Abstract
Background and aim
Accurately predicting microvascular invasion (MVI) before surgery is beneficial for surgical decision-making, and some high-risk hepatocellular carcinoma (HCC) patients may benefit from postoperative adjuvant transarterial chemoembolization (PA-TACE). The purpose of this study was to develop and validate a novel nomogram for predicting MVI and assessing the survival benefits of selectively receiving PA-TACE in HCC patients.
Methods
The 1372 HCC patients who underwent hepatectomy at four medical institutions were randomly divided into training and validation datasets according to a 7:3 ratio. We developed and validated a nomogram for predicting MVI using preoperative clinical data and further evaluated the survival benefits of selective PA-TACE in different risk subgroups.
Results
The nomogram for predicting MVI integrated alpha-fetoprotein, tumor diameter, tumor number, and tumor margin, with an area under the curve of 0.724, which was greater than that of any single predictive factor. The calibration curve, decision curve, and clinical impact curve demonstrated that the nomogram had strong predictive performance. Risk stratification based on the nomogram revealed that patients in the low-risk group did not achieve better DFS and OS with PA-TACE (all p > 0.05), while patients in the medium-to-high risk groups could benefit from higher DFS (Medium-risk, p = 0.039; High-risk, p = 0.027) and OS (Medium-risk, p = 0.001; High-risk, p = 0.019) with PA-TACE.
Conclusions
The nomogram predicting MVI demonstrated strong predictive performance, and its risk stratification aided in identifying different subgroups of HCC patients who may benefit from PA-TACE with improved survival outcomes.
Keywords: Microvascular invasion, Hepatocellular carcinoma, Postoperative, Transarterial chemoembolization, Hepatectomy, Nomogram
1. Introduction
Microvascular invasion (MVI) is one of the important risk factors that severely affect the survival outcome of patients with hepatocellular carcinoma (HCC), and it is closely related to tumor diameter, tumor number, and serum tumor markers [1,2]. Its presence is increasingly recognized as a reflection of increased local infiltration and distant metastatic capacity of the tumor [[1], [2], [3]]. Partial hepatectomy and liver transplantation are potentially curative treatments in selected patients with HCC [4]. However, some scholars have found little benefit from liver transplantation in patients with MVI [5]. A reasonable criterion to include patients with HCC for liver transplantation should achieve an optimal balance between good surgical outcomes and donor shortage [6]. When both procedures were evidently suitable, liver resection was more preferred for patients with MVI because of similar 5-year survival rates in these two procedures [5,6]. Thus, the ability to predict the risk of MVI preoperatively facilitates surgical decisions. It should not be overlooked that patients with MVI who undergo liver resection are still not immune to the high rate of tumor recurrence, which significantly reduces long-term survival outcomes [1,2,4,6]. Moreover, some patients with high-risk HCC in clinical practice may benefit from postoperative adjuvant transarterial chemoembolization (PA-TACE) [[7], [8], [9], [10]]. This study developed and validated a novel nomogram for predicting MVI using large-scale clinical data from multiple medical institutions, and further evaluated the survival benefits of selectively receiving PA-TACE in different subgroups of patients.
2. Methods
2.1. Patients
Clinical data of HCC patients from four medical institutions between January 2018 and September 2021 were retrospectively analyzed. Inclusion criteria: (1) Patients underwent liver resection surgery with confirmed negative tumor margin on pathology; (2) Postoperative pathology confirmed HCC; (3) Preoperative imaging examination did not reveal portal vein tumor invasion, lymph node metastasis, or extrahepatic metastasis. Exclusion criteria: (1) Patients with missing clinical data or incomplete follow-up data; (2) Patients confirmed by pathology to have other malignant liver tumors; (3) Patients who died within 30 days after surgery; (4) Patients with a history of other malignant tumors. This study was approved by the ethics committees of all participating medical institutions and followed the guidelines of the Declaration of Helsinki.
2.2. Assessment of MVI
The "7-point" baseline sampling method was used to collect pathological specimens during surgery: 1. Samples were collected 1:1 at the junction of cancer and adjacent tissues at 12, 3, 6 and 9 points of the tumor; 2. At least one sample is collected inside the tumor; 3. One piece of liver tissue was taken at a distance of ≤1 cm and >1 cm from the tumor border, respectively. MVI is defined as the presence of tumor cells in the portal vein, hepatic vein or blood vessels of liver tissue near the tumor margin visible under the microscope. Two senior pathologists confirmed and interpreted the pathological specimens for the diagnosis of MVI by means of hematoxylin-eosin staining and immunohistochemistry.
2.3. Treatment of PA-TACE
The risk of recurrence of HCC is evaluated by doctors based on the preoperative clinical data and postoperative pathological indicators of the patient. Patients with a high risk of recurrence (with one or more of the following features: advanced tumor staging, tumor diameter ≥ 5 cm, multiple tumors, alpha-fetoprotein(AFP) ≥ 400, MVI, Edmondson-Steiner III-IV grade and satellite nodules) are recommended to receive PA-TACE about 4 weeks after hepatectomy. However, patients decide whether to follow the recommendation based on their medical adherence, financial status or other social factors. Prior to receiving PA-TACE, patients need to undergo laboratory tests including liver function tests, coagulation function tests, etc., to confirm their good physical condition. During the operation, a catheter was placed through the femoral artery via the Seldinger technique into the hepatic artery on the side where the tumor was removed, and finally an appropriate amount of mixed emulsion consisting of chemotherapeutic agents and embolic agents was injected into the residual liver. The dose of chemotherapeutic agents (Fluorouracil, 400–500 mg/m2; Epirubicin, 40–70 mg/m2; Lobaplatin, about 50 mg/m2) and embolic agents (lipiodol and gelatin sponge, 3–5 mL) to be injected needs to be determined by a comprehensive assessment of body surface area, physical status and remaining liver volume [[7], [8], [9], [10]].
2.4. Follow-up
All patients were followed up either outpatient or inpatient. Patients were followed up every 1–2 months for six months after discharge and every 3–6 months for the subsequent period. Tumor recurrence was defined as a new tumor nodule confirmed by enhanced CT or/and enhanced MRI or biopsy. The current study used disease-free survival (DFS) and overall survival (OS) as study endpoints. DFS was defined as the time from hepatectomy to diagnosis of tumor recurrence, while OS was defined as the time from hepatectomy to death or last follow-up. All patients were followed up until April 1, 2022.
2.5. Statistical analysis
Patients were randomly assigned to the training and validation datasets in a 7:3 ratio. For continuous data that follow normal distribution, between-group comparisons were conducted using independent sample t-test (mean ± standard deviation); for continuous data that do not follow normal distribution, between-group comparisons were conducted using Mann-Whitney U test [median (quartile distance)]; The chi-square test was used to detect classified data, which was expressed as numbers (n) and proportions (%); Independent prognostic factors for DFS and OS were determined by univariate and multivariate Cox regression analysis; The independent predictive factors for MVI were identified through univariate and multivariate Logistic regression analysis, and all independent predictive factors were further integrated into a nomogram for predicting MVI; Variables with P < 0.05 in the univariate analysis were used in the multivariate analysis; Kaplan-meier survival analysis was used to assess DFS and OS, and the difference between curves was estimated by logarithmic rank test; Model fit was assessed with a calibration plot by means of 1000 boot-strap resamples; Evaluation of the predictive performance and clinical utility of the nomogram was conducted using ROC curve, decision curve, and clinical impact curve; Risk stratification was performed based on the total points of the nomogram; Different subgroups of patients were analyzed for survival outcomes after receiving PA-TACE using COX regression analysis. The above data were statistically analyzed by R software (Version 4.2.1; http://www.r-project.org) and X-title software (version 3.6.1; http://tissuearray.org; Yale University School of Medicine, New Haven, Conn). P < 0.05 was indicative of a statistically significant difference.
3. Results
3.1. Clinical characteristics and risk factors for survival outcomes
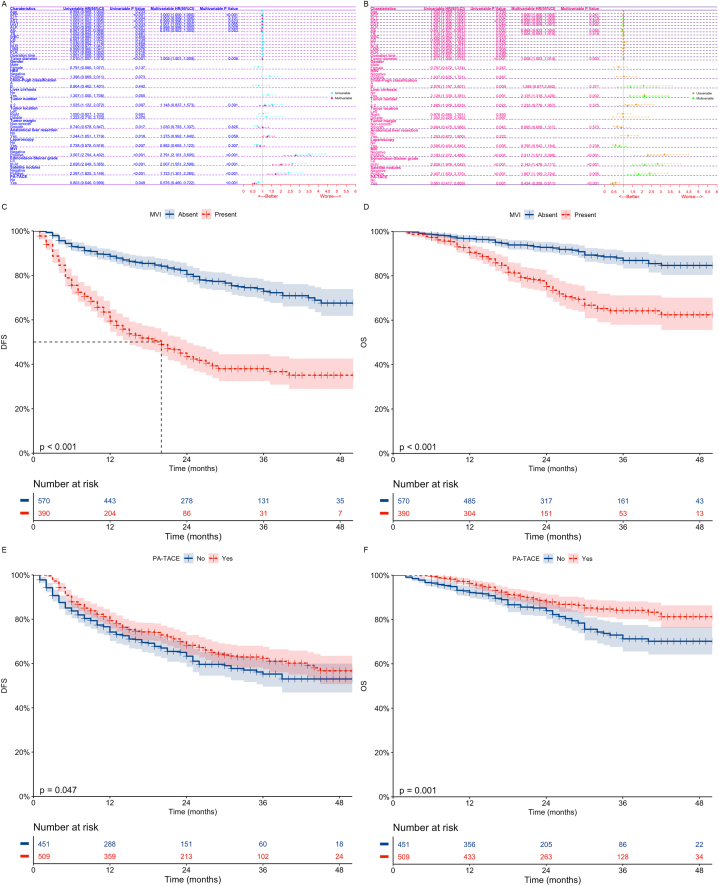
1372 HCC patients who underwent radical hepatectomy were randomly assigned to a training dataset (n = 960) and a validation dataset (n = 412) in a 7:3 ratio. The comparison of baseline clinical characteristics between the two datasets was considered not significantly different (Table .1, all p > 0.05). Through Cox regression analysis and Kaplan-Meier survival analysis (Fig. 1, Training dataset; Fig. S1, Validation dataset), we found that MVI is an independent risk factor affecting patients' DFS (Training dataset; p < 0.001; Validation dataset; p < 0.001) and OS (Training dataset; p < 0.001; Validation dataset; p = 0.002), while PA-TACE is an independent protective factor affecting patients' DFS (Training dataset; p < 0.001; Validation dataset; p < 0.001) and OS (Training dataset; p < 0.001; Validation dataset; p < 0.001). Patients with MVI have significantly lower DFS (Training dataset; p < 0.001; Validation dataset; p < 0.001) and OS (Training dataset; p < 0.001; Validation dataset; p < 0.001), while those who receive PA-TACE can achieve higher DFS (Training dataset, p = 0.047; Validation dataset, p = 0.003) and OS (Training dataset, p = 0.001; Validation dataset, p < 0.001).
Table 1.
Comparison of clinical characteristics of MVI patients between different datasets.
| Clinical characteristics |
MVI absent |
MVI present |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n = 815) | Training dataset (n= 570) | Validation dataset (n = 245) | P | Total (n = 557) | Training dataset (n = 390) | Validation dataset (n = 167) | P | ||
| Age (years) | 57.00 (49.00, 65.00) | 57.00 (49.00, 65.00) | 57.00 (48.00, 65.00) | 0.626 | 55.00 (46.00, 64.00) | 55.00 (47.00, 64.00) | 55.00 (46.00, 64.00) | 0.913 | |
| AFP (ng/mL) | 17.47 (4.30, 186.25) | 17.145 (4.00, 229.88) | 18.200 (4.80, 131.40) | 0.834 | 224.50 (16.40, 1000.00) | 223.90 (16.43, 1000.00) | 224.50 (16.95, 1000.00) | 0.938 | |
| ALT (U/L) | 30.00 (21.10, 44.67) | 30.00 (21.00, 45.00) | 30.10 (21.66, 43.00) | 0.863 | 31.00 (22.12, 47.00) | 31.00 (23.00, 48.54) | 32.00 (20.98, 45.50) | 0.441 | |
| AST (U/L) | 32.57 (25.71, 44.81) | 32.89 (25.50, 45.00) | 32.00 (26.00, 42.81) | 0.861 | 38.89 (28.07, 56.37) | 37.90 (28.20, 54.85) | 40.00 (28.04, 60.10) | 0.818 | |
| GGT (U/L) | 44.45 (27.00, 81.95) | 44.23 (26.67, 84.00) | 44.70 (27.00, 78.00) | 0.969 | 63.71 (35.23, 118.00) | 65.21 (36.00, 118.94) | 62.00 (35.00, 99.02) | 0.386 | |
| ALP (U/L) | 91.00 (72.81, 115.44) | 92.00 (73.75, 114.40) | 88.00 (71.00, 115.64) | 0.270 | 100.00 (78.02, 129.12) | 101.18 (80.00, 131.00) | 98.49 (77.65, 125.51) | 0.369 | |
| Alb(g/L) | 41.56 (38.41, 44.25) | 41.56 (38.33, 44.32) | 41.57 (38.70, 43.85) | 0.939 | 40.80- (37.80, 43.40) | 40.63 (37.63, 43.10) | 41.08 (38.30, 44.21) | 0.060 | |
| TB (mol/L) | 14.48 (10.73, 19.80) | 14.24 (10.70, 19.59) | 14.80 (10.90, 20.05) | 0.460 | 14.80 (10.94, 19.70) | 14.66 (10.97, 19.39) | 14.80 (10.76, 20.46) | 0.617 | |
| WBC (109/L) | 5.24 (4.16, 6.47) | 5.21 (4.14, 6.40) | 5.34 (4.20, 6.56) | 0.406 | 5.33 (4.38, 6.51) | 5.29 (4.32, 6.48) | 5.54 (4.50, 6.76) | 0.265 | |
| CR (μmol/L) | 73.00 (62.20, 83.62) | 72.90 (62.06, 83.55) | 73.00 (63.40, 84.00) | 0.594 | 73.10 (62.35, 82.23) | 73.15 (62.36, 82.04) | 73.00 (62.58, 82.85) | 0.863 | |
| PT (s) | 11.80 (11.20, 12.60) | 11.80 (11.20, 12.68) | 11.80 (11.30, 12.40) | 0.884 | 12.00 (11.40, 12.60) | 12.00 (11.40, 12.60) | 11.90 (11.40, 12.60) | 0.495 | |
| NLR | 2.10 (1.52, 3.01) | 2.06 (1.50, 2.95) | 2.19 (1.60, 3.08) | 0.130 | 2.32 (1.69, 3.36) | 2.35 (1.69, 3.38) | 2.24 (1.60, 3.34) | 0.377 | |
| LMR | 3.79 (2.80, 5.10) | 3.82 (2.78, 5.19) | 3.66 (2.86, 5.00) | 0.630 | 3.24 (2.44, 4.56) | 3.29 (2.39, 4.49) | 3.20 (2.51, 4.71) | 0.615 | |
| PLR | 101.50 (78.13, 140.36) | 102.39 (78.25, 139.79) | 100.00 (78.02, 142.00) | 0.797 | 115.79 (88.50, 165.04) | 117.26 (88.47, 167.10) | 109.26 (90.36, 158.39) | 0.306 | |
| Operation time (mins) | 202.00 (150.00, 260.00) | 201.00 (150.00, 260.00) | 205.00 (150.00, 270.00) | 0.858 | 230.00 (180.00, 288.00) | 232.50 (180.00, 295.00) | 225.00 (172.50, 275.00) | 0.265 | |
| Tumor diameter (mm) | 33.00 (22.00, 53.00) | 34.00 (22.00, 54.00) | 31.00 (23.00, 49.00) | 0.189 | 57.00 (38.00, 82.00) | 59.50 (36.25, 82.00) | 56.00 (40.00, 82.50) | 0.635 | |
| Gender [n(%)] | male | 674 (82.70) | 464 (81.40) | 210 (85.71) | 0.164 | 479 (86.00) | 334 (85.64) | 145 (86.83) | 0.813 |
| female | 141 (17.30) | 106 (18.60) | 35 (14.29) | 78 (14.00) | 56 (14.36) | 22 (13.17) | |||
| HBV [n(%)] | Negative | 113 (13.87) | 85 (14.91) | 28 (11.43) | 0.227 | 68 (12.21) | 42 (10.77) | 26 (15.57) | 0.149 |
| Positive | 702 (86.13) | 485 (85.09) | 217 (88.57) | 489 (87.79) | 348 (89.23) | 141 (84.43) | |||
| Child–Pugh classification [n(%)] | A | 782 (95.95) | 543 (95.26) | 239 (97.55) | 0.185 | 529 (94.97) | 370 (94.87) | 159 (95.21) | 1.000 |
| B | 33 (4.05) | 27 (4.74) | 6 (2.45) | 28 (5.03) | 20 (5.13) | 8 (4.79) | |||
| Liver cirrhosis [n(%)] | No | 219 (26.87) | 147 (25.79) | 72 (29.39) | 0.329 | 129 (23.16) | 86 (22.05) | 43 (25.75) | 0.402 |
| Yes | 596 (73.13) | 423 (74.21) | 173 (70.61) | 428 (76.84) | 304 (77.95) | 124 (74.25) | |||
| Tumor number [n(%)] | 1 | 760 (93.25) | 526 (92.28) | 234 (95.51) | 0.125 | 463 (83.12) | 323 (82.82) | 140 (83.83) | 0.866 |
| ≥ 2 | 55 (6.75) | 44 (7.72) | 11 (4.49) | 94 (16.88) | 67 (17.18) | 27 (16.17) | |||
| Tumor location [n(%)] | left | 249 (30.55) | 176 (30.88) | 73 (29.80) | 0.226 | 178 (31.96) | 124 (31.79) | 54 (32.34) | 0.990 |
| right | 534 (65.52) | 376 (65.96) | 158 (64.49) | 346 (62.12) | 243 (62.31) | 103 (61.68) | |||
| double | 32 (3.93) | 18 (3.16) | 14 (5.71) | 33 (5.92) | 23 (5.90) | 10 (5.99) | |||
| Tumor margin [n(%)] | Non-smooth | 128 (15.71) | 93 (16.32) | 35 (14.29) | 0.532 | 201 (36.09) | 136 (34.87) | 65 (38.92) | 0.415 |
| Smooth | 687 (84.29) | 477 (83.68) | 210 (85.71) | 356 (63.91) | 254 (65.13) | 102 (61.08) | |||
| Anatomical liver resection [n(%)] | No | 290 (35.58) | 202 (35.44) | 88 (35.92) | 0.959 | 143 (25.67) | 106 (27.18) | 37 (22.16) | 0.255 |
| Yes | 525 (64.42) | 368 (64.56) | 157 (64.08) | 414 (74.33) | 284 (72.82) | 130 (77.84) | |||
| Laparoscopy [n(%)] | No | 425 (52.15) | 301 (52.81) | 124 (50.61) | 0.618 | 374 (67.15) | 269 (68.97) | 105 (62.87) | 0.192 |
| Yes | 390 (47.85) | 269 (47.19) | 121 (49.39) | 183 (32.85) | 121 (31.03) | 62 (37.13) | |||
| Edmondson-Steiner grade [n (%)] | I-II | 735 (90.18) | 509 (89.30) | 226 (92.24) | 0.243 | 420 (75.40) | 294 (75.38) | 126 (75.45) | 1.000 |
| III-IV | 80 (9.82) | 61 (10.70) | 19 (7.76) | 137 (24.60) | 96 (24.62) | 41 (24.55) | |||
| Satellite nodules [n (%)] | Negative | 759 (93.13) | 525 (92.11) | 234 (95.51) | 0.107 | 439 (78.82) | 314 (80.51) | 125 (74.85) | 0.166 |
| Positive | 56 (6.87) | 45 (7.89) | 11 (4.49) | 118 (21.18) | 76 (19.49) | 42 (25.15) | |||
| PA-TACE [n (%)] | No | 431 (52.88) | 298 (52.28) | 133 (54.29) | 0.653 | 229 (41.11) | 153 (39.23) | 76 (45.51) | 0.199 |
| Yes | 384 (47.12) | 272 (47.72) | 112 (45.71) | 328 (58.89) | 237 (60.77) | 91 (54.49) | |||
MVI, Microvascular invasion; AFP, Alpha-fetoprotein; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; GGT, Gamma-glutamyltransferase; ALP, Alkaline phosphatase; Alb, Albumin; TB, Total bilirubin; WBC, White blood cell; CR, Creatinine; PT, Prothrombin time; NLR, Neutrophil-to-lymphocyte ratio; LMR, Lymphocyte-to-monocyte ratio; PLR, Platelet-to-lymphocyte ratio; HBV, Hepatitis B virus; PA-TACE, Postoperative adjuvant transarterial chemoembolization.
Fig. 1.
Univariate and multivariate cox regression analysis of DFS (A) and OS (B) after hepatectomy in HCC patients in the training dataset; Kaplan-Meier analysis of DFS and OS in patients with MVI (CD) and those receiving PA-TACE (EF) in the training dataset.
DFS, Disease-free survival; OS, Overall survival; HCC, Hepatocellular carcinoma; HR, Hazard ratio; Cl, Confidence interval; AFP, Alpha-fetoprotein; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; GGT, Gamma-glutamyltransferase; Alb, Albumin; TB, Total bilirubin; WBC, White blood cell; CR, Creatinine; PT, Prothrombin time; NLR, Neutrophil-to-lymphocyte ratio; LMR, Lymphocyte-to-monocyte ratio; PLR, Platelet-to-lymphocyte ratio; HBV, Hepatitis B virus; MVI, Microvascular invasion; PA-TACE, Postoperative adjuvant transarterial chemoembolization.
3.2. Development and validation of predictive MVI models
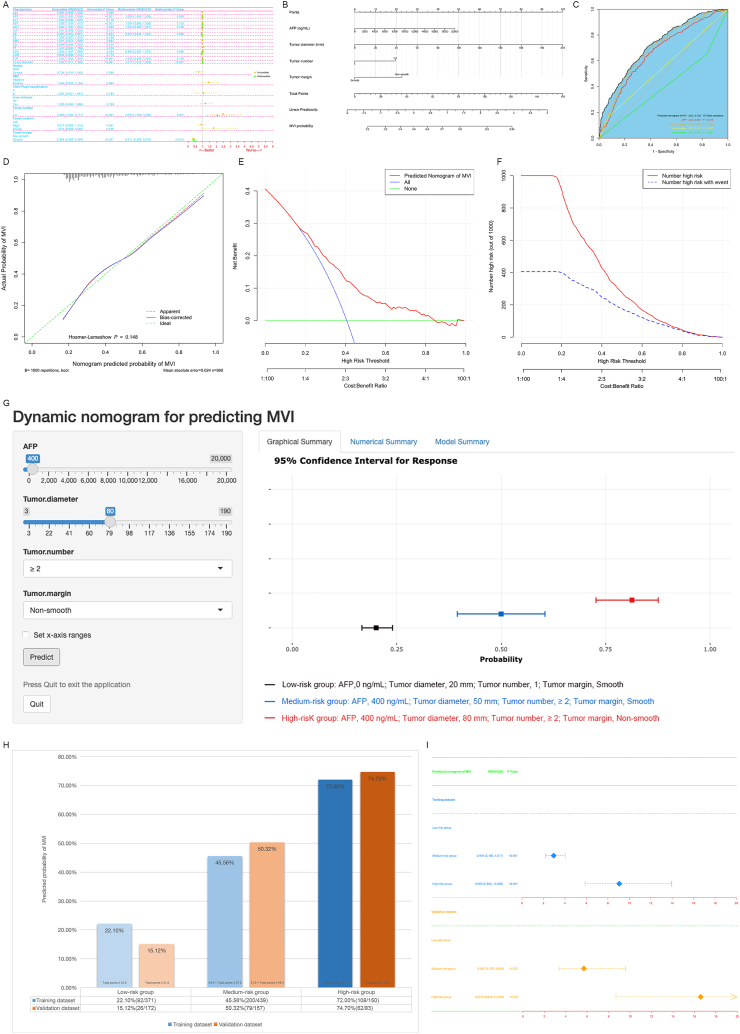
AFP, tumor diameter, tumor number and tumor margin were identified as independent predictors of MVI by logistic regression analysis (Fig. 2A, Training dataset; Fig. S2A, Validation dataset). We developed a nomogram for predicting MVI by integrating all independent predictive factors (Fig. 2B, Training dataset; Fig. S2B, Validation dataset), with an area under the curve (AUC) of 0.724, which was higher than that of any single predictive factor (Fig. 2C, Training dataset; Fig. S2C, Validation dataset). The calibration curve of the nomogram showed excellent agreement between predicted and observed outcomes (Fig. 2D, Training dataset; Fig. S2D, Validation dataset). Furthermore, the decision curve analysis and clinical impact curve revealed strong predictive performance and clinical utility of the nomogram (Fig. 2EF, Training dataset; Fig. S2EF, Validation dataset). Divide into low (Training dataset, ≤24.5; Validation dataset, ≤21.9), medium (Training dataset, >24.5 and ≤57.9; Validation dataset, >21.9 and ≤48.4), and high-risk (Training dataset, >57.9; Validation dataset, >48.4) groups based on the total points of the nomogram (Fig. S3, The average value as a critical point). By adding the points of each variable and placing them on the total points scale, we can easily obtain the predicted probability of MVI for patients (Fig. 3G, Online tools are available at https://dynamic-nomogram-model.shinyapps.io/DNPMVI/). According to the Kaplan-Meier analysis, significant differences were observed in both the DFS (Fig. S4A, Training dataset, p < 0.001; Fig. S4C, Validation dataset, p < 0.001) and OS (Fig. S4B, Training dataset, p < 0.001; Fig. S4D, Validation dataset, p < 0.001) among different risk strata. Furthermore, there were significant differences in the histograms (Fig. 2H) and odds ratio (Fig. 2I) of different risk stratifications in predicting the probability of MVI.
Fig. 2.
Univariate and multivariate Logistic regression analysis of predicted MVI in the training dataset (A); Nomogram for predicting MVI based on selected clinical factors in the training dataset (B). Roc curve analysis of predicted nomogram for MVI and other independent predictors in the training dataset (C); The calibration curve (D), decision curve (E), and clinical impact curve (F) of the nomogram in the training dataset.The threshold probabilities for the decision curves range from 17 % to 85 % and the corresponding net returns range from 0.293 to 0.003. It indicates that the nomogram improves the benefit compared with the measures that treat all patients and treat none patient when threshold probability is 17 %–85 %. In the clinical impact curve of 1000 individuals, the red curve (Number high risk) represents the number of patients classified by the nomogram as having a high risk of MVI under each threshold probability; The blue curve (Number high risk with event) represents the actual number of patients with MVI at each threshold probability; Online tools are available at https://dynamic-nomogram-model.shinyapps.io/DNPMVI/(G, Dynamic nomogram for predicting MVI). The black bar represents the low-risk group (AFP,0 ng/mL; Tumor diameter, 20 mm; Tumor number, 1; Tumor margin, Smooth); The blue bar represents the medium-risk group (AFP, 400 ng/mL; Tumor diameter, 50 mm; Tumor number, ≥2; Tumor margin, Smooth); The red bar represents the high-risk group (AFP, 400 ng/mL; Tumor diameter, 80 mm; Tumor number, ≥2; Tumor margin, Non-smooth); Histogram (H) and odds ratio (I) of different risk strata for the nomogram.
MVI, Microvascular invasion; ROC, Receiver operating characteristic; AUC, Area under the curve; OR, Odds ratio; Cl, Confidence interval; AFP, Alpha-fetoprotein; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; GGT, Gamma-glutamyltransferase; Alb, Albumin; TB, Total bilirubin; WBC, White blood cell; CR, Creatinine; PT, Prothrombin time; NLR, Neutrophil-to-lymphocyte ratio; LMR, Lymphocyte-to-monocyte ratio; PLR, Platelet-to-lymphocyte ratio; HBV, Hepatitis B virus.
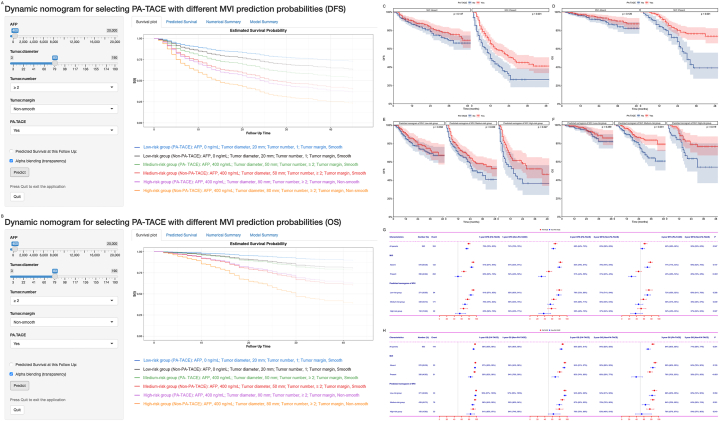
Fig. 3.
Prediction of DFS (A, Online tools are available at https://hyz1002250215.shinyapps.io/DN-PATACE-MVI-DFS/) and OS (B, Online tools are available at https://hyz1002250215.shinyapps.io/DN-PATACE-MVI-DFS/) of different subgroups of patients receiving PA-TACE using dynamic nomograms; Subgroup Kaplan-Meier analysis of DFS (C) and OS (D) of patients with MVI receiving PA-TACE in the training dataset; Subgroup Kaplan-meier analysis of DFS (E) and OS (F) of patients with different predicted risk stratification of MVI receiving PA-TACE in the training dataset; Subgroup forest plots of DFS (G) and OS (H) of different subgroups of patients receiving PA-TACE at 1, 2, and 3 years in the training dataset.
DFS, Disease-free survival; OS, Overall survival; MVI, Microvascular invasion; PA-TACE, Postoperative adjuvant transarterial chemoembolization.
3.3. Subgroup survival analysis
Based on risk stratification using a nomogram predicting MVI, we constructed dynamic nomograms to predict DFS (Fig. 3A, Online tools are available at https://hyz1002250215.shinyapps.io/DN-PATACE-MVI-DFS/) and OS (Fig. 3B, Online tools are available at https://hyz1002250215.shinyapps.io/DN-PATACE-MVI-OS/) of different subgroups receiving PA-TACE. PA-TACE can improve DFS (Fig. 3C, Training dataset, Median, 26 months vs 12 months, 1-, 2-, and 3-year, 66%-51%–45 % vs 50%-31%–26 %, p < 0.001; Fig. S5A, Validation dataset, Median, 29 months vs 8 months, 1-, 2-, and 3-year, 72%-62%–42 % vs 39%-25%–19 %, p < 0.001) and OS (Fig. 3D, Training dataset, Median, NA vs 30 months, 1-, 2-, and 3-year, 95%-80%–76 % vs 84%-68%–39 %, p < 0.001; Fig. S5B, Validation dataset, Median, NA vs 24 months, 1-, 2-, and 3-year, 100%-87%–79 % vs 75%-48%–38 %, p < 0.001) in patients with MVI, but it is ineffective for patients without MVI (Training dataset, DFS, p = 0.147, OS, p = 0.126; Validation dataset, DFS, p = 0.275, OS, p = 0.253). Patients in the medium-to high-risk group who received PA-TACE had higher DFS (Fig. 3E–G, Training dataset, Median, NA vs 31 months, 43 months vs 15 months, 1-, 2-, and 3-year, 76%-64%–58 % vs 69%-55%–45 %, 65%-58%–52 % vs 55%-37%–37 %, p = 0.039, p = 0.027; Fig. S5CE, Validation dataset, Median, NA vs 16 months, 25 months vs 5 months, 1-, 2-, and 3-year, 84%-72%–57 % vs 62%-45%–30 %, 73%-50%–39 % vs 38%-23%–23 %, p < 0.001, p = 0.001) and OS (Fig. 3FH, Training dataset, Median, NA vs NA, NA vs NA,1-, 2-, and 3-year, 96%-87%–84 % vs 90%-79%–64 %, 91%-79%–76 % vs 84%-63%–54 %, p = 0.001, p = 0.019; Fig. S5DF, Validation dataset, Median, NA vs NA, NA vs 24 months, 1-, 2-, and 3-year, 100%-94%–82 % vs 85%-67%–55 %, 100%-84%–74 % vs 75%-45%–40 %, p < 0.001, p = 0.001), while patients in the low-risk group did not show significant survival outcomes with PA-TACE (Training dataset, DFS, p = 0.358, OS, p = 0.359; Validation dataset, DFS, p = 0.277, OS, p = 0.291).
4. Discussion
MVI generally reflects the high invasive and metastatic capacity of the tumor, and its presence significantly worsens the surgical outcome of HCC [1,2,[4], [5], [6]]. Even in HCC with a diameter smaller than 3 cm, the incidence of microvascular invasion remains high, exceeding 20 % [11,12]. Studies have shown that patients with MVI often face the risk of early tumor recurrence within 1–2 years after surgery [[4], [5], [6],11,12]. International scholars have emphasized that MVI is an important indicator for assessing the risk of liver cancer recurrence and selecting treatment options, and should be included as a routine pathological examination [[1], [2], [3], [4], [5], [6]]. In this study, approximately 40 % of patients had MVI, and their DFS and OS were significantly lower than those without MVI. Currently, the presence of MVI can only be confirmed by postoperative pathological examination. However, it is well known that accurate preoperative prediction of MVI is crucial for surgical decision making [[4], [5], [6],13]. Early reports have confirmed the importance of preoperative assessment of MVI in the selection of transplant candidates, and patients predicted to have MVI should be prioritized for hepatectomy over liver transplantation [5,6]. Therefore, accurate prediction of MVI to optimize treatment plans is a major challenge for surgeons.
There is currently no unified scheme or standard in China and other countries for predicting preoperative MVI. Previous studies confirmed that tumor diameter, tumor number, tumor markers, and inflammation-related indicators were independent predictors of MVI, but univariate analysis lacked sensitivity and specificity for predicting MVI, and clinical application was apparently limited [[14], [15], [16]]. Among them, the larger multiple tumor diameters and unsmooth tumor margins represent a highly aggressive presentation of HCC, further indicating that such tumors are more prone to MVI [[15], [16], [17]]. Furthermore, AFP is a widely recognized tumor marker closely associated with HCC, not only with its malignant potential and high incidence, but also seen in patients with chronic hepatitis or cirrhosis, which by itself has little correlation with the presence of MVI. Instead, it was one of the predictors of the presence of MVI in this study, showing that high levels of AFP may also reflect the aggressive effect of tumor cells [18,19]. In the present study, the proposed nomogram, which incorporated 4 comprehensive and easily available preoperative clinical variables, performed well as supported by the C-index values of 0.724 and 0.789 in the training and validation datasets, respectively, and the optimal calibration curves demonstrating the agreements between prediction and actual observation.
The proposed nomogram can be a useful guide for selecting postoperative adjuvant therapy [20,21]. However, many studies are limited to constructing preoperative predictive models for MVI without providing recommendations for treating MVI patients with a poor prognosis [[14], [15], [16], [17]]. Previous studies have shown that PA-TACE is beneficial only to some MVI patients [10,[22], [23], [24]]. Wang et al. [7] found that the HCC patients with intermediate (tumor size >5 cm) or high risk of recurrence (single tumor with MVI as well as 2 or 3 tumors) after curative liver resection could benefit from PA-TACE (3-year OS, PA-TACE vs Non-PA-TACE, 85.2 % vs 77.4 %; P = 0.040). This study shows that PA-TACE is beneficial for patients (with MVI) in the medium to high risk group. Moreover, PA-TACE did not improve DFS and OS in patients without MVI and in the low-risk group. The findings of this study lead to the following 2 recommendations: (1) Patients in the medium-high risk group of nomogram should receive PA-TACE, while those in the low-risk group do not; (2) Patients with MVI are more deserving of adjuvant TACE than those without MVI.
The present study should be noted for several limitations. First, the study was conducted as a retrospective analysis, which made it impossible to completely avoid patient selection bias. Secondly, the drugs and dosages of PA-TACE could vary across medical centers. Furthermore, the standard of PA-TACE considering both efficacy and safety should be formulated in the future. Finally, it is hoped that more large, multicenter, prospective clinical trials will emerge in the future to validate the arguments associated with the present study.
5. Conclusions
In summary, PA-TACE may be a potential treatment modality to improve survival outcomes in patients with MVI, but is not effective for patients without MVI. The nomogram predicting MVI demonstrated strong predictive performance, and its risk stratification aided in identifying different subgroups of HCC patients who may benefit from PA-TACE with improved survival outcomes.
Funding statement
This work was funded by Zhongshan Science and Technology Plan Project of Guangdong Province (Project Number: 2021B1040), Key research and development projects of Jiangxi Provincial Department of Science and Technology (Project Number: 20202BBGL73092), Natural Science Foundation of Jiangxi Provincial (Project Number: 20171BAB205064) and National Natural Science Foundation of China (Project Number: 81860432) that have no role in the collection, analysis, interpretation of results or writing of the manuscripts.
Data availability statement
Data will be made available on request.
Additional information
No additional information is available for this paper.
Ethical approval statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the ethics committees of the First Affiliated Hospital of Nanchang University, the Second Affiliated Hospital of Nanchang University, Shenzhen People's Hospital and Zhongshan People's Hospital (Ethics number:2022-CDYFYYLK-08-015). Written informed consent was obtained from all patients for their data to be used for scientific purposes.
CRediT authorship contribution statement
Shuju Tu: Conceptualization, Data curation, Formal analysis, Resources, Software, Validation. Yongzhu He: Conceptualization, Data curation, Formal analysis, Methodology, Validation. Xufeng Shu: Conceptualization, Data curation, Formal analysis, Methodology, Software, Validation. Shiyun Bao: Conceptualization, Data curation, Formal analysis, Resources, Software. Zhao Wu: Conceptualization, Data curation, Formal analysis, Resources, Software. Lifeng Cui: Conceptualization, Data curation, Formal analysis, Resources, Software. Laihui Luo: Conceptualization, Data curation, Formal analysis, Methodology, Resources. Yong Li: Conceptualization, Data curation, Formal analysis, Software, Writing – original draft, Writing – review & editing. Kun He: Conceptualization, Data curation, Formal analysis, Funding acquisition, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e36770.
Contributor Information
Shiyun Bao, Email: baomi94@163.com.
Yong Li, Email: dryongli@163.com.
Kun He, Email: hekun80@126.com.
Abbreviations
- HCC
Hepatocellular carcinoma
- MVI
Microvascular invasion
- PA-TACE
Postoperative adjuvant transarterial chemoembolization
- AFP
Alpha-fetoprotein
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- GGT
Gamma-glutamyltransferase
- ALP
Alkaline phosphatase
- Alb
Albumin
- TB
Total bilirubin
- WBC
White blood cell
- CR
Creatinine
- PT
Prothrombin time
- NLR
Neutrophil-to-lymphocyte ratio
- LMR
Lymphocyte-to-monocyte ratio
- PLR
Platelet-to-lymphocyte ratio
- HBV
Hepatitis B virus
- ROC
Receiver operating characteristic
- AUC
Area under the receiver operating characteristic curve
- OR
Odds ratio
- HR
Hazard ratio
- Cl
Confidence interval
- DFS
Disease-free survival
- OS
Overall survival
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lee S., Kang T.W., Song K.D., Lee M.W., Rhim H., Lim H.K., et al. Effect of microvascular invasion risk on early recurrence of hepatocellular carcinoma after surgery and radiofrequency ablation. Ann. Surg. 2021;273(3):564–571. doi: 10.1097/SLA.0000000000003268. [DOI] [PubMed] [Google Scholar]
- 2.Nitta H., Allard M.A., Sebagh M., Ciacio O., Pittau G., Vibert E., et al. Prognostic value and prediction of extratumoral microvascular invasion for hepatocellular carcinoma. Ann. Surg Oncol. 2019;26(8):2568–2576. doi: 10.1245/s10434-019-07365-0. [DOI] [PubMed] [Google Scholar]
- 3.Endo Y., Alaimo L., Lima H.A., Moazzam Z., Ratti F., Marques H.P., et al. A novel online calculator to predict risk of microvascular invasion in the preoperative setting for hepatocellular carcinoma patients undergoing curative-intent surgery. Ann. Surg Oncol. 2023;30(2):725–733. doi: 10.1245/s10434-022-12494-0. [DOI] [PubMed] [Google Scholar]
- 4.Kloeckner R., Galle P.R., Bruix J. Local and regional therapies for hepatocellular carcinoma. Hepatology. 2021:137–149. doi: 10.1002/hep.31424. Baltimore, Md. [DOI] [PubMed] [Google Scholar]
- 5.Mazzaferro V., Llovet J.M., Miceli R., Bhoori S., Schiavo M., Mariani L., et al. Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10(1):35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 6.Vitale A., Cucchetti A., Qiao G.L., Cescon M., Li J., Ramirez Morales R., et al. Is resectable hepatocellular carcinoma a contraindication to liver transplantation? A novel decision model based on "number of patients needed to transplant" as measure of transplant benefit. J. Hepatol. 2014;60(6):1165–1171. doi: 10.1016/j.jhep.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z., Ren Z., Chen Y., Hu J., Yang G., Yu L., et al. Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: a randomized controlled study. Clin. Cancer Res. 2018;24(9):2074–2081. doi: 10.1158/1078-0432.CCR-17-2899. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz J.D., Schwartz M., Mandeli J., Sung M. Neoadjuvant and adjuvant therapy for resectable hepatocellular carcinoma: review of the randomised clinical trials. Lancet Oncol. 2002;3(10):593–603. doi: 10.1016/s1470-2045(02)00873-2. [DOI] [PubMed] [Google Scholar]
- 9.Liang L., Li C., Wang M.D., Wang H., Zhou Y.H., Zeng Y.Y., et al. Development and validation of a novel online calculator for estimating survival benefit of adjuvant transcatheter arterial chemoembolization in patients undergoing surgery for hepatocellular carcinoma. J. Hematol. Oncol. 2021;14(1):165. doi: 10.1186/s13045-021-01180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H., Du P.C., Wu M.C., Cong W.M. Postoperative adjuvant transarterial chemoembolization for multinodular hepatocellular carcinoma within the Barcelona Clinic Liver Cancer early stage and microvascular invasion. Hepatobiliary Surg. Nutr. 2018;7(6):418–428. doi: 10.21037/hbsn.2018.09.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pawlik T.M., Delman K.A., Vauthey J.N., Nagorney D.M., Ng I.O., Ikai I., et al. Tumor size predicts vascular invasion and histologic grade: implications forselection of surgical treatment for hepatocellular carcinoma. Liver Transplant. 2005;11:1086–1092. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 12.Onaca N., Davis G.L., Jennings L.W., Goldstein R.M., Klintmalm G.B. Improved results of transplantation forhepatocellular carcinoma: a report from the international registry of hepatic tumors in LiverTransplantation. Liver Transplant. 2009;15:574–580. doi: 10.1002/lt.21738. [DOI] [PubMed] [Google Scholar]
- 13.Cucchetti A., Qiao G.L., Cescon M., Li J., Xia Y., Ercolani G., et al. Anatomic versusnonanatomic resection in cirrhotic patients with early hepatocellular carcinoma. Surgery. 2014;155:512–521. doi: 10.1016/j.surg.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Cucchetti A., Piscaglia F., Grigioni A.D., Ravaioli M., Cescon M., Zanello M., et al. Preoperative prediction of hepatocellular carcinoma tumour grade and microvascular invasion by means of artificial neural network: a pilot study. J. Hepatol. 2010;52(6):880–888. doi: 10.1016/j.jhep.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 15.Wang L., Jin Y.X., Ji Y.Z., Mu Y., Zhang S.C., Pan S.Y. Development and validation of a prediction model for microvascular invasion in hepatocellular carcinoma. World J. Gastroenterol. 2020;26(14):1647–1659. doi: 10.3748/wjg.v26.i14.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan A.W.H., Zhong J., Berhane S., Toyoda H., Cucchetti A., Shi K., et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J. Hepatol. 2018;69(6):1284–1293. doi: 10.1016/j.jhep.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 17.He Y.Z., He K., Huang R.Q., Wang Z.L., Ye S.W., Liu L.W., et al. Preoperative evaluation and prediction of clinical scores for hepatocellular carcinoma microvascular invasion: a single-center retrospective analysis. Ann. Hepatol. 2020;19(6):654–661. doi: 10.1016/j.aohep.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Ryu T., Takami Y., Wada Y., Tateishi M., Hara T., Yoshitomi M., et al. A clinical scoring system for predicting microvascular invasion in patients with hepatocellular carcinoma within the milan criteria. J. Gastrointest. Surg. 2019;23(4):779–787. doi: 10.1007/s11605-019-04134-y. [DOI] [PubMed] [Google Scholar]
- 19.Nitta H., Allard M.A., Sebagh M., Ciacio O., Pittau G., Vibert E., et al. Prognostic value and prediction of extratumoral microvascular invasion for hepatocellular carcinoma. Ann. Surg Oncol. 2019;26(8):2568–2576. doi: 10.1245/s10434-019-07365-0. [DOI] [PubMed] [Google Scholar]
- 20.Peng Z., Chen S., Xiao H., Wang Y., Li J., Mei J., et al. Microvascular invasion as a predictor of response to treatment with sorafenib and transarterial chemoembolization for recurrent intermediate-stage hepatocellular carcinoma. Radiology. 2019;292(1):237–247. doi: 10.1148/radiol.2019181818. [DOI] [PubMed] [Google Scholar]
- 21.Sumie S., Nakashima O., Okuda K., Kuromatsu R., Kawaguchi A., Nakano M., et al. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann. Surg Oncol. 2014;21(3):1002–1009. doi: 10.1245/s10434-013-3376-9. [DOI] [PubMed] [Google Scholar]
- 22.Qi Y.P., Zhong J.H., Liang Z.Y., Zhang J., Chen B., Chen C.Z., Li L.Q., Xiang B.D. Adjuvant transarterial chemoembolization for patients with hepatocellular carcinoma involving microvascular invasion. Am. J. Surg. 2019;217(4):739–744. doi: 10.1016/j.amjsurg.2018.07.054. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z.H., Zhang X.P., Zhou T.F., Wang K., Wang H., Chai Z.T., et al. Adjuvant transarterial chemoembolization improves survival outcomes in hepatocellular carcinoma with microvascular invasion: a systematic review and meta-analysis. Eur. J. Surg. Oncol. 2019;45(11):2188–2196. doi: 10.1016/j.ejso.2019.06.031. [DOI] [PubMed] [Google Scholar]
- 24.Wang L., Ke Q., Lin N., Zeng Y., Liu J. Does postoperative adjuvant transarterial chemoembolization benefit for all patients with hepatocellular carcinoma combined with microvascular invasion: a meta-analysis. Scand. J. Gastroenterol. 2019;54(5):528–537. doi: 10.1080/00365521.2019.1610794. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.