Abstract
Nucleotide sequencing and phylogenetic analysis of 10 recognized prototype strains of the porcine enterovirus (PEV) cytopathic effect (CPE) group I reveals a close relationship of the viral genomes to the previously sequenced strain F65, supporting the concept of a reclassification of this virus group into a new picornavirus genus. Also, nucleotide sequences of the polyprotein-encoding genome region or the P1 region of 28 historic strains and recent field isolates were determined. The data suggest that several closely related but antigenically and molecular distinct serotypes constitute one species within the proposed genus Teschovirus. Based on sequence data and serological data, we propose a new serotype with strain Dresden as prototype. This hitherto unrecognized serotype is closely related to porcine teschovirus 1 (PTV-1, former PEV-1), but induces type-specific neutralizing antibodies. Sequencing of field isolates collected from animals presenting with neurological disorders prove that other serotypes than PTV-1 may also cause polioencephalomyelitis of swine.
The porcine enteroviruses (PEVs) were described as causative agents of severe and mild neurological disorders known as Teschen/Talfan disease (16, 39), reproductive failure (10), pneumonia (27), diarrhea (13), and dermal lesions of swine (25). Due to the physicochemical properties of their virions, they were previously classified as enteroviruses. Several distinct serotypes have been described (3, 5, 6, 12, 17, 23, 36). Based on parameters such as (i) cytopathic effect (CPE), (ii) replication properties in various host cell lines, and (iii) serological assays, the PEVs were divided into three CPE groups: I (serotypes 1 to 7 and 11 to 13), II (serotype 8), and III (serotypes 9 and 10) (23, 42). Recently, partial genome regions of members of each CPE group—Porcine teschovirus 1 (PTV-1) strains Talfan and F65 (9, 20), PEV-8 (J. H. Peng, R. P. Kitching, and N. J. Knowles, unpublished data), and PEV-9 (J. H. Peng, J. W. McCauley, R. P. Kitching, and N. J. Knowles, unpublished data)—were cloned and sequenced. Analysis of available genome information revealed that PEV-9 and PEV-10 are typical enteroviruses, while PEV-8 appears to be closely related to both enteroviruses and rhinoviruses. However, the genome of strain F65 (a member of CPE group I) exhibits unique features suggesting the reclassification into a new picornavirus taxon. The new genus was named Teschovirus, with F65 as a member of the species Porcine teschovirus (22). In this study, we will use the proposed name “teschovirus” as synonymous to “PEV CPE group I.”
Since the molecular properties of other members of PEV CPE group I and their relationship to F65 are still unclear, we aimed to gain further information on these viruses. For this purpose, the genomes of the prototype strains of PEV-1 to -7 and PEV-11 to -13 (proposed names PTV-1 to -10 [Table 1]) were sequenced and compared to genomes of other members of the family Picornaviridae. Moreover, several historic strains and recent field isolates were included to examine evolutionary aspects of the PTVs and facilitate an unequivocal classification of these strains. At present, serotyping using hyperimmune sera is difficult due to the significant cross-reactivity of PTV-specific antibodies. This property of the PTVs led to the description of untypeable strains (e.g., references 8 and 36). Another recent approach addressed this question by the generation of monoclonal antibodies (MAbs) (8).
TABLE 1.
PTV strains and field isolates
| Proposed name | Former name | Sequenced genome region | Isolated from: | GenBank accession no. | Reference | Sourcec |
|---|---|---|---|---|---|---|
| Prototype strains | ||||||
| PTV-1 Talfan | PEV-1 | Polyprotein | Brain | AF231769 | 16 | BFAV |
| PTV-2 T80 | PEV-2 | Polyprotein | Tonsils | AF296087 | 4 | IAHPL |
| PTV-3 O 2b | PEV-3 | Polyprotein | Brain | AF296088 | 21 | ATCC |
| PTV-4 PS 36 | PEV-4 | Polyprotein | Fetus | AF296089 | 10 | ATCC |
| PTV-5 F 26 | PEV-5 | Polyprotein | Feces | AF296090 | 1 | ATCC |
| PTV-6 PS 37 | PEV-6 | Polyprotein | Fetus | AF296091 | 10 | ATCC |
| PTV-7 F 43 | PEV-7 | Polyprotein | Feces | AF296092 | 1 | BFAV |
| PTV-8 UKG 173/74 | PEV-11 | Polyprotein | Feces | AF296093 | 23 | IAHPL |
| PTV-9 Vir 2899/84 | PEV-12 | Polyprotein | CNSd | AF296094 | 3 | SVUA |
| PTV-10 Vir 460/88 | PEV-13 | Polyprotein | CNS | AF296095 | 3 | SVUA |
| PTV-11 Dresden | PEV-1 | Polyprotein | CNS | AF296096 | 15 | BFAV |
| Field isolates | ||||||
| PTV-1 Teschen-Konratice | PEV-1 | Polyprotein | Brain | AF231768 | 28 | BFAV |
| PTV-1 Teschen-Bozen 654 | PEV-1 | Polyprotein | Brain | AF231767 | 28 | SVUA |
| PTV-1 Teschen-Tirol | PEV-1 | P1 region | Brain | AF296097 | 28 | BFAV |
| PTV-1 Teschen-199 | PEV-1 | P1 region | Brain | AF296098 | Unpublished | BFAV |
| PTV-1 IBRSV-VII | PEV-1 | P1 region | Cell culture | AF296099 | Unpublished | BFAV |
| PTV-1 DS 562/91 | PEV-1 | Polyprotein | CNS | AF296100 | Unpublished | LVUSH |
| PTV-1 Sek 549/98 | NDa | P1 region | Organ poolb | AF296101 | Unpublished | ITT |
| PTV-1 Vir 2236/99 | ND | Polyprotein | CNS | AF296102 | Unpublished | SVUA |
| PTV-1 Vir 1626/89 | PEV-1 | Polyprotein | Brain | AF296103 | 3 | SVUA |
| PTV-1 Vir 1627/89 | PEV-1 | Polyprotein | Brain | AF296104 | 3 | SVUA |
| PTV-1 PS 34 | PEV-1 | P1 region | Fetus | AF296105 | 10 | ATCC |
| PTV-1 5-DVIII | ND | P1 region | Rectal swab | AF296106 | 36 | TMLVUA |
| PTV-2 Vir 6711-12/83 | PEV-2 | Polyprotein | Brain | AF296107 | 3 | SVUA |
| PTV-2 Vir 6793/83 | PEV-2 | Polyprotein | Brain | AF296108 | 3 | SVUA |
| PTV-2 Vir 480/87 | PEV-2 | Polyprotein | Brain | AF296109 | 3 | SVUA |
| PTV-2 Sek 49/99 | ND | P1 region | Organ pool | AF296110 | Unpublished | ITT |
| PTV-4 Vir 918-19/85 | PEV-4 | Polyprotein | Brain | AF296111 | 3 | SVUA |
| PTV-4 Vir 3764/85 | PEV-4 | Polyprotein | Brain | AF296112 | 3 | SVUA |
| PTV-4 Vir 2500/99 | ND | Polyprotein | CNS | AF296113 | Unpublished | SVUA |
| PTV-5 Vir 1806/89 | PEV-5 | P1 region | Organ pool | AF296114 | 3 | SVUA |
| PTV-6 Vir 3634/85 | PEV-6 | Polyprotein | Brain, spleen | AF296115 | 3 | SVUA |
| PTV-6 Vir 289/89 | PEV-6 | P1 region | Brain | AF296116 | 3 | SVUA |
| PTV-6 21-SZ | ND | P1 region | Rectal swab | AF296117 | 36 | TMLVUA |
| PTV-8 25-TVII | ND | P1 region | Rectal swab | AF296118 | 36 | TMLVUA |
| PTV-10 Vir 461/88 | PEV-13 | Polyprotein | CNS | AF296119 | 3 | SVUA |
| PTV-11 UKG 53/81 | ND | P1 region | Feces | AF296120 | Unpublished | IAHPL |
| PTV-11 DS 1696/91 | ND | P1 region | Brain | AF296121 | 2 | LVUSH |
| Strains used for neutralization and indirect immunofluorescence assay | ||||||
| PTV-2 O 3b | PEV-2 | Brain | 21 | ATCC | ||
| PTV-5 Sek 254/97 | PEV-5 | Organ pool | Unpublished | ITT | ||
| PTV-7 WR 2 | PEV-7 | Feces | 30 | BFAV | ||
| PTV-7 UKG 163/79 | PEV-7 | Feces | 24 | IAHPL | ||
| PTV-8 DS 805/92 | PEV-11 | Brain | Unpublished | LVUSH | ||
| PTV-8 DS 756/93 | PEV-11 | Brain | Unpublished | LVUSH | ||
| PTV-9 D 77/94 | PEV-12 | Brain | Unpublished | SVLUA | ||
| PTV-9 UKG 158/80 | PEV-12 | Feces | 24 | IAHPL | ||
| PTV-10 UKG 170/80 | PEV-13 | Feces | 24 | IAHPL |
ND, serotype not determined.
Organ pools were prepared from lymph nodes, spleen, liver, and lungs.
BFAV, Bundesforschungsanstalt für Viruskrankheiten der Tiere (Insel Riems, Tübingen, Germany); IAHPL, Institute for Animal Health Pirbright Laboratory (Pirbright, England); ATCC, American Type Culture Collection (Manassas, Va.); SVUA, Staatliches Veterinäruntersuchungsamt (Arnsberg, Germany); LVUSH, Lebensmittel- und Veterinäruntersuchungsamt des Landes Schleswig-Holstein (Neumünster, Germany); ITT, Institut für Tierzucht, Tierhaltung und Tiergesundheit (Oldenburg, Germany); TMLVUA, Thüringer Medizinal-, Lebensmittel- und Veterinäruntersuchungsamt (Bad Langensalza, Germany); SVLUA, Staatliches Veterinär- und Lebensmitteluntersuchungsamt (Frankfurt/Oder, Germany).
CNS, central nervous system.
MATERIALS AND METHODS
Sources of prototype viruses and clinical specimens.
All PTV prototype strains used in this study are listed in Table 1. Isolates from brain and spinal cord were collected from animals showing symptoms of neurological disorders (Teschen/Talfan disease). Isolates from fetuses and organ pools (lymph nodes, spleen, liver, and lungs) were collected from animals showing clinical symptoms such as reproductive failure and pneumonia. Isolates from feces and rectal swabs were collected from healthy animals. PTV-1 IBRSV-VII is the only strain of this collection of unknown origin, as it was isolated from an apparently contaminated cell culture.
Cell culture and propagation of viruses.
PEV and PTV strains were propagated in porcine kidney (PS-EK and PK-15) or porcine embryonic testes (EFH-R, CCLV, and RIE170) cells. PS-EK and PK-15 cells were maintained in Dulbecco modified Eagle medium and EFH-R cells were maintained in a mixture of Hanks and Eagle minimal essential media with nonessential amino acids and sodium pyruvate. The media were supplemented with 10% fetal bovine serum. There were no fundamental differences in susceptibility of these cells to various viruses. Time of harvest for the propagated virus strains was dependent on the extent of CPE. Incubation was ended when 90% of the monolayers were destroyed, a time point which varied from 18 h to 4 days postinfection. The number of virus passages was kept as low as possible.
Indirect immunofluorescence (IIF) assay.
Monolayers of EFH-R cells grown on multispot slides were infected with virus at a multiplicity of infection of 0.5 to 2.0 and incubated until slight CPE was observed, generally 16 to 20 h postinfection. The cells were washed in isotonic buffer and air dried before immersion in acetone. Anti-PEV/PTV MAbs were applied and incubated for 1 h. A Cy3 (indocarbocyanin)-conjugated goat anti-mouse immunoglobulin G secondary antibody (Dianova) was used to detect specifically bound MAbs. Fluorescence intensity was rated from not detectable (−) to strong (+++) by microscopic examination.
Neutralization assays.
For neutralization assays, neutralizing hyperimmune sera specific to different virus strains were generated in rabbits. To propagate these sera, virus was partially purified by separation of cell debris and concentrated by ultracentrifugation. Virus was administered to rabbits intramuscularly starting with 1 ml of infectious virus (≥108.8 50% tissue culture infective doses [TCID50]) suspended in complete Freund's adjuvant. Animals were boosted three to four times, with slightly increasing doses of virus every 4 weeks. Furthermore, a PTV-1 (Swiss strain Märvil)-specific antiserum produced in specific pathogen-free pigs was used. Virus neutralization assays were performed by two methods. (i) Virus was adjusted to 100 to 1,000 TCID50/50 μl and neutralized with antisera of a known type specificity diluted in twofold steps. The maximum serum dilution which neutralized the virus completely was determined. The reaction value of a serum neutralizing its reference virus strain was set at 100% (SNT). (ii) Virus was diluted in 10-fold steps. Equal volumes of a constant dilution of an immune serum were added and allowed to react with the virus for 1 h at 37°C. Subsequently, the mixtures were transferred to EFH-R cells grown in 96-well plates. For controls, immune sera were replaced by normal rabbit sera. After 3 to 5 days, cells were surveyed for CPE and infectivity titers were calculated. The degree of neutralization was expressed as the neutralization index (NI) indicating the difference in neutralization of the virus by an immune serum in relation to a normal serum. The NI was calculated as negative decadal logarithm (VNT). Neutralization of virus was considered significant if NI was ≥1.7 (29).
Sample preparation, reverse transcription-PCR (RT-PCR), and sequencing of amplicons.
RNA of PTV-infected PS-EK cells was prepared by the method of Chomczynski and Sacchi (7). Five micrograms of total RNA was reverse transcribed with 20 pmol of oligo(dT)20 primer and 40 U of Superscript (RNase H-free) reverse transcriptase (Gibco) in a total volume of 40 μl. Two microliters of the cDNA was used for high-fidelity Expand long PCR (Roche Diagnostics) employing different specific primer sets (not shown). Prior to sequencing, PCR products were purified with either a Qiaquick gel extraction kit or Qiaquick PCR purification kit (Qiagen). Sequencing was done according to the cycle sequencing protocol of ABI Perkin-Elmer. Sequencing products were run on an ABI Prism 310 genetic analyzer.
Nucleotide and amino acid sequences, sequence alignments, and phylogenetic trees.
The nucleotide and amino acid sequences (with EMBL or Genbank sequence library accession numbers given in parentheses) of the following virus strains were used for sequence comparisons: poliovirus type 1 (PV-1) Mahoney (J02281), coxsackievirus A type 16 (CVA-16) G-10 (U05876), CVA-21 Coe (D00538), CVB-3 Nancy (M33854), human enterovirus 70 (EV-70) J670/71 (D00820), bovine enterovirus type 1 (BEV-1) VG(5)27 (D00214), PEV-8 V13 (AJ001391), PEV-9 UKG 410/73 (Y14459), PEV-10 LP 54 (complete genomic sequence [R. Zell and A. Krumbholz, unpublished data]), A-2 plaque virus (AF201894), human rhinovirus 1B (HRV-1B) B632 (D00239), HRV-14 1059 (L05355, K02121, and X01087), foot-and-mouth disease virus (FMDV) A22 (X74812), Aichi virus (AB010145), avian encephalomyelitis virus (AEV) (AJ225173), equine rhinitis A virus (ERAV; former equine rhinovirus 1 [ERV-1]) (L43052), equine rhinitis B virus (ERBV; former ERV-2) (X96871), hepatitis A virus (HAV) LA (K02990), encephalomyocarditis virus (EMCV) B (M22457, J04335), parechovirus 1 (former echovirus 22) Harris (L02971), PTV-1 (formerly PEV-1) F65 (AJ011380), and Theiler murine encephalomyelitis virus (TMEV) DA (M20301).
Sequences were aligned manually or with the ClustalW program (38) and compared to the corresponding genome regions of other picornaviruses. Neighbor-joining trees were calculated with the quartet puzzling method (34, 35) using the JTT substitution model for amino acid sequences (19) and the Tamura-Nei model for nucleotide sequences (37). The reliability of the clustering was tested by 10,000 iterations in the quartet puzzling method. For tree construction, maximum-likelihood branch lengths were computed. Consistency of branching was also tested with the maximum-parsimony algorithm (data not shown) using the PHYLIP program package (14).
RNA secondary predictions and free energy calculations were performed with the mfold program, version 3.0 (43).
Nucleotide sequence accession numbers.
The nucleotide sequence data reported in this paper are available from the GenBank nucleotide sequence database under accession no. AF231767 to AF231769 and AF296087 to AF296121.
RESULTS
Sequencing of PTV prototype strains.
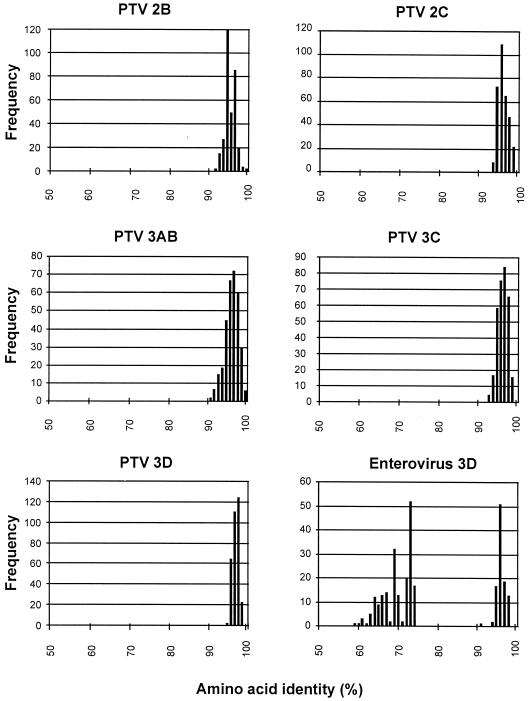
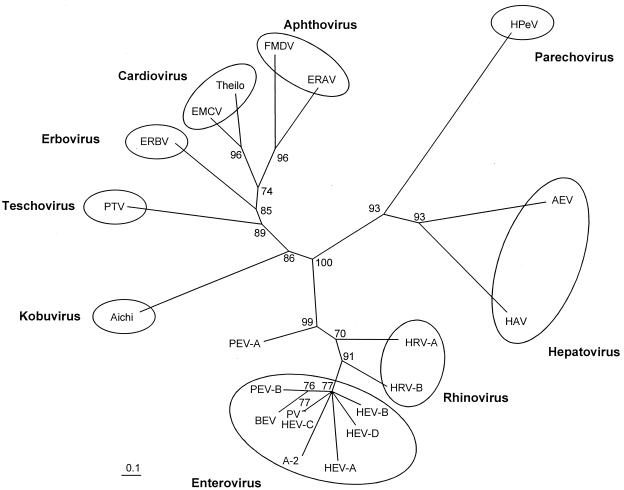
Of the 13 previously described serotypes of PEV, 10 were subclassified into CPE group I (3, 23). Molecular data describing their relation to PTV-1 strain F65 are missing. Using long RT-PCR, amplicons of more than 7 kbp of these viruses were generated and directly sequenced. With this approach, the complete polyprotein-encoding nucleotide sequences of the PTV prototype strains were determined and compared to the genomes of other picornaviruses. The organization of the teschovirus genome is shown in Fig. 1. The teschovirus RNA has a length of at least 7,100 nucleotides (nt) containing one long open reading frame. The deduced polyproteins range from 2,203 to 2,207 amino acids (aa). For PTV-1 strain F65, two AUG initiator codons, at nt 336 and 432, were suggested (9). However, the first start codon is not conserved among the PTVs (Fig. 2). More likely, viral translation starts at nt 432 (the second in-frame AUG initiator codon of F65). There is a Kozak consensus sequence around this start codon (UCACCAUGG) which is supposed to bind to the 18S rRNA (26). Up to nine base pairs may be formed at this site. Twenty-two nucleotides upstream of the A432UG initiator codon is a short oligopyrimidine tract (CUUU) which together with neighboring nucleotides may interact with the 3′ end of the 18S rRNA. Here, up to 10 nucleotides may be involved in base pairing (Fig. 2). Such an oligopyrimidine tract was reported to be important for proper translation initiation. Prerequisite is a distance of approximately 22 bp (for a review, see reference 33). For the other start codon, an oligopyrimidine tract is located 15 nt upstream of the A336UG triplet (9) at a distance comparable to those found in hepatoviruses (33). The nucleotide sequence 3′ to this A336UG triplet is extremely well conserved. In contrast, synonymous third-base substitutions are observed 3′ to nt 432 (Fig. 2). Assuming a translation start at nt 432, the PTV polyprotein contains a highly conserved leader protein of 86 aa. This leader protein seems to be released from the polyprotein by the 3C proteinase, as concluded from the presence of a conserved consensus QG cleavage site. Like the leader protein, the nonstructural proteins of the P2 and P3 regions show considerable sequence conservation. This high degree of sequence homology does not allow the differentiation of distinct serotypes. The teschovirus 2A proteinase is FMDV-like; i.e., the putative 2A proteinase is an oligopeptide with a C-terminal NPG↓P consensus cleavage site. The processing site at the N terminus is unclear, since both an asparagine residue (at aa 953 of the consensus sequence) which is characteristic for the cleavage of FMDV 2A and a nearby QG cleavage site (at aa 947/948) is conserved. Thirteen of 16 amino acids are identical to the FMDV 2A sequence. Pairwise comparison of the nonstructural proteins reveal high (>90%) amino acid identity. Previously, application of quantitative taxonomy to all members of the potyviruses and enteroviruses provided strong evidence for species distinction (31, 40). To determine whether the PTV serotypes belong to one or more species, we analyzed pairwise similarities between the P2 and P3 nonstructural proteins of 26 teschoviruses and, as a control, 25 sequences of enterovirus 3D polymerases belonging to six different species (Fig. 3). While the frequency distribution of pairwise amino acid identity scores of each teschovirus P2-P3 protein accumulates in a single peak, there is a discontinuous distribution of pairwise similarities for the enterovirus 3D polymerases which is characteristic for a multispecies genus. From this result, we concluded that all known PTV serotypes belong to one species. Construction of neighbor-joining trees of selected P2 and P3 proteins supports the results of pairwise comparisons and demonstrates the unique position of the porcine teschoviruses within the picornavirus family (Fig. 4). In all examples examined, cardioviruses, aphthoviruses, and ERBV (formerly ERV-2) are their closest relatives.
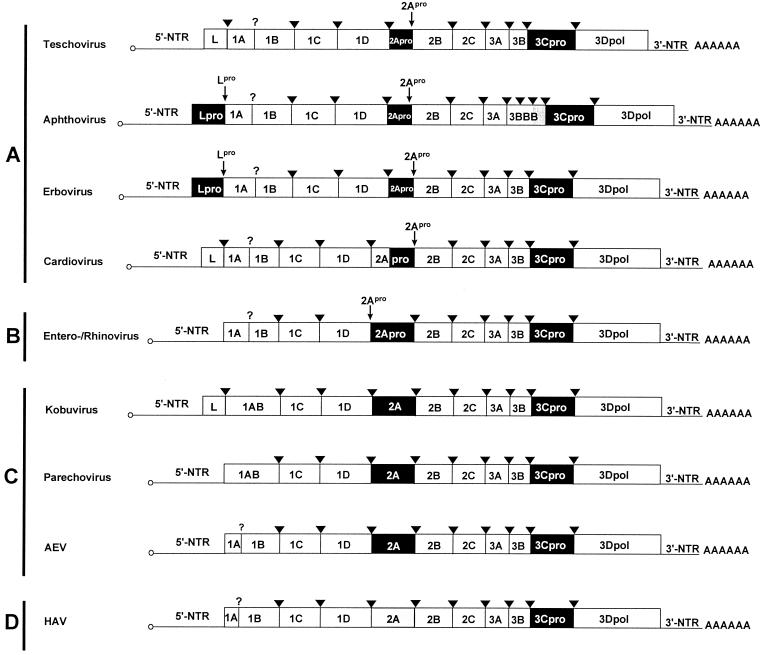
FIG. 1.
Genome organization of teschovirus and other picornavirus genera. The open reading frames are flanked on either sides by NTRs. Names of gene regions (not drawn to scale) encoding viral proteins are presented. In terms of 2A function, four virus groups (A to D) can be distinguished. Gene regions coding for proteinases (2Apro, 3Cpro, Lpro) and the nonproteolytic leader proteins (L) are highlighted in black and grey, respectively. The cardiovirus 2A protein has an N-terminal extension (indicated in grey) of unknown function. Among the aphthoviruses, FMDV has three copies of the 3B gene (as indicated), while ERAV (formerly ERV-1) does not. The genome-linked 3B peptides at the 5′ end and the poly(A) tails at the 3′ ends are indicated. The processing sites of 3Cpro (arrowheads) and 2Apro and Lpro (small arrows) are also shown. A question mark symbolizes unknown proteolytic activity responsible for the maturation cleavage of the 1AB precursor.
FIG. 2.
Alignment of partial nucleotide sequences around the start codon of PTV-1 strain F 65 and the PTV prototype strains. The consensus initiator codon at nt 432 is underlined. The AUG triplet at nt 336 of F 65 (also underlined) is not conserved among the teschovirus strains. Deviating nucleotides are highlighted. Nucleotide numbering refers to the sequence of strain F 65 (GenBank accession no. AJ 011380). The 3′ ends of 18S rRNA sequences are aligned to viral sequences which are thought to direct the ribosome to the initiation codon. Nucleotides of the 18S rRNA which allow the formation of base pairs with viral RNA are underlined.
FIG. 3.
Frequency distribution of pairwise amino acid identity scores of P2 and P3 proteins. Twenty-six sequences of teschovirus nonstructural proteins (2B to 3D) were investigated and compared to 25 enterovirus 3D polymerase sequences. The amino acid identity scores of 60 to 75% of the enterovirus genus are characteristic for comparisons of heterologous species, while amino acid identity scores greater 90% of enteroviruses and PTVs indicate comparisons of (i) different strains of homologous serotypes or (ii) heterologous serotypes of homologous species.
FIG. 4.
Unrooted neighbor-joining tree of the 3D polymerases of 21 picornavirus species. Representative serotypes of the enterovirus and rhinovirus genera: CVA-16 (HEV-A), CVB-3 (HEV-B), PV-1/CVA-21 (PV/HEV-C), EV-70 (HEV-D), A-2 plaque virus, PEV-10 (PEV-B), PEV-8 (PEV-A), HRV-1b (HRV-A), and HRV-14 (HRV-B). Amino acid sequences were aligned with the ClustalW program. Maximum-likelihood branch lengths were calculated using the quartet puzzling method. Branch lengths are proportional to genetic divergence. The scale bar indicates amino acid substitutions per site. Circles indicate acknowledged picornavirus genera. Numbers at nodes represent percentages of bipartitions in intermediate trees that have been generated in 10,000 puzzling steps. Note that the three CPE groups of PEVs belong to three distinct taxa (CPE 1 = PTV, CPE II = PEV-A, CPE III = PEV-B). PEV-A is a tentative species of the enterovirus genus.
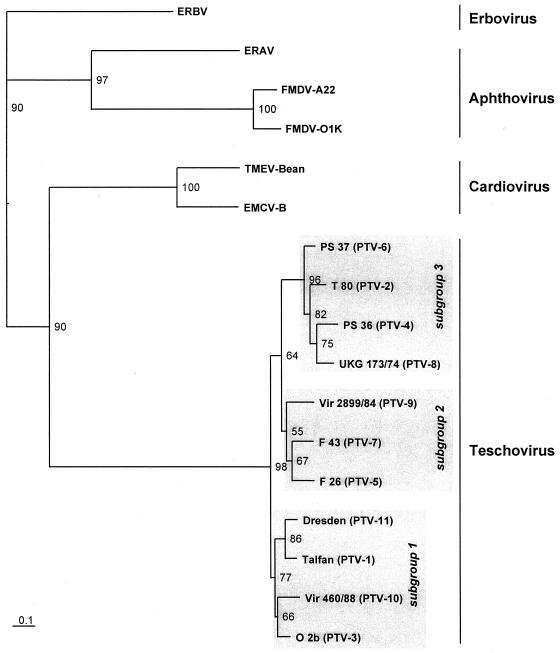
In contrast to the nonstructural proteins, the capsid proteins 1A to 1D are the most divergent proteins of the PTV serotypes (1AB, 352 to 354 aa; IC, 242 aa, 1D, 262 to 264 aa). Extents of pairwise amino acid identity range from 79 to 87% for 1AB, 76 to 91% for 1C, and 66 to 82% for 1D. Phylogenetic tree construction using the Puzzle program supports the existence of distinct serotypes, which were demonstrated with serological methods (3, 23) (Tables 2 and 3). For this analysis, representative members of the most closely related genera cardiovirus, aphthovirus, and erbovirus (ERBV) were included to illustrate both the genetic distance of the PTV serotypes to the heterologous genera and the close relationship within the teschoviruses (Fig. 5). The proposed PTV species can be divided into three subgroups consisting of three to four serotypes each.
TABLE 2.
Cross-neutralization
| Antigen | SNT value with indicated antiserum
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PTV-1 Märwil | PTV-2 O 3b | PTV-3 O 2b | PTV-4 PS 36 | PTV-5 F 26 | PTV-6 PS 37 | PTV-7 WR 2 | PTV-8 UKG173/74 | PTV-9 Vir2899/84 | PTV-10 Vir 460/88 | PTV-11 Dresden | |
| PTV-1 Konratice | 100 | 0.4 | 0.4 | 0.2 | 0.4 | 0.2 | 0.8 | 1.1 | 1.6 | 0.2 | 7.1 |
| Vir 1626/89 | 100 | 0.8 | 0.8 | 0.0 | 0.4 | 0.6 | 0.8 | 0.8 | 1.6 | 0.4 | 0.1 |
| PS 34 | 100 | 1.1 | 1.4 | 0.5 | 0.1 | 0.3 | 0.1 | 0.4 | 1.1 | 0.2 | 1.0 |
| Talfan | 100 | 0.2 | 3.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.4 | 0.4 | 0.2 | 0.5 |
| Teschen 199 | 25.0 | 0.2 | 0.6 | 0.1 | 0.2 | 0.1 | 1.1 | 0.4 | 1.1 | 0.2 | 6.2 |
| Tirol | 50.0 | 0.4 | 6.3 | 0.6 | 0.4 | 0.3 | 0.1 | 0.4 | 2.8 | 0.2 | 4.9 |
| PTV-2 O 3b | 0.8 | 100 | 0.1 | 0.2 | 0.4 | 0.2 | 0.8 | 0.4 | 3.1 | 0.1 | 0.6 |
| PTV-3 O 2b | 0.8 | 0.4 | 100 | 0.2 | 0.8 | 0.8 | 0.8 | 0.4 | 1.6 | 0.4 | 0.6 |
| PTV-4 PS 36 | 0.8 | 0.5 | 0.1 | 100 | 0.4 | 0.1 | 0.8 | 0.4 | 0.8 | 0.2 | 1.0 |
| PTV-5 F 26 | 1.6 | 0.5 | 0.4 | 0.2 | 100 | 1.6 | 0.8 | 0.4 | 3.1 | 0.4 | 0.8 |
| PTV-6 PS 37 | 0.8 | 0.5 | 0.1 | 0.1 | 0.4 | 100 | 0.8 | 0.4 | 0.1 | 0.2 | 0.4 |
| PTV-7 WR 2 | 0.8 | 0.5 | 0.8 | 0.1 | 0.4 | 0.2 | 100 | 0.6 | 0.8 | 0.4 | 0.8 |
| PTV-8 UKG 173/74 | 0.8 | 0.5 | 0.8 | 0.1 | 0.3 | 0.1 | 0.1 | 100 | 0.6 | 0.1 | 0.8 |
| PTV-9 Vir 2899/84 | 1.6 | 0.5 | 0.8 | 0.1 | 3.6 | 1.4 | 0.1 | 0.8 | 100 | 0.4 | 0.8 |
| PTV-10 Vir 460/88 | 3.1 | 0.5 | 1.6 | 0.0 | 3.3 | 0.7 | 0.1 | 0.8 | 0.8 | 100 | 1.6 |
| PTV-11 Dresden | 3.1 | 0.5 | 0.8 | 0.0 | 1.1 | 0.5 | 2.1 | 0.4 | 0.4 | 0.0 | 100 |
| PTV-11 DS 1696/91 | 3.1 | NTa | NT | NT | NT | NT | NT | NT | 2.2 | NT | 100 |
NT, not tested.
TABLE 3.
IIF assay
| Virus | Strain | Fluorescence intensity with MAb:
|
||
|---|---|---|---|---|
| 040/4B1 | 158/5D2 | 041/3C3 | ||
| PTV-1 | Talfan | +++ | +++ | − |
| PS 34 | +++ | +++ | − | |
| Vir 1626/89 | +++ | +++ | − | |
| Tirol | +++ | +++ | − | |
| Konratice | +++ | +++ | − | |
| Bozen 654 | +++ | +++ | − | |
| DS 562/91 | +++ | +++ | − | |
| Teschen 199 | +++ | +++ | − | |
| PTV-2 | T80 | +++ | − | − |
| O 3b | +++ | − | − | |
| Vir 6711-12/83 | +++ | − | − | |
| PTV-3 | O 2b | +++ | − | − |
| PTV-4 | PS 36 | +++ | − | − |
| Vir 918-19/85 | +++ | − | − | |
| Vir 3634/85 | +++ | − | − | |
| PTV-5 | F 26 | +++ | − | − |
| Sek 254/97 | +++ | − | − | |
| Vir 1806/89 | +++ | − | − | |
| PTV-6 | PS 37 | +++ | − | − |
| Vir 3634/85 | +++ | − | − | |
| Vir 289/89 | +++ | − | − | |
| PTV-7 | F 43 | +++ | − | − |
| WR 2 | +++ | − | − | |
| UKG 163/79 | +++ | − | − | |
| PTV-8 | UKG 173/74 | +++ | − | − |
| DS 805/92 | +++ | − | − | |
| DS 756/93 | +++ | − | − | |
| PTV-9 | Vir 2899/84 | +++ | − | − |
| D 77/94 | +++ | − | − | |
| UKG 158/80 | +++ | − | − | |
| PTV-10 | Vir 461/88 | +++ | − | − |
| UKG 170/80 | +++ | − | − | |
| PTV-11 | Dresden | +++ | − | +++ |
| DS 1696/91 | +++ | − | +++ | |
| UKG 53/81 | +++ | − | +++ | |
FIG. 5.
Neighbor-joining tree based on the P1 region of 17 picornaviruses. Deduced amino acid sequences of the capsid proteins of 11 PTV serotypes and their closest picornavirus relatives (Fig. 4) were aligned with the ClustalW program. Maximum-likelihood branch lengths were calculated using the quartet puzzling method. Branch lengths are proportional to genetic divergence. The scale bar indicates amino acid substitutions per site. Numbers at nodes represent the percentage of bipartitions in intermediate trees that have been generated in 10,000 puzzling steps. Grey rectangles indicate subgroups within the teschovirus genus.
So far, the 5′ end of the PTV genome has not been determined. However, the presence of an oligo(C) stretch was demonstrated for F65 (9). The 5′ and 3′ nontranslated regions (NTRs) are the most conserved genome regions of the PTVs. For example, a stretch of 250 nt at the 3′ part of the supposed internal ribosome entry site (IRES) has a nucleotide identity score of 99% (see also Fig. 2). To determine the nucleotide sequence of the 5′ NTR up to this C tract, PCR with a negative-strand-specific oligo(dC)20 primer and a positive-strand-specific primer was performed. However, this approach was successful in only 3 of 25 assayed strains (Talfan, Bozen, and Vir 1626/89 [data not shown]).
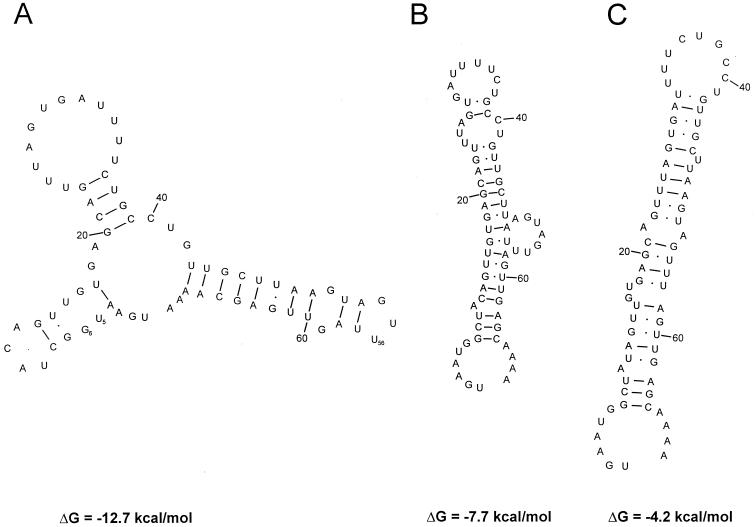
Twenty-eight 3′-NTR sequences of all serotypes were determined. As a result, the length of the 3′ NTR ranges from 65 to 67 nt. The overall sequence is highly conserved (nucleotide identity greater 94%). However, all sequenced strains lack the two 3′ cytosine residues of F65 which results in significant changes of the proposed RNA secondary structure (9). Also, some strains have an insertion of an uridine residue at nt 56 of the 3′ NTR as well as exchanges at nt 5/6 and 47/48 (data not shown). Due to these differences, both of the stem-loops proposed by Doherty and coworkers appear to be less conserved among the PTVs. Using the mfold 3.0 program, conserved RNA secondary structures consisting of three stem-loops are suggested (Fig. 6). The free energy of this proposal ranges from −10.0 (F65) to −13.4 (Vir 2899/84) kcal/mol. While the above-mentioned nucleotide substitutions and the insertion increase the amount of free energy in the proposed three-stem-loop model, they lead to a decrease of the previous proposals' free energies.
FIG. 6.
Proposals of conserved RNA secondary structures of the PTV 3′ NTR. The predictions shown apply to strains Talfan, T80, PS 36, F 26, F 43, UKG 173/80, and 25-TVII. The putative secondary structures of the other PTVs (not shown) are equivalent but have nucleotide substitutions at positions 5/6 (resulting in two additional Watson-Crick base pairs) and 47/48 (leading to an A/C mismatch), an insertion at position 56 (which increases the negative free energy of the loop), and two additional cytosine residues prior to the poly(A) tail (only F65). Proposed structure A has a free energy of −12.7 kcal/mol. The free energies of the other PTVs range from −10.0 (F 65 [not shown]) to −13.4 (Vir 2899/84 [not shown]) kcal/mol. The predictions of structures B and C are based on proposals suggested for strain F 65 (not shown). For F 65, free energies of −10.1 kcal/mol for proposed structure B and −6.6 kcal/mol for proposed structure C were calculated. All tested PTV strains yielded significant lower amounts of free energy than F 65, ranging from −7.7 to −3.2 kcal/mol for structure B and −4.2 to +2.6 kcal/mol for structure C (data not shown). The predictions of the secondary structures and the free energies were calculated with the mfold 3.0 program (43).
Sequencing of PTV field isolates.
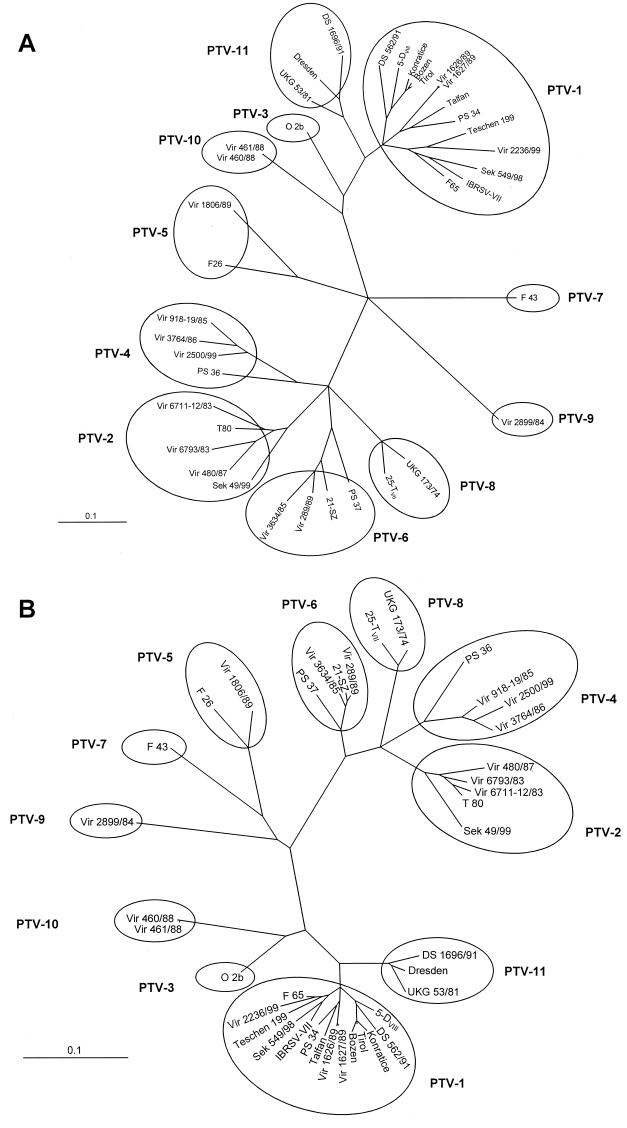
Due to a significant cross-reaction of polyclonal antisera used for IIF assays, serotyping of PTVs often leads to ambiguous results. To facilitate unequivocal serotyping and identify circulating genotypes, the genomes of several historical strains and recent field isolates (Table 1) were sequenced by the same approach as used for the prototype strains. At least the nucleotide sequence of the P1 region which encodes the capsid proteins, the flanking 5′ NTR, and part of the P2 region was determined. As a result, field isolates can be easily typed on the basis of capsid protein sequences (Fig. 7). Due to the nucleotide substitutions, pairwise amino acid identities of homologous serotypes range from 90 to 99% (for capsid protein 1AB), while those for heterologous serotypes are below 90%. Some genotypes of PTV-4, -5, and -6 (PS 36, PS 37, and Vir 1806/89) exhibit up to 15% divergence from the consensus sequence (Fig. 7A). For PTV-1, three main genotypes are observed in both nucleotide and amino acid sequence phylogenies. All three genotypes were found to be identical with both approaches. Tree topology was also essentially identical using a maximum parsimony algorithm (data not shown). The genotypes contain neurotropic and nonneurotropic viruses, virulent and avirulent strains, as well as recent and historic isolates. As shown in Fig. 7, three fields isolates (Dresden, UKG 53/81, and DS 1696/91) are clearly distinct from PTV-1 and the other PTV serotypes, indicating the existence of a hitherto unrecognized serotype.
FIG. 7.
Unrooted neighbor-joining trees of the P1 region of 39 porcine teschoviruses. Nucleotide sequences (A) and deduced amino acid sequences (B) of the capsid protein-encoding genome regions were aligned with the ClustalW program. Maximum-likelihood branch lengths were calculated using the quartet puzzling method. Branch lengths are proportional to genetic divergence. The scale bar indicates nucleotide substitutions and amino acid substitutions per site; circles indicate serotypes within the teschovirus genus.
Phenotypical characterization of the Dresden serotype.
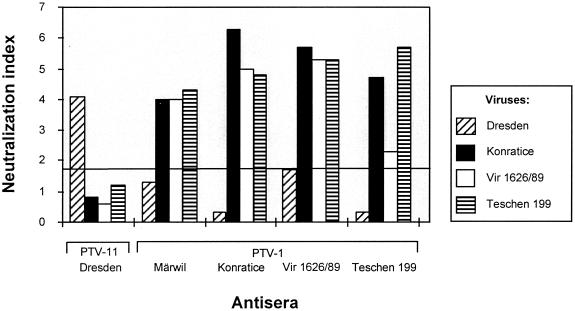
Reaction patterns of cross-neutralization in SNT revealed a remarkable difference between the Dresden isolate and all other PTV serotypes, although a relation to some PTV-1 strains seems to exist (Table 2). A second approach using the more sensitive VNT confirms this result (Fig. 8). There is no significant neutralization of the Dresden strain by the tested PTV-1 serotype-specific hyperimmune sera raised against Märwil and the strains Konratice, Vir 1626/89, and Teschen 199, each of which represents one of the main PTV-1 genotypes. Only for the PTV-1 strain Vir 1626/89 was some borderline neutralization observed (Fig. 8). On the other hand, a Dresden-specific rabbit hyperimmune serum neutralized Dresden virus even if strongly diluted but none of the PTV-1 strains. Furthermore, MAbs generated against most of the PTV serotypes were used in an IIF assay (M. Dauber, unpublished data) (8). The Dresden isolate, UKG 53/81, and DS 1696/91 were strongly recognized by the PTV group-specific MAb 040/4B1 and the Dresden-specific MAb 041/3C3 (Table 3). Neither the PTV-1-specific MAb 158/5D2 nor other serotype-specific MAbs showed any reaction with these virus strains (for the PTV-1 specific MAb, see Table 3). These results indicate that strain Dresden and the closely related strains UKG 53/81 and DS 1696/91 may be considered as a distinct serotype.
FIG. 8.
Neutralization assays of virus strains Dresden (PTV-11), Konratice (PTV-1), Vir 1626/89 (PTV-1), and Teschen 199 (PTV-1) using type-specific hyperimmune sera. The antisera were raised against strains Dresden, Märwil, Konratice, Vir 1626/89, and Teschen 199. Only neutralization scores greater 1.7 (indicated by a line) are considered to be specific.
DISCUSSION
Despite being initially described in the 1930s, the molecular genetic properties of the PEVs remained unclear for a long time. Application of classical virological methods revealed the heterogeneity within this virus group: While some physicochemical properties (e.g., acid stability) suggested classification as enteroviruses, other features (e.g. thermostability in the presence of 1 M MgCl2) were incompatible with this view. These characteristics in combination with the growth properties in different host cell lines and the cell type specificity were the basis of a subclassification into three CPE groups (23, 42). Ten acknowledged serotypes comprise CPE group I, the main group (3, 23). However, significant cross-reaction of serotype-specific antibodies led sometimes to ambiguous serotyping. Some isolates were just untypeable (8, 36). Considerable progress was made recently, when the polyprotein-encoding region of a member of CPE group I was cloned and sequenced (9, 20). Because of their distinct nucleotide sequences, they were suggested to be a new picornavirus genus, and the name “Teschovirus” was suggested (22). Sequencing of the polyprotein-encoding genome region of 10 prototype strains and of more than 29 field isolates of CPE group I reveals that PTVs are a uniform group which is clearly separated from the CPE groups II (PEV-8) and III (PEV-9 and -10). Consequently, renaming of all members of this group seems to be warranted. A proposal is presented in Table 1. The proposed genus Teschovirus is characterized by a nonproteolytic leader protein and an FMDV-like 2A proteinase (Fig. 1). A leader protein with no apparent proteinase activity was also described for the cardioviruses and Kobuvirus. However, a close phylogenetic relationship of the three leader proteins was not observed (data not shown). Pairwise comparison of the nonstructural protein sequences of each sequenced teschovirus strain and the 3D polymerase sequences of 25 enteroviruses indicate that all PTV serotypes belong to the same species whereas six species comprise the enterovirus genus (Fig. 3). This is concluded from the pairwise amino acid identity scores which distribute in a single peak. Previously, it was shown that a discontinuous distribution of pairwise similarities (as observed for the enterovirus 3D polymerases) is characteristic for a multispecies genus (31, 40).
Although the 5′ end of the teschovirus genome has not been determined, a striking degree of sequence conservation of the putative PTV IRES region is evident (Fig. 2). No other picornavirus group has a comparable nucleotide identity up to 99%. PTV-1 strains have a poly(C) tract followed by unique IRES sequences. The precise location of putative secondary structures awaits detailed analysis. Two possible translation initiation sites were suggested (9). However, the A336UG triplet is not conserved among all PTVs, and the highly conserved nucleotide sequences 3′ to this triplet show no third-base substitutions (Fig. 2). Moreover, the oligopyrimidine tract thought to interact with the 18S rRNA is quite close to the A336UG (distance of 15 nt). Hepatoviruses have an oligopyrimidine sequence 16 nt upstream of the AUG initiator codon. A second start codon is 6 nt downstream. Previously, it was hypothesized that an inappropriate distance might reduce translation efficiency (33) leading to the nonlytic phenotype of these slowly reproducing viruses. The other PTV start codon, A432UG, is absolutely conserved among 50 sequenced PTV strains (data not shown) and part of a Kozak consensus sequence which is in the optimal distance (23 nt downstream) from a rather short pyrimidine stretch. However, the coding sequences contain numerous third-base changes (Fig. 2), indicating accumulation of synonymous substitutions. In a previous study (31), analysis of the 5′-NTR sequences of seven clinical coxsackievirus B5 isolates revealed little or no genetic linkage between 5′-NTR sequences and serotype. It was hypothesized that this observation might be the result of a high frequency of recombination. Likewise, no such correlation could be found for the teschoviruses. Determination of whether this can be attributed to recombination events must await a detailed study including more field isolates.
The 3′ NTR has a conserved nucleotide sequence which allows the formation of three putative stem-loops. Calculation of free energy yields values ranging from −10.0 to −13.4 kcal/mol. Previously, Doherty et al. (9) suggested two alternative folds, which appear to be unlikely since they are less conserved (data not shown). The three-stem-loop model is supported by the observation that exchanges at nt 5/6 and 47/48 of the 3′ NTR as well as an insertion at position 56 and only one C residue prior to the poly(A) tail fit the suggested model well while leading to a decrease of the free energy of the previous proposals.
Picornavirus serotypes are defined by the ability to induce neutralizing type-specific antisera. According to the SNT and VNT results presented in Table 2 and Fig. 8, strains Dresden, UKG 53/81, and DS 1696/91 should be considered as a distinct, albeit hitherto unrecognized serotype. This view is supported by the results of IIF assays employing a set of PTV type-specific MAbs (Table 3) and the sequence data (Fig. 7). Therefore, we propose the existence of an 11th PTV serotype with strain Dresden as a prototype.
In terms of the 2A protein, picornaviruses consist of four groups (Fig. 1). (i) In cardioviruses, aphthoviruses, teschoviruses, and erbovirus (ERBV), the 2A protein is involved in an unusual proteolytic activity at the conserved NPGP sequence motif of its C terminus. (ii) The 2A protein of enteroviruses and rhinoviruses is a trypsin-like proteinase with a cysteine residue in the catalytic center; it cleaves the polyprotein at its N terminus (for a review, see reference 32). (iii) For parechoviruses, Aichi virus, and the hepatovirus-like AEV, conserved sequence motifs were described which were also found in the H-rev107 family of proteins (18). (iv) The HAV 2A protein is unrelated to known proteins. Other characteristic features of the teschovirus genome organization are also common to aphthoviruses, cardioviruses, and erbovirus, i.e., the presence of a poly(C)/oligopyrimidine tract and a leader protein (Fig. 1). Assuming a common ancestor, all proteins of these four picornavirus genera have diverged except for the highly conserved 2A proteinase and the L proteinases of aphthoviruses and erbovirus, which are papain-like cysteine proteinases. Within the teschovirus genus, one species has evolved so far. This is concluded from the frequency distribution of pairwise sequence comparisons of 26 PTV sequences including all serotypes. In previous studies, this approach was found useful for the demarcation of potyvirus and enterovirus species (31, 40). However, the PTV speciation process seems to be still in progress since the existence of three PTV subgroups is observed: the suggested serotypes PTV-1, -3, -10, and -11 comprise group 1, group 2 includes PTV-2, -4, -6, and -8, and PTV-5, -7 and -9 belong to group 3 (Fig. 5). The genetic distance of the PTV serotypes within each subgroup is quite similar, suggesting that the evolution of teschoviruses proceeded in two steps. The first step led to the development of three subgroups which then subdivided into 11 serotypes. Moreover, the suggested serotypes PTV-4, -5, and -6 have evolved genotypes which exhibit a remarkable diversity from the sequences of the other members of their serotype (up to 19% of the nucleotide sequence). For PTV-1, the Teschen strains were previously considered to include two subtypes (28): subtype 1 contained strains Konratice and Bozen, while strains Tirol and Talfan belong to the subtype 2. Although this differentiation was based on physicochemical properties (electrophoresis with DEAE-cellulose), such differences are not reflected by nucleotide and amino acid sequences. Instead, Konratice, Bozen, and Tirol are genetically very similar. Three main PTV-1 genotypes can be described, each of which contains neurotropic strains and nonneurotropic field isolates (Fig. 7).
Another aspect concerning evolutionary changes of teschoviruses is the observation that the virulence of these viruses is gradually changing. In Europe, many thousand outbreaks of severe polioencephalomyelitis caused by PTV-1 Teschen strains occurred in the 1930s to 1950s. This epizootic was associated with considerable economic losses. Subsequently, the manifestation index of severe polioencephalomyelitis decreased and the highly virulent Teschen strains were replaced by less virulent Talfan strains (15, 16). Today, PTV-1 is still frequently isolated from the feces, tonsils, and other nonneural organs of apparantly unaffected pigs. On the other hand, other serotypes than PTV-1 are increasingly identified to be the cause of neurologic disorders of swine (3). Determination of whether this observation reflects changes of virus prevalence or is the result of improved methods of virus detection (41) and virus isolation awaits further investigations.
ACKNOWLEDGMENTS
The excellent technical assistance of Eveline Ball, Veronika Güntzschel, and Sabine Wachsmuth is acknowledged. We thank Michelle Doherty and Elizabeth Hoey for communication of the PTV-1 F65 nucleotide sequence (GenBank accession no. AJ 011380) and Nick J. Knowles for communication of the PEV-8 and PEV-9 sequences (GenBank accession no. AJ001391 and Y14459) prior to publication. We also thank Rudolf Ahl (Bundesforschungsanstalt für Viruskrankheiten der Tiere, Tübingen, Germany), Nigel Ferris (Institute for Animal Health Pirbright Laboratory, Pirbright, England), Marlies Klopries (Institut für Tierzucht, Tierhaltung und Tiergesundheit, Oldenburg, Germany), and Christine Ludwig (Thüringer Medizinal-, Lebensmittel- und Veterinäruntersuchungsamt, Bad Langensalza, Germany) for providing strains and Martin Hofmann (Institut für Viruskrankheiten und Immunprophylaxe, Mittelhäusern, Switzerland) for providing serum.
REFERENCES
- 1.Alexander T J, L, Betts A O. Further studies on porcine enteroviruses isolated at Cambridge. II. Serological grouping. Res Vet Sci. 1967;8:330–337. [PubMed] [Google Scholar]
- 2.Appel G, Steinhagen P, Ohlinger V F, Ewald C. Enzephalomyopathie bei Sauen und Mastschweinen infolge einer Infektion mit porzinem Enterovirus (PEV) Tierärztl Umschau. 1995;50:326–336. [Google Scholar]
- 3.Auerbach J, Prager D, Neuhaus S, Loss U, Witte K H. Grouping of porcine enteroviruses by indirect immunofluorescence and description of two new serotypes. Zentbl Vet Med B. 1994;41:277–282. doi: 10.1111/j.1439-0450.1994.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 4.Betts A O. Studies on enteroviruses of the pig. I. The recovery in tissue culture of two related strains of a swine polio-encephalomyelitis virus from the tonsils of “normal” pigs. Res Vet Sci. 1960;1:57–65. [Google Scholar]
- 5.Betts A O, Kelly D F, Lamont P H, Sheffy B E. The isolation and characterization of some enteroviruses from pigs. Vet Rec. 1961;73:752–755. [Google Scholar]
- 6.Bohl E H, Singh K V, Hancock B B, Kasza L. Studies on five porcine enteroviruses. Am J Vet Res. 1960;21:99–103. [PubMed] [Google Scholar]
- 7.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 8.Dauber M. Identification of group I porcine enteroviruses by monoclonal antibodies in cell culture. Vet Microbiol. 1999;67:1–12. doi: 10.1016/s0378-1135(99)00024-3. [DOI] [PubMed] [Google Scholar]
- 9.Doherty M, Todd D, McFerran N, Hoey E M. Sequence analysis of a porcine enterovirus serotype 1 isolate: relationships with other picornaviruses. J Gen Vir. 1999;80:1929–1941. doi: 10.1099/0022-1317-80-8-1929. [DOI] [PubMed] [Google Scholar]
- 10.Dunne H W, Gobble J L, Hokanson J F, Kradel D C, Bubash G R. Porcine reproductive failure associated with a newly identified “SMEDI” group of picornavirus. Am J Vet Res. 1965;26:1284–1297. [PubMed] [Google Scholar]
- 11.Dunne H W, Kradel D C, Clark C D, Bubash G R, Ammermann E H. Porcine enteroviruses: a serologic comparison of thirty-eight Pennsylvania isolates with other reported North American strains, Teschen, Talfan, and T80 serums. A progress report. Am J Vet Res. 1967;28:557–568. [PubMed] [Google Scholar]
- 12.Dunne H W, Wang J T, Ammerman E H. Classification of North American porcine enteroviruses: a comparison with European and Japanese strains. Infect Immun. 1971;4:619–631. doi: 10.1128/iai.4.5.619-631.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edington N, Christofinis G J, Betts A O. Pathogenicity of Talfan and Konratice strains of Teschen virus in gnotobiotic pigs. J Comp Pathol. 1972;82:393–399. doi: 10.1016/0021-9975(72)90038-2. [DOI] [PubMed] [Google Scholar]
- 14.Felsenstein J. PHYLIP: phylogeny inference package, version 3.57c. Seattle, Wash: University of Washington; 1995. [Google Scholar]
- 15.Hahnefeld H, Hahnefeld E, Wittig W. Talfan disease der Schweine in Deutschland. I. Mitteilung: Isolierung und Charakterisierung von Teschenvirus Subtyp Talfan bei Saugferkeln im Bezirk Dresden. Arch Exp Veterinaermed. 1965;12:185–218. [PubMed] [Google Scholar]
- 16.Harding J D J, Done J T, Kershaw G F. A transmissible polio-encephalomyelitis of pigs (Talfan disease) Vet Rec. 1957;69:824–832. [Google Scholar]
- 17.Honda E, Kimata A, Hattori I, Kumagai T, Tsuda T, Tokui T. A serological comparison of 4 Japanese isolates of porcine enteroviruses with the international reference strains. Jpn J Vet Sci. 1990;52:49–54. doi: 10.1292/jvms1939.52.49. [DOI] [PubMed] [Google Scholar]
- 18.Hughes P, Stanway G. The 2A proteins of three diverse picornaviruses are related to each other and to the H-rev107 family of proteins involved in the control of cell proliferation. J Gen Virol. 2000;81:201–207. doi: 10.1099/0022-1317-81-1-201. [DOI] [PubMed] [Google Scholar]
- 19.Jones D T, Taylor W R, Thornton J M. The rapid generation of mutation data matrices from protein sequences. CABIOS. 1992;8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- 20.Kaku Y, Yamada S, Murakami Sequence determination and phylogenetic analysis of RNA-dependent RNA polymerase (RdRp) of the porcine enterovirus 1 (PEV-1) Talfan strain. Arch Virol. 1999;144:1845–1852. doi: 10.1007/s007050050709. [DOI] [PubMed] [Google Scholar]
- 21.Kasza L. Swine polioencephalomyelitis viruses isolated from the brain and intestines of pigs. Am J Vet Res. 1965;26:131–137. [PubMed] [Google Scholar]
- 22.King A M Q, Brown F, Christian P, Hovi T, Hyypiä T, Knowles N J, Lemon S M, Minor P D, Palmenberg A C, Skern T, Stanway G. Picornaviridae. In: Van Regenmortel M H V, Fauquet C M, Bishop D H L, Calisher C H, Carsten E B, Estes M K, Lemon S M, Maniloff J, Mayo M A, McGeoch D, Pringle C R, Wickner R B, editors. Virus taxonomy. Seventh Report of the International Committee for the Taxonomy of Viruses. New York, N.Y: Academic Press; 2000. pp. 657–673. [Google Scholar]
- 23.Knowles N J, Buckley L S, Pereira H G. Classification of porcine enteroviruses by antigenic analysis and cytopathic effects in tissue culture: description of 3 new serotypes. Arch Virol. 1979;62:201–208. doi: 10.1007/BF01317552. [DOI] [PubMed] [Google Scholar]
- 24.Knowles N J. Isolation and identification of porcine enteroviruses in Great Britain, 1979 to 1980. Brit Vet J. 1983;139:19–22. doi: 10.1016/s0007-1935(17)30584-5. [DOI] [PubMed] [Google Scholar]
- 25.Knowles N J. The association of group III porcine enteroviruses with epithelial tissue. Vet Rec. 1988;122:441–442. doi: 10.1136/vr.122.18.441. [DOI] [PubMed] [Google Scholar]
- 26.Kozak M. An analysis of 5′ non-coding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15:8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liebke H, Schlenstedt D. Eine Enterovirus (ECSO)-Infektion bei Schweinen mit nervösen Störungen und einer gleichzeitig vorhandenen Rhinitis. Tierärztl Umschau. 1971;26:287–291. , 324–330. [Google Scholar]
- 28.Mayr A. Degrees of variation of the virus of Teschen disease and relationship to other enteroviruses of swine. Bull Off Int Epizoot. 1961;56:106. [Google Scholar]
- 29.Mayr A, Bachmann P A, Bibrack B, Wittmann G. Virologische Arbeitsmethoden. II. Jena, Germany: Gustav Fischer Verlag; 1977. pp. 469–473. [Google Scholar]
- 30.McConnell S, Spertzel R O, Shively J N. Isolation, characterization, and serologic comparison of selected porcine enteroviruses by plaque reduction test. Am J Vet Res. 1968;29:245–251. [Google Scholar]
- 31.Oberste M S, Maher K, Kilpatrick D R, Pallansch M A. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J Virol. 1999;73:1941–1948. doi: 10.1128/jvi.73.3.1941-1948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan M D, Flint M. Virus-encoded proteinases of the picornavirus super-group. J Gen Virol. 1997;78:699–723. doi: 10.1099/0022-1317-78-4-699. [DOI] [PubMed] [Google Scholar]
- 33.Scheper G C, Voorma H O, Thomas A A M. Basepairing with 18S ribosomal RNA in internal initiation of translation. FEBS Lett. 1994;352:271–275. doi: 10.1016/0014-5793(94)00975-9. [DOI] [PubMed] [Google Scholar]
- 34.Strimmer K, von Haeseler A. Quartet puzzling: a quartet maximum likelihood method for reconstructing tree topologies. Mol Biol Evol. 1996;13:964–969. [Google Scholar]
- 35.Strimmer K, Goldman N, von Haeseler A. Bayesian probabilities and quartet puzzling. Mol Biol Evol. 1997;14:210–211. [Google Scholar]
- 36.Szent-Ivanyi T. Studies on swine enteroviruses. I. Isolation and serological grouping of strains. Acta Microbiol Acad Sci Hung. 1963;10:125–128. [PubMed] [Google Scholar]
- 37.Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- 38.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trefny L. Hromadna onemocnemi vepru na Tesinsku. Zverolek Obz. 1930;23:235–236. [Google Scholar]
- 40.Van Regenmortel M H V, Bishop D H L, Fauquet C M, Mayo M A, Maniloff J, Calisher C H. Guidelines to the demarcation of virus species. Arch Virol. 1997;142:1505–1518. [PubMed] [Google Scholar]
- 41.Zell R, Krumbholz A, Henke A, Birch-Hirschfeld E, Stelzner A, Doherty M, Hoey E, Dauber M, Prager D, Wurm R. Detection of porcine enteroviruses by nRT-PCR: differentiation of CPE groups I-III with specific primer sets. J Virol Methods. 2000;88:205–218. doi: 10.1016/s0166-0934(00)00189-0. [DOI] [PubMed] [Google Scholar]
- 42.Zoletto R. Caratteristiche differenzialii degli enterovirus suini. Vet Ital. 1965;6:3–20. [Google Scholar]
- 43.Zuker M, Mathews D H, Turner D H. Algorithms and thermodynamics for RNA secondary structure prediction: a practical guide. In: Barciszewski J, Clark B F C, editors. RNA biochemistry and biotechnology. NATO ASI Series. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1999. pp. 11–43. [Google Scholar]