Abstract
Microbial interactions are widespread and important processes that support the link between disease and microbial ecology. The gut microbiota is a major source of microbial stimuli that can have detrimental or beneficial effects on human health. It is also an endocrine organ that maintains energy homeostasis and host immunity. Obesity is a highly and increasingly prevalent metabolic disease and the leading cause of preventable death worldwide. An imbalance in the gut microbiome is associated with several diseases including obesity-related metabolic disorders. This review summarizes the complex association between the gut microbiome and obesity-associated metabolic diseases and validates the role and mechanisms of ecological dysregulation in the gut in obesity-associated metabolic disorders. Therapies that could potentially alleviate obesity-associated metabolic diseases by modulating the gut microbiota are discussed.
Keywords: Gut microbiome, Obesity, Microbiota, Metabolic disorders, Mechanisms, Clinical applications
1. Introduction
Obesity is a globally prevalent condition that increases the risk of metabolic conditions such as type 2 diabetes, cardiovascular disease, and chronic kidney disease [1,2]. Its multifactorial etiology involves genetic predisposition, environmental influences, and lifestyle patterns, with precise pathophysiological pathways yet to be elucidated. Recent evidence underscores the significant role of the gut microbiota and its metabolites, such as short-chain fatty acids and bile acids, in obesity and metabolic diseases [3]. The gut microbiome, which comprises trillions of bacteria, is crucial for human homeostasis [4]. Bäckhed et al. [5] found a notable reduction in adiposity in germ-free mice compared to their conventional counterparts, with subsequent microbiota transplantation from conventional mice increasing fat content in germ-free subjects. These observations underscore the influence of the microbiome on obesity, prompting further investigations into its exact contribution to this condition. However, the association between alterations in the microbiome and obesity remains unclear. Moreover, which microbes are beneficial or harmful in obesity is also unclear. Studies have explored the gut microbiota in obese versus lean individuals, noting differences in bacterial species such as those in the Firmicutes and Bacteroidetes phyla [[6], [7], [8], [9]]. However, the results are inconsistent and their functional implications remain unclear [10]. Interventions such as dietary changes, probiotics, and prebiotics have the potential to modulate obesity outcomes; however, their efficacy and mechanisms of action are still under investigation. Gut microbes may influence obesity through mechanisms such as energy extraction from food, regulation of fat storage genes, and inflammation modulation [11,12]. Despite this progress, identifying specific microbes that protect against or contribute to obesity remains challenging [13,14]. This review provides a thorough examination of the mechanisms by which the gut microbiome modulates obesity. Additionally, we discuss specific gut microbes that exhibit protective effects in humans, both in relation to obesity and related metabolic conditions. We systematically searched PubMed, Web of Science, and Embase using keywords related to obesity-associated metabolic diseases and the gut microbiome. Duplicate entries were removed to ensure the accuracy of the analysis [15].
2. Gut microbiome
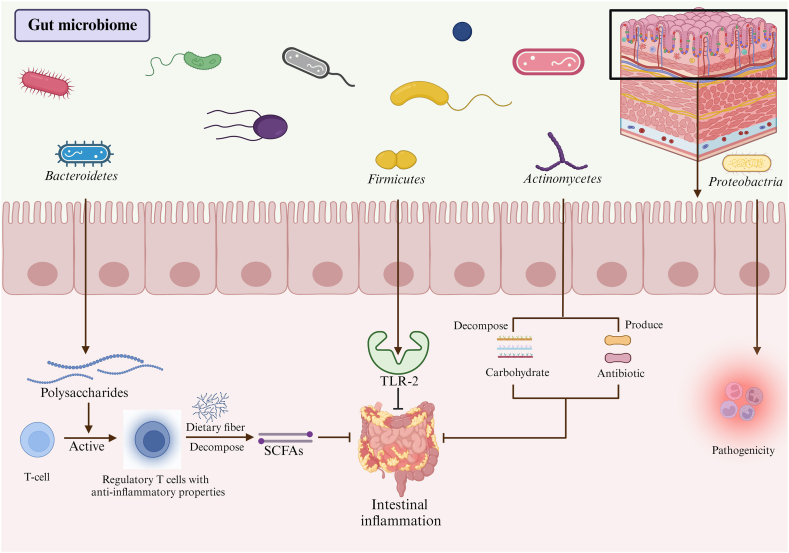
The human body contains a large number of microorganisms, most of which are found in the gastrointestinal tract [16]. The intestine is a complex ecosystem with a microenvironment consisting of a monolayer of epithelial cells, a local immune system, and microorganisms. These three intestinal tract components interact to maintain a dynamic balance [17,18] (Fig. 1). The gut microbiome in the human intestine comprises more than 40 genera of commensal bacteria. The major microorganisms were Bacteroidetes and Firmicutes [19], with essential contributions from Proteobacteria and Actinobacteria [20]. Bacteroidetes are involved in many essential metabolic activities in the human colon and may help suppress inflammation, although there is some evidence suggesting that they also have the potential to promote inflammation [21]. Among these is Bacteroides fragilis, a strain that suppresses intestinal inflammation by secreting polysaccharides that encourage T cells to differentiate into regulatory T cells with anti-inflammatory properties [22,23]. Faecalibacterium prausnitzii breaks down dietary fibers in food and produces abundant short-chain fatty acids (SCFAs), which help reduce the inflammatory response in the intestine [[24], [25], [26]]. Akkermansia muciniphila has been highlighted for its role in preserving the intestinal mucus layer, protecting the epithelial cells, and dampening inflammation [27,28]. The Bacteroides vulgatus strain can secrete endotoxins that stimulate intestinal inflammatory responses [22,29]. Prevotella copri can damage intestinal mucosal epithelial cells and stimulate intestinal inflammatory response [30]. Escherichia coli can produce toxins and cause intestinal inflammation [31]. Many types of Firmicutes are beneficial, with Lactobacillus species being common probiotics [32], whereas only a small number are pathogenic. Some strains of Firmicutes can produce toxins, and their metabolic byproducts may lead to intestinal inflammation and other health issues. An example of a pathogenic Firmicutes strain is Clostridium difficile, a resistant bacterium that can cause gastrointestinal infections [33,34]. Many types of Proteobacteria are pathogenic, including E. and Helicobacter pylori [35]. Although Actinomycetes have been shown to have potential health benefits, such as aiding in the breakdown of complex carbohydrates and producing antibiotics, the precise relationship between Actinomycetes and diseases such as obesity and diabetes mellitus remains unclear. Some studies have suggested that alterations in the gut microbiota, including decreases in Actinomycetes abundance, may be associated with these conditions; however, further investigation is necessary to confirm and comprehensively elucidate these associations [36] (Table 1).
Fig. 1.
The gut microenvironment.
Table 1.
Species and functions of the gut microbiome.
| Gut microbiome species | Function | References |
|---|---|---|
| Bacteroidetes |
Mycobacterium avium: Basic metabolic activities, suppression of inflammation. Bacteroides fragilis Intestinal inflammation suppression, polysaccharide secretion, regulatory T cell differentiation. Faecalibacterium prausnitzii Dietary fiber breakdown, abundant SCFA production, reduction of inflammatory response. Akkermansia muciniphila Inhibition of intestinal inflammation, reduction of mucus layer spoilage, protection of intestinal epithelial cells. Potential for promoting inflammation. Bacteroides vulgatus Endotoxin secretion, stimulation of intestinal inflammatory response prevotella copri Damage to intestinal mucosal epithelial cells, stimulation of intestinal inflammatory response. Escherichia coli Toxin production, induction of intestinal inflammation |
[[21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31]] |
| Firmicutes | Beneficial bacteria, lactic acid bacteria, common probiotic. Clostridium difficile, Resistant bacterium, gastrointestinal infections. |
[[32], [33], [34]] |
| Proteobacteria | Includes many pathogenic bacteria, such as Escherichia coli and Helicobacter pylori | [35] |
| Actinobacteria | Health benefits, breakdown of complex carbohydrates, antibiotic production, unclear relationship with metabolic disease | [36] |
Emerging evidence suggests a robust association between the gut microbiome and the development of obesity, with individual microbiomes potentially exerting varying effects on obesity relative to overall gut microbial diversity. Walker et al. [37] reported no alteration in the Firmicutes/Bacteroidetes ratio with the onset of obesity, challenging the purported link between gut microbiome composition and obesity. Notably, the Verrucomicrobia phylum, especially Akkermansia muciniphila, which resides in the mucus layer, has been identified for its anti-obesity effects [38,39]. Plovier et al. [40] found that the administration of A. muciniphila in mice reduced the occurrence of obesity and related metabolic disorders. The bacterium reduced weight gain and fat mass from a high-fat diet while improving glucose intolerance and insulin sensitivity. Additionally, A. muciniphila engages with Toll-like receptor (TLR)-2, a member of the TLR family involved in microbial detection and metabolic regulation, to enhance gut barrier function and combat obesity [40]. Lactobacillus acidophilus has been shown to mitigate body weight, adiposity, inflammation, and insulin resistance in mice fed a high-fat diet by modulating the AMPK-SREBP-1c/PPARα pathway while also activating brown adipose tissue (BAT) and enhancing energy, glucose, and lipid metabolism [41,42]. Although some animal studies have confirmed that an inappropriate proportion of the gut microbiome is associated with obesity, few clinical studies have demonstrated that the gut microbiome causes obesity.
2.1. The gut Microbiome's role in obesity-related metabolic disorders
2.1.1. Inflammation
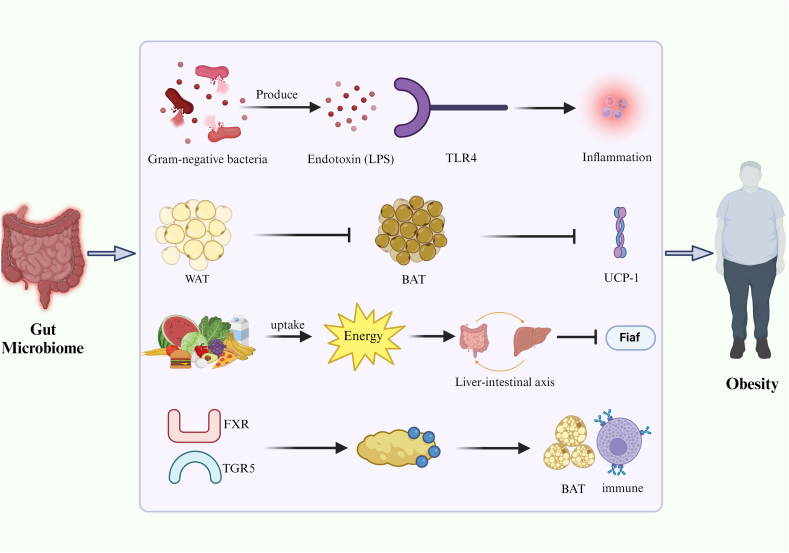
Obesity is linked to persistent low-grade inflammation and is often associated with increased levels of the endotoxin lipopolysaccharide (LPS) levels in the outer membrane of Gram-negative bacteria [43,44]. When these bacteria die, LPS enters the bloodstream, translocates across the intestinal mucosa, and triggers chronic inflammation, which contributes to obesity [45,46]. Elevated circulating endotoxin levels enhanced adipose tissue inflammation by promoting the secretion of pro-inflammatory cytokines [46,47] (Fig. 2, Table 2).
Fig. 2.
Pathways through which the gut microbiome influences obesity via endotoxins, inflammation, energy balance, and adipose tissue regulation.
Table 2.
Mechanisms of obesity caused by the gut microbiome and treatment.
| Mechanisms | Treatments | References |
|---|---|---|
| Endotoxin-induced chronic inflammation | Dietary interventions: high dietary fiber, tea polyphenols | [[43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60],61,[62], [63], [64], [65], [66]] |
| Adipose tissue browning interference | Probiotic supplementation | [[67], [68], [69], [70], [71], [72],[73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85]] |
| microbiome imbalances | Antibiotic application (β-lactams, macrolides and vancomycin) | [[86], [87], [88], [89], [90], [91],[92], [93], [94]] |
| Gut microbiome-induced energy accumulation | Metformin, acarbose | [95,96,[97], [98], [99], [100]] |
| Regulation of metabolic pathways | Fecal microbiota transplantation | [[101], [102], [103],[104], [105], [106],107,108,109] |
| Regulation of bile acid anabolism | [110,[111], [112], [113]] |
Bacterial LPS has limited benefits but produces high levels of endotoxic shock [48]. LPS is instrumental in fat accumulation [20], with gut receptors that modulate inflammation and metabolic dysregulation. Scheithauer et al. [49] observed that mice with reduced expression of the LPS receptor CD14 exhibited lower levels of inflammatory markers and fewer macrophages in fat stores. The intestinal lining serves as a critical barrier against bacterial elements [50]. However, in the presence of obesity and increased levels of fatty acids, the intestinal epithelium may become disrupted, allowing the passage of LPS across the gut wall, leading to a moderate increase in LPS levels in the circulation [[51], [52], [53]].
TLR4 binds to LPS from Gram-negative bacteria and is crucial for LPS signaling in immune cells [50,[54], [55], [56]]. LPS activates the inflammatory response via the nuclear factor-kappa B pathway through TLR4, leading to metabolic disorders [46,57,58]. Clemente-Postigo et al. [59] found increased serum LPS and chylomicron levels in patients with morbid obesity after a high-fat diet, with postprandial LPS levels correlated with hypertriglyceridemia. González-Sarrías et al. [60] explored the effect of gut microbiota manipulation on plasma LPS-binding protein levels, which are indicative of endotoxemia, in obese individuals. Their analysis using 16S rDNA sequencing revealed that fecal microbiota transplantation (FMT) could reshape the gut microbiota, potentially mitigating the risk of endotoxemia.
2.1.2. Browning of adipose tissue
The body contains three types of adipose tissue: white adipose tissue (WAT), beige adipose tissue, and BAT. WAT stores excess energy in triglycerides, which can lead to obesity [67]. BAT increases energy consumption and accelerates lipolysis by producing heat, thereby preventing excess body fat [67]. Beige adipose tissue, which is browned WAT, also helps avoid obesity [68]. The gut microbiome can influence the activation of BAT and browning of WAT, thereby affecting the development of obesity [69] (Table 2). Mestdagh et al. [70] observed increased BAT activity and higher energy expenditure in mice with a certain composition of the gut microbiota, resulting in lower body weight and improved glucose tolerance. Their study provided the first evidence that the gut microbiota affects the functioning of adipose tissue by increasing the production of BAT and interfering with lipolysis. Suárez-Zamorano et al. [71] found that depletion of the microbiota promoted browning of WAT and increased the expression of Ucp1, a marker of BAT. These findings support the conclusion that gut microbes hinder browning. They also found that mice treated with microbiota transplantation developed glucose intolerance, increased white fat weight, and decreased brown gene levels [71] (Table 2). Kong et al. [72] used pyrosequencing to examine the gut microbiota in morbidly obese subjects before and after Roux-en-Y gastric bypass and noted associations between microbial alterations and changes in gene expression in WAT.
2.1.3. Energy absorption and distribution
The gut microbiome plays a crucial role in energy absorption and distribution, significantly affecting body weight and metabolic health [114,115]. Obesity has been shown to cause alterations in the gut microbiome, including a reduction in bacterial diversity [86,87]. Specifically, Rahat-Rozenbloom et al. [88] observed an increase in the Firmicutes/Bacteroidetes ratio in obese individuals. This observation was corroborated by Hills et al. [89] who noted that obese individuals had increased Firmicutes levels and decreased Bacteroidetes levels relative to lean individuals. Further experiments by Duca et al. [86] showed that transplanting a microbiota with a high Firmicutes/Bacteroidetes ratio from obese rats into germ-free mice could induce obesity. This suggests that Firmicutes may have a more efficient mechanism of extracting energy from the diet, thus enhancing calorie absorption and promoting weight gain [6,7,90,91]. Additionally, such an alteration in the gut microbiome composition can suppress fasting-induced adipose factor (Fiaf). Fiaf normally helps regulate lipid metabolism by inhibiting lipoprotein lipase, an enzyme that promotes triglyceride accumulation in adipocytes [101]. When Fiaf is suppressed, lipoprotein lipase activity increases, leading to greater triglyceride storage in fat cells [5] (Table 2).
Moreover, gut microbial metabolites can enter the bloodstream through various mechanisms including absorption, enterohepatic circulation, and increased intestinal permeability. These metabolites further modulate energy absorption and distribution [102,103]. Certain components of the gut microbiome may inhibit Fiaf, thereby reducing triacylglycerol metabolism, increasing fat storage, and contributing to obesity [5]. They also process dietary polysaccharides, which leads to increased hepatic lipogenesis and fat cell triglyceride storage [5]. Another way by which the gut microbiota can exacerbate obesity is through the production of excess butyrate, which is a major energy source in the colon. Elevated butyrate levels increase energy accumulation, thereby contributing to weight gain [95]. In summary, the interplay between the gut microbiome, its metabolites, and energy distribution is complex but crucial for understanding and managing obesity. The gut microbiome plays a significant role in metabolic health and weight regulation by influencing energy absorption and fat storage.
2.1.4. Bile acids
Bile acids play a crucial role in metabolic and inflammatory processes. Alterations in bile acid metabolism affect lipid metabolism, energy homeostasis, and intestinal hormone production. The gut microbiome modulates bile acid synthesis and influences metabolic pathways through receptors such as the farnesoid X receptor (FXR) and Takeda G protein-coupled receptor 5 (TGR5) [110,116]. Bile acids are known to increase the production of intestinal hormones, such as glucagon-like peptide-1, which enhances insulin secretion and improves glucose metabolism [[117], [118], [119]]. Bile acids affect BAT and immune reactions through TGR5 [116]. Additionally, increased intestinal bile acid levels and decreased fecal elimination have been observed in germ-free subjects. Zheng et al. [111] demonstrated a significant increase in the body weight of normal mice fed a diet supplemented with bile acids, and that the structure of the gut microbiome in these mice resembled that of obese mice. These findings suggest that the gut microbiome participates in the development of obesity by regulating bile acid metabolism (Table 2).
Moreover, clinical data increasingly suggest that the metabolically varied microbiota in the human gut may influence obesity through bile acid metabolism. Ryan et al. [112] found that dysregulation of the gut microbiome–host metabolic axis and bile-mediated signaling pathways may increase the risk of developing metabolic diseases. Allegretti et al. [113] explored the effect of FMT on metabolically healthy obese patients. These findings indicate lasting alterations in the gut microbiome and bile acid profiles following treatment with FMT from a lean donor, reinforcing the link between microbiota changes and obesity via bile acid pathways.
2.2. SCFAs
SCFAs such as acetate, propionate, and butyrate are produced by gut bacteria during the fermentation of dietary fibers. These metabolites play a crucial role in regulating glucose and lipid metabolism by stimulating insulin secretion and incretin hormones and enhancing glucose uptake in peripheral tissues [[104], [105], [106]]. SCFAs also influence the expression of genes involved in lipid metabolism and energy expenditure and contribute to the regulation of body weight and metabolic health [120]. Elevated SCFA production, which is often observed in obese individuals, potentially leads to increased energy harvesting and fat storage [88]. Furthermore, SCFAs increase the production of intestinal hormones, decrease inflammation by maintaining intestinal permeability, and prevent fat accumulation through the activation of G protein-coupled receptor 43 [121].
2.3. Amino acids and related metabolites
Gut bacteria generate various metabolites from amino acids, such as phenylacetylglutamine, indoxyl sulfate, and p-cresol sulfate, which are implicated in insulin resistance, inflammation, and oxidative stress [107,108]. These metabolites disrupt metabolic pathways and contribute to obesity-related complications. For instance, tryptophan metabolites produced by the gut microbiota regulate the expression of miR-181 in WAT, affecting energy expenditure and glucose homeostasis [96]. An abnormal gut microbiota-miR-181 axis is crucial for WAT inflammation, insulin resistance, and obesity development, and metabolites such as indole-3-carboxylic acid and indole sulfate can influence energy accumulation by regulating miR-181 expression in WAT. Gut microbiota-derived tryptamine and phenethylamine impair insulin sensitivity in metabolic syndrome [122] (Table 2).
2.4. Gut microbiome-based obesity treatments
2.4.1. Dietary interventions
Dietary fiber improves microbial diversity in the gut and maintains a balanced glucolipid metabolism. Zhao et al. [61] found that high dietary fiber causes an increase in beneficial bacteria. Their study showed that 8 weeks of resistant starch supplementation decreased abdominal fat, promoted weight loss, and improved insulin resistance in overweight individuals by modulating the gut microbiota [123]. Other studies have shown that high-fiber foods can increase the production of indole propionate in the gut, thereby promoting lipid metabolism and maintaining beta cell function [62]. Benítez-Páez et al. [63] observed that obesity may be alleviated through caloric restriction and fiber supplementation, which is potentially linked to alterations in gut microbes and their metabolite production, such as SCFAs. Diets high in fat and animal protein and low in fiber are associated with reduced Bacteroidetes and increased Firmicutes [64]. Specifically, increased high-fat diet intake is associated with a reduction in Lactobacillus spp. and increased secretion of pro-inflammatory products. High-fat diets increase endotoxemia and inflammation, increase energy intake, and induce obesity [65]. Finally, polyphenols in tea have been found to regulate the gut microbiota in diabetic mice, reduce fasting blood glucose levels and mesenteric fat, and prevent damage to beta cells [66] (Table 2).
2.4.2. Probiotics
Probiotics are live microorganisms that ameliorate intestinal microbiota disorders, reduce mucosal permeability, decrease inflammation, and stimulate the growth and multiplication of beneficial intestinal bacteria upon entry into the intestines [73,74]. In clinical trials, probiotics and commensal bacteria (such as Lactobacillus gaseri, L. casei, L. rhamnosus, L. bulgaricus, Streptococcus thermophilus, Bifidobacterium breve, and B. longum) are beneficial for patients with obesity, mainly affecting body mass index (BMI) and fat mass. Some probiotics (e.g., L. plantarum) are helpful in reducing insulin resistance and cell adhesion molecule-1 levels, whereas synbiotics (e.g., L. rhamnosus and L. acidophilus) reduce plasma lipid levels [[75], [76], [77], [78]].
In a meta-analysis of 15 randomized controlled trials, Borgeraas et al. [79] evaluated the effect of probiotics on overweight or obese subjects. Outcomes from these studies included body weight changes in 13 trials and changes in fat mass percentage in seven trials. The results of these studies indicated the efficacy of probiotics in obesity management. Musazadeh et al. [80] conducted a meta-analysis revealing that probiotics significantly reduced BMI, body weight, and waist circumference, with interventions exceeding 8 weeks showing pronounced weight loss in obese participants. There was a significant change in BMI in participants with concomitant metabolic syndrome and in those in whom the intervention was continued for more than 12 weeks. Therefore, the authors recommended probiotic supplementation as an effective intervention for obese patients [80].
Park et al. [81] identified only four randomized controlled trials in their meta-analysis that indicated a negligible effect of probiotics on body weight or BMI reduction. Suzumura et al. [82] reached a similar conclusion, noting minimal effects on waist circumference and no significant changes in body weight or BMI. These findings suggest that the limited number of studies and potential methodological limitations could hinder definitive conclusions. Consequently, further comprehensive randomized controlled trials are necessary to more precisely elucidate the metabolic effects of probiotics.
Everard et al. [83] demonstrated a reduction in Firmicutes and an increase Bacteroidetes secretion in genetically (ob/ob) or diet-induced obese and diabetic mice following probiotic administration. Probiotics improve glucose tolerance and reduce adipogenesis, oxidative stress, and low-grade inflammation. Parnell et al. [84] showed that the combined effect of probiotics significantly altered the gut microbiome of obese and non-obese rats by increasing Bacteroidetes and decreasing the relative abundance of Firmicutes. In addition, prebiotic intake significantly alters energy metabolism and reduces fat accumulation in rats. These results suggest that probiotics improve obesity and energy metabolism by modulating the gut microbiome. In a randomized, double-blind, placebo-controlled clinical trial, Kassaian et al. [85] noted a marked increase in the Bacteroides fragilis to E. coli ratio and a decline in the Firmicutes to Bacteroidetes ratio following probiotic use. This implies that modulating gut microbial ratios using probiotics could be a viable strategy for obesity and metabolic disease management (Table 2).
2.4.3. Antibiotics
Antibiotic therapy is another feasible method of regulating the gut microbiome. A study establishing a mouse model simulating antibiotic use showed that treatment with beta-lactams or macrolides altered microbiota metabolism [92,93]. Murphy et al. [94] studied vancomycin's impact on the microbiome of male C57BL/J6 mice on a high-fat diet and found significant reductions in Firmicutes and Bacteroidetes, indicating antibiotics might influence the gut microbiome and obesity-related metabolic irregularities. However, it is important to note that antibiotics should only be used in specific situations and should not be considered a primary method for regulating the microbiome in individuals with metabolic disorders. It is crucial to consider the potential risks associated with the use of antibiotics and ensure safe handling and use of any specific antibiotics developed for this purpose in the future (Table 2).
2.4.4. Other pharmacological agents
Antidiabetic drug therapy has recently been shown to modulate the gut microbiota (Table 2). Metformin exerts hypoglycemic effects via the gut microbiome. Forslund et al. [97] in their meta-analysis involving 199 patients with type 2 diabetes and 544 healthy subjects found that metformin significantly altered the gut microbiome composition. Bauer et al. [98] showed that metformin modifies the microbiota, enhances glucagon-like peptide-1, and improves glucose regulation via sodium-glucose co-transporter protein-1 in the small intestine. Ejtahed et al. [99] conducted a randomized, double-blind clinical trial involving obese patients taking metformin in conjunction with a low-calorie diet. A significant increase in Ehrlichia/Shigella abundance in this group. In addition, acarbose administration led to a higher relative abundance of Lactobacillus and Bifidobacterium in the gut microbiota. These microbes are associated with improved intestinal health, reduced blood glucose and lipid levels, reduced inflammation, and enhanced metabolic well-being [100].
2.4.5. FMT
In FMT, the functional microbiota from healthy human feces is transplanted into the gastrointestinal tract of a recipient to establish a new gut microbiome. In one study, FMT improved insulin sensitivity and increased gut microbiome diversity in subjects with obesity and metabolic disorders [124]. Aron-Wisnewsky et al. [109] summarized the current data on the metabolic effects of FMT that improve the metabolic status, particularly insulin sensitivity. These findings provide a rationale for the use of FMT in the treatment of obesity, metabolic syndrome, and diabetes [124]. However, few studies have been conducted on FMT, and its specific mechanism of action remains unclear and requires further in-depth analysis. Nevertheless, FMT is expected to be a useful therapeutic modality in certain conditions (Table 2).
3. Microbial adaptation and advanced characterization techniques
The gut microbes adapt to obesity-related metabolic disorders in several ways. To optimize nutrient utilization, these microbes make metabolic adjustments that allow them to process diverse substrates more efficiently. They also form protective biofilms that help them adhere to gut surfaces, provide a shield against harsh conditions, and influence the local environment. Additionally, these microorganisms dynamically modulate gene expression in response to changes in nutrient availability and host immune response. In the context of obesity, these adaptations can affect the balance of the microbial community, thereby affecting the host's metabolic processes and contributing to inflammatory responses and insulin resistance.
Advancements in metagenomics and artificial intelligence (AI) have revolutionized our understanding of gut microbiota. Metagenomics analysis involves collecting fecal samples, extracting DNA, and using high-throughput sequencing to generate detailed genomic data. Thesedata are then processed to identify the microbial species and predict their functional capabilities. AI enhances this analysis by applying machine-learning algorithms to detect patterns and correlations within complex data. AI models can predict health outcomes and personalize interventions based on an individual's unique microbiota profile. In addition, AI aids in visualizing and interpreting data and reveals significant patterns and anomalies. By integrating metagenomics with AI, researchers can gain a deeper understanding of the gut microbiota and its impact on health and disease, leading to more precise diagnostics and tailored therapeutic strategies.
4. Future perspective
The evolving understanding of the role of the gut microbiome in obesity-related metabolic disorders has opened several promising avenues for research and therapeutic development. Personalized medicine is expected to benefit significantly from tailored treatments based on individual microbiome profiles. Future studies should focus on elucidating the specific mechanistic pathways through which microbial metabolites influence host metabolism.
Further clinical trials are required to investigate the efficacy and safety of probiotic and prebiotic therapies. Additionally, exploring microbiome-drug interactions and their impact on drug metabolism and effectiveness is crucial. Dietary interventions such as plant-based diets and intermittent fasting merit further examination to understand their influence on the gut microbiota. The identification of microbial biomarkers for early diagnosis and prognosis has the potential to transform patient management. Integrating microbiome research with omics technologies could offer a comprehensive view of host-microbe interactions. Finally, public health strategies that incorporate microbiome research can significantly enhance gut health and overall wellbeing.
5. Conclusion
Although the gut microbiome is diverse, certain microbiota may play a role in the development of obesity through various mechanisms. Increasing evidence supports the strong association between the gut microbiome and obesity. This review analyzes the role of the gut microbiome in obesity-related metabolic disorders, focusing on mechanisms such as chronic low-grade inflammation, adipose tissue browning, microbiome imbalances, energy absorption and distribution, bile acids, SCFAs, amino acids, and related metabolites. Moreover, we address microbial adaptation and advanced characterization techniques, offering new insights into gut microbiota dynamics. Correcting the gut microbiome has emerged as a promising approach for addressing obesity-induced metabolic disorders. Therapeutic interventions for these disorders involving the gut microbiome include dietary modifications, probiotic supplementation, antibiotics, other pharmacological agents, and FMT. Currently, the few clinical therapies available for the regulation of intestinal microbiota have a limited effect. Hence, there is an immediate need for additional research to elucidate the intricate link between the gut microbiome and obesity with the aim of identifying new strategies for modifying the gut microbiota to treat obesity.
Statements and declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Data availability statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Funding
This work was supported by the National Natural Science Foundation of China (82170865), Natural Science Foundation of Shandong Province of China (ZR2020MH106; ZR2021QD132), Taishan Scholars Project of Shandong Province (tsqn202211365), Yuandu scholars (2021), Public Domestic Visiting Program of Weifang Medical University and Education, Teaching and Research Projects of Weifang Medical University (2022YB033).
CRediT authorship contribution statement
Kexin Zhang: Writing – original draft, Methodology, Data curation, Conceptualization. Qi Zhang: Writing – original draft, Methodology, Data curation, Conceptualization. Hongyan Qiu: Investigation, Data curation. Yanhui Ma: Investigation, Data curation. Ningning Hou: Investigation, Data curation. Jingwen Zhang: Investigation, Data curation. Chengxia Kan: Investigation, Data curation. Fang Han: Investigation, Data curation. Xiaodong Sun: Writing – review & editing, Supervision. Junfeng Shi: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Xiaodong Sun, Email: xiaodong.sun@sdsmu.edu.cn.
Junfeng Shi, Email: jfshi@sdsmu.edu.cn.
References
- 1.Roberto C.A., Swinburn B., Hawkes C., et al. Patchy progress on obesity prevention: emerging examples, entrenched barriers, and new thinking. Lancet. 2015;385(9985):2400–2409. doi: 10.1016/S0140-6736(14)61744-X. [DOI] [PubMed] [Google Scholar]
- 2.Afshin A., Forouzanfar M.H., Reitsma M.B., et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017;377(1):13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu J., Wang K., Wang X., Pang Y., Jiang C. The role of the gut microbiome and its metabolites in metabolic diseases. Protein Cell. 2021;12(5):360–373. doi: 10.1007/s13238-020-00814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.John G.K., Mullin G.E. The gut microbiome and obesity. Curr. Oncol. Rep. 2016;18(7):45. doi: 10.1007/s11912-016-0528-7. [DOI] [PubMed] [Google Scholar]
- 5.Bäckhed F., Ding H., Wang T., et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101(44):15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi J., Qiu H., Xu Q., Ma Y., Ye T., Kuang Z., Qu N., Kan C., Hou N., Han F., Sun X. Integrated multi-omics analyses reveal effects of empagliflozin on intestinal homeostasis in high-fat-diet mice. iScience. 2023;26(1) doi: 10.1016/j.isci.2022.105816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu H., Kan C., Han F., Luo Y., Qu N., Zhang K., Ma Y., Hou N., Wu D., Sun X., Shi J. Metagenomic and metabolomic analysis showing the adverse risk-benefit trade-off of the ketogenic diet. Lipids Health Dis. 2024;23(1):207. doi: 10.1186/s12944-024-02198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 9.Walters W.A., Xu Z., Knight R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014;588(22):4223–4233. doi: 10.1016/j.febslet.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finucane M.M., Sharpton T.J., Laurent T.J., Pollard K.S. A taxonomic signature of obesity in the microbiome? Getting to the guts of the matter. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0084689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 12.Rastelli M., Cani P.D., Knauf C. The gut microbiome influences host endocrine functions. Endocr. Rev. 2019;40(5):1271–1284. doi: 10.1210/er.2018-00280. [DOI] [PubMed] [Google Scholar]
- 13.Musso G., Gambino R., Cassader M. Obesity, diabetes, and gut microbiota: the hygiene hypothesis expanded. Diabetes Care. 2010;33(10):2277–2284. doi: 10.2337/dc10-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sze M.A., Schloss P.D. Looking for a signal in the noise: revisiting obesity and the microbiome. mBio. 2016;7(4) doi: 10.1128/mBio.01018-16. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stasi A., Mir T., Pellegrino A., Wani A.K., Shukla S. Forty years of research and development on forensic genetics: a bibliometric analysis. Forensic Sci Int Genet. 2023;63 doi: 10.1016/j.fsigen.2023.102826. [DOI] [PubMed] [Google Scholar]
- 16.Cani P.D. Human gut microbiome: hopes, threats and promises. Gut. 2018;67(9):1716–1725. doi: 10.1136/gutjnl-2018-316723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fay K.T., Ford M.L., Coopersmith C.M. The intestinal microenvironment in sepsis. Biochim. Biophys. Acta, Mol. Basis Dis. 2017;1863(10 Pt B):2574–2583. doi: 10.1016/j.bbadis.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen Q., Huang Z., Yao J., Jin Y. Extracellular vesicles-mediated interaction within intestinal microenvironment in inflammatory bowel disease. J. Adv. Res. 2022;37:221–233. doi: 10.1016/j.jare.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bäckhed VTF. Functional Interactions between the Gut Microbiota and Host Metabolism. [DOI] [PubMed]
- 20.Du L, Lei X, Wang J, et al. Lipopolysaccharides Derived from Gram-Negative Bacterial Pool of Human Gut Microbiota Promote Inflammation and Obesity Development. [DOI] [PubMed]
- 21.Stojanov S., Berlec A., Štrukelj B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. 2020;8(11) doi: 10.3390/microorganisms8111715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Round J.L., Mazmanian S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009;9(5):313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atarashi K., Tanoue T., Shima T., et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331(6015):337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez-Siles M., Khan T.M., Duncan S.H., Harmsen H.J., Garcia-Gil L.J., Flint H.J. Cultured representatives of two major phylogroups of human colonic Faecalibacterium prausnitzii can utilize pectin, uronic acids, and host-derived substrates for growth. Appl. Environ. Microbiol. 2012;78(2):420–428. doi: 10.1128/AEM.06858-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miquel S., Martín R., Rossi O., et al. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013;16(3):255–261. doi: 10.1016/j.mib.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Quévrain E., Maubert M.A., Michon C., et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn's disease. Gut. 2016;65(3):415–425. doi: 10.1136/gutjnl-2014-307649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belzer C., de Vos W.M. Microbes inside--from diversity to function: the case of Akkermansia. ISME J. 2012;6(8):1449–1458. doi: 10.1038/ismej.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Everard A., Belzer C., Geurts L., et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wexler H.M. Bacteroides: the good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 2007;20(4):593–621. doi: 10.1128/CMR.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsen J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology. 2017;151(4):363–374. doi: 10.1111/imm.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Croxen M.A., Law R.J., Scholz R., Keeney K.M., Wlodarska M., Finlay B.B. Recent advances in understanding enteric pathogenic Escherichia coli. Clin. Microbiol. Rev. 2013;26(4):822–880. doi: 10.1128/CMR.00022-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amabebe E., Robert F.O., Agbalalah T., Orubu E. Microbial dysbiosis-induced obesity: role of gut microbiota in homoeostasis of energy metabolism. Br. J. Nutr. 2020;123(10):1127–1137. doi: 10.1017/S0007114520000380. [DOI] [PubMed] [Google Scholar]
- 33.Lawley T.D., Clare S., Walker A.W., et al. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathog. 2012;8(10) doi: 10.1371/journal.ppat.1002995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theriot C.M., Young V.B. Interactions between the gastrointestinal microbiome and Clostridium difficile. Annu. Rev. Microbiol. 2015;69:445–461. doi: 10.1146/annurev-micro-091014-104115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGEE D.J., Coker C., Testerman T.L., Harro J.M., Gibson S.V., Mobley H. The Helicobacter pylori flbA flagellar biosynthesis and regulatory gene is required for motility and virulence and modulates urease of H. pylori and Proteus mirabilis. J. Med. Microbiol. 2002;51(11):958–970. doi: 10.1099/0022-1317-51-11-958. [DOI] [PubMed] [Google Scholar]
- 36.Song Z., Li S., Li R. An investigation into the correlation of intestinal flora with obesity and gestational diabetes mellitus. Comput. Math. Methods Med. 2022;2022 doi: 10.1155/2022/5677073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker A.W., Ince J., Duncan S.H., et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5(2):220–230. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhai Q., Feng S., Arjan N., Chen W. A next generation probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. Nutr. 2019;59(19):3227–3236. doi: 10.1080/10408398.2018.1517725. [DOI] [PubMed] [Google Scholar]
- 39.Ondee T., Pongpirul K., Visitchanakun P., et al. Lactobacillus acidophilus LA5 improves saturated fat-induced obesity mouse model through the enhanced intestinal Akkermansia muciniphila. Sci. Rep. 2021;11(1):6367. doi: 10.1038/s41598-021-85449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plovier H., Everard A., Druart C., et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23(1):107–113. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 41.Park S.S., Lee Y.J., Song S., et al. Lactobacillus acidophilus NS1 attenuates diet-induced obesity and fatty liver. J. Endocrinol. 2018;237(2):87–100. doi: 10.1530/JOE-17-0592. [DOI] [PubMed] [Google Scholar]
- 42.Kang Y., Kang X., Yang H., et al. Lactobacillus acidophilus ameliorates obesity in mice through modulation of gut microbiota dysbiosis and intestinal permeability. Pharmacol. Res. 2022;175 doi: 10.1016/j.phrs.2021.106020. [DOI] [PubMed] [Google Scholar]
- 43.Russo S., Kwiatkowski M., Govorukhina N., Bischoff R., Melgert B.N. Meta-inflammation and metabolic reprogramming of macrophages in diabetes and obesity: the importance of metabolites. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.746151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rasuli L., Dehghani M.H., Aghaei M., Mahvi A.H., Mubarak N.M., Karri R.R. Occurrence and fate of bacterial endotoxins in the environment (air, water, wastewater) and remediation technologies: an overview. Chemosphere. 2022;303(Pt 2) doi: 10.1016/j.chemosphere.2022.135089. [DOI] [PubMed] [Google Scholar]
- 45.Schneier M., Razdan S., Miller A.M., Briceno M.E., Barua S. Current technologies to endotoxin detection and removal for biopharmaceutical purification. Biotechnol. Bioeng. 2020;117(8):2588–2609. doi: 10.1002/bit.27362. [DOI] [PubMed] [Google Scholar]
- 46.Lad N., Murphy A.M., Parenti C., et al. Asthma and obesity: endotoxin another insult to add to injury. Clin Sci (Lond). 2021;135(24):2729–2748. doi: 10.1042/CS20210790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Creely S.J., McTernan P.G., Kusminski C.M., et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2007;292(3):E740–E747. doi: 10.1152/ajpendo.00302.2006. [DOI] [PubMed] [Google Scholar]
- 48.Caroff M., Karibian D. Structure of bacterial lipopolysaccharides. Carbohydr. Res. 2003;338(23):2431–2447. doi: 10.1016/j.carres.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 49.Scheithauer T., Rampanelli E., Nieuwdorp M., et al. Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.571731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schoeler M., Caesar R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019;20(4):461–472. doi: 10.1007/s11154-019-09512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.distribution and kinetics of lipopro source infect immun so 2001 may 69 5 2821 8. [DOI] [PMC free article] [PubMed]
- 52.Cani P.D., Amar J., Iglesias M.A., et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 53.Erridge C., Attina T., Spickett C.M., Webb D.J. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am. J. Clin. Nutr. 2007;86(5):1286–1292. doi: 10.1093/ajcn/86.5.1286. [DOI] [PubMed] [Google Scholar]
- 54.Lu Y.C., Yeh W.C., Ohashi P.S. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42(2):145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 55.Yang L., Song H.L. [Toll-like receptor 4 in liver ischemia-reperfusion injury. Zhonghua Gan Zang Bing Za Zhi. 2019;27(6):473–476. doi: 10.3760/cma.j.issn.1007-3418.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 56.Bayan N., Yazdanpanah N., Rezaei N. Role of toll-like receptor 4 in diabetic retinopathy. Pharmacol. Res. 2022;175 doi: 10.1016/j.phrs.2021.105960. [DOI] [PubMed] [Google Scholar]
- 57.Guo S., Al-Sadi R., Said H.M., Ma T.Y. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am. J. Pathol. 2013;182(2):375–387. doi: 10.1016/j.ajpath.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manco M., Putignani L., Bottazzo G.F. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr. Rev. 2010;31(6):817–844. doi: 10.1210/er.2009-0030. [DOI] [PubMed] [Google Scholar]
- 59.Clemente-Postigo M., Queipo-Ortuño M.I., Murri M., et al. Endotoxin increase after fat overload is related to postprandial hypertriglyceridemia in morbidly obese patients. J. Lipid Res. 2012;53(5):973–978. doi: 10.1194/jlr.P020909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.González-Sarrías A., Romo-Vaquero M., García-Villalba R., Cortés-Martín A., Selma M.V., Espín J.C. The endotoxemia marker lipopolysaccharide-binding protein is reduced in overweight-obese subjects consuming pomegranate extract by modulating the gut microbiota: a randomized clinical trial. Mol. Nutr. Food Res. 2018;62(11) doi: 10.1002/mnfr.201800160. [DOI] [PubMed] [Google Scholar]
- 61.Zhao L., Zhang F., Ding X., et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359(6380):1151–1156. doi: 10.1126/science.aao5774. [DOI] [PubMed] [Google Scholar]
- 62.de Mello V.D., Paananen J., Lindström J., et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci. Rep. 2017;7 doi: 10.1038/srep46337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benítez-Páez A., Hess A.L., Krautbauer S., et al. Sex, food, and the gut microbiota: disparate response to caloric restriction diet with fiber supplementation in women and men. Mol. Nutr. Food Res. 2021;65(8) doi: 10.1002/mnfr.202000996. [DOI] [PubMed] [Google Scholar]
- 64.Harakeh S.M., Khan I., Kumosani T., et al. Gut microbiota: a contributing factor to obesity. Front. Cell. Infect. Microbiol. 2016;6:95. doi: 10.3389/fcimb.2016.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen W., Gaskins H.R., McIntosh M.K. Influence of dietary fat on intestinal microbes, inflammation, barrier function and metabolic outcomes. J. Nutr. Biochem. 2014;25(3):270–280. doi: 10.1016/j.jnutbio.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 66.Chen T., Liu A.B., Sun S., et al. Green tea polyphenols modify the gut microbiome in db/db mice as Co-abundance groups correlating with the blood glucose lowering effect. Mol. Nutr. Food Res. 2019;63(8) doi: 10.1002/mnfr.201801064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheng L., Wang J., Dai H., et al. Brown and beige adipose tissue: a novel therapeutic strategy for obesity and type 2 diabetes mellitus. Adipocyte. 2021;10(1):48–65. doi: 10.1080/21623945.2020.1870060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rui L. Brown and beige adipose tissues in health and disease. Compr. Physiol. 2017;7(4):1281–1306. doi: 10.1002/cphy.c170001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yin T., Bayanjargal S., Fang B., et al. Lactobacillus plantarum Shinshu N-07 isolated from fermented Brassica rapa L. attenuates visceral fat accumulation induced by high-fat diet in mice. Benef. Microbes. 2020;11(7):655–667. doi: 10.3920/BM2020.0009. [DOI] [PubMed] [Google Scholar]
- 70.Mestdagh R., Dumas M.E., Rezzi S., et al. Gut microbiota modulate the metabolism of brown adipose tissue in mice. J. Proteome Res. 2012;11(2):620–630. doi: 10.1021/pr200938v. [DOI] [PubMed] [Google Scholar]
- 71.Suárez-Zamorano N., Fabbiano S., Chevalier C., et al. Microbiota depletion promotes browning of white adipose tissue and reduces obesity. Nat Med. 2015;21(12):1497–1501. doi: 10.1038/nm.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kong L.C., Tap J., Aron-Wisnewsky J., et al. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am. J. Clin. Nutr. 2013;98(1):16–24. doi: 10.3945/ajcn.113.058743. [DOI] [PubMed] [Google Scholar]
- 73.Hill C., Guarner F., Reid G., et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 74.Thomas L.V., Suzuki K., Zhao J. Probiotics: a proactive approach to health. A symposium report. Br. J. Nutr. 2015;114(Suppl 1):S1–S15. doi: 10.1017/S0007114515004043. [DOI] [PubMed] [Google Scholar]
- 75.Kadooka Y., Sato M., Imaizumi K., et al. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur. J. Clin. Nutr. 2010;64(6):636–643. doi: 10.1038/ejcn.2010.19. [DOI] [PubMed] [Google Scholar]
- 76.Asemi Z., Zare Z., Shakeri H., Sabihi S.S., Esmaillzadeh A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann. Nutr. Metab. 2013;63(1–2):1–9. doi: 10.1159/000349922. [DOI] [PubMed] [Google Scholar]
- 77.Samah S., Ramasamy K., Lim S.M., Neoh C.F. Probiotics for the management of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2016;118:172–182. doi: 10.1016/j.diabres.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 78.Ardeshirlarijani E., Tabatabaei-Malazy O., Mohseni S., Qorbani M., Larijani B., Baradar Jalili R. Effect of probiotics supplementation on glucose and oxidative stress in type 2 diabetes mellitus: a meta-analysis of randomized trials. Daru. 2019;27(2):827–837. doi: 10.1007/s40199-019-00302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Borgeraas H., Johnson L.K., Skattebu J., Hertel J.K., Hjelmesaeth J. Effects of probiotics on body weight, body mass index, fat mass and fat percentage in subjects with overweight or obesity: a systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2018;19(2):219–232. doi: 10.1111/obr.12626. [DOI] [PubMed] [Google Scholar]
- 80.Musazadeh V., Zarezadeh M., Ghalichi F., et al. Anti-obesity properties of probiotics; a considerable medical nutrition intervention: findings from an umbrella meta-analysis. Eur. J. Pharmacol. 2022;928 doi: 10.1016/j.ejphar.2022.175069. [DOI] [PubMed] [Google Scholar]
- 81.Park S., Bae J.H. Probiotics for weight loss: a systematic review and meta-analysis. Nutr. Res. 2015;35(7):566–575. doi: 10.1016/j.nutres.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 82.Suzumura E.A., Bersch-Ferreira Â.C., Torreglosa C.R., et al. Effects of oral supplementation with probiotics or synbiotics in overweight and obese adults: a systematic review and meta-analyses of randomized trials. Nutr. Rev. 2019;77(6):430–450. doi: 10.1093/nutrit/nuz001. [DOI] [PubMed] [Google Scholar]
- 83.Everard A., Lazarevic V., Derrien M., et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60(11):2775–2786. doi: 10.2337/db11-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Parnell J.A., Reimer R.A. Prebiotic fibres dose-dependently increase satiety hormones and alter Bacteroidetes and Firmicutes in lean and obese JCR:LA-cp rats. Br. J. Nutr. 2012;107(4):601–613. doi: 10.1017/S0007114511003163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kassaian N., Feizi A., Rostami S., Aminorroaya A., Yaran M., Amini M. The effects of 6 mo of supplementation with probiotics and synbiotics on gut microbiota in the adults with prediabetes: a double blind randomized clinical trial. Nutrition. 2020;79–80 doi: 10.1016/j.nut.2020.110854. [DOI] [PubMed] [Google Scholar]
- 86.Duca F.A.S.Y., Lepage P.D.F., Langelier B.D.J., Covasa M. 2014. Replication of Obesity and Associated Signaling Pathways through Transfer of Microbiota from Obese Prone Rat; pp. 1624–1636. [DOI] [PubMed] [Google Scholar]
- 87.Petriz B.A., Castro A.P., Almeida J.A., et al. Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats. BMC Genom. 2014;15(1):511. doi: 10.1186/1471-2164-15-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rahat-Rozenbloom S., Fernandes J., Gloor G.B., Wolever T.M. Evidence for greater production of colonic short-chain fatty acids in overweight than lean humans. Int. J. Obes. 2014;38(12):1525–1531. doi: 10.1038/ijo.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hills RD Jr, Pontefract B.A., Mishcon H.R., Black C.A., Sutton S.C., Theberge C.R. Gut microbiome: profound implications for diet and disease. Nutrients. 2019;11(7) doi: 10.3390/nu11071613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Turnbaugh P.J., Hamady M., Yatsunenko T., et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krajmalnik-Brown R., Ilhan Z.E., Kang D.W., DiBaise J.K. Effects of gut microbes on nutrient absorption and energy regulation. Nutr. Clin. Pract. 2012;27(2):201–214. doi: 10.1177/0884533611436116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cox L.M., Yamanishi S., Sohn J., et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158(4):705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nobel Y.R., Cox L.M., Kirigin F.F., et al. Metabolic and metagenomic outcomes from early-life pulsed antibiotic treatment. Nat. Commun. 2015;6:7486. doi: 10.1038/ncomms8486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murphy E.F., Cotter P.D., Hogan A., et al. Divergent metabolic outcomes arising from targeted manipulation of the gut microbiota in diet-induced obesity. Gut. 2013;62(2):220–226. doi: 10.1136/gutjnl-2011-300705. [DOI] [PubMed] [Google Scholar]
- 95.Goffredo M., Mass K., Parks E.J., et al. Role of gut microbiota and short chain fatty acids in modulating energy harvest and fat partitioning in youth. J. Clin. Endocrinol. Metab. 2016;101(11):4367–4376. doi: 10.1210/jc.2016-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Virtue A.T., McCright S.J., Wright J.M., Jimenez M.T., Mowel W.K., Kotzin J.J., Joannas L., Basavappa M.G., Spencer S.P., Clark M.L., Eisennagel S.H., Williams A., Levy M., Manne S., Henrickson S.E., Wherry E.J., Thaiss C.A., Elinav E., Henao-Mejia J. The gut microbiota regulates white adipose tissue inflammation and obesity via a family of microRNAs. Sci. Transl. Med. 2019;11(496) doi: 10.1126/scitranslmed.aav1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Forslund K., Hildebrand F., Nielsen T., Falony G., Le Chatelier E., Sunagawa S., Prifti E., Vieira-Silva S., Gudmundsdottir V., Pedersen H.K., Arumugam M., Kristiansen K., Voigt A.Y., Vestergaard H., Hercog R., Costea P.I., Kultima J.R., Li J., Jørgensen T., Levenez F., Dore J., Nielsen H.B., Brunak S., Raes J., Hansen T., Wang J., Ehrlich S.D., Bork P., Pedersen O. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bauer P.V., Duca F.A., Waise T., et al. Metformin alters upper small intestinal microbiota that impact a glucose-SGLT1-sensing glucoregulatory pathway. Cell Metab. 2018;27(1):101–117.e5. doi: 10.1016/j.cmet.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 99.Ejtahed H.S., Tito R.Y., Siadat S.D., et al. Metformin induces weight loss associated with gut microbiota alteration in non-diabetic obese women: a randomized double-blind clinical trial. Eur. J. Endocrinol. 2019;180(3):165–176. doi: 10.1530/EJE-18-0826. [DOI] [PubMed] [Google Scholar]
- 100.Gu Y., Wang X., Li J., et al. Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat. Commun. 2017;8(1):1785. doi: 10.1038/s41467-017-01682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim H.K., Youn B.S., Shin M.S., et al. Hypothalamic Angptl4/Fiaf is a novel regulator of food intake and body weight. Diabetes. 2010;59(11):2772–2780. doi: 10.2337/db10-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wikoff W.R., Anfora A.T., Liu J., et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106(10):3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zheng X., Xie G., Zhao A., et al. The footprints of gut microbial-mammalian co-metabolism. J. Proteome Res. 2011;10(12):5512–5522. doi: 10.1021/pr2007945. [DOI] [PubMed] [Google Scholar]
- 104.Kimura I., Inoue D., Maeda T., et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41) Proc Natl Acad Sci U S A. 2011;108(19):8030–8035. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.den Besten G., van Eunen K., Groen A.K., Venema K., Reijngoud D.J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013;54(9):2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Canfora E.E., Meex R., Venema K., Blaak E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019;15(5):261–273. doi: 10.1038/s41574-019-0156-z. [DOI] [PubMed] [Google Scholar]
- 107.Mardinoglu A., Shoaie S., Bergentall M., et al. The gut microbiota modulates host amino acid and glutathione metabolism in mice. Mol. Syst. Biol. 2015;11(10):834. doi: 10.15252/msb.20156487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zheng P., Zeng B., Zhou C., et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Mol Psychiatry. 2016;21(6):786–796. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- 109.Aron-Wisnewsky J., Clément K., Nieuwdorp M. Fecal microbiota transplantation: a future therapeutic option for obesity/diabetes. Curr. Diab. Rep. 2019;19(8):51. doi: 10.1007/s11892-019-1180-z. [DOI] [PubMed] [Google Scholar]
- 110.Chávez-Talavera O., Tailleux A., Lefebvre P., Staels B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology. 2017;152(7):1679–1694.e3. doi: 10.1053/j.gastro.2017.01.055. [DOI] [PubMed] [Google Scholar]
- 111.Zheng X., Huang F., Zhao A., et al. Bile acid is a significant host factor shaping the gut microbiome of diet-induced obese mice. BMC Biol. 2017;15(1):120. doi: 10.1186/s12915-017-0462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ryan P.M., Stanton C., Caplice N.M. Bile acids at the cross-roads of gut microbiome-host cardiometabolic interactions. Diabetol Metab Syndr. 2017;9:102. doi: 10.1186/s13098-017-0299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Allegretti J.R., Kassam Z., Mullish B.H., et al. Effects of fecal microbiota transplantation with oral capsules in obese patients. Clin. Gastroenterol. Hepatol. 2020;18(4):855–863.e2. doi: 10.1016/j.cgh.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 114.De Siena M., et al. Food emulsifiers and metabolic syndrome: the role of the gut microbiota. Foods. 2022;11(15):2205. doi: 10.3390/foods11152205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sasidharan Pillai S., Gagnon C.A., Foster C., Ashraf A.P. Exploring the gut microbiota: key insights into its role in obesity, metabolic syndrome, and type 2 diabetes. The Journal of clinical endocrinology and metabolism. 2024 doi: 10.1210/clinem/dgae499. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thomas C., Gioiello A., Noriega L., Strehle A., Oury J., Rizzo G., Macchiarulo A., Yamamoto H., Mataki C., Pruzanski M., Pellicciari R., Auwerx J., Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metabol. 2009;10(3):167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yan Y., Niu Z., Sun C., Li P., Shen S., Liu S., Wu Y., Yun C., Jiao T., Jia S., Li Y., Fang Z.Z., Zhao L., Wang J., Xie C., Jiang C., Li Y., Feng X., Hu C., Jiang J., Ying H. Hepatic thyroid hormone signalling modulates glucose homeostasis through the regulation of GLP-1 production via bile acid-mediated FXR antagonism. Nat. Commun. 2022;13(1):6408. doi: 10.1038/s41467-022-34258-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen C., Liu L., Zhong Y., Wang M., Ai Y., Hou Y., Chen H., Lin X., Zhang Y., Ding M., Luo T., Li J., Li X., Xiao X. Gut microbiota-bile acids-glucagon like peptide-1 axis contributes the resistance to high fat diet-induced obesity in mice. The Journal of nutritional biochemistry. 2023;117 doi: 10.1016/j.jnutbio.2023.109358. [DOI] [PubMed] [Google Scholar]
- 119.Morimoto K., Watanabe M., Sugizaki T., Irie J., Itoh H. Intestinal bile acid composition modulates prohormone convertase 1/3 (PC1/3) expression and consequent GLP-1 production in male mice. Endocrinology. 2016;157(3):1071–1081. doi: 10.1210/en.2015-1551. [DOI] [PubMed] [Google Scholar]
- 120.He J., Zhang P., Shen L., Niu L., Tan Y., Chen L., Zhao Y., Bai L., Hao X., Li X., Zhang S., Zhu L. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int. J. Mol. Sci. 2020;21(17):6356. doi: 10.3390/ijms21176356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tolhurst G., Heffron H., Lam Y.S., Parker H.E., Habib A.M., Diakogiannaki E., Cameron J., Grosse J., Reimann F., Gribble F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61(2):364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhai L., et al. Gut microbiota-derived tryptamine and phenethylamine impair insulin sensitivity in metabolic syndrome and irritable bowel syndrome. Nat. Commun. 2023;14(1):4986. doi: 10.1038/s41467-023-40552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li H., et al. Resistant starch intake facilitates weight loss in humans by reshaping the gut microbiota. Nat. Metab. 2024;6(3):578–597. doi: 10.1038/s42255-024-00988-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alang N., Kelly C.R. Weight gain after fecal microbiota transplantation. Open Forum Infect. Dis. 2015;2(1) doi: 10.1093/ofid/ofv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.