Abstract
Background
The reference values of eNO have certain differences among people of different countries and races. We aimed to obtain the reference value of eNO in healthy children and adolescents (6–18 years old) in China and to explore the associations between the reference values with ages, gender, heights, BMI, and regions.
Methods
We measured FeNO50 levels in 5949 healthy Chinese children and adolescents, FeNO200 and CaNO levels in 658 participants from 16 provinces of 7 administrative areas in China aged 6–18. All persons were studied after obtaining informed consent from children and their parents.
Results
The mean FeNO50 of 5949 Chinese children and adolescents aged 6–18 years was 14.1 ppb, with a 95% confidence interval of 1-38.1 ppb. The mean FeNO200 of 658 persons was 6.9 ppb with a 95% upper confidence interval of 15.0 ppb, and the mean CaNO was 3.0 ppb with a 95% upper confidence interval of 11.2 ppb. In the 6–11 age group, age and height were correlated with the logarithm of FeNO50 (P < 0.001, P < 0.05). There was no significant correlation between the logarithm of FeNO200 and gender, age, height and BMI (all P > 0.05). The logarithm of CaNO was correlated with gender (P < 0.05). In the 12–18 age group, gender, height, and region were correlated with the logarithm of FeNO50 (all P < 0.001). There was only a weak correlation between the logarithm of FeNO200 and height (P < 0.001). The logarithm of CaNO was negatively correlated with age (P < 0.05).
Conclusions
Higher FeNO50, FeNO200 and CaNO values were found in healthy children and adolescents in China compared with foreign reports, and is affected by age, height, gender, and region. This study provides useful references for clinical application of eNO in children, especially Asian children.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-024-02938-4.
Keywords: FeNO50, FeNO200, CaNO, Reference values, Children and adolescents
Background
Nitric oxide (NO), a gas signaling molecule of endogenous origin, is synthesized within the airway epithelial cells and its production is enhanced during eosinophilic inflammatory conditions. Fractional Exhaled Nitric Oxide (FeNO) has been acknowledged as a biomarker for type 2 inflammation in the airway, which plays a crucial role in bronchial hyper-responsiveness, mucus secretion, goblet cell hyperplasia, T-helper type 2 polarization, as well as allergic and eosinophilic inflammation [1]. In 2005, the American Thoracic Society (ATS) and the European Respiratory Society (ERS) jointly published the technical guidelines for the quantification of exhaled NO [2]. In 2011, the ATS endorsed the use of FeNO as a diagnostic tool for eosinophilic airway inflammation and as a reference for the administration of inhaled corticosteroids [3]. Similarly, the Global Initiative for Asthma (GINA) guidelines, the National Institute for Health and Care Excellence (NICE) guidelines, and other national asthma guidelines also advocate for the utilization of FeNO in the diagnosis and assessment of asthma in both adults and children [4–7].
FeNO serves as an indicator of eosinophilic airway inflammation, with its concentration varying across different segments of the airway and detectable at various flow rates, thereby providing insights into the inflammation present in the large airway, small airway, and upper airway [8]. The 2011 AST guidelines have suggested the use of FeNO50 as a reference value for assessing NO levels in children. However, it is important to note that FeNO50, which measures NO levels at a low flow rate of 50 ml/s, only reflects the NO levels in the large airways. Recent clinical studies have shown that patients with asthma often experience inflammation in the small airways. As a result, current guidelines recommend the use of FeNO200 and CaNO as markers for small airway inflammation [8, 9]. However, there is still a lack of established reference values for these small airway inflammation indicators (FeNO200, CaNO).
The determination of reference values for exhaled NO (eNO) and the identification of influencing factors are crucial in the assessment of asthma. Despite the recommendation of ATS standards for the reference value of FeNO50, variations in the reference value of eNO persist among individuals from different countries and races. Notably, studies conducted in North America and Europe have provided insights into the normal values of FeNO200 and CaNO as markers of small airway inflammation, yet there is a lack of description regarding these values in Asian children.
Hence, the Shanghai Children’s Medical Center assumed the leading role in a collaborative effort involving 19 branch centers affiliated with the Respiratory department of children’s hospitals or the Pediatric department of general hospitals across 16 provinces in China. This multi-center study, conducted from May 2018 to May 2021, aimed to establish the baseline levels of eNO in healthy children aged 6–18 years. The ultimate objective was to ascertain the normal range of eNO in Chinese children, thereby furnishing a valuable reference for the clinical diagnosis and utilization of eNO in pediatric healthcare settings in China and potentially throughout Asia.
Materials and methods
Study participants and design
This is a cross-sectional study of children and adolescents aged 6–18 in China from May 2018 to May 2021. Informed consent was obtained from the parents or legal guardians of the children. The inclusion criteria were as follows: ① The ethnicity is Han, and the age range is 6 to 18 years old; ② Born in China, both parents are Chinese Han nationality; ③ No history of respiratory tract infections within 4 weeks before; ④ No history of recurrent respiratory tract infections; ⑤ No allergic diseases such as asthma and rhinitis; ⑥ No family history of asthma; ⑦ No neurological disorders; ⑧ Normal intellectual development; ⑨ No smoking in the family environment; ⑩ No congenital lung and heart disease. A total of 8000 children were enrolled in the study. This study was approved by the Ethics Committee of Shanghai Children’s Medical Center (Ethics No: SCMCIRB-K2017007), and each subcenter follows the master research unit ethics. Clinical Trial: ChiCTR1800019029 (Registration date was October 22, 2018).
Here, 20 centers from 16 provinces, seven areas in China (North, East, Northeast, Middle, South, Southwest, and Northwest), participated in this study. Of the 400 healthy children were recruited from each center and divided into two age groups: group I (age: 6–11 years old) had 200 cases, with 100 males and 100 females; Group II (age: 12–18 years old) had 200 cases, with 100 males and 100 females. Each center recruited healthy volunteers through local primary and secondary schools. After obtaining the informed consent of children and parents, the study was conducted.
Procedures
Physical growth index measurement
All of the subjects’ heights and weights were recorded by fixed staff. The participants removed their shoes and wore shirts or light sweaters. Their weight was determined by scale that was accurate to 0.1 kg and their height by a vertical altimeter that was accurate to 0.1 cm. The children’s birth dates and genders were noted simultaneously.
Determination of exhaled NO
All enrolled children were screened for healthy children by spirometry and then tested for exhaled NO. FeNO50 was measured in children from seven areas of China, FeNO200 and CaNO were measured in children from East China. The eNO was measured by the Nacoulomb breath analyzer. The subjects were tested for eNO according to the 2005 ATS/ERS and the 2017 ERS guidelines [1, 8]. Eating, strenuous exercise, or pulmonary function tests were banned 1 h before the test. Before the test, the operator will explain the test method and precautions to the subject, consult and fill in the subject’s information (including age, sex, height and weight).
The test was performed with the subject in a seated position, holding the inspiratory filter, exhaling the remaining air, then covering the mouth with the filter, inhaling first and then exhaling at a flow rate of 50 ml/s and 200 ml/s, respectively, as required by the test. The values of FeNO50 and FeNO200 were recorded in ppb. Two tests were attempted for each subject at a single flow rate with an error of less than 10%, and unsuccessful attempts were recorded as test failures. CaNO values were calculated from FeNO50 and FeNO200.
According to the 2017 ERS guideline, the calculation of CaNO requires exhalation detection at 3 flow rates. However, this method is operationally difficult in younger children. In 2014, Peter J Barnes suggested that a low flow rate (50 ml/s) and a high flow rate (200 ml/s) could be used to differentiate NO in the large and small airways [9]. Therefore, the method of CaNO calculation was simplified using a two-compartment linear model in this study. To ensure the accuracy of the instruments, two standard gas mixtures with NO concentrations of 60 ppb and 250 ppb were used to test and calibrate the instruments before the start of the project in each subcenter.
Statistical analysis
The Kolmogorov-Smirnov method was used to test the normality of FeNO50, FeNO200 and CaNO. Data that failed the normality test were logarithmically transformed into data with a normal distribution. FeNO50, FeNO200 and CaNO were skewed distributions, so the normal reference values were calculated using the 95% upper limit value in the logarithmic state, and the actual values were calculated after taking the logarithm, corresponding to the 95% upper limit value, which is the upper limit of the normal values of the measured values.
Multiple stepwise regression and multiple linear regression analysis were performed on factors that may affect FeNO50, FeNO200 and CaNO: age, sex, height, Body-Mass-Index (BMI), and region (for FeNO50 only) as independent variables. VIF was used to test interactions between variables. Weight was not counted in the analysis because it was covariate with BMI. For gender comparisons between males and females, t-tests were used for comparisons between groups conforming to normally distributed data, and nonparametric tests were used for comparisons between groups with nonnormally distributed data. All statistical analyses were performed using SPSS 22.0(IBM, Armonk, NY, USA). P < 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 8000 children were completing the enrollment questionnaire. The reasons for exclusion were (1) incomplete questionnaire (n = 231), (2) failure to fall within the predetermined age range (n = 13), (3) having a history of eating within 1 h before measurement (n = 344), (4) having strenuous exercise within 1 h before measurement (n = 208), (5) inability to complete the FeNO50 measurement (n = 467) and spirometry(n = 582), (6) abnormal pulmonary ventilation function (n = 206). Finally, a total of 5949 healthy children were included in the statistical analysis (Fig. 1). Subject characteristics, including the total study sample with eNO measurements, are presented in Table 1. FeNO50 measurements were performed in 5949 children. Of the 3534 children (1881 males and 1653 females) aged 6–11 years, and 2415 children (1220 males and 1195 females) aged 12–18 years. A total of 800 children from two centers in East China participated in FeNO200/CaNO determination, and only 658 cases (359 males and 299 females) completed the determination of FeNO200/CaNO. 345 children (199 males and 146 females) aged 6–11 years, and 313 children (160 males and 153 females) aged 12–18 years.
Fig. 1.
Schematic presentation of the recruitment of healthy children
Table 1.
The demographic data of the participants
| FeNO50 | FeNO200/CaNO | ||||||
|---|---|---|---|---|---|---|---|
| All (N = 5949) |
6–11 years (N = 3534) |
12–18 years (N = 2415) |
All (N = 658) |
6–11 years (N = 345) |
12–18 years (N = 313) |
||
| Age, years, M (P25, P75) | 10.0 (8.0, 13.0) | 9.0 (7.0, 10.0) | 14.0 (13.0, 16.0) | 11.0 (9.0, 15.0) | 9.0 (8.0, 10.0) | 15.0 (13.0, 17.0) | |
| Sex | |||||||
| Male | 3101 (52.1%) | 1881 (53.2%) | 1220 (50.5%) | 359 (54.6%) | 199 (57.7%) | 160 (51.1%) | |
| Female | 2848 (47.9%) | 1653 (46.8%) | 1195 (49.5%) | 299 (45.4%) | 146 (42.3%) | 153 (48.9%) | |
| Height, cm, M (P25, P75) | 147.0 (133.0, 161.0) | 135.0 (128.0, 144.0) | 163.0 (157.0, 170.0) | 150.5 (137.0, 165.0) | 137.0 (131.0, 144.0) | 165.0 (159.0, 172.0) | |
| Weight, kg, M (P25, P75) | 39.9 (28.0, 53.0) | 30.0 (25.0, 38.0) | 54.0 (47.0, 62.8) | 45.0 (32.0, 58.0) | 33.0 (27.0, 41.0) | 58.0 (50.0, 67.5) | |
| BMI, kg/m2, M (P25, P75) | 18.0 (15.6, 20.8) | 16.3 (14.9, 18.9) | 20.0 (18.1, 22.6) | 19.2 (16.6, 22.0) | 17.4 (15.5, 20.3) | 20.7 (18.7, 24.0) | |
Normal ranges of FeNO50, FeNO200 and CaNO in healthy children
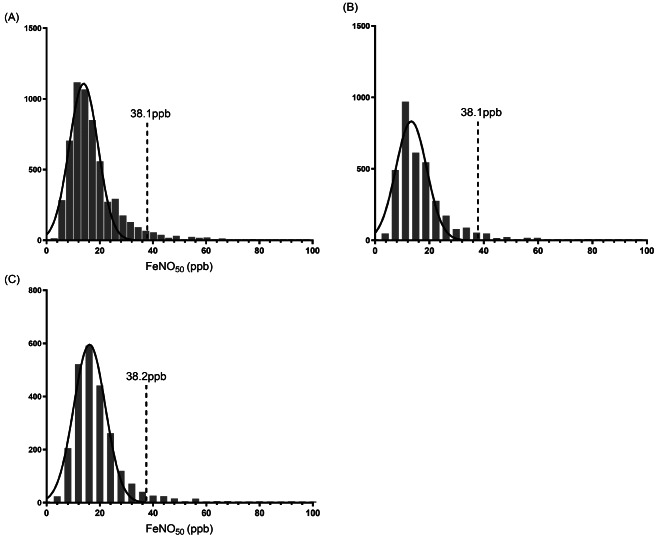
In this study, the geometric mean of FeNO50 in 5949 children aged 6–18 years was 14.1ppb (P25-P75:13.9-14.3ppb), and the 95% upper limit was 38.1ppb. The 95% upper limit of FeNO50 in children aged 6–11 years was 38.1ppb, and the geometric mean was 13.1ppb (P25-P75:12.9-13.4ppb), which respectively were 38.2ppb and 15.9ppb (P25-P75:13.4-13.9ppb) in children aged 12–18 years (Fig. 2).
Fig. 2.
Distribution of FeNO50. (A) FeNO50 in children aged 6–18 years; (B) FeNO50 in children aged 6–11 years; (C) FeNO50 in children aged 12–18 years
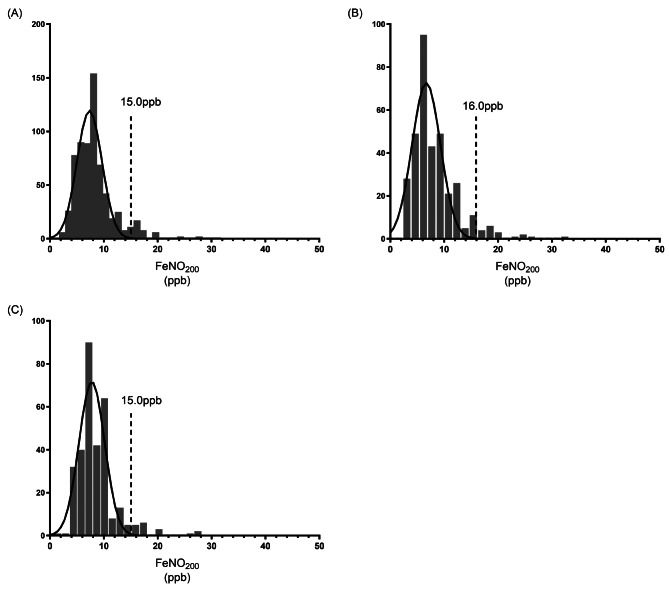
The range of FeNO200 in 658 children aged 6–18 years was 1-31ppb, with the geometric mean being 6.9ppb (P25-P75:6.9-7.2ppb) and the 95% upper limit being 15.0ppb. The 95% upper limit of FeNO200 in children aged 6–11 years was 16.0ppb, and the geometric mean was 6.6ppb (P25-P75:6.3-7.0ppb), which respectively were 15.0ppb and 7.3ppb (P25-P75:6.3-7.0ppb) in children aged 12–18 years (Fig. 3).
Fig. 3.
Distribution of FeNO200. (A) FeNO200 in children aged 6–18 years; (B) FeNO200 in children aged 6–11 years; (C) FeNO200 in children aged 12–18 years
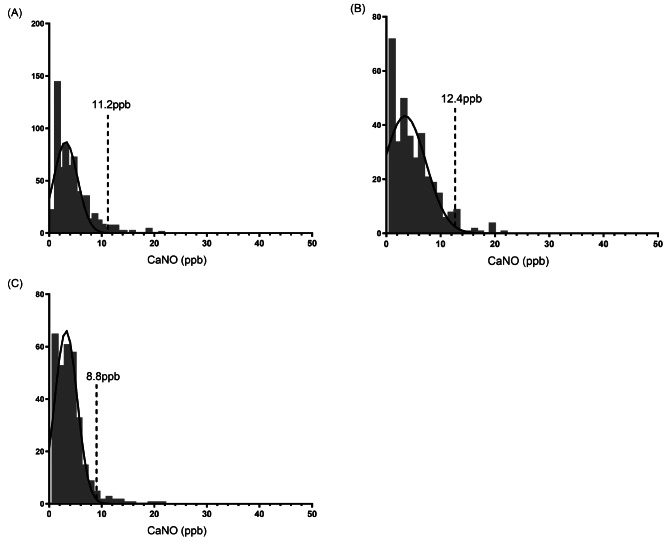
The range of CaNO in 658 children aged 6–18 years was 0.5-21.1ppb, with the geometric mean being 3.0ppb (P25-P75:2.9-3.2ppb) and the 95% upper limit was 11.2ppb. The 95% upper limit of CaNO in children aged 6–11 years was 12.4ppb, and the geometric mean was 3.6ppb (P25-P75:3.3-4.0ppb), which respectively were 8.8ppb and 2.8ppb (P25-P75:2.6-3.1ppb) in children aged 12–18 years (Fig. 4).
Fig. 4.
Distribution of CaNO. (A) CaNO in children aged 6–18 years; (B) CaNO in children aged 6–11 years; (C) CaNO in children aged 12–18 years
Factors affecting FeNO50 values
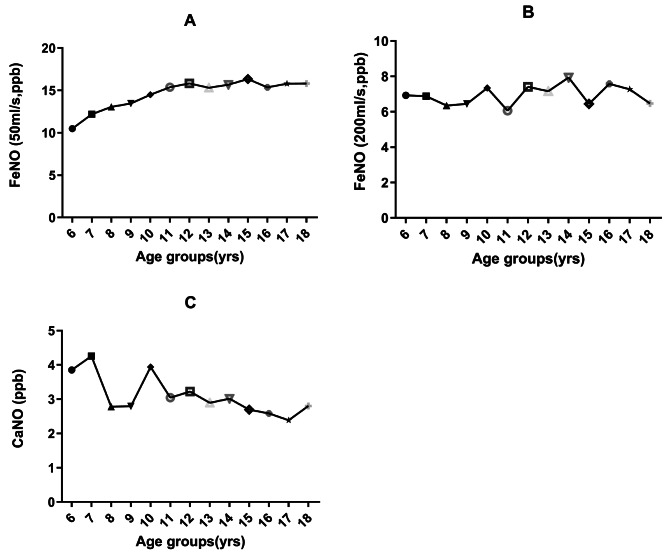
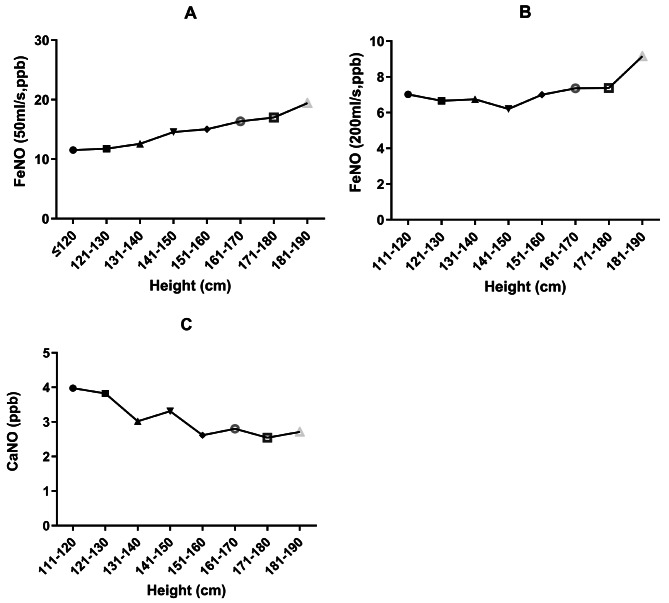
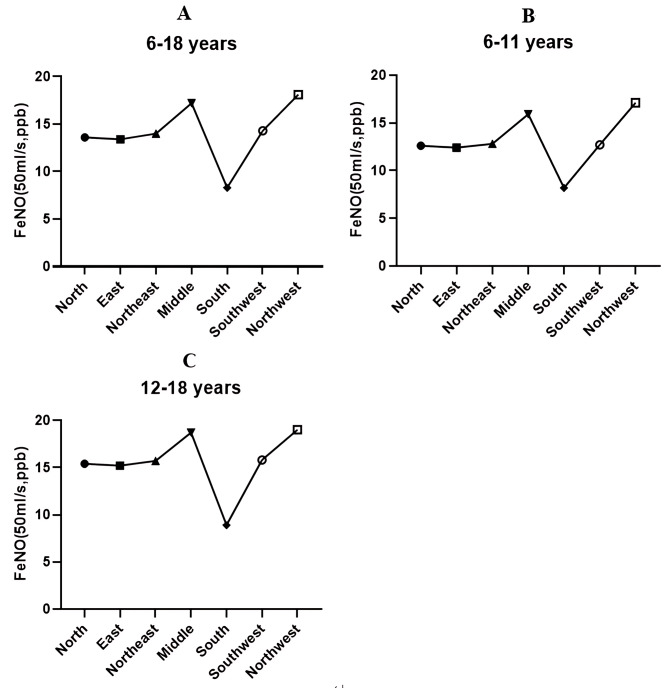
Only taking into account independent variables, the logarithm of FeNO50 was significantly correlated with age, height, and BMI in children aged 6 to 18 (Figs. 5A and 6A, all P < 0.001). FeNO50 in males was significantly higher than that in females, and there were significant differences among different regions (Fig. 7A, P < 0.05). According to the results of multivariate regression analysis, the logarithm of FeNO50 has a significant relationship with height, region, and gender (all P < 0.05), but not to age and BMI in children aged 6–18 years (all P>0.05).
Fig. 5.
FeNO50, FeNO200 and CaNO values in children of all ages
Fig. 6.
FeNO50, FeNO200 and CaNO values of children with different heights
Fig. 7.
FeNO50 values of children with different regions
For children aged 6–11 years, the logarithm of FeNO50 was significantly correlated with age, height and BMI when only independent factors were considered. There was no significant difference in FeNO50 values between males and females, but there were significant differences in different regions (Fig. 7B, P < 0.05). The results of multivariate regression analysis showed that age and height were significantly correlated with the logarithm of FeNO50, while gender, BMI and regions were not (Table 2).
Table 2.
Regression coefficients (β) and p-values of the multiple regression models for eNO parameters in age group 6–11 years
| Interceptβ | Age | Gender | Height | BMI | Regions | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p-value | β | p-value | β | p-value | β | p-value | β | p-value | R 2 | R2boot | ||||||
| FeNO50 | 1.718 | 0.054 | < 0.001 | -0.014 | 0.412 | 0.003 | < 0.05 | -0.001 | 0.958 | 0.026 | 0.116 | 0.036 | 0.036 | ||||
| FeNO200 | 2.308 | 0.011 | 0.704 | -0.061 | 0.259 | -0.001 | 0.784 | -0.014 | 0.122 | - | - | 0.013 | 0.001 | ||||
| CaNO | 2.750 | 0.014 | 0.786 | -0.199 | < 0.05 | -0.012 | 0.148 | 0.012 | 0.485 | - | - | 0.020 | 0.009 | ||||
The R2 is the unadjusted coefficient of determination of the models and R2 boot is the corresponding optimism- corrected R2 values as estimated by bootstrapping
For children and adolescents aged 12–18 years old, the logarithm of FeNO50 was significantly correlated with height, but not with age or BMI when only independent factors were considered. The FeNO50 value of males was significantly higher than that of females, and there were significant differences in different regions (Fig. 7C, P < 0.05). The results of multivariate regression analysis showed that the logarithm of FeNO50 was significantly correlated with gender, height and region, but not with age and BMI (Table 3).
Table 3.
Regression coefficients (β) and p-values of the multiple regression models for eNO parameters in age group 12–18 years
| Interceptβ | Age | Gender | Height | BMI | Regions | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p-value | β | p-value | β | p-value | β | p-value | β | p-value | R 2 | R2boot | ||||||
| FeNO50 | 2.396 | -0.010 | 0.638 | -0.123 | < 0.001 | 0.003 | < 0.05 | -0.026 | 0.195 | 0.023 | < 0.001 | 0.023 | 0.022 | ||||
| FeNO200 | 0.501 | -0.115 | 0.052 | -0.077 | 0.187 | 0.009 | < 0.001 | -0.082 | 0.142 | - | - | 0.043 | 0.040 | ||||
| CaNO | 1.740 | -0.050 | < 0.05 | -0.005 | 0.929 | -0.008 | 0.896 | 0.038 | 0.514 | - | - | 0.017 | 0.014 | ||||
The R2 is the unadjusted coefficient of determination of the models and R2 boot is the corresponding optimism- corrected R2 values as estimated by bootstrapping
Factors affecting FeNO200 values
Only taking into account independent variables, the logarithm of FeNO200 was significantly correlated with height (Fig. 6B, P < 0.05), but not with age and BMI (Fig. 5B). FeNO200 in males was significantly higher than that in females (P < 0.05). Multivariate regression analysis showed that the logarithm of FeNO200 was correlated with height and BMI, but not with gender and age.
For children aged 6–11 years, there was no significant correlation between the logarithm of FeNO200 and age, height and BMI when independent factors were considered. There was no significant difference in FeNO200 values between males and females (P>0.05). The results of multivariate regression analysis showed that gender, age, height and BMI were not significantly correlated with the logarithm of FeNO200 (Table 2).
For children and adolescents aged 12–18 years, the logarithm of FeNO200 was significantly correlated with height, but not with age and BMI when only independent factors were considered. The FeNO200 value of males was significantly higher than that of females. Multivariate regression analysis showed that for the logarithm of FeNO200 was significantly correlated with height, but not with age, gender, and BMI (Table 3).
Factors affecting CaNO values
For children aged 6–18 years, the logarithm of CaNO was negatively correlated with age and height when only independent factors were considered (Figs. 5C and 6C, all P < 0.05) and had no correlation with BMI (P>0.05). There was no significant difference between males and females in the values of CaNO. Multivariate regression analysis showed that the logarithm of CaNO was negatively correlated with height, but not with gender, age and BMI.
For children aged 6–11 years, there was no significant correlation between the logarithm of CaNO and age, height and BMI when independent factors were considered. There was no significant difference in CaNO values between males and females (P>0.05). Multivariate regression analysis showed that the logarithm of CaNO was related to gender, but not age, height or BMI (Table 2).
For children and adolescents aged 12–18 years, the logarithm of CaNO was negatively correlated with age, but not with height and BMI when only independent factors were considered. There was no difference in CaNO values between males and females (P>0.05). Multivariate regression analysis showed that the logarithm of CaNO was negatively correlated with age, but not with height, gender, and BMI (Table 3).
Discussion
In assessing airway inflammation, diagnosing asthma, assessing asthma control, directing treatment, and estimating the likelihood of recurrence, the detection of eNO is crucial according to the ERS [8]. The FeNO combined with GINA guideline group helps to reduce the daily dose of ICS and treatment costs [10]. However, given the differences in race, location, region and environment, the reference value of eNO varies. Current international guidelines are based predominantly on data collected from white populations. If the interpretation is based on unsuitable reference values, it will cause incorrect results and mislead clinicians. Therefore, it is crucial to clarify the normal reference values held by Chinese children.
To our knowledge, this study is the largest sample research to explore the normal reference values of eNO in healthy children until now. The results showed that the geometric mean of FeNO50 aged 6–18 years was 14.1 ppb with an upper 95% confidence interval of 38.1 ppb, which is similar to the previous study for 9–22 years people in China [11], and to those in the United States and Canada, but higher than those in Europe (Italy, Finland, Spain, Slovakia, and France) (e-Table 1) [12–21]. According to the study, Asian and African populations have higher FeNO50 values than Whites. This.
may be the reason for this phenomenon because the United States and Canada are large immigrant countries with a larger population of other races besides Whites. The geometric mean of FeNO200 in 658 children was 6.9 ppb, with an upper 95% limit of 15.0 ppb and the mean of CaNO was 3.0 ppb, with an upper 95% limit of 11.2 ppb, which are higher than the previous studies from the UK, Finland, USA, and Spain (e-Table 2) [22–27]. The reasons may be the same as FeNO50, FeNO200 and CaNO are higher in populations of Asian origin than in European populations, but also need a large sample clinical study to verify.
Age and height are known to be associated independently with FeNO values [28, 29]. In this study, we found that FeNO50 was positively correlated with age and height in children aged 6–11 years, FeNO50 and FeNO200 were positively correlated with height in children aged 12–18, VIF for age and height were below 3, threshold for collinearity diagnostics. This showed that there is no multicollinearity effect between age and height. Those findings are consistent with the previous studies. The age-dependent eNO may be due to two reasons. One is that the airway surface area increases with age, increasing NO diffusion in the lungs [30]. Second, repeated immune stimulation during growth increased the formation of inducible NO synthase [31]. In children aged 12–18, CaNO was inversely associated with age, similar to a previous study, which found that CaNO decreased with age in people under 20 years old. The reason for this correlation needs further investigation.
Gender, as one of the important influencing factors of eNO, our study found that FeNO50 and FeNO200 in males were significantly higher than those in females in children aged 12–18, and CaNO was significantly higher in males than females for children aged 6–11. The findings are consistent with some clinical studies [32, 33]. The difference in airway surface area and diameter between males and females may be the cause of the gender disparity. The small airway space in females leads to different NO diffusion, thus the eNO values of female children are low, which is consistent with the results of the 2007–2010 National Health and Nutrition Examination Survey of the United States, in which, no gender difference in FeNO50 value was found in children aged 6–11 years old, and the FeNO50 value of 12–80 years old also showed that males were higher than females [8].
BMI, as an important confounding factor of eNO, our study found that no significant correlation between eNO and BMI or obesity (e-Table 3). In the Third National Health and Nutrition Survey, there was a significant positive correlation between BMI and asthma [34]. Some cross-sectional studies have also found that obesity is associated with asthma diagnosis, respiratory symptoms and airway hyperresponsiveness [35, 36]. Therefore, it is important to clarify whether BMI or obesity affects eNO. Our study found no significant correlation between eNO and BMI. According to the Chinese childhood obesity standard, the subjects were divided into obese group and non-obese group, and the effect of obesity on eNO was further analyzed. The results showed that the eNO did not differ between obese and non-obese children, similar to the previous study [37]. Nonetheless, we could not exclude the possibility that airway inflammation in obese subjects may be caused by immune mechanisms that are independent of NO.
Our study found that the value of FeNO50 was significantly correlated with regions, which was significantly higher in Middle and Northwest China. The reasons may be related to China’s vast surface area, environmental pollution, and regional differences in eating preferences. Environmental pollution has an important impact on the value of FeNO50 [33]. Previous studies found that the FeNO50 level of Asian people is more significantly correlated with PM2.5 [38]. The air pollution in southern China is better than in other areas, which may make the FeNO50 value of children in southern China lower than in other areas. Diet also has a significant effect on the values of eNO. NO is produced both by oxygen-dependent NO synthases catalyzing its production from L-arginine and an alternative nitrate (NO3−)–nitrite (NO2−)–NO pathway [39]. The latter can be influenced by supplementation with exogenous dietary NO3−. Studies have shown that dietary nitrate supplementation, such as spinach, lettuce, broccoli, radish and so on, can effectively increase the concentration of nitrite and nitrate, and can increase the concentration of FeNO50 [40, 41]. In contrast, the middle and northwestern regions of China were more prone to eating preserved food, which may also be one of the reasons for the increase in the value of FeNO50.
This study had several limitations. First, the healthy children in this study were defined through the questionnaire survey and the pulmonary function measurement, without the measurement of eosinophil count or total IgE level, which could not completely exclude children with allergic diseases. Second, FeNO200 and CaNO tests have been performed on children from two centers in East China, which cannot fully reflect the level of children in the whole of China. Third, research indicates that latitude and longitude, which have a significant impact on temperature and environment, may also have an impact on the reference value of eNO due to the higher risk of asthma at low latitudes [42, 43]. Therefore, these issues should be considered in future studies.
Conclusion
In summary, we investigated the normal values and influencing factors of FeNO50, FeNO200 and CaNO in healthy children and adolescents aged 6–18 years in China. Age, height and gender, as important physiological indicators, were associated with FeNO50, FeNO200 and CaNO. The influence of regional differences on eNO was not only reflected in the differences among regions of China, but also in the differences in expiratory values between Chinese, North American and European children, further confirming that the eNO values of the Asian population are higher than those of European and American population. Although, there some several limitations in this study, it is still the largest study of normal eNO in healthy children so far, which can provide some recommendations for the clinical application of eNO in international children, particularly in Asian children.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge all the participants, technicians at the pulmonary function laboratories and administrators of this study.
Abbreviations
- NO
Nitric oxide
- eNO
Exhaled nitric oxide
- FeNO
Fractional Exhaled Nitric Oxid
- FeNO50
Fractional concentration of exhaled nitric oxide at a 50 ml/s flow rat
- FeNO200
Fractional concentration of exhaled nitric oxide at a 200 ml/s flow rat
- CaNO
Concentration of nitric oxide of the alveolar or acinar region
- ATS
American Thoracic Society
- ERS
European Respiratory Societ
- GINA
Global Initiative for Asthma
- NICE
National Institute for Health and Care Excellence
- BMI
Body-Mass-Index
- ICS
Inhaled Corticosteroids
- PM
Particulate matter
- IgE
Immunoglobulin E
Author contributions
Yazun Liu drafted the manuscript. Hao Zhang designed the study, and contributed to the manuscript revision of the manuscript draft. Acquisition, analysis, or interpretation of data: All authors (Yazun Liu, Hao Zhang, Jinrong Wang, Yuling Han, Chunhong Pan, Wenhui Jiang, Chunyan Ma, Yongsheng Shi, Chunmei Jia, Yuehua Zhang, Ming Li, Fei Wang, Yanyan Yu, Yong Feng, Li Liu, Aihong Liu, Qiaoling Zhang, Zhen Long, Fuli Dai, Yanli Zhang, Minghong Ji, Dongjun Ma). All authors read and approved the final manuscript.
Funding
Shanghai Medical Guidance, Science and Technology Support project of Traditional Chinese Medicine and Western Medicine (No. 19401931800); Innovation. Fund for Combination of Chinese Traditional and Western Medicine, Shanghai. Jiao Tong University School of Medicine (No. 18zxy007); Shanghai Shenkang Clinical Management Optimization Project (No. SHDC12018602).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Shanghai Children’s Medical Center (Ethics No: SCMCIRB-K2017007), and each subcenter follows the master research unit ethics. All participants from this study (Trial registration: Chinese Clinical Trial Registry ChiCTR 1800019029) signed an informed consent form.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chung Kian Fan. Increasing utility of FeNO as a biomarker of type-2 inflammation in severe asthma. Lancet Respiratory Med. 2021;9(10):1083–4. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society, European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respri Crit Med. 2005;171(8):912–30. [DOI] [PubMed] [Google Scholar]
- 3.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin AC, Plummer AL, Taylor. DR. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FeNO) for clinical applications. AM J RESP CRIT CARE. 2011;184(5):602–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjermer L, Alving K, Diamant Z, Magnussen H, Pavord I, Piacentini G, Price D, Roche N, Sastre J, Thomas M. Usmani, O. current evidence and future research needs for FeNO measurement in respiratory diseases. RESP MED. 2014;108(6):830–41. [DOI] [PubMed] [Google Scholar]
- 5.Global Initiative For Asthma. Global strategy for asthma management and prevention [EB/OL]. [2022-05-04]. http://www.ginasthma.org
- 6.Wise J. Use clinical tests to diagnose asthma and to avoid overdiagnosis, says NICE. BMJ. 2015;350:h522. [DOI] [PubMed] [Google Scholar]
- 7.Lung Function Cooperation Group, Group R, Branch of Science, Chinese Medical Association. A series of guidelines for pulmonary function and noninvasive airway inflammation in children (7): exhaled nitric oxide monitoring. Clin J Appl Clin Pediatr. 2017;32(21):1622–7. [Google Scholar]
- 8.Horváth I, Barnes PJ, Loukides S, Sterk PJ, Högman M, Olin AC, Amann A, Antus B, Baraldi E, Bikov A et al. A European respiratory Society technical standard: exhaled biomarkers in lung disease. EUR RESPIR J, 2017;49 (4). [DOI] [PubMed]
- 9.Paredi P, Kharitonov SA, Meah S, Barnes PJ, Usmani OS. A novel approach to partition central and peripheral airway nitric oxide. Chest. 2014;145(1):113–9. [DOI] [PubMed] [Google Scholar]
- 10.You S, Zhang J, Bai Y, Ji L, Wang H. Normal values of nasal NO and exhaled NO in young Chinese people aged 9–22 years. World J Otorhinolaryngol Head Neck Surg. 2016;2(1):22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchvald F, Baraldi E, Carraro S, Gaston B, De Jongste J, Pijnenburg MW, Silkoff PE, Bisgaard H. Measurements of exhaled nitric oxide in healthy subjects age 4 to 17 years. J ALLERGY CLIN IMMUN. 2005;115(6):1130–6. [DOI] [PubMed] [Google Scholar]
- 12.Malmberg LP, Petäys T, Haahtela T, Laatikainen T, Jousilahti P, Vartiainen E, Mäkelä MJ. Exhaled nitric oxide in healthy nonatopic school-age children: determinants and height-adjusted reference values. PEDIATR PULM. 2006;41(7):635–42. [DOI] [PubMed] [Google Scholar]
- 13.Kovesi T, Kulka R, Dales R. Exhaled nitric oxide concentration is affected by age, height, and race in healthy 9- to 12-year-old children. Chest. 2007;133(1):169–75. [DOI] [PubMed] [Google Scholar]
- 14.Hervás D, Milán JM, Garde J. Differences in exhaled nitric oxide in atopic children. ALLERGOL IMMUNOPATH. 2008;36(6):331–5. [DOI] [PubMed] [Google Scholar]
- 15.Banovcin P, Jesenak M, Michnova Z, Babusikova E, Nosal S, Mikler J, Fabry J. Barreto, M. factors attributable to the level of exhaled nitric oxide in asthmatic children. EUR J MED RES. 2009;14(Suppl 4):9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janahi I, Saadoon A, Tuffaha A, Panneerselvam B. Effects of age, gender, and environmental exposures on exhaled nitric oxide level in healthy 12 to 18 years Qatari children. ANN THORAC MED. 2012;7(2):98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rouatbi S, Alqodwa A, Ben Mdella S, Saad B. Fraction of exhaled nitric oxide (FeNO) norms in healthy north African children 5–16 years old. PEDIATR PULM. 2012;48(10):981–95. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Shu L, Cai X, Wang Z, Jiao X, Liu F, Hou P, Wang L, Shan L, Chen N, et al. Gender and age affect the levels of exhaled nitric oxide in healthy children. EXP THER MED. 2013;5(4):1174–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.See KC, Christiani DC. Normal values and thresholds for the clinical interpretation of exhaled nitric oxide levels in the US general population: results from the National Health and Nutrition Examination Survey 2007–2010. Chest. 2013;143(1):107–16. [DOI] [PubMed] [Google Scholar]
- 20.Menou A, Babeanu D, Paruit HN, Ordureau A, Guillard S, Chambellan A. Normal values of offline exhaled and nasal nitric oxide in healthy children and teens using chemiluminescence. J BREATH RES. 2017;11(3):036008. [DOI] [PubMed] [Google Scholar]
- 21.Paraskakis E, Brindicci C, Fleming L, Krol R, Kharitonov SA, Wilson NM, Barnes PJ, Bush A. Measurement of bronchial and alveolar nitric oxide production in normal children and children with asthma. AM J RESP CRIT CARE. 2006;174(3):260–7. [DOI] [PubMed] [Google Scholar]
- 22.Sepponen A, Lehtimäki L, Huhtala H, Kaila M, Kankaanranta H, Moilanen E. Alveolar and bronchial nitric oxide output in healthy children. PEDIATR PULM. 2008;43(12):1242–8. [DOI] [PubMed] [Google Scholar]
- 23.Puckett JL, Taylor RW, Leu SY, Guijon OL, Aledia AS, Galant SP. George, SC. Clinical patterns in asthma based on proximal and distal airway nitric oxide categories. Respir Res. 2010;11:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linkosalo L, Lehtimäki L, Holm K, Kaila M, Moilanen E. Relation of bronchial and alveolar nitric oxide to exercise-induced bronchoconstriction in atopic children and adolescents. Volume 23. PEDIAT ALLERG IMM-UK; 2011. pp. 360–6. 4. [DOI] [PubMed]
- 25.Corcuera-Elosegui P, Sardón-Prado O, Aldasoro-Ruiz A, Korta-Murua J, Mintegui-Aramburu J, Emparanza-Knorr J, Pérez-Yarza E. Inflammatory patterns in Asthmatic Children based on alveolar nitric. Oxide Determ ARCH BRONCONEUMOL. 2015;51(6):279–84. [DOI] [PubMed] [Google Scholar]
- 26.Högman M, Thornadtsson A, Liv P, Hua-Huy T, Dinh-Xuan AT, Tufvesson E, Dressel H, Janson C, Koskela K, Oksa P, Sauni R, et al. Effects of growth and aging on the reference values of pulmonary nitric oxide dynamics in healthy subjects. J BREATH RES. 2017;11(4):047103. [DOI] [PubMed] [Google Scholar]
- 27.Latzin P, Beck J, Griese M. Exhaled nitric oxide in healthy children: variability and a lack of correlation with atopy. Volume 13. PEDIAT ALLERG IMM-UK; 2002. pp. 37–46. 1. [DOI] [PubMed]
- 28.Olin AC, Rosengren A, Thelle DS, Lissner L, Bake B, Toren K. Height, age, and atopy are associated with fraction of exhaled nitric oxide in a large adult general population sample. Chest. 2006;130(5):1319–25. [DOI] [PubMed] [Google Scholar]
- 29.Brody DJ, Zhang X, Kit BK, Dillon CF. Reference values and factors associated with exhaled nitric oxide: U.S. youth and adults. Respir Med. 2013;107(11):1682–91. [DOI] [PubMed] [Google Scholar]
- 30.Avital A, Uwyyed K, Berkman N, Bar-Yishay E, Godfrey S. Springer, C. exhaled nitric oxide is age-dependent in asthma. PEDIATR PULM. 2003;36(5):433–8. [DOI] [PubMed] [Google Scholar]
- 31.Taylor DR, Mandhane P, Greene JM, Hancox RJ, Filsell S, McLachlan CR, Williamson AJ, Cowan JO, Smith AD. Sears, MR. factors affecting exhaled nitric oxide measurements: the effect of sex. Respir Res. 2007;8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Travers J, Marsh S, Aldington S, Williams M, Shirtcliffe P, Pritchard A, Weatherall M, Beasley R. Reference ranges for exhaled nitric oxide derived from a random community survey of adults. AM J RESP CRIT CARE. 2007;176(3):238–42. [DOI] [PubMed] [Google Scholar]
- 33.Chen X, Liu F, Niu Z, Mao S, Tang H, Li N, Chen G, Liu S, Lu Y, Xiang H. The association between short-term exposure to ambient air pollution and fractional exhaled nitric oxide level: a systematic review and meta-analysis of panel studies. ENVIRON POLLUT. 2020;265:114833. (Pt A). [DOI] [PubMed] [Google Scholar]
- 34.von Mutius E, Schwartz J, Neas LM, Dockery D, Weiss ST. Relation of body mass index to asthma and atopy in children: the National Health and Nutrition Examination Study III. Thorax. 2001;56(11):835–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carpio C, Villasante C, Galera R, Romero D, de Cos A, Hernanz A, García-Río F. Systemic inflammation and higher perception of dyspnea mimicking asthma in obese subjects. J ALLERGY CLIN IMMUN. 2016;137(3):718–e264. [DOI] [PubMed] [Google Scholar]
- 36.Cetlin AA, Gutierrez MR, Bettiol H, Barbieri MA, Vianna EO. Influence of asthma definition on the asthma-obesity relationship. BMC Public Health, 2012, 12 844. [DOI] [PMC free article] [PubMed]
- 37.Leung TF, Li CY, Lam CW, Au CS, Yung E, Chan IH, Wong GW, Fok TF. The relation between obesity and asthmatic airway inflammation. Volume 15. PEDIAT ALLERG IMM-UK; 2004. pp. 344–50. 4. [DOI] [PubMed]
- 38.Lin W, Huang W, Zhu T, Hu M, Brunekreef B, Zhang Y, Liu X, Cheng H, Gehring U, Li C, et al. Acute respiratory inflammation in children and black carbon in ambient air before and during the 2008 Beijing olympics. ENVIRON HEALTH PERSP. 2011;119(10):1507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feelisch M, Fernandez BO, Bryan NS, Garcia-Saura MF, Bauer S, Whitlock DR, Ford PC, Janero DR, Rodriguez J, Ashrafian H. Tissue processing of nitrite in hypoxia: an intricate interplay of nitric oxide-generating and -scavenging systems. J BIOL CHEM. 2008;283(49):33927–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Capper TE, Siervo M, Clifford T, Taylor G, Iqbal W, West D, Stevenson EJ. Pharmacokinetic Profile of incremental oral doses of Dietary Nitrate in Young and older adults: a crossover Randomized Clinical Trial. J NUTR. 2022;152(1):130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pavitt MJ, Lewis A, Buttery SC, Fernandez BO, Mikus-Lelinska M, Banya WAS, Feelisch M, Polkey MI, Hopkinson NS. Dietary nitrate supplementation to enhance exercise capacity in hypoxic COPD: EDEN-OX, a double-blind, placebo-controlled, randomised cross-over study. Thorax. 2021;77(10):968–75. [DOI] [PubMed] [Google Scholar]
- 42.Oktaria V, Dharmage SC, Burgess JA, Simpson JA, Morrison S, Giles GG, Abramson MJ, Walters EH, Matheson MC. Association between latitude and allergic diseases: a longitudinal study from childhood to middle-age. ANN ALLERG ASTHMA IM, 2013;110 (2), 80 – 5.e1. [DOI] [PubMed]
- 43.Cong X, Xu X, Zhang Y, Wang Q, Xu L, Huo X. Temperature drop and the risk of asthma: a systematic review and meta-analysis. ENVIRON SCI POLLUT R. 2017;24(28):22535–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.