Abstract
Galanin is a neuropeptide implicated in the regulation of feeding, reproduction, cognition, nociception, and seizure susceptibility. There are three known galanin receptor (GALR) subtypes (GALR1, GALR2, and GALR3), which bind to galanin with different affinities and have their own unique distributions, signaling mechanisms, and putative functions in the brain and peripheral nervous system. To gain further insight into the possible physiological significance of GALR2, we created mutant mice that were deficient in GALR2 and compared their phenotype to that of wild-type (WT) littermate or age-matched controls, with respect to basic motor and sensory function, feeding behavior, reproduction, mood, learning and memory, and seizure susceptibility. Phenotypic analysis revealed that animals bearing a deletion of GALR2 did not differ significantly from their WT controls in any of the measured variables. We conclude that either GALR2 plays no role in these physiological functions or through redundancy or compensation these mutant animals can adapt to the congenital absence of GALR2. It is also conceivable that GALR2 plays only a subtle role in some of these functions and that the impact of its loss could not be detected by the analytical procedures used here.
Galanin is a neuropeptide comprising 29 to 30 amino acids and is widely distributed throughout the central and peripheral nervous system (35, 36, 45, 46, 49). Since its discovery over 20 years ago, galanin has been shown to have a role in numerous physiological processes, including the neuroendocrine regulation of gonadotropin and growth hormone secretion, nociception, cognition, and seizure susceptibility (reviewed in references 1, 3, and 19). The molecular actions of galanin are thought to be mediated by one or more galanin receptor (GALR) subtypes, GALR1, GALR2, and GALR3, all of which are G-protein-coupled receptors (reviewed in references 6, 7, and 25). Despite their partial sequence homology, these receptors differ with respect to the signaling pathways activated by galanin. Activation of GALR1 and GALR3 inhibits adenylate cyclase and the opening of potassium channels (reviewed in references 6, 29, and 47), whereas activation of GALR2 increases intracellular calcium through stimulation of phospholipase C (6, 41, 53, 55). Differences among their signaling mechanisms are likely to contribute to their unique physiological actions. In addition, these different GALRs also have distinctive patterns of distribution in the nervous system, sustaining the notion that these receptors have diverse physiological functions (6, 9, 11, 16, 17, 21, 29, 37, 39, 47). However, beyond the diversity of their signaling mechanisms and distributions, we understand relatively little about the physiological role played by any of the different galanin receptor subtypes. This is partly attributable to the fact there are few (if any) receptor-specific ligands available (2, 31, 43, 57), and to date, only one of the receptors (GALR1) has been genetically targeted for ablation for the purpose of studying the resultant phenotype (4, 18, 23, 26, 34, 56).
GALR2 has been implicated in the mediation of galanin's effect on jejunal contraction (54), stimulation of growth hormone and prolactin secretion (11, 40), myometrial contraction (38), seizure susceptibility (34), peripheral nerve regeneration (8, 32), hippocampal neuroprotection (12, 33), and the response to axotomy in motor and sensory neurons (48, 58). In the case of GALR2, its mRNA has been localized to hypothalamic nuclei, hippocampus, amygdala, several regions of the cortex, and the dorsal root ganglion (reviewed in references 11, 20, 29, and 39) and has been shown to be regulated following peripheral nerve axotomy (48, 58). Considering the distribution of GALR2 and its mRNA in such areas as the hypothalamus, hippocampus, amygdala, cortex, and dentate gyrus (11, 16, 20, 29, 39) and its unique signaling mechanism (7), we postulated that GALR2 plays an important role in regulating feeding behavior, growth, several aspects of reproduction, behavior, learning and memory, and seizure susceptibility. To identify physiological processes for which GALR2 has a vital function, we generated mice that were deficient in GALR2 and compared their phenotype to that of normal wild-type (WT) controls.
MATERIALS AND METHODS
Experimental animals.
All animals used in these studies were housed in the animal care facilities at the University of Washington Department of Comparative Medicine or at Nura, Inc. (Seattle, WA). All procedures were approved by the University of Washington or the Nura Institutional Animal Care and Use Committee, in accordance with the National Institutes of Health Guide to Care and Use of Laboratory Animals. Animals were group housed except during actual experimental procedures, when single housing was required. All animals were kept on a constant light/dark cycle at all times (12:12 with lights on at 6:00 [University of Washington] or 7:00 [Nura]). Mice were weaned onto Pico Lab Rodent 20 5058 (20% protein, 9% fat) and then later switched to Pico Lab Rodent 20 5053 (20% protein, 4.5% fat).
Generation of GALR2−/− (null) mice.
GALR2−/− knockout (KO) mice were produced at Nura, Inc., by retroviral mutagenesis as described previously (30). Briefly, an embryonic stem (ES) cell library was constructed by infecting 129/SvImJ ES cells with a retroviral vector. The vector included a selection marker, termination codons in all reading frames, a splice acceptor, and a transcription terminator. Mutations in the GALR2 gene were found in the library by PCR analysis of genomic DNAs using vector-specific and gene-specific primers. Mutant clones isolated from the library were used for animal production using standard injection methods. In brief, mutant ES cells were injected into blastocysts of C57BL/6J mice before being transferred into the uteri of day 2.5 pseudopregnant CD-1 females. Chimeric mice were bred with 129S1/SvImJ mice to generate knockouts on an inbred background. The resulting progeny were genotyped by PCR of tail DNA to identify pups containing a disruption in the GALR2 gene. Heterozygous animals bearing the mutation were bred together to obtain homozygous GALR2 KO mice and wild-type control mice.
Reverse transcriptase (RT) PCR was employed to confirm inactivation of the GALR2 gene. Brains were dissected and stored in RNALater (Ambion, Austin, TX) at 4°C until RNA isolation. RNA was isolated from homogenized tissue by phenol extraction and LiCl precipitation using Totally RNA (Ambion). RNA was treated with DNase I (Ambion) for 1 h at 37 C. Equal amounts (∼100 ng) of RNA were used in each sample for reverse transcription reactions using a Super-Script first-strand synthesis kit (Invitrogen, Carlsbad, CA). Each reaction was run in duplicate with reverse transcriptase or without to control for possible genomic DNA contamination. cDNA was amplified for 40 cycles (94 C for 60 s, 58 C for 60 s, 72 C for 90 s, final extension 72 C for 10 min) with GALR2-specific primers (5′-TCACTGCTCTGCAAGGCCGTTCA-3′ and 5′-AGATTGGCCAGCTGCGACTGACTGT-3′) that were predicted to produce a 233-bp PCR fragment. The primer binding regions are located in exon 1 and exon 2 of the GALR2 gene, with the inactivating retroviral insertion located in the intron separating the two exons. Verification of deletion of the GALR2 gene was previously published by Krasnow et al. (30).
All WT control mice used were littermate controls or age-matched to the GALR2 knockout mice from Het × Het breedings. Food and body weight measurements were taken daily just prior to lights out.
Behavioral testing. (i) Home cage activity.
Home cage activity was monitored by a MicroMax photobeam system (Accuscan Instruments, Columbus, OH) that was exterior to the cage. Animals in their home cage were placed in the photobeam boxes and tested for activity over a 3-day period. Food consumption was also measured during this same time frame (test days 1 to 3) (n = 22 per genotype [11 males and 11 females]).
(ii) Open field activity.
Open field activity was monitored in VersaMax chambers (Accuscan Instruments) measuring 40 by 40 cm and detected by photobeam breaks. Measurements used to assess locomotor activity include horizontal activity, total number of rearing events, and distance traveled in the center compared to total distance traveled (center to total distance ratio) (n = 22 per genotype [11 males and 11 females]).
(iii) Nociception.
Nociception was measured using the standard hot plate test for nociception (pain) carried out by placing a mouse on a 55°C hot plate (Accuscan Instruments) and measuring the latency of a hind limb response (shake or lick). The maximum time allowed on the hot plate was 30 s; if the animal did not respond within this time; the time of latency was recorded as 30 seconds (n = 22 per genotype [11 males and 11 females]).
(iv) Tail suspension assay.
The tail suspension assay involved the use of an automated tail suspension apparatus (Med Associates, St. Albans, VT). The load cell amplifier picked up the animal's movements (struggle to escape), and the data were collected over a 6-min test session (n = 22 per genotype [11 males and 11 females]). The time spent struggling is a measure of learned helplessness behavior or behavioral despair, and the latency to the onset of the end of the struggling can be increased by antidepressants.
(v) The light-dark exploration test measures the conflict between the natural tendencies of mice to explore a novel environment and their tendencies to avoid the aversive properties of a brightly lit (anxiety-provoking) open area.
The brightly lit compartment (27 cm by 20 cm by 30 cm) comprises two-thirds of the surface area, while the dark compartment (18 cm by 20 cm by 30 cm) comprises one-third of the surface area. An opening is designed to allow the mouse access to both compartments. This test was used in conjunction with the stress-induced hypothermia test.
(vi) Stress-induced hyperthermia test.
The stress-induced hyperthermia test measures anxiety and reflects an unconditioned physiological response where the rectal temperature of a mouse increases in response to stress. The basal temperature (T0) of mice was measured rectally (Physitemp). A few seconds later the mouse was placed in the light-dark box for 6 min. The timing of each transition from the dark to light or light to dark compartments was recorded during the period. Immediately after the completion of the light-dark box test, the mouse was removed from the box and the stressed temperature (T1) was determined. Measurements used to assess anxiety-related responses are the total number of transitions in the light-dark box and the change in body temperature (T1 − T0) from baseline over the 6-min test (n = 22 per genotype [11 males and 11 females]).
(vii) PPI.
Prepulse inhibition of the acoustic startle response (PPI) was tested using the SR-Lab system (San Diego Instruments, San Diego, CA). A test session consisted of six trial types. One type used a 40-ms, 120-dB sound as the startle stimulus. Four types used acoustic prepulses followed by acoustic startle stimulus. The 20-ms prepulse sounds of 73, 76, 79, and 82 dB were presented 100 ms before the startle stimulus. Finally, there were 70-dB trials where no startle stimulus was presented to measure baseline reaction. Six blocks of the six trial types were presented in pseudorandom order. The startle response was recorded for 65 ms starting with the onset of the startle stimulus. Measurements used to assess PPI were the maximum startle amplitude and the percent time each of the four prepulses inhibits the startle response (n = 22 per genotype [11 males and 11 females]).
(viii) Contextual fear conditioning test.
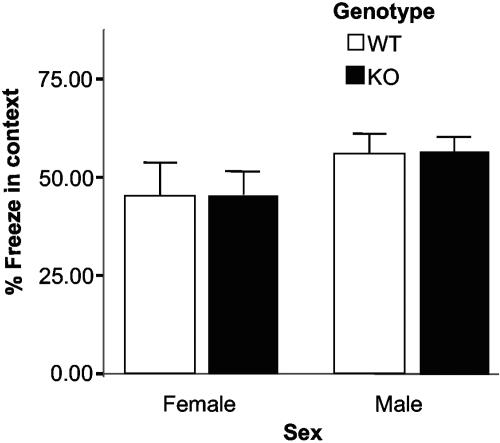
The contextual fear conditioning test, which measures emotion-based learning and memory, involved placing a mouse in an enclosed chamber (Med Associates) with the floor made up of metal rods equipped to deliver a mild electric shock. Electric shock was paired with a noise such that the shock was delivered immediately when the noise turned off. In training session the mouse was placed in the chamber and allowed to explore the environment. Then a 75- to 80-dB white noise was turned on. A foot shock was paired with the noise cessation. The mouse was tested 24 h later by assaying the amount of freezing it showed in the context (the chamber) in which it was shocked (context test) (n = 22 per genotype [11 males and 11 females]). Freezing was automatically measured using the FreezeFrame system (Actimetrics, Wilmette, IL). This paradigm of “background” contextual fear conditioning is a measure of both amygdala and hippocampal functions (42).
(ix) Tolerance and sensitivity to ethanol.
Tolerance and sensitivity to ethanol were tested by examining core body temperature of the mice before and after an intraperitoneal (i.p.) injection of ethanol. Core body temperature was measured rectally (T0) (Physitemp), and then the mice were administered an i.p. dose of 2.5 g/kg of body weight and placed in a dosing chamber. Body temperature was measured rectally 30 min post injection (T1). On the next day mice were treated the same way. Sensitivity to ethanol was measured as the difference in body temperature (T1 − T0) after injection, while tolerance was measured as the difference in the temperature changes between the two testing days (n = 22 per genotype [11 males and 11 females]).
References for the above behavioral tests can be found in Current Protocols in Neuroscience (10).
Reproductive physiology. (i) Estrous cyclicity.
Female mice were housed individually and allowed to acclimate for 7 days while being handled daily. After the 7th day, a daily vaginal smear was obtained from mature females, just after lights came on for 28 days. Smears were placed onto microscope slides and allowed to dry and stained using a Giemsa stain. Smears were then analyzed blindly for stage of estrus. After stage of estrus was determined for each of the smears, they were then evaluated for estrous-cycle length and amount of time spent at each stage of estrus (n = 9 per genotype).
(ii) Fertility and lactation.
To determine fertility and mammary gland function (lactation), KO female mice were bred to WT male mice and KO male mice were bred to WT female mice (n = 5 females per genotype). The number of pups born and number of pups weaned were noted. Maternal behaviors were noted for any deviation from normal maternal behavior (including cannibalization and abandonment).
Feeding and body weight regulation. (i) Body weight and food intake.
Daily body weight and food intake measurements were taken just prior to lights out for 12 days prior to any experimental procedures (after being housed singly for >7 days; n = 9 per genotype). Food consumption was measured after animals were housed singly for a minimum of 7 days to stabilize body weight and food consumption. For the fasting experiment and leptin injection study, cages were cleaned prior to experiments to remove potential spillage and cages were examined for residual food at the end of the study.
(ii) Leptin administration.
Leptin used in these studies was supplied by Zymogenetics Inc., Seattle, WA. Weight-matched WT and KO littermates (n = 10 per genotype) were housed individually and monitored until body weight and food consumption stabilized. Mice were treated intraperitoneally with either leptin (100 μg/mouse in 500 μl saline) or saline (500 μl) for 14 days. Injections were given 1 h prior to lights out (22).
(iii) Fasting.
Weight-matched WT and KO littermates (n = 9 per genotype) were housed individually and monitored until food intake and body weight stabilized. At 11:00 on day 1, food was removed from 48 h. Mice were provided with water at all times. At 11:00 on day 3, mice were refed. Body weight of mice was monitored at 24 h, 36 h, and 48 h of fasting to be sure that body weight did not drop below 20% of normal weight. Body weight and food intake were monitored for the next 48 h after the fast.
Seizure susceptibility. (i) Pentylenetetrazole-induced seizure susceptibility.
Pentylenetetrazole (PTZ) (25 to 50 mg/kg) was dissolved in water and administered (4 ml/kg, i.p.) to seizure-naive mice (n = 9 [KO]; n = 8 [WT]). After injection, animals were placed into a clear container and closely monitored for 10 min. Latencies to first myoclonic jerk (focal seizure) and clonic-tonic (generalized) seizure served as measures of seizure susceptibility. Animals not having seizures were assigned latencies of 10 min.
(ii) Flurothyl-induced seizure susceptibility.
Seizure-naive mice were placed in an airtight Plexiglas chamber, and flurothyl (2,2,2-trifluroethylether; Aldrich, Milwaukee, WI) was infused (20 μl/min) onto filter paper inside the container, from which it vaporized (n = 8 per genotype). Mice were removed immediately from the chamber after the onset of a generalized seizure. Each mouse was tested individually and received only one exposure to flurothyl. Latencies to first myoclonic jerk and to clonic-tonic seizure were measured.
Statistics.
Statistical analyses of behavioral tests were performed by a 2-way analysis of variance (gender × genotype), with repeated measures for tests with multiple time points. Data from the other tests were analyzed by Student's t test or by two-way analysis of variance if a treatment was involved. The criterion for a statistically significant difference was a P value of <0.05.
RESULTS
Behaviors.
There were no significant differences between genotypes in home cage activity (daytime activity, 5,043 ± 537 beam breaks [WT] versus 6,426 ± 524 beam breaks [KO]; nighttime activity, 10,518 ± 893 beam breaks [WT] versus 12,201 ± 870 beam breaks [KO]) (see Fig. S1 in the supplemental material), suggesting that the GALR2 mutation did not disrupt circadian activity rhythms. There were no significant differences between genotypes in open field activity (i.e., hypo- or hyperactive) (total activity, 948 ± 147 cm [WT] versus 1,025 ± 143 cm [KO]; center to total distance ratio, 0.037 [WT] versus 0.034 [KO]) (see Fig. S2 in the supplemental material). There were no significant differences between genotypes in latency to response to the hot plate (i.e., nociception) (16.3 ± 1.2 s [WT] versus 16.3 ± 1.2 s [KO]) (Fig. 1). There were no significant differences in stress-induced hyperthermia (body temperature baseline, 36.04 ± 0.09°C [WT] versus 35.9 ± 0.09°C [KO]; change in body temperature, 1.41 ± 0.09°C [WT] versus 1.44 ± 0.09°C [KO]) (Fig. 2) observed between genotypes, and there were no significant differences between genotypes in the tail suspension assay (total immobility, 182 ± 6 s [WT] versus 191 ± 6 s [KO]) (see Fig. S3 in the supplemental material), indicating no significant differences in anxiety- and depression-based behaviors between WT and GALR2 KO mice. Furthermore there were no significant differences between genotypes for the contextual fear conditioning assay (percent in freezing time, 50.9 ± 4.2% [WT] versus 51.6 ± 4.1% [KO]) (Fig. 3), indicating that learning behavior was similar between the genotypes. Also, there was no significant differences between genotypes for prepulse inhibition of the acoustic startle response (PPI) (73 dB, 32.5 ± 7% inhibition [WT] versus 37.6 ± 3.6% inhibition [KO]; 76 dB, 63.9 ± 3.9% inhibition [WT] versus 67.7 ± 3.6% inhibition [KO]; 79 dB, 69 ± 4.5% inhibition [WT] versus 74.3 ± 2.8% inhibition [KO]; 82 dB, 81.9 ± 3.3% inhibition [WT] versus 85.3 ± 1.9% inhibition [KO]) (see Fig. S4 in the supplemental material), indicating that the sensory motor gating function that is often disrupted in psychotic conditions is normal in the GALR2 KO mice. Finally, there were no differences between genotypes in sensitivity (change in temperature, −2.86 ± 0.16°C [WT] versus −2.94 ± 0.15°C [KO]) and tolerance (change in temperature, −0.76 ± 0.20°C [WT] versus −0.92 ± 0.19°C change in temperature [KO]) to ethanol (see Fig. S5 in the supplemental material).
FIG. 1.
Nociception. Latency (in seconds) to hind-limb response (shake or lick) during a standard hot plate test is shown. There were no differences between genotypes (P = 0.98; n = 22 per genotype [11 males, 11 females]).
FIG. 2.
Anxiety and stress. Results of the stress-induced hyperthermia test are shown. (A) Baseline body temperature of GALR2 KO and WT littermates (P = 0.2); (B) change in body temperature from baseline body temperature during stress-induced hyperthermia (P = 0.84; n = 22 per genotype [11 males, 11 females]).
FIG. 3.
Learning and memory. Results of the contextual fear conditioning test are shown. There were no significant differences in the percentage of freezing time to the context between genotypes (P = 0.91; n = 22 per genotype [11 males, 11 females]).
Reproductive physiology.
There was no significant difference in reproductive phenotype observed in either male or female GALR2 knockout mice compared to WT controls. Estrous cyclicity was similar between genotypes, with an average cycle lasting between 4 and 5 days (4.1 ± 0.9 days [WT] versus 4.8 ± 0.1 days [KO]) (Fig. 4A). Both genotypes had similar numbers of pups with their first litter (4.5 ± 1.9 pups [WT] versus 4.7 ± 1.5 pups [KO]) (Fig. 4B) and weaned similar numbers of pups (4.2 ± 2.3 pups [WT] versus 4.2 ± 1.9 pups [KO]) (Fig. 4C). No significant differences in maternal behaviors were observed between genotypes. All GALR2 KO males were able to impregnate WT females (data not shown), as were their WT controls.
FIG. 4.
Reproductive function. (A) Estrous-cycle length (n = 9 per genotype); (B) fertility (pups/litter; n = 5 litters/genotype); (C) lactation (number of pups weaned; n = 5 litters/genotype). There were no significant differences between genotypes in estrous cyclicity (P = 0.12), number born (P = 0.50), or number weaned (P = 0.62). All GALR2 KO males were able to impregnate WT females (data not shown) (n = 5 per genotype).
Feeding and body weight regulation.
There were no significant differences between genotypes in either body weight (males, 27.5 ± 4.2 g [WT] versus 30.1 ± 3.0 g [KO]; females, 21.8 ± 2.9 g [WT] versus 21.6 ± 2.3 [KO]) (Fig. 5A) or food intake (3.2 ± 0.6 g [WT] versus 3.3 ± 0.4 g [KO]) (Fig. 5B). Leptin treatment did not significantly affect food intake (data not shown) but did significantly reduce body weight (data not shown) in both genotypes; however, there were no significant differences between genotypes in either food intake (4.02 ± 0.44 g [WT] versus 3.55 ± 0.56 g [KO]) (see Fig. S6B in the supplemental material) or change in body weight (−1.86 ± 0.58 g [WT] versus −1.39 ± 0.51 g [KO]) (see Fig. S6A in the supplemental material) while the animals received leptin. Fasting produced a significant reduction in body weight in both genotypes, and there were no discernible differences between genotypes at 0 h (22.1 ± 2.9 g [WT] versus 21.6 ± 1.8 g [KO]), 24 h (20.9 ± 2.6 g [WT] versus 20.6 ± 2.7 g [KO]), 36 h (21.2 ± 5.2 g [WT] versus 19.9 ± 2.7 g [KO]), or 48 h (19.5 ± 2.5 g [WT] versus 19.6 ± 2.9 g [KO]) of the fast and no significant difference between genotypes in the overall change in body weight at 48 h of the fast (−2.5 ± 1.0 g [WT] versus −2.0 ± 1.3 g [KO]) (Fig. 5C). At 24 h and 48 h postfast there was no significant difference between genotypes in body weight (24 h postfast, 22.0 ± 3.1 g [WT] versus 21.8 ± 2.0 g [KO]; 48 h postfast, 22.2 ± 2.9 g [WT] versus 21.5 ± 1.8 g [KO]) (Fig. 5C) or food intake (24 h postfast, 6.7 ± 3.6 g [WT] versus 6.7 ± 1.5 g [KO]; 48 h postfast, 3.9 ± 0.9 g [WT] versus 3.4 ± 0.8 g [KO]) (Fig. 5D).
FIG. 5.
Food intake and body weight. (A) Mean body weight (P = 0.67; n = 9 per genotype). (B) Mean 24-h food intake (P = 0.66; n = 9 per genotype). (C) Body weight response to a 48-h fast and refeeding (0 h, P = 0.67; 24 h, P = 0.82; 36 h, P = 0.85; 48 h, P = 0.97; n = 9 per genotype). (D) Food intake 24 and 48 h after fast. Body weight, P = 0.89 24 h postfast and P = 0.53 48 h postfast. Food intake, P = 0.96 24 h postfast and P = 0.26 48 h postfast (n = 9 per genotype). There were no significant differences between genotypes for any of the measured parameters.
Seizure susceptibility.
There were no significant differences between genotypes in either the latency to myotonic jerk (226 ± 155 s [WT] versus 154 ± 38 s [KO]) (Fig. 6A) or the latency to clonic-tonic seizures (340 ± 169 s [WT] versus 356 ± 163 s [KO]) (Fig. 6A) following administration of PTZ. There were no significant differences between genotypes in the number of mice that showed tonic extension or tonic extension coupled with death (Fig. 6B).
FIG. 6.
Seizure susceptibility. (A) Latency to myotonic jerk (MJ) induced by PTZ (P = 0.20; n = 8 WT mice and 9 KO mice) and latency to clonic-tonic seizure (C/T) induced by PTZ (P = 0.83; n = 8 WT mice and 9 KO mice). (B) Number of mice that experienced MJ, C/T, tonic extension, or tonic extension with death after seizure induction with PTZ. (C) Latency to MJ induced by flurothyl (P = 0.87; n = 8 per genotype) and latency to C/T induced by flurothyl (P = 0.78; n = 8 per genotype). (D) Number of mice that experienced MJ, C/T, tonic extension, or tonic extension with death after seizure induction with flurothyl.
There were no significant differences between genotypes in the latency to myotonic jerk (347 ± 25 s [WT] versus 349 ± 20 s [KO]) (Fig. 6C) or in the latency to clonic-tonic seizure (374 ± 17 s [WT] versus 371 ± 28 s [KO]) (Fig. 6C) following flurothyl administration. There were no significant differences between genotypes in the number of mice that underwent tonic extension or tonic extension coupled with death (Fig. 6D).
DISCUSSION
Galanin has been widely implicated in many diverse processes (reviewed in references 1 and 3), and GALR2 is expressed throughout the central and peripheral nervous system, notably in areas of the brain that control complex physiological processes (16, 17, 24, 39). Thus, we anticipated that mice deficient in GALR2 would show phenotypic alterations in key physiological functions and behaviors, such as reproduction, body weight homeostasis, and learning and memory. However, GALR2 KO mice were indistinguishable from their littermate or age-matched WT controls in the tests we performed. These results expand our earlier limited observations in GALR2 KO mice (30). Our results show that GALR2 KO mice are not all that different from GALR1 KO mice except that GALR1 mice exhibit anxiety behaviors (23), seizure susceptibility (26, 34), lower serum concentrations of insulin-like growth factor 1 (26), and some other subtle phenotypes (4, 56), all of which were not evident in our studies of the GALR2 KO mouse.
We had expected that deletion of GALR2 would result in reproductive dysfunction, perhaps being reflected by failure of pregnancy or parturition. Galanin and GALR2 mRNAs are both expressed in myometrium (38), and alterations in myometrial contractility produced by galanin are thought to be attributable to GALR2. Furthermore, the expression of galanin increases in the gravid myometrium (38, 51, 52), suggesting that galanin and GALR2 may have a role in pregnancy and parturition. However, no effect of GAL2 deficiency was evident in any reproductive parameter. Although the mutant and WT females delivered at similar dates, parturition was not observed. Thus, it is conceivable that the mutant animals may have experienced dystocia, but even if this was the case, it did not influence the number of live pups produced.
We also thought that deletion of GALR2 might result in disruption of the homeostatic regulation of body weight, as might be reflected in differences in growth rates and the response to fasting and leptin treatment. GALR2 is highly expressed in the arcuate nucleus (39), which is a nodal point for the neuroendocrine regulation of body weight (44). GALR2 is also colocalized in the arcuate nucleus with proopiomelanocortin, which plays a key role in the regulation of body weight (5, 44). Moreover, mice that are deficient in galanin are more sensitive to chronic leptin treatment (22), all of which would suggest that galanin and GALR2 play a role in the homeostatic regulation of feeding and body weight. However, we observed no difference in any measures of feeding or body weight regulation between WT and GALR2-deficient mice.
We also anticipated that GALR2-deficient mice might show alterations in anxiety- and depression-related behaviors and tasks involving learning and memory, since galanin has been implicated in these complex behaviors and GALR2 is expressed in regions of the brain where these behaviors are coordinated (39). Yet we observed no discernible effect of GALR2 deficiency on anxiety (stress-induced hyperthermia), depression (tail suspension assay), learning and memory (contextual fear conditioning), psychosis (prepulse inhibition), activity (open field and home cage activity tests), or nociception (hot plate test). We also thought GALR2-deficient mice might show alterations in their susceptibility to seizure induction, especially in light of the new findings by Mazarati et al. in which reduction of GALR2 receptor binding via complementary antisense peptide infusion increased seizure susceptibility in rats (33, 34). However, again we observed no difference in seizure susceptibility between WT and GALR2-deficient animals.
Thus, despite the strong inference that would implicate galanin/GALR2 signaling in the coordination of various complex behaviors, analysis of the GALR2 null mutants did not reveal a discernible phenotype. It is conceivable that developmental compensation or some redundancy in the function of another galanin receptor subtype masks what would otherwise appear in the phenotype of these mutants. It is also possible that GALR2 plays only a subtle modulatory role in these complex behaviors and that its absence is physiologically insignificant and potentially discoverable only with more sophisticated analyses. It is not unprecedented that deletion of a single important neuropeptide or receptor gene produces an unremarkable or subtle phenotype (13, 27). However, when animals with such a single deletion were challenged pharmacologically or studied under special circumstances, a previously unseen phenotype was discovered (13, 50). Moreover, it has been shown that when animals with a single mutation are crossed with animals with a different mutation, both of which have unremarkable phenotypes, the apparent significance of the gene may become manifest. For example, although neuropeptide Y-deficient animals do not exhibit any remarkable feeding phenotype (14), when they are crossed with ob/ob mice, the obesity syndrome in the ob/ob mouse is partially corrected (15). This demonstrates the importance of neuropeptide Y in controlling feeding behaviors, at least in the pathological state characteristic of the ob/ob mouse. It is possible that different pharmacological manipulations of the GALR2 KO mouse, or crossing the GALR2 KO with another mutant animal, may reveal the significance and importance of GALR2.
None of our tests revealed a difference between the WT and GALR2-deficient animals; however, it is possible that for some (or all) of these measures, there actually is a difference between the genotypes but our analysis lacked sufficient statistical power to identify them—i.e., false negatives. Conclusively ruling out false negatives can be problematic. In the work presented here, it is possible that we missed a difference in one or more of the variables because the assay(s) lacked sufficient statistical power; however, we determined that with a power of 0.8, it would have been possible to detect a difference of 25% or less with most of the tests, including the hot plate test (23%), body temperature (0.7%), the tail suspension test (12%), ethanol sensitivity (19%), body weight (14%), food intake (14.5%), body weight after fasting (17%), food intake after fasting (25%), food intake while receiving leptin (15%), estrous-cycle length (23%), and seizure susceptibility induced by flurothyl (9%). Thus, we would conclude that any real effect of genotype on these various behaviors is likely to be subtle and physiologically insignificant, at least under laboratory conditions. Recently, Kinney et al. reported preliminary findings on an independent line of GALR2-deficient mice, demonstrating that GALR2 KO mice are normal compared to WT controls in a wide battery of tests, with the single exception of a subtle difference in a reflex index (28).
In summary, mice that are deficient in GALR2 exhibit normal growth, reproduction, body weight regulation, learning and memory, and susceptibility to seizure induction. We conclude that developmental mechanisms compensate for the congenital lack of GALR2 signaling or that redundant pathways mask the phenotype of the null mutation in GALR2. Alternatively, it is conceivable that GALR2 plays only a subtle role in these complex behaviors and that genetic ablation of GALR2 produces a phenotype that falls below detectable limits of the assays used to analyze these physiological processes.
Supplementary Material
Acknowledgments
We thank Jeremy Smith, Matthew Cunningham, Stephanie Krasnow, and Heather Dungan for their critical review of earlier versions of this paper. We also thank Dawit Teklemichael and Blake Acohido for their invaluable help with establishment of the GALR2 KO colony at the University of Washington.
This work was supported by grants from the National Institutes of Health (R01 HD27142, SCCPRR U54 HD12629, R01 DK61517) and the National Science Foundation (IBN 0110686).
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Bartfai, T., G. Fisone, and U. Langel. 1992. Galanin and galanin antagonists: molecular and biochemical perspectives. Trends Pharmacol. Sci. 13:312-317. [DOI] [PubMed] [Google Scholar]
- 2.Bartfai, T., X. Lu, H. Badie-Mahdavi, A. M. Barr, A. Mazarati, X. Y. Hua, T. Yaksh, G. Haberhauer, S. C. Ceide, L. Trembleau, L. Somogyi, L. Krock, and J. Rebek, Jr. 2004. Galmic, a nonpeptide galanin receptor agonist, affects behaviors in seizure, pain, and forced-swim tests. Proc. Natl. Acad. Sci. USA 101:10470-10475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bedecs, K., M. Berthold, and T. Bartfai. 1995. Galanin—10 years with a neuroendocrine peptide. Int. J. Biochem. Cell Biol. 27:337-349. [DOI] [PubMed] [Google Scholar]
- 4.Blakeman, K. H., J. X. Hao, X. J. Xu, A. S. Jacoby, J. Shine, J. N. Crawley, T. Iismaa, and Z. Wiesenfeld-Hallin. 2003. Hyperalgesia and increased neuropathic pain-like response in mice lacking galanin receptor 1 receptors. Neuroscience 117:221-227. [DOI] [PubMed] [Google Scholar]
- 5.Bouret, S., V. Prevot, D. Croix, A. Howard, E. Habert-Ortoli, S. Jegou, H. Vaudry, J. C. Beauvillain, and V. Mitchell. 2000. Expression of GalR1 and GalR2 galanin receptor messenger ribonucleic acid in proopiomelanocortin neurons of the rat arcuate nucleus: effect of testosterone. Endocrinology 141:1780-1794. [DOI] [PubMed] [Google Scholar]
- 6.Branchek, T., K. E. Smith, and M. W. Walker. 1998. Molecular biology and pharmacology of galanin receptors. Ann. N. Y. Acad. Sci. 863:94-107. [DOI] [PubMed] [Google Scholar]
- 7.Branchek, T. A., K. E. Smith, C. Gerald, and M. W. Walker. 2000. Galanin receptor subtypes. Trends Pharmacol. Sci. 21:109-117. [DOI] [PubMed] [Google Scholar]
- 8.Burazin, T. C., and A. L. Gundlach. 1998. Inducible galanin and GalR2 receptor system in motor neuron injury and regeneration. J. Neurochem. 71:879-882. [DOI] [PubMed] [Google Scholar]
- 9.Burgevin, M. C., I. Loquet, D. Quarteronet, and E. Habert-Ortoli. 1995. Cloning, pharmacological characterization, and anatomical distribution of a rat cDNA encoding for a galanin receptor. J. Mol. Neurosci. 6:33-41. [DOI] [PubMed] [Google Scholar]
- 10.Crawley J. 2005. Behavioral neuroscience, p. 8.0.1-8.18.15. .In J. Crawley, C. Gerfen, R. McKay, M. Rogawski, D. Sibley, and P. Skolnick (ed.), Current protocols in neuroscience. John Wiley, New York, N.Y.
- 11.Depczynski, B., K. Nichol, Z. Fathi, T. Iismaa, J. Shine, and A. Cunningham. 1998. Distribution and characterization of the cell types expressing GALR2 mRNA in brain and pituitary gland. Ann. N. Y. Acad. Sci. 863:120-128. [DOI] [PubMed] [Google Scholar]
- 12.Elliott-Hunt, C. R., B. Marsh, A. Bacon, R. Pope, P. Vanderplank, and D. Wynick. 2004. Galanin acts as a neuroprotective factor to the hippocampus. Proc. Natl. Acad. Sci. USA 101:5105-5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erickson, J. C., K. E. Clegg, and R. D. Palmiter. 1996. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature 381:415-421. [DOI] [PubMed] [Google Scholar]
- 14.Erickson, J. C., R. S. Ahima, G. Hollopeter, J. S. Flier, and R. D. Palmiter. 1997. Endocrine function of neuropeptide Y knockout mice. Regul. Pept. 70:199-202. [DOI] [PubMed] [Google Scholar]
- 15.Erickson, J. C., G. Hollopeter, and R. D. Palmiter. 1996. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science 274:1704-1707. [DOI] [PubMed] [Google Scholar]
- 16.Fathi, Z., Cunningham, A. M., L. G. Iben, P. B. Battaglino, S. A. Ward, K. A. Nichol, K. A. Pine, J. Wang, M. E. Goldstein, T. P. Iismaa, and I. A. Zimanyi. 1997. Cloning, pharmacological characterization and distribution of a novel galanin receptor. Brain Res. Mol. Brain Res. 51:49-59. [DOI] [PubMed] [Google Scholar]
- 17.Fathi, Z., A. M. Cunningham, L. G. Iben, P. B. Battaglino, S. A. Ward, K. A. Nichol, K. A. Pine, J. Wang, M. E. Goldstein, T. P. Iismaa, and I. A. Zimanyi. 1998. Cloning, pharmacological characterization and distribution of a novel galanin receptor. Brain Res. Mol. Brain Res. 53:348. [DOI] [PubMed] [Google Scholar]
- 18.Fetissov, S. O., A. S. Jacoby, P. R. Brumovsky, J. Shine, T. P. Iismaa, and T. Hokfelt. 2003. Altered hippocampal expression of neuropeptides in seizure-prone GALR1 knockout mice. Epilepsia 44:1022-1033. [DOI] [PubMed] [Google Scholar]
- 19.Finn, P. D., D. K. Clifton, and R. A. Steiner. 1998. The regulation of galanin gene expression in gonadotropin-releasing hormone neurons. Mol. Cell. Endocrinol. 140:137-142. [DOI] [PubMed] [Google Scholar]
- 20.Gundlach, A. L., T. C. Burazin, and J. A. Larm. 2001. Distribution, regulation and role of hypothalamic galanin systems: renewed interest in a pleiotropic peptide family. Clin. Exp. Pharmacol. Physiol. 28:100-105. [DOI] [PubMed] [Google Scholar]
- 21.Gustafson, E. L., K. E. Smith, M. M. Durkin, C. Gerald, and T. A. Branchek. 1996. Distribution of a rat galanin receptor mRNA in rat brain. Neuroreport 7:953-957. [DOI] [PubMed] [Google Scholar]
- 22.Hohmann, J. G., S. M. Krasnow, D. N. Teklemichael, D. K. Clifton, D. Wynick, and R. A. Steiner. 2003. Neuroendocrine profiles in galanin-overexpressing and knockout mice. Neuroendocrinology 77:354-366. [DOI] [PubMed] [Google Scholar]
- 23.Holmes, A., J. W. Kinney, C. C. Wrenn, Q. Li, R. J. Yang, L. Ma, J. Vishwanath, M. C. Saavedra, C. E. Innerfield, A. S. Jacoby, J. Shine, T. P. Iismaa, and J. N. Crawley. 2003. Galanin GAL-R1 receptor null mutant mice display increased anxiety-like behavior specific to the elevated plus-maze. Neuropsychopharmacology 28:1031-1044. [DOI] [PubMed] [Google Scholar]
- 24.Howard, A. D., C. Tan, L. L. Shiao, O. C. Palyha, K. K. McKee, D. H. Weinberg, S. D. Feighner, M. A. Cascieri, R. G. Smith, L. H. Van Der Ploeg, and K. A. Sullivan. 1997. Molecular cloning and characterization of a new receptor for galanin. FEBS Lett. 405:285-290. [DOI] [PubMed] [Google Scholar]
- 25.Iismaa, T. P., and J. Shine. 1999. Galanin and galanin receptors. Results Probl. Cell Differ. 26:257-291. [DOI] [PubMed] [Google Scholar]
- 26.Jacoby, A. S., Y. J. Hort, G. Constantinescu, J. Shine, and T. P. Iismaa. 2002. Critical role for GALR1 galanin receptor in galanin regulation of neuroendocrine function and seizure activity. Brain Res. Mol. Brain Res. 107:195-200. [DOI] [PubMed] [Google Scholar]
- 27.Kieffer, B. L., and C. Gaveriaux-Ruff. 2002. Exploring the opioid system by gene knockout. Prog. Neurobiol. 66:285-306. [DOI] [PubMed] [Google Scholar]
- 28.Kinney J. W., A. M. Barr, B. Conti, M. Behrens, and T. Bartfai. 2004. Initial behavioral characterization of galanin receptor subtype 2 knockout mice, abstr. 1008.13. In Abstr. 34th Annu. Meet. Soc. Neurosci., Society for Neuroscience, Washington, D.C.
- 29.Kolakowski, L. F., Jr., G. P. O'Neill, A. D. Howard, S. R. Broussard, K. A. Sullivan, S. D. Feighner, M. Sawzdargo, T. Nguyen, S. Kargman, L. L. Shiao, D. L. Hreniuk, C. P. Tan, J. Evans, M. Abramovitz, A. Chateauneuf, N. Coulombe, G. Ng, M. P. Johnson, A. Tharian, H. Khoshbouei, S. R. George, R. G. Smith, and B. F. O'Dowd. 1998. Molecular characterization and expression of cloned human galanin receptors GALR2 and GALR3. J. Neurochem. 71:2239-2251. [DOI] [PubMed] [Google Scholar]
- 30.Krasnow, S. M., J. G. Hohmann, A. Gragerov, D. K. Clifton, and R. A. Steiner. 2004. Analysis of the contribution of galanin receptors 1 and 2 to the central actions of galanin-like peptide. Neuroendocrinology 79:268-277. [DOI] [PubMed] [Google Scholar]
- 31.Ma, X., Y. G. Tong, R. Schmidt, W. Brown, K. Payza, L. Hodzic, C. Pou, C. Godbout, T. Hokfelt, and Z. Q. Xu. 2001. Effects of galanin receptor agonists on locus coeruleus neurons. Brain Res. 919:169-174. [DOI] [PubMed] [Google Scholar]
- 32.Mahoney, S. A., R. Hosking, S. Farrant, F. E. Holmes, A. S. Jacoby, J. Shine, T. P. Iismaa, M. K. Scott, R. Schmidt, and D. Wynick. 2003. The second galanin receptor GalR2 plays a key role in neurite outgrowth from adult sensory neurons. J. Neurosci. 23:416-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazarati, A., X. Lu, K. Kilk, U. Langel, C. Wasterlain, and T. Bartfai. 2004. Galanin type 2 receptors regulate neuronal survival, susceptibility to seizures and seizure-induced neurogenesis in the dentate gyrus. Eur. J. Neurosci. 19:3235-3244. [DOI] [PubMed] [Google Scholar]
- 34.Mazarati, A., X. Lu, S. Shinmei, H. Badie-Mahdavi, and T. Bartfai. 2004. Patterns of seizures, hippocampal injury and neurogenesis in three models of status epilepticus in galanin receptor type 1 (GalR1) knockout mice. Neuroscience 128:431-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melander, T., T. Hokfelt, and A. Rokaeus. 1986. Distribution of galaninlike immunoreactivity in the rat central nervous system. J. Comp. Neurol. 248:475-517. [DOI] [PubMed] [Google Scholar]
- 36.Melander, T., T. Hokfelt, A. Rokaeus, J. Fahrenkrug, K. Tatemoto, and V. Mutt. 1985. Distribution of galanin-like immunoreactivity in the gastro-intestinal tract of several mammalian species. Cell Tissue Res. 239:253-270. [DOI] [PubMed] [Google Scholar]
- 37.Mennicken, F., C. Hoffert, M. Pelletier, S. Ahmad, and D. O'Donnell. 2002. Restricted distribution of galanin receptor 3 (GalR3) mRNA in the adult rat central nervous system. J. Chem. Neuroanat. 24:257-268. [DOI] [PubMed] [Google Scholar]
- 38.Niiro, N., J. Nishimura, K. Hirano, H. Nakano, and H. Kanaide. 1998. Mechanisms of galanin-induced contraction in the rat myometrium. Br. J. Pharmacol. 124:1623-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Donnell, D., S. Ahmad, C. Wahlestedt, and P. Walker. 1999. Expression of the novel galanin receptor subtype GALR2 in the adult rat CNS: distinct distribution from GALR1. J. Comp. Neurol. 409:469-481. [PubMed] [Google Scholar]
- 40.Ottlecz, A., G. D. Snyder, and S. M. McCann. 1988. Regulatory role of galanin in control of hypothalamic-anterior pituitary function. Proc. Natl. Acad. Sci. USA 85:9861-9865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pang, L., T. Hashemi, H. J. Lee, M. Maguire, M. P. Graziano, M. Bayne, B. Hawes, G. Wong, and S. Wang. 1998. The mouse GalR2 galanin receptor: genomic organization, cDNA cloning, and functional characterization. J. Neurochem. 71:2252-2259. [DOI] [PubMed] [Google Scholar]
- 42.Phillips, R. G., and J. E. LeDoux. 1994. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn. Mem. 1:34-44. [PubMed] [Google Scholar]
- 43.Saar, K., A. M. Mazarati, R. Mahlapuu, G. Hallnemo, U. Soomets, K. Kilk, S. Hellberg, M. Pooga, B. R. Tolf, T. S. Shi, T. Hokfelt, C. Wasterlain, T. Bartfai, and U. Langel. 2002. Anticonvulsant activity of a nonpeptide galanin receptor agonist. Proc. Natl. Acad. Sci. USA 99:7136-7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz, M. W., and G. J. Morton. 2002. Obesity: keeping hunger at bay. Nature 418:595-597. [DOI] [PubMed] [Google Scholar]
- 45.Skofitsch, G., and D. M. Jacobowitz. 1986. Quantitative distribution of galanin-like immunoreactivity in the rat central nervous system. Peptides 7:609-613. [DOI] [PubMed] [Google Scholar]
- 46.Skofitsch, G., and D. M. Jacobowitz. 1985. Immunohistochemical mapping of galanin-like neurons in the rat central nervous system. Peptides 6:509-546. [DOI] [PubMed] [Google Scholar]
- 47.Smith, K. E., C. Forray, M. W. Walker, K. A. Jones, J. A. Tamm, J. Bard, T. A. Branchek, D. L. Linemeyer, and C. Gerald. 1997. Expression cloning of a rat hypothalamic galanin receptor coupled to phosphoinositide turnover. J. Biol. Chem. 272:24612-24616. [DOI] [PubMed] [Google Scholar]
- 48.Sten Shi, T. J., X. Zhang, K. Holmberg, Z. Q. Xu, and T. Hokfelt. 1997. Expression and regulation of galanin-R2 receptors in rat primary sensory neurons: effect of axotomy and inflammation. Neurosci. Lett. 237:57-60. [DOI] [PubMed] [Google Scholar]
- 49.Tatemoto, K., A. Rokaeus, H. Jornvall, T. J. McDonald, and V. Mutt. 1983. Galanin—a novel biologically active peptide from porcine intestine. FEBS Lett. 164:124-128. [DOI] [PubMed] [Google Scholar]
- 50.Thiele, T. E., G. I. Miura, D. J. Marsh, I. L. Bernstein, and R. D. Palmiter. 2000. Neurobiological responses to ethanol in mutant mice lacking neuropeptide Y or the Y5 receptor. Pharmacol. Biochem. Behav. 67:683-691. [DOI] [PubMed] [Google Scholar]
- 51.Vrontakis, M. E., I. C. Schroedter, H. Cosby, and H. G. Friesen. 1992. Expression and secretion of galanin during pregnancy in the rat. Endocrinology 130:458-464. [DOI] [PubMed] [Google Scholar]
- 52.Vrontakis, M., I. Schroedter, V. Leite, and H. G. Friesen. 1993. Estrogen regulation and localization of galanin gene expression in the rat uterus. Biol. Reprod. 49:1245-1250. [DOI] [PubMed] [Google Scholar]
- 53.Wang, S., T. Hashemi, S. Fried, A. L. Clemmons, and B. E. Hawes. 1998. Differential intracellular signaling of the GalR1 and GalR2 galanin receptor subtypes. Biochemistry 37:6711-6717. [DOI] [PubMed] [Google Scholar]
- 54.Wang, S., L. Ghibaudi, T. Hashemi, C. He, C. Strader, M. Bayne, H. Davis, and J. J. Hwa. 1998. The GalR2 galanin receptor mediates galanin-induced jejunal contraction, but not feeding behavior, in the rat: differentiation of central and peripheral effects of receptor subtype activation. FEBS Lett. 434:277-282. [DOI] [PubMed] [Google Scholar]
- 55.Wittau, N., R. Grosse, F. Kalkbrenner, A. Gohla, G. Schultz, and T. Gudermann. 2000. The galanin receptor type 2 initiates multiple signaling pathways in small cell lung cancer cells by coupling to G(q), G(i) and G(12) proteins. Oncogene 19:4199-4209. [DOI] [PubMed] [Google Scholar]
- 56.Wrenn, C. C., J. W. Kinney, L. K. Marriott, A. Holmes, A. P. Harris, M. C. Saavedra, G. Starosta, C. E. Innerfield, A. S. Jacoby, J. Shine, T. P. Iismaa, G. L. Wenk, and J. N. Crawley. 2004. Learning and memory performance in mice lacking the GAL-R1 subtype of galanin receptor. Eur. J. Neurosci. 19:1384-1396. [DOI] [PubMed] [Google Scholar]
- 57.Wu, W. P., J. X. Hao, L. Lundstrom, Z. Wiesenfeld-Hallin, U. Langel, T. Bartfai, and X. J. Xu. 2003. Systemic galnon, a low-molecular weight galanin receptor agonist, reduces heat hyperalgesia in rats with nerve injury. Eur. J. Pharmacol. 482:133-137. [DOI] [PubMed] [Google Scholar]
- 58.Zhang, X., Z. O. Xu, T. J. Shi, M. Landry, K. Holmberg, G. Ju, Y. G. Tong, L. Bao, X. P. Cheng, Z. Wiesenfeld-Hallin, A. Lozano, J. Dostrovsky, and T. Hokfelt. 1998. Regulation of expression of galanin and galanin receptors in dorsal root ganglia and spinal cord after axotomy and inflammation. Ann. N. Y. Acad. Sci. 863:402-413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.