Abstract
The development of the cardiovascular system and the development of the early hematopoietic systems are closely related, and both require signaling through the Tie2 receptor tyrosine kinase. Although endothelial cells and hematopoietic cells as well as their precursors share common gene expression patterns during development, it remains completely unknown how Tie2 signaling coordinately regulates cardiovascular development and early hematopoiesis in vivo. We show here that mice with a targeted mutation in tyrosine residue 1100 in the carboxyl-terminal tail of Tie2 display defective cardiac development and impaired hematopoietic and endothelial cell development in the paraaortic splanchnopleural mesoderm similar to that seen in Tie2-null mutant mice. Surprisingly, however, unlike Tie2-null mutant mice, mice deficient in signaling through this tyrosine residue show a normal association of perivascular cells with nascent blood vessels. These studies are the first to demonstrate the physiological importance of a single tyrosine residue in Tie2, and they suggest that multiple tyrosine residues in the receptor may coordinate cardiovascular development and early hematopoietic development.
The development and integrity of the early cardiovascular system are essential for embryonic growth and survival. The formation of the primitive heart and primary vascular plexus involves the in situ differentiation of endothelial cells from mesenchymal precursors, a process known as vasculogenesis (39). In developing blood vessels, vasculogenesis is followed by angiogenesis, a process consisting of expansion or remodeling of preexisting blood vessels by sprouting, intercalated, or intussusceptive growth. Periendothelial support cells, such as pericytes, and smooth muscle cells then are recruited to the nascent vessels to surround the endothelial tubes and stabilize the vessels (4). During heart development, the primitive myocardial and endocardial rudiments interact during differentiation of the heart, resulting in the formation of complex myocardial trabeculations filling the ventricle (20). The development of the early hematopoietic system is intimately related to angiogenesis (24), suggesting the possible existence of a common progenitor cell, the hemangioblast, that can give rise to both endothelial cells and hematopoietic stem cells (HSCs) (8, 15).
The cellular events involved in cardiovascular development and hematopoiesis are tightly regulated processes controlled by paracrine signals, many of which are initiated by the binding of growth factor ligands to their cognate transmembrane receptor tyrosine kinases (RTKs) expressed on the surfaces of both endothelial and hematopoietic cells (49, 53). Tie1 and Tie2 are members of a family of RTKs expressed on both endothelial and hematopoietic cells. Although the ligand for Tie1 remains to be identified, the angiopoietins (Ang1 to Ang4) modulate Tie2 kinase activity specifically. Interestingly, these ligands appear to have opposing actions on Tie2 activation, as Ang1 and Ang4 stimulate theautophosphorylation of Tie2, while Ang2 and Ang3 can inhibit this phosphorylation in certain cellular contexts (6, 28, 50). Specifically, Ang2 blocks the ability of Ang1 to activate Tie2 in endothelial cells (28), while both Ang1 and Ang2 can activate Tie2 expressed in hematopoietic precursor cells (41).
The disruption of Tie2 receptor signaling results in embryonic lethality by embryonic day 9.5 (E9.5) to E12.5 as a consequence of vascular hemorrhage and impaired cardiac development, with few myocardial trabeculations and retraction of the endocardial lining from the myocardial wall (7, 35, 42, 44). In addition, embryos lacking Tie2 exhibit angiogenic remodeling defects, a lack of recruitment of periendothelial cells, and impaired hematopoiesis in the paraaortic splanchnopleural mesoderm (P-Sp) region (7, 35, 42, 46). Chimeric analysis has shown that HSCs deficient in both Tie1 and Tie2 fail to be maintained in the adult microenvironment (37); more recent studies have demonstrated that signaling through Tie2 is essential for the maintenance of HSCs in the quiescent state in the adult bone marrow, possibly through its role in inducing the adhesion of stem cells to osteoblasts (1). Together, these observations strongly support an intimate relationship between hematopoietic and endothelial cell developmental functions and the dual role of Tie2 in these processes.
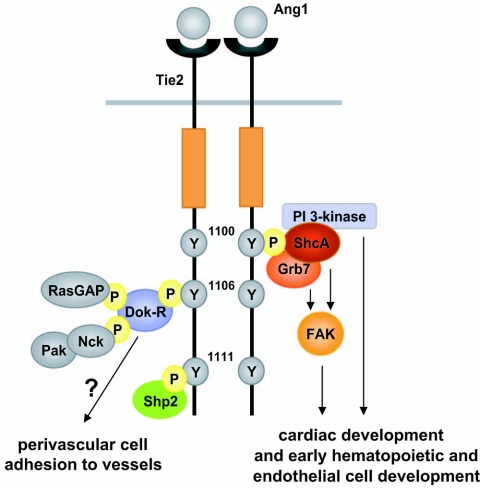
Insight into the molecular mechanisms that control the function of Tie2 has been provided by the identification of a series of signaling proteins that associate with the cytoplasmic tail of the activated receptor. The autophosphorylation of distinct tyrosine residues on RTKs following ligand binding allows the recruitment of downstream signaling molecules via their Src homology 2 (SH2) and phosphotyrosine binding domains (36). The cytoplasmic tail of Tie2 contains three conserved tyrosine residues which appear to function as autophosphorylation sites for the recruitment of downstream signaling proteins. Tyrosine residue 1100 (Tyr1100) appears to be a major autophosphorylation site on Tie2; binding of the p85 regulatory subunit of phosphatidylinositol 3-kinase (PI3-kinase) to this site results in the activation of PI3-kinase, which in turn regulates endothelial cell survival and motility (11, 19, 22, 34). More recently, the ShcA adaptor protein was also shown to bind to this site via its SH2 domain, and the recruitment of ShcA to Tie2 was shown to affect endothelial cell migration and sprouting but not cell survival (2). An additional autophosphorylation site on Tie2, tyrosine residue 1106 (Tyr1106), mediates binding to the phosphotyrosine binding domain of docking protein Dok-R, which in turn becomes tyrosine phosphorylated to allow the assembly of a signaling complex with RasGAP and Nck/Pak; the latter potentiates Ang1-induced endothelial cell migration (17, 29). Finally, the Grb7 and Grb14 adaptor proteins associate with Tie2 via Tyr1100 and Tyr1106, respectively, and Shp2 binds to the receptor at tyrosine residue 1111; however, the functional significance of these interactions remains unclear (14, 19).
Although the binding sites of signal transduction molecules have been mapped to specific tyrosine residues on the Tie2 receptor, the unique roles of these individual tyrosine residues in the physiological and developmental functions of Tie2 remain completely unknown. In attempt to define the in vivo significance of particular signaling pathways emanating from Tie2, we generated mice harboring site-specific engineered mutations in Tie2. This approach provides a physiologically relevant context in which to examine the relationship between Tie2 and key signaling partners, because both ligands and receptors are expressed at normal levels and in the correct spatial and temporal patterns. In this study, we focused on Tyr1100 in Tie2, since this residue is an apparent major autophosphorylation site and its role in vitro has been well characterized.
To explore the possible physiological function of Tyr1100 in vivo, we generated cDNA “knock-in” mice, in which tyrosine 1100 was mutated to phenylalanine (Y1100F). Mice homozygous for this mutation (Tie2F1100/F1100) succumb to embryonic lethality at the same stage as Tie2-null embryos, with similar defects in the development of the heart and angiogenesis. Moreover, hematopoietic development and endothelial cell development in the P-Sp region are similarly impaired in Tie2F1100/F1100 embryos. Surprisingly, however, vascular integrity and perivascular cell adhesion to the nascent vessels in the head region are not affected in Tie2F1100/F1100 embryos. The present results suggest that Tie2 signaling emerging from Tyr1100 is selectively required for heart development as well as hematopoietic and endothelial cell development in the P-Sp region and that alternative tyrosine residues may be functionally required for specific aspects of vessel stability.
MATERIALS AND METHODS
Animals.
The Tie2 knock-in targeting constructs are depicted in Fig. 1A. The neo cassette was flanked by loxP sites for Cre-mediated excision. Site-directed mutagenesis to alter tyrosine residue 1100 to phenylalanine was performed with Tie2 cDNA, and the mutation was confirmed by DNA sequencing (17). Targeting constructs were linearized with KpnI and transfected into R1 embryonic stem (ES) cells (31) by electroporation. DNA from clones resistant to both G418 and ganciclovir was analyzed for homologous recombination by Southern blot hybridization. Subsequently, the neo cassette was excised from the targeted allele in the homologous recombinant ES clones by transient expression of Cre recombinase (pCAGGS-NLS-Cre). After screening of neo-excised ES clones by Southern blot hybridization, ES clones bearing wild-type Tie2 or Y1100F cDNA knock-in alleles were used for aggregation with CD1 morulas. Chimeric mice were crossed to CD1 mice, and germ line transmission was confirmed on the basis of PCR analysis of DNA extracted from tissues. PCR primers used for genotyping were as follows: wild-type allele, 5′-CCACGGTCTCTCCCAGGACTGA-3′ and 5′-ATAATGTGTCTGTGATCTGCAG-3′; and wild-type Tie2 or Y1100F cDNA knock-in allele, 5′-ACACTGTATGAGAAGTTTACCT-3′ and 5′-ATAATGTGTCTGTGATCTGCAG-3′. The Tie2-null mice used were described previously (7). Heterozygous wild-type Tie2 or Y1100F cDNA knock-in and Tie2-null mice were backcrossed with CD1 mice over six generations. Embryonic stages were determined according to the number of somites found. ShcA mutant mice were generated as described previously (26).
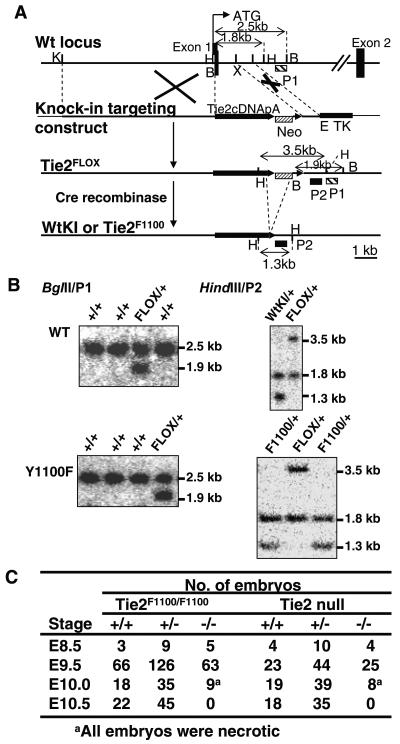
FIG. 1.
Generation of Tie2 cDNA knock-in mice expressing either wild-type or Y1100F Tie2. (A) Strategy for Tie2 knock-in mutation. The knock-in targeting construct contains either wild-type (Wt) or Y1100F Tie2 cDNA followed by a simian virus 40 poly(A) sequence, the phosphoglycerate kinase (PGK) promoter driving neomycin resistance (neo) (PGK-neo is flanked by loxP sites), and the thymidine kinase (TK) gene. The Tie2FLOX gene is generated by homologous recombination of the knock-in targeting construct and the mouse Tie2 locus. After exposure to Cre recombinase, the Tie2-WtKI or Tie2F1100 allele (bottom) is produced. K, KpnI; H, HindIII; B, BglII; X, XbaI; E, EcoRI. (B) (Left panels) BglII digests of targeted Tie2FLOX ES clones for wild-type (WT) or Y1100F Tie2. Probe P1, a 0.25-kb EcoRI-AccI genomic DNA fragment, was used. (Right panels) HindIII digest of targeted Tie2-WtKI or Tie2F1100 ES clones after excision of the neo cassette. Probe P2, a 0.8-kb XbaI-EcoRI fragment, was used. (C) Tie2F1100/F1100 and Tie2-null embryos succumb at a similar stage between E10.0 and E10.5.
Biochemical analysis.
Mutant Tie2 receptor cDNAs in pcDNA3.1 (17) were introduced into subconfluent human embryonic kidney 293T (HEK293T) cells by polyethyleneimine-based transfection. Tie2 immunoprecipitates were washed three times with PLC lysis buffer (18) and Western blotted with anti-Tie2 (clone 33; BD Pharmingen, San Diego, CA) or anti-phosphotyrosine (4G10; Upstate Biotechnology, Lake Placid, NY) antibodies. For the in vitro kinase assay, Tie2 immunoprecipitates were washed three times with PLC lysis buffer followed by three washes with kinase reaction buffer (50 mM HEPES [pH 7.5], 2.5 mM MgCl2, 4 mM MnCl2, 0.1 mM sodium orthovanadate). Kinase reactions were performed with kinase reaction buffer in the presence of 5 μCi of [γ-32P]ATP and 1 μg of glutathione S-transferase-Dok-R substrate (18) for 30 min at 37°C. Reactions were terminated by boiling with sodium dodecyl sulfate sample buffer, and reaction mixtures were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and exposed to film.
Immunohistochemical analysis.
The procedures for immunohistochemical analysis of whole embryos were described previously (54, 55). In brief, anti-PECAM1 antibody (rat anti-mouse monoclonal antibody MEC13.3; BD Pharmingen, San Diego, CA) or anti-c-Kit antibody (rat anti-mouse monoclonal antibody 2B8; BD Pharmingen) was developed with goat horseradish peroxidase-conjugated anti-rat immunoglobulin antibody (Biosource, Camarillo, CA). In the final step of staining, samples were soaked with phosphate-buffered saline containing 250 μg/ml diaminobenzidine (Sigma, St. Louis, MO) and 0.08% NiCl2 for 30 min, and hydrogen peroxide was added to 0.075% for the enzymatic reaction. For immunohistochemical analysis of P-Sp explants, cells were fixed with 4% paraformaldehyde in phosphate-buffered saline for 10 min at room temperature. Anti-PECAM1 antibody (MEC13.3) and Cy3-labeled donkey anti-rat immunoglobulin G (Jackson ImmunoResearch Laboratories, West Grove, PA) were used for staining.
Cell preparation and flow cytometry.
Single cell suspensions were prepared as described previously (32). In brief, E9.5 embryos were dissociated by incubation with 2.4 U/ml dispase II (Roche, Mannheim, Germany) for 20 min at 37°C and cell dissociation buffer (GIBCO-BRL, Rockville, MD) for 20 min followed by gentle pipetting. Large cell clumps were removed with nylon mesh. Cells were labeled with fluorescein isothiocyanate-conjugated antibody against PECAM1 (MEC13.3), mouse monoclonal antibody against Tie2 (clone 33), and APC-conjugated goat antibody against mouse immunoglobulin G (all from BD Pharmingen). Dead cells were excluded with propidium iodide.
In vitro culturing of P-Sp.
P-Sp culture conditions were described previously (46). In brief, P-Sp explants of E9.5 embryos were cultured on OP9 stromal cells in RPMI 1640 with 10% fetal calf serum (Wisent Inc., St. Bruno, Quebec, Canada) and 10−5 M β-mercaptoethanol supplemented with interleukin 6 (20 ng/ml) (Stem Cell Technologies, Vancouver, British Columbia, Canada), interleukin 7 (20 ng/ml) (R&D Systems, Minneapolis, MN), SCF (50 ng/ml) (R&D Systems, Minneapolis, MN), and Epo (2 U/ml) (Roche, Mannheim, Germany) at 37°C in humidified 5% CO2-containing air. On day 8 of culturing, hematopoietic cells were counted and stained with May-Grünwald Giemsa stain, and the remaining cells on the culture plates were fixed for immunostaining.
Electron microscopic analysis.
Freshly dissected E9.5 embryos were fixed with 2% glutaraldehyde (Canemco, Inc.) in 0.1 M Sorensen's phosphate buffer (pH 7.3) (Canemco, Inc.). Samples were washed with the buffer, postfixed with 1% osmium tetroxide in 0.1 M Sorensen's phosphate buffer, dehydrated in graded ethanols, and embedded in Spurr epoxy resin (Canemco, Inc.). Thin sections were cut with an RMC 6000 ultramicrotome, stained with uranyl acetate and lead citrate, and examined with a Philips CM 100 electron microscope (Philips Electron Optics).
RESULTS
Generation of mice with a mutant Tie2 receptor.
To investigate the physiological function of Tyr1100 in Tie2 signaling, gene targeting was performed to introduce a tyrosine to phenylalanine mutation at position 1100 (Y1100F) into the tie2 locus. The Y1100F mutation prevents the binding and/or activation of the p85 subunit of PI3-kinase, Grb7 and ShcA, but preserves the ability to phosphorylate Dok-R (2, 17, 19). The targeting vectors contained either the wild-type or Y1100F Tie2 cDNA followed by a simian virus 40 poly(A) sequence, a neomycin resistance gene (neo) flanked by loxP sites, and a thymidine kinase gene (Fig. 1A). Homologous recombination in R1 ES cells results in insertion of the Tie2 cDNA into the first coding exon of Tie2, thereby replacing the signal peptide encoding sequences and allowing the cDNA to be expressed from the endogenous Tie2 promoter. Doubly resistant clones that had undergone homologous recombination for wild-type or Y1100F cDNA knock-in were identified by Southern blotting and designated FLOX (Fig. 1B, left panels). The neo cassette was then excised by electroporation of Cre vector and clones with a deleted neo cassette for either wild-type or Y1100F cDNA knock-in were identified by Southern blotting (Fig. 1B, right panels). Two independent ES cell clones were used for the generation of germ line chimeras. Interbreeding of Tie2 wild-type knock-in (Tie2-WtKI) heterozygous animals yielded viable, healthy, and fertile homozygous offspring at the Mendelian ratios indicating that no detrimental effects were caused by the cDNA knock-in approach. Mice heterozygous for the Y1100F mutation were healthy and fertile and were intercrossed to generate homozygous offspring. At E9.5, Mendelian ratio of wild-type, heterozygous and homozygous embryos was observed; however, there was a drastic decrease in the number of homozygotes between E10.0 and E10.5, and no live homozygous mutant (Tie2F1100/F1100) embryos were found at E10.5, similar to that observed in Tie2-null embryos (Fig. 1C).
Level of expression of Tie2 is preserved in Y1100F mutant mice.
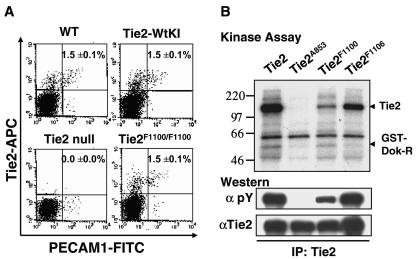
To confirm that the surface expression level of Tie2 was maintained at endogenous levels in Tie2-WtKI and Tie2F1100/F1100 mice, E9.5 (24sp) embryos were analyzed by flow cytometric analysis. Using antibodies specific for platelet and endothelial cell adhesion molecule 1 (PECAM1) and Tie2, Tie2-positive endothelial cells were scored in wild-type, Tie2-WtKI, Tie2-null, and Tie2F1100/F1100 mice. This analysis revealed that both the number of Tie2+ endothelial cells and the levels of Tie2 expression in Tie2-WtKI and Tie2F1100/F1100 embryos at E9.5 were comparable to that in wild-type embryos (Fig. 2A and data not shown).
FIG. 2.
Tie2 expression in Tie2F1100/F1100 mutants and biochemical analysis of the Y1100F mutation. (A) A fluorescence-activated cell sorting analysis of E9.5 embryos with antibodies for PECAM1 and Tie2 shows that the numbers of Tie2+ endothelial cells were comparable among wild-type (WT), Tie2-WtKI, and Tie2F1100/F1100 embryos at E9.5. The mean ± standard deviation percentages of PECAM1+ Tie2+ cells are displayed (n = 5). (B) Full-length wild-type Tie2, Tie2A853, Tie2F1100, and Tie2F1106 cDNAs were transiently expressed in HEK293T cells. Lysates were immunoprecipitated (IP) with anti-Tie2 antibody. Parallel immunoprecipitates were subjected to an in vitro kinase assay in the presence of glutathione S-transferase-Dok-R substrate or Western blotted with antibodies for either phosphotyrosine (pY) or Tie2. Tie2F1100 retains kinase activity toward Dok-R similar to that of wild-type Tie2. The reduced level of phosphorylation of Tie2F1100 likely reflects the importance of tyrosine 1100 as a major autophosphorylation site on the receptor.
Y1100F mutation retains kinase activity.
Activation of the Tie2 receptor has previously been reported in E10.5 mouse embryos (21) and we next sought to analyze the level of phosphorylation on Tie2 in embryos at E9.5, the stage immediately prior to when Tie2-null and Tie2F1100/F1100 embryos die. Tie2 immunoprecipitates from lysate prepared from ten wild-type embryos revealed a faint band of tyrosine phosphorylated Tie2 that comigrated with the ectopically expressed cDNA, suggesting that only a small fraction of Tie2 RTK may be activated in vivo at this stage (data not shown). This observation, as well as only small numbers of Tie2+ endothelial cells (1.5%) in E9.5 whole embryos from wild-type, Tie2-WtKI, or Tie2F1100/F1100 mice (Fig. 2A), precluded us from conducting biochemical analysis using embryos. Thus, HEK293T cells expressing wild-type Tie2, kinase-inactive Tie2 (Tie2A853), Tie2F1100, and Tie2F1106 (19) were used for further biochemical analysis. We have previously demonstrated that tyrosine phosphorylation of the Y1100F mutant is greatly reduced when compared to wild-type Tie2 and the Tyr1106 mutant (19). To ensure that Tie2F1100 does not exhibit any weaknesses in kinase activity which might contribute to hypophosphorylation of the receptor, we subjected Tie2 immunoprecipitates to an in vitro kinase assay with Dok-R as a substrate of Tie2. Although Tie2F1100 itself is phosphorylated to a lesser extent than wild-type Tie2, phosphorylation of Dok-R by Tie2F1100 is comparable to that seen with wild-type Tie2 (Fig. 2B). Phosphorylation of Dok-R was not observed with the kinase inactive Tie2 (Tie2A853) or with Tie2F1106, a mutant which disrupts binding to Dok-R in vivo (17). The reduction in tyrosine phosphorylation of Tie2F1100 likely reflects the loss of a major autophosphorylation site on the receptor since the Y1100F mutant retains full kinase activity.
Tie2F1100/F1100 homozygous embryos display defects in cardiovascular development similar to those displayed by Tie2-null embryos.
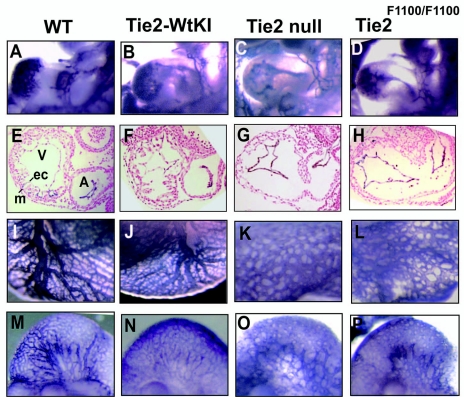
Tie2-null embryos display defective cardiovascular development, including impaired myocardial trabeculation in the heart and defective angiogenic remodeling (7, 42). Since Tie2F1100/F1100 homozygous embryos succumb to lethality at the same point as Tie2-null embryos, we sought to examine whether similar cardiovascular defects might be present in these embryos. The most prominent defect in embryos lacking Tie2 involves the heart (7, 42). The primitive myocardial and endocardial rudiments of the heart interact during differentiation of the heart, resulting in the formation of complex myocardial trabeculations filling the ventricle. By use of whole-mount staining with antibodies to PECAM1 to visualize endothelial cell patterning, wild-type, Tie2-WtKI, Tie2-null, and Tie2F1100/F1100 embryos were analyzed. At E9.5 (24sp), both Tie2-null and Tie2F1100/F1100 embryos exhibited a less intricately folded endothelium lining the myocardium in the heart, compared to wild-type and Tie2-WtKI embryos (Fig. 3A to D). Cross-sectional analysis of the heart region with anti-PECAM1 antibodies revealed that both mutant hearts are devoid of myocardial trabeculations and the endocardial lining appears collapsed and retracted from the myocardial wall (Fig. 3E to H).
FIG. 3.
Defective cardiovascular development in both Tie2-null and Tie2F1100/F1100 mutants. (A to D) Tie2-null (C) and Tie2F1100/F1100 (D) embryos show a less complex structure of the endocardium of the heart than do wild-type (WT) (A) and Tie2-WtKI (B) embryos, as determined by whole-mount anti-PECAM1 staining at E9.5. (E to H) Cross-sections through the heart regions of E9.5 embryos were immunostained with anti-PECAM1 antibodies to highlight the endocardium and counterstained with nuclear fast red. Note the absence of myocardial trabeculation in both Tie2-null and Tie2F1100/F1100 mutants. (I to L) Defects in yolk sac vessel remodeling can be seen in both Tie2-null (K) and Tie2F1100/F1100 (L) mutants compared with wild-type (I) and Tie2-WtKI (J) embryos following whole-mount anti-PECAM1 staining at E9.5. (M to P) Whole-mount anti-PECAM1 staining shows arrested vessel remodeling in the heads of both Tie2-null (O) and Tie2F1100/F1100 (P) mutants compared with wild-type (M) and Tie2-WtKI (N) embryos at E9.5. A, atrium; V, ventricle; ec, endocardium; m, myocardium.
Other notable defects in Tie2-null embryos include decreased complexity in the vascular network, as well as defects in vascular branching and sprouting. In the yolk sac, the initially homogeneous capillary network, which normally reorganizes into a highly branched and intricate vascular network by E10.5, instead maintained an immature dilated and honeycomb appearance in both Tie2-null and Tie2F1100/F1100 embryos at E9.5 (24sp) (Fig. 3I to L). In the head region at E9.5, the fine branches of the vasculature that are normally visible in wild-type and Tie2-WtKI embryos (Fig. 3M and N) were not observed in Tie2-null and Tie2F1100/F1100 embryos and instead there appeared to be extensive fusion of the capillary network (Fig. 3O and P). Formation of the dorsal aorta and anterior cardinal veins were normal in both Tie2-null and Tie2F1100/F1100 embryos (data not shown).
Pericyte coverage and vessel integrity are normal in Tie2F1100/F1100 mutants.
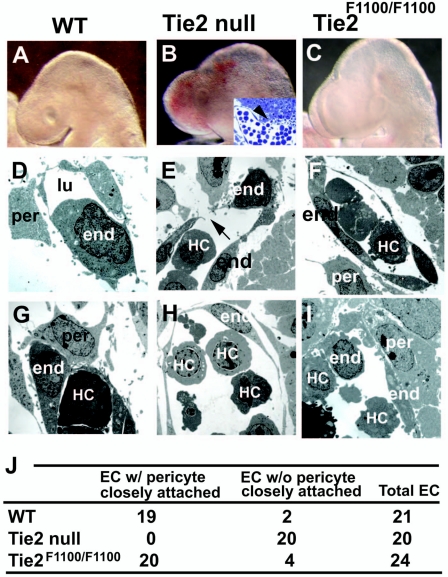
In addition to the cardiovascular patterning defects, another obvious phenotype in Tie2-null embryos is the presence of hemorrhages in the head (18 of 18 samples) (Fig. 4A to C) (7). Cross-sectional analysis of this region displayed hemorrhaging through a gap in the blood vessel wall in Tie2-null embryos (Fig. 4B, inset). However, hemorrhages were only rarely observed in Tie2F1100/F1100 (1 of 19 samples) (Fig. 4C) and never in wild-type (0 of 18 samples) (Fig. 4A) embryos at E9.5 (24sp). To analyze the origin of these hemorrhages, electron microscopic analysis was performed. Tie2-null embryos displayed some gaps in the vascular endothelial cells of the perineural plexus in the head region (Fig. 4E). In contrast, no gaps were observed in the endothelial cells of Tie2F1100/F1100 embryos and wild-type embryos (Fig. 4D and F). Furthermore, endothelial cells were surrounded by pericytes in wild-type (19 of 21 samples) (Fig. 4G and J) and Tie2F1100/F1100 (20 of 24 samples) (Fig. 4I and J) embryos, whereas no pericyte coverage of endothelial cells was observed in Tie2-null embryos (0 of 20 samples) (Fig. 4H and J).
FIG. 4.
Intact vessel integrity and pericyte coverage in Tie2F1100/F1100 embryos. (A to I) No hemorrhages were seen in wild-type (WT) (A) or Tie2F1100/F1100 (C) embryos, whereas Tie2-null embryos displayed hemorrhages in the head (B) at E9.5. A cross-section of Tie2-null embryos with toluidine blue staining shows hemorrhaging (arrowhead) through a gap in the blood vessel wall (inset in B). Ultrastructural analysis depicts gaps in the vascular endothelial cells of the perineural plexus in the head region of Tie2-null embryos (arrow in E), whereas no gaps were observed in wild-type (D) or Tie2F1100/F1100 (F) embryos. Furthermore, endothelial cells were surrounded by pericytes in wild-type (19 of 21 samples) (G) and Tie2F1100/F1100 (20 of 24 samples) (I) embryos, whereas no pericyte coverage along endothelial cells was observed in Tie2-null embryos (0 of 20 samples) (H). (J) Sections of blood vessels were scored for pericytes closely attached to endothelial cells. end, endothelium; per, pericytes; lu, lumen; HC, hematopoietic cells; EC, endothelial cells.
Hematopoiesis and endothelial cell development in the P-Sp region are impaired in Tie2F1100/F1100 and Tie2-null embryos.
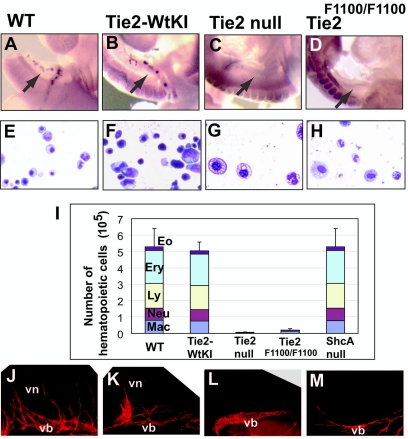
Impaired hematopoiesis and vascular development in the P-Sp region comprising the omphalomesenteric artery and vitelline artery was previously reported in Tie2-null embryos (46). This finding prompted us to analyze hematopoiesis and vascular development in Tie2F1100/F1100 mutants in situ and/or using a P-Sp explant culture system. First, we analyzed the existence of c-Kit+ hematopoietic cell clusters in the omphalomesenteric artery and vitelline artery, that are able to differentiate into multilineage hematopoietic cells (46). Both Tie2-null and Tie2F1100/F1100 embryos lacked c-Kit+ cell clusters in the omphalomesenteric artery and vitelline artery, while aggregations of these cells were detected in wild-type and Tie2-WtKI embryos (Fig. 5A to D). Next, we utilized an established in vitro coculture system where a P-Sp explant from an E9.5 embryo is cultured on stromal cells in the presence of growth factors to allow growth of both hematopoietic and endothelial cells (46). Hematopoietic cells developed in this assay system (Fig. 5E to H) were quantified and further characterized. Notably, the total number of hematopoietic cells was dramatically reduced in explants from Tie2-null and Tie2F1100/F1100 embryos (Fig. 5I). Analysis of specific cell populations in the explant cultures revealed that only small numbers of macrophages and neutrophils grew from P-Sp explants of the Tie2-null and Tie2F1100/F1100 embryos, whereas erythroblasts, lymphoid cells, neutrophils and eosinophils developed from explants of wild-type and Tie2-WtKI embryos (Fig. 5E to I). Endothelial cell development in P-Sp explants from Tie2 wild-type and mutant embryos was analyzed by PECAM1 immunohistochemical staining. In this culture system of E9.5 embryo explants, endothelial cells first proliferate to form a sheet-like vascular bed and subsequently sprout from the periphery of the sheet to form a vascular network (46). Analysis of PECAM1 staining in the P-Sp explants revealed that wild-type and Tie2-WtKI explants developed vascular networks (Fig. 5J and K) while Tie2-null and Tie2F1100/F1100 mutant explants developed vascular beds without sprouting (Fig. 5L and M). Expression of the Tie2 binding partner ShcA has been demonstrated in the P-Sp region (26); thus, we set out to determine whether defects in hematopoiesis in Tie2F1100/F1100 mutant embryos might be as a consequence of impaired recruitment of this signaling molecule to Tyr1100 on Tie2. Surprisingly, examination of hematopoietic cell growth from P-Sp explants from E9.5 ShcA-null embryos revealed no defects in hematopoiesis (Fig. 5I).
FIG. 5.
Suppression of hematopoiesis and endothelial cell development in the P-Sp region in both Tie2-null and Tie2F1100/F1100 mutants at E9.5. (A to D) Whole-mount immunostaining with anti-c-Kit antibody. c-Kit+ hematopoietic clusters were found in the omphalomesenteric artery (arrows) and vitelline artery in wild-type (WT) embryos, whereas no c-Kit+ cells were present in the corresponding regions of Tie2-null and Tie2F1100/F1100 embryos. (E to H) May-Grünwald Giemsa staining. A variety of hematopoietic cell lineages, including erythroblasts, lymphoid cells, and neutrophils, were generated after 8 days of culturing of explants from wild-type (E) and Tie2-WtKI (F) embryos, while only macrophages and neutrophils were generated from Tie2-null (G) and Tie2F1100/F1100 (H) mutants. (I) Quantitation of the number of hematopoietic cells grown from Tie2-null and Tie2F1100/F1100 explants revealed that substantially fewer cells were present in the Tie2 mutants than in the wild-type and Tie2-WtKI embryos. The number of hematopoietic cells from P-Sp explants of ShcA-null embryos was comparable to that for the wild type. The mean and standard deviation of data derived from five experiments are shown. (J to M) P-Sp cultures stained with anti-PECAM1 antibody. Poor vascular network formations were observed in cultures of Tie2-null (L) and Tie2 F1100/F1100 (M) mutant explants compared with wild-type (J) and Tie2-WtKI (K) explants. Eo, eosinophils; Ery, erythroblasts; Ly, lymphocytes; Neu, neutrophils; Mac, macrophages; vb, vascular bed; vn, vascular network.
DISCUSSION
In this study, we have determined that Tyr1100 in the Tie2 receptor is essential for aspects of cardiac development and hematopoiesis in the mouse embryo. Embryos lacking Tyr1100 develop numerous overlapping defects with those found in Tie2-null embryos, demonstrating that signaling pathways mediated through this tyrosine residue are crucial for normal embryonic development. Defects in the formation of myocardial projections to form trabeculae as well as adhesion of the endocardial lining to the underlying myocardium are found in both Tie2-null and Tie2F1100/F1100 embryos. Disruption of the interaction between these two cell layers is thought to arise as a consequence of impaired signal exchange between the Ang1-expressing myocardium and the Tie2-expressing endocardium (44). A strict requirement for Tie2 in the developing heart has also been demonstrated through chimeric analysis wherein cells lacking Tie2 were excluded from the endocardium by E10.5 (38). The inability of endocardial cells lacking Tyr1100 on Tie2 to rescue the cardiac defects observed in Tie2-null embryos underscores the physiological importance of this docking site in mediating aspects of Tie2 function in vivo.
In addition to the role in cardiac development, Tyr1100 on Tie2 is also essential for angiogenic sprouting and/or remodeling in the yolk sac and the head region. Interestingly however we show that this same tyrosine is not required for periendothelial cell recruitment and vessel integrity. This latter observation is of interest in light of previous mosaic analyses involving chimeric mice between normal embryonic cells and cells lacking both Tie1 and Tie2 in which we demonstrated that these receptors are dispensable for sprout formation during embryogenesis (38). We had previously concluded that impaired angiogenic sprouting and remodeling observed in Tie2-null embryos was a secondary consequence of defective cardiac function and poor circulation (38). In the targeted Tie2F1100/F1100 mutants, development of the heart was impaired to the same extent as in Tie2-null embryos, suggesting that impaired blood vessel sprouting and/or remodeling is a secondary consequence of perturbed circulation, but defective pericyte recruitment and vessel integrity in Tie2-null embryos are directly dependent on intact Tie2 function. One way to reconcile these two seemingly contradictory observations is to hypothesize that in the mosaic analysis, extrinsic factors necessary for pericyte recruitment and/or adhesion to nascent blood vessels might be provided by wild-type endothelial cells that are missing or dysfunctional in homozygous-null embryos.
Defects in development of the heart region and angiogenic sprouting in both Tie2-null and Tie2F1100/F1100 embryos are reminiscent of those seen in embryos lacking the ShcA adaptor protein. ShcA-null embryos display an elongated endocardium with few myocardial trabeculae as well as defective angiogenic remodeling in the yolk sac and the head region (26). Although the p85 regulatory subunit of PI3-kinase has also been shown to bind Tie2 at Tyr1100, mice with a targeted gene disruption of p85 alpha do not display embryonic lethality and have no defective heart development (10, 45). Together these data raise the possibility that binding of the SH2 domain of ShcA to Tie2 at Tyr1100 (2) might have an important role in the regulation of development of the heart and angiogenic sprouting. Signaling through other docking sites on Tie2 such as Tyr1106 likely function to preserve cell adhesion and perivascular cell recruitment in Tie2F1100/F1100 embryos.
One of the most striking observations in Tie2F1100/F1100 embryos is that despite the defects in cardiac cell adhesion, periendothelial support cells appear to adhere normally to endothelial cells in other vascular beds to preserve vessel integrity. In the absence of Ang1, endothelial cells are poorly associated with their underlying matrix and there are fewer pericytes associated with the vasculature (44). Accordingly, Ang1 expression has been shown in animal models to reduce vascular leakage, possibly by promoting attachment of endothelial cells to the surrounding matrix (47, 48). Although it remains to be determined which signaling pathways mediate adhesion downstream of Tie2, stabilization of the vasculature in Tie2F1100/F1100 embryos suggests that endocardial-myocardial cell interactions and vascular endothelial cell periendothelial cell interactions are governed by signaling pathways mediated by distinct tyrosine residues in Tie2. Endocardial cells of the heart and vascular endothelial cells share common gene expression patterns; however, they have been shown to derive from distinct precursor populations in Xenopus (52) and it follows that these populations might therefore differ in their ability to regulate myocardial and periendothelial cells. During heart development, primitive myocardial and endocardial rudiments develop in situ from the pericardial wall and subadjacent mesenchyme, respectively, at about the same time (3, 20). Signal transduction pathways initiated by Tie2 in the endocardium may then provide extrinsic factors such as neuregulin-1 necessary for cardiomyocyte proliferation and formation of myocardial trabeculations (23, 30, 56). By contrast, in developing blood vessels, vasculogenesis and angiogenesis occur first, resulting in nascent blood vessels. Periendothelial cells are then recruited and attached to the nascent vessels to surround and stabilize the vessel (4). In this stabilizing step, Tie2-expressing endothelial cells may secrete extrinsic factors, such as PDGFB or HB-EGF, necessary for periendothelial support cell migration, differentiation and/or adhesion to form the vessel wall (9, 13). Origin of Tie2 expression may therefore influence distinct signaling pathways and the release of specific growth factors which affect development of the heart and blood vessels. Moreover, context dependent actions of angiopoietins may further contribute to these differences.
In addition to the role of Tyr1100 in development of the heart and angiogenic sprouting, we have also demonstrated that early hematopoiesis is similarly impaired in both Tie2-null and Tie2F1100/F1100 embryos. c-Kit+ hematopoietic clusters are not detected in both Tie2-null and Tie2F1100/F1100 embryos at E9.5. Recently, it has been shown that emergence of hematopoietic progenitors in the P-Sp region or dorsal aorta is intimately related to development of adjacent endothelial cells (25, 33). Moreover, defects in hematopoiesis in VEGFR-3-deficient P-Sp explants have been shown to be due to abnormal vascular development (12) and endothelial cells isolated from the P-Sp can support hematopoiesis in vitro (27). Thus, we infer that emergence of c-Kit+ hematopoietic progenitors is perturbed or delayed in Tie2-null and Tie2F1100/F1100 mutants due to abnormal endothelial development in the P-Sp region. This would lead to impaired hematopoietic cell differentiation in the P-Sp culture system since a poorly developed endothelial microenvironment would affect the ability of these progenitors to respond to the appropriate differentiation and migration cues. These findings are consistent with our previous report that Tie1/Tie2 doubly deficient cells can contribute to fetal liver hematopoiesis and Tie receptors are not an intrinsic requirement for developmental hematopoiesis (37). Generating endothelial or hematopoietic cell specific Tie2 mutant mice could resolve the issue whether impaired hematopoiesis in Tie2 mutant P-Sp depends on endothelial development.
Our findings raise questions about which signaling pathways emerging from Tyr1100 are responsible for P-Sp hematopoiesis. We demonstrate here that, despite recruitment of the ShcA adaptor protein to Tyr1100 on Tie2 (2), early hematopoiesis in the P-Sp region is normal in ShcA-deficient explant cultures. Interestingly however, mice lacking the p85 alpha regulatory subunit of PI3-kinase had impaired definitive hematopoiesis (10, 16, 45). Although the explant culture conditions described here did not allow us to specifically examine the requirement of PI3-kinase in P-Sp hematopoiesis mediated by Tie2, the role of Tie2 signaling through PI3-kinase in vascular development and hematopoiesis has been extensively examined. Tie2-PI3-kinase signaling mediates endothelial cell migration and stimulates tube formation (11, 19, 22, 34) and both Tie2 signaling and PI3-Kinase activation are required for the maintenance of cultured hematopoietic cells (5, 40, 51). A role for the adaptor protein Grb7 in cell migration is also well characterized although the in vivo function of Tie2-Grb7 signaling remains unknown (43). Taken together, these observations suggest that Tie2-PI3-kinase or Tie2-Grb7 signaling, but not Tie2-ShcA signaling, is required for P-Sp hematopoiesis.
In summary, through selective ablation of a single tyrosine residue on Tie2 in vivo, we demonstrated that signal transduction pathways emerging from Tyr1100 on the receptor are required for the development of the heart and for early hematopoietic and endothelial cell development in the P-Sp region, while signaling pathways stemming from residues other than tyrosine 1100 may govern perivascular cell recruitment and/or adhesion to nascent vessels to promote vascular integrity (Fig. 6). These studies illustrate how early hematopoiesis and how endocardial and vascular endothelial cell function are distinctly regulated by a single cell surface receptor and provide some insight into how such a receptor exerts control over many different cellular and developmental pathways.
FIG. 6.
Proposed model for the selective in vivo role of Tie2 signaling pathways. Signal transduction pathways emanating from Tyr1100 on Tie2 are required for cardiac development and early hematopoietic and endothelial cell development in the P-Sp region. PI3-kinase activation in response to Ang1 is mediated through Tyr1100. Additionally, the adaptor proteins ShcA and Grb7, both of which can associate with the cytosolic FAK kinase, are also recruited to Tyr1100. In contrast, signaling from residues other than Tyr1100, such as Tyr1106, may govern perivascular cell recruitment and/or adhesion to nascent vessels to promote vascular integrity. The formation of a signaling complex of Dok-R, RasGAP, Nck, and Pak occurs at Tyr1106. The SH2 domain containing tyrosine phosphatase Shp2 has been proposed to bind to Tyr1111, although the functional significance of this interaction remains unclear. Additional important tyrosine residues on Tie2, including juxtamembrane tyrosine residue 814 (which may recruit Shp2 and/or Grb14) and tyrosine residue 990 in the activation loop, are not shown.
Acknowledgments
We thank S. Tondat and S. MacMaster for ES cell aggregations; M. Peralta for histological sections; D. P. Holmyard, M. Hirashima, and K. Chawengsaksophak for assistance with electron microscopic, fluorescence-activated cell sorting, and fluorescence immunostaining analyses, respectively; N. Takakura and T. Yokomizo for advice on P-Sp culturing and whole-mount immunostaining of c-Kit+ clusters, respectively; W. R. Hardy and T. Pawson for providing ShcA mutant mice; Y. Yamanaka for critical reading of the manuscript; and all members of the Bernstein Laboratory for helpful discussions.
This work was supported in part by the National Cancer Institute of Canada (A.B.), a Canadian Institutes of Health research fellowship and a Uehara Memorial Foundation fellowship (K.T.), a National Cancer Institute of Canada/Terry Fox Run research fellowship (N.J.), and a scientist award from the Canadian Institutes of Health Research (D.J.D.).
The authors declare that they have no competing financial interests.
REFERENCES
- 1.Arai, F., A. Hirao, M. Ohmura, H. Sato, S. Matsuoka, K. Takubo, K. Ito, G. Y. Koh, and T. Suda. 2004. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118:149-161. [DOI] [PubMed] [Google Scholar]
- 2.Audero, E., I. Cascone, F. Maniero, L. Napione, M. Arese, L. Lanfrancone, and F. Bussolino. 2004. Adaptor ShcA protein binds tyrosine kinase Tie2 receptor and regulates migration and sprouting but not survival of endothelial cells. J. Biol. Chem. 279:13224-13233. [DOI] [PubMed] [Google Scholar]
- 3.Brutsaert, D. L., P. Fransen, L. J. Andries, G. W. De Keulenaer, and S. U. Sys. 1998. Cardiac endothelium and myocardial function. Cardiovasc. Res. 38:281-290. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet, P. 2000. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 6:389-395. [DOI] [PubMed] [Google Scholar]
- 5.Cascone, I., E. Audero, E. Giraudo, L. Napione, F. Maniero, M. R. Philips, J. G. Collard, G. Serini, and F. Bussolino. 2003. Tie-2-dependent activation of RhoA and Rac1 participates in endothelial cell motility triggered by angiopoietin-1. Blood 102:2482-2490. [DOI] [PubMed] [Google Scholar]
- 6.Davis, S., T. H. Aldrich, P. F. Jones, A. Acheson, D. L. Compton, V. Jain, T. E. Ryan, J. Bruno, C. Radziejewski, P. C. Maisonpierre, and G. D. Yancopoulos. 1996. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 87:1161-1169. [DOI] [PubMed] [Google Scholar]
- 7.Dumont, D. J., G. Gradwohl, G. H. Fong, M. C. Puri, M. Gertsenstein, A. Auerbach, and M. L. Breitman. 1994. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 8:1897-1909. [DOI] [PubMed] [Google Scholar]
- 8.Eichmann, A., C. Corbel, V. Nataf, P. Vaigot, C. Breant, and N. M. Le Douarin. 1997. Ligand-dependent development of the endothelial and hemopoietic lineages from embryonic mesodermal cells expressing vascular endothelial growth factor receptor 2. Proc. Natl. Acad. Sci. USA 94:5141-5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folkman, J., and P. A. D'Amore. 1996. Blood vessel formation: what is its molecular basis? Cell 87:1153-1155. [DOI] [PubMed] [Google Scholar]
- 10.Fruman, D. A., S. B. Snapper, C. M. Yballe, L. Davidson, J. Y. Yu, F. W. Alt, and L. C. Cantley. 1999. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85alpha. Science 283:393-397. [DOI] [PubMed] [Google Scholar]
- 11.Fujikawa, K., I. de Aos Scherpenseel, S. K. Jain, E. Presman, R. A. Christensen, and L. Varticovski. 1999. Role of PI 3-kinase in angiopoietin-1-mediated migration and attachment-dependent survival of endothelial cells. Exp. Cell Res. 253:663-672. [DOI] [PubMed] [Google Scholar]
- 12.Hamada, K., Y. Oike, N. Takakura, Y. Ito, L. Jussila, D. J. Dumont, K. Alitalo, and T. Suda. 2000. VEGF-C signaling pathways through VEGFR-2 and VEGFR-3 in vasculoangiogenesis and hematopoiesis. Blood 96:3793-3800. [PubMed] [Google Scholar]
- 13.Hellstrom, M., M. Kalen, P. Lindahl, A. Abramsson, and C. Betsholtz. 1999. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126:3047-3055. [DOI] [PubMed] [Google Scholar]
- 14.Huang, L., C. W. Turck, P. Rao, and K. G. Peters. 1995. GRB2 and SH-PTP2: potentially important endothelial signaling molecules downstream of the TEK/TIE2 receptor tyrosine kinase. Oncogene 11:2097-2103. [PubMed] [Google Scholar]
- 15.Huber, T. L., V. Kouskoff, H. J. Fehling, J. Palis, and G. Keller. 2004. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature 432:625-630. [DOI] [PubMed] [Google Scholar]
- 16.Huddleston, H., B. Tan, F. C. Yang, H. White, M. J. Wenning, A. Orazi, M. C. Yoder, R. Kapur, and D. A. Ingram. 2003. Functional p85alpha gene is required for normal murine fetal erythropoiesis. Blood 102:142-145. [DOI] [PubMed] [Google Scholar]
- 17.Jones, N., S. H. Chen, C. Sturk, Z. Master, J. Tran, R. S. Kerbel, and D. J. Dumont. 2003. A unique autophosphorylation site on Tie2/Tek mediates Dok-R phosphotyrosine binding domain binding and function. Mol. Cell. Biol. 23:2658-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones, N., and D. J. Dumont. 1998. The Tek/Tie2 receptor signals through a novel Dok-related docking protein, Dok-R. Oncogene 17:1097-1108. [DOI] [PubMed] [Google Scholar]
- 19.Jones, N., Z. Master, J. Jones, D. Bouchard, Y. Gunji, H. Sasaki, R. Daly, K. Alitalo, and D. J. Dumont. 1999. Identification of Tek/Tie2 binding partners. Binding to a multifunctional docking site mediates cell survival and migration. J. Biol. Chem. 274:30896-30905. [DOI] [PubMed] [Google Scholar]
- 20.Kaufman, M. H., and V. Navaratnam. 1981. Early differentiation of the heart in mouse embryos. J. Anat. 133:235-246. [PMC free article] [PubMed] [Google Scholar]
- 21.Koblizek, T. I., A. S. Runting, S. A. Stacker, A. F. Wilks, W. Risau, and U. Deutsch. 1997. Tie2 receptor expression and phosphorylation in cultured cells and mouse tissues. Eur. J. Biochem. 244:774-779. [DOI] [PubMed] [Google Scholar]
- 22.Kontos, C. D., T. P. Stauffer, W. P. Yang, J. D. York, L. Huang, M. A. Blanar, T. Meyer, and K. G. Peters. 1998. Tyrosine 1101 of Tie2 is the major site of association of p85 and is required for activation of phosphatidylinositol 3-kinase and Akt. Mol. Cell. Biol. 18:4131-4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kramer, R., N. Bucay, D. J. Kane, L. E. Martin, J. E. Tarpley, and L. E. Theill. 1996. Neuregulins with an Ig-like domain are essential for mouse myocardial and neuronal development. Proc. Natl. Acad. Sci. USA 93:4833-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubo, H., and K. Alitalo. 2003. The bloody fate of endothelial stem cells. Genes Dev. 17:322-329. [DOI] [PubMed] [Google Scholar]
- 25.Kumano, K., S. Chiba, A. Kunisato, M. Sata, T. Saito, E. Nakagami-Yamaguchi, T. Yamaguchi, S. Masuda, K. Shimizu, T. Takahashi, S. Ogawa, Y. Hamada, and H. Hirai. 2003. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity 18:699-711. [DOI] [PubMed] [Google Scholar]
- 26.Lai, K. M., and T. Pawson. 2000. The ShcA phosphotyrosine docking protein sensitizes cardiovascular signaling in the mouse embryo. Genes Dev. 14:1132-1145. [PMC free article] [PubMed] [Google Scholar]
- 27.Li, W., S. A. Johnson, W. C. Shelley, M. Ferkowicz, P. Morrison, Y. Li, and M. C. Yoder. 2003. Primary endothelial cells isolated from the yolk sac and para-aortic splanchnopleura support the expansion of adult marrow stem cells in vitro. Blood 102:4345-4353. [DOI] [PubMed] [Google Scholar]
- 28.Maisonpierre, P. C., C. Suri, P. F. Jones, S. Bartunkova, S. J. Wiegand, C. Radziejewski, D. Compton, J. McClain, T. H. Aldrich, N. Papadopoulos, T. J. Daly, S. Davis, T. N. Sato, and G. D. Yancopoulos. 1997. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277:55-60. [DOI] [PubMed] [Google Scholar]
- 29.Master, Z., N. Jones, J. Tran, J. Jones, R. S. Kerbel, and D. J. Dumont. 2001. Dok-R plays a pivotal role in angiopoietin-1-dependent cell migration through recruitment and activation of Pak. EMBO J. 20:5919-5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer, D., and C. Birchmeier. 1995. Multiple essential functions of neuregulin in development. Nature 378:386-390. [DOI] [PubMed] [Google Scholar]
- 31.Nagy, A., J. Rossant, R. Nagy, W. Abramow-Newerly, and J. C. Roder. 1993. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc. Natl. Acad. Sci. USA 90:8424-8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishikawa, S. I., S. Nishikawa, H. Kawamoto, H. Yoshida, M. Kizumoto, H. Kataoka, and Y. Katsura. 1998. In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity 8:761-769. [DOI] [PubMed] [Google Scholar]
- 33.North, T. E., M. F. de Bruijn, T. Stacy, L. Talebian, E. Lind, C. Robin, M. Binder, E. Dzierzak, and N. A. Speck. 2002. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity 16:661-672. [DOI] [PubMed] [Google Scholar]
- 34.Papapetropoulos, A., G. Garcia-Cardena, T. J. Dengler, P. C. Maisonpierre, G. D. Yancopoulos, and W. C. Sessa. 1999. Direct actions of angiopoietin-1 on human endothelium: evidence for network stabilization, cell survival, and interaction with other angiogenic growth factors. Lab. Investig. 79:213-223. [PubMed] [Google Scholar]
- 35.Patan, S. 1998. TIE1 and TIE2 receptor tyrosine kinases inversely regulate embryonic angiogenesis by the mechanism of intussusceptive microvascular growth. Microvasc. Res. 56:1-21. [DOI] [PubMed] [Google Scholar]
- 36.Pawson, T., and J. D. Scott. 1997. Signaling through scaffold, anchoring, and adaptor proteins. Science 278:2075-2080. [DOI] [PubMed] [Google Scholar]
- 37.Puri, M. C., and A. Bernstein. 2003. Requirement for the TIE family of receptor tyrosine kinases in adult but not fetal hematopoiesis. Proc. Natl. Acad. Sci. USA 100:12753-12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puri, M. C., J. Partanen, J. Rossant, and A. Bernstein. 1999. Interaction of the TEK and TIE receptor tyrosine kinases during cardiovascular development. Development 126:4569-4580. [DOI] [PubMed] [Google Scholar]
- 39.Risau, W., H. Sariola, H. G. Zerwes, J. Sasse, P. Ekblom, R. Kemler, and T. Doetschman. 1988. Vasculogenesis and angiogenesis in embryonic-stem-cell-derived embryoid bodies. Development 102:471-478. [DOI] [PubMed] [Google Scholar]
- 40.Saito, M., M. Hamasaki, and M. Shibuya. 2003. Induction of tube formation by angiopoietin-1 in endothelial cell/fibroblast co-culture is dependent on endogenous VEGF. Cancer Sci. 94:782-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato, A., A. Iwama, N. Takakura, H. Nishio, G. D. Yancopoulos, and T. Suda. 1998. Characterization of TEK receptor tyrosine kinase and its ligands, angiopoietins, in human hematopoietic progenitor cells. Int. Immunol. 10:1217-1227. [DOI] [PubMed] [Google Scholar]
- 42.Sato, T. N., Y. Tozawa, U. Deutsch, K. Wolburg-Buchholz, Y. Fujiwara, M. Gendron-Maguire, T. Gridley, H. Wolburg, W. Risau, and Y. Qin. 1995. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 376:70-74. [DOI] [PubMed] [Google Scholar]
- 43.Shen, T. L., and J. L. Guan. 2004. Grb7 in intracellular signaling and its role in cell regulation. Front. Biosci. 9:192-200. [DOI] [PubMed] [Google Scholar]
- 44.Suri, C., P. F. Jones, S. Patan, S. Bartunkova, P. C. Maisonpierre, S. Davis, T. N. Sato, and G. D. Yancopoulos. 1996. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87:1171-1180. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki, H., Y. Terauchi, M. Fujiwara, S. Aizawa, Y. Yazaki, T. Kadowaki, and S. Koyasu. 1999. Xid-like immunodeficiency in mice with disruption of the p85alpha subunit of phosphoinositide 3-kinase. Science 283:390-392. [DOI] [PubMed] [Google Scholar]
- 46.Takakura, N., X. L. Huang, T. Naruse, I. Hamaguchi, D. J. Dumont, G. D. Yancopoulos, and T. Suda. 1998. Critical role of the TIE2 endothelial cell receptor in the development of definitive hematopoiesis. Immunity 9:677-686. [DOI] [PubMed] [Google Scholar]
- 47.Thurston, G., J. S. Rudge, E. Ioffe, H. Zhou, L. Ross, S. D. Croll, N. Glazer, J. Holash, D. M. McDonald, and G. D. Yancopoulos. 2000. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat. Med. 6:460-463. [DOI] [PubMed] [Google Scholar]
- 48.Thurston, G., C. Suri, K. Smith, J. McClain, T. N. Sato, G. D. Yancopoulos, and D. M. McDonald. 1999. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 286:2511-2514. [DOI] [PubMed] [Google Scholar]
- 49.Traver, D., and L. I. Zon. 2002. Walking the walk: migration and other common themes in blood and vascular development. Cell 108:731-734. [DOI] [PubMed] [Google Scholar]
- 50.Valenzuela, D. M., J. A. Griffiths, J. Rojas, T. H. Aldrich, P. F. Jones, H. Zhou, J. McClain, N. G. Copeland, D. J. Gilbert, N. A. Jenkins, T. Huang, N. Papadopoulos, P. C. Maisonpierre, S. Davis, and G. D. Yancopoulos. 1999. Angiopoietins 3 and 4: diverging gene counterparts in mice and humans. Proc. Natl. Acad. Sci. USA 96:1904-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wakabayashi, M., H. Miwa, M. Shikami, A. Hiramatsu, T. Ikai, E. Tajima, H. Yamamoto, K. Miura, A. Satoh, M. Itoh, A. Imamura, H. Mihara, Y. Katoh, and M. Nitta. 2004. Autocrine pathway of angiopoietins-Tie2 system in AML cells: association with phosphatidyl-inositol 3 kinase. Hematol. J. 5:353-360. [DOI] [PubMed] [Google Scholar]
- 52.Walmsley, M., A. Ciau-Uitz, and R. Patient. 2002. Adult and embryonic blood and endothelium derive from distinct precursor populations which are differentially programmed by BMP in Xenopus. Development 129:5683-5695. [DOI] [PubMed] [Google Scholar]
- 53.Ward, N. L., and D. J. Dumont. 2002. The angiopoietins and Tie2/Tek: adding to the complexity of cardiovascular development. Semin. Cell Dev. Biol. 13:19-27. [DOI] [PubMed] [Google Scholar]
- 54.Yokomizo, T., M. Ogawa, M. Osato, T. Kanno, H. Yoshida, T. Fujimoto, S. Fraser, S. Nishikawa, H. Okada, M. Satake, T. Noda, and Y. Ito. 2001. Requirement of Runx1/AML1/PEBP2alphaB for the generation of haematopoietic cells from endothelial cells. Genes Cells 6:13-23. [DOI] [PubMed] [Google Scholar]
- 55.Yoshida, H., T. Kunisada, M. Kusakabe, S. Nishikawa, and S. I. Nishikawa. 1996. Distinct stages of melanocyte differentiation revealed by anlaysis of nonuniform pigmentation patterns. Development 122:1207-1214. [DOI] [PubMed] [Google Scholar]
- 56.Zhao, Y. Y., D. R. Sawyer, R. R. Baliga, D. J. Opel, X. Han, M. A. Marchionni, and R. A. Kelly. 1998. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J. Biol. Chem. 273:10261-10269. [DOI] [PubMed] [Google Scholar]