Abstract
Human signaling lymphocytic activation molecule (SLAM; also known as CDw150) has been shown to be a cellular receptor for measles virus (MV). Chinese hamster ovary cells transfected with a mouse SLAM cDNA were not susceptible to MV and the vesicular stomatitis virus pseudotype bearing MV envelope proteins alone, indicating that mouse SLAM cannot act as an MV receptor. To determine the functional domain of the receptor, we tested the abilities of several chimeric SLAM proteins to function as MV receptors. The ectodomain of SLAM comprises the two immunoglobulin superfamily domains (V and C2). Various chimeric transmembrane proteins possessing the V domain of human SLAM were able to act as MV receptors, whereas a chimera consisting of human SLAM containing the mouse V domain instead of the human V domain no longer acted as a receptor. To examine the interaction between SLAM and MV envelope proteins, recombinant soluble forms of SLAM were produced. The soluble molecules possessing the V domain of human SLAM were shown to bind to cells expressing the MV hemagglutinin (H) protein but not to cells expressing the MV fusion protein or irrelevant envelope proteins. These results indicate that the V domain of human SLAM is necessary and sufficient to interact with the MV H protein and allow MV entry.
Measles virus (MV), a member of the Morbillivirus genus in the Paramyxoviridae family, causes an acute childhood disease which still claims roughly 1 million lives a year. MV is a nonsegmented negative-strand RNA virus with two envelope glycoproteins, the hemagglutinin (H) and fusion (F) proteins (10). CD46 has been shown to be a cellular receptor for vaccine strains of MV such as the Edmonston and Halle strains (9, 25). These strains are capable of infecting all CD46-positive primate cell lines. However, recent clinical isolates of MV, which were usually isolated in the marmoset B-cell line B95a or human B-cell lines, were found to grow only in some primate B- and T-cell lines and human dendritic cells (11, 12, 14, 16, 32, 33, 37, 38). By using vesicular stomatitis virus (VSV) pseudotypes bearing MV envelope proteins, we showed that virus entry is a major determinant of cell tropism of the Edmonston and B95a-isolated MV strains (38).
Recently, expression cloning, combined with the VSV pseudotype system, allowed us to identify signaling lymphocytic activation molecule (SLAM; also known as CDw150) as a cellular receptor for MV (39). We showed that the cell surface expression of human SLAM (hSLAM) rendered rodent cells susceptible to all MV strains examined, including the Edmonston strain, B95a-isolated strains, peripheral blood mononuclear cell-isolated strains, and MV present in throat swabs from measles patients (39). SLAM, an important costimulatory molecule in lymphocyte activation, is expressed on some T and B cells (2, 3, 7, 22, 30, 31, 34), consistent with MV tropism and pathology including lymphopenia and immunosuppression (10).
SLAM contains two highly glycosylated immunoglobulin (Ig) superfamily domains and has structural features placing it within the CD2 family, which includes CD2, CD48, CD58, 2B4, and Ly-9 (8). Like other members of the CD2 family, SLAM comprises an N-terminal membrane-distal V-set domain and a membrane-proximal C2-set domain, followed by the transmembrane segment (TM) and cytoplasmic tail (CY). A recent study showed that mouse SLAM (mSLAM) also shares molecular and functional characteristics with the human counterpart, with its predicted amino acid sequence exhibiting 58% similarity to that of hSLAM (6).
In this study, we first examined whether mSLAM can act as a cellular receptor for MV, in an attempt to explain MV's inability to infect mice. We then defined the region of hSLAM that interacts with MV, by constructing various chimeric molecules. We also examined the interaction between MV envelope proteins and recombinant soluble forms of SLAM. The results indicated that the V domain of hSLAM, which binds the MV H protein, is essential for its function as an MV receptor.
MATERIALS AND METHODS
Cells and viruses.
Derivations and culture conditions of cell lines used have been described elsewhere (38). The Edmonston (American Type Culture Collection) and KA (37) strains of MV were grown and titrated on Vero and B95a cells, respectively. Pseudotype viruses (VSVΔG∗, VSVΔG∗-EdHF, VSVΔG∗-KAHF, and VSVΔG∗-G) were prepared by infecting with VSVΔG∗-G the human kidney cell line 293T which had been transfected with the appropriate expression plasmids encoding envelope proteins, as previously described (38).
Constructions of expression plasmids.
Isolation of the hSLAM cDNA has been described elsewhere (39). Primers used for PCR amplification of hSLAM were 5′-CCCGAATTCCAGACAGCCTCTGCTGCATGAC-3′ (SF1) and 5′-AAAGCGGCCGCCCTTCAGAAAAGTCCCTTTGTTGG-3′ (SB1). The mSLAM cDNA was obtained by reverse transcription (RT) of total RNA from phorbol 12-myristate 13-acetate-stimulated BALB/c mouse splenic cells followed by PCR. Primers used for the PCR of mSLAM were 5′-CCAGAATTCTGGCTAATGGATCCC-3′ (muSF1) and 5′-AGGCGGCCGCCTTTCACTGGGTAT-3′ (muSB1). The sequences underlined are sites for restriction enzymes. The PCR products were subcloned into the eukaryotic expression vector pCAGGS (27) and sequenced. The plasmids encoding the membrane-bound form of SLAM with the complete CY were selected and named pCAG-hSLAM and pCAG-mSLAM, respectively. The cDNAs encoding human CD4 (17) and CD46 (the C2 isoform) (29) were also subcloned into pCAGGS (pCAG-CD4 and pCAG-CD46). Plasmids encoding chimeric molecules (pCAG-mSLAM-hV, pCAG-hSLAM-mV, and pCAG-CD4-hV) were constructed by gene splicing by overlap extension (SOEing) as described elsewhere (40). The V domains of hSLAM and mSLAM are amino acid positions 1 to 138 and 1 to 139, respectively. The two templates and primers for the first and second PCRs used to prepare each product are listed in Table 1. The PCR products were cloned into pCAGGS. The plasmid encoding a truncated form of SLAM (pCAG-hV) was prepared by amplifying DNA fragments in two separate PCRs and digesting them with appropriate restriction enzymes. The desired fragments were isolated from agarose gels and then ligated in the same mixture with pCAGGS predigested with EcoRI and NotI (the three molecules were ligated together). The templates, primers, and restriction enzymes used are listed in Table 2. pDisplay (Invitrogen) contains the sequences encoding the c-Myc epitope and platelet-derived growth factor receptor (PDGFR) TM. Soluble forms of SLAM were produced as fusion proteins between the extracellular domain of SLAM and the rabbit IgG Fc fragment (4). Plasmids encoding the soluble forms of SLAM (pCAG-shSLAM-rIgG, pCAG-smSLAM-rIgG, and pCAG-smSLAM-hV-rIgG) were prepared by PCRs amplifying the DNA fragments, followed by restriction enzyme digestion and ligation with the predigested pCAGGS, as described above. The templates, primers, and restriction enzymes used are described in Table 2. pSK100, which encodes the constant region of rabbit IgG, was kindly provided by John A. T. Young. All constructs were verified by DNA sequencing.
TABLE 1.
PCR templates and primers used to prepare plasmids encoding chimeric molecules
| Product | Template | PCR primer(s)
|
||
|---|---|---|---|---|
| 1 st
|
2nd | |||
| Sense | Antisense | |||
| mSLAM-hV | pCAG-hSLAM | CCCGAATTCCAGACAGCCTCTGCTGCATGAC (SF1) | GGAGGGGAGACCTGTTCATAAAGCC | SF1 + muSB1 |
| pCAG-mSLAM | AACAGGTCTCCCCTCC | AGGCGGCCGCCTTTCACTGGGTAT (muSB1) | ||
| hSLAM-mV | pCAG-mSLAM | CCAGAATTCTGGCTAATGGATCCC (muSF1) | GTGGAGACCTGCTCATAAAGC | muSF1 + SB1 |
| pCAG-hSLAM | GCTTTATGAGCAGGTCTCCAC | AAAGCGGCCGCCCTTCAGAAAGTCC CTTTGTTGG (SB1) | ||
| CD4-hV | pCAG-hSLAM | SF1 | CATAAAGCCTCAACTGC | SF1 + rCD4 |
| pCAG-CD4 | GAGGCTTTATGGAGCCACTCAGC | GGCCTCGTGCCTCAAATG (rCD4) | ||
TABLE 2.
PCR templates and primers used to prepare plasmids encoding truncated and soluble forms of SLAM
| Product | Template | Primer
|
Restriction enzyme(s) used to cut PCR products | |
|---|---|---|---|---|
| Sense | Antisense | |||
| hV | pCAG-hSLAM | SF1 | CATAAAGCCTCAACTGC | EcoRI |
| pDisplay | TAATACGACTCACTATAGGG (T7) | TAGAAGGCACGTCGAG (BGHr) | SmaI + NotI | |
| shSLAM-rIgG | pCAG-hSLAM | SF1 | GAAGCTAGCTTCTGAGGGGTCTG | EcoRI + NheI |
| pSK100 | CATGCTAGCATGATCTCACGCACC | TCACTAAAGGGAAGCGG | NheI + NotI | |
| smSLAM-rIgG | pCAG-mSLAM | muSF1 | GAAGCTAGCTTCTGAGGAGGATTCC | EcoRI + NheI |
| pSK100 | CATGCTAGCATGATCTCACGCACC | TCACTAAAGGGAAGCGG | NheI + NotI | |
| smSLAM-hV-rIgG | pCAG-mSLAM-hV | SF1 | GAAGCTAGCTTCTGAGGAGGATTCC | EcoRI + NheI |
| pSK100 | CATGCTAGCATGATCTCACGCACC | TCACTAAAGGGAAGCGG | NheI + NotI | |
Immunofluorescence staining.
Chinese hamster ovary (CHO) cells were transfected with plasmid DNAs by using Lipofectamine Plus reagent (Life Technologies). The transfected cells were stained with mouse anti-hSLAM monoclonal antibody (MAb) IPO-3 (34) (Kamiya Biochemical) or rat anti-mSLAM MAb 12F12 (6), followed by staining with fluorescein isothiocyanate (FITC)-labeled secondary antibody. The stained cells were analyzed on a FACScan machine (Becton Dickinson). Dead cells (those that positively stained with propidium iodide) were excluded from the analysis.
Infection of cells with MV and pseudotypes.
CHO cells (2 × 104 cells/well) were transfected with 0.05 μg each of plasmid DNA by using Lipofectamine Plus reagent. At 24 h after transfection, the cells were infected with the Edmonston or KA strain of MV at a multiplicity of infection of 0.1. The cells were examined for cytopathic effect (CPE) at 24 h after infection. In different experiments, the CHO cells were infected with VSV pseudotype viruses at 24 h after transfection as described above, and infectious titers of the pseudotype viruses were determined by counting the number of green fluorescent protein (GFP) expressing cells under a fluorescence microscope at 24 h after infection.
Preparation of soluble molecules.
293T cells were transfected with pCAGGS, pCAG-shSLAM-rIgG, pCAG-smSLAM-rIgG, or pCAG-smSLAM-hV-rIgG by using Lipofectamine Plus reagent. At 24 h after transfection, the culture medium was replaced with serum-free medium comprising 50% Dulbecco modified Eagle medium and 50% Cosmedium (Cosmo Bio). The supernatants containing soluble molecules were recovered after a further 72 h of cell culture and concentrated in a Centricon-30 (Amicon). The supernatant from 293T cells transfected with pCAGGS was used as a control (mock supernatant).
Western blot analysis.
The concentrated soluble molecules were separated on sodium dodecyl sulfate (SDS)–10% polyacrylamide gels and then transferred to nitrocellulose membranes. After being blocked in phosphate-buffered saline with 5% nonfat dried milk and 0.02% polyoxyethylenesorbitan monolaurate (Tween 20), the membranes were incubated in the blocking buffer containing biotinylated goat anti-rabbit IgG antibody (Wako) or IPO-3. After washing, membranes were incubated with horseradish peroxidase-conjugated streptavidin (Zymed) or goat anti-mouse IgG (Bio-Rad) and then treated with ECL (enhanced chemiluminescence) Western blotting detection reagent (Amersham Pharmacia Biotech).
Soluble SLAM binding to cells.
293T cells were transiently transfected with the expression vector pCXN2 (27) as a control or an expression plasmid encoding the Edmonston H protein (pCXN2H) (37), Edmonston F protein (pCXN2F) (37), or human T-cell leukemia virus type 1 (HTLV-1) Env protein (pCAGHTLV-1 env) (28). The transfected 293T cells were harvested at 24 h after transfection by treatment with phosphate-buffered saline containing 5 mM EDTA. The cells were incubated in the serum-free medium with 20 μg of rabbit IgG per ml or the concentrated supernatants containing the soluble molecules on ice for 60 min. The amounts of soluble molecules were adjusted according to the enzyme-linked immunosorbent assay so that approximately the same amounts were incubated with the cells. After washing, the cells were incubated with FITC-labeled anti-rabbit IgG (Dako) for 30 min and analyzed by flow cytometry. In the blocking study, the transfected 293T cells were incubated with 100 μg of anti-MV H protein MAb C-1 (21) or mouse IgG per ml at 4°C for 30 min. After centrifugation, the cells were incubated with shSLAM-rIgG in the presence of 100 μg of anti-MV H protein MAb or mouse IgG per ml for 60 min, and binding was analyzed as described above.
RESULTS
mSLAM cannot act as a receptor for MV.
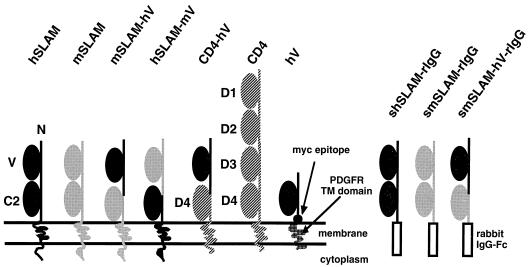
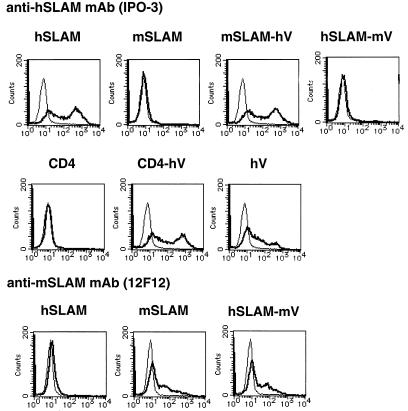
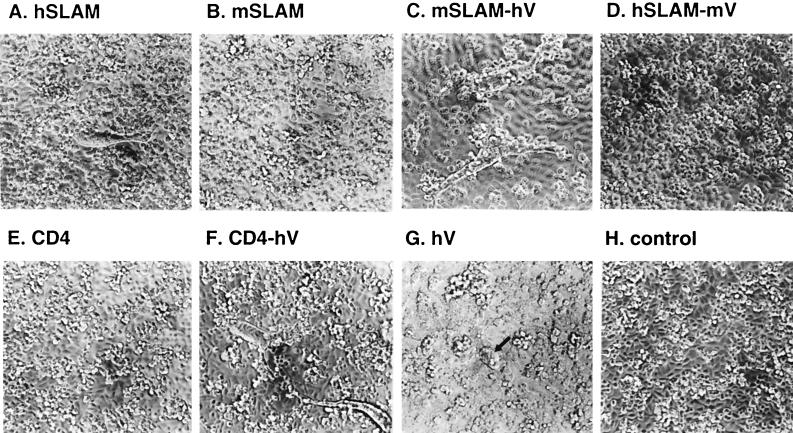
We first examined whether mSLAM can act as a receptor for MV. The structures of mSLAM and other molecules examined in this study are shown in Fig. 1. A cDNA clone encoding the membrane bound form of mSLAM with the long CY was obtained from total RNA of BALB/c mouse splenic cells by RT-PCR and was cloned into a eukaryotic expression vector pCAGGS (pCAG-mSLAM). DNA sequencing showed that the cDNA clone had a single nucleotide difference from but the same predicted amino acid sequence as the reported sequences of mSLAM (6). CHO cells were transiently transfected with pCAG-mSLAM or the expression plasmid encoding hSLAM (pCAG-hSLAM) (39), and cell surface expression of SLAM on the transfected CHO cells was confirmed by flow cytometry using anti-mSLAM MAb 12F12 or anti-hSLAM MAb IPO-3 (Fig. 2). We also found that 12F12 does not recognize hSLAM, while IPO-3 does not react to mSLAM. These CHO cells were infected with MV. While hSLAM-expressing CHO cells developed CPE upon infection with the MV KA strain isolated in B95a cells, those expressing mSLAM did not show any sign of CPE, like CHO cells transfected with pCAGGS (Fig. 3A, B, and H). Syncytia observed were not extensive, probably because SLAM was expressed transiently, and MV does not replicate very efficiently in CHO cells (9). Infection with the Edmonston strain gave the same results (data not shown). The finding indicated that mSLAM does not act as an MV receptor.
FIG. 1.
Structures of the membrane-bound and soluble forms of SLAM and chimeric molecules. Ig superfamily V-set and C2-set domains of SLAM and D1 to D4 of CD4 are indicated. hV comprises the hSLAM V domain, Myc epitope, and platelet-derived growth factor receptor (PDGFR) TM domain.
FIG. 2.
Flow cytometry analysis of cell surface expression of SLAM and chimeric molecules. CHO cells were transfected with expression plasmids encoding the indicated molecules; at 24 h after transfection, they were stained with IPO-3 (thick line, upper and middle rows) or 12F12 (thick line, lower row), followed by FITC-labeled secondary antibody. Thin lines indicate staining with mouse or rat IgG control antibody followed by FITC-labeled secondary antibody.
FIG. 3.
Development of CPE on CHO cells transiently transfected with expression plasmids encoding the indicated molecules (A to G) or control vector pCAGGS (H). The transfected CHO cells were infected with the KA strain of MV at a multiplicity of infection of 0.1 at 24 h after transfection and then observed 24 h after infection. CPE on hV-expressing CHO cells is indicated by an arrow (G). Longer incubation (up to 72 h) did not affect the presence or absence of CPE.
The V domain of hSLAM is essential for MV infection.
To determine the region(s) of hSLAM important for the MV receptor function, we constructed various chimeric molecules (Fig. 1). SLAM is a member of Ig superfamily, and its extracellular region comprises the V and C2 domains. We prepared chimeric molecules between hSLAM and mSLAM in which the V domains were reciprocally exchanged and examined whether these chimeric molecules could act as MV receptors. One plasmid expressing the hSLAM V domain and mSLAM C2 domain, TM, and CY (pCAG-mSLAM-hV) and another expressing the mSLAM V domain and hSLAM C2 domain, TM, and CY (pCAG-hSLAM-mV) were produced.
CHO cells were transiently transfected with pCAG-mSLAM-hV or pCAG-hSLAM-mV, and cell surface expression of the chimeric molecules was confirmed by flow cytometry using IPO-3 or 12F12, respectively (Fig. 2). These findings in turn indicated that the epitopes recognized by these MAbs reside in the V domains of the respective SLAMs. CHO cells transfected with pCAG-mSLAM-hV were shown to develop CPE upon infection with MV (Fig. 3C). By contrast, CHO cells transfected with pCAG-hSLAM-mV did not develop CPE after MV infection (Fig. 3D). Thus, the V domain of hSLAM is necessary and probably sufficient for its function as an MV receptor.
It is, however, possible that in addition to the V domain of hSLAM, some other region(s) of mSLAM homologous to hSLAM is involved in the receptor function of mSLAM-hV. To test whether the V domain of hSLAM alone can confer to the cell surface molecule the MV receptor function, we prepared plasmid pCAG-CD4-hV, which expressed the chimeric molecule comprising the hSLAM V domain and human CD4 domain 4 (D4), TM, and CY (Fig. 1). CD4 itself did not act as an MV receptor (Fig. 3E). CHO cells transiently transfected with pCAG-CD4-hV expressed the chimeric molecule on the cell surface (Fig. 2) and developed CPE upon infection with MV (Fig. 3F). Thus, regions of hSLAM other than the V domain were not required for the MV receptor function.
We also prepared a plasmid expressing a truncated molecule whose ectodomain consisted only of the V domain of hSLAM and Myc epitope (pCAG-hV) (Fig. 1). This construct directed cell surface expression of the molecule, albeit at a low level (Fig. 2), and allowed the transfected CHO cells to develop weak CPE after MV infection (Fig. 3G).
VSV pseudotypes bearing MV envelope proteins infect cells expressing the V domain of hSLAM.
To quantitatively evaluate the MV receptor function of the chimeric molecules, we used the pseudotype system in which the recombinant VSV containing GFP in lieu of the VSV G protein (VSVΔG∗) was complemented with envelope glycoproteins provided in trans (36). We prepared the following VSV pseudotypes: VSVΔG∗-EdHF, bearing the H and F proteins of the Edmonston strain; VSVΔG∗-KAHF, bearing the H protein of the KA strain and the F protein of the Edmonston strain; and VSVΔG∗, bearing no envelope proteins. CHO cells were transfected with various expression plasmids encoding the authentic and chimeric molecules, and then infected with the pseudotype viruses. Infectivity titers of the pseudotype viruses, which were determined by counting the number of GFP-expressing cells, are shown in Fig. 4. (In this system, quantitative comparison of the infectivity titers on each cell type can be made between VSVΔG∗-EdHF and VSVΔG∗ or between VSVΔG∗-KAHF and VSVΔG∗ but not between VSVΔG∗-EdHF and VSVΔG∗-KAHF. This is because the envelope proteins provided in trans may be incorporated into VSV pseudotype particles at different efficiencies.)
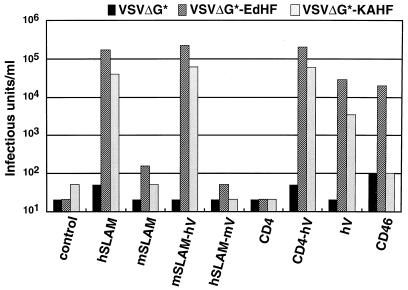
FIG. 4.
Infectivity titers of pseudotype viruses on CHO cells transiently transfected with expression plasmids encoding the indicated molecules or pCAGGS (control). The transfected CHO cells were infected with pseudotype viruses (VSVΔG∗, VSVΔG∗-EdHF, and VSVΔG∗-KAHF) at 24 h after transfection, and infectivity titers were measured by counting the number of GFP-expressing cells at 24 h after infection.
VSVΔG∗ had negligible infectivity titers in all transfected cells. Infectivity titers of VSVΔG∗-EdHF and VSVΔG∗-KAHF were more than 500-fold higher on CHO cells transfected with pCAG-hSLAM, pCAG-mSLAM-hV, or pCAG-CD4-hV than on CHO cells transfected with pCAGGS; they were also significant but lower on CHO cells transfected with pCAG-hV. The lower titers on these cells probably reflected the low expression level of the truncated SLAM on the cell surface (Fig. 2). Those CHO cells that did not express the V domain of hSLAM (the cells transfected with pCAG-mSLAM, pCAG-hSLAM-mV, or pCAG-CD4) were not susceptible to VSVΔG∗-EdHF and VSVΔG∗-KAHF. CD46-expressing CHO cells were susceptible to VSVΔG∗-EdHF but not to VSVΔG∗-KAHF. These results are consistent with the development of CPE after MV infection.
The V domain of hSLAM binds to the MV H protein.
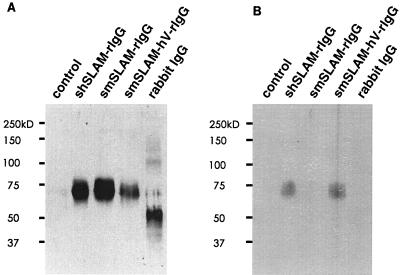
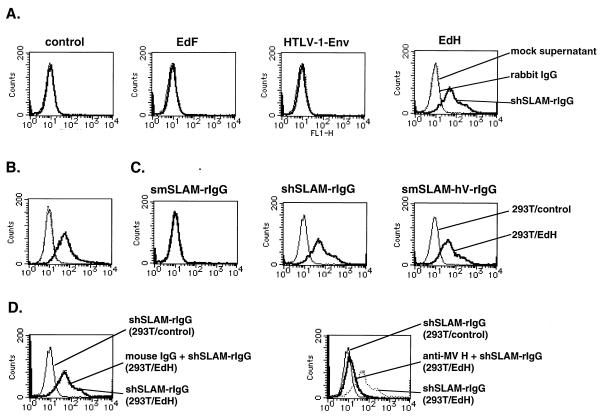
It is thought that the H protein of MV mediates receptor binding and that the F protein has membrane fusion activity. To demonstrate that hSLAM actually interacts with the H protein, we prepared a DNA construct encoding the soluble form of hSLAM fused with the rabbit IgG Fc fragment and cloned it in pCAGGS (pCAG-shSLAM-rIgG). We also prepared similar plasmid constructs for producing the soluble forms of mSLAM (pCAG-smSLAM-rIgG) and the chimeric molecule comprising the V domain of hSLAM and the C2 domain of mSLAM (pCAG-mSLAM-hV-rIgG). 293T cells were transfected with these plasmids, and soluble molecules were recovered from the supernatants. Production of the soluble molecules was confirmed by Western blot analysis using IPO-3 and anti-rabbit IgG, in which the expected bands were detected (Fig. 5). To examine interactions between recombinant soluble forms of SLAM and envelope proteins, 293T cells were transiently transfected with the expression plasmid encoding the MV H, MV F or HTLV-1 Env protein and then incubated with concentrated supernatants containing the soluble molecules. Soluble hSLAM was shown to bind to cells expressing the MV H protein but not to cells expressing the MV F or HTLV-1 Env protein (Fig. 6A). Surface expression of the MV H protein on transfected 293T cells is shown in Fig. 6B. The chimeric molecule possessing the hSLAM V domain also exhibited binding to cells expressing the MV H protein, but soluble mSLAM did not (Fig. 6C). The binding of soluble hSLAM to the MV H protein-expressing cells was inhibited by anti-MV H protein MAb but not by control mouse IgG, confirming the specificity of the binding (Fig. 6D). These results indicate that the V domain of hSLAM binds to the ectodomain of the MV H protein.
FIG. 5.
Western blot analysis of soluble molecules. Concentrated supernatants of 293T cells transfected with expression plasmids encoding the indicated molecules or pCAGGS (control) were separated together with rabbit IgG on SDS-polyacrylamide gels under reducing conditions and transferred to membranes, which were incubated with anti-rabbit IgG (A) or IPO-3 (B) to detect soluble molecules.
FIG. 6.
Binding of soluble molecules to 293T cells transiently transfected with pCXN2 (control) or an expression plasmid encoding the MV Edmonston H protein (EdH), Edmonston F protein (EdF), or HTLV-1 Env protein. (A) Transfected 293T cells were incubated with soluble hSLAM (shSLAM-rIgG), mock supernatant, or rabbit IgG at 24 h after transfection. The mock supernatant was obtained from 293T cells transfected with pCAGGS. (B) At 24 h after transfection, 293T cells transfected with the expression plasmid encoding the MV H protein were stained with anti-MV H protein MAb C-1 (solid thick line) or control mouse IgG (dotted line), followed by staining with FITC-labeled anti-mouse IgG. 293T cells transfected with pCXN2 were stained with C-1 and then with FITC-labeled anti-mouse IgG (solid thin line). (C) 293T cells transfected with pCXN2 (293T/control) or the plasmid encoding the MV H protein (293T/EdH) were incubated with soluble molecules (smSLAM-rIgG, shSLAM-rIgG, or smSLAM-hV-rIgG). (D) 293T/EdH cells were pretreated with C-1 or mouse IgG and then incubated with shSLAM-rIgG in the presence of C-1 or mouse IgG. After washing, the samples in panels A, C, and D were stained with FITC-labeled anti-rabbit IgG.
DISCUSSION
In this study, we first showed that mSLAM, which has structural and functional similarities to the human homologue (6), was unable to act as an MV receptor, consistent with the observation that mice are not susceptible to MV (26). We then demonstrated that the cell surface transmembrane proteins possessing the V domain of hSLAM could act as MV receptors, whereas the chimeric hSLAM whose V domain was replaced with the mouse counterpart could not. These results indicate that the V domain of hSLAM is necessary and sufficient for its MV receptor function and that the other regions of hSLAM, including the C2 domain, TM, and CY (two CY forms by alternate splicing [7]) were not required. We also showed that soluble hSLAM was capable of binding to cells expressing the MV H protein but not to cells expressing the MV F or HTLV-1 Env protein. Binding to cells expressing the H protein also occurred with the soluble molecule comprising the V domain of hSLAM and the C2 domain of mSLAM. Thus, we conclude that the V domain of hSLAM can interact with the ectodomain of the H protein in the absence of the F protein.
Previous studies have shown that many viruses use as receptors cell surface glycoproteins which possess Ig superfamily domains. In these cases, viruses tend to bind to the N-terminal (most membrane-distal) domains of the molecules; human immunodeficiency virus interacts with D1 of CD4 (1, 23), major group human rhinoviruses interact with the first C2 domain of intercellular adhesion molecule 1 (20, 35), and poliovirus interacts with the V domain of the poliovirus receptor (15). Among viruses whose cellular receptors do not possess the Ig superfamily domains, vaccine strains of MV bind to the short consensus repeats (SCRs) 1 and 2 of CD46 (13, 18), and Epstein-Barr virus binds to the two outermost SCRs of CD21 (24). Our results showed that like these receptors, the N-terminal V domain is responsible for the interaction between MV and hSLAM. It is likely that the membrane-distal domains of these molecules are more accessible to viruses and thus can act as virus-binding sites.
To further localize the functional domain of hSLAM, we produced other chimeric molecules on an mSLAM backbone in which only an N-terminal (amino acid positions 1 to 67) or C-terminal (positions 68 to 139) part of the V domain was replaced with the corresponding region of hSLAM (data not shown). Although these molecules were expressed on the cell surface, they were unable to function as MV receptors. Thus, we conclude that the whole V domain of hSLAM is required for the MV receptor function; studies using site-directed mutagenesis and synthetic peptides may further define the residues within the V domain of hSLAM involved in this function.
The truncated molecule whose ectodomain is composed mostly of the V domain of hSLAM did not act as an MV receptor as efficiently as the chimeric molecules with two Ig superfamily domains including the V domain of hSLAM. Although a low level of cell surface expression of this molecule seems to be responsible for this finding, the distance of the MV binding site from the membrane may also influence the receptor function (5).
We have previously showed that IPO-3, a MAb directed against hSLAM, can inhibit the development of CPE in MV-infected cells (39). Reactivities of IPO-3 to various chimeric molecules indicated that IPO-3 recognizes an epitope on the V domain of hSLAM. These results are consistent with the finding that the V domain of hSLAM interacts with MV. It is likely that IPO-3 inhibits MV infection, either by directly blocking the MV-binding site on the V domain of hSLAM or by steric hindrance. A12, another MAb specific for hSLAM (7), also recognized an epitope on the V domain of hSLAM (data not shown).
SLAM is considered to play an important role in bidirectional signaling during T-cell and B-cell activation (2, 3, 7, 22, 30, 31). Since ligation of SLAM with A12 or IPO-3 induces activation and proliferation of T and B cells (3, 7, 22, 34), SLAM must interact with its natural ligand also through the V domain. It has been shown that CD2, the most extensively studied member of the CD2 family, also possesses the ligand-binding site on the V domain of the molecule (8). SLAM has been reported to be homophilic (a self-ligand) (2, 30), but a recent report showed that SLAM self-associates with very low affinity (19), raising questions regarding the physiological role of such interactions. Though the nature of SLAM-ligand interaction remains to be characterized, binding sites for both the natural ligand and the H protein of MV appear to be located on the V domain of SLAM. This is in contrast with another MV receptor, CD46, on which binding sites for MV and natural ligands C3b and C4b are distinct (13, 18). Although the residues on SLAM involved in MV binding may not be exactly the same as those involved in binding the natural ligand, MV binding to SLAM may affect the signal transduction pathways induced through SLAM. Considering the immunosuppression observed during MV infection, it is tempting to speculate that such an interaction leads to inhibition of SLAM-mediated lymphocyte activation.
ACKNOWLEDGMENTS
We thank M. A. Whitt for allowing us to use the VSVΔG∗-GFP system and F. Kobune and J. A. T. Young for providing the KA strain of MV and for pSK100, respectively.
This work was supported by grants from the Ministry of Education, Science and Culture of Japan and from the Organization for Drug ADR Relief, R&D Promotion and Product Review of Japan.
REFERENCES
- 1.Arthos J, Deen K C, Chaikin M A, Fornwald J A, Sathe G, Sattentau Q J, Clapham P R, Weiss R A, McDougal J S, Pietropaolo C, Axel R, Truneh A, Maddon P J, Sweet R W. Identification of the residues in human CD4 critical for the binding of HIV. Cell. 1989;57:469–481. doi: 10.1016/0092-8674(89)90922-7. [DOI] [PubMed] [Google Scholar]
- 2.Aversa G, Carballido J, Punnonen J, Chang C-C J, Hauser T, Cocks B G, de Vries J E. SLAM and its role in T cell activation and Th cell responses. Immunol Cell Biol. 1997;75:202–205. doi: 10.1038/icb.1997.30. [DOI] [PubMed] [Google Scholar]
- 3.Aversa G, Chang C-C, Carballido J M, Cocks B G, de Vries J E. Engagement of the signaling lymphocytic activation molecule (SLAM) on activated T cells results in IL-2-independent, cyclosporin A-sensitive T cell proliferation and IFN-gamma production. J Immunol. 1997;158:4036–4044. [PubMed] [Google Scholar]
- 4.Brojatsch J, Naughton J, Rolls M M, Zingler K, Young J A T. CAR1, a TNFR-related protein, is a cellular receptor for cytopathic avian leukosis-sarcoma viruses and mediates apoptosis. Cell. 1996;87:845–855. doi: 10.1016/s0092-8674(00)81992-3. [DOI] [PubMed] [Google Scholar]
- 5.Buchholz C J, Schneider U, Devaux P, Gerlier D, Cattaneo R. Cell entry by measles virus: long hybrid receptors uncouple binding from membrane fusion. J Virol. 1996;70:3716–3723. doi: 10.1128/jvi.70.6.3716-3723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro A G, Hauser T M, Cocks B G, Abrams J, Zurawski S, Churakova T, Zonin F, Robinson D, Tangye S G, Aversa G, Nichols K E, de Vries J E, Lanier L L, O'Garra A. Molecular and functional characterization of mouse signaling lymphocytic activation molecule (SLAM): differential expression and responsiveness in Th1 and Th2 cells. J Immunol. 1999;163:5860–5870. [PubMed] [Google Scholar]
- 7.Cocks B G, Chang C-C J, Carballido J M, Yssel H, de Vries J E, Aversa G. A novel receptor involved in T-cell activation. Nature. 1995;376:260–263. doi: 10.1038/376260a0. [DOI] [PubMed] [Google Scholar]
- 8.Davis S J, van der Merwe P A. The structure and ligand interactions of CD2: implications for T-cell function. Immunol Today. 1996;17:177–187. doi: 10.1016/0167-5699(96)80617-7. [DOI] [PubMed] [Google Scholar]
- 9.Dorig R E, Marcil A, Chopra A, Richardson C D. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 10.Griffin D E, Bellini W J. Measles virus. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1267–1312. [Google Scholar]
- 11.Grosjean J, Caux C, Bella C, Berger I, Wild F, Banchereau J, Kaiserlian D. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J Exp Med. 1997;186:801–812. doi: 10.1084/jem.186.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu E C, Sarangi F, Iorio C, Sidhu M S, Udem S A, Dillehay D L, Xu W, Rota P A, Bellini W J, Richardson C D. A single amino acid change in the hemagglutinin protein of measles virus determines its ability to bind CD46 and reveals another receptor on marmoset B cells. J Virol. 1998;72:2905–2916. doi: 10.1128/jvi.72.4.2905-2916.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwata K, Seya T, Yanagi Y, Pesando J M, Johnson P M, Okabe M, Ueda S, Ariga H, Nagasawa S. Diversity of sites for measles virus binding and for inactivation of complement C3b and C4b on membrane cofactor protein CD46. J Biol Chem. 1995;270:15148–15152. doi: 10.1074/jbc.270.25.15148. [DOI] [PubMed] [Google Scholar]
- 14.Kobune F, Sakata H, Sugiura A. Marmoset lymphoblastoid cells as a sensitive host for isolation of measles virus. J Virol. 1990;64:700–705. doi: 10.1128/jvi.64.2.700-705.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koike S, Ise I, Nomoto A. Functional domains of poliovirus receptor. Proc Natl Acad Sci USA. 1991;88:4104–4108. doi: 10.1073/pnas.88.10.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lecouturier V, Fayolle J, Caballero M, Carabana J, Celma M L, Fernandez-Munoz R, Wild T F, Buckland R. Identification of two amino acids in the hemagglutinin glycoprotein of measles virus (MV) that govern hemadsorption, HeLa cell fusion, and CD46 downregulation: phenotypic markers that differentiate vaccine and wild-type MV strains. J Virol. 1996;70:4200–4204. doi: 10.1128/jvi.70.7.4200-4204.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maddon P J, Littman D R, Godfrey M, Maddon D E, Chess L, Axel R. The isolation and nucleotide sequence of a cDNA encoding the T cell surface protein T4: a new member of the immunoglobulin gene family. Cell. 1985;42:93–104. doi: 10.1016/s0092-8674(85)80105-7. [DOI] [PubMed] [Google Scholar]
- 18.Manchester M, Valsamakis A, Kaufman R, Liszewski M K, Alvarez J, Atkinson J K, Lublin D M, Oldstone M B A. Measles virus and C3 binding sites are distinct on membrane cofactor protein(CD46) Proc Natl Acad Sci USA. 1995;92:2303–2307. doi: 10.1073/pnas.92.6.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mavaddat N, Mason D W, Atkinson P D, Evans E J, Gilbert R J, Stuart D I, Fennelly J A, Barclay A N, Davis S J, Brown M H. Signaling lymphocytic activation molecule (CDw150) is homophilic but self-associates with very low affinity. J Biol Chem. 2000;275:28100–28109. doi: 10.1074/jbc.M004117200. [DOI] [PubMed] [Google Scholar]
- 20.McClelland A, deBear J, Yost S C, Meyer A M, Marlor C W, Greve J M. Identification of monoclonal antibody epitopes and critical residues for rhinovirus binding in domain 1 of intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1991;88:7993–7997. doi: 10.1073/pnas.88.18.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McFarlin D E, Bellini W J, Mingioli E S, Behar T N, Trudgett A. Monospecific antibody to the haemagglutinin of measles virus. J Gen Virol. 1980;48:425–429. doi: 10.1099/0022-1317-48-2-425. [DOI] [PubMed] [Google Scholar]
- 22.Mikhalap S V, Shlapatska L M, Berdova A G, Law C-L, Clark E A, Sidorenko S P. CDw150 associates with Src-homology 2-containing inositol phosphatase and modulates CD95-mediated apoptosis. J Immunol. 1999;162:5719–5727. [PubMed] [Google Scholar]
- 23.Moebius U, Clayton L K, Abraham S, Harrison S C, Reinherz E L. The human immunodeficiency virus gp120 binding site on CD4: delineation by quantitative equilibrium and kinetic binding studies of mutants in conjunction with a high-resolution CD4 atomic structure. J Exp Med. 1992;176:507–517. doi: 10.1084/jem.176.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molina H, Brenner C, Jacobi S, Gorka J, Carel J C, Kinoshita T, Holers V M. Analysis of Epstein-Barr virus-binding sites on complement receptor 2 (CR2/CD21) using human-mouse chimeras and peptides. At least two distinct sites are necessary for ligand-receptor interaction. J Biol Chem. 1991;266:12173–12179. [PubMed] [Google Scholar]
- 25.Naniche D, Varior-Krishnan G, Cervoni F, Wild T F, Rossi B, Rabourdin-Combe C, Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neighbour P A, Rager-Zisman B, Bloom B R. Susceptibility of mice to acute and persistent measles infection. Infect Immun. 1978;21:764–770. doi: 10.1128/iai.21.3.764-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants by a novel eukaryotic vector. Gene. 1991;108:193–200. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 28.Okuma K, Nakamura M, Nakano S, Niho Y, Matsuura Y. Host range of human T-cell leukemia virus type I analyzed by a cell fusion-dependent reporter gene activation assay. Virology. 1999;254:235–244. doi: 10.1006/viro.1998.9530. [DOI] [PubMed] [Google Scholar]
- 29.Post T W, Liszewski M K, Adams E M, Tedja I, Miller E A, Atkinson J P. Membrane cofactor protein of the complement system: alternative splicing of serine/threonine/proline-rich exons and cytoplasmic tails produces multiple isoforms that correlate with protein phenotype. J Exp Med. 1991;174:93–102. doi: 10.1084/jem.174.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Punnonen J, Cocks B G, Carballido J M, Bennett B, Peterson D, Aversa G, de Vries J E. Soluble and membrane-bound forms of signaling lymphocytic activation molecule (SLAM) induce proliferation and Ig synthesis by activated human B lymphocytes. J Exp Med. 1997;185:993–1004. doi: 10.1084/jem.185.6.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sayos J, Wu C, Morra M, Wang N, Zhang X, Allen D, van Schaik S, Notarangelo L, Geha R, Roncarolo M G, Oettgen H, De Vries J E, Aversa G, Terhorst C. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 1998;395:462–469. doi: 10.1038/26683. [DOI] [PubMed] [Google Scholar]
- 32.Schneider-Schaulies J, Dunster L M, Kobune F, Rima B, Ter Meulen V. Differential downregulation of CD46 by measles virus strains. J Virol. 1995;69:7257–7259. doi: 10.1128/jvi.69.11.7257-7259.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider-Schaulies J, Schnorr J-J, Brinckmann U, Dunster L M, Baczko K, Liebert U G, Schneider-Schaulies S, ter Meulen V. Receptor usage and differential downregulation of CD46 by measles virus wild-type and vaccine strains. Proc Natl Acad Sci USA. 1995;92:3943–3947. doi: 10.1073/pnas.92.9.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sidorenko S P, Clark E A. Characterization of a cell surface glycoprotein IPO-3, expressed on activated human B and T lymphocytes. J Immunol. 1993;151:4614–4624. [PubMed] [Google Scholar]
- 35.Staunton D E, Dustin M L, Erickson H P, Springer T A. The arrangement of the immunoglobulin-like domains of ICAM-1 and the binding sites for LFA-1 and rhinovirus. Cell. 1990;61:243–254. doi: 10.1016/0092-8674(90)90805-o. [DOI] [PubMed] [Google Scholar]
- 36.Takada A, Robinson C, Goto H, Sanchez A, Murti K G, Whitt M A, Kawaoka Y. A system for functional analysis of Ebola virus glycoprotein. Proc Natl Acad Sci USA. 1997;94:14764–14769. doi: 10.1073/pnas.94.26.14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka K, Xie M, Yanagi Y. The hemagglutinin of recent measles virus isolates induces cell fusion in a marmoset cell line, but not in other CD46-positive human and monkey cell lines, when expressed together with the F protein. Arch Virol. 1998;143:213–225. doi: 10.1007/s007050050281. [DOI] [PubMed] [Google Scholar]
- 38.Tatsuo H, Okuma K, Tanaka K, Ono N, Minagawa H, Takade A, Matsuura Y, Yanagi Y. Virus entry is a major determinant of cell tropism of Edmonston and wild-type strains of measles virus as revealed by vesicular stomatitis virus pseudotypes bearing their envelope proteins. J Virol. 2000;74:4139–4145. doi: 10.1128/jvi.74.9.4139-4145.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tatsuo H, Ono N, Tanaka K, Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406:893–897. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- 40.Vallejo A N, Pogulis R J, Pease L R. Mutagenesis and synthesis of novel recombinant genes using PCR. In: Dieffenbach C W, Dveksler G S, editors. PCR primer: a laboratory manual. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1995. pp. 603–612. [Google Scholar]