Key Points
Question
Can microplastics reach the olfactory bulb in the human brain?
Findings
This case series analyzed the olfactory bulbs of 15 deceased individuals via micro-Fourier transform infrared spectroscopy and detected the presence of microplastics in the olfactory bulbs of 8 individuals. The predominant shapes were particles and fibers, with polypropylene being the most common polymer.
Meaning
The presence of microplastics in the human olfactory bulb suggests the olfactory pathway as a potential entry route for microplastics into the brain, highlighting the need for further research on their neurotoxic effects and implications for human health.
This case series investigates microplastics in the olfactory bulbs of deceased individuals and examines their size, morphology, color, and polymeric composition.
Abstract
Importance
Microplastic (MP) pollution is an emerging environmental and health concern. While MPs have been detected in various human tissues, their presence in the human brain has not been documented, raising important questions about potential neurotoxic effects and the mechanisms by which MPs might reach brain tissues.
Objective
To determine the presence of MPs in the human olfactory bulb and to analyze their characteristics such as size, morphology, color, and polymeric composition.
Design, Setting, and Participants
This case series study used a cross-sectional design involving the analysis of olfactory bulb tissues obtained from deceased individuals during routine coroner autopsies. The sampling procedures were conducted at São Paulo City Death Verification Service, with laboratory analysis carried out at the Brazilian Synchrotron Light Laboratory (LNLS). Participants included 15 adult individuals who had been residents of São Paulo for more than 5 years and underwent coroner autopsies. Exclusion criteria included previous neurosurgical interventions. Data analysis was performed in April 2024.
Exposure
The primary exposure assessed was the presence of MPs in the olfactory bulb, analyzed through direct tissue examination and digested tissue filtration followed by micro-Fourier transform infrared spectroscopy.
Main Outcomes and Measures
The main outcomes were the identification and characterization of MPs within the olfactory bulb, including their size, morphology, color, and polymeric composition.
Results
The median age of the 15 deceased individuals was 69.5 years, ranging from 33 to 100 years, with 12 males and 3 females. MPs were detected in the olfactory bulbs of 8 out of 15 individuals. A total of 16 synthetic polymer particles and fibers were identified, with 75% being particles and 25% being fibers. The most common polymer detected was polypropylene (43.8%). Sizes of MPs ranged from 5.5 μm to 26.4 μm for particles, and the mean fiber length was 21.4 μm. Polymeric materials were absent in procedural blank and negative control filters, indicating minimal contamination risk.
Conclusions and Relevance
This case series provides evidence of MPs found in the human olfactory bulb, suggesting a potential pathway for the translocation of MPs to the brain. The findings underscore the need for further research on the health implications of MP exposure, particularly concerning neurotoxicity and the potential for MPs to bypass the blood-brain barrier.
Introduction
The ubiquity of microplastic (MP) pollution has become a pervasive environmental concern,1 raising questions about its occurrence within the human body and its harmful effects.2 While MPs have been detected in various organs of the human body, such as the lungs,3,4 large and small intestines,5 liver,6 placenta,7,8 semen,9 and bloodstream,10 to our knowledge, there have been no published studies to date reporting their presence in the human brain.
The presence of the blood-brain barrier (BBB) is likely an important limiting factor for the access of MPs to the human brain via hematogenous translocation. Despite this, some animal studies have shown that MPs can impair the BBB and reach the brain via oral ingestion, leading to neurotoxic effects.11,12,13 Another potential entry site for micro- and nanoplastics (MNPs) in the human brain is the olfactory pathway.14 This pathway involves olfactory neurons in the nasal that transmit information about odors to the central olfactory system of the brain. Olfactory axons pass through the cribriform plate (CP) of the ethmoid bone and reach the olfactory bulbs (OB), which are connected to the limbic system of the brain.
There are different levels of evidence suggesting that the olfactory pathway might allow the translocation of exogenous particles to the brain. Environmental black carbon particles have been detected in various human brain regions, with one of the highest concentrations found in the OB, measuring 420.8 particles/mm3.15 Rarely, the 15- to 30-μm–sized ameboid form of Naegleria fowleri penetrates the brain via the nose, causing amebic meningoencephalitis.16 Affected individuals typically present with the disease after contact with contaminated freshwater bodies or after rinsing the nose with nonsterile tap water.17 Furthermore, the permeability of this barrier has been evoked as a possible quicker and safer drug delivery route to the brain,18,19 as well as access to cerebrospinal fluid through nasal lymphatic vessels.20
In this study, given the ubiquitous presence of MPs in the air21 and their previous identification in the human nasal cavity,22,23 we hypothesized that the smallest-size fraction of MPs could reach the OB. Therefore, we conducted an investigation into the presence of MPs within human OB obtained from 15 deceased individuals during coroner autopsies. We identified and analyzed various characteristics of the MPs, including their size, morphology, color, and polymeric composition.
Methods
This case series study was approved by the ethical board of the São Paulo University Medical School, in compliance with the Helsinki Declaration. Written informed consent was provided by the deceased individuals’ next of kin. The study was conducted from February 2023 to May 2024 and followed the Reporting Guideline for Case Series.46
Study Population
We obtained the bilateral OBs from 15 adult individuals who underwent routine coroner autopsies at the São Paulo City Death Verification Service of University of São Paulo to determine the cause of death. All individuals had been residents of São Paulo for more than 5 years. Cases in which the deceased had previously undergone neurosurgical interventions were not selected for the study. Information regarding previous occupations and underlying diseases was obtained through questionnaires administered to the next of kin. Additionally, autopsy reports were reviewed. We also collected samples from the OB of 2 stillbirths at 7 months gestation, as a negative control for the study. The collection of OBs took place between February 2023 and February 2024.
Quality Control and Quality Assurance and Evaluation of Sample Processing
We implemented a plastic-free approach to safeguard the integrity of our results. This strategy facilitated a thorough assessment of potential sources of variability and error, thereby enhancing the reliability of our collected data. All procedures, from the OB sampling to the micro-Fourier transform infrared (μFTIR) spectroscopy analysis, followed the protocols recommended by several studies.24,25,26 Briefly, all solutions were prefiltered through a Whatman cellulose filters with a mesh size of 0.45 μm. Stainless steel materials, glassware, and samples were covered with aluminum foil (before and after processing) to avoid airborne sample contamination. Ultrapure water with a resistivity of 18.2 mΩ was obtained from a Milli-Q purification device (Millipore Corp). Glass and stainless-steel materials were washed thoroughly using the purified water 3 times and then using acetone P.A. to remove any particles or fibers that have adhered to the glass. The scientific staff responsible for handling samples wore exclusively 100% cotton laboratory coats and were required to remove any plastic or textile bracelets, rings, and watches to minimize the risk of sample contamination. Clean latex gloves were used for all procedures. The samples were processed in a clean laminar flow cabinet (ISO class 5, SKU330313, Hipperquímica, SP, Brazil). Blank filters (47 mm) were used from the OB collection to the sample filtering to assess possible airborne contamination. A clean filter was also used as a negative control. Access to the μFTIR spectroscopy and the digestion/filtration room was restricted to the operators only, to avoid air flow in the room and the suspension/resuspension of possible atmospheric contaminants.
Sample Processing
The presence of MPs in the OB was assessed in 2 ways: directly on the tissue and a digested assessment. The cryo-cuts method preserves the spatial context of MPs within the tissue, allowing their proximity to anatomical structures such as blood vessels to be observed. This is crucial for understanding potential pathways of MPs translocation and accumulation within the OB. The digestion method ensures that MPs that are deeply embedded in the tissue are not overlooked. Postdigestion, MPs are concentrated on filters, which can then be analyzed for a more accurate quantification and identification without interference from the tissue matrix. By combining these 2 methods, the study maximized the probability of detecting and characterizing MPs within the OB.
OB Cryo-Cuts
The left OB of each case was horizontally cryo-sectioned using a Leica CM1860 UV cryostat (Leica Biosystems) at 10 μm thickness and thaw-mounted onto 5 mm × 5 mm gold/chromium-coated silicon dioxide/silicon substrates. No fixatives were used for the tissue sections. The samples were then freeze-dried for 48 hours (Freezone 6 [Labconco Corp]) and examined by optical microscopy (Eclipse LV100ND [Nikon Instruments Inc]). The freeze-drying process maintains the integrity of biological tissues by extracting water without substantially compromising their structure. Futhermore, the presence of water molecules, characterized by strong hydrogen bonding, poses a considerable challenge in FTIR measurements, as they mask specific signals indicative of chemical compositions.27
The procedures took place in a biosafety level 2 room in the Cryogenic Preparations Laboratory (LCRIO) at the Brazilian Synchrotron Light Laboratory (LNLS), National Center for Energy and Materials Research (CNPEM).
Sample Digestion and Filtering
Immediately after sampling, the right OBs from 10 selected cases were individually frozen at −20 °C in glass vials, covered with aluminum foil, and sealed with a glass lid until the digestion. For 5 patients, there was no available tissue for digestion. The tissues were then incubated for 12 hours at 40 °C using the enzyme mixture Corolase 7089 (20 UHb/mL)4 inside the laminar flux hood.
The solution was then filtered using a glass vacuum filtration system (Sigma-Aldrich) and silver membrane filters (25 mm in diameter and 0.45 microns pore size [Millipore]). Subsequently, the filters were kept individually in closed Petri dishes inside a glass dissector until the spectroscopy analysis. Due to the material characteristics, a recovery test was not feasible.
Micro-Fourier Transform Infrared Spectroscopy
We performed single-point μFTIR microspectroscopy measurements in reflection mode using a diffraction-limited IR microscope (Cary 620 [Agilent Technologies]). The IR microscope is coupled to a Michelson interferometer responsible for the frequency demultiplexing of the mid-IR broadband response. We used a 1000 K Globar source and illumination and interferograms detection was done by using a high-sensitivity cryo-cooled Mercury–Cadmium-Telluride (MCT [Infrared Associates Inc]). After the interferometer, the IR beam was directed to a 25 × objective that produced an illumination spot of 420 μm × 420 μm on the sample’s surface. This field of view was further reduced to 50 to 100 μm by slits to concentrate the analysis around specific particles. The reflected light was collected through a confocal arrangement by the same objective lens and then directed to the MCT detector. FTIR spectra were generated by calculating the Fourier transforms of the recorded interferograms. The spectral resolution was configured at 16 cm−1, encompassing the range from 4000 to 700 cm−1. Each μFTIR spectrum was normalized to the spectrum of a clean gold surface, which served as a reference background. The cryo-cuts and digested filters were fully analyzed. The μFTIR analyses took place in the IMBUIA beamline at the Brazilian Synchrotron Light Laboratory (LNLS), National Center for Research in Energy and Materials (CNPEM).
The acquired spectra were processed manually using the KnowItAll Informatics System 2024 (John Wiley and Sons Inc). The comparative analysis was performed with the help of FTIR spectra libraries developed for MPs research, including the FTIR Library of Plastic Particles (FLOPP),28 FTIR Library of Plastic Particles Sourced from the Environment (FLOPP-e),28 siMPLe database,29 and KnowItAll IR Spectral Library. We adopted a Hit Quality Index greater than 75% of agreement between characteristic bands of polymers observed in reference materials with bands observed in unknown particles or fibers.30,31
Microphotograph Analysis
We determined particle sizes by analyzing microphotographs obtained through μFTIR spectroscopy. ImageJ 1.54g software (US National Institutes of Health) was used for accurate measurements.
Statistical Analysis
Descriptive analyses were performed using SPSS Statistics 26.0 software (IBM Inc). These analyses were performed in April 2024.
Results
The median (range) age of the 15 deceased individuals was 69.5 (33-100) years. They included 12 males and 3 females. Demographic information is detailed in Table 1. Apart from the 2 cases with histological evidence of previous ischemic cerebral infarction and 1 case with a subarachnoid hematoma due to a ruptured aneurysm of the middle cerebral artery, there were no cerebral histological abnormalities in the remaining cases. The mean (SD) mass of the OB (left or right) was 0.187 (0.050) g, ranging from 0.100 to 0.273 g.
Table 1. Demographic and Autopsy Findings of the Decedents.
| Decedent | Demographic data | Underlying diseases | Cause of death | ||
|---|---|---|---|---|---|
| Age at death, y | Sex | Occupation | |||
| 1 | 69 | Male | Driver | Lung cancer | Pulmonary tuberculosis |
| 2 | 76s | Female | Cook | Systemic arterial hypertension | Acute pulmonary edema |
| 3 | 70 | Male | Mechanic | Acute myocardial infarction, prostate cancer | Pulmonary edema |
| 4 | 33 | Male | Freelancer | Epilepsy, chronic alcoholism | Pneumonia |
| 5 | 71 | Male | Waiter | Ruptured thoracic aortic dissection, systemic arterial hypertension, chronic alcoholism | Hemothorax |
| 6 | 78 | Male | Manager | Atherosclerosis | Acute myocardial infarction |
| 7 | 65 | Male | Tinker | Pleural empyema, systemic arterial hypertension | Septic shock |
| 8 | 71 | Female | Cook | Middle cerebral artery aneurysm, systemic arterial hypertension, neuralgia | Subarachnoid hemorrhage |
| 9 | 62 | Male | Tinker | Systemic arterial hypertension, chronic alcoholism | Acute myocardial infarction |
| 10 | 77 | Male | Bricklayer | Ischemic heart disease, systemic arterial hypertension, diabetes, chronic alcoholism | Acute pulmonary edema |
| 11 | 57 | Male | Unemployed | Systemic arterial hypertension, heart disease, chronic alcoholism, congenital muscular dystrophy | Unknown |
| 12 | 70 | Female | Pensioner | Hypertrophic heart disease, asthma | Pulmonary edema |
| 13 | 66 | Male | Cleaner | Hypertensive heart disease, systemic arterial hypertension, chronic alcoholism | Pulmonary edema |
| 14 | 78 | Male | Concierge | Stroke, Chagas disease | Bronchopneumonia |
| 15 | 100 | Male | Tinker | Myeloid leukemia | Bronchopneumonia |
A total of 16 synthetic polymer particles and fibers were identified in 8 out of the 15 deceased individuals, with a range from 1 to 4 MPs per OB. Of these, 75% were particles, of which 83.4% were fragments and 16.6% were spheres, while 25% were fibers with a length-to-width ratio exceeding 3. The particles had a mean (SD) length of 12.1 (7.2) μm, ranging from 5.5 to 26.4 μm, and a mean (SD) width of 8.9 (6.4) μm, ranging from 3.0 to 25.4 μm. The fibers exhibited a mean (SD) length of 21.4 (2.6) μm, ranging from 19.0 to 24.5 μm, and a mean (SD) width of 3.8 (1.8) μm, ranging from 3.0 to 6.0 μm.
In the procedural blank filters, we detected 2 cotton fibers, 2 silica beads, and 1 silicate fragment. Polymeric materials were absent in both the procedural blank and negative control filters. From the 2 collected samples in stillborn, we were able to analyze 1 case, which did not show the presence of MPs. The other case had insufficient material for analysis.
Polypropylene was the most prevalent polymer (43.8%), followed by polyamide, nylon, and polyethylene vinyl acetate (12.5%). This was followed by polyethylene, perlon polyamide, and wool-polypropylene, which accounted for 6.3%). Upon comparison with the reference spectral library of plastic materials, the identified MP particles and fibers exhibited indications of weathering. The μFTIR spectra of the weathered MPs differed substantially from those of pristine standard samples; multiple peaks in the spectra of weathered MPs were attenuated or entirely absent.
Microphotographs and μFTIR point-spectra showing the main types of MP detected in the OB are shown in Figure 1 and Figure 2. The complete μFTIR point-spectra results of the digested OB are presented in the eFigure in Supplement 1. Table 2 provides details regarding the morphology, color, and chemical characterization of the particles and fibers.
Figure 1. Microphotographs and Micro-Fourier Transform Infrared (μFTIR) Spectra of the Microplastics Found in the Olfactory Bulb Tissue.
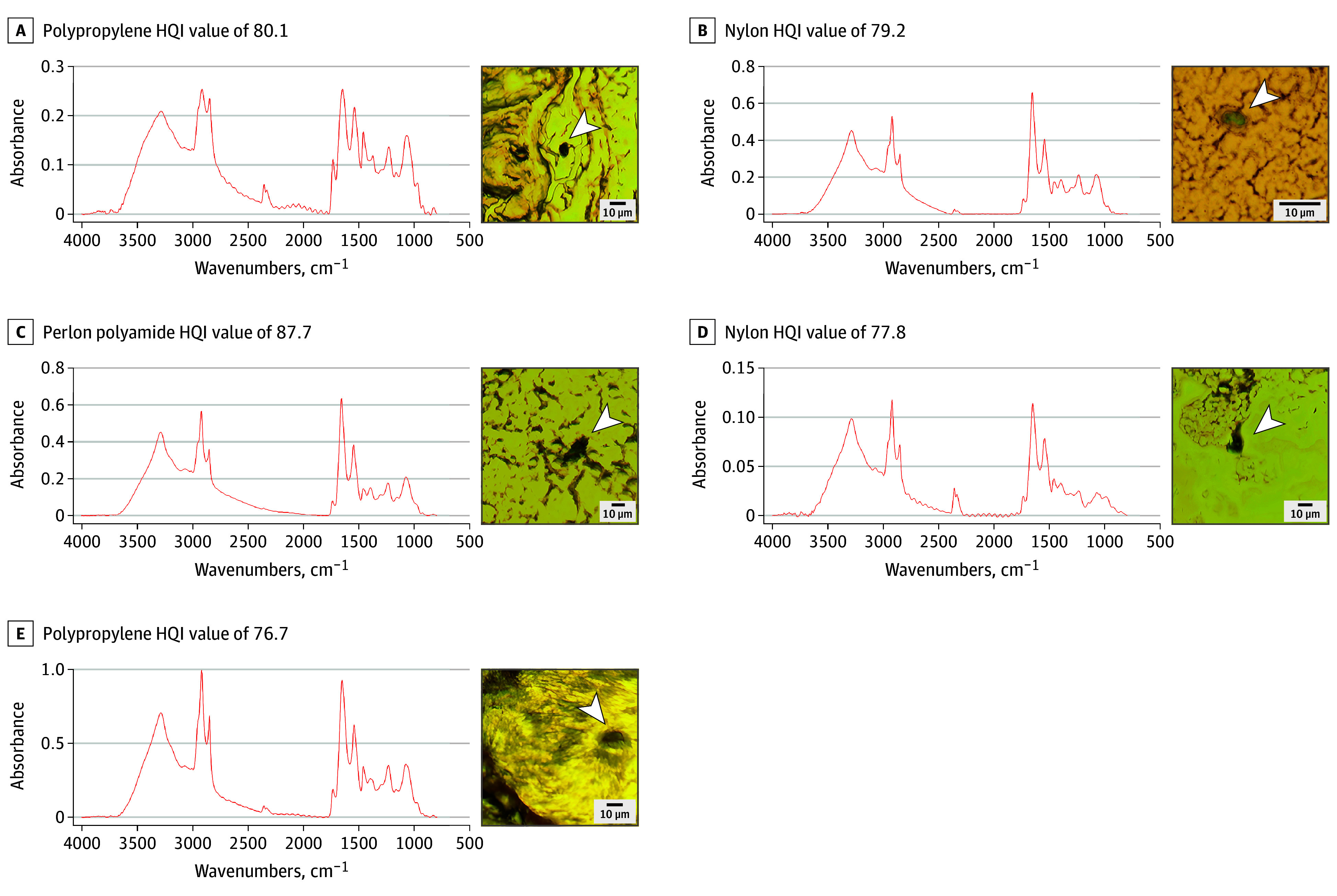
HQI indicates hit quality index.
Figure 2. Microphotographs and Micro-Fourier Transform Infrared (μFTIR) Spectra of the Main Microplastics Found in the Digested Olfactory Bulb.
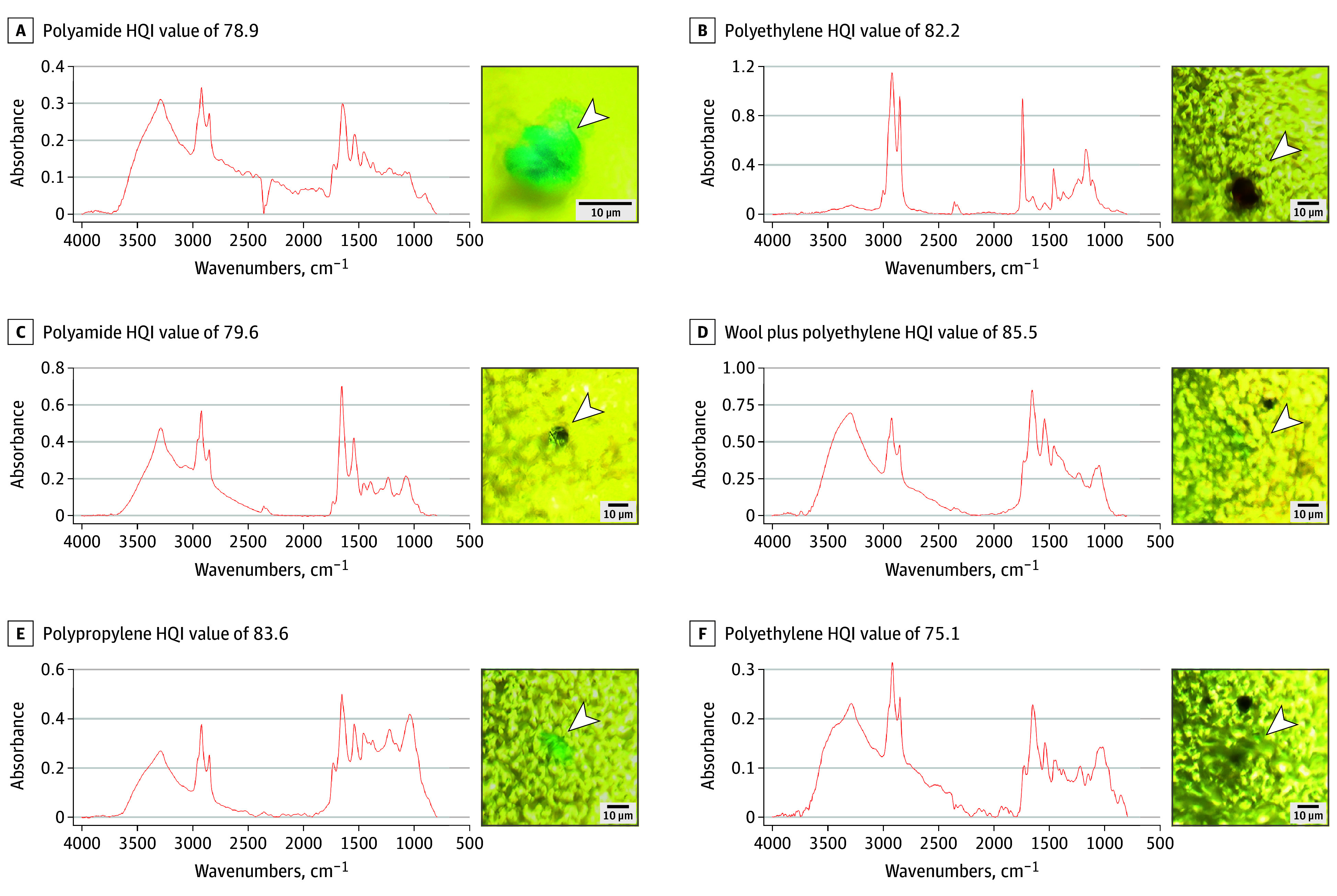
HQI indicates hit quality index.
Table 2. Morphology and Polymeric Matrix of the Identified Particles and Fibers.
| Decedent | Cryo-cuts (left OB) | Digested Filters (right OB) | ||||||
|---|---|---|---|---|---|---|---|---|
| Format | Size, μma | Polymer matrix | Color | Format | Size, μma | Polymer matrix | Color | |
| 1 | Particle | 5.5 × 5.1 | Polypropylene | Black | Particle | 13.6 × 8.9 | Polyamide | Blue |
| 2 | Particle | 13.4 × 5.0 | Nylon | Green | NA | NA | NA | NA |
| 5 | Particle | 8.5 × 4.0 | Perlon polyamide | Gray | NA | NA | NA | NA |
| 6 | NA | NA | NA | NA | Particle | 18.1 × 10.6 | Polyethylene vinyl acetate | White |
| NA | NA | NA | NA | Fiber | 19.6 × 6.0 | Polypropylene | Green | |
| NA | NA | NA | NA | Particle | 15.2 × 8.6 | Polyethylene vinyl acetate | Brown | |
| NA | NA | NA | NA | Fiber | 24.5 × 2.6 | Wool + polypropylene | Green | |
| 7 | NA | NA | NA | NA | Particle | 26.4 × 25.4 | Polypropylene | Blue/gray |
| NA | NA | NA | NA | Particle | 17.0 × 9.1 | Polypropylene | Blue/gray | |
| NA | NA | NA | NA | Fiber | 19.0 × 4.4 | Polypropylene | Pink | |
| 11 | NA | NA | NA | NA | Particle | 8.3 × 7.0 | Polyamide | Gray |
| 12 | Particle | 6.6 × 3.0 | Polypropylene | Gray | Particle | 5.9 × 4.4 | Polyethylene | Light blue |
| Particle | 19.2 × 16.7 | Polypropylene | Green | |||||
| 15 | Fiber | 22.6 × 3.0 | Nylon | Blue | NA | NA | NA | NA |
Abbreviations: NA, not applicable; OB, olfactory bulb.
Particles are listed as length × width; fibers are listed as length × diameter.
Discussion
To our knowledge, this is the first study in which the presence of MPs in the human brain was identified and characterized using μFTIR, allowing quantification and characterization of the morphology and polymeric matrix. Specifically, we detected particles as the predominant shape in the OB in 8 out of 15 individuals who underwent autopsy in Sao Paulo. Our data extend the notion that not only black carbon15 but also MP accumulate in the OB in humans.
We believe that the anatomy of the cribriform plate of the ethmoid bone may serve as a gateway in the nasal passages from within the skull. This plate, situated between the frontal and sphenoid bones, lies horizontally and contains multiple foramina, each less than 1 mm in diameter.32 The OB lies directly above it, and the olfactory neurons of the nasal mucosa reach the OB via the foramina of the cribriform plate. Recent studies have shown that part of the cerebrospinal fluid outflow occurs via lymphatic vessels that surround the olfactory axons, reaching the nasal mucosa and extending toward the nasal lymphoid tissue.33 Ossification of the CP occurs by 1 year of age,34 and the total area of the perforations is age-dependent; it is 3.79 to 3.99 mm2 in those over 50 years of age and 5.61 to 7.91 mm2 in those under 50 years of age. This decrease in the area over time, causing compression and dysfunction of the olfactory nerves, is thought to explain the decreased olfactory sensation in older individuals.35 Furthermore, in mice, paracellular spaces in the olfactory epithelium can reach 5 to 20 μm in the medial-lateral dimension of the transport and a 10- to 100-μm range observed in the rostral-caudal dimension.36 If a similar situation is observed in humans, this could represent another factor facilitating entry of larger particles in the brain via the cribriform plate.
Given the widespread presence of MPs in the air, some of which are associated with PM2.5,37 the identification of MPs in the nose45 and now in the OB, along with the vulnerable anatomical pathways, reinforces the notion that the olfactory pathway is an important entry site for exogenous particles to the brain. In previous epidemiological studies, exposure to PM2.5 has been associated with neurological and psychiatric adverse outcomes, such as dementia.38,39 Some neurodegenerative diseases, such as Parkinson disease, seem to have a connection with nasal abnormalities as initial symptoms.40 In experimental studies, both exposures to PM2.5 and MPs have shown to cause several neurotoxic effects, including disturbances on the brain development.41,42 The cribriform plate reaches maturation at 1 to 2 years of age, which is a critical time window during which MP penetration into the brain could have negative effects on the organ maturation.
In this study, the MP polymeric matrix found in the OB corresponds to the most produced and manufactured plastics, such as polypropylene, nylon/polyamide, polyethylene and polyethylene vinyl acetate, present in packaging, clothes and home accessories, suggesting indoor environments as a major source of inhaled MPs.21,43
Limitations
This study has certain limitations. Although the olfactory pathway seems a likely exposure route, we cannot dismiss the possibility of multiple entry routes. MPs might have reached the OB either through systemic circulation, crossing the BBB, or via the respiratory pathway through the trigeminal nerve.44 The biologic matrix of the OB tissues can be a confounding factor when analyzing MP spectra due to its similarity to some polymeric materials. Therefore, we were cautious to consider suspect particles as polymeric material only when spectral bands highly matched with weathered bands from MP libraries (HQI >75%). In the filtered samples, the biological matrix was previously digested, not being an issue. Given the maximum spatial resolution (3 μm) of μFTIR spectroscopy setup and the limited capacity of analysis for other techniques, we were unable to detect nanoplastics. It is likely that the number of plastics in the submicron range with the potential to cause substantial biological damage would be far more numerous.
Avoiding contamination is one of the biggest challenges when analyzing MP. Due to the presence of MP fibers and particles in the air, we have used blank samples in all methodological procedures to detect contamination of the air. We found no MP in our procedural blanks, which supports the validity of our results. Furthermore, we had the opportunity to analyze the brains of 2 stillbirths. However, the status of brain tissue maceration made the analysis challenging due to difficulties in sampling and processing.
Conclusions
This case series describes the presence of MPs in the OB, mainly particles of the most commonly produced/processed polymers for clothing and packaging such as polypropylene and nylon. Our data support the idea that the olfactory pathway is an important entry site for environmental air pollutants. Considering the potential neurotoxic effects caused by MPs in the brain, and the widespread environmental contamination with plastics, our results should raise concern in the context of increasing prevalence of neurodegenerative diseases. Noninvasive imaging technologies, such as magnetic resonance imaging, are needed to overcome the current limitations in tissue analysis of different human organs and to improve the understanding of the health hazards of MPs.
eFigure. Microphotographs and µFTIR Spectra of the Microplastics Found in the Digested Olfactory Bulb
Data Sharing Statement
References
- 1.Li Y, Tao L, Wang Q, Wang F, Li G, Song M. Potential health impact of microplastics: a review of environmental distribution, human exposure, and toxic effects. Environ Health. 2023;1(4):249-257. doi: 10.1021/envhealth.3c00052 [DOI] [Google Scholar]
- 2.Marfella R, Prattichizzo F, Sardu C, et al. Microplastics and nanoplastics in atheromas and cardiovascular events. N Engl J Med. 2024;390(10):900-910. doi: 10.1056/NEJMoa2309822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jenner LC, Rotchell JM, Bennett RT, Cowen M, Tentzeris V, Sadofsky LR. Detection of microplastics in human lung tissue using μFTIR spectroscopy. Sci Total Environ. 2022;831:154907. doi: 10.1016/j.scitotenv.2022.154907 [DOI] [PubMed] [Google Scholar]
- 4.Amato-Lourenço LF, Carvalho-Oliveira R, Júnior GR, Dos Santos Galvão L, Ando RA, Mauad T. Presence of airborne microplastics in human lung tissue. J Hazard Mater. 2021;416:126124. doi: 10.1016/j.jhazmat.2021.126124 [DOI] [PubMed] [Google Scholar]
- 5.Zhu L, Kang Y, Ma M, et al. Tissue accumulation of microplastics and potential health risks in human. Sci Total Environ. 2024;915:170004. doi: 10.1016/j.scitotenv.2024.170004 [DOI] [PubMed] [Google Scholar]
- 6.Horvatits T, Tamminga M, Liu B, et al. Microplastics detected in cirrhotic liver tissue. EBioMedicine. 2022;82:104147. doi: 10.1016/j.ebiom.2022.104147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halfar J, Čabanová K, Vávra K, et al. Microplastics and additives in patients with preterm birth: the first evidence of their presence in both human amniotic fluid and placenta. Chemosphere. 2023;343:140301. doi: 10.1016/j.chemosphere.2023.140301 [DOI] [PubMed] [Google Scholar]
- 8.Ragusa A, Svelato A, Santacroce C, et al. Plasticenta: First evidence of microplastics in human placenta. Environ Int. 2021;146:106274. doi: 10.1016/j.envint.2020.106274 [DOI] [PubMed] [Google Scholar]
- 9.Montano L, Giorgini E, Notarstefano V, et al. Raman microspectroscopy evidence of microplastics in human semen. Sci Total Environ. 2023;901:165922. doi: 10.1016/j.scitotenv.2023.165922 [DOI] [PubMed] [Google Scholar]
- 10.Leslie HA, van Velzen MJM, Brandsma SH, Vethaak AD, Garcia-Vallejo JJ, Lamoree MH. Discovery and quantification of plastic particle pollution in human blood. Environ Int. 2022;163:107199. doi: 10.1016/j.envint.2022.107199 [DOI] [PubMed] [Google Scholar]
- 11.Kopatz V, Wen K, Kovács T, et al. Micro- and nanoplastics breach the blood-brain barrier (BBB): biomolecular corona’s role revealed. Nanomaterials (Basel). 2023;13(8):1404. doi: 10.3390/nano13081404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin H, Yang C, Jiang C, et al. Evaluation of neurotoxicity in BALB/c mice following chronic exposure to polystyrene microplastics. Environ Health Perspect. 2022;130(10):107002. doi: 10.1289/EHP10255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shan S, Zhang Y, Zhao H, Zeng T, Zhao X. Polystyrene nanoplastics penetrate across the blood-brain barrier and induce activation of microglia in the brain of mice. Chemosphere. 2022;298:134261. doi: 10.1016/j.chemosphere.2022.134261 [DOI] [PubMed] [Google Scholar]
- 14.Hua T, Kiran S, Li Y, Sang QA. Microplastics exposure affects neural development of human pluripotent stem cell-derived cortical spheroids. J Hazard Mater. 2022;435:128884. doi: 10.1016/j.jhazmat.2022.128884 [DOI] [PubMed] [Google Scholar]
- 15.Vanbrabant K, Van Dam D, Bongaerts E, et al. Accumulation of ambient black carbon particles within key memory-related brain regions. JAMA Netw Open. 2024;7(4):e245678. doi: 10.1001/jamanetworkopen.2024.5678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoder JS, Straif-Bourgeois S, Roy SL, et al. Primary amebic meningoencephalitis deaths associated with sinus irrigation using contaminated tap water. Clin Infect Dis. 2012;55(9):e79-e85. doi: 10.1093/cid/cis626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siddiqui R, Khan NA. Contemporary approaches to treat Naegleria fowleri: a patent overview. Pharm Pat Anal. 2021;10(3):99-101. doi: 10.4155/ppa-2020-0023 [DOI] [PubMed] [Google Scholar]
- 18.Agrawal M, Saraf S, Saraf S, et al. Nose-to-brain drug delivery: an update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J Control Release. 2018;281:139-177. doi: 10.1016/j.jconrel.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 19.Crowe TP, Greenlee MHW, Kanthasamy AG, Hsu WH. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018;195:44-52. doi: 10.1016/j.lfs.2017.12.025 [DOI] [PubMed] [Google Scholar]
- 20.Chae J, Choi M, Choi J, Yoo SJ. The nasal lymphatic route of CSF outflow: implications for neurodegenerative disease diagnosis and monitoring. Anim Cells Syst (Seoul). 2024;28(1):45-54. doi: 10.1080/19768354.2024.2307559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amato-Lourenço LF, Dos Santos Galvão L, Wiebeck H, Carvalho-Oliveira R, Mauad T. Atmospheric microplastic fallout in outdoor and indoor environments in São Paulo megacity. Sci Total Environ. 2022;821:153450. doi: 10.1016/j.scitotenv.2022.153450 [DOI] [PubMed] [Google Scholar]
- 22.Torres-Agullo A, Karanasiou A, Lacorte S. Nasal lavage technique reveals regular inhalation exposure of microplastics, not associated from face mask use. Environ Int. 2023;178:108129. doi: 10.1016/j.envint.2023.108129 [DOI] [PubMed] [Google Scholar]
- 23.Tuna A, Taş BM, Başaran Kankılıç G, et al. Detection of microplastics in patients with allergic rhinitis. Eur Arch Otorhinolaryngol. 2023;280(12):5363-5367. doi: 10.1007/s00405-023-08105-7 [DOI] [PubMed] [Google Scholar]
- 24.Gao W, Deng XJ, Zhang J, Qi L, Zhao XQ, Zhang PY. Assessment of quality control measures in the monitoring of microplastic: a critical review. Environmental Pollutants and Bioavailability. 2023;35(1). doi: 10.1080/26395940.2023.2203349 [DOI] [Google Scholar]
- 25.Gwinnett C, Miller RZ. Are we contaminating our samples? A preliminary study to investigate procedural contamination during field sampling and processing for microplastic and anthropogenic microparticles. Mar Pollut Bull. 2021;173(Pt B):113095. doi: 10.1016/j.marpolbul.2021.113095 [DOI] [PubMed] [Google Scholar]
- 26.Rochman, CM, Brookson, C, Bikker, J, et al. Rethinking microplastics as a diverse contaminant suite. Environ Toxicol Chem. 2019;38(4):703-711. doi: 10.1002/etc.4371 [DOI] [PubMed] [Google Scholar]
- 27.Jackson M, Mantsch HH. The use and misuse of FTIR spectroscopy in the determination of protein structure. Crit Rev Biochem Mol Biol. 1995;30(2):95-120. doi: 10.3109/10409239509085140 [DOI] [PubMed] [Google Scholar]
- 28.De Frond H, Rubinovitz R, Rochman CM. μATR-FTIR spectral libraries of plastic particles (FLOPP and FLOPP-e) for the analysis of microplastics. Anal Chem. Published online November 23, 2021. doi: 10.1021/acs.analchem.1c02549 [DOI] [PubMed] [Google Scholar]
- 29.Primpke S, Cross RK, Mintenig SM, et al. Toward the systematic identification of microplastics in the environment: evaluation of a new independent software tool (siMPle) for spectroscopic analysis. Appl Spectrosc. 2020;74(9):1127-1138. doi: 10.1177/0003702820917760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weisser J, Pohl T, Heinzinger M, Ivleva NP, Hofmann T, Glas K. The Identification of Microplastics Based on Vibrational Spectroscopy Data—A Critical Review of Data Analysis Routines. TrAC. Trends Analyt Chem. 2022;148:116535. doi: 10.1016/j.trac.2022.116535 [DOI] [Google Scholar]
- 31.Morgado V, Palma C, Bettencourt da Silva RJN. Microplastics identification by infrared spectroscopy - Evaluation of identification criteria and uncertainty by the Bootstrap method. Talanta. 2021;224:121814. doi: 10.1016/j.talanta.2020.121814 [DOI] [PubMed] [Google Scholar]
- 32.Abolmaali N, Gudziol V, Hummel T. Pathology of the olfactory nerve. Neuroimaging Clin N Am. 2008;18(2):233-42. doi: 10.1016/j.nic.2007.10.002 [DOI] [PubMed] [Google Scholar]
- 33.Spera I, Cousin N, Ries M, et al. Open pathways for cerebrospinal fluid outflow at the cribriform plate along the olfactory nerves. EBioMedicine. 2023;91:104558. doi: 10.1016/j.ebiom.2023.104558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krmpotić-Nemanić J, Padovan I, Vinter I, Jalsovec D. Development of the cribriform plate and of the lamina mediana. Ann Anat. 1998;180(6):555-559. doi: 10.1016/S0940-9602(98)80065-4 [DOI] [PubMed] [Google Scholar]
- 35.Kalmey JK, Thewissen JG, Dluzen DE. Age-related size reduction of foramina in the cribriform plate. Anat Rec. 1998;251(3):326-329. doi: [DOI] [PubMed] [Google Scholar]
- 36.Kincaid AE, Ayers JI, Bartz JC. Specificity, size, and frequency of spaces that characterize the mechanism of bulk transepithelial transport of prions in the nasal cavities of hamsters and mice. J Virol. 2016;90(18):8293-8301. doi: 10.1128/JVI.01103-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akhbarizadeh R, Dobaradaran S, Amouei Torkmahalleh M, Saeedi R, Aibaghi R, Faraji Ghasemi F. Suspended fine particulate matter (PM2.5), microplastics (MPs), and polycyclic aromatic hydrocarbons (PAHs) in air: their possible relationships and health implications. Environ Res. 2021;192:110339. doi: 10.1016/j.envres.2020.110339 [DOI] [PubMed] [Google Scholar]
- 38.Braithwaite I, Zhang S, Kirkbride JB, Osborn DPJ, Hayes JF. Air pollution (particulate matter) exposure and associations with depression, anxiety, bipolar, psychosis and suicide risk: a systematic review and meta-analysis. Environ Health Perspect. 2019;127(12):126002. doi: 10.1289/EHP4595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kioumourtzoglou MA, Schwartz JD, Weisskopf MG, et al. Long-term PM2.5 exposure and neurological hospital admissions in the northeastern United States. Environ Health Perspect. 2016;124(1):23-29. doi: 10.1289/ehp.1408973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fullard ME, Morley JF, Duda JE. Olfactory dysfunction as an early biomarker in Parkinson’s disease. Neurosci Bull. 2017;33(5):515-525. doi: 10.1007/s12264-017-0170-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu XQ, Huang J, Song C, Zhang TL, Liu YP, Yu L. Neurodevelopmental toxicity induced by PM2.5 exposure and its possible role in neurodegenerative and mental disorders. Hum Exp Toxicol. Published online August 3, 2023. doi: 10.1177/09603271231191436 [DOI] [PubMed] [Google Scholar]
- 42.Prüst M, Meijer J, Westerink RHS. The plastic brain: neurotoxicity of micro- and nanoplastics. Part Fibre Toxicol. 2020;17(1):24. doi: 10.1186/s12989-020-00358-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu J, Zhang X, Liao K, Wu P, Jin H. Microplastics in dust from different indoor environments. Sci Total Environ. 2022;833:155256. doi: 10.1016/j.scitotenv.2022.155256 [DOI] [PubMed] [Google Scholar]
- 44.Jeong SH, Jang JH, Lee YB. Drug delivery to the brain via the nasal route of administration: exploration of key targets and major consideration factors. J Pharm Investig. 2023;53(1):119-152. doi: 10.1007/s40005-022-00589-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taş BM, Tuna A, Başaran Kankılıç G, et al. Role of microplastics in chronic rhinosinusitis without nasal polyps. Laryngoscope. 2024;134(3):1077-1080. doi: 10.1002/lary.30926 [DOI] [PubMed] [Google Scholar]
- 46.Kempen JH. Appropriate use and reporting of uncontrolled case series in the medical literature. Am J Ophthalmol. 2011;151(1):7-10.e1. doi: 10.1016/j.ajo.2010.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Microphotographs and µFTIR Spectra of the Microplastics Found in the Digested Olfactory Bulb
Data Sharing Statement


