Abstract
Background
To examine the relationship between neutrophil-to-lymphocyte ratio (NLR), age, and mortality rates after emergency surgery.
Methods
In this observational study, a total of 851 patients undergoing emergency surgery between January 2022 and January 2023 were retrospective examined. Using 30 and 180 days mortality data, NLR differences and receiver operating characteristic (ROC) curves were analyzed using a 65-year threshold. A multiple logistic regression model was constructed incorporating age and NLR. Finally, Kaplan–Meier curves were constructed for mortality.
Results
Among 851 patients, the 30 and 180 days mortality rates were 5.2% and 10.8%, respectively. Median NLR in 30 days was 5.6 (3.1 to 9.6) in survivors and 8.7 (4.6 to 13.4) in deceased patients (p < 0.0001); in 180 days, it was 5.5 (3.1 to 9.8) and 8.8 (4.8 to 14.5), respectively (p < 0.0001). In the 30- and 180-days mortality analyses, median NLRs were 5.1 (2.9 to 8.9) and 4.9 (2.9 to 8.8) in survivors and 10.6 (6.9 to 16.6) and 9.3 (5.4 to 14.9) in deceased patients aged < 65 years, respectively. The ROC AUC in patients younger than 65 years was higher for 30 days (AUC 0.75; 95% CI 0.72 to 0.87) and 180 days (AUC 0.73; 95% CI 0.64 to 0.81). Multivariate logistic regression revealed that the NLR (odds ratio, 1.03 [95% CI 1.005 to 1.053; p = 0.0133) and age (odds ratio, 1.05 [95% CI 1.034 to 1.064; p < 0.0001) significantly contributed to the model. Survival analysis revealed differences in the 180 days mortality (p = 0.0006).
Conclusion
We observed differences in preoperative NLR between patients who survived and those who died after emergency surgery. Age impacts the use of NLR as a mortality risk factor.
Trial registration
NCT06549101, retrospectively registered.
Keywords: Neutrophil-to-lymphocyte ratio, Emergency surgery, Mortality, Prediction, Anesthesia, Preoperative care
Introduction
Immune system and inflammatory responses are crucial in systemic reactions to surgical stress and tissue repair [1]. However, it is not considered a significant factor in the preoperative assessment of patients before surgery.
One way to assess the inflammatory state and components of a patient’s innate immune response is the neutrophil-to-lymphocyte ratio (NLR), derived from the ratio between the number of neutrophils and the total lymphocyte count [2]. The NLR is a systemic inflammatory response index that reflects the balance between innate (neutrophils) and adaptive (lymphocytes) immune responses [3, 4]. This simple yet informative index can be readily obtained from routine preoperative complete blood count [5]. However, despite its clinical convenience, the role of the NLR in emergency surgery remains relatively underexplored.
The role of inflammation has been delineated using NLR in various health conditions such as acute kidney injury [4], ischemic stroke [6], cardiovascular mortality [7, 8], sepsis [9], chemotherapy outcomes in cancer [10, 11], and overall mortality [12]. Additionally, neutrophils are related to cardiovascular complications as one of the primary cellular components of the host immune response against pathogens and tissue damage repair [2, 13, 14].
Here, we hypothesized that an increased NLR reflects a subjacent inflammatory state predisposing patients to adverse postoperative outcomes. Therefore, we retrospectively analyzed patients who underwent emergency surgeries at our center. We aimed to address the relationship between the NLR and mortality rates after non-cardiac emergency surgery.
Materials and methods
Ethical approval and study design
Following approval from the Research Ethics Board of our hospital (Ref: OAIC 37/22), we conducted a retrospective observational analysis of all patients admitted to our center’s emergency operating room between January 2022 and January 2023. Patient consent was not required by the ethics committee because of the retrospective design of the study. Personal information was kept confidential.
Data collection
Data collected from electronic patient records included age, preoperative blood count, and the American Society of Anesthesiologists physical status classification. Additionally, comorbidities, complete blood count results and the type of surgery were documented. Using the national registry database, patients who died within 6 months were identified. Mortality analyses were conducted after 30 and 180 days. Additionally, a sub-analysis utilizing 65 years of age as the threshold was performed to assess the differences between younger and older patients.
Statistical analysis
Categorical variables were summarized using absolute and relative frequencies. Percentage comparisons were performed using Fisher’s exact test. Continuous variables were presented as medians and interquartile ranges. Median non-paired comparisons were assessed using the Mann–Whitney U test. Receiver operating characteristic (ROC) curves for mortality were generated using NLR data and 95% confidence intervals were computed [15]. Finally, we conducted multiple logistic regression analysis employing age and NLR as independent quantitative variables, while mortality was treated as a dichotomized outcome variable. We calculated a pseudo R2 (Tjur’s R2) and evaluated the goodness-of-fit using the Hosmer–Lemeshow test and log-likelihood ratio. Multicollinearity was ruled out using the Variance Inflation Factor (VIF) and R2 with other variables. The resulting model was further tested against a complex model with additional variables, incorporating comorbidities that were found to be significantly different between survivors and deceased patients. Statistical significance was set at p < 0.05. Data was analyzed using the GraphPad Prism software, version 10.1 (La Jolla, CA, USA).
Results
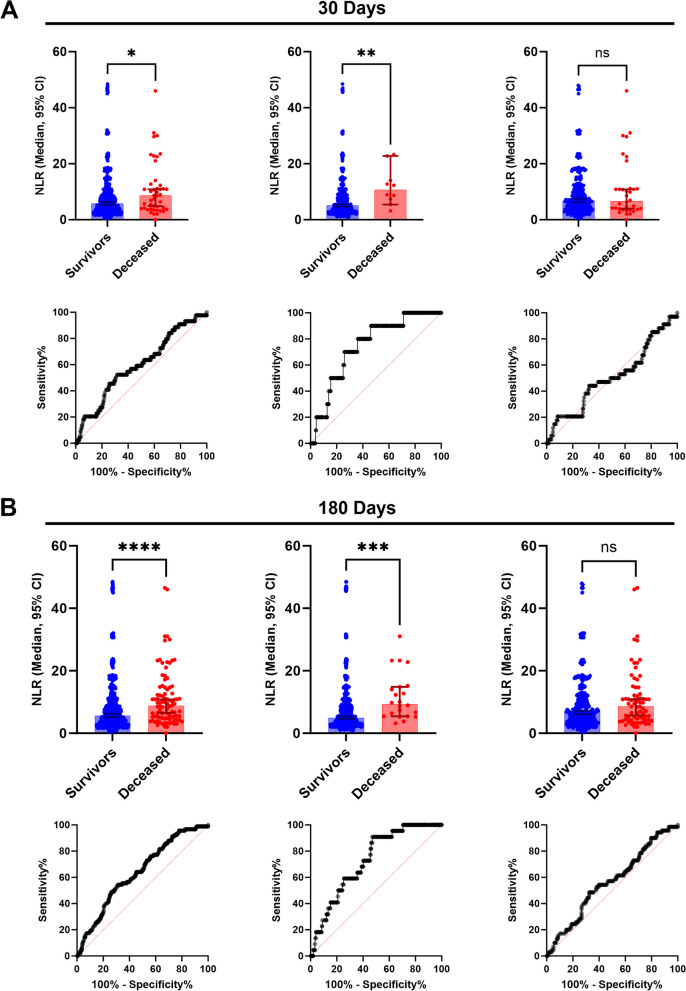
A total of 851 patients who underwent emergency surgery during the screening period were included. Mortality at 30 days was 5.2% (n = 44), which increased to 10.8% (n = 92) at 6 months. (Table 1.). Most of the patients undergoing emergency surgery had intraperitoneal pathology. Within the subgroup analysis, patients with vascular pathology had higher mortality (p = 0.0022). We found a difference in the median NLR at 30 days between survivor patients (n = 807) with a median value of 5.6 (first and third quartiles 3.1 to 9.6) and deceased patients (n = 44) with a median value of 8.7 (4.6 to 13.4) (p < 0.0001) (Fig. 1A, upper panel); in the 180 days analysis, a significant difference was observed between groups, with an NLR of 5.5 (3.1 to 9.8) in survivors (n = 759) and 8.8 (4.8 to 14.5) in deceased (n = 92) patients (p < 0.0001) (Fig. 1B, upper panel). After age subgroup analysis in patients younger than 65 years, we found a median NLR of 5.1 (2.9 to 8.9) in survivors (n = 497) and 10.6 (6.9 to 16.6) in deceased (n = 10) patients (p = 0.0064) for 30 days mortality. Individuals aged 65 years and above exhibited no difference in the median NLR, with values of 6.8 (3.9 to 12.1) and 6.7 (3.7 to 11.0) between survivors (n = 310) and deceased (n = 34) patients (p = 0.9068). In the 180 days mortality analysis, age differences were observed. Among patients younger than 65 years, survivors (n = 485) exhibited a median NLR of 4.9 (2.9 to 8.8), while deceased patients (n = 22) had a median NLR of 9.3 (5.4 to 14.9) (p = 0.0002). Individuals aged 65 years and above exhibited no difference in the median NLR, with values of 6.6 (3.7 to 11.8) and 8.6 (4.3 to 13.1) between survivors (n = 274) and deceased (n = 70) patients (p = 0.0991). Gender differences found that the median NLR was higher between male patients with an NLR of 6.1 (3.4 to 10.2) and female patients with an NLR of 5.1 (2.7 to 8.8) in the survivors’ group (p = 0.006) and an NLR of 9.1 (4.8 to 14.7) in male patients and an NLR of 8.6 (4.4 to 12.7) in female patients in the deceased group (p = 0.0029). The total NLR in the sample was also higher in males (6.5; 3.5 to 10.8) than in females (5.3; 2.9 to 9.4) (p = 0.0053). Differences in the 180 days mortality were observed between the male (p = 0.0006) and female (p = 0.0029) groups (Table 2).
Table 1.
Preoperative demographics
| Demographics | ||||
|---|---|---|---|---|
| Total | Survivors | Deceased | P value | |
| 851 | 742 (87.2%) | 109 (12.8%) | ||
| Age - years | ||||
| Median (IQR) | 60 (41-73) | 57 (39-70) | 73 (64-82) | <0.0001 |
| Range | 87 | 87 | 69 | |
| Sex - n (%) | ||||
| Female | 408/851 (48%) | 361/742 (49%) | 47/109 (43%) | 0.3052 |
| Male | 443/851 (52%) | 381/742 (51%) | 62/109 (57%) | 0.3052 |
| ASA Classification – n(%) | ||||
| I | 100/851 (11.8%) | 99/742 (13.3%) | 1/109 (0.9%) | <0.0001 |
| II | 390/851 (45.8%) | 386/742 (52.0%) | 4/109 (3.7%) | <0.0001 |
| III | 252/851 (29.6%) | 235/742 (31.7%) | 17/109 (15.6%) | 0.0004 |
| IV | 109/851 (12.8%) | 22/742 (3.0%) | 87/109 (79.8%) | <0.0001 |
| Comorbidities - n (%) | ||||
| Hypertension | 365/851 (42.9%) | 291/742 (79.7%) | 74/109 (20.3%) | <0.0001 |
| Diabetes | 201/851 (23.6%) | 165/742 (82.1%) | 36/109 (17.9%) | 0.0158 |
| Obesity | 155/851 (18.2%) | 147/742 (94.8%) | 8/109 (5.2%) | 0.0008 |
| Chronic Kidney Disease | 56/851 (6.6%) | 43/742 (76.8%) | 13/109 (23.2%) | 0.0223 |
| Chronic liver disease | 42/851 (4.9%) | 27/742 (64.3%) | 15/109 (35.7%) | <0.0001 |
| Cerebrovascular disease | 41/851 (4.8%) | 30/742 (73.2%) | 11/109 (26.8%) | 0.0133 |
| Heart failure | 40/851 (4.7%) | 28/742 (70.0%) | 12/109 (30.0%) | 0.0026 |
| Coronary artery disease | 37/851 (4.3%) | 27/742 (73.0%) | 10/109 (27.0%) | 0.0191 |
| Chronic Obstructive Pulmonary Disease | 25/851 (2.9%) | 19/742 (76.0%) | 6/109 (24.0%) | 0.1190 |
| Complete blood count - Median (IQR) | ||||
| Hemoglobin (g/dL) | 13.0 (11.0 - 14.3) | 13.2 (11.5 - 14.4) | 10.2 (8.4 - 12.6) | <0.0001 |
| White blood cells (n/mL) | 12060 (8530 - 15660) | 12165 (8710 - 15655) | 10980 (7350 - 16295) | 0.1349 |
| Platelets (n/µL) | 260000 (203000 - 321000) | 262000 (207750 - 322000) | 257000 (152000 - 317500) | 0.0356 |
| Erythrocyte Sedimentation Rate (mm/Hr) | 30 (14 - 51) | 29 (14 - 51) | 33 (13 - 52) | 0.7470 |
| Type of surgery - n (%) | ||||
| Intraperitoneal | 568/851 (66.7%) | 501/742 (67.5%) | 67/109 (61.5%) | 0.2312 |
| Vascular | 80/851 (9.4%) | 60/742 (8.1%) | 20/109 (18.3%) | 0.0022 |
| Trauma | 58/851 (6.8%) | 54/742 (7.3%) | 4/109 (3.7%) | 0.2205 |
| Urological | 56/851 (6.6%) | 51/742 (6.9%) | 5/109 (4.6%) | 0.2938 |
| Neurological | 48/851 (5.6%) | 39/742 (5.3%) | 9/109 (8.3%) | 0.262 |
| Superficial | 15/851 (1.8%) | 15/742 (2.0%) | 0/109 (0%) | 0.2393 |
| Head and Neck | 14/851 (1.6%) | 14 /742(1.9%) | 0/109 (0%) | 0.2366 |
| Intrathoracic | 12/851 (1.4%) | 8/742 (1.1%) | 4/109 (3.7%) | 0.0557 |
The table displays age, gender, ASA classification performed by attending anesthesiologist during preoperative evaluation, distribution of comorbidities (Columns do not sum up to 100% as a patient may have one or more comorbidities), complete blood count values (including hemoglobin, hematocrit, platelet count, and white blood cell count), and type of emergency surgery. Totals and differences between survivors and deceased are described. P value <0.05 is considered significant
Fig. 1.
A 30 days mortality analysis between survivor and deceased patients. Upper panel, neutrophil-to- lymphocyte ratio median differences: Total population (left), patients younger than 65 years (Middle) and, 65 years and above patients (Right). * 0.0302, ** p=0.0064, ns p=0.9068; Mann–Whitney U test. Lower panel, ROC curves: Total population AUC 0.60 (left), patients younger than 65 years AUC 0.75 (Middle) and, 65 years and above patients AUC 0.51 (Right). B 180 days mortality analysis between survivor and deceased patients. Upper panel, neutrophil-to- lymphocyte ratio median differences: Total population (left), patients younger than 65 years (Middle) and, 65 years and above patients (Right). **** <0.0001, *** p=0.0002, ns p=0.0991; Mann–Whitney U test. Lower panel, ROC curves: Total population AUC 0.64 (left), patients younger than 65 years AUC 0.73 (Middle) and, 65 years and above patients AUC 0.56 (Right)
Table 2.
Preoperative neutrophil-to-lymphocyte ratio characteristics
| Type of surgery NLR - Median (IQR) | Survivors | Deceased | P value |
|---|---|---|---|
| Neutrophil-to-Lymphocyte Ratio (NLR) | |||
| Intraperitoneal | 5.7 (3.3 - 9.5) | 10.0 (5.6 - 14.7) | <0.0001 |
| Vascular | 6.0 (2.8 - 9.8) | 4.5 (3.4 - 5.2) | 0.8535 |
| Trauma | 4.4 (3.1 - 8.8) | 4.5 (3.4 - 5.2) | 0.7759 |
| Urological | 6.8 (3.7 - 11.25) | 15.2 (5.0 - 25.2) | 0.1916 |
| Neurological | 4.1 (2.3 - 10.8) | 5.3 (3.6 - 10.1) | 0.2943 |
| Superficial | 3.7 (2.5 - 8.2) | - | - |
| Head and Neck | 4.3 (2.6 - 10.0) | - | - |
| Intrathoracic | 7.44 (2.4 - 20.7) | 12.8 (10.1 - 21.3) | 0.4606 |
| 30 days mortality | |||
| NLR - Median (IQR) | 5.8 (3.2-10.26) | 8.7 (3.9-12.6) | 0.0302 |
| Range | 48.1 | 46.0 | |
| NLR < 65 years - Median (IQR) | 5.1 (2.9-8.9) | 10.6 (6.8-16.2) | 0.0064 |
| Range | 47.8 | 20.1 | |
| NLR ≥ 65 years - Median (IQR) | 6.8 (3.9-12.1) | 6.7 (3.7-11.0) | 0.9068 |
| Range | 47.6 | 46.0 | |
| 180 days mortality | |||
| NLR - Median (IQR) | 5.6 (3.1-9.8) | 8.8 (4.8-14.5) | <0.0001 |
| Range | 48.1 | 46.5 | |
| NLR < 65 years - Median (IQR) | 4.9 (2.8- 8.8) | 9.3 (5.4-14.9) | 0.0002 |
| Range | 47.8 | 27.8 | |
| NLR ≥ 65 years - Median (IQR) | 6.6 (3.7-11.8) | 8.6 (4.3-13.1) | 0.0991 |
| Range | 47.6 | 46.5 | |
| Sex - 180 days mortality | |||
| NLR Female | 5.1 (2.7-8.8) | 8.6 (4.4-12.7) | 0.0006 |
| NLR Male | 6.1 (3.4-10.2)a | 9.1 (4.8-14.7)b | 0.0029 |
The table shows the difference in Neutrophil to Lymphocyte Ratio between survivors and deceased. Differences between types of surgery are described. Both the distribution of NLR is observed for mortality at 30 and 180 days for the total number of patients and in those older and younger than 65 years of age. P-value of 0.05 is considered significant
Female and Male Mann–Whitney U test: a0.006, b0.8395
ROC analysis was conducted to assess the overall discrimination performance of NLR [16]. The area under the curve (AUC) of NLR was 0.60 (95% CI 0.51 to 0.68) for 30 days mortality and 0.64 (95% CI 0.58 to 0.70) for 180 days mortality. Interestingly, the ROC AUC in patients younger than 65 years was higher for 30 days (AUC 0.75; 95% CI 0.72 to 0.87) and 180 days (AUC 0.73; 95% CI 0.64 to 0.81) compared to 65 years and older patients (30 days: AUC 0.51; 95% CI 0.40 to 0.62; and 180 days (AUC 0.56; 95% CI 0.50 to 0.64) (Fig. 1A and B, lower panels; Table 3).
Table 3.
Receiver Operating Characteristic (ROC) curve analysis
| Cut-Off | Sensitivity % | 95% CI | Specificity% | 95% CI | Likelihood ratio | Youden´s Index | AUC | |
|---|---|---|---|---|---|---|---|---|
| 30 days mortality ROC | ||||||||
| Total population | > 8.551 | 52,27 | 37,94% to 66,25% | 68,4 | 65,11% to 71,52% | 1,654 | 0,21 | 0,60 |
| Younger than 65 years | > 8.660 | 70 | 39,68% to 89,22% | 74,04 | 70,02% to 77,70% | 2,697 | 0,44 | 0,75 |
| 65 years and above patients | > 19.80 | 20,59 | 10,35% to 36,80% | 91,61 | 87,99% to 94,21% | 2,455 | 0,13 | 0,51 |
| 180 days mortality ROC | ||||||||
| Total population | > 8.423 | 54,35 | 44,20% to 64,15% | 68,64 | 65,26% to 71,84% | 1,733 | 0,23 | 0,64 |
| Younger than 65 years | > 5.233 | 90,91 | 72,19% to 98,38% | 52,99 | 48,54% to 57,39% | 1,934 | 0,44 | 0,73 |
| 65 years and above patients | > 9.385 | 48,57 | 37,25% to 60,05% | 67,52 | 61,76% to 72,79% | 1,495 | 0,16 | 0,56 |
The table displays Cut-Off values for 30- and 180-days mortality based on the highest Youden's index. Sensitivity, specificity with 95% confidence interval (CI) are provided. Likelihood ratio ((1 - sensitivity) / specificity), Youden's index (sensitivity + specificity - 1), and the area under the curve (AUC) for each analysis are presented
Multivariate logistic regression analysis was conducted to explore the association between NLR and age with respect to mortality. Upon analyzing the data over a 180-day period, both NLR (odds ratio, 1.03 [95% CI 1.005 to 1.053; p = 0.0133) and age (odds ratio, 1.05 [95% CI 1.034 to 1.064; p < 0.0001) significantly contributed to the regression model, exhibiting an AUC of 0.75 (95% CI 0.70 to 0.80; p < 0.0001; Tjur’s R2 = 0.071) (Fig. 2). Additionally, the Hosmer–Lemeshow did not show evidence against the null hypothesis that the model’s predictions fit well the data (X2 = 11.77; df = 8; p = 0.16), while the log-likelihood ratio test rejected the null hypothesis that a model without predictors fitted as well as the model with NLR and age as predictors (X2 = 62.56; df = 2; p < 0.0001) (Table 4). The specificity for mortality was 89.19%, whereas its sensitivity could not be estimated as all observations were predicted to be alive up to 180 days after surgery. No multicollinearity was observed between the NLR and age (VIF = 1.016, R2 = 0.02). The inclusion of sex as a variable did not improve the model.
Fig. 2.
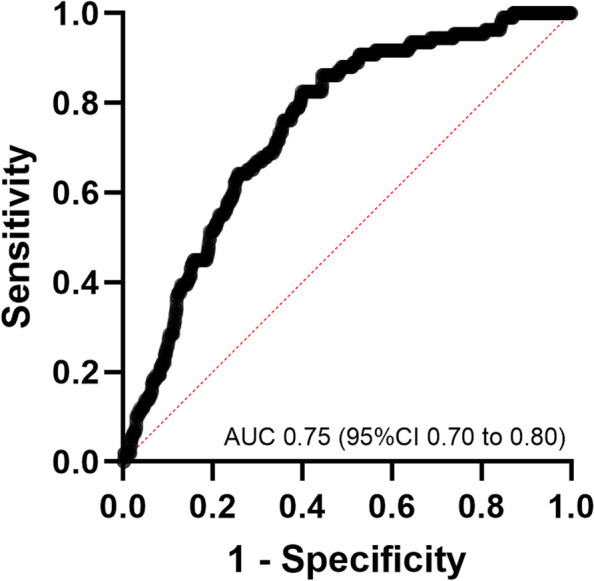
Receiver Operating Characteristic (ROC) Curve from Multivariate Logistic Regression between NLR and Age with Respect to Mortality. ROC curve AUC 0.75 (95% CI 0.70 to 0.80, p <0.0001)
Table 4.
Results of Logistic Regression on 180-day Mortality
| Predictor | Coefficient, β | SE | |Z| | P value | OR = exp(β) | 95% CIa |
|---|---|---|---|---|---|---|
| Intercept | -5,3950 | 0,53060 | 10,17 | <0,0001 | 0,005 | 0.0015, 0.01216 |
| Age | 0,0472 | 0,00733 | 6,44 | <0,0001 | 1,048 | 1.034, 1.064 |
| NLR | 0,0294 | 0,01187 | 2,48 | 0,0133 | 1,030 | 1.005, 1.053 |
| Overall model-fitting | Value | df | P value | |||
| Hosmer-Lemeshow test | 11,77 | 8 | 0,1617 | |||
| LR (G-squared) test | 62,56 | 2 | <0.0001 | |||
| Tjur's R-squared | 0,0707 | |||||
The variables used in the multivariate regression model are presented. Overall model fitting analyses are described. A p-value less than 0.05 is considered significant
Acronyms: CI Confidence interval, df Degrees of freedom, NLR Neutrophil-to-lymphocyte ratio, LRT Log-likelihood ratio test, SE Standard error, OR Odds ratio
aBased on profile likelihood
We developed a more complex model incorporating all comorbidities found to be significantly different in the univariable analysis (Table 1.). The addition of hypertension (p = 0.0870), diabetes (p = 0.2722), obesity (p = 0.1921), chronic kidney disease (p = 0.6240), chronic liver disease (p = 0.9564), cerebrovascular disease (p = 0.1326), heart failure (p = 0.7005), and coronary disease (p = 0.8150) did not significantly improve the regression model compared to the simpler model including only age and NLR.
When testing each variable individually to enhance the regression model, we found that hypertension combined with age and NLR resulted in a significantly different model (p = 0.0441), but this did not improve our ROC curve analysis (AUC of 0.7484; 95% CI 0.70 to 0.79; p < 0.0001; Tjur’s R² = 0.083). Therefore, the simpler model using age and NLR performed better than models with additional covariates. Interestingly, the inclusion of the ASA classification introduced multicollinearity into the model (VIF = 1.3, R2 = 0.23) and did not improve the results. Although the VIF indicates low multicollinearity, the ASA classification, which stratifies multiple comorbidities, did not enhance the model’s predictive power. Therefore, we decided not to pursue further analysis with the ASA classification.
Finally, a Kaplan–Meier Survival analysis was conducted, employing an NLR threshold of 3 to distinguish between normal patients and those considered to exhibit signs of inflammation. This analysis revealed a significant difference in 180-day mortality between the groups (p = 0.0006) (Fig. 3).
Fig. 3.
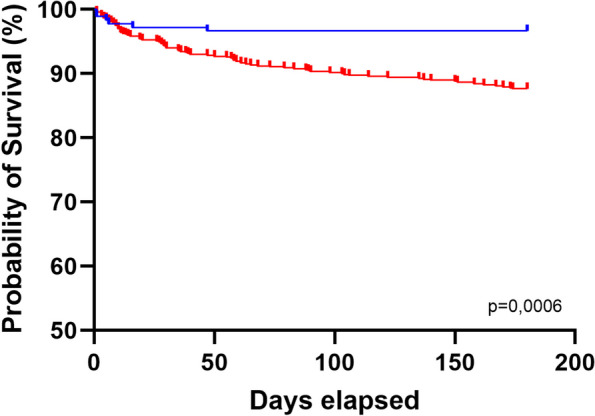
Kaplan–Meier survival and 180-day mortality analysis of NLR with a threshold of 3. Time (Days) versus estimated probability of survival (Percent). Censored point corresponds to deceased patients. No follow up lost was observed
Discussion
The inflammatory and immune response statuses before emergency surgery are not a component of any preoperative algorithm. Here, we identified an association between preoperative NLR and mortality. We observed that deceased patients at 30 and 180 days after surgery had a higher NLR upon admission for surgery than those who remained alive at these time points. Additionally, patients with an NLR > 3 exhibited decreased survival rates after 180 days. Through receiver characteristic curve analysis, we noted that NLR demonstrated acceptable discriminatory capacity. Predictive efficacy was higher in the age group < 65 years. Accordingly, the results of the multivariate logistic regression indicated that incorporating age into the model enhanced the predictive capacity of NLR for 180 days mortality.
Immune response is crucial in tissue repair and healing [17]. The assessment of the inflammatory status is often omitted in preoperative non-cardiac surgery evaluation algorithms, although the system response manifesting as sepsis is part of The American College of Surgery National Surgical Quality Improvement Program risk calculator [18]. In patients without sepsis, the inflammatory status or immune cellular response is not considered in the categorization or management algorithms of surgical patients.
Surgery involves controlled tissue damage, and its outcomes are related to the regenerative capacity of the tissue [19]. Activation of macrophages and neutrophils is required at the site of surgical damage [20, 21]. Neutrophils are the predominant immune cells in human blood and protect against pathogens and other harmful agents [22]. During injury, neutrophils are readily available after damage-associated molecular patterns are released from the damaged cells [23]. Upon recruitment, these cells undergo phenotypic changes, giving rise to distinct subpopulations aimed at various functions, such as clearing debris, releasing effectors such as growth factors and metalloproteinases, and engaging in processes related to angiogenesis, regeneration, anti-inflammatory responses, and secretion of reparative cytokines, all of which contribute to the resolution of injury [24, 25]. Despite their role in tissue repair, neutrophil-associated tissue injury is observed due to the amplification of the inflammatory response and the direct release of reactive oxygen species and proteolytic enzymes [26].
Additionally, a novel mechanism known as neutrophil extracellular trap-induced tissue damage has been proposed as an additional tissue injury factor [23, 27, 28]. This finding emphasizes the significance of neutrophils and their role in maintaining tissue homeostasis. Hence, understanding and integrating NLR as a preoperative factor could incorporate the balance in cellular immune status using preoperative algorithms.
The NLR has been studied in acute kidney injury [4], ischemic stroke [6], cardiovascular mortality [7], and chemotherapy outcomes in cancer [10]. Although preoperative evaluation is simple and available in most preoperative emergency and non-emergency settings at a low cost, it has not been implemented. Age-related differences appear to be important factors. We found that NLR may predict mortality more effectively in younger patients. Chronological age is not always correlated with biological age and is more difficult to assess using common algorithms. Although 65 years is a frequently recommended threshold to differentiate between young and older patients, it has been reported that older patients are more prone to complications after non-cardiac surgery [18]. Variations in the NLR values across different life stages have been described previously. In a healthy adult population, excluding geriatric individuals, NLR values are lower than 3.5 [2, 29]. The exact cutoff value remains elusive, and an NLR < 3 is considered to be within the normal range [30]. The cutoff value in older individuals may be affected by immune senescence, which could complicate utilizing the NLR in preoperative settings [31].
Our study has a few limitations. In addition to its retrospective design, our study was a single-center analysis over a one-year period, thereby limiting the external validity of our findings. Second, we found differences in the use of the NLR across different ages. After analyzing the values from our entire sample, we used 65 years as the cutoff to define age differences. This value, employed by preoperative evaluation guidelines owing to its association with cardiovascular complications, may not necessarily define the function and utility of NLR in predicting outcomes, as chronological and biological ages are not always related. Further studies are necessary to define this variable. Finally, owing to the small sample size, we did not obtain a clear cutoff value in our receiver operating curve analysis, and an NLR value of 3 was used according to the literature. Our analysis suggests that patients with an NLR greater than 8.5 are at increased risk of mortality at both 30 and 180 days. However, larger cohorts are necessary to elucidate this further. Confounding factors, such as the number of patients who developed sepsis, shock, or other inflammatory states related to the surgical intervention, were not analyzed in this retrospective study. This introduces an analysis bias that should be addressed in prospective studies.
Here, we found differences between patients who survived for 30 and 180 days regarding the preoperative NLR. When combined with age, it enhances the prediction of mortality; however, in older patients, NLR alone may not reflect postsurgical outcomes, as immune senescence may be a contributing factor.
It is also noteworthy that our NLR analysis was conducted among patients with no differences in total white blood cells count between those with higher or lower mortality. Introducing the component of innate and cellular immune response, as implied by the NLR, distinguishes mortality in patients undergoing emergency surgery and may be useful for clinicians as an accessible additional parameter beyond solely using leukocyte count as a marker of inflammation. This suggests that the NLR could enhance preoperative evaluation algorithms.
In conclusion, our study observed differences in preoperative NLR between patients who survived and those who died after emergency surgery. Patients with an NLR greater than 3 had lower survival rates at 180 days. Differences in NLR values in the younger and older than 65 years groups impacted the use of the NLR as a mortality risk factor. Further studies are necessary to validate the use of NLR in the preoperative evaluation of patients undergoing emergency surgery.
Acknowledgements
None.
Authors’ contributions
FM and MC conceived the study, drafted the work. FM, MA, IE, CE, HC, LC, CDP, RG performed the data collection. Data were analyzed and interpreted by FM, RG, FMd, and MC. All the authors revised the work, commented on previous versions of the manuscript, approved the version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This work was funded by ANID - Millennium Science Initiative Program – ICN2021_45 (MC): Millennium Institute on Immunology and Immunotherapy (ICN2021_45).
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Board of the Hospital Clínico de la Universidad de Chile (Ref: OAIC 37/22). Accordingly, informed consent has been waived by the Research Ethics Board. Personal information was kept confidential.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Felipe Maldonado, Email: fmaldonado@uchile.cl.
Mónica Cáceres, Email: monicacaceresll@uchile.cl.
References
- 1.Caballero-Sánchez N, Alonso-Alonso S, Nagy L. Regenerative inflammation: when immune cells help to re-build tissues. FEBS J. 2022. 10.1111/febs.16693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buonacera A, Stancanelli B, Colaci M, Malatino L. Neutrophil to lymphocyte ratio: an emerging marker of the relationships between the immune system and diseases. Int J Mol Sci. 2022;23:3636. 10.3390/ijms23073636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshida Y, Iwasa H, Kim H, Suzuki T. Association between neutrophil-to-lymphocyte ratio and physical function in older adults: a Community-based cross-sectional study in Japan. Int J Environ Res Public Health. 2022;19:8996. 10.3390/ijerph19158996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu Alfeilat M, Slotki I, Shavit L. Single emergency room measurement of neutrophil/lymphocyte ratio for early detection of acute kidney injury (AKI). Intern Emerg Med. 2018;13:717–25. 10.1007/s11739-017-1715-8. [DOI] [PubMed] [Google Scholar]
- 5.Faria SS, Fernandes PC, Silva MJB, Lima VC, Fontes W, Freitas-Junior R, et al. The neutrophil-to-lymphocyte ratio: a narrative review. Ecancermedicalscience. 2016;10:702. 10.3332/ecancer.2016.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celikbilek A, Ismailogullari S, Zararsiz G. Neutrophil to lymphocyte ratio predicts poor prognosis in ischemic cerebrovascular disease. J Clin Lab Anal. 2013;28:27–31. 10.1002/jcla.21639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azab B, Zaher M, Weiserbs KF, Torbey E, Lacossiere K, Gaddam S, et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol. 2010;106:470–6. 10.1016/j.amjcard.2010.03.062. [DOI] [PubMed] [Google Scholar]
- 8.Serra R, Ielapi N, Licastro N, Provenzano M, Andreucci M, Bracale UM, et al. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as biomarkers for cardiovascular surgery procedures: a literature review. Rev Recent Clin Trials. 2021;16:173–9. 10.2174/1574887115999201027145406. [DOI] [PubMed] [Google Scholar]
- 9.Huang Z, Fu Z, Huang W, Huang K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: a meta-analysis. Am J Emerg Med. 2020;38:641–7. 10.1016/j.ajem.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 10.Chua W, Charles KA, Baracos VE, Clarke SJ. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer. 2011;104:1288–95. 10.1038/bjc.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takenaka Y, Oya R, Takemoto N, Inohara H. Neutrophil-to-lymphocyte ratio as a prognostic marker for head and neck squamous cell carcinoma treated with immune checkpoint inhibitors: meta-analysis. Head Neck. 2022;44:1237–45. 10.1002/hed.26997. [DOI] [PubMed] [Google Scholar]
- 12.Song M, Graubard BI, Rabkin CS, Engels EA. Neutrophil-to-lymphocyte ratio and mortality in the United States general population. Sci Rep. 2021;11:464. 10.1038/s41598-020-79431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mortaz E, Alipoor SD, Adcock IM, Mumby S, Koenderman L. Update on neutrophil function in severe inflammation. Front Immunol. 2018;9:2171. 10.3389/fimmu.2018.02171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–76. 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 15.Gagnon RC, Peterson JJ. Estimation of confidence intervals for area under the curve from destructively obtained pharmacokinetic data. J Pharmacokinet Biopharm. 1998;26:87–102. 10.1023/a:1023228925137. [DOI] [PubMed] [Google Scholar]
- 16.Nahm FS. Receiver operating characteristic curve: overview and practical use for clinicians. Korean J Anesthesiol. 2022;75:25–36. 10.4097/kja.21209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raziyeva K, Kim Y, Zharkinbekov Z, Kassymbek K, Jimi S, Saparov A. Immunology of acute and chronic wound healing. Biomolecules. 2021;11:700. 10.3390/biom11050700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halvorsen S, Mehilli J, Cassese S, Hall TS, Abdelhamid M, Barbato E, et al. 2022 ESC guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. Eur Heart J. 2022;43:3826–924. 10.1093/eurheartj/ehac270. [DOI] [PubMed] [Google Scholar]
- 19.Guillamat-Prats R. The role of MSC in wound healing, scarring and regeneration. Cells. 2021;10:1729. 10.3390/cells10071729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillipson M, Kubes P. The healing power of neutrophils. Trends Immunol. 2019;40:635–47. 10.1016/j.it.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Brazil JC, Quiros M, Nusrat A, Parkos CA. Innate immune cell-epithelial crosstalk during wound repair. J Clin Invest. 2019;129:2983–93. 10.1172/JCI124618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–75. 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 23.Wang J. Neutrophils in tissue injury and repair. Cell Tissue Res. 2018;371:531–9. 10.1007/s00441-017-2785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pillay J, Hietbrink F, Koenderman L, Leenen LPH. The systemic inflammatory response induced by trauma is reflected by multiple phenotypes of blood neutrophils. Injury. 2007;38:1365–72. 10.1016/j.injury.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 25.Brancato SK, Albina JE. Wound macrophages as key regulators of repair: origin, phenotype, and function. Am J Pathol. 2011;178:19–25. 10.1016/j.ajpath.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilgus TA, Roy S, McDaniel JC. Neutrophils and wound repair: positive actions and negative reactions. Adv Wound Care (New Rochelle). 2013;2:379–88. 10.1089/wound.2012.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 28.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–9. 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 29.Forget P, Khalifa C, Defour J-P, Latinne D, Van Pel M-C, De Kock M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res Notes. 2017;10:12. 10.1186/s13104-016-2335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farkas JD. The complete blood count to diagnose septic shock. J Thorac Dis. 2020;12:S16–21. 10.21037/jtd.2019.12.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lagnado A, Leslie J, Ruchaud-Sparagano M-H, Victorelli S, Hirsova P, Ogrodnik M, et al. Neutrophils induce paracrine telomere dysfunction and senescence in ROS-dependent manner. EMBO J. 2021;40:e106048. 10.15252/embj.2020106048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.