Abstract
Introduction
Physical frailty is associated with increased mortality and poor quality of life (QoL) before and after liver transplantation (LT). Evidence is lacking on how to tailor exercise and behavioural techniques in this patient population.
Methods and analysis
Home-based EXercise and motivAtional programme before and after Liver Transplantation (EXALT) is a phase 2b, open-label, two-centre randomised controlled clinical trial designed to investigate whether a remotely monitored ‘home-based exercise and theory-based motivation support programme (HBEP)’ before and after LT improves QoL in LT recipients. Adult patients awaiting a primary LT will be assessed for eligibility at two LT centres (Birmingham, Royal Free London). Participants will be randomly assigned (1:1) to receive either an HBEP while on the LT waiting list through to 24 weeks after LT (Intervention) or a patient exercise advice leaflet (Control). Using a standard method of difference in means (two-sided significance level 0.05; power 0.90) and accounting for a 35% attrition/withdrawal rate, a minimum of 133 patients will be randomised to each treatment group. The primary outcome measure will be assessed using intention-to-treat analysis of the difference in the Physical Component Score of Short form-36 version 2.0 health-related QoL questionnaire between the groups at 24 weeks post-LT.
Ethics and dissemination
The protocol was approved by the South Central-Hampshire A National Research Ethics Committee. Recruitment into the EXALT trial started in May 2022 and is due to end in June 2024, with 217/266 patients randomised to date. The intervention follow-up is due to finish in May 2026. The findings of this trial will be disseminated through peer-reviewed publications, conferences and social media.
Trial registration number
ISRCTN13476586.
Keywords: LIVER TRANSPLANTATION, CIRRHOSIS, LIVER FAILURE, QUALITY OF LIFE, PSYCHOLOGY
WHAT IS ALREADY KNOWN ON THIS TOPIC
Physical frailty is associated with increased morbidity, mortality and poor health-related quality of life (QoL) before and after liver transplantation (LT).
Exercise interventions are effective at improving clinical outcomes in other chronic disease states and prior to elective surgery.
However, due to multiple physical and psychological barriers to exercise therapy in patients with advanced chronic liver disease (AdvCLD), evidence is lacking on how to tailor exercise and behavioural techniques to this unique patient population.
WHAT THIS STUDY ADDS
This will be the first randomised controlled trial to investigate whether a pre-LT and post-LT physiotherapy-led, individualised home-based exercise and motivation programme in patients AdvCLD improves QoL after LT.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The study will improve our understanding of (1) the safety and efficacy of a remotely monitored home-based exercise programme and (2) the impact of theory-based motivation support (ie, Empowering Physio) in patients with AdvCLD before and after LT.
The study will improve our understanding of how to prescribe exercise (frequency, intensity, time and type) and improve motivation towards exercise in patients with AdvCLD, previously perceived to be ‘too sick to exercise.’
Introduction
Advanced chronic liver disease (AdvCLD) is the third most common cause of death in the UK and liver transplantation (LT) remains the only cure, with over 1000 operations per year.1 Despite a new organ allocation system and advances in clinical/surgical management, 5%–10% of patients die on the LT waiting list, with a further 5% of patients dying within 6 months after LT.2 LT exerts a significant physiological and psychological stress on recipients, who are already physically and mentally frail as a consequence of AdvCLD. Increasingly, physical frailty has been associated with poor clinical outcomes, including increased hospitalisation and intensive care unit (ICU) utilisation,3,5 a 50% risk of severe postoperative complications6 and a twofold increase in pre-LT and post-LT mortality.7,10 Furthermore, physical frailty both before and after LT is associated with poor psychological and physical health-related quality of life (QoL),11,13 which is itself an independent predictor of mortality.14 QoL post-LT significantly lags behind that of the general population15 and although the majority of recipients are under 65 years old, fewer than 50% return to employment, which is largely attributed to prolonged disability/frailty.16
Exercise interventions have been shown to be effective in other fields of medicine, including prior to elective major surgery. In 2014, the American Society for Transplantation set out a research agenda for exercise interventions in patients awaiting solid-organ transplantation.17 Despite the higher numbers of LT, as compared with heart and lung transplantation, the application of exercise training in this population has been virtually non-existent. There are notable physical and psychological barriers to exercise therapy in patients with AdvCLD, including the presence of ascites, encephalopathy, sarcopenia and fatigue, as well as anxiety/depression and a lack of motivation. Not only are patients with AdvCLD perceived to be ‘too sick’ to exercise by healthcare professionals and the patient/carers themselves, but there remains little evidence to guide the individualisation of exercise and appropriate application of behavioural/motivation techniques in this unique patient population. The majority of studies to date are limited by small sample size, heterogeneity of interventions (aerobic vs resistance, hospital vs home-based, pre-LT vs post-LT), lack of behavioural/motivational support and mostly exclude the ‘sickest’ patients (ie, those with significant liver failure).18,21 Even though supervised hospital-based exercise interventions may best support engagement and adherence, the large geographical catchment area means that for many patients the time and cost required to travel to their LT centre several times each week is prohibitive.22,24 There are clear advantages to home-based exercise programmes,25 26 including increased flexibility and reduced travel burdens for patients. However, it is essential that future studies focus on patients’ motivation to engage and on psychobehavioural barriers (eg, low self-efficacy, competence and individualised support) to exercise in order to optimise such interventions delivered at home.27
On this basis, we hypothesised that a remotely monitored ‘home-based exercise and theory-based motivation support programme’ (HBEP) delivered by physiotherapists before and after LT, will result in significant improvements in QoL of LT recipients. To test this hypothesis, we designed a phase 2b open-label, two-centre randomised controlled trial (RCT), entitled ‘Home-based EXercise and motivAtional programme before and after Liver Transplantation (EXALT).’ In addition to investigating the effect of the intervention on morbidity (complications and physical frailty) and mortality, the EXALT trial will aim to determine how the theory-based motivation support plus exercise intervention affects engagement with, motivation to participate in and adherence to the home-based exercise programme.
Methods
Study design overview
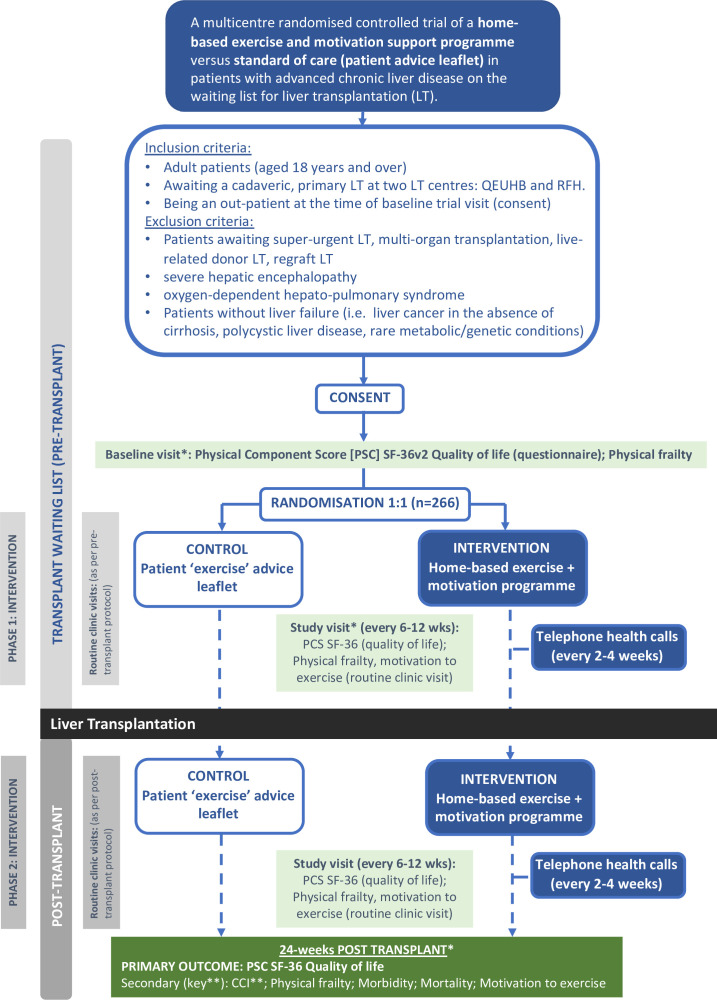
EXALT is a phase 2b open-label two-centre RCT of an HBEP in adult patients with AdvCLD on the LT waiting list. Patients who satisfy the eligibility criteria will be randomly assigned (1:1) to receive a remotely monitored, physiotherapist-led HBEP (intervention arm) or a patient exercise advice leaflet (control arm) for up to 52 weeks before LT and 24 weeks after LT. After which, a 24-week follow-up period will be scheduled.
The primary outcome measure will be assessed using a modified intention-to-treat analysis of the difference in the Physical Component Score (PCS) from the Short Form-36 version 2.0 (SF-36v2) health-related QoL questionnaire between the intervention and control groups at 24 weeks post-LT. The primary outcome analysis will only include patients who have an LT within 52 (+2) weeks of randomisation. A schematic of the trial design is summarised in figure 1.
Figure 1. EXALT trial design schematic. *At baseline, at 6 weeks (pretransplant) and 24 weeks after transplant (mechanistic ‘muscle’ substudy; n=100; optional): Cardiopulmonary exercise tests, muscle ultrasound and biomarkers. CCI, Comprehensive Complications Index; EXALT, EXercise and motivAtional programme before and after Liver Transplantation; PCS, Physical Component Score; RFH, Royal Free Hospital; SF-36, Short form-36; QEUHB, Queen Elizabeth University Hospital Birmingham.** represents Key Secondary Outcome.
Patient selection
Eligible adults (≥18 years old) will be identified and recruited to the two participating trial centres between April 2022 and June 2024 (estimation). The two UK trial centres will be the supraregional LT units at the Queen Elizabeth University Hospital Birmingham (QEUHB, opened April 2022) and Royal Free Hospital, London (RFH, opened November 2022).
Inclusion criteria
To be eligible to participate in the EXALT Trial, adult patients wil have to be listed for a cadaveric primary LT at either QEUHB or RFH and be an outpatient at the time of visit 1 (ie, when written consent was obtained).
Exclusion criteria
Patients listed for super urgent, multiorgan transplantation, live-related donor LT and/or regraft LT will be excluded. In addition, patients with an inability to safely comply with exercise interventions (eg, oxygen-dependent hepatopulmonary syndrome, grade 3/4 hepatic encephalopathy) and/or those without chronic liver failure (eg, non-cirrhotic hepatocellular carcinoma (HCC); polycystic liver disease, rare metabolic/genetic conditions including glycogen storage disorders) will be excluded.
Patients, who are on the LT waiting list and are potentially eligible for the EXALT trial, will be identified by a member of the direct care, multidisciplinary LT team and will receive an invitation letter. All patients who declare an interest to partake in the trial, will be given a ‘patient information sheet (PIS)’, either in person at their LT waiting list clinic or via post. Participants will be given a minimum of 24 hours to read the PIS and ask any questions to the local trial team. If the patient then agrees to enrol in the trial, the baseline trial (visit 1) will be arranged. All trial participants will give informed written consent at the beginning of visit 1 prior to undergoing any trial-specific investigations, examinations or interventions. At this stage, the patient will nominate a ‘personal consultee(s)’, to act in their best interests, in the event they lack mental capacity (ie, hepatic encephalopathy) at any stage during the trial.
Patients who satisfy the eligibility for the main EXALT trial at the QEUHB site will be given the option to participate in a ‘muscle mechanistic’ substudy. The substudy (n=100) will involve additional investigations, namely a cardiopulmonary exercise test (CPET), a muscle ‘quadricep’ ultrasound, a 6 min walk test (6MWT) and blood samples that will be stored for analysis of biomarkers of inflammation, oxidative stress associated with muscle injury. These assessments will be undertaken at pre-LT visits 1, 2 and post-LT visit 9 (end of treatment). A detailed summary of the substudy will be published separately (online supplemental methods). A patient’s decision to participate in or withdraw from the substudy will not affect their participation in the main EXALT trial.
Study visit overview
In total, the trial schedule will consist of a maximum of 10 outpatient visits, with up to 6 visits pre-LT (phase 1=baseline, weeks 6, 12, 24, 36 and 48) and 4 visits post-LT (phase 2=weeks 6, 12, 24 and 48 post-LT). The participants will enter the post-LT phase 2 of the trial on the day of LT, irrespective of how many trial visits the complete pre-LT. In addition to visits listed above, the participants will be reviewed by the local study team on the day of admission for LT (immediately pre-LT), post-LT and within 48–72 hours prior to discharge from hospital. Table 1 summarises the trial schedule.
Table 1. EXALT trial schedule.
| Study visits | Baseline | End-of-intervention | Follow-up | |||||||||
| Preliver transplant (LT) (variable time-line) | Transplant | Post-LT (fixed time-line) | ||||||||||
| Visit 1 | Visit 2 | Visit 3 | Visit 4 | Visit 5 | Visit 6 | Inpatient stay | Visit 7 | Visit 8 | Visit 9 | Visit 10 | ||
| Time points | 0 weeks | 6 weeks | 12 weeks | 24 weeks | 36 weeks | 48 weeks | 52 week cut-off* | 6 weeks post | 12 weeks post | 24 weekspost | 48 weekspost | |
| (±7 days) | (±7 days) | (±7 days) | (±14 days | (±14 days) | day 10±3 days | (±7 days) | (±7 days) | (±14 days) | (±14 days) | |||
| Consent | X | |||||||||||
| Randomisation (intervention vs control)) | X | |||||||||||
| Clinical examination and review (routine clinic) | X | X | X | X | X | X | X | X | X | X | X | X |
| Standard bloods pre-LT (FBC, U+E, LFTs, AST, GGT, INR, CRP, nutrition, ammonia) | X | X | X | X | X | X | X | |||||
| Standard bloods post-LT (FBC, U+E, LFTs, AST, GGT, INR, CRP, nutrition, tacrolimus) | X | X | X | X | X | |||||||
| Standard clinic observations (BP, dry BMI, weight, hand grip strength, MAMC) | X | X | X | X | X | X | X | X | X | X | X | X |
| Frailty/functionality assessment (LFI, DASI) | X | X | X | X | X | X | X | X | X | X | X | X |
| Primary outcome SF-36v2 questionnaire | X | X | X | X | X | X | X | X | X | X | X | |
| Behavioural/psychological questionnaires (HCCQ, PNES, BREQ-2) | X | X | X | X | X | X | X | X | X | X | X | |
| Serious adverse events (complications/morbidity) | X | X | X | X | X | X | X | X | X | X | X | X |
| LT data (date, donor details, organ support, ICU stay) | X | |||||||||||
| Exercise adherence: review participant ‘exercise’ diary | X | X | X | X | X | X | X | X | X | X | ||
| Exercise Adherence: Acceleromter | Weeks 0–2 | 4–6 | 10–12 | 22–24 | Weeks 4–6 | 10–12 | 22–24 | |||||
| Mechanistic 'muscle' substudy (n=100, optional): | ||||||||||||
| CPET, muscle USS, 6MWT, specialist biomarkers | X | X | (X) | X | ||||||||
| Interventions: | ||||||||||||
| Intervention: home-based exercise and theory-based motivation support programme | X | X | X | X | X | X | X | X | X | X | ||
| Intervention: Telecall (15–30 min) | Week 2,4 | 8, 10 | 16,20 | 4 | 8 | 16 | ||||||
| Control: standard of care patient ‘exercise’ advice leaflet | X | X | ||||||||||
52 (+2) week cut-off: study intervention will stop if participant has not had their (LT) by 52 weeks. (X) optional substudy consent if have not undergone LT within the 1-year-year time- frame.Key:
AST, aspartate transferase; BMI, body mass index; BP, blood pressure; BREQ-2, Behavioural Regulation in Exercise Questionaire-2; CPET, cardiopulmonary exercise test; CRP, C reactive protein; DASI, Duke Activity Status Index; EXALTEXercise and motivAtional programme before and after Liver TransplantationFBC, full blood count; GGT, gamma-glutamyl transferase; HCCQ, Healthcare Climate Questionnaire; ICU, intensive care unit; INR, international normalised ratio; LFI, Liver frailty index; LFTsliver function testsMAMC, mid-arm muscle circumference; 6MWT6 min walk testPNES, Psychological Need Satisfaction in Exercise Scale; U+E, urea and electrolyteUSSUltrasound
Due to the unpredictable nature of the timing of LT, the duration of the study intervention will range from a minimum of 24 weeks+1 day (1-day pre-LT; 24 weeks post-LT) to a maximum of 76 weeks (52 weeks pre-LT; 24 weeks post-LT). The maximum duration of the trial for an individual participant, including screening, intervention and the follow-up visit, will be approximately 2 years (100 weeks). In the event that a participant is transplanted after 52 (+2) weeks, the physiotherapy-led HBEP they will be randomised to will be terminated. However, with the participant’s ongoing willingness to continue in the study, their data will be collected until the trial end date.
Randomisation
Participants who meet all the eligibility criteria and provide written informed consent will be randomly assigned on a 1:1 basis to either:
-
Group 1: Intervention group. A remotely monitored, physiotherapist-delivered home-based exercise and motivational support programme while on the LT waiting list (maximum 52 weeks) through to 24 weeks post-LT,
OR
Group 2: Control group. Patient exercise advice leaflet before and after LT.
Randomisation will be undertaken using a secure, online computer-generated system (Research Electronic Data Capture (REDCap)28) at the Birmingham Clinical Trials Unit (BCTU). A minimisation algorithm will be used within the randomisation system to ensure balance in the allocation over the following variables: gender (male, female), age (≤55 years, >55 years), UKELD score (≤54, >54), trial centre (QEUHB, RFH) and enrolment into the ‘muscle mechanistic’ substudy (yes, no). The latter will become a default ‘no’ for all randomised patients once the target sample size of 100 patients enrolled in the substudy has been reached. A ‘random element’ will be included in the minimisation algorithm, so that each participant has a probability (unspecified here), of being randomised to the opposite treatment that they would have otherwise received. Trial participants will be allocated a unique trial identification number to preserve patient confidentiality.
Treatment groups
Group 1: HBEP (intervention arm)
The pre-LT and post-LT HBEP intervention will be delivered by trained study physiotherapists and comprise two core components:
A remotely monitored, personalised, home-based exercise programme.
A bespoke autonomous motivation promotion programme, that is, ‘Empowering Physio’, delivered to physiotherapists to support them in introducing and delivering the home-based exercise to the participant.
Physiotherapist training
Prior to commencing the study, the physiotherapists will receive formal face-to-face training on physical ‘liver’ frailty and delivering the HBEP (group 1) intervention by FRW. Training by JLD will also incorporate the principles and strategies of ‘Empowering Physio’ in order to deliver the motivation support programme. ‘Empowering Physio’ is an evidence-based training programme designed by JLD for physiotherapists, grounded particularly in self-determination theory and achievement goal theory. The face-to-face training will take place over a 3-day structured course. The details of the programme content and course structure can be found in online supplemental methods.
Intervention schedule
Pre-LT phase 1 of the HBEP will commence the day after baseline visit 1 (maximum 3 days post visit 1) and end on either (a) the day of LT or (b) 52 (+2) weeks postrandomisation if LT has not taken place. After LT, the participant will initially undergo physiotherapist delivered walking and basic exercise programmes (supported in concordance with Empowering Physio principles) until discharged from hospital. After LT, the HBEP will recommence on discharge from hospital and will be adapted according to the patient’s up-to-date Liver Frailty Index (LFI) and Duke Activity Score Index (DASI). Visit 9 (24 weeks post-LT) will mark the end of the trial intervention. The intervention schedule is summarised in table 2.
Table 2. HBEP intervention (group 1) schedule.
| Trial visitsTrial intervention | Visit 1Week 0 | THCWeeks 2 and 4 | DevicesWeeks 4–6 | Visit 2Week 6 | THCWeeks 8 and 10 | DevicesWeeks 10–12 | Visit 3Week 12 | THCWeeks 16 and 20 | DevicesWeeks 22–24 | Visit 4Week 24 | Visit 5Week 36 | Visit 6Week 48 | LT | IP stay | THCWeek 4 | DevicesWeeks 4–6 | Visit 7Week 6 | THCWeeks 8, 16 | DevicesWeeks 10–12 | Visit 8Week 12 | DevicesWeeks 22–24 | Visit 9Week 24 | Visit 10Week 48 |
| Education | |||||||||||||||||||||||
| Patient education session | X | ||||||||||||||||||||||
| Devices and Handouts | |||||||||||||||||||||||
| Accelerometer | X | X | X | X | X | X | X | ||||||||||||||||
| Participant Diary issued | X | X | |||||||||||||||||||||
| Exercise instruction | |||||||||||||||||||||||
| Pre-LT HBEP plan | X | ||||||||||||||||||||||
| Review and adaptation of HBEP | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Review of participant diary | X | X | X | X | X | X | X | X | X | ||||||||||||||
| Post-LT HBEP plan | X | ||||||||||||||||||||||
| ‘Empowering Physio’ | |||||||||||||||||||||||
| Identify knowledge about benefits of exercise | X | ||||||||||||||||||||||
| Link exercise to personally meaningful goals/events | X | ||||||||||||||||||||||
| Decisional balance patient centred goal setting | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||
| Supports attempts to change behaviour | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||
| Normalise failed attempts | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||
| Problem solving | X | X | X | X | X | X | X | X | X | X | X | X | X |
HBEP, home-based exercise programme; IP, inpatient; LT, liver transplant; THC, telephone health call; XtelecallXface-face visit
Intervention content
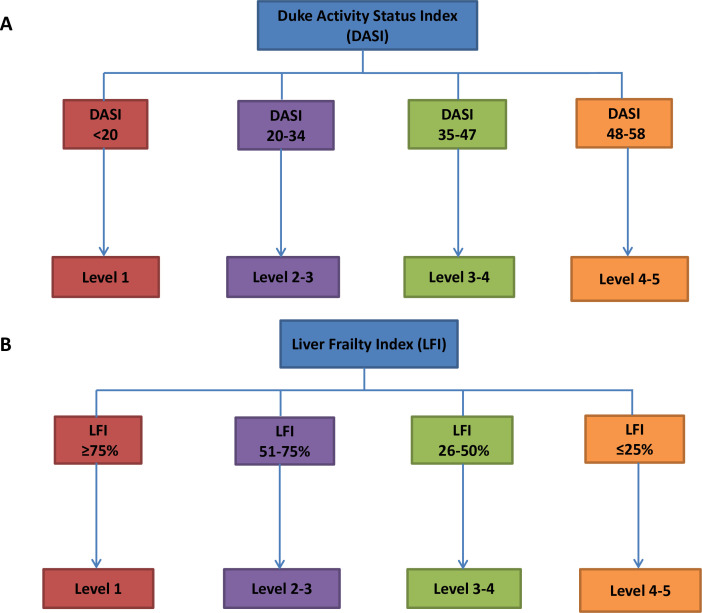
The HBEP will consist of a combination of aerobic and resistance exercise sessions. The initial level (duration, recovery period, intensity) of aerobic exercise sessions will be determined from the baseline DASI (figure 2A) while accounting for any exercise limiting comorbidities, such as ascites, peripheral oedema and/or hepatic encephalopathy. It will be recommended to the participants that they: (A) aim to complete three sessions of aerobic exercise per week; (B) select their exercise modality of choice per week (walking, cycling, swimming, cross-trainer, rowing ergo or running) and (C) alternate ‘work’ (moderate intensity, rate of perceived exertion (RoPE) score 12–14) and ‘active rest’ (RoPE 9–11) periods in each exercise session (table 3). In addition, participants will be asked to participate in a 20 min circuit of bodyweight resistance exercises twice weekly on alternate days to the aerobic sessions. The circuit will consist of four cycles of 8–12 repetitions of five exercises, chosen by the patient (table 4) with 2 min of ‘active rest’ (walking slowly on the spot) between each exercise and each cycle. The programme and entry level will be developed according to LFI (figure 2B), and a trial of 8–12 repetitions exercises within the designated entry level. The participant will be instructed to terminate each set of an exercise when they reach a ‘repetitions in reserve’ (RIR) of 1–2 (ie, they feel they could complete 1 or 2 additional repetitions, but no more). The participant will be advised to progress to each level of difficulty once they can achieve 12 repetitions with 1–2 RIR and depending on feedback from the Telecalls (table 4). A session template is summarised below:
Figure 2. The programme and entry level of HBEP. (A) The initial level (duration, recovery period, intensity) of aerobic exercise sessions was determined from the baseline DASI. (B) The resistance exercise sessions will be developed according to the LFI.
Table 3. Aerobic exercise component of HBEP (group 1).
| Level of difficulty | Exercise | Intensity | Duration (min) |
| 1 | Walking/cycling/swimming/cross-trainer/running | 1×5 min @ RoPE 12–14 | 13 |
| 1×3 min recovery @ RoPE 9–11 | |||
| 1×5 min @ RoPE 12–14 | |||
| 2 | Walking/cycling/swimming/cross-trainer/running | 1×7 min @ RoPE 12–14 | 17 |
| 1×3 min recovery @ RoPE 9–11 | |||
| 1×7 min @ RoPE 12–14 | |||
| 3 | Walking/cycling/swimming/cross-trainer/running | 1×10 min @ RoPE 12–14 | 23 |
| 1×3 min recovery @ RoPE 9–11 | |||
| 1×10 min @ RoPE 12–14 | |||
| 4 | Walking/cycling/swimming/cross-trainer/running | 1×12 min @ RoPE 12–14 | 27 |
| 1×3 min recovery @ RoPE 9–11 | |||
| 1×12 min @ RoPE 12–14 | |||
| 5 | Walking/cycling/swimming/cross-trainer/running | 1×10 min @ RoPE 12–14 | 33 |
| 1×3 min recovery @ RoPE 9–11 | |||
| 1×10 min @ RoPE 12–14 | |||
| 1×3 min recovery @ RoPE 9–11 | |||
| 1×10 min @ RoPE 12–14 | |||
| 6 | Walking/cycling/swimming/cross-trainer/running | 1×15 min @ RoPE 12–14 | 33 |
| 1×3 min recovery @ RoPE 9–11 | |||
| 1×15 min @ RoPE 12–14 | |||
| 7 | Walking/cycling/swimming/cross-trainer/running | 1×20 min @ RoPE 12–14 | 33 |
| 1×3 min recovery @ RoPE 9–11 | |||
| 1×10 min @ RoPE 12–14 | |||
| 8 | Walking/cycling/swimming/cross-trainer/running | 1×30 min @ RoPE 12–14 | 30 |
| 9 | Walking/cycling/swimming/cross-trainer/running | 1×35 min @ RoPE 12–14 | 35 |
HBEPhome-based exercise and theory-based motivation support programmeRoPErate of perceived exertion (range 6–20)
Table 4. Resistance exercise component of HBEP (group 1).
| Muscle group | Exercise | 1 | 2 | 3 | 4 | 5 | 6 |
| Upper limb press | Horizontal press | Wall press-up | Press-up on knees | Hands-elevated press-up | Progressively lower hands-elevated press-up | Press-up | Press-up with progressive band resistance |
| Vertical press | Overhead press, arms only | Overhead press with light weight (eg, soup cans) | Overhead press with heavier weight (eg, water bottles) | Pike push-up, hands on raised surface | Pike push up | Pike push-up, feet elevated to knee height | |
| Upper limb pull | Horizontal pull | Two-arm row with light weight (eg, soup can) | One-arm row with light weight (eg, soup cans) | Two-arm row with heavier weight (eg, water bottles) | One-arm row with heavier weight (eg, water bottles) | Two-arm row with progressive band resistance | One-arm row with progressive band resistance |
| Lateral/vertical pull | Lateral rotation with yellow TB | Bilateral abduction with TB | Diagonal TB pull | Vertical pull down with yellow TB | Vertical pull down with red TB | Vertical pull down with green TB | |
| Lower limb | Squat | Raised surface chair stands | Wall squat | Chair stands | Full squat | Squat with light weight (eg, soup cans) | Squat with progressive band resistance |
| Lunge | Static lunge with support | Static lunge without support | Dynamic half lunge | Dynamic full lunge | Walking lunge | Walking lunge with progressive load | |
| Step ups | Low step-up (eg, 1 stair) | Low step-up with knee raise | Low step-up with knee raise and light weight (eg, soup cans) | High step-up with high knee | High step-up with high knee and light weight (eg, soup cans) | High weighted step up with high knee and progressive load | |
| Core stability | Anti-anterior flexion | Four-point kneeling holds | Four-point kneeling with leg raises | Four point kneeling alternate arm and leg raises | Kneeling plank | Plank | Plank with progressive load |
| Glute med/anti-lateral flexion | Clams | Clams heels raised | Straight leg clam | Elbows-elevated side plank | Elevated side plank | Side plank | |
| Extension | Pelvic tilt in crook lying | Bridges | Bridges with yellow TB | Bridges with red TB | Bridges with red TB and heel raise | Bridges with red TB straight leg reps |
HBEPhome-based exercise and theory-based motivation support programmeTBtheraband resistance bands
| No. of exercises | No of circuits | Repetitions | Rest period between circuits (min) | Total time (min) |
| 1×upper limb push | 4 | 8–12 | 2 | 26 |
| 1×upper limb pull | ||||
| 2×lower limb | ||||
| 1×core/balance |
Intervention personalisation
After obtaining consent and completion of baseline assessments, participants will meet the study physiotherapist at visit 1. The baseline assessments (DASI and LFI), along with Empowering Physio strategies to increase feelings of autonomy, competence and relatedness towards exercise, will be used to design a personalised, written HBEP for the participant. In addition, to using the DASI and LFI, the entry level of difficulty for the exercises will be influenced by discussions with the participant in regard to benefits and consequences for the patient in terms of optimising fitness/physical capacity and exercise adoption. The participants will then attend an individualised physiotherapist-delivered training session, which will consist of (a) patient education (benefits, pacing, breathless management, RoPE, nutrition (pre/post exercise); (b) exercise familiarisation and (c) issuing of devices (wrist accelerometer) and written information (written HBEP, participant diary) (table 2). At each trial visit (pre-LT visits 2–6, post-LT visits 7 and 8), the results of the physical frailty assessments (DASI, LFI), review of the participant exercise diary and discussions with the participant themselves will be used to progress exercises and revise goals of their HBEP. As per Empowering Physio principles, the active role of the participant in the decision-making process, regarding progression and goal revision, will aim to support more autonomous reasons for engagement in the HBEP.
Remote monitoring of the intervention
In addition to face-to-face trial visits, participants will receive a 15–30 min telephone health call (‘Telecall’) from the trial physiotherapist every 2–4 weeks pre-LT and post-LT (table 2). The purpose of these calls will be to identify any adverse events (AEs) or areas of concern, receive feedback regarding the HBEP, provide motivational support, empower the patient and guide progression of HBEP. Post-LT, the Empowering Physio delivery will shift to employ strategies for the participant to foster autonomous motivation and maintenance of exercise behaviour.
Group 2: patient exercise advice leaflet (control group)
Participants will receive a standardised patient exercise advice leaflet, which includes standard written advice on physical activity and exercise before and after LT (online supplemental figure). Following baseline assessment, participants will receive a 20 min face-to-face consultation with the physiotherapist at visit 1. During the consultation, they will receive written (patient leaflet) and verbal advice on the generic benefits of exercise pre-LT, alongside the physiotherapist demonstrating four standard resistance exercise that would be safe to complete at home. Post-LT, participants will receive standard physiotherapy input during the admission, and prior to discharge home, have a 30 min inpatient consultation with the physiotherapist. During this consultation, they will be provided with the post-LT patient exercise advice leaflet and advised to gradually increase their exercise post-LT.
Outcome measures
Primary outcome measure
The primary outcome measure will be the PCS from the SF-36v2 health-related QoL questionnaire at 24 weeks post-LT.
Secondary outcome measures
The key secondary outcome measure will be the Comprehensive Complication Index (CCI), assessed at 24 weeks post-LT. The other secondary outcome measures that will be assessed at 24 weeks post-LT (unless stated) include (1) Mental Component Score (MCS) of SF-36v2 health-related QoL questionnaire; (2) physical frailty evaluation with the LFI and DASI; (3) pre-LT morbidity evaluation with UKELD, MELD-Na, frequency/duration of hospital admissions and mortality (*assessed up to day of LT); (4) post-LT length of ICU/hospital stay and hospital readmissions (frequency, duration (days)); (5) post-LT 30, 90, 180 and 365 days mortality; (6) habitual daily physical activity levels; (7) perceptions of need support provided by the physiotherapist (Health Care Climate Questionnaire (HCCQ)); (8) feelings of competence, autonomy and relatedness in regard to engagement in the exercise programme (Basic Psychological Need Satisfaction in Exercise Scale (PNSE)) and (9) motivation to engage in exercise (Behavioural Regulation in Exercise Questionaire-2 (BREQ-2).
Analytical methods
QoL assessment
QoL will be assessed by the SF‐36v2 health-related QOL questionnaire (QualityMetric Health Outcomes Solutions, Lincoln, USA). The SF-36v2 questionnaire is a practical, reliable and valid measure of physical and mental health that can be completed in 5–10 min. It consists of 36 questions composed of eight multi‐item scales, which reflect the impact of health problems on both the physical and mental condition of the patient.29 30 A higher score reflects a better QoL. Two summary subscores will be calculated which are weighted combinations of the eight scales, one to reflect the impact on physical function (PCS; primary outcome measure) and one to reflect the impact on psychological function (MCS).14 Scoring of the SF-36v2 questionnaire will based on the instructions provided in the SF-36v2 user’s manual.31
Psychological (motivation-based) questionnaires
The HCCQ32 will be used to measure the patients’ perceptions of the degree to which they feel their interactions with their physiotherapist empowers them to engage in exercise during the HBEP (ie, healthcare climate created promoted feelings of competence, autonomy and relatedness). Patients will be asked to respond to each of the 15 items, indicating the extent to which they agreed with each statement, on a Likert scale ranging from 1 (strongly disagree) to 7 (strongly agree) and a mean composite score will be calculated for use in analysis. The PNSE will be used to examine participants’ basic psychological need satisfaction in relation to their engagement in exercise during the HBEP.33 The PNSE comprises of 18 items, capturing the three basic psychological needs of autonomy (6 items), competence (6 items) and relatedness (6 items). Participants will be asked to respond to each item, indicating the degree with which they agree with each statement, on a Likert scale from 1 (false) to 6 (true). The BREQ-234 will be used to measure the participants’ degree of self-determined motivation to engage in exercise during the HBEP. Participants will be asked to respond to 19 items assessing their intrinsic regulation (4 items), identified regulation (4 items), introjected regulation (3 items), external regulation (4 items) and amotivation (4 items) in respect to the exercise programme. Participants will be asked to rate their agreement with each statement on a 5-point Likert scale from 0 (not true for me) to 4 (very true for me). These psychological questionnaires will be used to assess the theory-based motivation support programme and to test the theoretically expected psychological mechanisms underlying engagement with and motivation to participate in the HBEP.
Physical frailty data
The LFI will be used to objectively assess physical frailty, as previously described in ambulatory patients with AdvCLD.35 It is a composite metric of hand grip strength (HGS), time to do five chair stands (seconds) and time holding three balance positions (feet side by side, semi-tandem and tandem). The LFI score (range 0.5–7.5) will be calculated using an on-line calculator (available at: http://liverfrailtyindex.ucsf.edu), with patient physical frailty categorised as robust, pre-frail and frail according to their index (index ≤3.2 (robust), 3.2–4.5 (prefrail), >4.5 (frail)). The DASI questionnaire will be used to assess functional exercise capacity. It is a 12-item self-reported assessment of functional capacity that requires 2 min to complete.36 It provides prognostic information in a variety of chronic diseases and can be used as an index of disease progression over time.37,39 Using the DASI Points (range 0–58.2), the VO2 peak (=0.43×DASI points+9.6 mL/kg) and metabolic equivalents (METs=VO2 peak/3.5) will be calculated.
Physical activity/exercise monitoring
Habitual (average daily) physical activity participation will be measured using a wrist-worn accelerometer (Actigraph GT9X; GT3XP-BTLE). Accelerometers will be initialised to ensure participants do not receive any feedback on their activity levels during their participation in the trial (ie, accelerometers are not part of the intervention). The accelerometers will be worn by the participants 24 hours/day for set 14-day periods, ensuring they will still be worn during their scheduled exercise. These 14-day time periods will be after visit 1 (baseline; 1–14 days) and prior to visit 2 (week 6), visit 3 (week 12), visit 4 (24 weeks), visit 7 (6 weeks post-LT), visit 8 (12 weeks post-LT) and visit 9 (24 weeks post-LT). Data captured during the 14-day periods will be analysed to assess the average acceleration (average daily volume of activity), intensity gradient (distribution of activity across intensities) and intensity-related activity patterns. The accelerometers will aid with assessing how much the participants engage with exercise, and their levels of overall physical activity, during the trial. Participants randomised to the HBEP (intervention arm) will also be provided with a paper ‘exercise’ diary to self-record all structured exercises undertaken during the trial. Using the diary, the study physiotherapist(s) will document how many structured HBEP sessions the participant had completed per week (maximum 5 per week) and will rate how adherent the participants have been with the HBEP (ie, classified as <33%, 33%–66% and >66% adherent).
Morbidity and mortality data
LT-related complications will be assessed using the CCI. CCI is a well-validated, reproducible tool in surgery and LT, which provides a 0–100 index (0=no complications, 100=death) using the frequency and grade (CTCAE grade) of surgical-related complications (note: all 26 types of complications listed in the case report form (CRF) will be used to calculate the CCI).6 40 The sample size will enable an accurate, representative comparison of the intervention and control arms 24 weeks post-LT to investigate if the HBEP significantly reduces surgical complications post-LT.
Pre-LT morbidity will be assessed by calculating the UKELD and MELD-Na scores, alongside the frequency/duration of non-elective hospital admissions and mortality of the LT waiting list. UKELD41 will be calculated using: UKELD=((5.395Xln(INR))+(1.485Xln(creatinine))+(3.13Xln(bilirubin))−(81.565Xln(Na)))+435. Similarly, MELD-Na42 will be calculated using: MELD-Na=MELD Score-Na–(0.025×MELD×(140-Na))+140.
Post-LT morbidity will be assessed by length of ICU stay (hours), length of hospital stay (days; immediately post-LT) and frequency/duration of hospital readmission (LT to 24 week, V9). Readmission to ICU on the same hospital admission for LT will be included, recorded and added to the total ICU length of stay (hours). In the rare event that a patient is transferred to their local non-LT hospital or an inpatient rehabilitation unit, these bed days will be included in the hospital length of stay. The date and cause of death will be documented using the death certificate for reference. The 30-day, 90-day, 180-day and 1-year mortality rates will be calculated.
Clinical and laboratory data
Patient demographics and clinical history/examination will be recorded, including; disease aetiology, comorbidities (ie, diabetes, mental health illness), complications of AdvCLD (ie, encephalopathy, portal hypertension, HCC), drug history/nutritional supplements and social history (ie, smoking, employment status). Data regarding LT will be collected including: LT indication; timing of LT listing, LT date, donor details (donor after brain death/donor after cardiac death, machine perfusion, age), need for organ support and immunosuppression regimen.
Measurements of ‘wet’ weight, height, systolic/diastolic blood pressure, resting heart rate and oxygen saturations on room air, dominant HGS and mid-arm muscle circumference will be recorded. ‘Wet’ body mass index (BMI) will be defined as ‘wet’ weight in kilograms (kg) divided by the square of the height in metres(kg/m2). The ‘dry’ weight (kg) will be estimated using the 5%/10%/15% reduction rule for mild/moderate/severe ascites and 5% for peripheral oedema .
Non-fasting blood samples will be analysed for full blood count, urea, creatinine and electrolytes, liver function tests, international normalised ratio, C reactive protein, nutrition markers (incuding vitamin D) and ammonia. In addition to UKELD and MELD-Na, Childs-Pugh score (5–15; A–C) will be calculated at the pre-LT visits.
Statistical justification and outcome analysis
Sample size justification
The mean PCS of the SF-36v2 health-related survey for patients with AdvCLD or on the LT waiting list is approximately 39–42, with an SD ranging from 8 to 24.1443,45 LT alone has been reported to improve PCS by +4 points (≈10%) compared with pre-LT. In contrast, small studies post-LT have highlighted that basic, supervised exercise interventions improve the PCS by +8–9 points (≈20%). However, no studies to date have incorporated a combined pre-LT and post-LT exercise programme, with the addition of theory-based motivational support.
For the sample size calculation of the EXALT trial, a +4 point (10%) improvement in the control arm and +12 point (30%) improvement in the intervention arm is proposed. Therefore, a meaningful clinically important difference (MCID) of 8 points with an SD of 16 is used. In order to detect an MCID of 8 points with SD of 16, 90% power and a type I error rate of 5%, using the standard method of difference in means (two sided), a total of 172 participants is required. NHS data report that approximately 30%–35% of patients on the waiting list for LT will not have their transplant within 1 year of randomisation (NHSBT database 2015–2020). Therefore, the sample size be inflated to account for this and other possible withdrawals. Adjusting for a 35% attrition/drop-out rate, the recruitment target is inflated from 172 (86 per group) to 266 participants (133 per group).
Analysis of primary outcome measures
The primary clinical question of interest is ‘Does HBEP (intervention) improve QoL (as measured using the PCS from the SF-36v2 health-related QoL questionnaire) at 24 weeks post-LT, in patients who underwent LT’. The population-level summary for the primary estimand is the difference in mean PCS scores between the groups at 24 weeks post-LT. The primary estimand population will, therefore, be only those patients with LT within 52 (+2) weeks of randomisation, that is, similar to ‘modified ITT’ analysis population which will exclude any patients who have not had an LT within 52 (+2) weeks of randomisation. The possible intercurrent events within the primary estimand population are those patients who drop-out (die/withdraw/lost to follow-up) before 24-week post-LT or those patients who do not adhere to the allocated intervention post-LT. To handle these intercurrent events, the chosen estimand strategy is treatment policy which is similar to doing an analysis as per the ‘ITT principle’ within the primary estimand population. The estimator proposed for the analysis of the primary estimand is mixed model for repeated measurements which will use all available data post-LT. Parameters allowing for participant, intervention arm, baseline score, time and the randomisation minimisation variables will be included (all as fixed effects). Time will be assumed to be a categorical (fixed) variable. To allow for a varying treatment effect over time, a time-by-treatment interaction parameter will be included in the model. As sensitivity analysis, a complier average causal effect analysis for the primary outcome only will also be carried out.
Patients who are randomised but do not end up having an LT within 52 (+2) weeks of being randomised will be initially excluded from the analysis detailed above. However, to ensure we account for all randomised patients, we will undertake a secondary analysis which will include all randomised patients, whether or not they received an LT. For this analysis, we will only include data for the PCS of SF36v2 collected at baseline and for the pre-LT time points (excluding any data collected post-LT). Given patients will have an LT at different time points, the time to LT between groups will be analysed. Patients who do not have an LT within 52 (+2) weeks postrandomisation will be censored at 52 (+2) weeks. A Cox proportional hazard model will be fitted on the time to LT data, and results will be expressed as the HR with 95% CIs. Kaplan-Meier curves will be constructed for visual presentation of time-to-event comparisons. As a further analysis, we will explore the time to LT and pre-LT SF36v2 data using a joint model approach, where we will jointly model the time to event (ie, LT) data with the longitudinal PCS data collected at pre-LT time points for all randomised patients. The results from these analyses will be interpreted alongside the findings from primary estimand.
Planned subgroup analyses
Analyses would have been limited to the same variables used in the minimisation algorithm except for the parameter ‘enrolled in the ‘muscle’ substudy (yes, no)’. The effects of these subgroups will be examined by including a treatment group by subgroup interaction parameter in the model. To allow for the possibility of differential changes over time within the different subgroups, time by subgroup and the three-way interaction between treatment, time and subgroup will also be included in the model. Differences between treatment groups within subgroups and over time will be generated by producing differences between groups through the model that includes the relevant interaction parameter. The subgroup analysis results will be presented for main primary outcome time point (ie, 24 weeks post-LT). The results of subgroup analysis will be treated with caution and will be used for the purposes of hypothesis generation only.
Missing data
Any missing data for PCS from SF36v2 at post-LT time points, at which SF36v2 is meant to be completed, will be imputed using multiple imputation with chained equations assuming that data will be missing not at random.
Planned final analysis
The final analysis for the trial will occur once: (A) The last randomised patient has had their LT and their 24 weeks follow-up assessment post-LT completed or (B) When the last randomised patient has not had their LT within 1 year (52+2 weeks) of being randomised. All corresponding outcome data must have been entered onto the trial database and validated as being ready prior to the final analysis.
Serious AE reporting and analysis
The recording and reporting of AEs will be in accordance with the UK Policy Framework for Health and Social Care Research, the Principles of Good Clinical Practice (GCP) as set out in the UK statutory instrument (2004/1031; and subsequent amendments) and the requirements of the Health Research Authority (HRA). The EXALT trial population will by definition have a life-threatening AdvCLD that requires major curative LT surgery, and as such, there is expected to be a high number of AEs in these patients. For this reason, as well as the low-risk nature of the trial intervention (ie, home-based exercise) to study participants, only serious AEs (SAEs) will be reported.
The reporting period for SAEs in EXALT will be from the day of randomisation (baseline visit 1) until the end of trial follow-up (365 days post-LT; visit 10). The safety profile for this trial population and interventions will be well characterised (predefined list of pre-LT and post-LT ‘expected’ SAEs, for example, encephalopathy requiring admission, acute LT graft rejection), to ensure a strategy of targeted reporting of SAEs will not affect the safety of the participants. Examples of SAEs that will require expedited reporting to the EXALT trial office within 24 hours include death, retransplantation and multiorgan failure requiring ICU support.
The BCTU will keep detailed records of all SAEs reported (nature, onset, duration, severity, outcome) and the chief investigator or delegate(s) will perform an evaluation with respect to seriousness, causality and expectedness. Interim analysis of safety data will be performed and presented to the independent data management committee (DMC) on a 6-monthly basis. The unblinded DMC will advise accordingly with regard to participant safety and specifically whether extra/new data monitoring will be required for the remainder of the EXALT trial. The DMC will operate in accordance with a trial-specific charter based on the template created by the Damocles Group. In addition, the EXALT trials office will report all events categorised as ‘unexpected’ and ‘related’ SAEs with the trial intervention to the NREC and the University of Birmingham’s Research Governance Team within 15 days of being notified.
Mechanistic muscle substudy (optional; n=100)
The main aim of the optional substudy is to undertake a detailed evaluation of the biological and physiological mechanisms that may underlie any HBEP-induced improvements in clinical outcomes, including QoL and physical function/frailty. A better understanding of how exercise works on cardiopulmonary fitness (CPET, 6MWT), muscle biology (muscle quadricep ultrasound, blood biomarkers) and their association with QoL (SF-36v2), will help guide and inform the exercise dose (‘frequency’, ‘intensity’, ‘duration’) required in future studies, to produce improvements in clinical outcomes and to maximise the efficiency and longevity of LT among patients with AdvCLD. The mechanistic substudy will aim to recruit 100 participants and will take place at 3 time points: pre-LT visit 1 (baseline, week 0), pre-LT visit 2 (week 6) and at the post-LT visit 9 (24 weeks post-LT; end of intervention). In the event that a participant is not transplanted by visit 6 (pre-LT phase 1) of the study intervention, with the participant’s ongoing willingness to continue in the study, they will be given the option of a final substudy visit (visit 6 (+6-week window); inclusive of CPET, muscle ultrasound, 6MWT) and their data will be collected until the main EXALT trial end date. See online supplemental methods for more information on the ‘mechanistic muscle’ substudy.
Storage of trial samples
Trial samples (blood) will be only stored at the University of Birmingham (NIHR BRC Immunology and infection laboratory) for participants who consent to the ‘mechanistic muscle’ substudy. Non-fasted blood (approximately 21 mL) will be collected, centrifuged, processed and stored at −80°C at the study site (if the site is outside Birmingham) before being transferred in batches to the University of Birmingham; where samples will be stored at minus 80°C prior to batch analysis. All plasma and serum samples will be labelled with unique Trial ID, site, date, visit number and patient initials. Batch analysis will take place for measures of oxidative stress, antioxidant capacity, myokines and inflammatory markers as listed in online supplemental methods. Any samples remaining at the end of the study will either be destroyed in accordance with laboratory procedures or if the patient has given consent for samples to be used in future research, remaining samples will be transferred to a licensed biobank for use in other ethically approved studies.
Data handling, quality assurance, record keeping and retention
Data management will be undertaken according to the standard operating procedures of the BCTU with regard to the Data Protection Act 2018 (plus subsequent amendments) and the International Conference on Harmonisation GCP (ICH GCP). BCTU will be responsible for monitoring the trial in accordance with the trial risk assessment/monitoring plan and providing reports to the trial steering committee, DMC and NREC (including notification of serious trial-related breaches). The trial will be registered with the Data Protection Act website (number Z6195856) at the University of Birmingham. Participants identifiable data will be shared only with the clinical team on a need-to-know basis to provide clinical care and to ensure safe and appropriate follow-up. All EXALT participants will provide specific written consent at trial entry to enable their data to be shared with the trial unit and other relevant, specified parties. On completion of the trial, data will be transferred to a secure archiving facility, where identifiable data will be held for a minimum of 10 years and then securely destroyed.
Case report forms
Trial sites will enter data directly onto the online, electronic CRFs in REDCap. The REDCap electronic database will include CRFs for eligibility criteria and randomisation; baseline/follow-up medical history, physical examinations (eg, ascites, encephalopathy) and key medications (ie, antidepressants, analgesia); clinical observations and physical frailty measures (eg, BMI, HGS, LFI); patient-reported outcomes measures (ie, DASI, SF-36v2; psychological questionnaires); laboratory tests; level of HBEP prescribed; LT data (ie, organ type) and SAE reporting. The CRFs will capture all of visits 1–10, day of LT and inpatient admission post-LT. Other CRFs will incorporate the ‘mechanistic muscle’ substudy and a change of trial status (ie, trial withdrawal, not transplanted with 52 weeks of randomisation). Missing and ambiguous data will be queried using a data clarification system in line with the EXALT data management plan.
Sponsorship, indemnity and monitoring
The University of Birmingham will be the sponsor of the trial. As sponsor the university will be responsible for the general conduct of the study and will indemnify the trial centre against any claims, arising from any negligent act or omission by the University in fulfilling the sponsor role in respect to the study. Both on-site and off-site monitoring of the trial will be performed as per the EXALT Trial Quality Management Plan. With respect to the conduct of the trial at site and other clinical care of the patient, responsibility for the care of the patients will remain with the NHS organisation (QEUHB or RFH) responsible for the clinical site and will, therefore, be indemnified through the NHS Litigation Authority.
Trial status
Recruitment into EXALT trial started in April 2022 and is due to end in June 2024, with 217/266 (82%) patients randomised from the two trial sites to date. The recruitment and intervention follow-up of EXALT participants is currently ongoing with the last trial visit (V10) due to take place in May 2026.
Discussion
Compliance with the trial protocol and safety profile of the HBEP will be reviewed on a biannual basis by an independent DMC, and to date (January 2024) no concerns have been raised.
Challenges in trial design
Study population
Previous studies of exercise in AdvCLD have mainly focused on patients with compensated (Childs-pugh A) cirrhosis, as those with AdvCLD and awaiting LT have been deemed ‘too sick’ to participate.21 AdvCLD is a multisystem disorder leading to physical frailty (muscle wasting, weakness, poor functional status, dependence of activities of daily living), cirrhotic cardiomyopathy, malnutrition, ascites, encephalopathy, anaemia and impaired pulmonary gas exchange; all of which limit a patient’s ability to exercise. Indeed, patients awaiting LT are some of the sickest and frailest patients in healthcare systems, to the extent that a 57-year old with AdvCLD has the predicted physical frailty of >80 years in the community.35 Therefore, safety and efficacy of exercise data is lacking in this patient population.
In the EXALT trial, patients with polycystic liver disease (ie, ‘variant’ listing criteria), rare metabolic/genetic conditions (ie, glycogen storage disorder) and non-cirrhotic liver cancer will be excluded due to the fact that they have a different pathophysiology (vs cirrhosis) and their average UK waiting times for LT exceed 1 year; thereby resulting in a high intervention withdrawal rate (ie, cut-off=52+2 weeks postrandomisation). Similarly, due to long LT waiting times and different surgical risk profiles (vs primary liver graft) patients awaiting multiorgan transplantation and regraft LT will be excluded. Patients with severe hepatic encephalopathy (ie, grade 3+) and hepatopulmonary syndrome will be also excluded due to an absence of any published exercise data, safety/compliance concerns and significant oxygen requirements, respectively.
To avoid selection bias, potential confounding factors such as age, gender, disease severity (UKELD) and trial site will be classified as minimisation variables at randomisation, to ensure a similar representation of patient demographics and clinical features in the intervention (group 1) and control arms (group 2).
Justification of primary outcome
The SF-36v2 (PCS) questionnaire is a validated, robust, reproducible patient-reported outcomes tool for assessing QoL in solid-organ transplantation and AdvCLD.1416 44,48 Consistent feedback from our pilot studies, patients and public involvement (PPI) groups and EXALT PPI team members (CP and KR) highlighted that patients felt that the QoL questionnaire accurately captures the whole LT experience from being on the waiting list through to the LT and the recovery afterwards. Fundamentally to the patients, their families and friends, QoL is the most important outcome to them in life (ie, in their own words ‘there is no point prolonging life with transplantation, if your QoL is not worth living afterwards’). The vast majority of patients undergoing LT are of working employment age with young families. If, however, they fail to recover their functional independence and physical activity levels post-LT,49 it has deleterious effects on their self-motivation, mental/physical health, ability to work, finances and family commitments. A low PCS, rather than MCS, of SF-36v2 has previously been associated with low survival, employment and functional status in our patient population.14 16 During trial design, it was deemed to be unfeasible (huge sample size, trial costs and duration) to perform a randomised controlled trial with post-LT survival (6–12 months) as the primary end-point, because post-LT 1-year survival rates consistently average >90% in the UK. For these reasons, SF-36v2 PCS was judged to be the best evidence-based outcome measure to investigate the efficacy of HBEP in patients before and after LT.
Trial intervention (safety and adherence)
Previous small, heterogeneous studies have highlighted that combined aerobic and resistance exercises yield the most promising improvements in aerobic capacity, muscle mass, strength, physical function and/or QoL.21 44 48 In addition, 6–12 weeks home-based interventions pre-LT have been reported as safe, feasible and cost-effective (ie, no travel time/expense) in patients with cirrhosis, but adherence and long-term efficacy remain a challenge.25 26 It was, therefore, essential that the EXALT trial focused on the patients’ motivation to engage and psychobehavioural barriers to exercise, in order to optimise exercise interventions delivered at home. Prior to EXALT, no studies to date have combined home-based exercises with motivational-behavioural strategies to increase engagement with and motivation to participate in exercise. In parallel to a remotely monitored, physiotherapy-delivered HBEP (consisting of combined aerobic and resistance exercise tailored to the patient’s physical fitness/morbidity), a bespoke Empowering Physio programme was developed previous to this trial (JLD) and used in EXALT to equip physiotherapists with the understanding of optimal motivation and skills to support each patient’s sense of autonomy, competence and relatedness in delivering the HBEP. By fostering more autonomous motivation towards the HBEP, the EXALT trial aims to maximise engagement with and motivation to participate in exercise overall in patients with AdvCLD before and after LT.
To ensure consistency of the physiotherapy-led HBEP (including motivational support) across the two sites, all study physiotherapists received a formal, interactive 3-day training course. In addition to frailty assessment and practical exercises, strategies and practical application of ‘Empowering Physio’ will be presented and discussed, including the opportunity to role-play (online supplemental methods). To test the fidelity of the physiotherapist-delivered HBEP, and in particular, that delivery was in accordance with the Empowering Physio programme and its underlying principles, videos of the physiotherapist-to-patient interactions will be undertaken and evaluated at various time points.
In the UK National Health Care (NHS) system, advice about exercise is recognised as ‘best practice’, with specific emphasis on the importance of communicating the benefits of exercise to patients (eg, ‘Moving Medicine’—Public Health England and Faculty of Sport and Exercise Medicine). Despite this, there remain no UK-wide standardised exercise advice leaflets for LT recipients. Therefore, in order to minimise any potential variation in standard exercise advice between the two trial sites, a trial-specific ‘generic’ patient exercise advice leaflet was developed by PPI members and the trial management group and will be used throughout the EXALT trial for the control arm at both hospitals (online supplemental figure).
Summary
To the best of our knowledge, the EXALT trial is the first RCT designed to investigate whether a pre-LT and post-LT physiotherapy-led, individualised home-based exercise and motivation programme in patients AdvCLD improves QoL after LT. The enrolment of the required sample size is due in June 2024 and the final results are expected by mid-2026. The full EXALT protocol (V.4.0) can be obtained from the EXALT trials website: https://www.birmingham.ac.uk/research/bctu/trials/portfolio-v/exalt/index.aspx.
supplementary material
Acknowledgements
The EXALT trial represents independent academic research funded by the National Institute of Health Research (NIHR) Efficacy and Mechanism Evaluation Programme (Ref: NIHR129318; awarded to MJA), University Hospital Birmingham (UHB) Charity and NIHR Birmingham Biomedical Research Centre (BRC). The EXALT trial team would like to express its gratitude to the patients enrolled into the trial and the Data Management Committee (DMC) consisting of Dr Ian Rowe (DMC Chair; independent liver/transplant medicine expert), Professor Trish Hepburn (Independent Senior Statistician) and Professor Stephen Wigmore (Independent transplant surgeon expert) for their time and input. In addition, EXALT trial team would like to thank the Trial Steering Committee (TSF) consisting of Professor Denny Levett (TSC Chair; Independent Critical Care/perioperative medicine expert), Dr Tasneem Pirani (Independent Critical Care/Liver medicine expert), Dr Kate Hallsworth (Independent Senior Physiotherapy expert), Dr Neil Corrigan (Senior Statistician) and Mark Lamond (Patient and public involvement representative). The EXALT trial team would also like to thank the nursing and administrative support of the NIHR Birmingham BRC, Wellcome Trust Clinical Research Facility (Birmingham), Royal Free Anaesthetics Department (London) and West Midlands Clinical Research Network (CRN).
The views expressed are those of the authors and not those of the NHS, the NIHR or the Department of Health.
Footnotes
Funding: The trial is funded by the National Institute of Health Research (NIHR) Efficacy and Mechanism Evaluation Programme (Ref: NIHR129318; awarded to MJA), UHB Charity and the NIHR Birmingham Biomedical Research Centre (BRC).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and was approved by South Central-Hampshire A National Research Ethics Committee (IRAS ID: 295426). Participants gave informed consent to participate in the study before taking part.
Collaborators: EXALT Trial Team:Nicholas Adams (NIHR Birmingham Biomedical Research Centre, University Hospital Birmingham NHS Foundation Trust and University of Birmingham, Birmingham, UK); Matthew J. Armstrong (Liver Transplant Unit, Queen Elizabeth University Hospitals Birmingham, Mindelsohn Way, Birmingham, B15 2TH, UK, NIHR Birmingham Biomedical Research Centre, University Hospital Birmingham NHS Foundation Trust and University of Birmingham, Birmingham, UK); Sharon Augustt (Hepatobiliary and Liver transplant Anaesthesia Department, Royal Free Hospital, London, UK); Shahida Begum (NIHR Birmingham Biomedical Research Centre, University Hospital Birmingham NHS Foundation Trust and University of Birmingham, Birmingham, UK); Liam Botfield (NIHR Birmingham Biomedical Research Centre, University Hospital Birmingham NHS Foundation Trust and University of Birmingham, Birmingham, UK); Dawn Brant (Birmingham Clinical Trials Unit (BCTU), University of Birmingham, Birmingham, UK) Brocklehurst Peter (Birmingham Clinical Trials Unit (BCTU), University of Birmingham, Birmingham, UK); Peter Brocklehurst (Birmingham Clinical Trials Unit (BCTU), University of Birmingham, Birmingham, UK); Emily Clibbens (Hepatobiliary and Liver transplant Anaesthesia Department, Royal Free Hospital, London, UK); Nigel Cope (Hepatobiliary and Liver transplant Anaesthesia Department, Royal Free Hospital, London, UK); Joan L. Duda (School of Sports, Exercise and Rehabilitation Sciences, University of Birmingham, Birmingham, UK) Fenton Sally AM (School of Sports, Exercise and Rehabilitation Sciences, University of Birmingham, Birmingham, UK); Sally A.M. Fenton (School of Sports, Exercise and Rehabilitation Sciences, University of Birmingham, Birmingham, UK); Alice Freer (Liver Transplant Unit, Queen Elizabeth University Hospitals Birmingham, Mindelsohn Way, Birmingham, B15 2TH, UK, NIHR Birmingham Biomedical Research Centre, University Hospital Birmingham NHS Foundation Trust and University of Birmingham, Birmingham, UK, Therapies Department, Queen Elizabeth University Hospitals Birmingham, Mindelsohn Way, Birmingham, UK); David J. Garside (Birmingham Clinical Trials Unit (BCTU), University of Birmingham, Birmingham, UK); Francesca Gowing (Hepatobiliary and Liver transplant Anaesthesia Department, Royal Free Hospital, London, UK); Ashlea Hargreaves (Liver Transplant Unit, Queen Elizabeth University Hospitals Birmingham, Mindelsohn Way, Birmingham, B15 2TH, UK, NIHR Birmingham Biomedical Research Centre, University Hospital Birmingham NHS Foundation Trust and University of Birmingham, Birmingham, UK, Therapies Department, Queen Elizabeth University Hospitals Birmingham, Mindelsohn Way, Birmingham, UK); William Leach (NIHR Birmingham Biomedical Research Centre, University Hospital Birmingham NHS Foundation Trust and University of Birmingham, Birmingham, UK); Daniel S. Martin (Peninsula Medical School, University of Plymouth, Plymouth, UK); Mehta Samir (Birmingham Clinical Trials Unit (BCTU), University of Birmingham, Birmingham, UK); Samir Mehta (Birmingham Clinical Trials Unit (BCTU), University of Birmingham, Birmingham, UK); Clare Melikian (Hepatobiliary and Liver transplant Anaesthesia Department, Royal Free Hospital, London, UK); Don Milliken (Hepatobiliary Surgery Anaesthesia Department, Royal Marsden Hospital, London, UK); Sonia Murray (Liver Transplant Unit, Queen Elizabeth University Hospitals Birmingham, Mindelsohn Way, Birmingham, B15 2TH, UK, NIHR Birmingham Biomedical Research Centre, University Hospital Birmingham NHS Foundation Trust and University of Birmingham, Birmingham, UK, Therapies Department, Queen Elizabeth University Hospitals Birmingham, Mindelsohn Way, Birmingham, UK); Chiemelie Ngonadi (Liver Transplant Unit, Queen Elizabeth University Hospitals Birmingham, Mindelsohn Way, Birmingham, B15 2TH, UK, NIHR Birmingham Biomedical Research Centre, University Hospital Birmingham NHS Foundation Trust and University of Birmingham, Birmingham, UK); Wendy Osborne (NIHR Birmingham Biomedical Research Centre, University Hospital Birmingham NHS Foundation Trust and University of Birmingham, Birmingham, UK); Christian Price (Patient and Public Involvement Group, NIHR BirminghamBiomedical Research Centre, Birmingham, UK); Karen Rockell (Patient and Public Involvement Group, NIHR Birmingham Biomedical Research Centre, Birmingham, UK); Sukhwant Sehmi (Birmingham Clinical Trials Unit (BCTU), University of Birmingham, Birmingham, UK); Gemma Slinn (Birmingham Clinical Trials Unit (BCTU), University of Birmingham, Birmingham, UK); Yongzhong Sun (Birmingham Clinical Trials Unit (BCTU), University of Birmingham, Birmingham, UK); Felicity R.Williams (Liver Transplant Unit, Queen Elizabeth University Hospitals Birmingham, Mindelsohn Way, Birmingham, B15 2TH, UK, NIHR Birmingham Biomedical Research Centre, University Hospital Birmingham NHS Foundation Trust and University of Birmingham, Birmingham, UK, School of Sports, Exercise and Rehabilitation Sciences, University of Birmingham, Birmingham, UK); Shu Xiaoyi.
Contributor Information
EXALT Trial Management Group:
Nicholas Adams, Shahida Begum, Liam Botfield, Alice Freer, David J Garside, Ashlea Hargreaves, William Leach, Sonia Murray, Wendy Osborne, Sukhwant Sehmi, Sharon Augustt, Emily Clibbens, Nigel Cope, Francesca Gowing, Shu Xiaoyi, Matthew J Armstrong, Dawn Brant, Peter Brocklehurst, Joan L Duda, Sally AM Fenton, Daniel S Martin, Samir Mehta, Clare Melikian, Chiemelie Ngonadi, Christian Price, Karen Rockell, Gemma Slinn, Yongzhong Sun, Felicity R Williams, and Don Milliken
EXALT Trial Management Group:
Chiemelie Ngonadi, Christian Price, Clare Melikian, Daniel S Martin, Dawn Brant, Don Milliken, Felicity R Williams, Gemma Slinn, Joan L Duda, Karen Rockell, Matthew J Armstrong, Peter Brocklehurst, Sally AM Fenton, Samir Mehta, and Yongzhong Sun
EXALT Trial Group:
Alice Freer, Ashlea Hargreaves, David J Garside, Emily Clibbens, Francesca Gowing, Liam Botfield, Nicholas Adams, Nigel Cope, Shahida Begum, Sharon Augustt, Sonia Murray, Sukhwant Sehmi, Wendy Osborne, and William Leach
EXALT Trial Team:
Nicholas Adams, Matthew J. Armstrong, Sharon Augustt, Shahida Begum, Liam Botfield, Dawn Brant, Peter Brocklehurst, Emily Clibbens, Nigel Cope, Joan L. Duda, Sally A.M. Fenton, Alice Freer, David J. Garside, Francesca Gowing, Ashlea Hargreaves, William Leach, Daniel S. Martin, Samir Mehta, Clare Melikian, Don Milliken, Sonia Murray, Chiemelie Ngonadi, Wendy Osborne, Christian Price, Karen Rockell, Sukhwant Sehmi, Gemma Slinn, Yongzhong Sun, Felicity R. Williams, and Shu Xiaoyi
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study at present.
References
- 1.Williams R, Alexander G, Aspinall R, et al. Gathering momentum for the way ahead: fifth report of the Lancet Standing Commission on Liver Disease in the UK. Lancet. 2018;392:2398–412. doi: 10.1016/S0140-6736(18)32561-3. [DOI] [PubMed] [Google Scholar]
- 2.Data from Rhiannon Taylor; NHSBT senior statistician. 2019. https://www.odt.nhs.uk/statistics-and-reports/organ-specific-reports/ Available.
- 3.Dunn MA, Josbeno DA, Tevar AD, et al. Frailty as tested by gait speed is an independent risk factor for cirrhosis complications that require hospitalization. Am J Gastroenterol. 2016;111:1768–75. doi: 10.1038/ajg.2016.336. [DOI] [PubMed] [Google Scholar]
- 4.Sinclair M, Poltavskiy E, Dodge JL, et al. Frailty is independently associated with increased hospitalisation days in patients on the liver transplant waitlist. World J Gastroenterol. 2017;23:899–905. doi: 10.3748/wjg.v23.i5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tandon P, Tangri N, Thomas L, et al. A rapid bedside screen to predict unplanned hospitalization and death in outpatients with cirrhosis: a prospective evaluation of the clinical frailty scale. Am J Gastroenterol. 2016;111:1759–67. doi: 10.1038/ajg.2016.303. [DOI] [PubMed] [Google Scholar]
- 6.Muller X, Marcon F, Sapisochin G, et al. Defining benchmarks in liver transplantation: a multicenter outcome analysis determining best achievable results. Ann Surg. 2018;267:419–25. doi: 10.1097/SLA.0000000000002477. [DOI] [PubMed] [Google Scholar]
- 7.Lai JC, Feng S, Terrault NA, et al. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant. 2014;14:1870–9. doi: 10.1111/ajt.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ney M, Haykowsky MJ, Vandermeer B, et al. Systematic review: pre- and post-operative prognostic value of cardiopulmonary exercise testing in liver transplant candidates. Aliment Pharmacol Ther. 2016;44:796–806. doi: 10.1111/apt.13771. [DOI] [PubMed] [Google Scholar]
- 9.Orman ES, Ghabril M, Chalasani N. Poor performance status is associated with increased mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2016;14:1189–95. doi: 10.1016/j.cgh.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams FR, Quinlan J, Freer A, et al. Duke activity status index and liver frailty index predict mortality in ambulatory patients with advanced chronic liver disease: a prospective, observational study. Aliment Pharmacol Ther. 2024;59:547–57. doi: 10.1111/apt.17834. [DOI] [PubMed] [Google Scholar]
- 11.Painter P, Krasnoff J, Paul SM, et al. Physical activity and health-related quality of life in liver transplant recipients. Liver Transpl. 2001;7:213–9. doi: 10.1053/jlts.2001.22184. [DOI] [PubMed] [Google Scholar]
- 12.Tapper EB, Baki J, Parikh ND, et al. Psychoactive medications, and cognitive dysfunction are associated with poor patient-reported outcomes in cirrhosis. Hepatology. 2019;69:1676–85. doi: 10.1002/hep.30336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Derck JE, Thelen AE, Cron DC, et al. Quality of life in liver transplant candidates: frailty is a better indicator than severity of liver disease. Transplantation. 2015;99:340–4. doi: 10.1097/TP.0000000000000593. [DOI] [PubMed] [Google Scholar]
- 14.Macdonald S, Jepsen P, Alrubaiy L, et al. Quality of life measures predict mortality in patients with cirrhosis and severe ascites. Aliment Pharmacol Ther. 2019;49:321–30. doi: 10.1111/apt.15084. [DOI] [PubMed] [Google Scholar]
- 15.Bownik H, Saab S. Health-related quality of life after liver transplantation for adult recipients. Liver Transpl. 2009;15 Suppl 2:S42–9. doi: 10.1002/lt.21911. [DOI] [PubMed] [Google Scholar]
- 16.Saab S, Wiese C, Ibrahim AB, et al. Employment and quality of life in liver transplant recipients. Liver Transpl. 2007;13:1330–8. doi: 10.1002/lt.21247. [DOI] [PubMed] [Google Scholar]
- 17.Mathur S, Janaudis-Ferreira T, Wickerson L, et al. Meeting report: consensus recommendations for a research agenda in exercise in solid organ transplantation. Am J Transplant. 2014;14:2235–45. doi: 10.1111/ajt.12874. [DOI] [PubMed] [Google Scholar]
- 18.Debette-Gratien M, Tabouret T, Antonini M-T, et al. Personalized adapted physical activity before liver transplantation: acceptability and results. Transplantation. 2015;99:145–50. doi: 10.1097/TP.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 19.Román E, García-Galcerán C, Torrades T, et al. Effects of an exercise programme on functional capacity, body composition and risk of falls in patients with cirrhosis: a randomized clinical trial. PLoS One. 2016;11:e0151652. doi: 10.1371/journal.pone.0151652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zenith L, Meena N, Ramadi A, et al. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol. 2014;12:1920–6. doi: 10.1016/j.cgh.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Williams FR, Berzigotti A, Lord JM, et al. Review article: impact of exercise on physical frailty in patients with chronic liver disease. Aliment Pharmacol Ther. 2019;50:988–1000. doi: 10.1111/apt.15491. [DOI] [PubMed] [Google Scholar]
- 22.Jones M, Jolly K, Raftery J, et al. 'DNA' may not mean 'did not participate': a qualitative study of reasons for non-adherence at home- and centre-based cardiac rehabilitation. Fam Pract. 2007;24:343–57. doi: 10.1093/fampra/cmm021. [DOI] [PubMed] [Google Scholar]
- 23.Morkane CM, Kearney O, Bruce DA, et al. An outpatient hospital-based exercise training programme for patients with cirrhotic liver disease awaiting transplantation: a feasibility trial. Transplantation. 2020;104:97–103. doi: 10.1097/TP.0000000000002803. [DOI] [PubMed] [Google Scholar]
- 24.Webb GJ, Hodson J, Chauhan A, et al. Proximity to transplant center and outcome among liver transplant patients. Am J Transplant. 2019;19:208–20. doi: 10.1111/ajt.15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kruger C, McNeely ML, Bailey RJ, et al. Home exercise training improves exercise capacity in cirrhosis patients: role of exercise adherence. Sci Rep. 2018;8:99. doi: 10.1038/s41598-017-18320-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams FR, Vallance A, Faulkner T, et al. Home-based exercise in patients awaiting liver transplantation: a feasibility study. Liver Transpl. 2019;25:995–1006. doi: 10.1002/lt.25442. [DOI] [PubMed] [Google Scholar]
- 27.National Institute for Health and Care Excellence Behaviour change: general approaches. Report no.: public health guideline 6. 2013
- 28.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research Informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ware JE. SF-36 health survey update. Spine (Phila Pa 1986) 2000;25:3130–9. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 30.SF-36V2 health survey. 2009. http://www.qualitymetric.com/WhatWeDo/GenericHealthSurveys/SF36v2HealthSurvey Available.
- 31.Ware JE, Kosinski M, Dewey J. How to score version 2 of the SF-36 health survey. 2001.
- 32.Heissel A, Pietrek A, Rapp MA, et al. Perceived health care climate of older people attending an exercise program: validation of the German short version of the health care climate questionnaire. J Aging Phys Act. 2020;28:276–86. doi: 10.1123/japa.2018-0350. [DOI] [PubMed] [Google Scholar]
- 33.Leisterer S, Gramlich L. Having a positive relationship to physical activity: basic psychological need satisfaction and age as predictors for students' enjoyment in physical education. Sports (Basel) 2021;9:90. doi: 10.3390/sports9070090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Markland D, Tobin V. A modification of the behavioral regulation in exercise questionnaire to include an assessment of amotivation. J Sport Exerc Psychol. 2004;26:191–6. doi: 10.1123/jsep.26.2.191. [DOI] [Google Scholar]
- 35.Lai JC, Covinsky KE, Dodge JL, et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology . 2017;66:564–74. doi: 10.1002/hep.29219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke activity status index) Am J Cardiol. 1989;64:651–4. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 37.Carter R, Holiday DB, Grothues C, et al. Criterion validity of the Duke activity status index for assessing functional capacity in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2002;22:298–308. doi: 10.1097/00008483-200207000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Tang WHW, Topol EJ, Fan Y, et al. Prognostic value of estimated functional capacity incremental to cardiac biomarkers in stable cardiac patients. J Am Heart Assoc. 2014;3:e000960. doi: 10.1161/JAHA.114.000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu J-R, Lennie TA, Frazier SK, et al. Health-related quality of life, functional status, and cardiac event-free survival in patients with heart failure. J Cardiovasc Nurs. 2016;31:236–44. doi: 10.1097/JCN.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schlegel A, Linecker M, Kron P, et al. Risk assessment in high- and low-MELD liver transplantation. Am J Transplant. 2017;17:1050–63. doi: 10.1111/ajt.14065. [DOI] [PubMed] [Google Scholar]
- 41.Neuberger J, Gimson A, Davies M, et al. Selection of patients for liver transplantation and allocation of donated livers in the UK. Gut. 2008;57:252–7. doi: 10.1136/gut.2007.131730. [DOI] [PubMed] [Google Scholar]
- 42.Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–26. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sciurba F, Criner GJ, Lee SM, et al. Six-minute walk distance in chronic obstructive pulmonary disease: reproducibility and effect of walking course layout and length. Am J Respir Crit Care Med. 2003;167:1522–7. doi: 10.1164/rccm.200203-166OC. [DOI] [PubMed] [Google Scholar]
- 44.Krasnoff JB, Vintro AQ, Ascher NL, et al. A randomized trial of exercise and dietary counseling after liver transplantation. Am J Transplant. 2006;6:1896–905. doi: 10.1111/j.1600-6143.2006.01391.x. [DOI] [PubMed] [Google Scholar]
- 45.Kabar I, Hüsing-Kabar A, Maschmeier M, et al. Pictorial representation of illness and self measure (PRISM): a novel visual instrument to quantify suffering in liver cirrhosis patients and liver transplant recipients. Ann Transplant. 2018;23:674–80. doi: 10.12659/AOT.910278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanikella R, Kawut SM, Brown RS, Jr, et al. Health-related quality of life and survival in liver transplant candidates. Liver Transpl. 2010;16:238–45. doi: 10.1002/lt.21984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garratt A, Schmidt L, Mackintosh A, et al. Quality of life measurement: bibliographic study of patient assessed health outcome measures. BMJ. 2002;324:1417. doi: 10.1136/bmj.324.7351.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moya-Nájera D, Moya-Herraiz Á, Compte-Torrero L, et al. Combined resistance and endurance training at a moderate-to-high intensity improves physical condition and quality of life in liver transplant patients. Liver Transpl. 2017;23:1273–81. doi: 10.1002/lt.24827. [DOI] [PubMed] [Google Scholar]
- 49.Lai JC, Segev DL, McCulloch CE, et al. Physical frailty after liver transplantation. Am J Transplant. 2018;18:1986–94. doi: 10.1111/ajt.14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing not applicable as no datasets generated and/or analysed for this study at present.