Abstract
Background:
As liver surgery continues to evolve, be it open, laparoscopic or robotic, it remains a procedure that can deteriorate in the blink of an eye. Liver surgery in patients with hepatoma is further complicated, as the vast majority have significant fibrosis, if not cirrhosis. Thus, parenchymal sparing resection is increasingly necessary. Effective and safe intracorporeal mobilization of the liver is essential for minimal access parenchymal-sparing and conventional resection.
Methods:
This retrospective review of over 150 cases performed provides a hands-on approach to laparoscopic hepatic mobilization with the use of an inexpensive technique using a 1” packing tape to “Sling” the liver in-order to divide the ligaments holding the liver in place and optimally position the liver for parenchymal transection.
Results:
Use of a 1” packing tape to “Sling” the liver intracorporeally is demonstrated to enable mobilization of the liver for tissue sparing non-anatomic, anatomic and major resections.
Conclusion:
Use of a 1” packing tape to “Sling” the liver intracorporeally can facilitate mobilization for resection. Surgeons hoping to master minimal access resection should also be well versed in the use of laparoscopic ultrasound and liver transplant “Piggyback” technique.
Keywords: Intracorporial liver mobilization, Laparoscopic liver resection
INTRODUCTION
Laparoscopic liver surgery has progressed significantly since the “International position on laparoscopic liver surgery during 2008.1 Planning for any liver resection requires a comprehensive review of body imaging, CAT scan or MRI, in order to use an organized, efficient approach to mobilize the liver and to avoid misadventure from aberrant anatomy or vasculature.2,3 To mobilize either lobe of the liver laparoscopically, one needs to recreate those actions used during an open case, with far less room, and less haptic sense.4,5 However, laparoscopy also allows visualization of multiple ligaments holding the liver in place, from angles not available during an open approach potentially resulting in an outcome benefit.6
METHODS
During mobilization for an open resection, the surgeon is often gently pulling the liver anteriorly or inferiorly in order to access and divide the triangular and coronary ligaments. Over the last decade, as experience was gained during resections with laparoscopic liver surgery from left lateral segmentectomy, segment 6 resection, posterior sectionectomy, segment 8 resection, left lobectomy, right lobectomy, caudate lobectomy, and isolated segment 7 resection.7 These actions were recreated laparoscopically using a sling made from 1-inch packing tape to manipulate, roll, and retract the liver anteriorly and inferiorly as needed. The sling was first used for left lateral segmentectomy then left hepatic lobectomy. Pulling the left lobe of the liver away from the diaphragm is understandably easier, as the left triangular and coronary ligaments are narrow and attached to the posterior cephalad portion of the diaphragm. The right lobe, however, is far more challenging, as the right coronary ligament is broad, extends posterior inferiorly from the diaphragm, and along the vena cava. The dome of the right lobe also can be an obstacle due to its size, limited space, and access posteriorly, particularly in patients with a narrow chest cavity. To maneuver the liver, something was needed that would be flexible, strong, and sleek. After going through approximately a dozen cases with bulky retraction devices, it was not until an umbilical tape was used to laparoscopically “Pringle” the liver that it was thought to “sling” an umbilical tape into the fissure of the ligamentum teres, extending through to the fissure of the ligamentum venosum, wrapping around the left lobe at the border of segments 2 and 3, with segment 4. It was also realized a sling could be used to move the liver in all directions. As more cases were performed, particularly for fatty livers, the umbilical tape proved too narrow, causing tears in the liver capsule. Use of 1-inch packing tape proved to have more grip, less likely to slip, tear, or cut into the liver. This is a descriptive review of how the “sling technique” can be used to mobilize the liver optimal parenchymal transection.
RESULTS
In general, we use a 5-mm, 30° angled laparoscope to start. While the 5-mm scope allows more flexibility in terms of placement, one needs to be aware that a nondigital 5-mm scope can be delicate and has more variability in visual quality from scope to scope. Although there are several ways to place laparoscopic ports, mobilization is performed using a 5-mm 30° laparoscope, and then the parenchymal transection is performed with a 10-mm 30° laparoscope. Thus, during mobilization, this allows easy switching of the 5-mm laparoscope from port to port, reducing operative time. It should be mentioned with the later generation operative robot, the scope can be switched from port to port, although using an 8-mm port and still takes more time.
It is important to remember port placement is dependent on anticipating where the parenchymal transection line will be. During transection, the laparoscope needs to be focused directly into the transection plan (Figure 5). The surgeon needs to give this considerable thought, because if bleeding occurs, the surgeon needs to see clearly into the transected liver to quickly control bleeding. To minimize bleeding, depending on the type of resection, a Pringle maneuver can be used.
Figure 5.
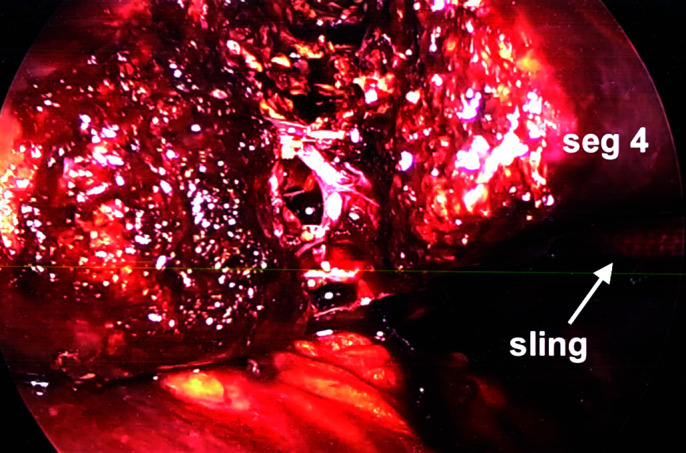
Parenchymal transection left lobectomy with sling on gentle traction. Gentle traction using sling opens parenchymal transection plane for left lobectomy using ultrasonic irrigator and aspirator. (Misonix)
Left Lateral Segmentectomy
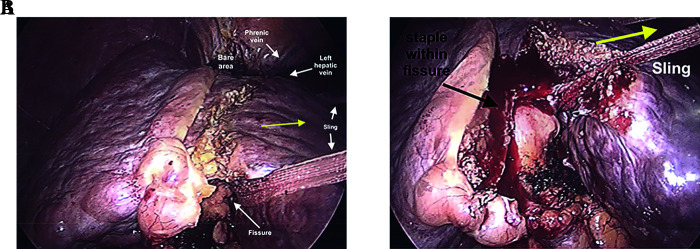
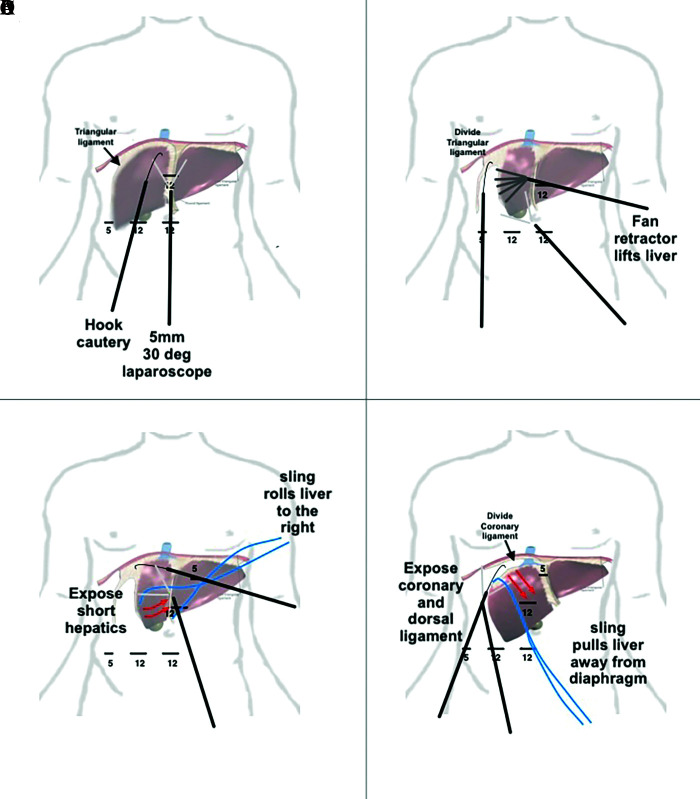
The left lateral segmentectomy is the easiest of liver resections and can be performed with 3 ports.8 After the bare area is divided to the extent one visualizes the middle hepatic vein, the sling is secured using the fissure of the ligamentum venosum and teres, then pulled laterally. The left triangular and coronary ligament are divided until the left hepatic vein is visualized where the phrenic vein ends (Figure 1A). To resect the left lateral segment, parenchymal transection can be performed anteriorly to about 2–3 cm deep with multiple energized cutting devices. An endovascular stapling device, using a 60-mm vascular load, is then placed in the fissure of the ligamentum venosum and teres, and fired. Two to four loads are usually required to resect the left lateral segment along the fissure (Figure 1B). Dissection of the left hepatic vein is usually not necessary, unless the tumor abuts it, and usually can be divided with the endovascular stapling device with ultrasound guidance. For a laparoscopic left lateral segmentectomy, a Pringle maneuver is not necessary. Figure 2 details the steps for left lobe mobilization used for left lateral segmentectomy and left lobectomy.
Figure 1.
Use of 1” sling for left lateral segmentectomy. (A) After division of the left triangular ligament, a 1” sling is passed along the fissure for ligmentum teres, extending through to the fissure for the ligamentum venosum. (B) Using an energy device to transect 1-2 cm of liver tissue, an endoscopic stapling device can be used to divide the structures within the fissure.
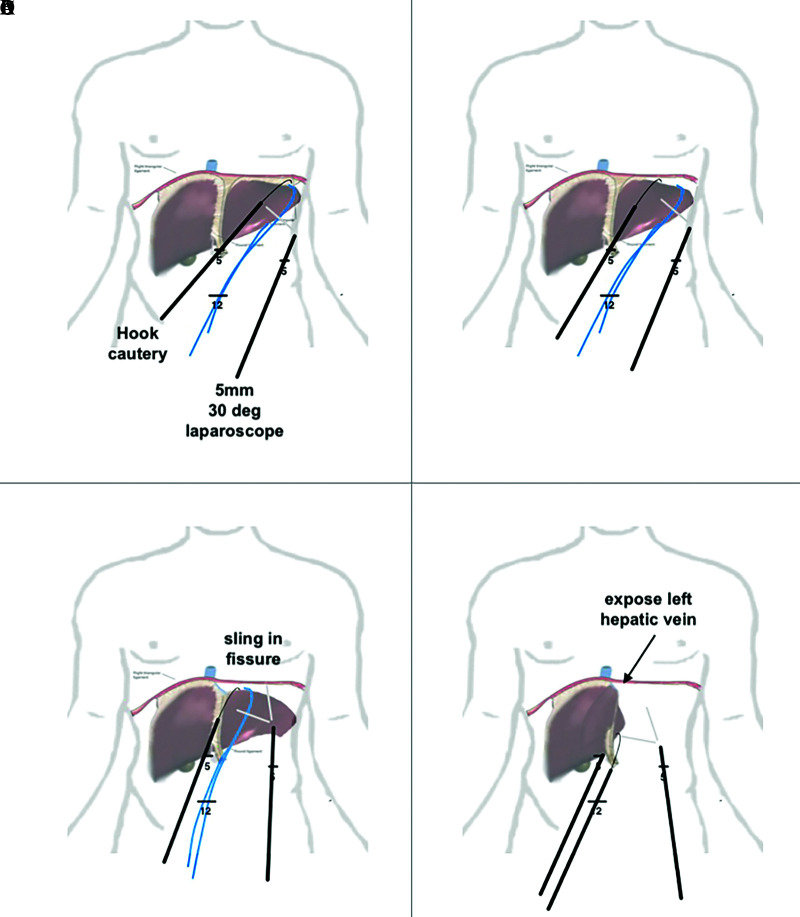
Figure 2.
Stepwise approach to mobilize left lobe of liver. (A) division of the right triangular ligament with a hook cautery visualized using a 30° 5-mm laparoscope. One can also use an umbilical tape or 1-inch packing tape to pull the liver away from the diaphragm. (B) extension of the dissection into the left coronary ligament. (C) exposure of the left hepatic vein. (D) liver being flipped anteriorly to right, dividing posterior coronary ligament.
Left Lobe Mobilization
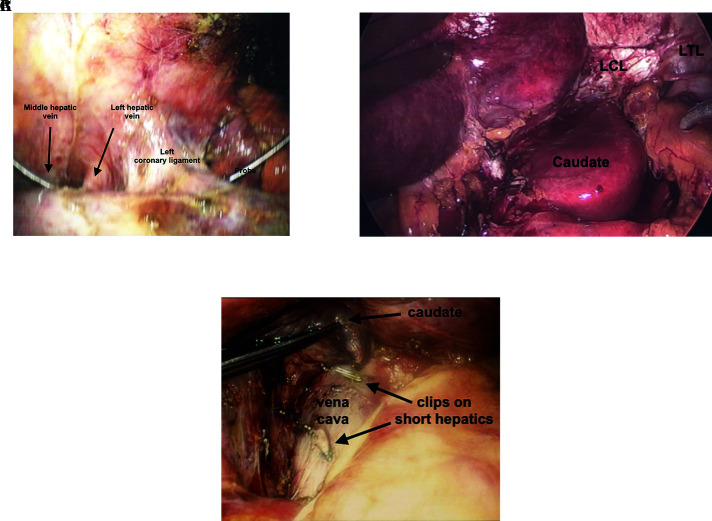
As the left lobe is usually less bulky and more flexible than the right, it can be retracted such that the left hepatic vein can be accessed anteriorly or posteriorly with relatively little effort. After the bare area is separated, the left coronary and triangular ligaments divided anteriorly, the left hepatic vein can be visualized (Figure 3A). This will also depend on whether the middle and left hepatic vein are fused or separate. Presurgical review is essential, and a laparoscopic ultrasound should be used to assess left hepatic vein prior to dissection. The left lateral segment can be reflected anteriorly to the right, exposing the caudate lobe posteriorly. As the caudate lobe is followed to the diaphragm, the left coronary ligament (LCL) is divided with a hook cautery (Figure 3B). Depending on the tumor location, it may be necessary to separate the caudate lobe from the vena cava and include with the surgical specimen (Figure 3C). One can use 5- or 10-mm clips.
Figure 3.
Division of left coronary ligament. (A) Exposure of left hepatic vein adjacent to left coronary ligament with probe between middle and left hepatic vein. (B) Exposure and division of posterior left triangular (LTL) and coronary ligament (LCL). This allows full visualization of the fissure, as well as can be used to mobilize the and resect the caudate lobe. (C) Caudate lobe can be lifted off vena cava after division of short hepatic veins with laparoscopic regular and micro clips.
Left Hepatic Lobectomy
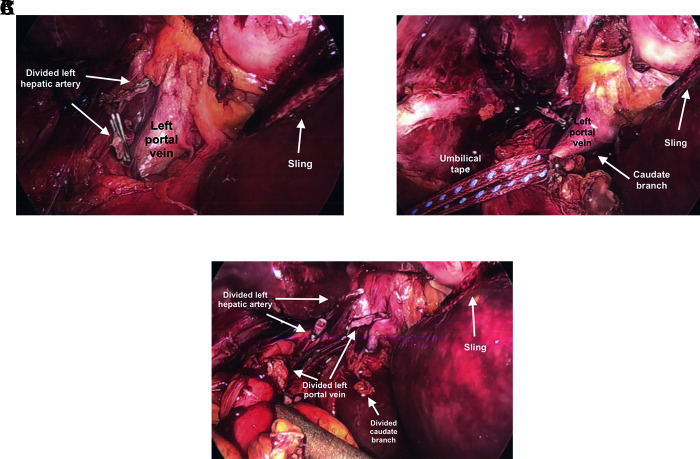
For a left hepatic lobectomy, laparoscopic hilar dissection can usually be facilitated by tacking the round ligament to the anterior abdominal wall at the level of the xiphoid and using a fan retractor to push the quadrate lobe anterior-cephalad, if needed. Dissection of the left hepatic artery and left portal can then be performed. The left hepatic artery is divided between 2–3 hemoclips proximally and distally (Figure 4A). The left portal vein is dissected circumferentially and freed. Umbilical tape is then passed around the left portal vein; gentle traction is applied (Figure 4B). The vein is divided with a 35- to 45-mm endovascular stapling device using a vascular load (Figure 4C). Using the 1-inch packing tape, a slip knot is used to wrap around the fissure of the ligamentum teres and venosum and gentle traction is applied laterally to the left. Using ultrasound guidance, parenchymal transection is then performed. As long as the middle and left hepatic veins are not involved by tumor, segment 4 can usually be pulled away from the right lobe to facilitate transection (Figure 5). To maintain traction, a Kelly clamp can be used to maintain traction on the packing or umbilical tape (Figure 6C).
Figure 4.
Laparoscopic hilar dissection for left lobectomy. (A) Divided left hepatic artery and exposure of left portal vein. Sling pulling left lateral segment. Traction on hilar plate allows easier dissection of left portal vein. (B) Umbilical tape applying traction to left portal vein prior to dividing. One needs to be mindful of caudate lobe branch off of left portal vein. (C) Divided left portal vein and caudate lobe branch.
Figure 6.
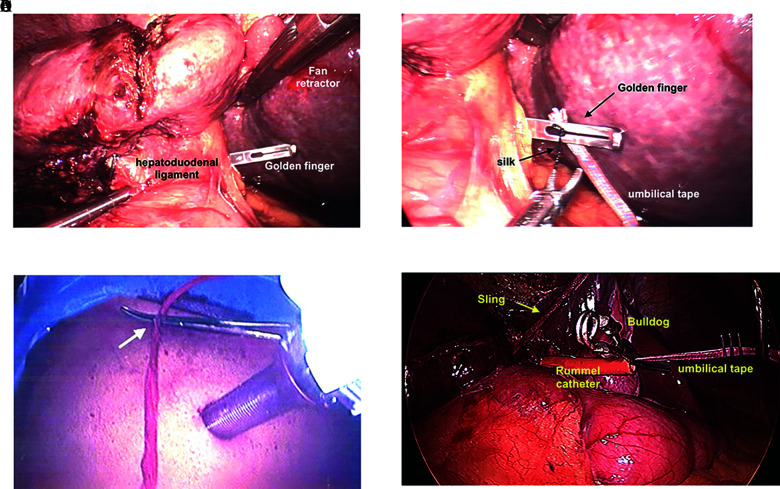
Laparoscopic “Pringle Maneuver.” (A) Golden finger is passed through the Foramen of Winslow. (B) 0 silk is tied to the umbilical tape and passed through the tip of the Golden finger. The umbilical tape is then pulled through the Foramen of Winslow and both ends are externalized. (C) Umbilical tape or sling through externalized through small stab and secured with a Kelly clamp to provide traction. (D) Umbilical tape is fed through a trimmed Rummel catheter, externalized through a stab wound. Sling is set up for right lobectomy (yellow arrow) and laparoscopic “Bulldog” is used to sinch down the Rummel catheter over the umbilical tape tourniqueting the hepatoduodenal ligament, causing inflow control.
Pringle Maneuver
To laparoscopically set up a “Pringle,” a “Golden Finger” is passed through the “Foramen of Winslow” and poked through the “Gastrohepatic Ligament” (Figure 6A). An umbilical tape is threaded through the tip and pulled through.9 To assist with threading the umbilical tape through the hole at the tip of the “Golden Finger,” a 0 silk is tied to the end of the umbilical tape, allowing easier passage through the hole at the tip of the “Golden. Finger” (Figure 6B). The ends of the umbilical tape are passed through a half-length Rummel (on the narrow side), and the ends of the umbilical tape are externalized through a 2-mm stab wound inferior to the lowest rib at the anterior axillary line. To “Pringle,” a laparoscopic Bull Dog is placed on the umbilical tape, synched down so the loop of umbilical tape around the hepatoduodenal is constricted to control inflow (Figure 6D). The externalized umbilical tape is gently held taught to stabilize and pull the hilum to the right or left as needed to expose the confluence of the right and left hepatic ducts and vascular structures. For right lobe resections, during transection, the right hilar branches are better exposed when the hepatoduodenal ligament is pulled to the left. This can be aided using indocyanine-green. The umbilical tape is controlled by applying gentle tension, then the umbilical tape is clamped flush to the skin (Figure 6C). The tension provided by the umbilical tape facilitates exposure of the hepatic duct allowing safer division. The right portal vein is divided with a 35- to 45-mm endoscopic stapling device using a vascular load and right hepatic artery between doubled clips.
Right Lobe Mobilization
Figures 7A–7D summarize the steps required to mobilize the right lobe of liver. There are 4 basic positions for the laparoscope and instruments commonly used to mobilize the liver. An extended approach can also be used to perform mobilization of the right lobe for segment 7 resection.10 To start, the 5-mm laparoscope is placed through the infraumbilical midline port. The falciform ligament is divided, extending into the bare area anteriorly until the middle and left hepatic veins are exposed anteriorly (Figure 7A). As the surgeon works towards the right, strict attention should be paid to the right phrenic vein, as it tracks to the vena cava and alerts the surgeon that the right hepatic vein is in close proximity. The right hepatic vein can usually be mobilized extrahepatically. One then partially divides the left coronary and triangular ligament. Due to the limited space during laparoscopy, in addition to pulling the right lobe of the liver away from the diaphragm and kidney, the liver may also need to slide to the left, such that the left lateral segment slides over the spleen. To fully slide the left lobe of the liver over the spleen, complete division of the left triangular ligament may be required.
Figure 7.
Stepwise approach to mobilize right lobe of liver. (A) division of round, falciform ligaments, and separation of bare area. (B) lift and rotate liver to the left using 10mm fan retractor and divide right triangular and coronary ligaments. (C) slide sling over right lobe and retract liver to the left exposing right coronary ligament along vena cava. (D) slide sling over dome of liver and retract inferiorly exposing coronary ligament and right hepatic vein.
Once the falciform ligament and anterior bare area are divided, a 10-mm articulating fan retractor is placed through an upper midline 12-mm port, placed posterior laterally to the right lobe and gently lifted (Figure 7B). This allows visualization and division of the right triangular ligament and inferior lateral aspect of the right coronary ligament. One needs to be aware of the right adrenal gland to avoid unnecessary bleeding when working towards the vena cava. As the liver is lifted anteriorly and rotated to the left, a hook cautery is used to divide the right triangular ligament laterally (Figures 8A and 8B). The motion of the hook cautery is to pull the hook out of the port with power on, and not swiping left to right or right to left. This becomes particularly relevant when working near the posterior dome of liver and along the vena cava. In these tight spaces, swiping left to right can result in a hole in the diaphragm and swiping right to left can result in a hole in the vena cava.
Figure 8.
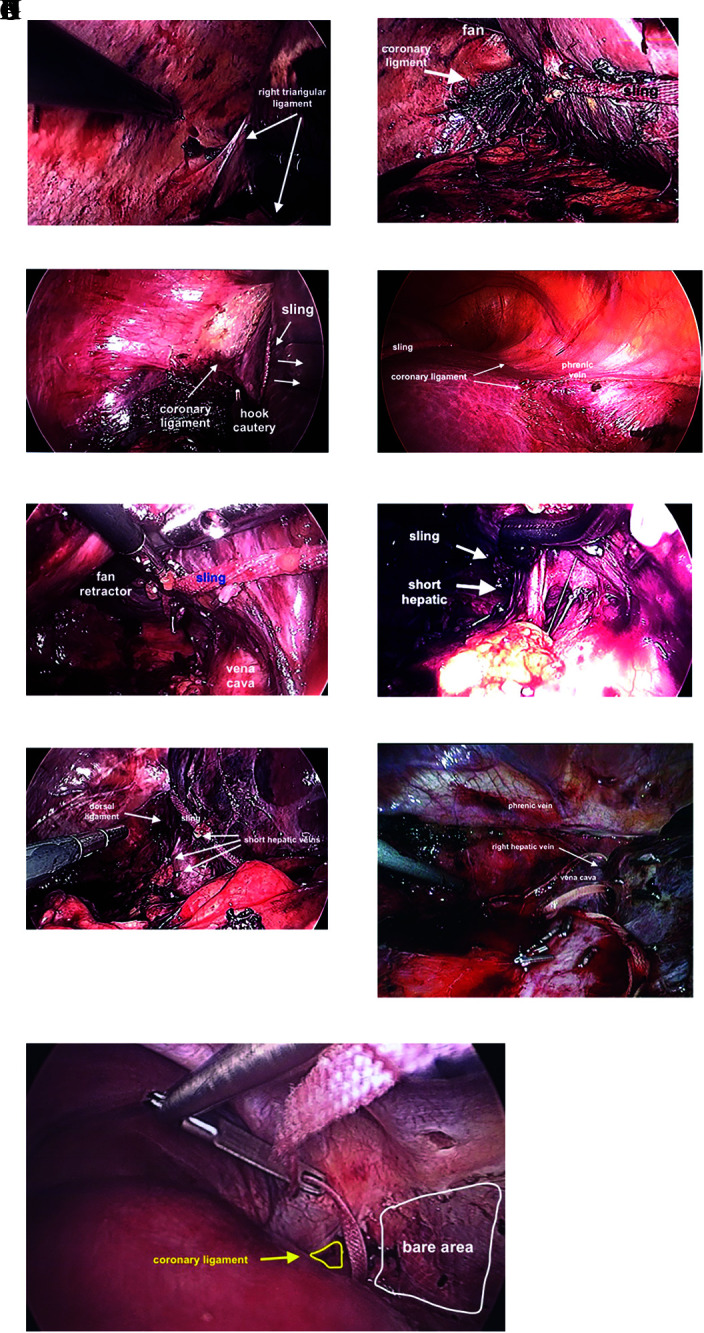
Mobilization of right lobe. (A) Right triangular ligament prior to dividing with a hook cautery. (B) Liver mobilized to the left facilitated with sling, fan retractor and division of coronary ligament. (C) Dome of liver retracted inferiorly opening coronary ligament aided by sling. (D) Division of anterior coronary ligament aided by retraction of liver to towards left foot aided by sling. (E) The “Batcave,” after coronary ligament divided as much as possible, the right lobe can be lifted off the vena cava aided by a fan retractor and sling allowing visualization the division of short hepatic branches. Care should be taken to prevent the sling from sliding into vena cava and tear a short hepatic vein. (F) Exposure short hepatic vein with right angle clamp. (G) Dorsal hepatic ligament prior to division. (H) Dorsal hepatic ligament after division, allowing full release of right lobe of liver. (I) Golden finger used to feed umbilical tape between right and middle hepatic veins.
Once the dome of the liver is well visualized and free laterally, 3 feet of 1-inch packing tape is placed intra-abdominally. Depending on the length of the right lobe and space available posterior to the rib cage, one can continue to roll the liver to the left with the fan retractor or wrap the 1-inch tape around the right lobe of the liver at the interface between segments 7/8 and 5/6 and externalize the free ends through a small (2–3 mm) incision at the left midclavicular line (Figure 7C). Depending on the shape of the rib cage, the stab is usually inferior to the lowest rib, or through the intercostal space between ribs 10 and 11, or 11 and 12. If possible, the 1-inch packing tape should lay within the renal impression. Using gentle traction on the 1-inch tape, the liver will slide and/or rotate to the left, further exposing the coronary ligament laterally. Retraction on the 1-inch tape is particularly useful in patients with a shallow and/or narrow chest cavity. Although one can use a 10-mm articulating fan retractor, it occupies a potential working port and has to be stabilized by a bulky external fixator or a surgical assistant that may lose positioning. The sling has the advantage of being less bulky, and it can be set in place by clamping the tape as it exits the skin incision (Figure 6C).
Using the 5- and 12-mm right lateral ports at the level of the umbilicus, the sling is placed over the dome of the liver and externalized alongside the upper midline abdominal or umbilical port (Figure 7D). Traction is applied slowly towards the left foot. The coronary ligament is spread apart and easily divided posteriorly (Figure 8C).
To divide the anterior superior portion of the right coronary ligament, a 5-mm 30° port is placed along the left side of the xiphoid process and the laparoscope is moved the 12-mm upper midline port (Figure 7C). A hook cautery is used to divide the anterior superior portion of the coronary ligament attached to the dome of the liver. During division of the anterior superior portion of the right coronary ligament, the phrenic vein again is used to gauge proximity to the right hepatic vein (Figure 8D). This can be the most difficult part of the mobilization, however using the 1-inch sling to retract the liver towards the left foot allows the dome of the liver to separate from the diaphragm, providing visualization and further mobilizing the right lobe. The surgeon may need to go back and forth between the lower lateral and upper midline ports to fully mobilize the right lobe.
Once the dome is free, it is easier to lift the liver away from the vena cava and begin to divide the short hepatic branches. Here a detailed assessment of preoperative imaging is critical here to avoid avulsing and or cutting a short or accessory right hepatic vein. As the right triangular and coronary ligaments are divided, segment 6 may flop posteriorly obstructing the surgeon’s view. This can be particularly annoying at first, as one has to essentially work in a cave, which we now affectionately refer to as the “Bat cave” (Figure 8E). While the liver is retracted anteriorly to the left, short hepatic veins can be visualized, isolated, and divided (Figure 8F).
After the right triangular, lateral, and superior portion of the coronary ligament is divided, the inferior portion of the coronary ligament is divided along the vena cava. As the coronary ligament approaches the right hepatic vein, it thickens as it approaches the dorsal ligament of the right lobe (Figure 8G). The dorsal ligament can have a sizable vein within and bleed vigorously if cut. Thus, the dorsal ligament should be divided between large endoclips, sutured directly, or stapled using an endovascular stapling device (Figure 8H). Division of the dorsal ligament is a key maneuver to fully mobilize the right lobe of the liver and usually divided after the short hepatic branches. Division of the dorsal hepatic ligament also allows better visualization and control of the right hepatic vein for lobectomy or isolated resection of segment 7. After the dorsal ligament is divided, the dome of the liver should be fully mobile.
To isolate and control the right hepatic vein a “Golden finger” is used to pass an umbilical tape between the right and middle hepatic veins (Figure 8I). To do so requires bouncing the “Golden finger” ever so gently through the space between the right and middle hepatic veins. The surgeon usually takes a deep breath, sets an intention, then gently creates a tunnel between the veins hugging the posterior surface of the liver. This is obviously easier for an extrahepatic right vein. It should be noted, fully exposing the right hepatic vein anteriorly facilitates creation of the tunnel and safer passage of the umbilical tape between the veins.
Use of the Sling to Facilitate Parenchymal Transection
Depending on the type of resection, a sling can be used to facilitate parenchymal transection via a “Hanging maneuver.”11,12 After full mobilization of the right lobe, for a right lobectomy, after the umbilical tape is passed between the right and middle hepatic vein, the umbilical tape can be externalized through the peri-xiphoid and inferior ports such that the liver flops over the tape as it lies anterior to the vena cava (Figure 9A). As the liver parenchyma is transected anteriorly, gravity causes an open book effect, widening the transection line (Figure 9B). Depending on where the liver needs to be retracted (pulled), the sling is pulled from right to left by externalizing the ends through a 2- to 3-mm stab along the left mid clavicular line. Manipulating the sling can rotate and elevate the liver, allowing better access and visualization for tissue transection, particularly of the posterior segments (Figures 9C and 9D). The transection technique can vary depending on the operator’s choice and whether the liver is normal, fatty, fibrotic, or cirrhotic.
Figure 9.
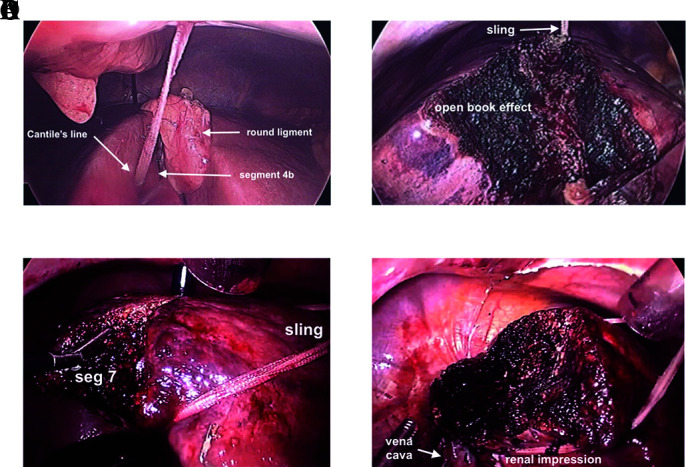
Intracorporeal sling of liver to facilitate tissue transection. (A) Right lobe sling prior to tissue transection within Cantile’s line after gallbladder removed. (B) Right lobe sling during tissue transection, open book effect. (C) Sling over dome, under renal impression, pulled counterclockwise, lifting liver allowing gravity to assist with tissue transection of segment 7. (D) Completed resection of segment 7.
DISCUSSION
In patients with hepatoma, the vast majority have significant fibrosis or cirrhosis. With the onset of fatty liver disease, hepatomas are more commonly observed in the setting fatty liver with fibrosis. The firmness, or softness of the liver affects the difficulty of laparoscopic mobilization. Fibrotic livers tend to be smaller, firmer, and more durable; fatty livers tend to be larger, softer, less durable, and more likely to crack. One needs to consider the shape of the chest, with narrow chests having less room to work. When instituted effectively, the sling not only is used to position the liver, but also counter force during liver transection, as described for open technique. Whether open or closed, adequate mobilization of the liver prior to resection allows for safer tissue transection, helps expose the hilum, and allows for control of unexpected bleeding. Anticipating an abnormal or fibrotic liver, tissue-sparing resection should be considered, if at all possible. That being said, it is equally important to recognize when hand-assist expedites a case, or when a laparoscopic approach offers no significant benefit.
Footnotes
Acknowledgements: Dr. Baburao Koneru for assistance with manuscript review.
Disclosure: none.
Funding sources: none.
Conflict of interests: none.
Informed consent: not applicable.
Contributor Information
Andrew N. de la Torre, CarePoint Health, Hudson County, New Jersey, USA.; New Jersey Medical School, Newark, New Jersey, USA.
Justin Adibi, CarePoint Health, Hudson County, New Jersey, USA.; Rowan School of Medicine, Stratford, New Jersey, USA.
Zaineb Zubair, Rowan School of Medicine, Stratford, New Jersey, USA..
References:
- 1.Buell JF, Cherqui D, Geller DA, et al. ; World Consensus Conference on Laparoscopic Surgery. The international position on laparoscopic liver surgery: the Louisville Statement, 2008. Ann Surg. 2009;250(5):825–830. [DOI] [PubMed] [Google Scholar]
- 2.Lamadé W, Glombitza G, Fischer L, et al. The impact of 3-dimensional reconstructions on operation planning in liver surgery. Arch Surg. 2000;135(11):1256–1261. [DOI] [PubMed] [Google Scholar]
- 3.Björnsson B, Lundgren L. A personal computer freeware as a tool for surgeons to plan liver resections. Scand J Surg. 2016;105(3):153–157. [DOI] [PubMed] [Google Scholar]
- 4.Ielpo B, Pittau G, Ciacio O, et al. Standardized laparoscopic right hepatic lobe mobilization. J Hepatobiliary Pancreat Sci. 2022;29(5):e30–e32. [DOI] [PubMed] [Google Scholar]
- 5.Choi SH, Choi GH, Han DH, Kwon SW, Choi JS. Laparoscopic right hepatectomy: toward protocolization and simplification. Ann Surg Oncol. 2017;24(2):554–555. [DOI] [PubMed] [Google Scholar]
- 6.Heinrich S, Tripke V, Huber T, Mittler J, Lang H. A match-pair analysis of open versus laparoscopic liver surgery. JSLS. 2017;21(4):e2017.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goh BKP, Han HS, Chen KH, et al. ; International Robotic and Laparoscopic Liver Resection Study Group Investigators. Defining global benchmarks for laparoscopic liver resections: an international multicenter study. Ann Surg. 2023;277(4):e839–e848. [DOI] [PubMed] [Google Scholar]
- 8.Sugawara T, Hashimoto M, Shindoh J. Laparoscopic left lateral sectionectomy: a three-port method. J Minim Access Surg. 2020;16(3):220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.SteinbrÜck K, Fernandes R, D'Oliveira M, et al. External pringle maneuver in laparoscopic liver resection: a safe, cheap and reproducible way to perform it. Arq Bras Cir Dig. 2021;33(4):e1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mashchenko I, Trtchounian A, Buchholz C, de la Torre AN. A sling technique for laparoscopic resection of segment seven of the liver. JSLS. 2018;22(2):e2018.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbassi F, Villiger RM, Villiger P. Laparoscopic liver-hanging maneuver for right hepatectomy - with video. J Visc Surg. 2017;154(4):295–296. [DOI] [PubMed] [Google Scholar]
- 12.Belghiti J, Guevara OA, Noun R, Saldinger PF, Kianmanesh R. Liver hanging maneuver: a safe approach to right hepatectomy without liver mobilization. J Am Coll Surg. 2001;193(1):109–111. [DOI] [PubMed] [Google Scholar]