Abstract
NTAL (non-T-cell activation linker, also called LAB) and LAT (linker for activation of T cells) are evolutionarily related transmembrane adaptor proteins that are phosphorylated upon immunoreceptor engagement. Using quantitative reverse transcription-PCR, both NTAL and LAT were found to be expressed in B cells. However, LAT expression was limited to early B cells, whereas NTAL expression typified mature B cells. To delineate their roles in B-cell development and function, Ntal-deficient mice were generated and crossed with Lat-deficient mice. B cells developed in Lat−/− Ntal−/− double-deficient mice and in mice lacking either of the two adaptors with the same efficiency as in wild-type mice. Upon B-cell antigen receptor cross-linking, Ntal−/− B cells exhibited slightly increased Ca2+ mobilization and proliferation. In addition, Ntal-deficient mice had increased levels of natural antibodies and slightly increased humoral response to a T-dependent antigen. Normal titers of serum-specific immunoglobulins were produced in response to a T-cell-independent antigen. Although NTAL is also expressed in plasma cells, its absence did not affect the hypergammaglobulinemia E and G1 that developed in mice with a mutation in tyrosine 136 of LAT. Therefore, NTAL does not play a role in B cells symmetric to the role played by LAT in T cells.
Transmembrane adaptor proteins recently emerged as a new family of molecules involved in immunoreceptor signaling. Some of them are palmitoylated and reside in lipid rafts whereas others are nonraft proteins (for review, see reference 9). A transmembrane adaptor protein called NTAL (for non-T-cell activation linker) (2), or LAB (for linker of activation of B cells) (11) has been recently identified as the product of the Wbscr5 gene. NTAL is structurally similar to LAT, a key component of the T-cell antigen receptor and high-affinity immunoglobulin E (IgE) receptor (FcɛRI) signaling pathways (17, 25). It possesses a short extracellular domain, a transmembrane region, two palmitoylated membrane proximal cysteine residues, and a long cytoplasmic tail with several tyrosine residues that are conserved between mouse and human. In B cells and mast cells, NTAL is rapidly tyrosine phosphorylated following ligation of BCR and FcɛRI. NK cells and mast cells coexpress NTAL and LAT, whereas other cell types such as mature B cells express only NTAL (2, 11).
Despite a remarkable conservation of the exon-intron organization of the Ntal and Lat genes and of the NTAL and LAT structural domains, suggesting that these two adaptors originate from the duplication of an ancestral gene (2), important differences exist in the intracytoplasmic partners capable of binding to LAT or to NTAL. For instance, none of the nine tyrosine residues found in NTAL is in a consensus binding motif for phospholipase Cγ1 or -2. As a consequence, NTAL does not bind to phospholipase Cγ and thus resembles a LAT molecule deprived of a phospholipase Cγ binding site. Indeed, when ectopically expressed in the T cells of LAT-deficient mice, NTAL behaved similarly to a LAT mutant that is deprived of its ability to interact with phospholipase Cγ1 and to trigger Ca2+ responses (10). Five of the NTAL tyrosines are potential binding sites for the cytosolic adaptor molecule Grb2. Therefore, NTAL has been hypothesized to relay signals from immunoreceptors to the Ras-mitogen-activated protein kinase pathway.
Recently, we and others have shown that FcɛRI-triggered secretory and Ca2+ responses are significantly enhanced in mast cells obtained from Ntal−/− mice (23, 26). Therefore, in mouse mast cells, NTAL primarily dampens the signaling pathway elicited by FcɛRI. In mast cells obtained from Lat−/− Ntal−/− double-deficient mice, FcɛRI-triggered events were, however, more inhibited than in mast cells from Lat−/− mice, suggesting that under some circumstances, NTAL may also exert some positive signaling role (23, 26). This last possibility is further supported by a recent observation made in an immature chicken B-cell line (DT-40) (20). In these transformed cells, NTAL is capable of up regulating B-cell receptor (BCR)-triggered Ca2+ responses, likely through the sequestration of Grb2-based inhibitory elements to lipid rafts. In contrast to mast cells, mature B lymphocytes do not express LAT and thus represent an interesting model to assess the function of NTAL in the absence of LAT. Preliminary data by Zhu and colleagues (26) suggest that B-cell development appears normal in NTAL-deficient mice, but the functional consequences of NTAL deficiency were not assessed. Therefore, the present study focused on the role of NTAL in B cells and aimed at determining whether NTAL constitutes a negative or a positive regulator of pre-BCR- and BCR-triggered events.
MATERIALS AND METHODS
Mice.
Mice deficient in recombination activation gene 1 (Rag1−/− mice) were originally obtained from E. Spanopoulou (Mount Sinai School of Medicine, New York) (19). Mice deficient in LAT (Lat−/−) and mice homozygous for a mutation that replaced tyrosine 136 of LAT with phenylalanine (LatY136F) have been described (1, 14). All mice used in this study were between 6 and 20 weeks of age and housed in a specific-pathogen-free animal facility. All experiments were in accordance with protocols approved by French laws and the European Directives.
Generation of Ntal-deficient mice.
The generation of Ntal-deficient mice has been reported (23). Screening of mice for the presence of the Ntal null allele was performed by PCR using the following oligonucleotides: a, 5′-CTA CGG AGC TGA GTG TTC TCA-3′; b, 5′-GAA CGG CTA GAA CTA CAC AGA G-3′; and c, 5′-GAG AGG AGG ATA AAG TGG ACC TC-3′. Wild-type Ntal allele was visualized as a 383bp fragment using the a-b pair of oligonucleotides, whereas Ntal null allele was visualized as a 450bp fragment using the a-c pair of oligonucleotides.
Purification of B cells.
Immature and mature B-cell fractions were identified and sorted following staining with combinations of antibodies specific for B220, CD43, IgM, and IgD. Bone marrow fractions A to C (B220+CD43+) were isolated from either Rag-1−/− or wild-type bone marrows. When analyzed for the presence of Ntal and Lat transcripts similar results were obtained. Fractions D and E were sorted from B220+CD43−-gated, wild-type bone marrow cells on the basis of IgM versus IgD cell surface expression (D: IgM−IgD− and E: IgM+IgD−). Transitional T1, T2 and mature B cells were isolated from B220+CD19+-gated, wild-type spleen cell population on the basis of IgM and IgD expression (T1: IgM+IgD−, T2: IgM+IgD+, mature B cells: IgM−IgD+). Marginal zone B cells were sorted from wild-type spleen on the basis of their B220+, CD19+, CD21/35hi, and CD23lo phenotypes. Plasma cells were sorted from the spleen of LatY136F mutant mice using a combination of anti-B220 and of anti-CD138 antibodies.
RNA preparation and quantitative RT-PCR.
Total cellular RNA, isolated from sorted cells using TRIzol (Invitrogen), was reverse transcribed using random primers and Superscript II reverse transcriptase (Life Technology). Real-time PCR was performed on cDNA samples using the QuantiTect SYBR Green PCR kit (QIAGEN) and the GeneAmp 5700 sequence detection System (PE Biosystems). The following pair of primers were used: Hprt sense, 5′-AGC CCT CTG TGT GCT CAA G G-3′, Hprt antisense, 5′-CTG ATA AAA TCT ACA GTC ATA GGA ATG GA-3′, Ntal sense, 5′-TCG GGA TTA TTG CTG CTG CT-3′, Ntal antisense, 5′-GTG CAT TTT CTT GCC GGT TC-3′, Lat sense, 5′-TCC CTG TTG TCT CCT CTG CT-3′ and Lat antisense, 5′-CTC TGC GCT CTC CTC ACT CT-3′. Cycling conditions were 1 cycle at 50°C for 2 min, 1 cycle at 95°C for 15 min, and 40 cycles corresponding to 30 s at 95°C and 1 min at 60°C. Analysis was performed using the sequence detection software supplied with the instrument. Relative expression values were expressed as 2-ΔcT, where ΔCT is the difference between the mean Ct value of duplicates of the test sample and of the endogenous HPRT control.
Proliferative responses of splenic B cells.
Spleen cell suspensions were depleted of erythrocytes by osmotic lysis, and B cells purified by negative selection using an AutoMacs and Microbeads coated with anti-CD43 monoclonal antibody (Miltenyi Biotech). The resulting preparations contained over 94% B220+ cells. Purified splenic B cells were cultured in 0.1 ml of culture medium at a density of 2.105 cells/well in 96-well flat-bottom plates. B cells were stimulated with graded doses of F(ab)′2 goat anti-mouse IgM antibody (Jackson ImmunoResearch), or 1 μg/ml lipopolysaccharide (Sigma). After 40 h of culture, 100 μl of CellTiter-Glo reagent (Promega) was added directly to each well. The resulting luminescence that is proportional to the ATP content of the culture, was measured using a Victor2 luminometer (Wallac, Perkin Elmer Life Science).
Ca2+ measurement.
Cells isolated from spleen or bone marrow were incubated at 37°C in 2 ml of IMDM medium containing 5 μM Indo-1 (Molecular probes) and 10 mM HEPES (pH 7). After 30 min, 2 ml of IMDM medium, 10 mM HEPES (pH 7), 5% fetal bovine serum were added, and incubation was continued for 30 min. After two washes, Indo-1-loaded cells were stained with an anti-B220 and/or anti-IgD monoclonal antibody and resuspended at 107 cells/ml. Baseline fluorescence was established using an LSR flow cytometer (Becton Dickinson), and BCR ligation initiated by the addition of F(ab)′2 goat anti-mouse IgM antibody (Jackson ImmunoResearch) to a final concentration of 10 μg/ml. The resulting calcium flux was followed in B220+-gated cells (spleen) or in B220+/IgD−-gated cells (bone marrow). In some experiments, prior to measurement, cells were resuspended in a solution that lacked CaCl2 and contained 0.5 mM EGTA. Following monitoring of BCR-induced release of intracellular Ca2+, the level of extracellular Ca2+ was restored to 1.3 mM, and the influx of Ca2+ through the plasma membrane recorded. Even Indo-1-loading was controlled by monitoring calcium flux in an aliquot of each sample treated with 1 μg/ml ionomycin (Sigma). Data acquisition and analysis were performed with CELLQUEST and FlowJo.
Flow cytometry.
Before being stained, cells were preincubated for 10 min with the 2.4G2 antibody to block Fc receptors. Single cell suspensions from bone marrow, lymph node, spleen or peritoneal cells were stained with antibodies against CD5, CD19, CD23, CD24, CD43, CD138, B220, BP1, CD21/35, IgM, and IgD (BD Pharmingen and ebioscience). Cells were analyzed on a FACSCalibur flow cytometer (Becton Dickinson). Data acquisition and analysis were performed with CELLQUEST.
Determination of serum isotype-specific immunoglobulin.
The titers of polyclonal IgM, IgG1, IgG3, and IgE antibodies were determined with isotype-specific enzyme-linked immunosorbent assays (Southern Biotechnology and P.A.R.I.S., Compiègne, France). The concentration of IgG1 and IgE were determined by comparison of test sample dilution series values with isotype control standards. In experiments measuring anti-ovalbumin antibodies and natural antibodies, the concentrations of polyclonal IgG1, IgG2a, IgG2b, and IgM antibodies were determined using ELISA based on isotype specific goat anti-mouse Ig polyclonals and rabbit anti-goat Ig peroxidase conjugate (Sigma). IgE antibody concentrations were determined using rat anti-mouse IgE polyclonal antibody in combination with rabbit anti-rat Ig peroxidase conjugate (Sigma). The concentration of total antigen-specific Ig was determined using goat anti-mouse Ig peroxidase (Sigma).
B-cell responses and ELISA.
To evaluate thymus-independent B-cell responses, Ntal−/− mice and wild-type littermates (8 to 10 per group) were immunized intravenously with 50 μg of TNP (trinitrolphenol)-Ficoll in phosphate-buffered saline (PBS). Mice were bled prior to and 7 days after immunization. To measure B-cell responses against thymus-dependent antigen, Ntal−/− mice and wild-type littermates (8 to 10 per group) were immunized intraperitoneally with 75 μg of TNP-ovalbumin (OVA) homogenized with complete Freund's adjuvant (1 to 1 volume ratio). Mice were bled prior to and 14 days after immunization. Serum antibody specific for TNP was measured using enzyme-linked immunosorbent assay (ELISA) plates coated overnight with TNP-OVA (10 μg/ml). Plates were developed with isotype-specific enzyme-linked immunosorbent assays (Southern Biotechnology). Absorbance was read at 450 nm on serial sample dilutions, and titers were calculated relative to control sera from unimmunized mice.
In other experiments, mice were immunized under suboptimal conditions with OVA (Sigma). Briefly, mice were primed intraperitoneally with 500 μg of OVA diluted in 0.5 ml PBS without adjuvant, boosted on day 45 with a similar antigen regimen. Serum was collected 7 days after the last immunization. Serum samples were diluted 100-fold, and OVA-specific antibodies measured using ELISA plates coated with OVA. Due to the intended suboptimal immunization conditions, the concentrations of OVA-specific antibodies present in the sera after the primary immunization were very low. Levels of natural antibodies were measured by ELISA using 100-fold diluted sera from nonimmunized mice, and ELISA plates coated with salmon DNA, DNP (dinitrophenol)-human albumin and gelatin (all from Sigma).
Western blot analysis.
They were performed as described before (4). Briefly, purified B cells were activated in vitro at 37°C by using 20 μg/ml affinity purified goat anti-mouse IgM F(ab)′2 fragment (Jackson ImmunoResearch). Following stimulation, cells were solubilized in a Nonidet P-40 (NP-40)/laurylmaltoside-based lysis buffer (50 mM Tris/HCl, pH 7.5; 165 mM NaCl; 10 mM EDTA; 10 mM NaF; 1 mM Na3VO4, 1% NP-40; 1% laurylmaltoside [n-dodecyl β-d-maltoside, Calbiochem]; 1 mM phenylmethylsulfonyl fluoride). Postnuclear supernatants were separated on 12% sodium dodecyl sulfate (SDS)-acrylamide gels, blotted onto nitrocellulose, and the blots were probed with anti-phosphotyrosine monoclonal antibody (4G10) or polyclonal antisera detecting the activated forms of JNK, ERK and p38 (Cell Signaling). A polyclonal rabbit antiserum directed at mouse NTAL was provided by J. Wienands (Institute of Immunology, University of Göttingen, Göttingen, Germany), and used for blot immunostaining (3). Immunoreactive proteins were visualized using peroxidase-labeled secondary antibodies and the enhanced chemiluminescence detection system (Amersham Biosciences).
RESULTS
NTAL expression in mouse B cells.
Previous studies have indicated that NTAL is primarily expressed in B cells and in myeloid cells (3, 11). To more precisely determine the pattern of NTAL expression during mouse B-cell development, populations corresponding to most of the identified stages of B-cell differentiation, including plasma cells, were prepared by cell sorting and immediately analyzed by quantitative RT-PCR for their relative levels of NTAL transcripts. Considering that early B cells have been reported to express LAT (16, 21), and that an important purpose of the present study was to determine whether some functional complementation exists between LAT and NTAL in B cells, individual B-cell subpopulations were also analyzed for the presence of LAT transcripts.
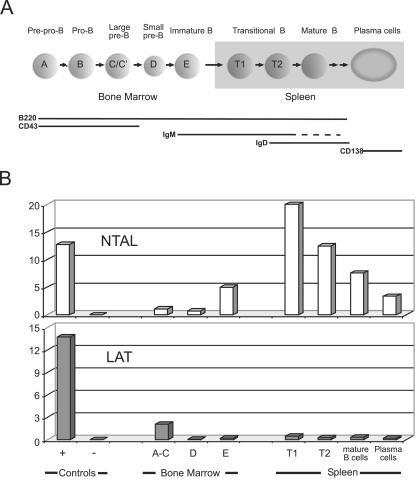
As depicted in Fig. 1A, early B-cell developmental stages occurring in the bone marrow can be delineated by flow cytometry, and populations corresponding to pro-B (fractions A to C/C′), pre-B (fractions D), and immature (fraction E) B cells defined using the expression of B220, CD43, CD24, BP1, IgM, and IgD (7). Consistent with recent reports (16, 21), LAT was found to be expressed during early stage of B-cell differentiation only, albeit at much lower levels than in T cells (Fig. 1B). In contrast to pro-B (fractions A to C/C′) and pre-B (fraction D) cells, which expressed little or no NTAL transcripts, immature B cells (fraction D) expressed high levels of NTAL transcripts (Fig. 1B).
FIG. 1.
Comparative expression of Ntal and Lat transcripts throughout mouse B-cell development. (A) Diagram of mouse B-cell development showing anatomic localizations and cell surface markers. Cell populations corresponding to the specified stages of B-cell development were sorted from either bone marrow or spleen (see Materials and Methods). RNA samples were prepared and the relative levels of Ntal and Lat mRNAs analyzed by quantitative RT-PCR. (B) Results are expressed as relative units of Ntal and Lat mRNA normalized using Hprt transcripts. Positive control samples (+) correspond to mature B cells (NTAL panel) or mature T cells (LAT panel) isolated from wild-type spleen, whereas negative control samples (−) corresponds to total spleen cells isolated from Ntal−/− × Lat−/− double-deficient mice. Data shown are representative of three independent experiments.
In the adult mouse, three populations of splenic B cells can be identified by staining for IgM and IgD: IgMhi IgDlo or transitional 1 (T1) cells are recent immigrant from the bone marrow that develop into IgMhiIgDhi transitional (T2) cells, some of which differentiate further into mature IgMloIgDhi, or follicular recirculating B cells (5). T1 B cells contained the highest levels of NTAL transcripts among the tested B-cell subpopulations, and NTAL transcript levels decayed during maturation into follicular B cells (Fig. 1B). Plasma cells also expressed NTAL transcripts, albeit at lower levels than mature follicular B cells. Therefore, NTAL expression typifies immature and mature B cells, and the sequential pattern of expression observed for LAT and NTAL during B-cell development precludes the occurrence of functional complementation between these two adaptors. Our data, using freshly isolated B-cell subpopulations, also match the differential pattern of NTAL and LAT expression previously observed in transformed mouse B-cell lines corresponding to various stages of B-cell development (20, 21).
B-cell development in Ntal−/− mice.
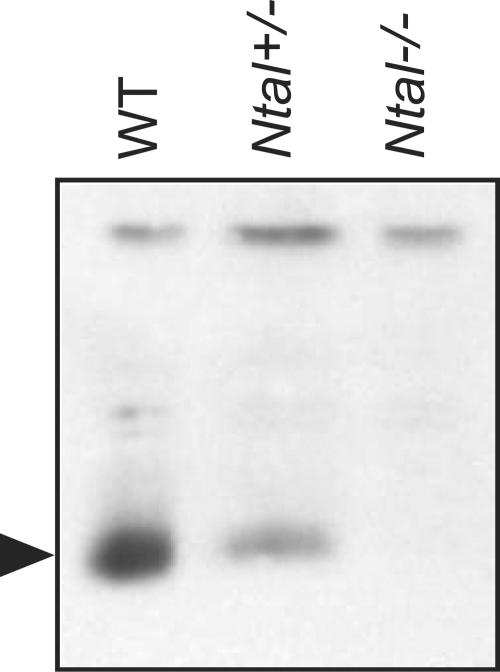
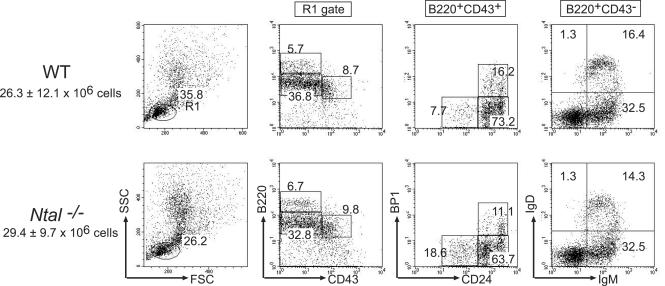
To address the role of Ntal in B cells, we generated knockout mice with a loxP sequence that replaced a central segment of the Ntal gene containing exons 2 to 9 (23). Mice homozygous for this mutation, Ntal−/−, were born at the expected Mendelian frequencies and lacked detectable NTAL protein (Fig. 2). B-cell development was examined in Ntal−/− mice and compared to that of wild-type littermates. Bone marrow cellularity was not affected in Ntal−/− mice. Moreover, when compared to wild-type controls, Ntal−/− mice showed similar percentages of cells corresponding to fractions A to E (Fig. 3). Therefore, consistent with the near absence of NTAL transcripts during the earliest stages of B-cell differentiation (Fig. 1), the cascade of developmental events induced through pre-BCR signaling was not altered by disruption of the Ntal gene.
FIG. 2.
Lack of NTAL expression in Ntal−/− splenocytes as detected by Western blotting. The arrow indicates the position of the band corresponding to NTAL (29 kDa). The upper band corresponds to an unidentified nonspecifically reactive protein. The intensity of this band demonstrates that each lane was loaded with comparable amounts of total proteins. The reduced amounts of NTAL found in Ntal+/− splenocytes compared to Ntal+/+ (WT) splenocytes denote the occurrence of a gene dosage effect.
FIG. 3.
Flow cytometric analysis of B-cell subpopulations in Ntal−/− bone marrow. Single-cell suspensions from bone marrow were stained with the indicated antibodies and analyzed by flow cytometry. Numbers indicate the percentages of cells in the specified quadrants or gates. The total number of cells found within each organ is shown for wild-type (WT) and Ntal−/− mice (averaged from six experiments).
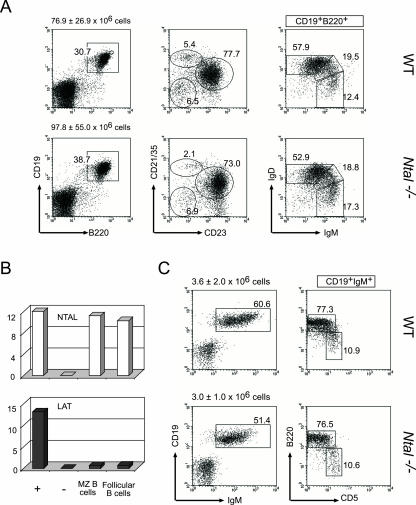
After leaving the bone marrow, immature B cells reach the spleen and differentiate into mature B cells. This maturation occurs through the T1 and T2 stages and depends in part on signals originating from the BCR (22). Although T1 and T2 transitional B cells expressed the highest levels of NTAL transcripts within the B-cell lineage (Fig. 1), disruption of the Ntal gene had no major effect on their absolute numbers, and on their ability to generate mature, follicular B cells, as defined on the basis of IgD and IgM, and of CD21/35 and CD23 profiles (Fig. 4A). Therefore, as hinted by Zhu and colleagues (26), the loss of NTAL does not block the generation of a large pool of conventional, mature B cells and does not cause changes in the surface level expression of the BCR and of coreceptors such as CD19 (Fig. 4).
FIG. 4.
Flow cytometric and quantitative RT-PCR analyses of B-cell subpopulations in Ntal−/− peritoneum and spleen. (A and C). Single-cell suspensions from spleen (A) and peritoneum (C) were stained with the indicated antibodies and analyzed by flow cytometry. Numbers indicate the percentages of cells in the specified quadrants or gates. The total number of cells found within each organ is shown for wild-type (WT) and Ntal−/− mice (averaged from 20 (spleen) and six (peritoneum) experiments). In the case of CD21/35 and CD23 profiles, windows indicate T1 (CD21/35lo CD23lo), follicular (CD21/35int CD23hi), and marginal zone (CD21/35hi, CD23lo) B cells. (B) Cell populations corresponding to marginal zone and follicular B cells were sorted from the spleen on the basis of CD21/35 and CD23 profiles, and RNA samples were prepared and the relative levels of Ntal and Lat mRNAs were analyzed by quantitative RT-PCR.
A fraction of bone marrow B-cell immigrants is found in the marginal zone, a specialized area of the spleen (13). Marginal zone B cells are nonrecirculating, long-lived B cells and constitute a crucial component of the early, T-independent response to blood-borne pathogens. Comparison of the CD21/35 and CD23 profile of Ntal−/− spleen B cells with that of wild-type littermates revealed the presence of twofold reduced numbers of marginal zone B cells (6.1 ± 0.9 × 106 in wild-type spleen versus 3.2 ± 0.6 × 106 in Ntal−/− spleen, Fig. 4A). Consistent with the view that NTAL play a role in the development and/or the maintenance of marginal zone B cells, when analyzed by quantitative RT-PCR for their relative levels of NTAL and LAT transcripts, marginal zone B cells were found to express little or no LAT transcripts and high levels of NTAL transcripts (Fig. 4B). B-1 cells constitute another minor subset of B cells that can be distinguished from follicular B cells, based on their surface phenotype and on their anatomical localization (6). Peritoneal B-1 cells play a pivotal role in T-cell-independent immune responses. B-1 B cells can be distinguished from follicular B cells based on their expression of CD5 and on their low levels of B220. As shown in Fig. 4C, normal numbers of B-1 cells were observed in the peritoneal cavity of Ntal−/− mice. Therefore, mice lacking NTAL showed twofold reduced numbers of marginal zone B cells and normal numbers of T1, T2, and follicular B cells in the spleen and of B-1 cells in the peritoneal cavity.
Stronger calcium responses in Ntal-deficient B cells.
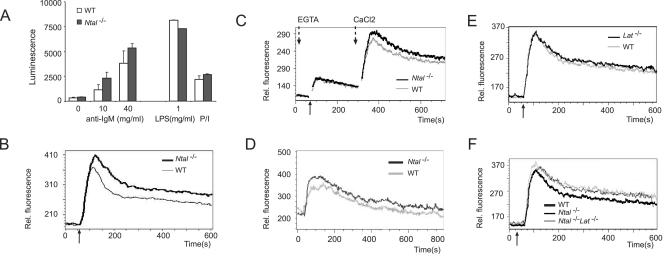
B lymphocytes were purified from spleens and stimulated with various mitogens. Compared to wild-type littermates B cells, proliferation of Ntal−/− B cells was normal and even slightly enhanced at all doses of anti-IgM tested. NTAL was also not required for proliferation induced through other receptors like CD40 or Toll-like receptor 4 (Fig. 5A). Occasionally, a slightly higher proliferative response of Ntal−/− B cells in response to CD40 stimulation was observed. In both NTAL-proficient and NTAL-deficient B cells, BCR cross-linking caused an initial spike in intracellular Ca2+ concentration followed by a sustained plateau of intermediate Ca2+ concentration that slowly decays to basal level. Compared to wild-type B cells, Ntal−/− B cells showed a systematic enhancement in the magnitude of the initial spike and of the sustained plateau (Fig. 5B).
FIG. 5.
Proliferative and Ca2+ responses in splenic B cells from Ntal−/− mice and Lat−/− Ntal−/− double-deficient mice. (A) Spleen B cells purified from wild-type (WT) or Ntal−/− mice were stimulated with increasing concentration of goat F(ab)′2 anti-mouse IgM antibody (0-40 μg/ml), with lipopolysaccharide (1 μg/ml), or with phorbol myristate acetate and ionomycin (P/I). After 40 h of culture, the ATP content of each culture, a value proportional to the extent of cell proliferation, was measured by luminescence. (B) Calcium flux analysis in response to BCR stimulation in wild-type and Ntal−/− mature B cells. The diagram depicts calcium flux (y axis) as a function of time (x axis). Arrow below the x axis indicates the time point of stimulation with F(ab)′2 goat anti-mouse IgM antibody. Data shown are representative of four independent experiments. (C) Intra- and extracellular Ca2+ mobilization was recorded (see Materials and Methods) in mature B cells from wild-type (WT) or Ntal−/− spleens. Same symbols as in panel B. The time point at which the extracellular Ca2+ concentration was restored to 1.3 mM is indicated by an arrow. (D) Calcium flux analysis in response to BCR stimulation in wild-type and Ntal−/− B220+ IgD−, immature B cells. Same symbols as in panel B. (E) Calcium flux analysis in response to BCR stimulation in wild-type, Ntal−/−, and Lat−/− Ntal−/− mature B cells. Same symbols as in panel B. (D) Calcium flux analysis in response to BCR stimulation in wild-type and Lat−/− mature B cells. Same symbols as in panel B.
To determine whether the absence of NTAL affects mobilization of Ca2+ from intra- and/or extracellular sources, BCR cross-linking was performed in the presence of EGTA to remove extracellular Ca2+ and thereby allow the monitoring of Ca2+ release from endoplasmic reticulum stores only. After restoring the extracellular Ca2+ concentration at later time points, entry of Ca2+ through the plasma membrane was also recorded. As shown in Fig. 5C, the absence of NTAL slightly increased Ca2+ influx across the plasma membrane only. Following BCR cross-linking, IgM+, IgD− immature B cells isolated from the bone marrow of Ntal−/− mice also showed a slightly enhanced Ca2+ influx compared to wild-type immature B cells (Fig. 5D).
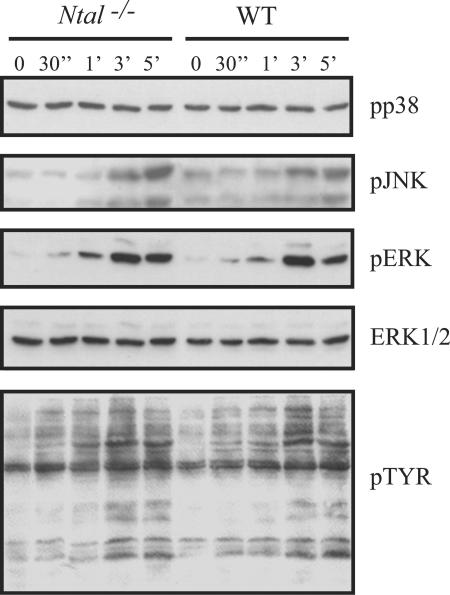
To determine whether the slightly enhanced calcium responses of Ntal−/− B cells correlated with enhanced activation of mitogen-activated protein kinases, purified wild-type and Ntal−/− mature B cells were stimulated for increasing periods of time with affinity-purified F(ab)′2 fragments of an anti-IgM antibody. Subsequently, total tyrosine phosphorylation and activation of the mitogen-activated protein kinase family members ERK, JNK, and p38 were assessed by Western blot using specific antibodies. As shown in Fig. 6, we did not observe any significant alteration in the overall pattern of tyrosine phosphorylation in several independent experiments. Similarly, BCR-mediated activation of JNK, ERK, and p38 was not significantly affected in Ntal−/− B cells (Fig. 6). It is, however, important to mention that, occasionally, phospho responses were slightly enhanced in Ntal−/− B cells compared to wild-type B cells.
FIG. 6.
Analysis of protein tyrosine phosphorylation and of mitogen-activated protein kinase phosphorylation in splenic B cells from Ntal−/− mice. Purified B cells of Ntal−/− (lanes 1-5) and wild-type mice (lanes 6-10) were either left untreated or activated with affinity purified F(ab)′2 fragments of an anti-IgM monoclonal antibody for the indicated periods of time. Postnuclear lysates were subsequently separated by 12% SDS-PAGE and the activation of the individual members of the mitogen-activated protein kinase family (ERK, JNK, p38) assessed using phosphospecific anti-JNK, anti-p38, and anti-ERK antibodies. For analysis of global tyrosine phosphorylation, the antiphosphotyrosine monoclonal 4G10 antibody was employed. To verify equal loading, the blots were stripped and reincubated with an anti-ERK1/2 polyclonal rabbit antiserum. The data shown are representative of seven independently performed experiments.
Humoral responses in Ntal−/− mice.
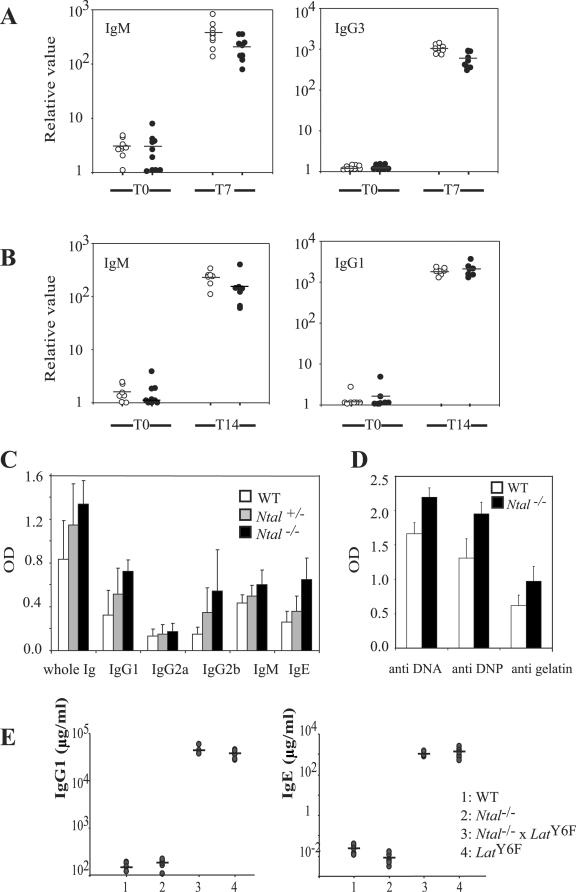
To check whether NTAL deficiency influenced the basal levels of Ig isotypes, their concentrations were determined by ELISA in the sera of unimmunized mice and found identical to those of wild-type littermates (data not shown). To determine whether NTAL is essential for B-cell responses in vivo, mice were immunized with the T-cell-independent antigen (TI) TNP-Ficoll, and two T-cell-dependent antigens, ovalbumin (OVA) and TNP-OVA, and the concentrations of anti-OVA or of antihapten antibodies was determined. The primary immune responses of mice immunized with TNP-Ficoll were similar between Ntal−/− and Ntal+/+ mice (Fig. 7A). We also did not find any difference in antibody responses to TNP-OVA, indicating that Ntal−/− mice also mount a normal immune response to T-cell-dependent antigens (Fig. 7B).
FIG. 7.
Humoral responses in Ntal−/− mice. (A) For measuring B-cell responses to T-independent antigen, group of 8-10 mice (wild-type, open circles; Ntal−/−, solid circles) were immunized with TNP-Ficoll. Mice were bled before (T0) and 7 days (T7) after immunization. TNP-specific immunoglobulins of the indicated isotypes (IgM and IgG3) were assessed in individual mice and plotted on a logarithmic scale. Values represent relative binding units obtained within the linear range of the titration curves compared with a standard nonimmunized control. (B) For measuring B-cell responses to the T-dependent antigen TNP-OVA, groups of 8-10 mice (wild-type, open circles; Ntal−/−, solid circles) were immunized with TNP-OVA and bled before (T0) and 14 days (T14) after immunization. Relative amounts of TNP-specific IgM and IgG1 were analyzed as described in A. Data shown are representative of two independent experiments. (C) Humoral immune response following suboptimal immunization against OVA. Wild-type (n = 6), Ntal+/− (n = 6), and Nta−/− (n = 6) mice were bled 7 days after the second immunization. Total Ig as well as Ig of the indicated isotypes and reactive with OVA were determined by ELISA. (D) Natural antibodies to DNA, DNP, and gelatin were assayed in preimmune sera from wild-type (n = 6) and Ntal−/− (n = 6) mice by ELISA. The concentration of total IgG reactive with the indicated antigens was measured. Differences in both C and D are significant at P < 0.01. (E) Ntal deficiency does not prevent the hypergammaglobulinemia E and G1 that develops in LATY136F mice. The concentrations of IgG1 and IgE in individual mice are plotted on a logarithmic scale. The mean of each distribution is indicated by an horizontal bar.
Previous results on mast cells showed that the regulatory effects of NTAL were best observable under suboptimal conditions of stimulation (23, 26). Along the same line, when immunization with TNP-OVA was performed in a suboptimal way, using intraperitoneal injection in the absence of adjuvant, Ntal−/− mice showed higher T-cell-dependent responses than Ntal+/+ mice (Fig. 7C). Finally, it should be noted that Ntal−/− mice had slightly increased levels of natural antibodies reactive in ELISA to three tested antigens (DNA, gelatin, and DNP) (Fig. 7D). Therefore, Ntal−/− B cells are proficient in TI responses and show slightly enhanced responses toward suboptimally delivered T-cell-dependent antigens.
NTAL and plasma cell differentiation.
Considering that NTAL transcripts were expressed in plasma cells (Fig. 1), we assessed next whether the lack of NTAL prevents the development of antibody secreting plasma cells. Mice homozygous for a mutation that replaced tyrosine 136 of LAT with a phenylalanine (LatY136F mice) develop, in the absence of any intentional immunization, a lymphoproliferative disorder involving polyclonal CD4+ T cells that chronically produced type 2 cytokines such as interleukin-4 (IL-4), IL-13, and IL-5. Over the first 7 weeks of life, this exaggerated T helper type 2 (Th2) differentiation caused a massive conversion of B cells into plasma cells secreting IgE and IgG1 (1, 18). The magnitude and the early onset of hypergammaglobulinemia E and G1 manifested by LatY136F mice allowed us to readily diagnose whether NTAL played a role in terminal B-cell differentiation and IgG1 and IgE isotype switching.
Analysis of mice deficient for the Ntal gene and homozygous for the LatY136F mutation showed that NTAL was dispensable for the unfolding of the lymphoproliferative disorder (data not shown). Monitoring of the IgG1 and IgE concentrations found in serum of 7-week-old Ntal−/− LatY136F mice showed that they contained concentrations of IgE and IgG1 that reached values similar to those of LatY136F homozygous mice (Fig. 7E). Therefore, NTAL appeared to be dispensable for the terminal differentiation of activated B cells into isotype-switched antibody secreting plasma cells, and its absence neither exacerbated, nor prevented the hypergammaglobulinemia that developed in LatY136F mice.
Normal B-cell development in Lat−/− Ntal−/− double-deficient mice.
LAT and NTAL are sequentially expressed in the B-cell lineage (Fig. 1). Therefore, if the gene corresponding to one of these two adaptors is knocked out, the product of the other likely cannot back up the function of the missing one. However, owing to the difficulty in assessing a priori whether the slightly above background NTAL transcript signals noted in pro-B cells (fractions A to C, Fig. 1) were functionally relevant, we aimed at genetically demonstrating that LAT-based and NTAL-based signaling complexes cannot complement each other during B-cell development.
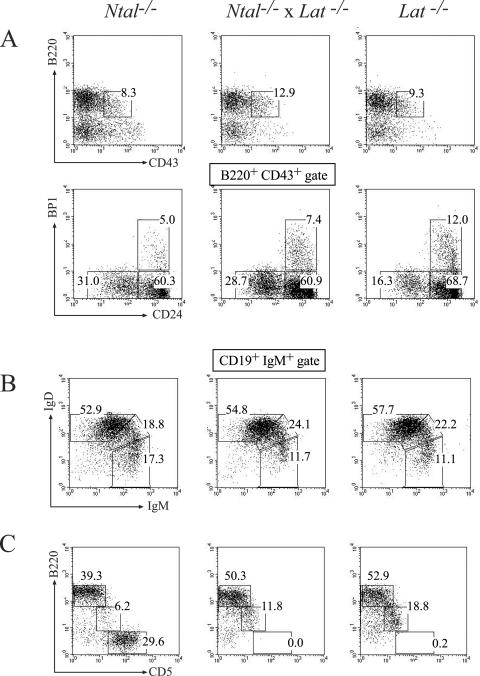
For that purpose, Ntal−/− and Lat−/−, mice were bred to generate double-deficient Ntal−/− Lat−/− mice. Analysis of B-cell development in Ntal−/− Lat+/+, Ntal+/+ Lat−/− and Ntal−/− Lat−/− mutant mice revealed no major difference with wild-type littermates (Fig. 8). Exposition of mice to 500 rad of ionizing irradiation removes all peripheral hematopoietic cells and bone marrow precursors. However, because stem cells in the bone marrow are resistant to this level of irradiation, the treated mice reconstitute their hematopoietic system 12 to 14 days postirradiation (15). Using such “autoreconstitution” conditions, at 12 days postirradiation, spleens from sublethally irradiated Ntal−/− Lat+/+, Ntal+/+ Lat−/−, and Ntal−/− Lat−/− mutant mice were found to contain B cells in numbers and with a surface phenotype identical to those present in sublethally irradiated wild-type littermates (data not shown). Thus, single or combined deletions of NTAL and LAT do not detectably affect early and late B-cell development.
FIG. 8.
Normal B-cell development in Lat−/− Ntal−/− double-deficient mice. Single-cell suspensions from bone marrow (A), spleen (B), and peritoneum (C) were stained with the indicated antibodies and analyzed by flow cytometry. The genotypes are specified above the dot plots. Numbers indicate the percentages of cells in the specified quadrants or gates. In the case of the peritoneum, windows indicate T (CD5hi B220−), B-1 (CD5int B220int), and follicular B (CD5− B220hi) cells. As expected, both Lat−/− and Lat−/− Ntal−/− mice are deprived of mature T cells.
As previously documented for NTAL-deficient mature B cells, B cells isolated from double-deficient Ntal−/− Lat−/− mice showed a similar slight increase in their ability to flux Ca2+ following BCR stimulation (Fig. 5E). In contrast, B cells deprived of LAT showed a Ca2+ influx comparable to that of wild-type B cells (Fig. 5F). Therefore, regardless of the presence or absence of LAT, NTAL-deficient mature B cells systematically showed a slightly enhanced Ca2+ influx upon BCR cross-linking.
DISCUSSION
A search for the existence of symmetry in the BCR and T-cell receptor signaling cassettes suggested that in B cells, the SLP-65 adaptor fulfilled functions requiring both SLP-76 and LAT in T cells. However, because SLP-65 lacks a palmitoylation site, a linker molecule allowing the targeting of SLP-65 to lipid rafts was thought to be still missing in the BCR signaling cassette. Based on its pattern of expression, and considering that it is primarily found in the lipid raft fraction of the plasma membrane, NTAL was thus hypothesized to constitute the B-cell homologue of LAT, thereby allowing the BCR-triggered localization of SLP-65 into lipid rafts (24).
We showed here that the lack of NTAL does not impede B-cell development and functions. Therefore, NTAL does not assume the same seminal role in the BCR signaling cassette as that played by LAT in the T-cell receptor signaling cassette. Consistent with our genetic data suggesting that NTAL does not act upstream of SLP-65, no receptor-stimulated association has been documented between NTAL and SLP-65 (3, 11). Moreover, recent data showed that a highly conserved leucine zipper in the SLP-65 N terminus is responsible for SLP-65 membrane association (12). Therefore, the functional association of SLP-65 with the membrane does not require a LAT-like protein, and, despite the structural homology existing between NTAL and LAT, NTAL is not a functional equivalent of LAT in B cells.
We have found that under some conditions, NTAL negatively regulates signaling through the BCR. However, in contrast to the readily detectable negative effects exerted by NTAL on FcɛRI-triggered signals, in the case of BCR-triggered events the enhanced responses resulting from the loss of NTAL were rather subtle. We noted slightly enhanced Ca2+ flux following stimulation of the BCR, slightly increased levels of natural antibodies, and increased responses to a T-cell-dependent antigen delivered under suboptimal conditions. In mast cells, the negative regulatory role of NTAL was explained by some kind of competition between LAT and NTAL for limited lipid rafts. Because LAT is absent from mature B cells, the rather minor negative regulatory effects exerted by NTAL in B cells cannot be explained, however, in the same way. Nevertheless, it remains possible that the absence of NTAL facilitates the access of other signaling molecules to lipid rafts.
The present results indicating a slightly increased Ca2+ influx in Ntal−/− mouse B cells are surprising in light of recent data obtained in the chicken pre-B-cell line DT40 (20). Overexpressing wild-type NTAL molecules in DT40 cells significantly enhanced Ca2+ influx following pre-BCR stimulation, whereas an NTAL mutant incapable of being targeted to the lipid rafts failed to influence Ca2+ influx. In DT40 cells, Grb2 appeared to play a negative regulatory role in BCR-induced Ca2+ responses, and NTAL likely contributed to relieve this negative effect by sequestering Grb2 to the lipid rafts. Based on these data, in mature mouse B cells, which express large amounts of NTAL, the loss of NTAL should have resulted in an inhibition rather than in an enhancement of BCR-triggered Ca2+ influx. However, NTAL-deficient mature B cells systematically showed a slightly enhanced Ca2+ influx upon BCR cross-linking.
It is possible that major differences exist in the regulation of Ca2+ influx between immature and mature B cells. However, even immature B cells from Ntal−/− mice showed an enhanced Ca2+ influx compared to wild-type immature B cells. Moreover, when membranes were isolated from unstimulated or BCR-stimulated splenic B cells from wild-type, Ntal−/−, and Ntal−/− Lat−/− mice, they showed the same content of Grb2 (data not shown). Altogether, these data suggests that in mouse B cells, NTAL is not involved in the regulation of Ca2+ influx through a Grb2-dependent pathway similar to the one described in DT40. This suggests that Ca2+ influx could be distinctly regulated in transformed chicken B cells (DT40) versus mouse primary B cells. However, it remains also possible that adaptive mechanisms have been set in motion in developing Ntal−/− B cells that compensate for the loss of NTAL.
Analysis of B-cell development in Ntal−/− Lat+/+, Ntal+/+ Lat−/− and Ntal−/− Lat−/− mutant mice revealed no major difference with wild-type littermates. In contrast to a recent report (21), we failed to document a reduction in the number of pro-B cells in Lat−/− mice. Consistent with our finding, it should be stressed that the levels of LAT transcripts expressed in pro-B cells were rather low compared to the one found in T cells, leading to the question of whether they are sufficient for pre-BCR function. Alternatively, and as recently documented for a deleterious mutation of the exon coding for the transmembrane segment of the Ig μ chain gene (8), in some mouse strains, early B-cell development may be more dependent on the presence of LAT than in others.
In this study, we have probed a rather extensive panel of events associated with B-cell differentiation and function and, except for a twofold reduction in the number of marginal zone B cells, failed to document any striking effect resulting from the loss of NTAL. Our study involved most of the B-cell subsets and encompassed activation events that span the whole life of a B-cell, starting with pre-BCR selection and going up to terminal differentiation into plasma cells. Therefore, the impact of the NTAL deficiency is less severe on pre-BCR and BCR signaling compared to its effect on FcɛRI-mediated signaling. Moreover, using Lat−/− Ntal−/− double-deficient mice, we showed that sequential expression and signaling by LAT and NTAL are not a prerequisite for B-cell development and function. These results obtained both in vivo and using freshly isolated B cells contrast with previous results obtained using transformed B-cell lines.
Acknowledgments
We thank M. Daëron and L. Leserman for discussions, N. Fuseri for his assistance, and P. Grenot and N. Brun for their advice.
Work at CIML was supported by INSERM, CNRS, ARC (program ARECA), and the European Communities (MUGEN Network of Excellence). Work at the Institute of Molecular Genetics was supported by the Center of Molecular and Cellular Immunology (project IM6837805001) and by project AV0Z50520514 awarded by the Academy of Sciences of the Czech Republic. The work at the Institute of Immunology of the Otto-von-Guericke University Magdeburg was supported by grants of the Deutsche Forschungsgemeinschaft (DFG-research group 521). Y.W. was supported by an exchange fellowship between Université de la Méditerranée and Shanghai Second Medical University.
REFERENCES
- 1.Aguado, E., S. Richelme, S. Nunez-Cruz, A. Miazek, A. M. Mura, M. Richelme, X. J. Guo, D. Sainty, H. T. He, B. Malissen, and M. Malissen. 2002. Induction of T helper type 2 immunity by a point mutation in the LAT adaptor. Science 296:2036-2040. [DOI] [PubMed] [Google Scholar]
- 2.Brdicka, T., M. Imrich, P. Angelisova, N. Brdickova, O. Horvath, J. Spicka, I. Hilgert, P. Luskova, P. Draber, P. Novak, N. Engels, J. Wienands, L. Simeoni, J. Osterreicher, E. Aguado, M. Malissen, B. Schraven, and V. Horejsi. 2002. Non-T cell activation linker (NTAL): a transmembrane adaptor protein involved in immunoreceptor signaling. J. Exp. Med. 196:1617-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brdicka, T., M. Imrich, P. Angelisova, N. Brdickova, O. Horvath, J. Spicka, I. Hilgert, P. Luskova, P. Draber, P. Novak, N. Engels, J. Wienands, L. Simeoni, J. Osterreicher, E. Aguado, M. Malissen, B. Schraven, and V. V. Horejsi. 2002. Non-T Cell Activation Linker (NTAL): A transmembrane adaptor protein involved in immunoreceptor signaling. J. Exp. Med. 196:1617-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brdicka, T., D. Pavlistova, A. Leo, E. Bruyns, V. Korinek, P. Angelisova, J. Scherer, A. Shevchenko, I. Hilgert, J. Cerny, K. Drbal, Y. Kuramitsu, B. Kornacker, V. Horejsi, and B. Schraven. 2000. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J. Exp. Med. 191:1591-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carsetti, R. 2004. Characterization of B-cell maturation in the peripheral immune system. Methods Mol. Biol. 271:25-35. [DOI] [PubMed] [Google Scholar]
- 6.Dal Porto, J. M., K. Burke, and J. C. Cambier. 2004. Regulation of BCR signal transduction in B-1 cells requires the expression of the Src family kinase Lck. Immunity 21:443-453. [DOI] [PubMed] [Google Scholar]
- 7.Hardy, R. R., and S. A. Shinton. 2004. Characterization of B lymphopoiesis in mouse bone marrow and spleen. Methods Mol. Biol. 271:1-24. [DOI] [PubMed] [Google Scholar]
- 8.Hasan, M., B. Polic, M. Bralic, S. Jonjic, and K. Rajewsky. 2002. Incomplete block of B-cell development and immunoglobulin production in mice carrying the muMT mutation on the BALB/c background. Eur. J. Immunol. 32:3463-3471. [DOI] [PubMed] [Google Scholar]
- 9.Horejsi, V., W. Zhang, and B. Schraven. 2004. Transmembrane adaptor proteins: organizers of immunoreceptor signalling. Nat. Rev. Immunol. 4:603-616. [DOI] [PubMed] [Google Scholar]
- 10.Janssen, E., M. Zhu, B. Craven, and W. Zhang. 2004. Linker for activation of B cells: a functional equivalent of a mutant linker for activation of T cells deficient in phospholipase C-gamma1 binding. J. Immunol. 172:6810-6819. [DOI] [PubMed] [Google Scholar]
- 11.Janssen, E., M. Zhu, W. Zhang, S. Koonpaew, and W. Zhang. 2003. LAB: A new membrane-associated adaptor molecule in B-cell activation. Nat. Immunol. 4:117-123. [DOI] [PubMed] [Google Scholar]
- 12.Kohler, F., B. Storch, Y. Kulathu, S. Herzog, S. Kuppig, M. Reth, and H. Jumaa. 2005. A leucine zipper in the N terminus confers membrane association to SLP-65. Nat. Immunol. 6:204-210. [DOI] [PubMed] [Google Scholar]
- 13.Lopes-Carvalho, T., and J. F. Kearney. 2004. Development and selection of marginal zone B cells. Immunol. Rev. 197:192-205. [DOI] [PubMed] [Google Scholar]
- 14.Nunez-Cruz, S., E. Aguado, S. Richelme, B. Chetaille, A. M. Mura, M. Richelme, L. Pouyet, E. Jouvin-Marche, L. Xerri, B. Malissen, and M. Malissen. 2003. LAT regulates gammadelta T cell homeostasis and differentiation. Nat. Immunol. 4:999-1008. [DOI] [PubMed] [Google Scholar]
- 15.Otero, D. C., and R. C. Rickert. 2003. CD19 function in early and late B-cell development. II. CD19 facilitates the pro-B/pre-B transition. J. Immunol. 171:5921-5930. [DOI] [PubMed] [Google Scholar]
- 16.Oya, K., J. Wang, Y. Watanabe, R. Koga, and T. Watanabe. 2003. Appearance of the LAT protein at an early stage of B-cell development and its possible role. Immunology 109:351-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saitoh, S., R. Arudchandran, T. S. Manetz, W. Zhang, C. L. Sommers, P. E. Love, J. Rivera, and L. E. Samelson. 2000. LAT is essential for Fc(epsilon)RI-mediated mast cell activation. Immunity 12:525-535. [DOI] [PubMed] [Google Scholar]
- 18.Sommers, C. L., C. S. Park, J. Lee, C. Feng, C. L. Fuller, A. Grinberg, J. A. Hildebrand, E. Lacana, R. K. Menon, E. W. Shores, L. E. Samelson, and P. E. Love. 2002. A LAT mutation that inhibits T cell development yet induces lymphoproliferation. Science 296:2040-2043. [DOI] [PubMed] [Google Scholar]
- 19.Spanopoulou, E., C. A. Roman, L. M. Corcoran, M. S. Schlissel, D. P. Silver, D. Nemazee, M. C. Nussenzweig, S. A. Shinton, R. R. Hardy, and D. Baltimore. 1994. Functional immunoglobulin transgenes guide ordered B-cell differentiation in Rag-1-deficient mice. Genes Dev. 8:1030-1042. [DOI] [PubMed] [Google Scholar]
- 20.Stork, B., M. Engelke, J. Frey, V. Horejsi, A. Hamm-Baarke, B. Schraven, T. Kurosaki, and J. Wienands. 2004. Grb2 and the non-t cell activation linker NTAL constitute a Ca2+-regulating signal circuit in b lymphocytes. Immunity 21:681-691. [DOI] [PubMed] [Google Scholar]
- 21.Su, Y. W., and H. Jumaa. 2003. LAT links the pre-BCR to calcium signaling. Immunity 19:295-305. [DOI] [PubMed] [Google Scholar]
- 22.Thien, M., T. G. Phan, S. Gardam, M. Amesbury, A. Basten, F. Mackay, and R. Brink. 2004. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity 20:785-798. [DOI] [PubMed] [Google Scholar]
- 23.Volna, P., P. Lebduska, L. Draberova, S. Simova, P. Heneberg, M. Boubelik, V. Bugajev, B. Malissen, B. S. Wilson, V. Horejsi, M. Malissen, and P. Draber. 2004. Negative regulation of mast cell signaling and function by the adaptor LAB/NTAL. J. Exp. Med. 200:1001-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wange, R. L. 2003. The missing link(er): a return to symmetry in antigen receptor signaling? Mol. Interv. 3:75-78, 50. [DOI] [PubMed] [Google Scholar]
- 25.Zhang, W., J. Sloan-Lancaster, J. Kitchen, R. P. Trible, and L. E. Samelson. 1998. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell 92:83-92. [DOI] [PubMed] [Google Scholar]
- 26.Zhu, M., Y. Liu, S. Koonpaew, O. Granillo, and W. Zhang. 2004. Positive and negative regulation of FcepsilonRI-mediated signaling by the adaptor protein LAB/NTAL. J. Exp. Med. 200:991-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]