Abstract
Background
Fibronectin glomerulopathy (FNG) is a rare autosomal dominant glomerulopathy that can lead to nephrotic syndrome. Here we report the case of an elderly patient diagnosed with FNG, exhibiting nephrotic-range proteinuria, with a 2-year follow-up.
Case presentation
A 75-year-old Korean female visited the nephrology clinic after experiencing generalized edema for 2 months. Her serum creatinine was 1.36 mg/dL, and urine protein-to-creatinine ratio was 3.99 g/g. Kidney biopsy revealed mesangial and subendothelial dense deposits, and immunohistochemistry for fibronectin showed strong positivity in the glomerulus. The patient’s family history included non-specific renal disease in her mother and two siblings. Genetic testing of the fibronectin 1 (FN1) gene showed Y973C mutation. She received conservative treatment, including angiotensin II receptor blockers (ARB). Two years after biopsy, the patient has preserved renal function and reduced proteinuria.
Conclusion
We report the case of a 75-year-old patient with nephrotic-range proteinuria, who was diagnosed with FNG, and found to harbor a FN1 gene mutation. In this case, conservative treatment including ARB yielded reduction of proteinuria and preservation of renal function.
Keywords: Fibronectin, Nephrotic syndrome, FN1, Glomerulopathy with Fibronectin Deposit, Elderly
Background
Fibronectin glomerulopathy (FNG) is a rare autosomal dominant glomerulopathy that manifests at various ages in both sexes [1], and is characterized by glomerular fibrillary deposits that show strong immune reactivity to fibronectin [2]. Upon kidney biopsy in FNG cases, light microscopy elucidates massive fibronectin deposition in the mesangium and along capillary walls, and electron microscopy reveals fibronectin deposition as finely granular or fibrillary substructures with randomly arranged fibrils of 12–16 nm [2, 3]. Patients with FNG exhibit clinical features, such as proteinuria, microscopic hematuria, hypertension, and renal impairment. Some patients show nephrotic-range proteinuria, and approximately 25–37% of FNG patients will progress to end-stage kidney disease (ESKD) [1, 4].
FNG is caused by mutations in the fibronectin 1 gene (FN1) on chromosome 2, and dominant mutations in FN1 reportedly account for 40% of FNG cases [5]. However, some genetically tested cases have not displayed any previously identified gene mutation [6].
Here, we report the case of a patient diagnosed with FNG at the age of 75 years, who exhibited nephrotic-range proteinuria. This case report includes the results of genetic analysis, and 2-year follow-up.
Case presentation
A 75-year-old Korean female visited the nephrology clinic for evaluation of azotemia and proteinuria, after experiencing generalized edema for 2 months. She had been diagnosed with hypertension 6 years before admission, and took a calcium channel blocker (S-Amlodipine besylate® 2.5 mg/day). Her blood pressure was 135/78 mmHg. Laboratory testing revealed elevated BUN (25.9 mg/dL) and serum creatinine (1.36 mg/dL). Her estimated glomerular filtration rate (eGFR) was 38 mL/min/1.73 m2, as calculated using the CKD-EPI equation. Urinalysis revealed proteinuria 3 + in dipstick and microscopic hematuria (urine RBC 10–20/HPF). The spot urine protein-to-creatinine ratio (PCR) was 3.99 g/g, and 24-hour urine collection showed urine total protein of 3629 mg/day. Here white blood cell count was 6,600/mm3, hemoglobin 13.0 g/dL, and platelet count 264,000/mm3. Results were negative in tests for anti-nuclear antibodies (ANA), anti-neutrophil cytoplasmic antibodies (ANCA), anti-glomerular basement membrane (anti-GBM) antibodies, anti-streptolysin O (ASO) antibodies, and rheumatoid factor (RF). Serologic test results were within normal limits—including IgA of 223 mg/dL (reference range, 70–400 mg/dL), IgG 844 mg/dL (reference range, 700-1,600 mg/dL), IgM 73 mg/dL (reference range, 40–230 mg/dL), C3 92 mg/dL (reference range, 90–180 mg/dL), and C4 26.9 mg/dL (reference range, 10–40 mg/dL). Negative results were obtained from serologic tests for hepatitis B, hepatitis C, and human immunodeficiency viruses. Serum M protein was not detected and serum protein electrophoresis was unremarkable. Renal ultrasound showed that the kidneys were of normal size and increased parenchymal echogenicity.
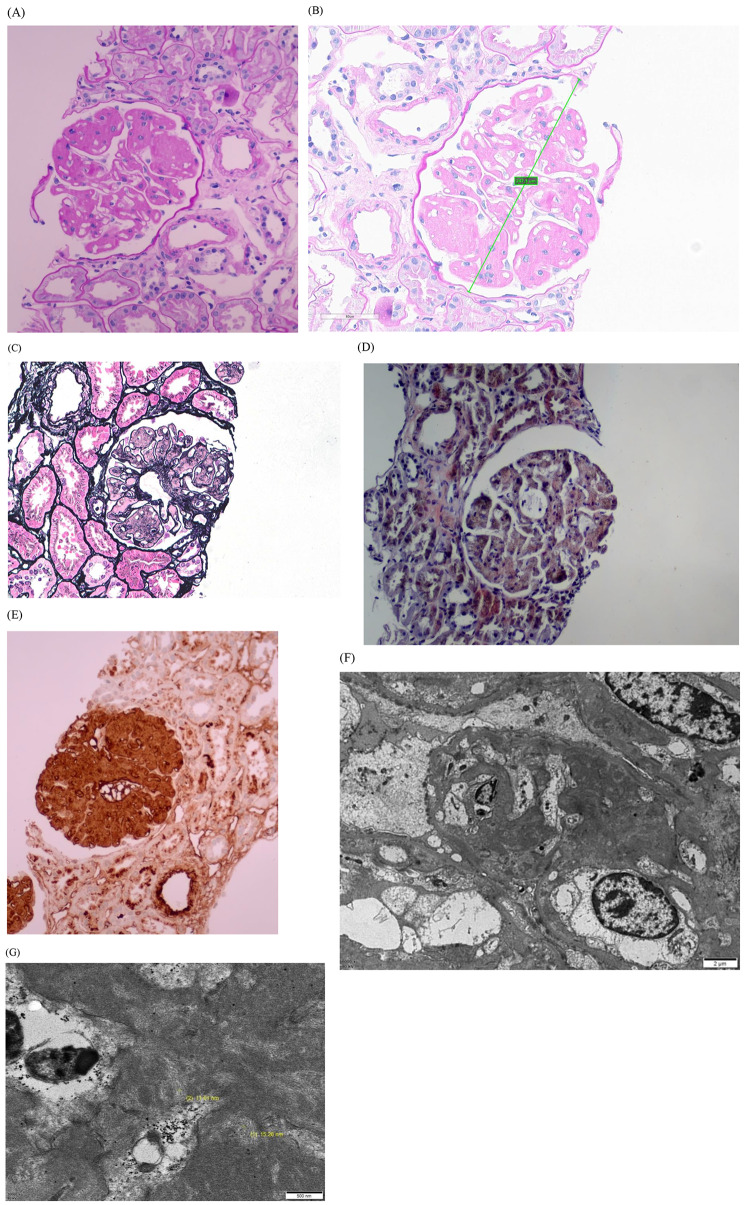
Renal biopsy was performed. The glomerulus exhibited mesangial expansion and lobular accentuation without mesangial hypercellularity upon PAS staining (Fig. 1A), the glomerular diameter was 247.3 micrometer (Fig. 1B). The mesangial expansion is negative staining in Periodic Acid Methenamine Silver (PAM) staining (Fig. 1C). Congo-red staining was negative for amyloid (Fig. 1D). Immunohistochemistry for fibronectin (A235, DAKO) showed strong positive staining in the glomerulus (Fig. 1E). Immunofluorescence results for IgG, IgA, IgM, C3, C1q, and fibrinogen were all negative in glomeruli and vessels. Electron microscopy revealed massive mesangial and subendothelial electron dense deposits, along with diffuse foot process effacement (Fig. 1F). These deposits exhibited a granular and fibrillary substructure, with fibril diameters ranging from 11 to 16 nm (Fig. 1G). Based on these findings, the patient was diagnosed with FNG.
Fig. 1.
Histologic findings of renal tissues. (A) Upon PAS staining, the glomerulus exhibits mesangial expansion and lobular accentuation without mesangial hypercellularity (x200), (B) the glomerular diameter was 247.3 micrometer (x400). (C) The mesangial expansion is negative staining in PAM staining (x400). (D) Congo red staining (x400) shows negative results. (E) Immunohistochemistry for fibronectin revealed strong positive staining in the glomerulus. (F) Electron microscopy revealed massive mesangial and subendothelial electron dense deposits, along with diffuse foot process effacement. (G) These deposits exhibited a granular and fibrillary substructure, with fibril diameters ranging from 11–16 nm
(A)
Family history was further investigated. The patient’s mother had clinical features of wax and wane generalized edema and suffered from kidney disease, although no specific disease name was known. The patient has two younger brothers and two younger sisters, of whom one brother and one sister are being treated for proteinuria. These two siblings had not undergone renal biopsy; therefore, their specific diagnoses were also unknown. None of the patient’s children or nephews showed renal disease or proteinuria. Genetic testing of whole-blood samples revealed an FN1 gene mutation—with an adenine changed to guanine at nucleotide 2,918 of the complementary DNA (c.2918 A > G)—causing a substitution in which the tyrosine at amino acid 973 is replaced by cysteine (Y973C).
The patient’s anti-hypertensive medication was changed to angiotensin II receptor blocker (Azilsartan® 20 mg/day), and torsemide (10 mg/day) was added for edema control. At 6 months after kidney biopsy, serum creatinine was 1.41 mg/dL (eGFR 36 mL/min/1.73 m2) and spot urine PCR was 2.35 g/g. At 1 year after biopsy, serum creatinine was 1.43 mg/dL (eGFR 35 mL/min/1.73 m2) and spot urine PCR was 0.48 g/g. At 2 years after renal biopsy, the patient’s renal function maintained stable (serum Cr 1.56 mg/dL, eGFR 32 mL/min/1.73 m2) and proteinuria was reduced (spot urine PCR 0.14 g/g).
Discussion
Here we report the case of a 75-year-old female patient who had been taking anti-hypertensive medication for high blood pressure for 6 years, developed generalized edema and nephrotic-range proteinuria, and was diagnosed with FNG, which was supported by genetic testing results.
FNG is a rare autosomal dominant disease with age-related penetrance, characterized by proteinuria, microscopic hematuria, hypertension, and massive glomerular fibronectin deposits that lead to ESKD [5]. Nephrotic-range proteinuria is reported in 35.9–73.6% of FNG patients [4, 6, 7]. In a brief analysis of a case series of 19 patients, those with nephrotic-range proteinuria had a significantly higher probability of renal function decline than patients with non-nephrotic-range proteinuria [4]. Although our presently described patient exhibited nephrotic-range proteinuria, her renal function was relatively well-preserved.
Pathologic features of FNG is lobular accentuation with mesangial expansion with minimal hypercellularity in light microscopy. Fibronectin immunochemistry stain is strongly positive in deposits, whereas immunofluorescence usually shows negative. In electron microscopy there are massive mesangial and subendothelial deposits, that could show focal fibrillary substructure with a fibril diameter 12–16 nm [3]. However, in this case, mesangial hypercellularity and glomerulomegaly were not observed because only two glomeruli were included in the light microscopy sample, which may be a limitation of this case. Although non-specific fibronectin deposition might be shown in elderly patients, immunohistochemistry for fibronectin revealed strong positive staining in the glomerulus of this patient, which was an important basis that allowed the diagnosis of FNG.
FNG manifests across various age groups, but few cases have been reported in patients over 65 years of age, and most of those patients have progressed to ESKD requiring dialysis (Table 1) [4, 7–9]. FNG generally exhibits a slow progression to ESKD over a period of 15–20 years, with ESKD developing in the 2nd to 6th decade of life [3, 10]. The reported duration of progression to ESKD varies from 71 to 93 months [1, 4]. Although our presently reported patient was 75 years old, her renal function remained stable, and proteinuria was reduced after 2 years of follow-up.
Table 1.
Fibronectin glomerulopathy in elderly patients
| Reference | Age at Bx | Sex | Ethnicity | Laboratory findings at diagnosis |
Family history | Genetic study | Outcome |
|---|---|---|---|---|---|---|---|
| Ishimoto et al.[8] | 78 | F | Japanese | Cr 1.14 mg/dL, UPCR 10.2 | None |
No FN1 missense mutations |
Dialysis 2 months after Bx |
| Yoshino et al.[9] | 67 | M | Japanese | Cr 1.7 mg/dL, UPCR 3.6 | Not examined | Not evaluated |
Cr 3.54 mg/dL 7 years after Bx |
| Cheng et al.[7] | 88 | M | Chinese | Cr 13.0 mg/dL, Anuria | None | Not evaluated |
Regular dialysis since Bx and died of heart attack half a year later |
| Zhang et al.[4] | 71 | M | Chinese | Cr 1.13 mg/dL, UPCR 1.21 |
2 sisters on dialysis |
Not evaluated | Dialysis 71 months after diagnosis |
Abbreviations: Bx, biopsy; Cr, creatinine; UPCR, urine protein-creatinine ratio
Castelletti et al. reported that FNG is associated with mutations in the locus of the gene encoding FN1 on chromosome 2q34. They identified three heterozygous missense mutations within the heparin-binding domains of FN1—Y973C, W1925R, and L1974R—which co-segregated with FNG in 40% of pedigrees [5]. Subsequently, Ohtsubo et al. identified six FN1 mutations from 12 families, including five novel FN1 mutations: p.Pro969Leu, p.Pro1472del, p.Trp1925Cys, p.Lys1953_Ilel961del, and p.Leu1974Pro [11]. Our patient has a family history of non-specific renal disease in her mother and two siblings. Whole-exome sequencing analysis of whole-blood samples revealed the mutation Y973C, which has been previously reported in the families of patients of various ethnicities, including from Italy, the Netherlands, Germany, Japan, and China [5, 10, 12]. This pathogenic variant in the FN1 gene is associated with FNG, an autosomal dominant condition with age-related penetrance. The observed phenotype of typical nephrotic syndrome confirms the presence of FNG.
Strategies for FNG treatment have not yet been established. Conservative treatment is generally applied, including blood pressure control and administration of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers (ARB). Steroid therapy has been tested, but its effectiveness is controversial. Prednisolone treatment decreased proteinuria in some patients [13], but did not yield a clear treatment response in other patients [9, 14]. Our presently described patient was treated with conservative treatment, including ARB, which achieved reduction of proteinuria and relative preservation of renal function. Randomized controlled trials might be unfeasible due to the rarity of FNG. However, the accumulation of reported experiences can suggest directions for FNG treatment.
Conclusions
In conclusion, here we report the case of a 75-year-old patient with nephrotic-range proteinuria, who was diagnosed with FNG, and found to harbor a FN1 gene mutation. In this case, conservative treatment including ARB yielded reduction of proteinuria and preservation of renal function.
Acknowledgements
The authors gratefully acknowledge professor Yong-Jin Kim and and Man-hoon Han (Department of pathology, School of Medicine, Kyungpook National University).
Abbreviations
- FNG
Fibronectin glomerulopathy
- FN1
Fibronectin 1
- ESKD
End-stage kidney disease
- eGFR
Estimated glomerular filtration rate
- ANA
Anti-nuclear antibodies
- ANCA
Anti-neutrophil cytoplasmic antibodies
- GBM
Glomerular basement membrane
- ASO
Anti-streptolysin O
- RF
Rheumatoid factor
- PCR
Protein-to-creatinine ratio
- ARB
Angiotensin II receptor blockers
Author contributions
Conceptualization: Choi JY. Data curation: Choi JY. Formal analysis: Choi JY, Kim Z. Investigation: Kim MS. Supervision: Choi JY, Kim Z. Visualization: Kim MS. Writing: Choi JY, Kim Z. Writing-review & editing: Choi JY, Kim Z. All authors have reviewed and agreed to the published version of the manuscript.
Funding
This work was supported by a 2024 Yeungnam University Research Grant.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Informed consent for publication was obtained from the patient.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gemperle O, Neuweiler J, Reutter FW, Hildebrandt F, Krapf R. Familial glomerulopathy with giant fibrillar (fibronectin-positive) deposits: 15-year follow-up in a large kindred. Am J Kidney Dis. 1996;28(5):668–75. [DOI] [PubMed] [Google Scholar]
- 2.Strom EH, Banfi G, Krapf R, Abt AB, Mazzucco G, Monga G, Gloor F, Neuweiler J, Riess R, Stosiek P, et al. Glomerulopathy associated with predominant fibronectin deposits: a newly recognized hereditary disease. Kidney Int. 1995;48(1):163–70. [DOI] [PubMed] [Google Scholar]
- 3.Lusco MA, Chen YP, Cheng H, Dong HR, Najafian B, Alpers CE, Fogo AB. AJKD Atlas of Renal Pathology: Fibronectin Glomerulopathy. Am J Kidney Dis. 2017;70(5):e21–2. [DOI] [PubMed] [Google Scholar]
- 4.Zhang T, Zhang W, Zuo K, Cheng Z. Clinicopathologic features and outcomes in Fibronectin Glomerulopathy: a Case Series of 19 patients. Front Med (Lausanne). 2020;7:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castelletti F, Donadelli R, Banterla F, Hildebrandt F, Zipfel PF, Bresin E, Otto E, Skerka C, Renieri A, Todeschini M, et al. Mutations in FN1 cause glomerulopathy with fibronectin deposits. Proc Natl Acad Sci U S A. 2008;105(7):2538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takii M, Suehiro T, Shima A, Yotsueda H, Hisano S, Katafuchi R. Fibronectin glomerulopathy complicated with persistent cloaca and congenital esophageal atresia: a case report and literature review. BMC Nephrol. 2017;18(1):288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng G, Wang Z, Yuan W, Dou Y, Liu D, Xiao J, Zhao Z. Fibronectin glomerulopathy in a 88 year-old male with acute kidney injury on chronic kidney disease: a case report and a review of the literature. Nefrologia. 2017;37(1):93–6. [DOI] [PubMed] [Google Scholar]
- 8.Ishimoto I, Sohara E, Ito E, Okado T, Rai T, Uchida S. Fibronectin glomerulopathy. Clin Kidney J. 2013;6(5):513–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshino M, Miura N, Ohnishi T, Suzuki K, Kitagawa W, Nishikawa K, Imai H. Clinicopathological analysis of glomerulopathy with fibronectin deposits (GFND): a case of sporadic, elderly-onset GFND with codeposition of IgA, C1q, and fibrinogen. Intern Med. 2013;52(15):1715–20. [DOI] [PubMed] [Google Scholar]
- 10.Nadamuni M, Piras R, Mazbar S, Higgins JP, Kambham N. Fibronectin glomerulopathy: an unusual cause of adult-onset nephrotic syndrome. Am J Kidney Dis. 2012;60(5):839–42. [DOI] [PubMed] [Google Scholar]
- 11.Ohtsubo H, Okada T, Nozu K, Takaoka Y, Shono A, Asanuma K, Zhang L, Nakanishi K, Taniguchi-Ikeda M, Kaito H, et al. Identification of mutations in FN1 leading to glomerulopathy with fibronectin deposits. Pediatr Nephrol. 2016;31(9):1459–67. [DOI] [PubMed] [Google Scholar]
- 12.Ertoy Baydar D, Kutlugun AA, Bresin E, Piras R. A case of familial glomerulopathy with fibronectin deposits caused by the Y973C mutation in fibronectin. Am J Kidney Dis. 2013;61(3):514–8. [DOI] [PubMed] [Google Scholar]
- 13.Goldman BI, Panner BJ, Welle SL, Gross MD, Gray DA. Prednisone-induced sustained remission in a patient with familial fibronectin glomerulopathy (GFND). CEN Case Rep. 2021;10(4):510–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hata M, Mori T, Hirose Y, Nishida Y, Mandai S, Ando F, Susa K, Iimori S, Naito S, Sohara E, et al. A case of unexpected diagnosis of fibronectin glomerulopathy with histological features of membranoproliferative glomerulonephritis. BMC Nephrol. 2024;25(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.