Abstract
IMPORTANCE:
Metabolic dysfunction-associated steatotic liver disease (MASLD) is an increasing cause of cirrhosis. Glucagon-like peptide-1 receptor agonists (GLP-1RA) are effective in improving liver inflammation in patients with MASLD.
OBJECTIVE:
To determine whether use of GLP-1 RAs is associated with lower risk of developing cirrhosis and its complications, including decompensation and hepatocellular cancer (HCC), among patients with MASLD.
DESIGN, SETTING, AND PARTICIPANTS:
This retrospective cohort study with an active comparator, new-user design used data from the national Veterans Health Administration Corporate Data Warehouse and Central Cancer Registry. Patients with MASLD and diabetes who were seen at 130 Veterans Health Administration hospitals and associated ambulatory clinics and who initiated either a GLP-1 RA or dipeptidyl peptidase 4 inhibitor (DPP-4i) between January 1, 2006, and June 30, 2022, were included. Patients were followed up from baseline until one of the study outcomes or the end of the study period (December 31, 2022), whichever came first.
EXPOSURES:
Each GLP-1 RA new user was propensity score matched in 1:1 ratio to a patient who initiated a DPP-4i during the same month. Separate analyses were conducted among patients without and with cirrhosis at baseline.
MAIN OUTCOMES AND MEASURES:
For patients without cirrhosis, the primary outcome was progression to cirrhosis defined by validated diagnoses codes or a non-invasive marker of liver fibrosis, and secondary outcomes were cirrhosis complications defined both as a composite and individual complications, including decompensation, HCC, or liver transplant, and all-cause mortality. For patients with cirrhosis, the primary outcome was a composite outcome of cirrhosis complications, and secondary outcomes were decompensation, HCC, and all-cause mortality.
RESULTS:
A total of 16,058 patients who initiated GLP-1 RAs, 14 606 did not have cirrhosis (mean [SD] age, 60.56 [10.31] years; 13 015 [89.1%] male), and 1452 had cirrhosis (mean [SD] age, 66.99 [7.09] years; 1360 [93.7%] male) at baseline. These patients were matched to an equal number of patients who initiated a DPP-4i. In patients without cirrhosis, GLP-1 RA use, compared with DPP-4i use, was associated with a lower risk of cirrhosis (9.98 vs 11.10 events per 1000 person-years; hazard ratio [HR], 0.86; 95% CI, 0.75–0.98). Similar results were seen for the secondary outcomes. GLP-1 RA use, compared with DPP-4i use, was associated with a lower risk of the composite outcome of cirrhosis complications (1.89 vs 2.55 events per 1000 person-years; HR, 0.78; 95% CI, 0.59–1.04) and mortality (21.77 vs 24.43 events per 1000 person-years; HR, 0.89; 95%CI, 0.81–0.98). There were no associations between GLP-1 RA use and outcomes in patients with cirrhosis.
CONCLUSIONS AND RELEVANCE:
CONCLUSIONS AND RELEVANCE In this cohort study, GLP-1 RA use was associated with a lower risk of progression to cirrhosis and mortality among patients with MASLD and diabetes. The protective association was not seen in patients with existing cirrhosis, underscoring the importance of treatment earlier in the disease course.
Background
Metabolic-dysfunction associated steatotic liver disease (MASLD) is the fastest growing cause of cirrhosis and its complications, including hepatocellular cancer (HCC).1 While antiviral treatments have greatly reduced cirrhosis-related sequalae of chronic viral hepatitis, studies have found the opposite trend for patients with MASLD.1,2 Clinical trial evidence for chemoprevention of cirrhosis or its complications in MASLD is currently lacking.
One candidate chemopreventive agent class is glucagon-like peptide-1 receptor agonists (GLP-1 RAs) currently used to treat diabetes and obesity. These medications reduce body weight, glycemia, and inflammation3 - actions that could reduce the risk of MASLD progression. GLP-1 RAs were associated with histological resolution of steatohepatitis in several randomized controlled trials.4–7 Some clinical trials on preventing cirrhosis are underway with results expected late in the 2020’s,8 and observational evidence could help inform decisions in the meantime. Even when the trials are complete, they may be underpowered to assess other outcomes like decompensation or HCC.
Patients with MASLD who have not yet developed cirrhosis represent an important population to conduct observational studies to investigate the associations of GLP-1 RAs. In 2 retrospective cohort studies from Europe, GLP-1 RA use was associated with slower progression to the composite major adverse liver outcome (i.e., cirrhosis, decompensated cirrhosis, HCC, or liver-related death). However, these studies were limited by inability to account for important confounders and lacked assessments of patients with and without MASLD cirrhosis separately.9,10 Most patients in these studies were treated with older GLP-1 RAs, leaving gaps in our knowledge about cirrhosis prevention with the newer agents.
In this study, we analyzed data from a large cohort of U.S. patients with MASLD and diabetes to comprehensively assess the associations between GLP-1 RA use and the risk of developing cirrhosis and its complications, including HCC.
Methods
Data Source
We used data from the national Veterans Heath Administration (VHA) Corporate Data Warehouse (CDW) and Central Cancer Registry. The VHA Corporate Data Warehouse includes all laboratory test results, diagnosis (International Statistical Classification of Diseases and Related Health Problems [ICD]), Current Procedural Terminology codes, annual Alcohol Use Disorders Identification Test screenings, and date of death.11,12 Central Cancer Registry is a repository for VHA patients with cancer and includes information on date of diagnosis, primary site, and histologic findings.
This study was approved by the institutional review boards at Baylor College of Medicine and the Michael E. DeBakey Veterans Affairs Medical Center. A waiver of consent was approved because of the retrospective nature of the study with a large number of patients and due to the research involving no more than minimal risk (including privacy risks) to the individuals.
Study Design
We designed this retrospective cohort study to emulate a target trial of GLP-1 RA compared to an active comparator treatment with dipeptidyl peptidase 4 inhibitors (DPP-4is) for prevention of cirrhosis and related complications in patients with MASLD and diabetes.13,14 Like GLP-1 RAs, DPP-4is are second to third-line antihyperglycemic drugs but with different mechanisms of actions and little to no effect on modifying the natural history of MASLD15, rendering it as an ideal control group. eTable 1 in Supplement 1 summarizes the key components of the target trial.
Eligibility criteria included an age of at least 18 years to 75 years, diagnosis of MASLD and type 2 diabetes, and no previously documented diagnosis of HCC or liver transplant. Patients needed to have at least 6 months between their first encounter in the VHA and study baseline.
We identified all adults with MASLD and diabetes who were seen in 130 VHA hospitals and associated ambulatory clinics and who initiated either a GLP-1 RA or DPP-4i between January 1, 2006 (GLP-1 RAs and DPP-4is were introduced in the U.S in 2005 and 2006, respectively) and June 30, 2022. We classified patients as having MASLD if they had two or more instances of elevated alanine aminotransferase (ALT) values (>40 IU/ml for men and >31 IU/ml for women) [to convert ALT to μkat/L, multiply by 0.0167]) in the ambulatory setting more than 6 months apart with evidence of at least 1 of the 5 metabolic traits16: diabetes, body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) of more than 252, hypertension, hypertriglyceridemia, and low high density lipoprotein (eTable 2 in Supplement 1). We selected the date of second elevated ALT value as MASLD diagnosis date. MASLD diagnosis was validated by review of electronic medical records by 2 physicians (R.S and B.A.) in a random sample of 150 patients (positive predictive value 91%, eTable 3 in Supplement 1). We identified type 2 diabetes based on any 2 ICD codes within 2 years of each other or any prescription for medications used to treat diabetes. No patient had type 1 diabetes.
We identified new users of GLP-1 RAs (exenatide, dulaglutide, liraglutide, semaglutide) and DPP-4is (sitagliptin, saxagliptin, linagliptin, and alogliptin) based on first dispensed prescription for 1 of these drugs in the VHA with a lookback period of 6 months. We defined the date of first prescription as the baseline. Patients in each treatment group had to meet the eligibility criteria at baseline to be included.
We divided patients based on their cirrhosis status at baseline and conducted 2 separate substudies in patients without and with cirrhosis. Cirrhosis was identified based on 2 or more ICD codes for cirrhosis or its complications (eTable 3 in Supplement 1), 1 or more ICD codes for cirrhosis or its complications with 1 or more prescription for medications used to manage cirrhosis complications17, or 2 or more Fibrosis- 4 (FIB-4) scores that were more than 2.67 within 1 year of each other.18–20 We examined the validity of this definition in a sample of 50 patients (positive predictive value 88%; eTable 3 in Supplement 1).
To ensure the balance of important characteristics across groups, for each sub-study, we matched eligible patients who initiated GLP-1 RA in a 1:1 ratio to eligible patients who started DPP4i within the same year and calendar month and using propensity scores. We followed patients from baseline until one of the study outcomes or the end of the study period (December 31, 2022), whichever came first.
Study Outcomes
Patients Without Cirrhosis
The primary outcome was a new diagnosis of cirrhosis. Secondary outcomes were a composite of new diagnosis of cirrhosis complications, including decompensated cirrhosis (ascites, hepatic encephalopathy, variceal bleeding, portal hypertension, or hepatorenal syndrome), HCC, or receipt of liver transplantation,21 the individual components of the composite outcome, and all-cause mortality.
We defined decompensated cirrhosis based on ICD codes that were previously validated in VHA database.17,22 We verified all HCC cases using Cancer Registry entries and electronic medical record reviews (eTable 3 in Supplement 1). We obtained all-cause mortality data from VHA Vital Status File that has a sensitivity of 98.3% and specificity of 99.8% relative to the National Death Index.12
Patients With Cirrhosis
The primary outcome was the composite of a new diagnosis of any of the cirrhosis complications. Secondary outcomes were a new diagnosis of decompensated cirrhosis, HCC, and all-cause mortality.
Statistical Analyses
We used propensity scores to adjust for confounding. The propensity score was calculated from a logistic regression model to estimate the probability of starting a GLP-1 RA vs a DPP4i. We included a range of confounders from the data sources measured at or before baseline including age (continuous), sex, race and ethnicity (Black, Hispanic or Latinx, White, other [grouped together owing to small sample sizes and including American Indian or Alaska Native, Asian, Native Hawaiian, and Other Pacific Islander], or unavailable), a VHA-specific variable based on income, disability, and eligibility for government aid (priority status of 1–3, 4–5, 6–8). We also included markers of diabetes severity, including duration (in years, [continuous]), complications (yes vs. no), hemoglobin A1c value (continuous), use (yes vs. no) and duration (in years, [continuous]) of diabetes medications (metformin, insulin, sulfonylureas, glitazones). Additionally, we considered elapsed time since MASLD diagnosis, time elapsed since cirrhosis diagnosis (for cirrhosis sub-study), BMI, (continuous), presence of other metabolic traits including hypertension, hypertriglyceridemia, and elevated high density lipoprotein (yes vs. no), alcohol use (Alcohol Use Disorders Identification Test screening score 0, 1, 2, or 3), smoking status (current, former, non-smoker), cardiovascular or chronic kidney disease (yes vs. no), comorbidity score (Deyo score, [continuous]), a comprehensive marker of overall mortality (Care Assessments Needs or CAN score, [continuous]).23,24 We included laboratory tests indicating activity (ALT, aspartate aminotransferase levels, [continuous]) and severity of liver disease (serum bilirubin, albumin, creatinine, platelet counts [continuous]) and a non-invasive marker of liver fibrosis (FIB-4 score, [continuous]). Last, we accounted for overall duration in care (in years, [continuous]), and propensity to use healthcare (number of outpatient visits in the 2 years before baseline, [continuous]). (eTables 4 and 5 in Supplement 1). We used 1:1 propensity score matching without replacement with the greedy algorithm, a caliper of 0.2 SD of the logit propensity scores, and simultaneous exact matching on the month of treatment initiation. An absolute standardized difference of less than 0.10 between the matched exposure groups was indicative of good balance.
We calculated incidence rates of the outcomes for each group, with 95% CIs. We used cause-specific proportional hazard models for each outcome to estimate the hazard ratios between matched GLP1 RAs and DPP4is with 95% CIs. We generated cumulative incidence curves for the matched groups over the follow-up period.
We used the SAS MI procedure (multiple imputations) to impute the few missing data, analyzed the 5 imputed datasets separately, and combined the results using MIANALYZE (eTable 6 in Supplement 1). All outcomes were pre-specified and interpreted separately and hence did not account for multiple comparisons in the statistical tests. SAS Enterprise Guide, version 8.3 (SAS Institute), was used for all data analyses.
Secondary and Sensitivity Analyses
The primary analyses applied an intention-to-treat design, with GLP-1 RA or DPP-4i use defined by the initial prescription. We conducted per-protocol analyses in which the follow-up began on the date of GLP-1 RA or DPP-4i prescriptions and ended on the day of the outcomes of interest, December 31, 2022, or initiation of a DPP4i among patients who entered the study on a GLP-1 RA or vice versa. We also assessed whether the associations varied by the type of GLP-1 RA (dulaglutide, liraglutide, semaglutide). We conducted subgroups analyses based on pre-specified risk factors including age, sex, BMI, degree of liver fibrosis (FIB-4 <1.3 vs. >1.3 among patients without cirrhosis) and diabetes severity (diabetes complications). We conducted a sensitivity analysis using an alternative, more restrictive specification of cirrhosis variable, defined based on 2 outpatient or 1 inpatient ICD codes for cirrhosis and excluded patients with FIB-4 score of less than 1.3 to further reduce any possible misclassification (eTable 6 in Supplement 1).25 To explore the possibility of residual confounding (e.g., by underlying health status or health care–seeking behavior), we used a negative outcome control by evaluating differences in risk of fractures during the follow up period (eTable 6 in Supplement 1).
Results
Patient Characteristics
Of 16,058 identified patients who initiated a GLP-1 RA, 14,606 did not have cirrhosis and 1,452 had cirrhosis at baseline. These patients were matched to an equal number of patients without and with cirrhosis who initiated DPP-4i (Figure 1).
Figure 1.
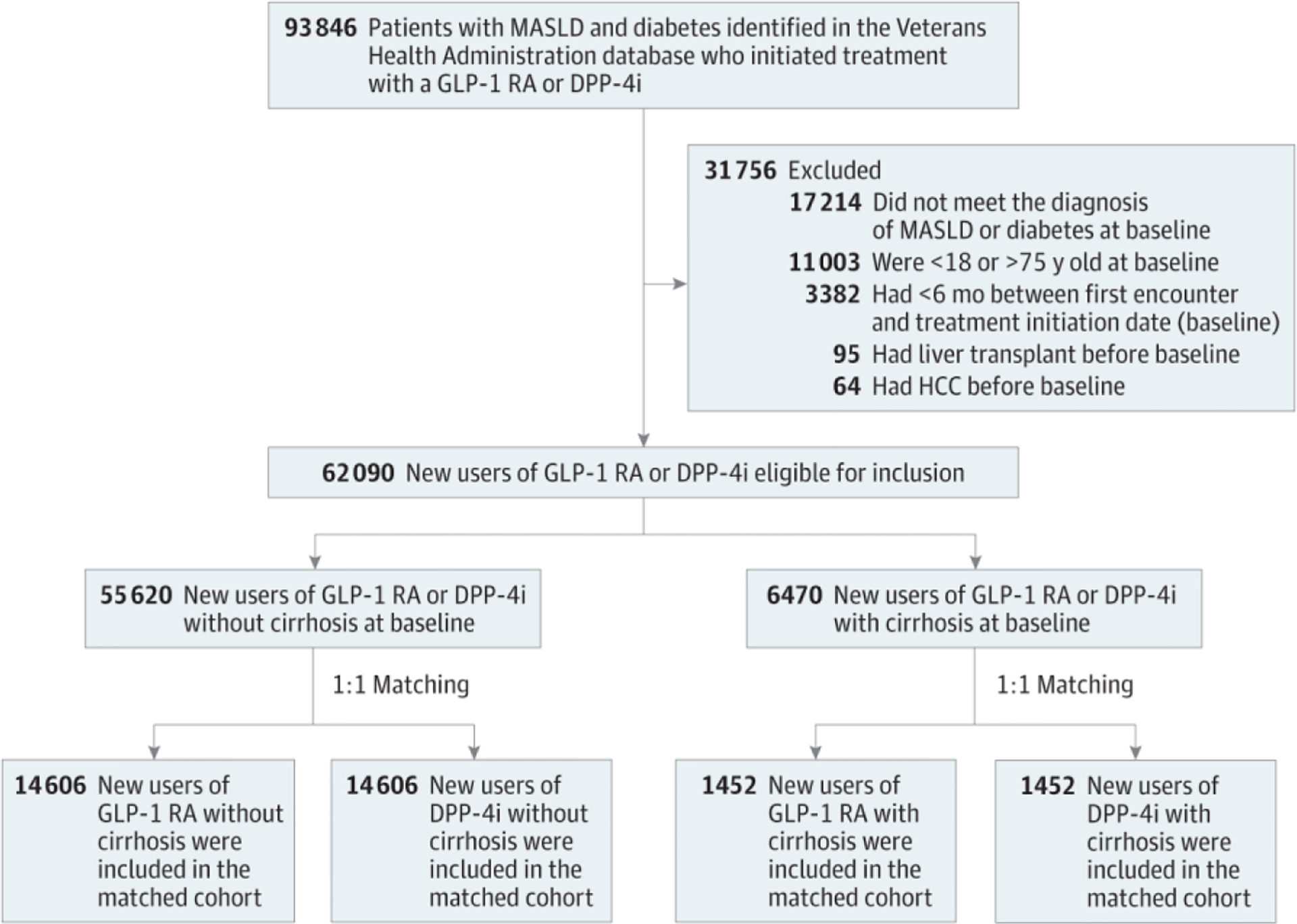
Study Flow DiagramDPP-4i indicates dipeptidyl peptidase 4 inhibitor; GLP-1 RA, glucagon-like peptide 1 receptor agonist; HCC, hepatocellular cancer; MASLD, metabolic dysfunction-associated steatotic liver disease.
In total, 1067 patients (6.6%) were prescribed exenatide, 4449 (27.7%) dulaglutide, 5733 (35.7%) liraglutide, and 12,148 (75.6%) were prescribed semaglutide. In total, 8848 patients (55.1%) switched from one GLP-1RA to another during study follow up.
Table 1 summarizes the characteristics of the GLP-1 RA and DPP-4i users after matching. The groups were well balanced across all covariates, with all standardized differences below 0.1.26 In the subgroup without cirrhosis, the mean (SD) age of GLP-1RA users was 60.47 (10.31) years and 13015 (89.1%) were men. Patients were in care for a mean SD of 10.80 (5.52) years before baseline. Patients were prescribed treatment a mean (SD) 7.93 (4.46) years following MASLD diagnosis. Patients with cirrhosis were older and in care longer than patients without cirrhosis (Table 1). (eTable 7 in Supplement 1) summarizes patient characteristics in the unmatched groups.
Table 1.
Characteristics Among Patients With Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) and Diabetes by Cirrhosis Status and Treatment Groupa,b
| Characteristic | Patients, No. (%) | |||||
|---|---|---|---|---|---|---|
| Without cirrhosis | With cirrhosis | |||||
| GLP-1 RA users (n = 14 606) |
DPP-4i users (n = 14 606) |
SMD | GLP-1 RA users (n = 1452) |
DPP-4i users (n = 1452) |
SMD | |
| Demographics | ||||||
| Age, mean (SD), y | 60.56 (10.31) | 60.47 (10.31) | 0.01 | 66.99 (7.09) | 67.01 (7.07) | <0.01 |
| Sex | ||||||
| Female | 1591 (10.9) | 1544 (10.6) | 0.01 | 92 (6.3) | 88 (6.1) | 0.01 |
| Male | 13 015 (89.1) | 13 062 (89.4) | 0.01 | 1360 (93.7) | 1364 (93.9) | 0.01 |
| Race and ethnicity | ||||||
| Black | 2107 (14.4) | 2171 (14.9) | 0.01 | 120 (8.3) | 117 (8.1) | 0.01 |
| Hispanic or Latinx | 799 (5.5) | 839 (5.7) | 0.01 | 72 (5.0) | 79 (5.4) | 0.02 |
| White | 11 057 (75.7) | 10 935 (74.9) | 0.02 | 1207 (83.3) | 1196 (82.4) | 0.02 |
| Otherc | 643 (4.4) | 661 (4.5) | 0.01 | 53 (3.6) | 60 (4.1) | 0.02 |
| Priority statusd | ||||||
| 1–3 | 9911 (67.8) | 9935 (68.0) | <0.01 | 1009 (69.5) | 997 (68.7) | <0.01 |
| 4–5 | 2522 (17.3) | 2528 (17.3) | <0.01 | 259 (17.8) | 263 (18.1) | <0.01 |
| 6–8 | 2173 (14.9) | 2143 (14.7) | <0.01 | 184 (12.7) | 192 (13.2) | <0.01 |
| Clinical factors | ||||||
| Diabetes duration, mean (SD), y | 7.77 (4.89) | 7.67 (4.70) | 0.02 | 9.54 (4.79) | 9.04 (4.78) | 0.10 |
| Diabetes complications | 11 199 (76.7) | 11 143 (76.3) | 0.01 | 1230 (84.7) | 1207 (83.1) | 0.04 |
| Hemoglobin A1c, mean (SD), % | 8.64 (1.70) | 8.65 (1.72) | 0.01 | 8.54 (1.63) | 8.52 (1.63) | 0.01 |
| Metformin use | 13 173 (90.2) | 13 262 (90.8) | 0.02 | 1318 (90.8) | 1309 (90.2) | 0.02 |
| Duration of metformin use, mean (SD), ye | 4.25 (3.93) | 4.19 (3.90) | 0.01 | 5.15 (4.29) | 4.90 (4.16) | 0.06 |
| Insulin use | 8577 (58.7) | 8317 (56.9) | 0.03 | 994 (68.5) | 962 (66.3) | 0.04 |
| Duration of insulin use, mean (SD), ye | 3.35 (5.03) | 2.98 (4.72) | 0.07 | 5.18 (6.17) | 4.55 (5.90) | 0.10 |
| Sulfonylurea use | 9807 (67.1) | 9919 (67.9) | 0.01 | 1049 (72.3) | 1031 (71.0) | 0.02 |
| Duration of sulfonylurea use, mean (SD), ye | 2.55 (3.30) | 2.57 (3.34) | 0.01 | 3.20 (3.65) | 3.07 (3.68) | 0.03 |
| Glitazone use | 2147 (14.7) | 1988 (13.6) | 0.03 | 229 (15.8) | 204 (14.1) | 0.04 |
| Duration of glitarone use, mean (SD), ye | 0.25 (0.96) | 0.23 (0.92) | 0.02 | 0.28 (1.07) | 0.26 (1.02) | 0.01 |
| BMI, mean (SD) | 36.05 (6.66) | 33.55 (6.47) | 0.07 | 35.40 (6.08) | 35.14 (6.53) | 0.04 |
| Hypertension | 13 120 (89.8) | 13 036 (89.2) | 0.01 | 1379 (95.0) | 1368 (94.2) | 0.03 |
| Hypertriglyceridemia | 14 165 (97.0) | 14 148 (96.9) | 0.01 | 1420 (97.8) | 1418 (97.7) | 0.01 |
| Low high-density lipoprotein | 14 116 (96.7) | 14 125 (96.7) | <0.01 | 1429 (98.4) | 1421 (97.9) | 0.04 |
| AUDIT-C score | ||||||
| 0 | 9412 (64.4) | 9432 (64.6) | <0.01 | 1048 (72.2) | 1058 (72.9) | 0.01 |
| 1 | 3815 (26.1) | 3775 (25.8) | 0.01 | 316 (21.8) | 311 (21.4) | 0.01 |
| 2 | 1049 (7.2) | 1053 (7.2) | <0.01 | 68 (4.7) | 64 (4.4) | 0.01 |
| 3 | 330 (2.3) | 346 (2.4) | 0.01 | 20 (1.3) | 19 (1.3) | 0.01 |
| Smoking status | ||||||
| Current | 2433 (16.7) | 2542 (17.4) | 0.01 | 212 (14.6) | 197 (13.6) | 0.02 |
| Former | 5682 (38.9) | 5572 (38.2) | 0.01 | 653 (45.0) | 666 (45.9) | 0.01 |
| Nonsmoker | 6491 (44.4) | 6492 (44.4) | 0.01 | 587 (40.4) | 589 (40.6) | <0.01 |
| Comorbidity | ||||||
| Deyo score, mean (SD) | 2.60 (1.86) | 2.53 (1.79) | 0.03 | 3.58 (2.34) | 3.37 (2.38) | 0.09 |
| Cardiovascular disease | 844 (5.8) | 858 (5.9) | <0.01 | 134 (9.2) | 128 (8.8) | 0.01 |
| Chronic kidney disease | 2339 (16.0) | 2205 (15.1) | 0.02 | 442 (30.4) | 391 (26.9) | 0.07 |
| CAN score, mean (SD)f | 61.60 (24.15) | 60.83 (24.31) | 0.03 | 73.66 (20.34) | 71.90 (21.56) | 0.08 |
| Baseline laboratory tests, mean (SD) | ||||||
| Creatinine, mg/dL | 2.43 (8.85) | 2.31 (8.45) | 0.01 | 2.19 (7.37) | 2.28 (7.48) | 0.01 |
| Alanine aminotransferase, U/mL | 41.09 (22.44) | 41.05 (24.68) | <0.01 | 42.67 (27.26) | 45.54 (31.56) | 0.09 |
| Aspartate aminotransferase, U/mL | 28.25 (14.75) | 28.21 (14.78) | <0.01 | 37.98 (22.62) | 40.19 (28.54) | 0.08 |
| Platelet count, ×103 per mm3 | 237.86 (62.09) | 237.60 (61.52) | <0.01 | 168.29 (61.53) | 170.26 (62.95) | 0.03 |
| Serum bilirubin, mg/dL | 0.59 (0.30) | 0.60 (0.30) | 0.01 | 0.70 (0.40) | 0.70 (0.37) | 0.01 |
| Serum albumin, g/dL | 4.06 (0.38) | 4.07 (0.37) | <0.01 | 3.98 (0.42) | 3.99 (0.44) | <0.01 |
| Fibrosis-4 scoreg | 1.22 (1.41) | 1.21 (0.80) | <0.01 | 2.65 (1.54) | 2.65 (1.47) | 0.01 |
| Health care use, mean (SD) | ||||||
| Duration in care, y | 10.80 (5.52) | 10.80 (5.44) | <0.01 | 11.81 (5.15) | 11.51 (5.07) | 0.05 |
| Duration between MASLD diagnosis and index, y | 7.93 (4.46) | 7.92 (4.39) | <0.01 | 9.04 (4.23) | 8.86 (4.23) | 0.04 |
| No. of outpatient visits | 45.0 (33.75) | 43.39 (32.95) | 0.05 | 57.08 (41.06) | 54.21 (39.83) | 0.07 |
Abbreviations: AUDIT-C. Alcohol Use Disorders Identification Test: BMI. body mass index (calculated as weight in kilograms divided by height in meters squared): CAN. Care Assessment Needs: DPP-4i. dipeptidyl peptidase 4 inhibitor: GLP-1RA. glucagon like peptide I receptor agonist; SMD. standardized mean difference; VA. US Department of Veterans Affairs.
SI conversion factors: To convert alanine aminotransferase or aspartate aminotransferase to μkat/L. multiply by 0.0167; albumin to g/L. multiply by 10; bilirubin to μmol/L multiply by 17.104; creatinine to μmol/L. multiply by 88.4: percentage of hemoglobin A1c to proportion of total hemoglobin, multiply by 0.01.
Operational definitions of all variables are listed in eTable 2 in Supplement 1. Values are after matching.
Prior to matching, data were missing for small percentages of patients without cirrhosis for GLP-1 RA vs DPP-4i users: race and ethnicity. 5.1% vs 6.0%; priority status. 09% vs 1.5%; smokimg status. 5.3% vs 7.9%; hemoglobin A1c. 0.9% vs 3.0%; CAN score. 1.6% vs 3.4%; BMI. 0.5% vs 1.6%; alanine aminotransferase. 0.9% vs 2.3%; aspartate aminotransferase. 1.4% vs 2.8%: platelet count. 2.1% vs 3.7%; bilirubin. 2.7% vs 4.7%; albumin. 4.2% vs 6.6%: Fibrosis-4 score. 3.0% vs 4.7%. and AUDIT-C score. 0.5% vs 2.9%. In the groups with cirrhosis, data were missing for small percentages of patients for the following variables for GLP-1 RA vs DPP-4i users: race and ethnicity. 5.1% vs 6.0%: priority status. 0.9% vs 1.5%: smoking status. 5.3% vs 7.9%; hemoglobin A1c. 0.9% vs 3.0%; CAN score. 1.6% vs 3.4%; BMI. 0.5% vs 1.6%: alanine aminotransferase. 0.9% vs 2.3%; aspartate aminotransferase. 1.4% vs 2.8%; platelet count. 2.1% vs 3.7%. bilirubin. 2.7% vs 4.7%; albumin. 4.2% vs 6.6%: Fibrosis-4 score. 3.0% vs 4.7%. and AUDIT-C score. 0.5% vs 2.9%. Multiple imputation was used to account for missing data in the propensity score-matched analysis.
The other category included American Indian or Alaska Native. Asian. Native Hawaiian. and Other Pacific Islander, and was grouped together owing to small sample sizes.
Priority status 1 through 3 indicates varying degrees of disability; 4. catastrophically disabled or receiving home benefits; 5. annual income below the VA pension benefits national income threshold or receiving Medicaid or VA pension; 6. World War I or different combat exposures; 7 and 8. income above the national income threshold or those who agree to pay the co-payment.
For the duration of treatment variables, patients without a given treatment were assigned a value of zero for the analysis.
The CAN score is a predictive analytic tool developed within the VA to help primary care physicians make management or care coordination decisions.22,26 We used the CAN score version designed to predict risk of death or hospitalization at 1 year. The methodology for calculation of the CAN score is consistent with the cumulative deficit model, similar to frailty scores, and the CAN score has been validated against other frailty scores.
The Fibrosis-4 score is a noninvasive marker of liver fibrosis in patients with chronic liver conditions, it can be calculated from age and 3 parameters obtained in routine laboratory assessments: alanine aminotransferase. aspartate aminotransferase, and platelet count.
The mean (SD) duration of GLP-1 agonist treatment was 3.20 (2.19) years and 2.96 (1.87) years in patients without and with cirrhosis, respectively. In patients without cirrhosis, 4565 (31.2%) switched from a DPP-4i to a GLP-1 RA, and 965 GLP-1 RA users (6.6%) switched to a DPP-4i during follow-up.
Study End Points
Tables 2 and 3 summarize the analyses for the primary and secondary outcomes.
Table 2.
Outcomes Comparing Glucagon-Like Peptide 1 Receptor Agonist (GLP-1 RA) and Dipeptidyl Peptidase 4 Inhibitor (DPP-4i) Use Among Patients With Metabolic Dysfunction-Associated Steatotic Liver Disease and Diabetes Without Cirrhosisa
| Exposure | No. of events | Person-years | Incidence per 1000 person-years (95% CI) | Hazard ratio (95% CI) |
|---|---|---|---|---|
| Primary outcome | ||||
| Cirrhosis | ||||
| GLP-1 RA | 477 | 47 770 | 9.98 (9.10–10.92) | 0.86 (0.75–0.98) |
| DPP-4i | 524 | 47 236 | 11.10 (10.16–12.09) | |
| Secondary outcomes | ||||
| Composite outcomc | ||||
| GLP-1 RA | 92 | 48 724 | 1.89 (1.52–2.32) | 0.78 (0.59–1.04) |
| DPP-4i | 123 | 48 200 | 2.55 (2.12–3.05) | |
| Decompensation | ||||
| GLP-1 RA | 88 | 48 816 | 1.80 (1.44–2.21) | 0.75 (0.55–1.01) |
| DPP-4i | 109 | 48 325 | 2.26 (1.82–2.72) | |
| Hepatocellular cancer | ||||
| GLP-1 RA | 12 | 48 944 | 0.24 (0.12–0.42) | 0.89 (0.40–2.01) |
| DPP-4i | 13 | 48 499 | 0.27 (0.14–0.45) | |
| All-cause mortality | ||||
| GLP-1 RA | 1066 | 48 954 | 21.77 (20.49–23.12) | 0.89 (0.81–0.98) |
| DPP-4i | 1185 | 48 513 | 24.43 (23.06–25.86) |
Results are from the intention-to-treat analysis.
Table 3.
Outcomes Comparing Glucagon-Like Peptide 1 Receptor Agonist (GLP-1RA) and Dipeptidyl Peptidase 4 Inhibitor (DPP-4i) Use Among Patients With Metabolic Dysfunction-Associated Steatotic Liver Disease, Diabetes, and Cirrhosisa
| Exposure | No. of events | Person-years | Incidence per 1000 person-years (95% CI) | Hazard ratio (95% CI) |
|---|---|---|---|---|
| Primary outcome | ||||
| Composite outcome | ||||
| GLP-1 RA | 68 | 3646 | 18.64 (14.48–23.64) | 1.18 (0.77–1.81) |
| DPP-4i | 55 | 3593 | 15.31 (11.53–19.92) | |
| Secondary outcomes | ||||
| Decompensation | ||||
| GLP-1 RA | 53 | 3665 | 14.45 (10.83–18.91) | 1.14 (0.72–1.82) |
| DPP-4i | 48 | 3604 | 13.32 (9.82–17.65) | |
| Hepatocellular cancer | ||||
| GLP-1 RA | 24 | 4428 | 5.32 (3.42–8.06) | 1.41 (0.69–2.90) |
| DPP-4i | 14 | 4378 | 3.20 (1.79–5.36) | |
| All-cause mortality | ||||
| GLP-1 RA | 244 | 4455 | 54.75 (48.10–62.08) | 0.88 (0.73–1.06) |
| DPP-4i | 272 | 4392 | 61.91 (54.78–69.73) |
Results are from the intention-to-treat analysis.
Patients without cirrhosis
Overall, the use of GLP-1 RAs was associated with a 14% lower risk of cirrhosis compared with the use of DPP-4is (9.98 vs 11.10 events per 1000 person-years; hazard ratio [HR], 0.86; 95% CI, 0.75–0.98; Table 2). Figure 2 shows a lower cumulative incidence of cirrhosis for patients taking GLP-1 RAs, with the curves diverging after 18 months of use.
Figure 2.
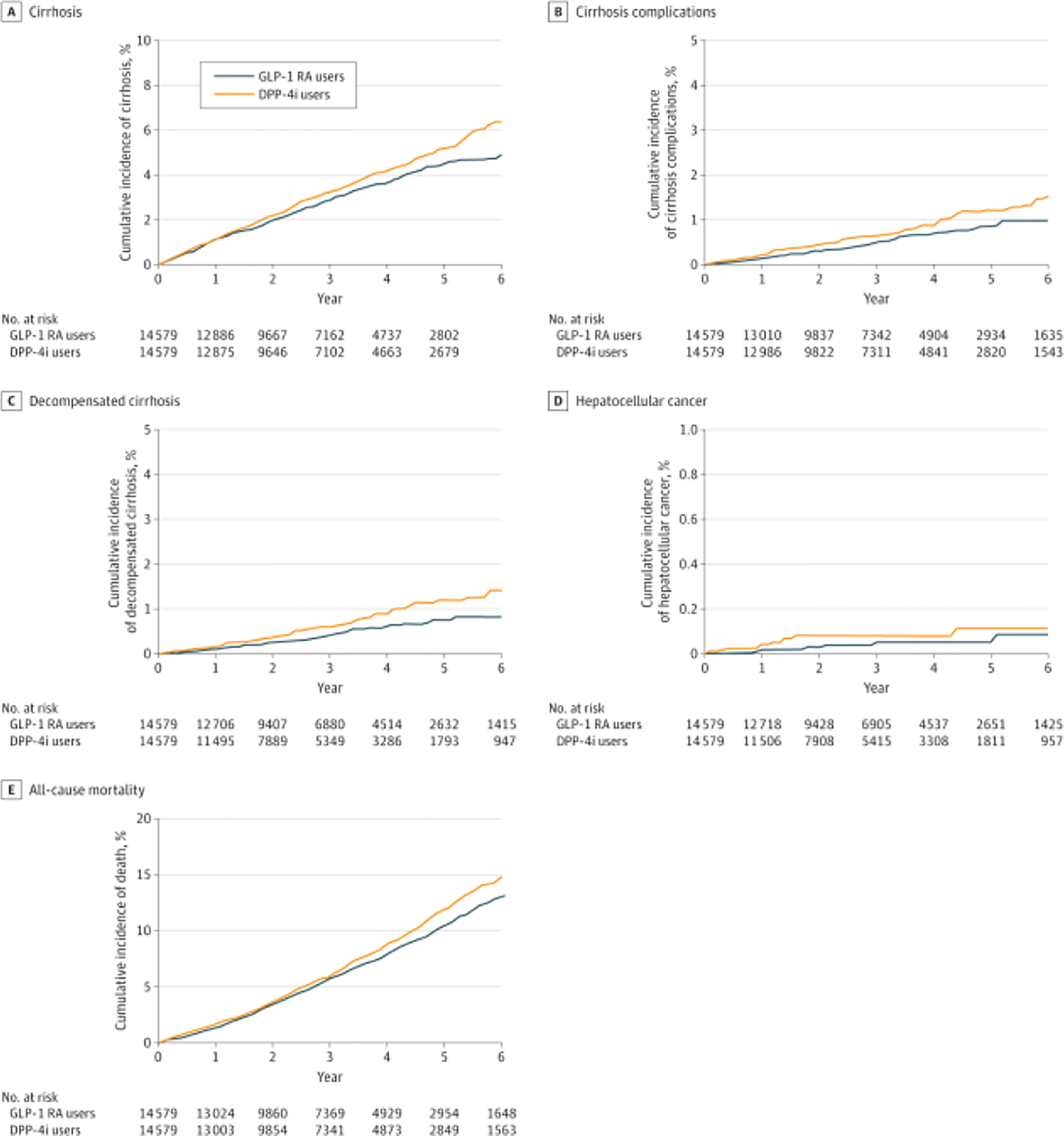
Cumulative Incidence of Cirrhosis and Complications in Patients Without CirrhosisCumulative incidence of cirrhosis (A) and composite end point of cirrhosis complications, including decompensated cirrhosis, hepatocellular cancer, and liver transplantation (B), as well as separate cumulative incidences of decompensated cirrhosis (C), hepatocellular cancer (D), and all-cause mortality (E) among patients with metabolic dysfunction-associated steatotic liver disease without cirrhosis taking glucagon-like peptide 1 receptor agonists (GLP-1 RAs) or dipeptidyl peptidase 4 inhibitors (DPP-4is).
The risk of the composite outcome was 22% lower in GLP-1 RA vs DPP-4i users (1.89 vs 2.55 events per 1000 person-years; HR, 0.78; 95% CI, 0.59–1.04). GLP-1 RA use compared with DPP-4i use was associated with a 30% lower risk of cirrhosis decompensation (1.80 vs 2.26 events per 1000 person-years; HR, 0.75; 95% CI, 0.55–1.01) and a 11% lower risk of all cause mortality (21.77 vs 24.43 events per 1000 person years; HR,0.89; 95% CI, 0.81–0.98). HCC risk was 0.24 vs 0.27 events per 1000 person-years in patients treated with GLP-1 RAs vs DPP-4is (HR, 0.89; 95% CI, 0.40–2.01).
The magnitude of the association was stronger for each outcome in per-protocol analyses (eTable 8 in Supplement 1). After stratification by specific GLP-1 RA, the use of semaglutide was associated with lower HRs for progression to cirrhosis, though with wide 95% CIs. There was no association between use of dulaglutide or liraglutide and MASLD outcomes (eFigure 1 in Supplement 1). There was no statistically significant interaction by age, sex, BMI, diabetes complications, or FIB-4 score. Using a restrictive definition of cirrhosis did not change the direction or magnitude of the association (HR,0.85; 95% CI, 0.66–1.07; eTable 9 in Supplement 1). There was a nearly identical pattern among the 2 groups in the risk of fractures during follow-up (HR, 1.03; 95% CI, 0.93–1.15; eFigure 2 in Supplement 1).
Patients with cirrhosis
Between GLP-1 RA and DPP-4i users, there were no statistically significant differences in the risk of cirrhosis complications (18.64 v. 15.31 events per 1000 person years; HR 1.18; 95% CI, 0.77–1.81), decompensated cirrhosis (14.45 vs. 13.32 events per 1000 person years, HR, 1.14; 95% CI, 0.72–1.82), HCC (5.32 v. 3.20 events per 1000 person-years; HR, 1.41; 95% CI, 0.69–2.90), or risk of all-cause mortality (54.75 v. 61.91 events per 1000 person-years; HR, 0.88; 95% CI, 0.73–1.06) (Table 3, eFigure 3 in Supplement 1). Per-protocol analyses generated similar results (eTable 10 in Supplement 1).
Discussion
In a large national cohort study of patients with MASLD and diabetes but without cirrhosis, we observed protective associations between GLP-1 RA use and subsequent development of cirrhosis, cirrhosis complications, and overall mortality. This chemopreventive activity became apparent 18 to 24 months after treatment initiation and increased over time. In contrast, there was limited benefit in patients with established cirrhosis. These data highlight the potential consequences of delaying treatment – either by lack of access or by patient or healthcare professional choice – on subsequent risk of cirrhosis complications.
These results fill an important gap in the understanding of cirrhosis chemoprevention among a large population of patients with MASLD but without cirrhosis. We were unable to assess whether there was an association with decreased HCC incidence due to the small number of cases. While prior work has established an association between cirrhosis and HCC,27 an independent establishment of the relationship between GLP-1 RA and reduced HCC will need further study. Notwithstanding, we did find a relationship with other complications and with overall mortality. Notably, the reduction in mortality associated with GLP-1 RA (2.6 per 1000 person-years) exceeded their effect on cirrhosis prevention (1.12/1000 person-years). The former is consistent with prior retrospective studies28 and randomized trials of patients with diabetes and obesity.29 The present data suggest that among patients with MASLD, GLP 1RAs may offer other survival benefits than those conferred by slowing the risk of liver disease progression.
We matched GLP-1 RA users with DPP4i users, and the 2 matched groups were similar across a broad range of covariates. The use of active comparator also mitigates the risk of confounding by indication and unmeasured clinical characteristics than when comparing GLP-1 RA user with non-users. However, both GLP-1 RA and DPP4i’s affect GLP levels, with the GLP-1 RAs being much more effective. It is possible that DPP4is have some protective effects on MASLD progression, rendering our results potentially conservative estimates. We also found a stronger association between semaglutide and outcomes than with other GLP-1 RAs, suggesting that the protective associations may become more pronounced as more effective GLP-1 RA or dual/triple agonists become available.
Using the VHA data enabled adjustment for important confounders, including factors which are often unavailable in other datasets. We also examined the possibility of surveillance bias, where GLP-1 RA users could have had more encounters, leading to faster diagnoses of cirrhosis and complications and found no evidence of this. We studied patients with and without MASLD cirrhosis separately, and found different associations, with clinical implications.
Limitations
This study has limitations. Although, we took several measures to minimize confounding, some amount of unmeasured confounding may still remain. Misclassification was also possible. We used validated definitions and confirmed robustness of findings in sensitivity analyses, but we could have missed outcomes if patients sought care outside the VHA. The definition for decompensated cirrhosis has not been validated in the study database, however we do not expect misclassification to be differential between the groups. The MASLD definition for this study relies on at least 2 elevated ALT levels and may have some degree of misclassification; it could also limit the generalizability of the present findings to patients with MASLD who have persistently normal liver enzymes. The absolute risk of some outcomes was low, such as HCC, resulting in imprecise estimates. We were also limited by relatively short duration of follow-up. The trends we observed warrant longer follow up to examine the protective association with HCC. The present sample included mostly male patients, but we had over 3000 female pateints without cirrhosis in the group, and the associations were similar in both sexes (eFigure 1 in Supplement 1). All patients we studied had diabetes in addition to MASLD and, thus, were potentially eligible for GLP-1 RA therapy. We did not examine harms associated with GLP-1 RA, which although uncommon, may not negligible. Future research should evaluate subgroups of higher risk individuals as well as individuals who may experience harms with GLP-1 RA to improve the risk-benefit profile for chemoprevention.
Conclusions
In this cohort study of patients with MASLD and diabetes, the use of GLP-1 RAs was associated with a lower risk of progression to cirrhosis and death. These results support the need for long-term randomized clinical trials to test the benefits of GLP-1 RA use for primary prevention of cirrhosis in patients with MASLD. While cirrhosis is a clear risk factor for HCC and reducing cirrhosis by GLP-1 RA use should prevent HCC, an independent confirmation of this relationship requires even larger studies than this one. In the meantime, the presence of MASLD can help with the prioritization of GLP-1 RA therapy in persons with diabetes.
Supplementary Material
Key Points.
Question:
Is use of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) associated with lower incidence of cirrhosis and its complications in patients with metabolic dysfunction-associated steatotic liver disease (MASLD)?
Findings:
In this cohort study of 16 058 patients (14 606 without and 1452 with cirrhosis), GLP-1 RA use was associated with a statistically significant reduction in the risk of progression to cirrhosis and its complications in patients with MASLD and diabetes than use of an active comparator treatment. The chemopreventive benefit was limited to patients who initiated GLP-1 RAs earlier in the disease course; patients who started GLP-1 RAs after they had already progressed to cirrhosis did not have lower rates of progression to hepatic decompensation or hepatocellular cancer.
Meaning:
If confirmed by clinical trials, GLP-1 RAs show promise as chemopreventive agents for cirrhosis and its complications in patients with MASLD and diabetes.
Acknowledgements
This work is supported by the National Cancer Institute (P01CA263025), and in part by grants from the NIH (R01CA186566, U01CA230997) and Cancer Prevention & Research Institute of Texas grant (RP150587). Drs. Kanwal and El-Serag are also supported in part by Center for Gastrointestinal Development, Infection and Injury (NIDDK P30 DK 56338) and the Veterans Administration Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413), Michael E. DeBakey VA Medical Center, Houston, Texas
References
- 1.Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. Apr 2021;18(4):223–238. doi: 10.1038/s41575-020-00381-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang DQ, Singal AG, Kono Y, Tan DJH, El-Serag HB, Loomba R. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab. Jul 5 2022;34(7):969–977.e2. doi: 10.1016/j.cmet.2022.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh YS, Jun HS. Effects of Glucagon-Like Peptide-1 on Oxidative Stress and Nrf2 Signaling. Int J Mol Sci. Dec 22 2017;19(1)doi: 10.3390/ijms19010026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. Feb 13 2016;387(10019):679–690. doi: 10.1016/s0140-6736(15)00803-x [DOI] [PubMed] [Google Scholar]
- 5.Khoo J, Hsiang JC, Taneja R, et al. Randomized trial comparing effects of weight loss by liraglutide with lifestyle modification in non-alcoholic fatty liver disease. Liver Int. May 2019;39(5):941–949. doi: 10.1111/liv.14065 [DOI] [PubMed] [Google Scholar]
- 6.Newsome PN, Buchholtz K, Cusi K, et al. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N Engl J Med. Mar 25 2021;384(12):1113–1124. doi: 10.1056/NEJMoa2028395 [DOI] [PubMed] [Google Scholar]
- 7.Yan J, Yao B, Kuang H, et al. Liraglutide, Sitagliptin, and Insulin Glargine Added to Metformin: The Effect on Body Weight and Intrahepatic Lipid in Patients With Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease. Hepatology. Jun 2019;69(6):2414–2426. doi: 10.1002/hep.30320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Research Study on Whether Semaglutide Works in People With Non-alcoholic Steatohepatitis (NASH) (ESSENCE). March 8, 2024. Updated March 05 2024. Accessed March 8, 2024. https://clinicaltrials.gov/study/NCT04822181?intr=semaglutide&cond=liver&rank=8#more-information
- 9.Wester A, Shang Y, Toresson Grip E, Matthews AA, Hagström H. Glucagon-like peptide-1 receptor agonists and risk of major adverse liver outcomes in patients with chronic liver disease and type 2 diabetes. Gut. Jan 30 2024;doi: 10.1136/gutjnl-2023-330962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engström A, Wintzell V, Melbye M, et al. Association of glucagon-like peptide-1 receptor agonists with serious liver events among patients with type 2 diabetes: A Scandinavian cohort study. Hepatology. Jun 1 2024;79(6):1401–1411. doi: 10.1097/hep.0000000000000712 [DOI] [PubMed] [Google Scholar]
- 11.Lapham GT, Achtmeyer CE, Williams EC, Hawkins EJ, Kivlahan DR, Bradley KA. Increased documented brief alcohol interventions with a performance measure and electronic decision support. Med Care. Feb 2012;50(2):179–87. doi: 10.1097/MLR.0b013e3181e35743 [DOI] [PubMed] [Google Scholar]
- 12.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. Apr 10 2006;4:2. doi: 10.1186/1478-7954-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernán MA, Robins JM. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am J Epidemiol. Apr 15 2016;183(8):758–64. doi: 10.1093/aje/kwv254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. Dec 2015;2(4):221–228. doi: 10.1007/s40471-015-0053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Budd J, Cusi K. Role of Agents for the Treatment of Diabetes in the Management of Nonalcoholic Fatty Liver Disease. Curr Diab Rep. Oct 5 2020;20(11):59. doi: 10.1007/s11892-020-01349-1 [DOI] [PubMed] [Google Scholar]
- 16.Rinella ME, Lazarus JV, Ratziu V, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. Dec 1 2023;78(6):1966–1986. doi: 10.1097/hep.0000000000000520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan DE, Dai F, Aytaman A, et al. Development and Performance of an Algorithm to Estimate the Child-Turcotte-Pugh Score From a National Electronic Healthcare Database. Clin Gastroenterol Hepatol. Dec 2015;13(13):2333–41.e1-6. doi: 10.1016/j.cgh.2015.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mózes FE, Lee JA, Selvaraj EA, et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut. May 2022;71(5):1006–1019. doi: 10.1136/gutjnl-2021-324243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siddiqui MS, Yamada G, Vuppalanchi R, et al. Diagnostic Accuracy of Noninvasive Fibrosis Models to Detect Change in Fibrosis Stage. Clin Gastroenterol Hepatol. Aug 2019;17(9):1877–1885.e5. doi: 10.1016/j.cgh.2018.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology. Nov 2017;66(5):1486–1501. doi: 10.1002/hep.29302 [DOI] [PubMed] [Google Scholar]
- 21.Wester A, Shang Y, Toresson Grip E, Matthews AA, Hagström H. Glucagon-like peptide-1 receptor agonists and risk of major adverse liver outcomes in patients with chronic liver disease and type 2 diabetes. Gut. Apr 5 2024;73(5):835–843. doi: 10.1136/gutjnl-2023-330962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanwal F, Kramer JR, Buchanan P, et al. The quality of care provided to patients with cirrhosis and ascites in the Department of Veterans Affairs. Gastroenterology. Jul 2012;143(1):70–7. doi: 10.1053/j.gastro.2012.03.038 [DOI] [PubMed] [Google Scholar]
- 23.Ruiz JG, Priyadarshni S, Rahaman Z, et al. Validation of an automatically generated screening score for frailty: the care assessment need (CAN) score. BMC Geriatr. May 4 2018;18(1):106. doi: 10.1186/s12877-018-0802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruiz JG, Rahaman Z, Dang S, Anam R, Valencia WM, Mintzer MJ. Association of the CAN score with the FRAIL scale in community dwelling older adults. Aging Clin Exp Res. Oct 2018;30(10):1241–1245. doi: 10.1007/s40520-018-0910-4 [DOI] [PubMed] [Google Scholar]
- 25.Mahmud N, Serper M, Taddei TH, Kaplan DE. The Association Between Proton Pump Inhibitor Exposure and Key Liver-Related Outcomes in Patients With Cirrhosis: A Veterans Affairs Cohort Study. Gastroenterology. Jul 2022;163(1):257–269.e6. doi: 10.1053/j.gastro.2022.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harder VS, Stuart EA, Anthony JC. Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychol Methods. Sep 2010;15(3):234–49. doi: 10.1037/a0019623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarao K, Nozaki A, Ikeda T, et al. Real impact of liver cirrhosis on the development of hepatocellular carcinoma in various liver diseases-meta-analytic assessment. Cancer Med. Mar 2019;8(3):1054–1065. doi: 10.1002/cam4.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yen FS, Hou MC, Wei JC, Shih YH, Hwu CM, Hsu CC. Effects of glucagon-like peptide-1 receptor agonists on liver-related and cardiovascular mortality in patients with type 2 diabetes. BMC Med. Jan 4 2024;22(1):8. doi: 10.1186/s12916-023-03228-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi Q, Nong K, Vandvik PO, et al. Benefits and harms of drug treatment for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. Bmj. Apr 6 2023;381:e074068. doi: 10.1136/bmj-2022-074068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Department of Veterans’ Affairs Table of Priority Groups. March 8, 2024. Updated 2024. Accessed March 8, 2024. https://www.payingforseniorcare.com/veterans/veterans_priority_groups
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


