Abstract
Background:
Children with tuberculous meningitis (TBM) present with diagnostic challenges as they often have atypical clinical features.
Objective:
To describe the baseline characteristic features of children diagnosed with central nervous system (CNS) TB (TBM and tuberculoma).
Design:
Retrospective descriptive study.
Methods:
Children less than 12 years presenting with neurological signs and symptoms were assessed for a therapeutic TBM trial eligibility. The results of their clinical, laboratory, neuroimaging, cerebrospinal fluid evaluations were analysed for TBM diagnosis.
Results:
Of 600 children evaluated, 61(10%) had CNS tuberculosis; TBM 47, tuberculoma 14. 20(33%) had definite TBM. Mean age of children with TBM was 5 ± 3.4 years. Of 47, 13(28%), 21(45%) and 13(28%) had grade I, II, and III disease respectively. Abnormalities suggestive of TBM in MRI and computed tomography brain were observed in 76% (26/34) and 77% (24/31) respectively. Abnormal cerebrospinal fluid white blood cell count, protein and glucose were observed in 56% (24/43), 49% (22/45), 47% (21/45) respectively. Among 41 patients with TBM followed up until discharge, five died.
Conclusion:
Younger children with TBM have severe forms. Confirmatory results may not be available in all. A holistic approach to care including addressing complications of hydrocephalus and strokes is needed.
Keywords: children, CNS tuberculosis, paediatric TBM, tuberculous meningitis
Plain language summary
Clinical features, results of brain imaging and other tests in the cerebrospinal fluid among children diagnosed with tuberculous meningitis – descriptive study
Why was the study done? What did the researchers do? Records of children aged between 6 months and 12 years who presented to the health care centre with signs and symptoms of central nervous system (CNS) disease and assessed for tuberculous meningitis (TBM) clinical trial eligibility were reviewed. The research team studied the signs and symptoms of the TBM, results of the CT/MRI brain scan and tests which were done in the cerebrospinal fluid (CSF) during hospitalization. What did the researchers find? Total number of children who presented to the health centre during the study period with CNS complaints and underwent lumbar puncture were 600. Among them 61 were diagnosed with CNS TB (47 had TBM and 14 had tuberculoma). Half of them were less than five years of age. Ten had neurological dysfunction. Fever, vomiting were the common complaints. Almost half of the children had vomiting, altered level of consciousness and seizures. Tests done in the CSF detected the bacteria causing TBM in half of the children. Abnormal cell counts or biochemical changes in the CSF specific to TBM were observed in half of the children. Abnormalities in CT/MRI imaging with features specific to the disease were observed in closer to three fourth of the children. What do the findings mean? Children with TBM often present late for care with severe forms and its complications. There would be diagnostic challenges as the symptoms were vague and might not present in a specific manner, specific tests in the CSF could be negative and if undiagnosed could lead to severe morbidity impacting the quality of life or death. Taking the overall picture of presenting complaints, results of CSF test and brain scan and with high degree of suspicion, TBM should be diagnosed early and managed appropriately.
Introduction
Paediatric tuberculosis (TB) is under-diagnosed and associated with significant morbidity and mortality with delays in treatment. Tuberculous meningitis (TBM) portends the highest morbidity and mortality, with the burden of paediatric TBM at global and national levels poorly defined. 1 Prevalence of TBM varies from 1% of TB cases in low prevalence population settings to 10% in high prevalence settings. 1 Younger children are at higher risk of TBM. 2 Children with TBM present with varied symptoms and signs and often with severe disease. Though children with TBM may improve after treatment, diagnostic and treatment delays are often associated with severe neurological and neurodevelopmental sequelae. 3 Mortality rates are between 5% and 23% and sequelae occur in at least 50% of children with TBM. 4 Poor nutritional status, disease stage, complications, and duration of the illness play an important role in influencing the treatment outcomes. 5 Mycobacterial culture is the gold standard for confirming diagnosis; however, results are often delayed, and too late for necessary real-time treatment decisions. Costly diagnostic tests, ancillary studies, including neuroimaging, expertise for cerebrospinal fluid (CSF) collection and testing may vary across different healthcare settings in hyperendemic areas. Another central nervous system (CNS) manifestation of TB, tuberculoma, is a well-circumscribed conglomerate of tubercles located in the brain parenchyma, commonly presenting as a mass lesion in neuroimaging, with or without fever and seizures. The symptoms often depend on the site of the location. 6 Tuberculoma and tuberculous abscess may present in isolation or along with TBM.
With many unanswered questions, diagnosis of CNS TB in children is often a challenge and delays lead to severe consequences. In this context, we aim to describe here the baseline clinical, laboratory and neuroimaging features of children diagnosed with CNS TB (TBM and tuberculoma) in a high-volume setting in India.
Methods
We performed a retrospective review of children with CNS TB who were assessed for TBM therapeutic trial participation. ICMR-National Institute for Research in Tuberculosis (ICMR-NIRT) was one of the study sites for TBM KIDS trial (NCT02958709) 6 and participants were recruited from the Institute of Child Health (ICH), a tertiary referral centre affiliated to Madras Medical College, Chennai, India. 6 Necessary approvals from the Institutional Ethics Committees of both the Institutes were obtained for the trial. Children aged between 6 months and 12 years, weighing more than 6 kg, admitted with neurological symptoms and underwent lumbar puncture (LP) as a diagnostic procedure between June 2017 and April 2021 were included for TBM KIDS trial screening. Parent(s) of the children provided written informed consent. The reporting of this study conforms to the strengthening of the reporting of observational studies in epidemiology statement.
Details of clinical characteristics, results of CSF test, neuroimaging and systemic evidence of TB were collected from the case records, laboratory and imaging reports. Seizures were diagnosed based on history, clinical evaluation and electroencephalogram. Weight loss was noted based on the history conveyed by the parent or caretaker. X-ray abnormalities were defined as infiltrates, cavitary lung lesions, adenopathy and pleural disease. Neuroimaging was done preferably within the first 2 days of admission prior to planning LP. CSF testing included gram stain, bacterial culture and sensitivity, acid-fast bacilli (AFB) smear, GeneXpert and mycobacterial liquid culture using mycobacterial growth inhibitor tube (MGIT). First and second-line drug sensitivity tests (DSTs) were done using MGIT. CSF profiles including cell counts, glucose and protein levels were performed. Tests in CSF is specific to TB were prioritised based on clinical condition and available CSF volume. GeneXpert was the preferred initial test in view of its faster turnaround time. Children were given the diagnosis of definite, probable, possible TBM or non-TBM based on the uniform case definition for use in clinical research diagnostic criteria. 7 Diagnosis of CNS tuberculoma was based on clinical criteria and neuroimaging which included computed tomography (CT) or magnetic resonance imaging (MRI) brain with contrast studies showing single or multiple ring-enhancing lesions and presence or absence of lipid lactate peaks in magnetic resonance (MR) spectroscopy.
Data analysis
Data was analysed using SPSS version 25 (Statistical Package for the Social Sciences Inc., Chicago, IL, USA). We performed descriptive statistics, presented continuous data as mean with standard deviation, and discrete data as proportions. Bivariate analysis using Chi-square was done for comparing proportions considering p-value less than 0.05 as significant. The number of children who underwent neuroimaging, different CSF tests and other procedures or for whom the results were available were presented as such (Table 1).
Table 1.
Baseline clinical, radiological and laboratory characteristics of children diagnosed with tuberculous meningitis (N = 47).
| Characteristics | n | N | % |
|---|---|---|---|
| Signs and symptoms | |||
| Fever | 39 | 47 | 83 |
| Vomiting | 31 | 47 | 66 |
| Headache | 26 | 47 | 55 |
| Lethargy/Altered sensorium | 31 | 47 | 66 |
| Seizures | 21 | 47 | 45 |
| Weight loss | 14 | 47 | 30 |
| irritability | 15 | 47 | 32 |
| Cough | 6 | 47 | 13 |
| Cranial nerve palsy* | 7 | 47 | 15 |
| FND $ | 10 | 47 | 21 |
| Meningeal signs | 18 | 47 | 38 |
| Modified BMRC grading | |||
| Grade I | 13 | 47 | 28 |
| Grade II | 21 | 47 | 45 |
| Grade III | 13 | 47 | 28 |
| Neuroimaging (CT/MRI/Both) | 44 | ||
| Hydrocephalus | 25 | 44 | 57 |
| Basal meningeal enhancement | 16 | 44 | 36 |
| Tuberculoma | 9 | 44 | 20 |
| Infarcts | 8 | 44 | 18 |
| Abnormal CT | 24 | 31 | 77 |
| Hydrocephalus | 17 | 31 | 55 |
| Tuberculoma | 3 | 31 | 10 |
| Basal meningeal enhancement | 9 | 31 | 29 |
| Infarcts | 2 | 31 | 6 |
| Abnormal MRI | 26 | 34 | 76 |
| Tuberculoma | 7 | 34 | 21 |
| Hydrocephalus | 13 | 34 | 36 |
| Basal meningeal enhancement | 9 | 34 | 26 |
| Infarcts | 6 | 34 | 18 |
| Abnormal CSF cytology ‡ | 24 | 43 | 56 |
| Abnormal CSF protein § | 22 | 45 | 49 |
| Abnormal CSF Sugar || | 21 | 45 | 47 |
| Underwent VP shunt | 15 | 59 | 25 |
| Previous H/O TB | 5 | 47 | 11 |
| Contact with TB | 8 | 46 | 17 |
| X-ray abnormalities | 6 | 41 | 15 |
| Gastric aspirate | 3 | 20 | 15 |
| Manteaux positive | 10 | 38 | 26 |
Common cranial nerve palsies – third, sixth and seventh nerve palsies.
Hemiparesis/hemiplegia-9, Paraparesis-1.
Clear appearance. Cells 10–500/µL, lymphocyte predominance (>50%).
Abnormal CSF protein >1 g/L.
Absolute glucose concentration <2.2 mmol/L.
BMRC, British Medical Research Council; CSF, cerebrospinal fluid; CT, computed tomography; FND, focal neurological deficit; MRI, magnetic resonance imaging; TB, tuberculous; VP, ventriculoperitoneal.
Results
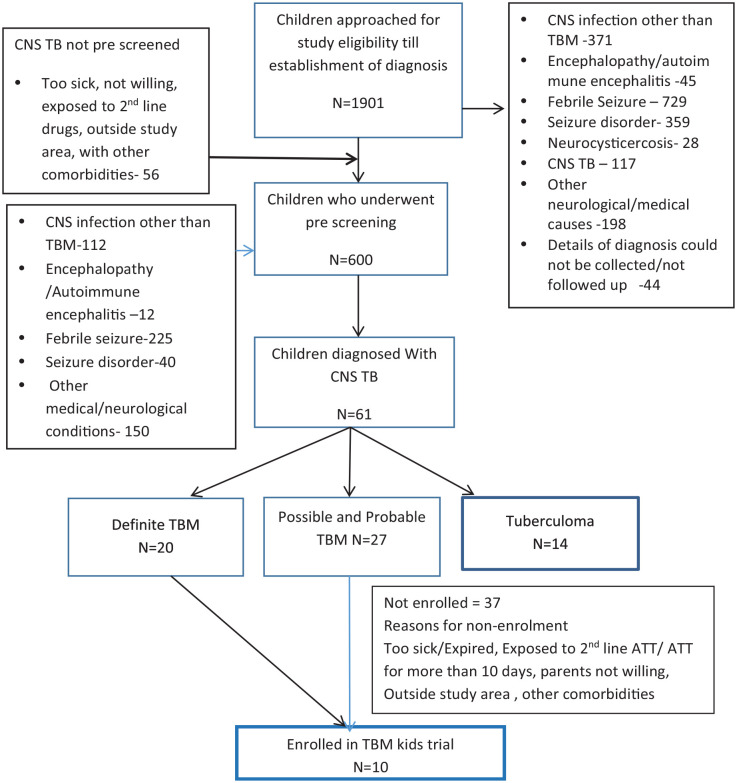
A total of 1901 children ages 6 months to 12 years admitted with neurological symptoms and signs consistent with possiblity TBM were followed until diagnosis was established. Among them, 600 with suspected acute meningoencephalitis/TBM underwent LP. Of the 600, 61 (10%) were diagnosed with CNS TB (TBM n = 47 and tuberculoma n = 14). Of 47 children with TBM, 20 had a positive confirmative diagnosis by GeneXpert or MGIT and 27 were diagnosed with probable or possible TBM (Figure 1).
Figure 1.
Flow chart of children with neurological manifestations approached for the clinical trial and diagnosed with CNS TB.
CNS, central nervous system; TB, tuberculosis.
Baseline characteristics
Baseline characteristics of children with TBM are presented in Table 1. The mean age of children with TBM was 5 ± 3.4 years and 26 (55%) were ⩽5 years of age. Eight of ten children who presented with focal neurological deficit (FND) were in the age group ⩽5 years and five had cranial nerve palsies as well. Among the 47 with TBM, 12(26%), 14(30%) and 21(45%) were in the age group of 6 months to <2 years, 2 to <6 years and 6 to 12 years respectively. Mean age of children with tuberculoma was 8 years ± 3.1 years. Of the 14 children with isolated tuberculoma, none were in the less than 2 years age group. Among the children in the 2 to <6 and 6 to 12 years age group, tuberculoma was present in 3 and 11 respectively. There was an increasing trend of isolated tuberculoma and a decreasing trend of TBM with increasing age.
Details of BCG immunisation were available for 48(78%) children and BCG scar was present in 39(81%). Of the 39 BCG vaccinated, 16(41%) had grade I, 15(38%) had grade II, 8(21%) had grade III disease. Altered sensorium/lethargy was seen in 15/20(75%) versus 7/27(56%) in definite TBM and other forms (probable and possible TBM) respectively (p-value < 0.005). Meningeal signs were noted in 11/20(55%) versus 7/27(26%) in definite TBM and other forms respectively (p-value 0.04). Seizures were observed among 11/20(55%) and 10/27(37%) children diagnosed with definite TBM and other forms respectively (p-value 0.22).
Neuroimaging in TBM
Of the 44/47 children with TBM who had undergone neuroimaging (34 MRI, 31 CT), 10 had only CT, 13 had only MRI and 21 had both. Among children who underwent MRI alone, hydrocephalus (8), basal meningeal enhancement (7), tuberculoma (6) and infarcts (6) were observed. Of the children, who had CT alone, features suggestive of TBM included hydrocephalus (12), basal meningeal enhancement (7) tuberculoma (2) and infarcts (2). Hydrocephalus (5), meningeal enhancement (2) and tuberculoma (1) were observed in both CT and MRI among children who underwent both investigations. Among TBM patients with tuberculoma, five had multiple lesions, three had single lesions and one had a tubercular abscess.
Mycobacterial tests
CSF AFB smear was negative in all children. MGIT culture was positive for M. tb growth in 20/35(57%) and Gene Xpert detected M. tb in 8/56 (14%) CSF samples. All eight CSF samples that detected M.tb in GeneXpert also showed growth in MGIT culture. DST in liquid culture showed resistance to antituberculous drugs in five children. All had INH resistance, Isolated H resistance in one and additional resistance to Rifampicin (1), Ethionamide (1), Ethambutol (1) and pyrazinamide (1). None of them had previous history of TB. None were contacts of TB patients except for one.
Ventriculoperitoneal shunt
Neuroimaging showed hydrocephalus in 25 children (8 in MRI, 12 in CT and 5 in both CT and MRI). Out of 47 children with TBM, 15 (32%), underwent VP shunt based on clinical condition and neuroimaging suggestive of hydrocephalus. Among them, 10 (67%) were ⩽ 5 years and five were less than 2 years of age. Of the 15, eight did not have a confirmatory test result as M. tb detected. CT/MRI brain showed basal leptomeningeal enhancement in seven, infarcts in three and granuloma in two in addition to hydrocephalus. Medical management was given to the other children who had not undergone VP shunt.
Isolated tuberculoma
Among children with tuberculoma, presenting features were headache (9), seizures and vomiting (8), fever (5) and lethargy/altered sensorium (4). Among them, four and six had undergone CT and MRI respectively, four had undergone both CT and MRI. Isolated tuberculoma/s were reported in all those imaging. Four had history of contact, none had any previous history of TB. Eight out of 12 for whom BCG vaccination details were known had BCG scar.
Outcome
Among the 41 with TBM with known outcomes at discharge, 5 died. All five had definite TBM, four had grade III and one had grade II disease, three had hydrocephalus and one had undergone VP shunt. Three of them were not vaccinated with BCG. One child also had lung involvement. No children with tuberculoma died at discharge.
Management
Of the 10 children enrolled in the TBM KIDS trial, four were randomised to high-dose Rifampicin (Rh30 mg/kg), levofloxacin, Isoniazid (H), Pyrazinamide (Z), four were randomised to Rh30 mg/kg, Ethambutol(E), HZ and two were randomised to standard WHO regimen (HREZ) in the intensive phase. All the children received standard WHO regimen (HR) in the continuation phase for 10 months. 6 All the other children diagnosed with TBM/tuberculoma, but ineligible for the trial were initiated on antituberculous therapy as per National TB programme.
Discussion
Paediatric TBM is a debilitating disease if not diagnosed early and treated appropriately. In our TBM cohort, almost half were less than 5 years of age. Mean age in studies reported by Savardekar et al. and Farihna et al. was 3 years.2,7 van Well et al. reported that 82% of children in their cohort were less than 5 years of age. 8 Higher risk for TBM in infants and gradual decrease in incidence with increasing age similar to our cohort has been reported in earlier studies as well. 9 The most common presenting symptom was fever in almost three-fourth of the children with TBM followed by vomiting. Fever and vomiting were the presenting symptoms in 79% and 70% of children with TBM in Turkey. 10 Almost half of the children presented with headache, altered sensorium and seizure in our TBM cohort. A study from western India observed fever (72%), altered sensorium (57%) and seizure (50%) similar to our study. However, headache (26%) and vomiting (17%) were observed in lesser number of children. 11 Higher number of Vietnamese children had fever (99%), headache (89%) and vomiting (76%). One-fourth of those children (27%) presented with unconsciousness whereas half of our children had altered sensorium. 12 Altered sensorium and meningeal signs were more commonly observed in children with definite TBM than probable/possible TBM. Miftode et al. 13 reported that MTB positivity is frequently reported in TBM patients with altered mental status. Varied nonspecific symptoms at the time of presentation to the health centre are a stumbling block in early diagnosis. More than three-fourth of the children had BCG vaccination. BCG given during infancy is shown to be more beneficial in prevention of TBM in younger children. 14
Abnormal findings on neuroimaging were observed in more than three-fourth of children with TBM. Hydrocephalus was commonly observed in both MRI and CT in TBM. Pienaar et al. 15 reported the superiority of MRI brain in detecting basal meningitis and infarcts. Similarly in our cohort, infarcts were detected more in MRI (6/34) versus (2/31). Basal meningitis was observed in MRI (9/34) versus CT (9/31) respectively. Almost 20% (9/44) of the children with TBM had tuberculoma in imaging in our cohort. Fatema et al. 16 reported hydrocephalus in 55%, tuberculoma in 50% and basal meningitis in 34% of their children. Among children with TBM who had undergone VP shunt as reported by Rohlwink et al. 3 while 50% had no neurological impairment, all had neurodevelopmental deficits. In our TBM cohort, almost one-third of the children underwent VP shunt and most of them were younger. Tuberculomas appear as ring-enhancing lesions in post-contrast CT/MRI brain, that is, round or irregular lesions surrounded by oedema. Elevated lipid lactate peak levels in MR spectroscopy are helpful in diagnosis. 6 None of the children in our cohort with isolated tuberculoma/s needed any surgical interventions.
CSF abnormalities including white blood cell count, glucose and protein were observed in almost half of our TBM cohort. Bang et al., reported that 97% of the children with TBM had CSF lymphocytosis. Similar to our study, AFB smear was negative in those children. 12 Mycobacterial positivity was 56% by liquid culture in our cohort. Rai et al. 17 reported CBNAAT positivity of 16% and AFB culture positivity of 27% among paediatric TBM. CSF Xpert positivity was around 37% in another setting from India. 18 Higher volume of CSF submitted for testing was shown to have an association with bacteriological confirmation in adults. 19 The challenges related to collection of CSF in sick children and the impact of volume available for testing need to be considered for these variations.
Limitations of our study include lack of outcome after hospitalisation as the main focus was to identify children for trial eligibility. This was a retrospective review done in a single centre among patients screened for clinical trial participation in a hyperendemic setting. The results may be generalisable to hyperendemic settings and may not be applicable to other settings. However, presenting the diagnostic features in a high TB burden setting might be useful to understand the strengths and gaps in TBM diagnosis. Given the retrospective nature of the study, missing data was significant in a number of cases.
Conclusion
Younger children are commonly affected with TBM and atypical presentations are common. Yield of available mycobacterial tests in CSF is low. Absence of confirmatory diagnosis (molecular diagnostics/AFB culture) does not rule out TBM. Strong suspicion in the presence of varied clinical features and holistic diagnostic work up, including CSF testing and neuroimaging, is the key to early diagnosis and management of paediatric TBM.
Supplemental Material
Supplemental material, sj-docx-1-tai-10.1177_20499361241274251 for Clinical and diagnostic features of central nervous system tuberculosis in Indian children – a descriptive study by Bella Devaleenal Daniel, Elilarasi Selladurai, Sarath Balaji, Arunagirinathan Venkatesan, Mythily Venkatesan, Prathiksha Giridharan, Sivakumar Shanmugam, Saravanan Natrajan, Ramesh Karunaianantham, Devika Kandasamy, Rajakumar Subramani, Kannan Muthuramalingam, Snegha K. Pramila, Syed Hissar, Kelly E. Dooley and Kiran T. Thakur in Therapeutic Advances in Infectious Disease
Acknowledgments
The authors acknowledge the following study team members for their contributions: Indian Council of Medical Research–National Institute for Research in Tuberculosis, Chennai (ICMR-NIRT): Mangalambal Ganesan, Gunasundari Arasan, Shakila Shankar, S. Stella Mary, Sureshwari Karuppaiah, Krishna Yadav, Leema Pauline, Priyadharshini Arul, Prabhavathy, Sankar Ganesh, Luke Elizabeth Hanna, Ruthra Vijayakumar, Surekha, S. Sathya, A. Radhakrishnan, A. R. Preethi, Kwasakar, M. Dharman, V. Sudha, Valarmathi Nagarajan, Linda Jennifer, R. Supriya, R. Manimegalai, Santhanam Kandan, Archana Maniselvi, Oli Puspha, S. Vaishnavi, R. Selvi, Logeswari Neelakandan. Additionally, we are thankful to Dr. Leeberk Raja and Dr. Aishwarya for their technical input.
Footnotes
ORCID iDs: Bella Devaleenal Daniel  https://orcid.org/0000-0001-6293-4990
https://orcid.org/0000-0001-6293-4990
Kiran T. Thakur  https://orcid.org/0000-0003-0050-0323
https://orcid.org/0000-0003-0050-0323
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Bella Devaleenal Daniel, ICMR – National Institute for Research in Tuberculosis, No 1, Mayor Satyamoorthy Road, Chetpet, Chennai 600031, India.
Elilarasi Selladurai, Institute of Child Health, Madras Medical College, Chennai, India.
Sarath Balaji, Institute of Child Health, Madras Medical College, Chennai, India.
Arunagirinathan Venkatesan, Institute of Child Health, Madras Medical College, Chennai, India.
Mythily Venkatesan, ICMR – National Institute for Research in Tuberculosis, Chennai, India.
Prathiksha Giridharan, ICMR – National Institute for Research in Tuberculosis, Chennai, India.
Sivakumar Shanmugam, ICMR – National Institute for Research in Tuberculosis, Chennai, India.
Saravanan Natrajan, ICMR – National Institute for Research in Tuberculosis, Chennai, India.
Ramesh Karunaianantham, ICMR – National Institute for Research in Tuberculosis, Chennai, India.
Devika Kandasamy, ICMR – National Institute for Research in Tuberculosis, Chennai, India.
Rajakumar Subramani, ICMR – National Institute for Research in Tuberculosis, Chennai, India.
Kannan Muthuramalingam, ICMR – National Institute for Research in Tuberculosis, Chennai, India.
Snegha K. Pramila, ICMR – National Institute for Research in Tuberculosis, Chennai, India
Syed Hissar, ICMR – National Institute for Research in Tuberculosis, Chennai, India.
Kelly E. Dooley, John Hopkins University School of Medicine, Baltimore, MD, USA
Kiran T. Thakur, Columbia University Irving Medical Center, New York Presbyterian Hospital, New York, NY, USA
Declarations
Ethics approval and consent to participate: The Institutional Ethics Committee of National Institute for Research in Tuberculosis (IEC study ID: 2015008) and the Institutional Ethics Committee of Madras Medical College (IEC study ID: 25062015) approved the TBM KIDS trial (NCT02958709) and the data collection for this study (pre-screening for TBM kids trial eligibility). Parents of all the children provided written informed consent for pre-screening the children for the trial. In addition to the parental consent, children more than 7 years of age provided assent, when their neurologic status permitted for the trial.
Consent for publication: Not applicable.
Author contributions: Bella Devaleenal Daniel: Conceptualisation; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Validation; Visualisation; Writing – original draft; Writing – review & editing.
Elilarasi Selladurai: Conceptualisation; Investigation; Methodology; Resources; Visualisation; Writing – original draft; Writing – review & editing.
Sarath Balaji: Data curation; Investigation; Resources; Visualisation; Writing – original draft; Writing – review & editing.
Arunagirinathan Venkatesan: Data curation; Methodology; Resources; Visualisation; Writing – original draft; Writing – review & editing.
Mythily Venkatesan: Conceptualisation; Data curation; Formal analysis; Methodology; Software; Validation; Visualisation; Writing – original draft; Writing – review & editing.
Prathiksha Giridharan: Formal analysis; Investigation; Methodology; Project administration; Visualisation; Writing – original draft; Writing – review & editing.
Sivakumar Shanmugam: Investigation; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Saravanan Natrajan: Investigation; Methodology; Supervision; Visualisation; Writing – original draft; Writing – review & editing.
Ramesh Karunaianantham: Investigation; Methodology; Validation; Visualisation; Writing – original draft; Writing – review & editing.
Devika Kandasamy: Investigation; Methodology; Visualisation; Writing – original draft; Writing – review & editing.
Rajakumar Subramani: Investigation; Methodology; Visualisation; Writing – original draft; Writing – review & editing.
Kannan Muthuramalingam: Investigation; Methodology; Visualisation; Writing – original draft; Writing – review & editing.
Snegha K. Pramila: Investigation; Methodology; Visualisation; Writing – original draft; Writing – review & editing.
Syed Hissar: Data curation; Methodology; Visualisation; Writing – original draft; Writing – review & editing.
Kelly E. Dooley: Conceptualisation; Data curation; Funding acquisition; Methodology; Project administration; Resources; Supervision; Visualisation; Writing – original draft; Writing – review & editing.
Kiran T. Thakur: Conceptualisation; Data curation; Methodology; Validation; Visualisation; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) at the National Institutes of Health (NIH) (award number R01HD074944).
Competing interests: The authors declare that there is no conflict of interest.
Availability of data and materials: The data contains potentially sensitive information, and the data access is governed by ICMR-NIRT. It can be made available through a data sharing according to Indian Government and NIH norms. Data are accessible to the interested upon request. ICMR data access request must be completed and reviewed. The form can be obtained from ICMR-NIRT, India –nirtdirector.ps@icmr.gov.in
References
- 1. Seddon JA, Tugume L, Solomons R, et al. The current global situation for tuberculous meningitis: epidemiology, diagnostics, treatment and outcomes. Wellcome Open Res 2019; 4: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Farinha N, Razali K, Holzel H, et al. Tuberculosis of the central nervous system in children: a 20-year survey. J Infect 2000; 41(1): 61–68. [DOI] [PubMed] [Google Scholar]
- 3. Rohlwink UK, Donald K, Gavine B, et al. Clinical characteristics and neurodevelopmental outcomes of children with tuberculous meningitis and hydrocephalus. Dev Med Child Neurol 2016; 58(5): 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Daniel BD, Grace GA, Natrajan M. Tuberculous meningitis in children: clinical management & outcome. Indian J Med Res 2019; 150(2): 117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abdella A, Deginet E, Weldegebreal F, et al. Tuberculous Meningitis in children: treatment outcomes at Discharge and its Associated factors in Eastern Ethiopia: a five years retrospective study. Infect Drug Res 2022: 15: 2743–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chatterjee S. Brain tuberculomas, tubercular meningitis, and post-tubercular hydrocephalus in children. J Pediatric Neurosci 2011; 6(Suppl. 1): S96–S100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Savardekar A, Chatterji D, Singhi S, et al. The role of ventriculoperitoneal shunt placement in patients of tubercular meningitis with hydrocephalus in poor neurological grade: a prospective study in the pediatric population and review of literature. Child’s Nervous Syst 2013; 29: 719–725. [DOI] [PubMed] [Google Scholar]
- 8. Van Well GT, Paes BF, Terwee CB, et al. Twenty years of pediatric tuberculous meningitis: a retrospective cohort study in the western cape of South Africa. Pediatrics 2009; 123(1): e1–e8. [DOI] [PubMed] [Google Scholar]
- 9. Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era [State of the Art]. Int J Tuberculosis Lung Dis 2004; 8(4): 392–402. [PubMed] [Google Scholar]
- 10. Güneş A, Uluca Ü, Aktar F, et al. Clinical, radiological and laboratory findings in 185 children with tuberculous meningitis at a single centre and relationship with the stage of the disease. Italian J Pediatr 2015; 41: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Israni AV, Dave DA, Mandal A, et al. Tubercular meningitis in children: clinical, pathological, and radiological profile and factors associated with mortality. J Neurosci Rural Pract 2016; 7(03): 400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bang ND, Caws M, Truc TT, et al. Clinical presentations, diagnosis, mortality and prognostic markers of tuberculous meningitis in Vietnamese children: a prospective descriptive study. BMC Infect Dis 2016; 16: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miftode EG, Dorneanu OS, Leca DA, et al. Tuberculous meningitis in children and adults: a 10-year retrospective comparative analysis. PLoS One 2015; 10(7): e0133477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 2006; 367(9517): 1173–1180. [DOI] [PubMed] [Google Scholar]
- 15. Pienaar M, Andronikou S, van Toorn R. MRI to demonstrate diagnostic features and complications of TBM not seen with CT. Child’s Nervous Syst 2009; 25: 941–947. [DOI] [PubMed] [Google Scholar]
- 16. Fatema K, Rahman MM, Akhter S, et al. Clinicoradiologic profile and outcome of children with tubercular meningitis in a tertiary care hospital in Bangladesh. J Child Neurol 2020; 35(3): 195–201. [DOI] [PubMed] [Google Scholar]
- 17. Rai A, Prasad R, Das B, et al. Cerebrospinal fluid Gene XPERT (CBNAAT) in children with tuberculous meningitis. J Clin Tuberculosis Mycobacterial Dis 2021; 24: 100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Soma SK, Lingappa L, Raju S, et al. Clinical profile, yield of cartridge-based nucleic acid amplification test (GeneXpert), and outcome in children with tubercular meningitis. J Pediatric Neurosci 2020; 15(3): 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heemskerk AD, Donovan J, Marais S, et al. Improving the microbiological diagnosis of tuberculous meningitis: a prospective, international, multicentre comparison of conventional and modified Ziehl–Neelsen stain, GeneXpert, and culture of cerebrospinal fluid. J Infect 2018; 77(6): 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tai-10.1177_20499361241274251 for Clinical and diagnostic features of central nervous system tuberculosis in Indian children – a descriptive study by Bella Devaleenal Daniel, Elilarasi Selladurai, Sarath Balaji, Arunagirinathan Venkatesan, Mythily Venkatesan, Prathiksha Giridharan, Sivakumar Shanmugam, Saravanan Natrajan, Ramesh Karunaianantham, Devika Kandasamy, Rajakumar Subramani, Kannan Muthuramalingam, Snegha K. Pramila, Syed Hissar, Kelly E. Dooley and Kiran T. Thakur in Therapeutic Advances in Infectious Disease