoptosis in this population of cells. In contrast, we found that Bim is induced between days 6 and 8 in both outer and inner cells (Fig. 2B). Bim induction would sensitize the acinar cells to stress conditions, possibly those resulting from loss of matrix attachment and failure of the inner cells, to activate intracellular signaling proteins that protect cells from apoptosis. It is of interest that centrally localized cells are separated from matrix proteins and fail to activate Akt from day 5 onward during morphogenesis (8); however, cell death is not detected until the period when Bim is induced, suggesting that Bim induction may be a critical trigger for death of the inner cell population. The temporal regulation of apoptosis after polarization of the outer acinar cells in the MCF-10A model shares features of apoptosis associated with cavitation of embryoid bodies (EBs) during development (5, 6). Centrally localized cells in the ectodermal mass of the EBs undergo cell death after polarization of the outer, matrix-attached cells due to a signal transduced from surrounding endoderm cells when they undergo differentiation. Our results raise the question whether the centrally localized EB cells become sensitized to the absence of matrix attachment due to induction of a proapoptotic protein such as Bim. Studies of EBs lacking basement membrane due to loss of laminin-1 indicate that the EB cells are not dependent on matrix attachment for survival until they differentiate after induction by the endodermal cells (27). Together, these results suggest that cells may not undergo death when deprived of matrix until they develop into a matrix-dependent state, possibly by induction of proapoptotic proteins, like Bim, that sense matrix deprivation.
It is not clear how outer acinar cells are protected from Bim-induced cell death. We examined the possibility that Bim apoptotic function may be differentially regulated between matrix-attached cells and matrix-deprived inner cells. Recently, BimEL has been shown to be phosphorylated by the MAPK pathway, and this phosphorylation reduced BimEL apoptotic activity (22, 23). Indeed, we show that BimEL is phosphorylated at the major Erk phosphorylation site and that this phosphorylation is lost after loss of adhesion. A mutant form of BimEL that is unable to be phosphorylated at this site displayed higher apoptotic activity; thus, loss of matrix attachment may facilitate Bim's apoptotic function by preventing Erk phosphorylation of Bim. Extrapolating to the acinar cultures, we propose that outer-matrix attached acinar cells are protected from Bim's apoptotic effects during morphogenesis by maintaining Bim in a phosphorylated state, whereas inner cells, in the absence of matrix signals, become sensitized to Bim's highly apoptotic unphosphorylated form. Consistent with this model, we found that Bim was phosphorylated on serine 69 in acini derived from late-stage cultures that consisted exclusively of matrix-attached cells. Previous studies have indicated that phosphorylation of Bim at serine 69 targets Bim for proteosome-mediated degradation. Although this could be contributing to the protection from Bim expression, we consistently detect high levels of Bim under conditions in which there is detectable phosphorylation. Thus, it is possible that phosphorylation of Bim may also interfere with its apoptotic activity by other mechanisms. Further studies addressing the ability of pS69Bim to bind to pro- and antiapoptotic Bcl-2 family members will address this.
We have previously shown that the ability of oncogenes to induce luminal filling requires not only enhancement of proliferation but also inhibition of apoptosis (8). The data presented here suggest that oncogenes such as ErbB2 homodimers and v-Src may escape luminal apoptosis by inhibiting expression of Bim. The block of Bim expression, coupled with reinitiation of proliferation, may be sufficient to induce luminal filling in activated ErbB2 and v-Src structures. In contrast to ErbB2 expressing MCF-10A structures, acini expressing the oncogene HPV E7, which also induces uncontrolled proliferation but not protection from apoptosis, was unable to block Bim expression. Inhibition of Bim expression in HPV E7 acinar structures using Bim siRNA oligonucleotides reduced the level of apoptosis in these structures, suggesting that HPV E7's inability to block luminal apoptosis is due, in part, to its inability to inhibit Bim levels. This result is consistent with our previous finding that HPV E7 structures coexpressing Bcl-2 contained filled lumen and few apoptotic cells (8). The inability of HPV E7 to activate the Erk MAPK pathway (data not shown) is probably responsible for its failure to prevent Bim expression. These results suggest that Bim may function as a key molecule in keeping cells expressing proliferative oncogenes from progressing to more advanced tumor phenotypes, i.e., one in which the luminal space is infiltrated with tumor cells. Indeed, recent data suggest that Bim can also keep the proliferative oncogene c-Myc in check since inactivation of a single allele of bim accelerated Myc-induced tumor development in vivo (12). Thus, oncogenic inactivation of Bim, i.e., by ErbB2, may increase the probability of acquiring additional oncogenic mutations that may allow for complete disruption of epithelial acinus architecture and eventually lead to metastasis.
Bim expression has been found to be regulated at the transcriptional level by the Erk MAPK (32, 41) or the Akt kinase (10) pathway. We previously showed that activation of Erk, but not Akt, regulates Bim mRNA and protein expression in monolayer or detached cultures of MCF-10A cells (32). We present here several lines of evidence implicating the Erk pathway in regulation of Bim expression in 3D acinar structures: (i) ErbB2 homodimer induced inhibition of Bim expression in large filled acinar structures was reversed with treatment with Mek inhibitors and (ii) cells expressing an active form of Mek significantly inhibited Bim expression during morphogenesis. In contrast, the PI 3-kinase pathway is not involved in regulating Bim expression in acinar structures since Bim expression was not altered after inhibition of the PI 3-kinase pathway in ErbB2 activated structures. In addition, activation of Akt in MCF-10A acini does not cause decrease luminal apoptosis or inhibit lumen formation (9), a finding which is consistent with Akt not regulating Bim expression in MCF-10A cells. However, in ErbB2 expressing acini both the Erk pathway and the PI 3-kinase pathway contribute to cell survival as inhibitors to both pathways induces apoptosis (Fig. 6A), suggesting that ErbB2-mediated survival effects may involve multiple signaling pathways.
Although the Erk MAPK pathway has been implicated in branching morphogenesis during development of a number of epithelial tissues, including the mouse salivary gland (21) and developing mouse kidney (13), little is known about the role of the Erk MAPK pathway in normal mammary morphogenesis. Our studies suggest that Erk activation has profound effects on cell survival during epithelial morphogenesis in vitro and that oncogenes, such as ErbB2, that activate this pathway may provide dual oncogenic functions by enhancing proliferation and blocking apoptosis. Indeed, a high level of activated Erk has been found in breast tumors (33), and elevated Erk kinase activity has been associated with ErbB2/Neu expression (36) in breast tumors. In addition, Bissell and coworkers have shown that inhibition of the hyperactive Erk kinase pathway in 3D cultures of transformed human mammary epithelial cells induces conversion from disorganized, highly proliferative structures to ones that morphologically resemble normal acini (37, 38), thus supporting our data showing that expression of an overactive Erk kinase pathway can significantly distort acinar architecture.
The data described here provide important new insights into the molecular mechanisms underlying the generation of luminal space in acinus-like structures via regulation of the proapoptotic protein Bim. Furthermore, these data also identify one pathway whereby oncogenes may induce filling of the luminal space during early stages of tumorigenesis in vivo, i.e., by blocking Bim expression via the Mek/Erk pathway. ErbB2 is overexpressed in 46 to 80% of primary ductal carcinoma in situ lesions in the breast (42). It will be of interest to determine whether the expression of Bim or other BH3-only Bcl-2 family members is altered in these early tumor lesions and, if so, whether this correlates with activation of the Erk MAPK pathway.
Acknowledgments
We thank Jessica K. Paulus for technical assistance, Jay Debnath for critical reading of the manuscript, and Mina Bissell for helpful discussions. We thank Phillippe Bouillet and Andreas Strasser for advice on Bim immunostaining and Sylvain Meloche, Stanley Korsmeyer, Martin McMahon, Phillippe Bouillet, Andreas Strasser, Denise Galloway, and Peter Howley for various reagents indicated in Materials and Methods. We thank ARIAD Pharmaceuticals for providing AP1510.
This study was supported by grants from NIH-NCI, Aventis Pharmaceuticals, and American Cancer Society (to J.S.B.); by a Susan Komen Breast Cancer Postdoctoral Fellowship (to M.J.R.); and by an NSF predoctoral fellowship (to K.R.M.).
REFERENCES
- 1.Biswas, S. C., and L. A. Greene. 2002. NGF down-regulates the BH3-only protein Bim and suppresses its proapoptotic activity by phosphorylation. J. Biol. Chem. 277:49511-49516. [DOI] [PubMed]
- 2.Blatchford, D. R., L. H. Quarrie, E. Tonner, C. McCarthy, D. J. Flint, and C. J. Wilde. 1999. Influence of microenvironment on mammary epithelial cell survival in primary culture. J. Cell Physiol. 181:304-311. [DOI] [PubMed] [Google Scholar]
- 3.Bouillet, P., S. Cory, L. C. Zhang, A. Strasser, and J. M. Adams. 2001. Degenerative disorders caused by Bcl-2 deficiency prevented by loss of its BH3-only antagonist Bim. Dev. Cell 1:645-653. [DOI] [PubMed] [Google Scholar]
- 4.Cheng, E. H., M. C. Wei, S. Weiler, R. A. Flavell, T. W. Mak, T. Lindsten, and S. J. Korsmeyer. 2001. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell 8:705-711. [DOI] [PubMed] [Google Scholar]
- 5.Coucouvanis, E., and G. R. Martin. 1999. BMP signaling plays a role in visceral endoderm differentiation and cavitation in the early mouse embryo. Development 126:535-546. [DOI] [PubMed] [Google Scholar]
- 6.Coucouvanis, E., and G. R. Martin. 1995. Signals for death and survival: a two-step mechanism for cavitation in the vertebrate embryo. Cell 83:279-287. [DOI] [PubMed] [Google Scholar]
- 7.Danial, N. N., and S. J. Korsmeyer. 2004. Cell death: critical control points. Cell 116:205-219. [DOI] [PubMed] [Google Scholar]
- 8.Debnath, J., K. Mills, N. Collins, M. Reginato, S. Muthuswamy, and J. Brugge. 2002. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell 111:29. [DOI] [PubMed] [Google Scholar]
- 9.Debnath, J., S. J. Walker, and J. S. Brugge. 2003. Akt activation disrupts mammary acinar architecture and enhances proliferation in an mTOR-dependent manner. J. Cell Biol. 163:315-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dijkers, P. F., K. U. Birkenkamp, E. W. Lam, N. S. Thomas, J. W. Lammers, L. Koenderman, and P. J. Coffer. 2002. FKHR-L1 can act as a critical effector of cell death induced by cytokine withdrawal: protein kinase B-enhanced cell survival through maintenance of mitochondrial integrity. J. Cell Biol. 156:531-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dijkers, P. F., R. H. Medema, J. W. Lammers, L. Koenderman, and P. J. Coffer. 2000. Expression of the proapoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr. Biol. 10:1201-1204. [DOI] [PubMed] [Google Scholar]
- 12.Egle, A., A. W. Harris, P. Bouillet, and S. Cory. 2004. Bim is a suppressor of Myc-induced mouse B-cell leukemia. Proc. Natl. Acad. Sci. USA 101:6164-6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher, C. E., L. Michael, M. W. Barnett, and J. A. Davies. 2001. Erk MAP kinase regulates branching morphogenesis in the developing mouse kidney. Development 128:4329-4338. [DOI] [PubMed] [Google Scholar]
- 14.Harris, J., M. Lippman, M. Morrow, and C. Osborne. 1999. Diseases of the breast. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa.
- 15.Hoffman, M. P., M. C. Kibbey, J. J. Letterio, and H. K. Kleinman. 1996. Role of laminin-1 and TGF-β3 in acinar differentiation of a human submandibular gland cell line (HSG). J. Cell Sci. 109(Pt. 8):2013-2021. [DOI] [PubMed] [Google Scholar]
- 16.Holbro, T., G. Civenni, and N. E. Hynes. 2003. The ErbB receptors and their role in cancer progression. Exp. Cell Res. 284:99-110. [DOI] [PubMed] [Google Scholar]
- 17.Huang, D. C., and A. Strasser. 2000. BH3-Only proteins-essential initiators of apoptotic cell death. Cell 103:839-842. [DOI] [PubMed] [Google Scholar]
- 18.Huang, J., J. D. Hardy, Y. Sun, and J. E. Shively. 1999. Essential role of biliary glycoprotein (CD66a) in morphogenesis of the human mammary epithelial cell line MCF10F. J. Cell Sci. 112(Pt. 23):4193-4205. [DOI] [PubMed] [Google Scholar]
- 19.Humphreys, R. C., M. Krajewska, S. Krnacik, R. Jaeger, H. Weiher, S. Krajewski, J. C. Reed, and J. M. Rosen. 1996. Apoptosis in the terminal endbud of the murine mammary gland: a mechanism of ductal morphogenesis. Development 122:4013-4022. [DOI] [PubMed] [Google Scholar]
- 20.Jaskoll, T., and M. Melnick. 1999. Submandibular gland morphogenesis: stage-specific expression of TGF-α/EGF, IGF, TGF-β, TNF, and IL-6 signal transduction in normal embryonic mice and the phenotypic effects of TGF-β2, TGF-β3, and EGF-r null mutations. Anat. Rec. 256:252-268. [DOI] [PubMed] [Google Scholar]
- 21.Kashimata, M., S. Sayeed, A. Ka, A. Onetti-Muda, H. Sakagami, T. Faraggiana, and E. W. Gresik. 2000. The ERK-1/2 signaling pathway is involved in the stimulation of branching morphogenesis of fetal mouse submandibular glands by EGF. Dev. Biol. 220:183-196. [DOI] [PubMed] [Google Scholar]
- 22.Ley, R., K. Balmanno, K. Hadfield, C. Weston, and S. J. Cook. 2003. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J. Biol. Chem. 278:18811-18816. [DOI] [PubMed] [Google Scholar]
- 23.Ley, R., K. E. Ewings, K. Hadfield, E. Howes, K. Balmanno, and S. J. Cook. 2004. Extracellular signal-regulated kinases 1/2 are serum-stimulated “Bim(EL) kinases” that bind to the BH3-only protein Bim(EL) causing its phosphorylation and turnover. J. Biol. Chem. 279:8837-8847. [DOI] [PubMed] [Google Scholar]
- 24.Lubarsky, B., and M. A. Krasnow. 2003. Tube morphogenesis: making and shaping biological tubes. Cell 112:19-28. [DOI] [PubMed] [Google Scholar]
- 25.Luciano, F., A. Jacquel, P. Colosetti, M. Herrant, S. Cagnol, G. Pages, and P. Auberger. 2003. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene 22:6785-6793. [DOI] [PubMed] [Google Scholar]
- 26.Marani, M., D. Hancock, R. Lopes, T. Tenev, J. Downward, and N. R. Lemoine. 2004. Role of Bim in the survival pathway induced by Raf in epithelial cells. Oncogene 23:2431-2441. [DOI] [PubMed] [Google Scholar]
- 27.Murray, P., and D. Edgar. 2000. Regulation of programmed cell death by basement membranes in embryonic development. J. Cell Biol. 150:1215-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muthuswamy, S. K., D. Li, S. Lelievre, M. J. Bissell, and J. S. Brugge. 2001. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat. Cell Biol. 3:785-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagata, M., H. Nakauchi, K. Nakayama, D. Loh, and T. Watanabe. 1996. Apoptosis during an early stage of nephrogenesis induces renal hypoplasia in bcl-2-deficient mice. Am. J. Pathol. 148:1601-1611. [PMC free article] [PubMed] [Google Scholar]
- 30.O'Connor, L., A. Strasser, L. A. O'Reilly, G. Hausmann, J. M. Adams, S. Cory, and D. C. Huang. 1998. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 17:384-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puthalakath, H., and A. Strasser. 2002. Keeping killers on a tight leash: transcriptional and posttranslational control of the proapoptotic activity of BH3-only proteins. Cell Death Differ. 9:505-512. [DOI] [PubMed] [Google Scholar]
- 32.Reginato, M. J., K. R. Mills, J. K. Paulus, D. K. Lynch, D. C. Sgroi, J. Debnath, S. K. Muthuswamy, and J. S. Brugge. 2003. Integrins and EGFR coordinately regulate the proapoptotic protein Bim to prevent anoikis. Nat. Cell Biol. 5:733-740. [DOI] [PubMed] [Google Scholar]
- 33.Salh, B., A. Marotta, C. Matthewson, M. Ahluwalia, J. Flint, D. Owen, and S. Pelech. 1999. Investigation of the Mek-MAP kinase-Rsk pathway in human breast cancer. Anticancer Res. 19:731-740. [PubMed] [Google Scholar]
- 34.Shinjyo, T., R. Kuribara, T. Inukai, H. Hosoi, T. Kinoshita, A. Miyajima, P. J. Houghton, A. T. Look, K. Ozawa, and T. Inaba. 2001. Downregulation of Bim, a proapoptotic relative of Bcl-2, is a pivotal step in cytokine-initiated survival signaling in murine hematopoietic progenitors. Mol. Cell. Biol. 21:854-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sorenson, C. M., S. A. Rogers, S. J. Korsmeyer, and M. R. Hammerman. 1995. Fulminant metanephric apoptosis and abnormal kidney development in bcl-2-deficient mice. Am. J. Physiol. 268:F73-F81. [DOI] [PubMed] [Google Scholar]
- 36.von Lintig, F. C., A. D. Dreilinger, N. M. Varki, A. M. Wallace, D. E. Casteel, and G. R. Boss. 2000. Ras activation in human breast cancer. Breast Cancer Res. Treatment 62:51-62. [DOI] [PubMed] [Google Scholar]
- 37.Wang, F., R. K. Hansen, D. Radisky, T. Yoneda, M. H. Barcellos-Hoff, O. W. Petersen, E. A. Turley, and M. J. Bissell. 2002. Phenotypic reversion or death of cancer cells by altering signaling pathways in three-dimensional contexts. J. Natl. Cancer Inst. 94:1494-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang, F., V. M. Weaver, O. W. Petersen, C. A. Larabell, S. Dedhar, P. Briand, R. Lupu, and M. J. Bissell. 1998. Reciprocal interactions between β1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc. Natl. Acad. Sci. USA 95:14821-14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang, P., A. P. Gilmore, and C. H. Streuli. 2004. Bim is an apoptosis sensor that responds to loss of survival signals delivered by epidermal growth factor but not those provided by integrins. J. Biol. Chem. 279:41280-41285. [DOI] [PubMed] [Google Scholar]
- 40.Wei, M. C., W. X. Zong, E. H. Cheng, T. Lindsten, V. Panoutsakopoulou, A. J. Ross, K. A. Roth, G. R. MacGregor, C. B. Thompson, and S. J. Korsmeyer. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weston, C. R., K. Balmanno, C. Chalmers, K. Hadfield, S. A. Molton, R. Ley, E. F. Wagner, and S. J. Cook. 2003. Activation of ERK1/2 by δRaf-1:ER* represses Bim expression independently of the JNK or PI3K pathways. Oncogene 22:1281-1293. [DOI] [PubMed] [Google Scholar]
- 42.Wilbur, D. C., and G. H. Barrows. 1993. Estrogen and progesterone receptor and c-erbB-2 oncoprotein analysis in pure in situ breast carcinoma: an immunohistochemical study. Mod. Pathol. 6:114-120. [PubMed] [Google Scholar]