Abstract
A 78-year-old woman presented to hospital with altered mental status. Hyponatremia was diagnosed and treated. She maintained mental confusion despite normal sodium. No headache, fever or focal signs were present. CSF analysis showed chronic meningitis; MRI demonstrated basilar enhancement. She was empirically treated for tuberculosis, with no improvement. PCR for Sporothrix in the CSF was positive. After treatment with Amphotericin followed by oral itraconazole, she completely recovered her cognitive abilities. Follow-up CSF was normal. This report illustrates the need to consider sporotrichosis in the differential diagnosis of chronic meningitis in immunocompetent, and the importance of PCR as a diagnostic tool.
Keywords: Sporothrichosis, Chronic meningitis, Fungal meningitis, Sporothrix brasiliensis
1. Introduction
Sporotrichosis is a mycosis caused by the universally distributed fungi belonging to the Sporothrix genus, which especially affects individuals who live in tropical and subtropical regions. These dimorphic fungi live in the soil and plants, from where they can inoculate and infect humans, as a sapronotic infection – hence the label “rose gardener's disease”. Zoonotic transmission, notably by cats, also happens, particularly in the Southeastern Brazil. Typically, sporotrichosis presents in its cutaneous or cutaneous-lymphatic forms, which account for more than 95 % of the cases [1]. Meningeal involvement, on the other hand, is rare, and generally affects immunocompromised hosts as part of disseminated infection by Sporothrix [2].
Most case series of central nervous system (CNS) sporotrichosis comprise only individuals with underlying immunosuppression, mainly HIV infection [3,4]. Meningeal sporotrichosis usually poses a diagnostic challenge, not only due to its rarity, but also because culture, the gold standard method, lacks the desirable sensitivity when performed in the cerebrospinal fluid (CSF). Ever more, however, mounting data show we can rely on other techniques, notably immunoassays and polymerase chain reaction (PCR) technique, to confirm sporotrichosis [5,6].
Here, we describe a case of sporotrichosis presenting as chronic meningitis in an immunocompetent host, with no concurrent skin lesion and no other organ involvement. Hyponatremia due to inappropriate antidiuretic hormone secretion was a prominent feature. In our patient, a positive PCR in the CSF was key to confirm the diagnosis. We believe exposing this case will raise awareness about the need to consider sporotrichosis in the differential diagnosis of chronic meningitis, even if there is no underlying immunosuppression. Hopefully, it will also draw attention to the role of PCR in making such diagnosis.
2. Case
A 78-year-old-woman, with no underlying medical condition, presented to the emergency department (ED) with fatigue and lethargy. According to her children, over the course of the previous 3 months, she had shown signs of cognitive disturbance, such as forgetfulness, fluctuating confusional states, disorientation, behavioral changes (mainly hypoactivity), and depressed mood. Given that she had lost her husband 9 months earlier, her cognitive and behavioral symptoms were initially attributed to a complicated grief. Sertraline had been started, with little response. For most of the time, however, she had still been able to carry on all her duties with occasional aid. Only in the 2 days prior to the ED visit, lethargy had become incapacitating. There were no seizures, headache, fever nor systemic symptoms.
Upon hospital admission (day 0), she was diagnosed with hyponatremia (Na 124 mmol/L), which then could account for all her symptoms. Serologies (syphilis, HIV, hepatitis B and C) were negative; her urinalysis was normal. Sertraline was suspended due to its potential sodium-lowering effect. Laboratory workup confirmed the syndrome of inappropriate antidiuretic hormone secretion (SIADH): high urinary sodium (78 mmol/L); high urine osmolality (525 mOsm/kg); low serum osmolality (260 mOsm/kg); low uric acid (2.1 mg/dL). CT scans of her head, chest, abdomen, and pelvis were unremarkable, and so were thyroid, adrenal, liver, heart and kidney functions tests – SIADH was then deemed idiopathic. The patient was treated with fluid restriction (limited to 500 mL of liquid intake per day) and oral replacement of sodium (2g of salt twice daily). At day 35, she was discharged from hospital after her sodium levels had gone back to normal (Na 135 mmol/L) and her mental status had partially improved.
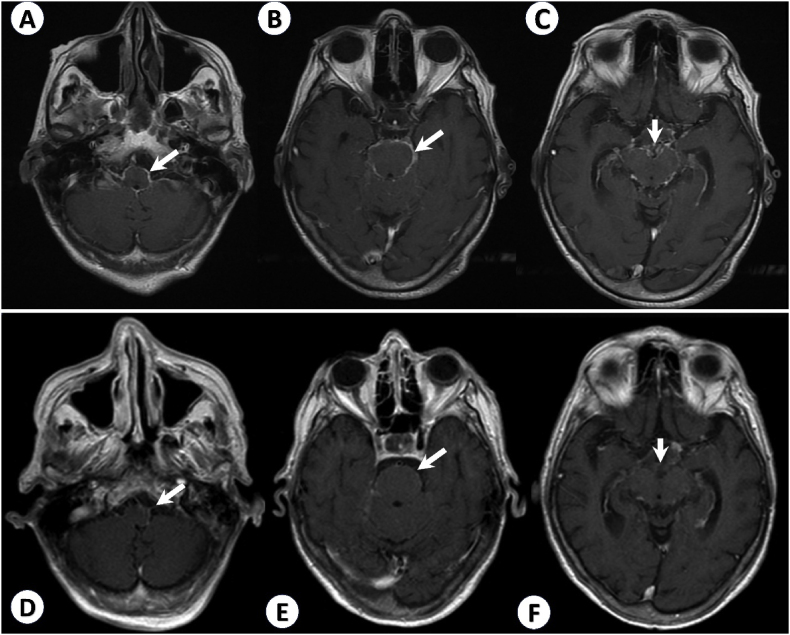
Two months later (at day 106), in an outpatient setting, she was referred to a Neurology consultation because of persistent cognitive decline despite controlled sodium levels. Her neurological examination revealed mild disorientation in time and space, marked loss in executive function and processing speed, slow and cautious gait, and no focal signs. She scored 7/30 on the Mini-mental state examination (MMSE). Once again, she denied having fever, seizures, headaches. She was then hospitalized for further investigation. She undergone a lumbar puncture, with normal opening pressure (12 cmH2O). The cerebrospinal fluid (CSF) analysis was consistent with chronic meningitis: 232 leukocytes/mm³ (95 % lymphocytes), remarkably high protein (3021 mg/dL) and consumed glucose (23 mg/dL); cultures and PCR specific for Mycobacterium were negative; adenosine deaminase (ADA) was slightly elevated (10 U/L). Brain magnetic resonance imaging (MRI) revealed intense basilar meningeal enhancement, and mild communicating hydrocephalus (Fig. 1A and B and C). Her clinical examination was unremarkable. CT scans of her chest, abdomen, and pelvis were repeated and remained normal.
Fig. 1.
T1 weighted, gadolinium-enhanced brain MRI. In the first MRI, performed upon hospital admission, there is intense basilar leptomeningeal contrast enhancement, around the medulla (A), pons (B) and midbrain (C) - arrows. After 1-month treatment with Amphotericin B, follow-up imaging showed less contrast enhancement at the same levels (D, E, and F) - arrows.
Even though microbiological tests were negative, she was empirically treated for meningeal tuberculosis (TB), considering the compatible clinical picture, CSF pattern, and MRI findings, together with epidemiology (TB is the most common cause of chronic meningitis in Brazil). After one month taking Rifampicin, Isoniazid, Pyrazinamide and Ethambutol (RHZE), the patient showed no clinical improvement. CSF samples were collected on a weekly basis, without any meaningful change thus far (see Table 1). PCR specific for Mycobacterium, mycobacterial cultures, cryptococcal antigens, direct mycological exams, and fungal cultures were negative in all such samples.
Table 1.
Serial CSF samples’ analyses.
| Date |
Opening pressure (cmH2O) |
Protein (mg/dL) |
Glucose (mg/dL) |
Leukocytes (/mm³) - % Lymphocytes |
ADA (U/L) |
PCR for mycobacteria |
Mycobacterial culture |
Fungal culture |
|
|---|---|---|---|---|---|---|---|---|---|
| Day 107 | 12 | 3021 | 23 | 232 (95 %) | – | – | – | Negative | |
| RHZE (Day 108 – day 137) | Day 112 | – | 2815 | 43 | 569 (62 %) | 10 | Negative | Negative | Negative |
| Day 117 | 10 | 572 | 15 | 439 (95 %) | – | Negative | Negative | Negative | |
| Day 124 | 10 | 406 | 12.5 | 307 (96 %) | 9 | Negative | Negative | Negative | |
| Day 134 | 12 | 602 | 16 | 182 (93 %) | – | Negative | Negative | Negative | |
| Amphotericin (Day 139 - day 181) | Day 143 | 10 | 437 | 37 | 117 (85 %) | – | – | – | Negative |
| Day 158 | 12 | 294 | 50 | 79 (90 %) | – | – | – | Negative | |
| Day 176 | 14 | 311 | 41 | 53 (95 %) | – | – | – | Negative | |
| Itraconazole | |||||||||
| Day 327 | 12 | 56 | 55 | 5(89 %) | – | – | – | Negative | |
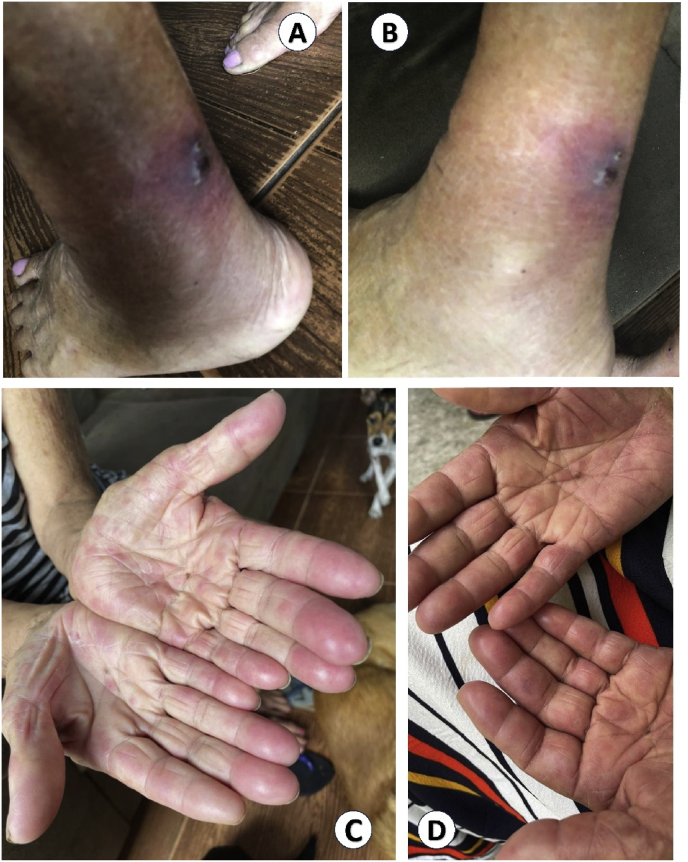
In a careful history review, one of her children revealed that more than 1 year earlier, the patient had been exposed to cats diagnosed with sporotrichosis (Fig. 2). 1 month after that, she had had a single crusted, ulcerated, nodular skin lesion just above her left ankle, which spontaneously disappeared (Fig. 3A and B), followed by polyarthritis, which also resolved without treatment (Fig. 3C and D). At the time of our evaluation, she had no cutaneous lesion nor signs of arthritis. In light of such new information, PCR using specific primers for Sporothrix genus was performed on the CSF and came out positive. Serum enzyme-linked immunosorbent assay (ELISA) for sporotrichosis was also reactive.
Fig. 2.
Homemade picture of the cat which belonged to the patient's daughter. Note the nodular lesion on the bridge of the nose and pinnae. The feline was diagnosed with sporotrichosis and treated accordingly. It had died 1 year before our patient presented to neurologic consultation.
Fig. 3.
A, B: Homemade pictures of a single crusted, ulcerated nodular skin lesion just above the patient's left ankle; it had disappeared spontaneously and entirely, almost 1 year prior to the first neurologic visit. C, D: Homemade pictures taken from our patient showing signs of arthritis (erythema and edema) at her interphalangeal joints; it had appeared circa 1 month after the skin nodule and also remitted completely with no treatment.
The technical procedures which yielded a positive PCR result may be summarized as follows: the genomic DNA was extracted from the CSF sample of the patient using the FavorPrep Fungi/Yeast Genomic DNA Extraction Mini Kit (Favorgen, Taiwan). Then, it was quantified by spectrophotometry using Nanovue Plus (GE Healthcare Life Sciences, Sweden). The DNA extracted and purified was submitted to the PCR reaction using the primers designed, according to Fernandes and colleagues [7]. The reaction was performed with a final volume of 10 μL in a solution containing PCR 1x buffer; 3 mM MgCl2; 0.2 μM dNTPs; 1.0 U/μL Taq DNA polymerase (all reagents of Ludwig, Brazil); 0.2 μM of each Forward and Reverse primer (Invitrogen, USA) and Milli-Q water; 1 μL of 20.0 ng/μL of DNA extracted was used for each reaction. The amplification cycles were: 1 cycle of 95 °C for 5 min; 40 cycles of 95 °C for 30 sec, 60 °C for 30 sec and 72 °C for 30 sec; finalized by a cycle of 72 °C for 5 min. Negative and positive PCR controls were included in each reaction. The positive control is DNA extracted from an international standard sample of S. schenkii ATCC MF765977, a reference for all reactions. Mixtures containing all the reaction components were used as the negative control, containing sterile ultrapure water in place of the extracted DNA. The final amplification product was analyzed on 7 % polyacrylamide gel stained by silver nitrate to visualize a fragment of 450bp expected for this amplicon.
Antifungal treatment was then started with intravenous liposomal Amphotericin B 5 mg/kg once daily. After 6 weeks on Amphotericin B, the patient had partial improvement of CSF features: 53 cells/mm³ (95 % lymphocytes); protein 311 mg/dL; glucose 41 mg/dL. In a second brain MRI, there was significantly less contrast enhancement (Fig. 1 D, E, F). She was discharged from hospital with oral itraconazole 200 mg daily. After 6 months on itraconazole, she returned for an outpatient visit. She had fully recovered her cognitive capacities (MMSE 28/30) and her gait had improved considerably. Follow-up CSF was nearly normal: 5 leukocytes/mm³ (89 % lymphocytes), protein 56 mg/dl, no glucose consumption (55 mg/dl). Her sodium levels also normalized with no further need of fluid restriction. Itraconazole was maintained for 12 months [8].
3. Discussion
Sporotrichosis is endemic in tropical regions, particularly in South America [9]. It is usually transmitted from the contact with contaminated moss, soil and plants. Zoonotic transmission by cats, however, is increasing, especially in the Southeastern Brazil, where it is the major cause of a large outbreak. Classically, the clinical picture starts with a nodular lesion at the site of inoculation, which ulcerates. Similar ulcerated nodules then appear, following the course of lymphatic vessels. Less frequently, the host may have a single cutaneous lesion. This is all what happens in the more common cutaneous and lymphocutaneous forms of the disease [1,9,10]. In less than 5 % of the cases, fungi spreading through the bloodstream may lead to cutaneous-disseminated and extracutaneous forms. In cutaneous-disseminated sporotrichosis, there is extensive involvement of skin and mucosae, bones and joints, internal organs, and rarely the CNS [1,2,11]. Merely extracutaneous infection may also be seen, as for example in sporotrichoid arthritis. Disseminated-cutaneous and extracutaneous forms of sporotrichosis are usually associated with immunosuppression, namely HIV infection, diabetes, malnutrition, and alcoholism [1,4].
We believe many lessons can be drawn by analyzing each aspect of the atypical sporotrichosis case we have just described. To begin with, meningeal sporotrichosis is in itself a rare condition. CNS involvement happens in less than 0.1 % of Sporothrix infections [9]. In parallel, sporotrichosis is an extremely unusual cause of chronic meningitis. Chronic meningitis by Sporothrix species may occur in isolation or as part of widespread disease; in both situations, it is generally linked to immunosuppression [10]. For instance, out of 1697 patients diagnosed with sporotrichosis in one reference center, 1680 were immunocompetent, none of whom developed meningitis. In contrast, the remainder 17 patients were HIV-positive and, among them, 2 presented CNS infection by Sporothrix [12]. A more recent case series from a 21-year sporotrichosis cohort described 17 cases of meningeal involvement, wherein all 17 had some immunocompromising condition (15 were HIV-positive, 1 was an alcoholic, and 1 had a history of chronic corticosteroid use) [3]. Yet in another study, the CNS was affected in 7 sporotrichosis patients; CNS infection was associated with HIV (5 out of 7), alcoholism, and diabetes [4]. In immunosuppressed hosts, meningeal sporotrichosis can be regarded as severe: in a literature review, mortality was estimated at 57 % [12].
Only seldom has CNS sporotrichosis been described in immunocompetent hosts [[13], [14], [15]]. Common to all the few published cases, there was considerable diagnostic delay. Frequently, empirical treatment for meningeal TB is started before the agent is found to be Sporothrix spp., as reported in 3 of 4 recent cases [[13], [14], [15]]. Having initially made the same therapeutic choice for our patient, we found that unsurprising, given that there are remarkable clinical, CSF, and radiological similarities between meningeal sporotrichosis and meningeal TB, and that the latter is infinitely more prevalent. Prognosis and response to treatment is variable in the immunocompetent: there have been instances of poor outcomes as death [14] and permanent neurologic sequelae [15]; but there has also been a patient who, just like ours, completely recovered after receiving itraconazole [13].
In several aspects, still, the case we describe adds novelties and nuances to the available literature on meningeal sporotrichosis of the immunocompetent. In other cases, skin lesions were also absent at the time of presentation [[13], [14], [15]], but unlike our case, there is no account of past cutaneous involvement. The lack of skin lesions has led to the hypothesis of inhalation being the probable route of infection in isolated meningeal sporotrichosis [14]. In contrast with this notion, our patient's history suggests she was contaminated by her cat, through her skin, where an inoculation lesion had formed and soon disappeared. Then the pathogen had spread to her interphalangeal joints, temporarily causing arthritis, and to her CNS, where it continued to cause disease.
Unlike ours, most published cases of meningeal sporotrichosis in the immunocompetent showed features of intracranial hypertension (ICH), in the form of either a high CSF opening pressure [13] or overt hydrocephalus with the need of ventricular shunt placement [14,15]. Despite a mild communicating hydrocephalus in MRI, our patient had never developed symptoms of ICH, nor did she have abnormal opening pressures. In our patient, SIADH and consequent hyponatremia were prominent clinical features. For many months, salt ingestion and extreme fluid restriction were required to control her sodium levels. The need for such measures disappeared once the chronic meningitis resolved. No other report mentioned water-electrolyte imbalance in association with meningeal sporotrichosis, either in immunocompetent [[13], [14], [15]] or in immunosuppressed hosts [[2], [3], [4],12,16]. Although it is known that chronic meningitis in general may lead to SIADH [17,18], it is the first time, to our knowledge, that SIADH is reported in connection with meningeal sporotrichosis specifically.
Finally, our case illustrates the importance of the PCR technique as a diagnostic tool in meningeal sporotrichosis. Albeit being the gold standard, CSF culture for Sporothrix spp. poses significant challenges in practice: the method is time-consuming and lacks enough sensitivity in specimens with low fungal burden, as often is the case with CSF [5]. When there are other sites of infection, the preemptive diagnosis of CNS sporotrichosis can be made combining the CSF pattern of chronic meningitis and a positive Sporothrix culture from another site [3]. Immunological and molecular methods, however, are especially useful in cases of isolated meningeal sporotrichosis, in which relying on culture alone might lead to a diagnostic delay of up to 6 months [19]. Serum ELISA has shown 90 % sensitivity and 80 % specificity for sporotrichosis in general [20]. For meningeal sporotrichosis, ELISA has been performed in the CSF, with good accuracy [5,19]. PCR technique for Sporothrix in the CSF is demonstrably reliable to confirm CNS sporotrichosis: it can be 92,9 % sensitive (nested PCR) and 91 % specific (quantitative PCR) [5,6].
We hope this case will help to draw attention to sporotrichosis as a possible cause of chronic meningitis, even in the immunocompetent. We believe it will add to current literature in describing a nuanced clinical presentation: the resolved cutaneous lesion; the marked hyponatremia; the lack of ICH; and the excellent response to treatment. It also demonstrates the importance of molecular methods to confirm, in a timely fashion, the diagnosis of a treatable and potentially curable disease.
Conflict of Interest
The authors declare that there is no Conflict of Interest.
Ethical approval
The authors confirm that the Ethics Policies of the journal, as noted in the author's guideline page, have been adhered to. Our study was approved by the Ethics Committee of the Santa Casa Hospital of Belo Horizonte, Brazil (CAAE 55549216.2.0000.5138).
CRediT authorship contribution statement
Victor Teatini Ribeiro: Writing – review & editing, Writing – original draft, Investigation, Conceptualization. Rachel Basques Caligiorne: Writing – review & editing, Resources, Investigation. Aldrin Pedroza Martins: Writing – review & editing, Investigation. Antônio Pereira Gomes Neto: Writing – review & editing, Supervision, Resources. Paulo Pereira Christo: Writing – review & editing, Supervision.
Acknowledgements
The authors thank the patient and her family members, who agreed to participate in this study. We also thank the technicians who helped with the diagnosis as well as the doctors and nurses who helped with the follow-up of this case.
The authors thank the funding agencies FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais, Brazil) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil).
Handling Editor: Dr Adilia Warris
References
- 1.Bonifaz A., Tirado-Sánchez A. Cutaneous disseminated and extracutaneous sporotrichosis: current status of a complex disease. Journal of Fungi. 2017;3 doi: 10.3390/jof3010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duani H., Palmerston M.F., Rosa Júnior J.F., Ribeiro V.T., Alcântara Neves P.L. Meningeal and multiorgan disseminated sporotrichosis: a case report and autopsy study. Med Mycol Case Rep. 2019;26:47–52. doi: 10.1016/j.mmcr.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lima M.A., Freitas D.F.S., Oliveira R.V.C., Fichman V., Varon A.G., Freitas A.D., Lamas C.C., Andrade H.B., Veloso V.G., Almeida-Paes R., Almeida-Silva F., Zancopé-Oliveira R.M., de Macedo P.M., Valle A.C.F., Silva M.T.T., Araújo A.Q.C., Gutierrez-Galhardo M.C. Meningeal sporotrichosis due to Sporothrix brasiliensis: a 21-year cohort study from a Brazilian reference center. Journal of Fungi. 2023;9 doi: 10.3390/jof9010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magalhães V.C.R., Colombo S.A., Peres N.T.A., Moura A.S., Lyon A.C., Lyon S., Dutra M.R.T., Fereguetti T.O., Andrade V.A., Azevedo M.I., Santos D.A. Clinical factors associated with systemic sporotrichosis in Brazil. Mycoses. 2024;67 doi: 10.1111/myc.13656. [DOI] [PubMed] [Google Scholar]
- 5.Almeida-Silva F., Almeida M. de A., Rabello V.B. de S., Zancopé-Oliveira R.M., Baeza L.C., Lamas C. da C., Lima M.A., de Macedo P.M., Gutierrez-Galhardo M.C., Almeida-Paes R., Freitas D.F.S. Evaluation of five non-culture-based methods for the diagnosis of meningeal sporotrichosis. Journal of Fungi. 2023;9 doi: 10.3390/jof9050535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliveira M.M.E., Muniz M. de M., Almeida-Paes R., Zancope-Oliveira R.M., Freitas A.D., Lima M.A., Gutierrez-Galhardo M.C., Freitas D.F.S. Cerebrospinal fluid PCR: a new approach for the diagnosis of CNS sporotrichosis. PLoS Negl Trop Dis. 2020;14:1–4. doi: 10.1371/journal.pntd.0008196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandes B., La Santrer E.F.R., de Figueiredo S.M., Rocha-Silva F., Assunção C.B., Abreu A.G., Santiago I.F., Johann S., Caligiorne R.B. Sporotrichosis: in silico design of new molecular markers for the Sporothrix genus. Rev. Soc. Bras. Med. Trop. 2023;56 doi: 10.1590/0037-8682-0217-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kauffman C.A., Bustamante B., Chapman S.W., Pappas P.G. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the infectious diseases society of America. Clin. Infect. Dis. 2007;45:1255–1265. doi: 10.1086/522765. [DOI] [PubMed] [Google Scholar]
- 9.Hernández-Castro R., Pinto-Almazán R., Arenas R., Sánchez-Cárdenas C.D., Espinosa-Hernández V.M., Sierra-Maeda K.Y., Conde-Cuevas E., Juárez-Durán E.R., Xicohtencatl-Cortes J., Carrillo-Casas E.M., Steven-Velásquez J., Martínez-Herrera E., Rodríguez-Cerdeira C. Epidemiology of clinical sporotrichosis in the americas in the last ten years. Journal of Fungi. 2022;8 doi: 10.3390/jof8060588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kauffman C.A. Central nervous system infection with other endemic mycoses: rare manifestation of blastomycosis, paracoccidioidomycosis, talaromycosis, and sporotrichosis. Journal of Fungi. 2019;5 doi: 10.3390/jof5030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandes B., Caligiorne R.B., Coutinho D.M., Gomes R.R., Rocha-Silva F., Machado A.S., La Santrer E.F.R., Assunção C.B., Guimarães C.F., Laborne M.S., Nunes M.B., Vicente V.A., de Hoog S. A case of disseminated sporotrichosis caused by Sporothrix brasiliensis. Med Mycol Case Rep. 2018;21:34–36. doi: 10.1016/j.mmcr.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galhardo M.C.G., Silva M.T.T., Lima M.A., Nunes E.P., Schettini L.E.C., De Freitas R.F., Paes R.D.A., Neves E.D.S., Do Valle A.C.F. Sporothrix schenckii meningitis in AIDS during immune reconstitution syndrome. J. Neurol. Neurosurg. Psychiatry. 2010;81:696–699. doi: 10.1136/jnnp.2009.173187. [DOI] [PubMed] [Google Scholar]
- 13.Hessler C., Kauffman C.A., Chow F.C. The upside of bias: a case of chronic meningitis due to Sporothrix schenckii in an immunocompetent host. Neurohospitalist. 2017;7:30–34. doi: 10.1177/1941874416641468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lima M.A., Vallier R., Silva M.M. Sporothrix brasiliensis meningitis in an immunocompetent patient. Pract Neurol. 2021;21:241–242. doi: 10.1136/practneurol-2020-002915. [DOI] [PubMed] [Google Scholar]
- 15.Mialski R., De Oliveira J.N., Da Silva L.H., Kono A., Pinheiro R.L., Teixeira M.J., Gomes R.R., De Queiroz-Telles F., Pinto F.G., Benard G. Chronic meningitis and hydrocephalus due to Sporothrix brasiliensis in immunocompetent adults: a challenging entity. Open Forum Infect. Dis. 2018;5 doi: 10.1093/ofid/ofy081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva-Vergara M.L., Maneira F.R.Z., De Oliveira R.M., Santos C.T.B., Etchebehere R.M., Adad S.J. Multifocal sporotrichosis with meningeal involvement in a patient with AIDS. Med. Mycol. 2005;43:187–190. doi: 10.1080/13693780500035904. [DOI] [PubMed] [Google Scholar]
- 17.Warren A.M., Grossmann M., Christ-Crain M., Russell N. Syndrome of inappropriate antidiuresis: from pathophysiology to management. Endocr. Rev. 2023;44:819–861. doi: 10.1210/endrev/bnad010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zunt J.R., Baldwin K.J. Chronic and subacute meningitis. CONTINUUM: Lifelong Learning in Neurology. 2012;18:1290–1318. doi: 10.1212/01.CON.0000423848.17276.21. [DOI] [PubMed] [Google Scholar]
- 19.Scott E.N., Kaufman L., Brown A.C., Muchmore H.G. Serologic studies in the diagnosis and management of meningitis due to Sporothrix schenckii. N. Engl. J. Med. 1987;317:935–940. doi: 10.1056/NEJM198710083171505. [DOI] [PubMed] [Google Scholar]
- 20.Bernardes-Engemann A.R., Orofino Costa R.C., Miguens B.P., Penha C.V.L., Neves E., Pereira B.A.S., Dias C.M.P., Mattos M., Gutierrez M.C., Schubach A., Oliveira Neto M.P., Lazéra M., Lopes-Bezerra L.M. Development of an enzyme-linked immunosorbent assay for the serodiagnosis of several clinical forms of sporotrichosis. Med. Mycol. 2005;43:487–493. doi: 10.1080/13693780400019909. [DOI] [PubMed] [Google Scholar]