Summary
Background
Chagas disease (CD), endemic in 21 Latin American countries, has gradually spread beyond its traditional borders due to migratory movements and emerging as a global health concern. We conducted a systematic review and meta-analysis of available data to establish updated prevalence estimates of CD in Latin American migrants residing in non-endemic countries.
Methods
A systematic search was conducted in MEDLINE/PubMed, Embase, Cochrane Library, Scopus, Web of Science, and LILACS via Virtual Health Library (Biblioteca Virtual em Saúde - BVS), including references published until November 1st, 2023. Pooled prevalence estimates and 95% confidence intervals (CI) were calculated using random effect models. Heterogeneity was assessed by the chi-square test and the I2 statistic. Subgroup analyses were performed to explore potential sources of heterogeneity among studies. The study was registered in the PROSPERO database (CRD42022354237).
Findings
From a total of 1474 articles screened, 51 studies were included. Studies were conducted in eight non-endemic countries (most in Spain), between 2006 and 2023, and involving 82,369 screened individuals. The estimated pooled prevalence of CD in Latin American migrants living in non-endemic countries was 3.5% (95% CI: 2.5–4.7; I2: 97.7%), considering studies in which screening was indicated simply because the person was Latin American. Per subgroups, the pooled CD prevalence was 11.0% (95% CI: 7.7–15.5) in non-targeted screening (unselected population in reference centers) (27 studies); in blood donors (4 studies), the pooled prevalence was 0.8% (95% CI: 0.2–3.4); among people living with HIV Latin American immigrants (4 studies) 2.4% (95% CI: 1.4–4.3) and for Latin American pregnant and postpartum women (14 studies) 3.7% (95 CI: 2.4–5.6). The pooled proportion of congenital transmission was 4.4% (95% CI: 3.3–5.8). Regarding the participants’ country of origin, 7964 were from Bolivia, of which 1715 (21,5%) were diagnosed with CD, and 21,304 were from other Latin American countries of which 154 (0,72%) were affected.
Interpretation
CD poses a significant burden of disease in Latin American immigrants in non-endemic countries, suggesting that CD is no longer a problem limited to the American continent and must be considered as a global health challenge.
Funding
This study was funded by the World Heart Federation, through a research collaboration with Novartis Pharma AG.
Keywords: Chagas disease, Prevalence, Latin american migrants, Non-endemic
Research in context.
Evidence before this study
During the conception of the study, an exhaustive search of published data was carried out, using the following public electronic databases: MEDLINE/PubMed, Embase, Cochrane Library, Scopus, Web of Science, and LILACS via Virtual Health Library (Biblioteca Virtual em Saúde - BVS). The descriptors and combinations used were “Chagas Disease” OR “Trypanosoma cruzi” OR “Triatominae” OR “Chagas Cardiomyopathy”) AND (“Serologic Tests” OR “Serological prevalence” OR “Seroepidemiologic Studies” OR “Seroprevalence” OR “Diagnostic Techniques and Procedures” and all those referring to endemic or non-endemic countries.
After the review we found only single study published in 2015 designed to estimate the prevalence of Chagas disease in Europe through a systematic review and meta-analysis.
Added value of this study
The epidemiology of Chagas disease has changed in recent years, and our study provides updated data taking into account recent migratory flows. Likewise, in this study we have included data from hospitals or specialized centers. Far from being considered a limitation, it provides very relevant information since allows us to estimate the pre-test probability of Chagas disease for individuals seeking consultation.
Moreover, the number of individuals included in our meta-analysis allowed for greater statistical precision of the estimates, as illustrated by the more precise CIs when compared with the previous meta-analysis.
Implications of all the available evidence
These data are crucial to update current estimates of the disease burden of CD in non-endemic regions, associated complications and even mortality worldwide in order to design appropriate control strategies, implement screening protocols and clinical management, which could help health authorities optimize existing resources.
Introduction
Chagas disease (CD), also known as American trypanosomiasis, is a neglected tropical disease caused by the protozoan parasite T. cruzi. Although considered endemic in 21 Latin American countries, CD has gradually spread beyond its traditional borders due to migratory movements, emerging as a global health concern. According to the World Health Organization, approximately 6–7 million people are estimated to be infected with T. cruzi worldwide, mostly in Latin America (estimated overall prevalence of 1.055%), with an increasing number of cases reported in non-endemic areas such as the United States, Canada, Europe, and Asia.1
In endemic areas, transmission primarily occurs through vectors, predominantly affecting rural areas with limited socio-economic resources.2 Other transmission routes, including vertical transmission, organ transplantation, and blood transfusion, can occur in both endemic and non-endemic areas.3 To mitigate these risks, endemic regions have implemented universal screening of blood and organ donors, while targeted programs have been established for some non-endemic areas, particularly those with a large number of Latin American population such as EUA and Europe (more specifically France, Italy, Portugal, Spain, Sweden, Switzerland, and the United Kingdom).4 Concerning fetal and maternal transmission, screening protocols for women of childbearing age have been implemented in endemic countries. However, despite the proven effectiveness of such programs in interrupting transmission, most non-endemic regions lack specific initiatives to address this issue.5,6
The majority of individuals with chronic CD infection remain asymptomatic, known as the chronic indeterminate form, whereas approximately 30–40% will develop the clinical forms of the disease, typically 10–30 years after the first contact with the infection.7 This, coupled with a lack of awareness among health professionals in non-endemic regions and barriers to healthcare access for migrant populations, contributes to the under-diagnosis of CD in such areas.8 Although it is currently recommended to screen people from high-risk areas, many regions lack established and adequately funded programs for this purpose.9 Therefore, understanding the prevalence of CD in non-endemic settings is essential for effective public health planning, resource allocation, and the development of targeted interventions, ultimately reducing the burden of this often-overlooked global health challenge.
Few studies have previously investigated the prevalence of CD in non-endemic countries. In 2009, a report based on aggregated data collected from the literature and official sources estimated between 68.000 and 122.000 cases in Europe, with 95% of cases remaining undiagnosed.10 In the United States, another study estimated a population of over 238.000 individuals with the infection.11 However, both estimates considered the national population prevalence of the countries of origin, disregarding potential biases resulting from specific geographical and socioeconomic characteristics of migrating individuals. In 2015, a systematic review and meta-analysis of studies reporting CD prevalence in European countries found a pooled prevalence of 4.2% among people born in Latin America residing in Europe, with the highest prevalence of around 18.1% observed among Bolivians.12
The scarcity of appropriate data on incidence, prevalence, and the frequency of complications - presents a major challenge to the comprehensive estimation of Chagas disease burden through efforts like the Global Burden of Disease study (GBD).13 This lack of data is particularly relevant in non-endemic countries, but endemic areas also suffer from this scarcity, with the most recent prevalence data being from 2015 based on 2010 data.1
Considering this need, we conducted a systematic review and meta-analysis of available literature to establish updated prevalence estimates of CD among people born in Latin America residing in non-endemic countries.
Methods
Protocol registration
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines14 (see attached checklist document, Appendix file S1). The study protocol was recorded in the International Prospective Register of Systemic Reviews (PROSPERO) database (CRD 42022354237).
This study is part of “The buRden of ChAgas dISEase in the contemporary world (RAISE)” project, a partnership among the Universidade Federal de Minas Gerais with the World Heart Federation, Novartis Global Health, and the University of Washington’s Institute of Health Metrics and Evaluation.
Eligibility criteria
Studies reporting the prevalence of CD in people born in Latin America living in non-endemic countries (according to the World Health Organization definition) were included in this review. Publications in English, Portuguese, or Spanish were included, without date restriction. Regarding study design, any observational study (cross-sectional and cohort studies) was considered eligible for inclusion in the meta-analysis. We excluded animal studies, case series, and reviews. Studies had to adequately describe the study site, the diagnostic method used to assess CD diagnosis, and the epidemiologic characteristics of the participating study subject population. In addition, the total numbers of subjects evaluated were required.
Sources and search strategy
Initially, a systematic review of available literature was performed to identify relevant reports assessing the prevalence of CD in people from Latin America living in non-endemic countries. A systematic search was conducted using the following public electronic databases: MEDLINE/PubMed, Embase, Cochrane Library, Scopus, Web of Science, and LILACS via Virtual Health Library (Biblioteca Virtual em Saúde - BVS). The descriptors and combinations used were: (“Chagas Disease” OR “T. cruzi” OR Triatominae OR “Chagas Cardiomyopathy”) AND (“Serologic Tests” OR “Serological prevalence” OR “Seroepidemiologic Studies” OR Seroprevalence OR “Diagnostic Techniques and Procedures”) AND (“Endemic region” OR “Endemic countries” OR Brasil∗ OR Brazil∗ OR Equador OR Ecuador OR Venezuela∗ OR Bolívia∗ OR Guiana OR Guyane OR Peru OR Surinam∗ OR Argentina∗ OR Colombia∗ OR “Guiana Francesa” OR “French Guiana” OR “Guyane francaise” OR Guatemala OR Mexico∗ OR Panama OR Paraguai OR Paraguay∗ OR “Costa Rica” OR “El Salvador” OR Honduras OR Nicaragua OR Belize OR Uruguai OR Uruguay∗ OR Chile∗ OR “North America” OR %2 2 United States” OR “South America” OR “Latin America” OR “Nonendemic region” OR “Non-endemic countries” OR Africa OR Antarctica OR Europe OR Asia OR Oceania OR Spain).
These descriptors were taken from the terminology of classification systems for indexing each database, MeSH (Medical Subject Headings) and DeCS (Health Sciences Descriptors). Published studies were identified in the electronic databases using the PECO strategy (Patient, Exposure, Comparator, and Outcome) to develop the descriptors. Where population was defined as individuals from CD endemic locations living in non-endemic countries, exposure was defined as CD or T. cruzi infection and the outcome by seroprevalence of CD, based on validated tests at the discretion of the local investigator. The comparator does not apply in this study. The search included references published until November 1st, 2023. In addition, the bibliography reference lists of articles selected for this review were evaluated for identifying potential additional relevant studies. Bibliographic citations from hand search of texts and associated citations, and from the databases’ “related articles” sections, were used to further identify potential articles.
Study selection
References obtained from each search were exported to Rayyan, a web tool designed to help researchers working on systematic reviews, compiling results from different databases, and facilitating inclusion and exclusion. Four independent reviewers (GNA, IM, PB, and FRM-M) carried out the selection of studies in two stages and at least two reviewers evaluated each study, with discrepancies resolved by a third tie-breaker. The first stage of selection screened the titles and abstracts of the publications for relevance and adequacy. The selected studies had full texts recovered for the second stage of selection. For this step we designed a Microsoft Excel database form, using the eligibility criteria as described, to assist in the archiving of eligible studies for the systematic review. Duplicate publications and papers reporting reanalysis of previously published data were excluded, as well as those with incomplete data reporting.
Data extraction
For each selected article, the reviewers extracted the following information, if available: full citation, year of publication, country and region where the study was conducted, study setting, characteristics of subjects (age, sex, and selection criteria), prevalence of CD, evaluated period, number of individuals evaluated, country of origin of the study subjects, and method used to diagnose the disease. The data extraction was carried out by two independent reviewers.
Study risk of bias assessment
To assess the quality of individual studies, we used the instrument proposed by JBI for systematic reviews of prevalence/incidence studies.15 The JBI tool includes nine questions addressing sample representativeness, participant recruitment, adequate sample size, study setting, data analysis, outcome measurement, and response rate. Each item was evaluated as either “yes”, “no”, “unclear”, or “not applicable”. Studies that received “yes” for seven or more questions were classified as of high quality and low risk of bias; those that received “yes” for five to six of the questions were classified as moderate risk of bias; and those that received “yes” for four or less were considered of low quality and high risk of bias. A summary of the quality assessment is presented in the Supplemental information file S2.
Quantitative meta-analysis
Comprehensive Meta-Analysis software version 4.0 (Biostat Inc., Englewood, USA), was used to calculate pooled prevalence estimates with their 95% confidence intervals (95% CI) using Der Simonian and Laird random-effects models. Summary data were visually presented by forest plots showing the pooled prevalence and 95% CI. To assess the odds of infection between the population of Bolivians and non-Bolivians, the odds ratio (OR) and 95% CI were used for the pooled analysis.
For the primary prevalence analysis, studies employing screening protocols within general representative Latin American population living in non-endemic countries were considered.
Heterogeneity and sensitivity analysis
Heterogeneity among studies was assessed using the Cochrane Q test (χ2 test, with significance assumed for p < 0.10), and Higgin’s and Thompson’s I2 statistic (I2 values rounds of 25%, 50%, and 75% show low, moderate, and high heterogeneity, respectively).16 To explore heterogeneity and factors that could potentially modify the pooled estimates, we performed subgroup analyses stratified by blood donors, pregnant and puerperal women, people living with HIV/AIDS (PLHIV), population under 18 years old, and unselected population. For the sensitivity analysis, the meta-analysis was re-tested with the exclusion of one study at a time to assess the possibility of a disproportionate impact of any individual study on summary estimates.
Publication bias
Assessment for potential publication bias was carried out by visual inspection of funnel plots, and statistically by calculating the Begg test.17
Role of the funding source
The funder did not have any relationship with the conduct of the study, the collection, analysis, and interpretation of the data. Novartis Pharma AG employees (YG, CD and MQ) participated in the review of this manuscript as coauthors.
Results
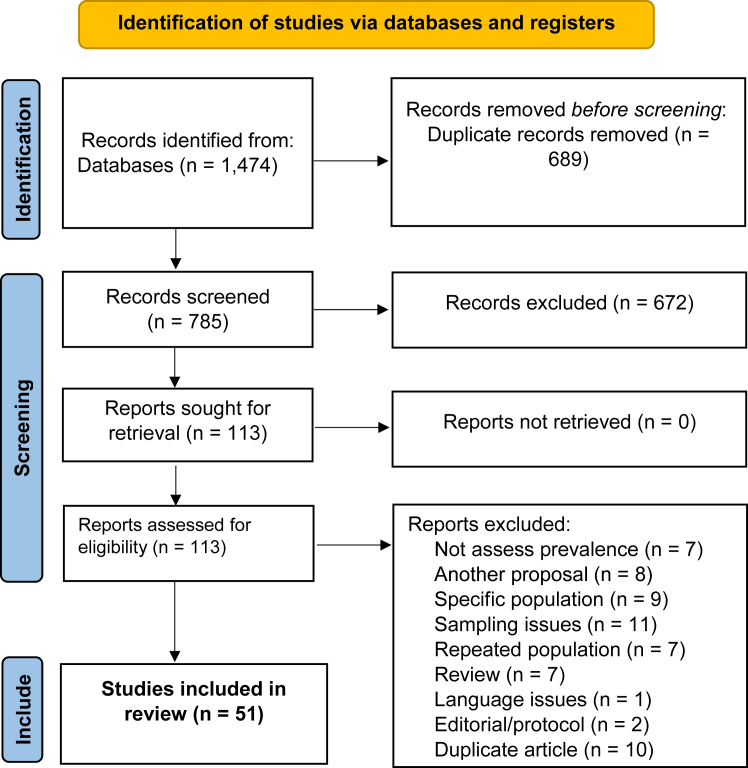
After removing duplicates, initial screening of the databases yielded 1474 study reports. After assessing titles and abstracts, 113 reports were selected for full-text review. In this first stage, publications were excluded because they were review studies, editorials, or research protocols, duplicated articles, did not assess prevalence, had different aims, included specific populations (studies only with individuals living with the disease), and had sampling problems, such as a very small sample and no capacity for representativeness, or repeated populations. As outlined in Fig. 1, after the second stage screening, a total of 51 reports were included in this systematic review and meta-analysis (S3 File).
Fig. 1.
Flow chart of study selection process.
The main characteristics of the selected studies are shown in Table 1. Publication dates ranged from 2006 to 2023 and enrolled a total of 82,369 individuals. Most of the studies estimated the prevalence of CD considering a non-selected Latin American population (understood as any adult individual born in Latin America evaluated) (n = 27), some studies included only pregnant and postpartum women (n = 14), PLHIV Latin American (n = 4), blood donors from Latin America and subjects under 18 years old (n = 6). Regarding the place of enrollment, subjects were located in the community, in tropical medicine units, or in primary health centers. Studies were conducted in eight non-endemic countries. Of these, 31 studies (60.8%) were from Spain, 8 (15.7%) from Italy, 4 (7.8%) from USA, 3 studies (5.9%) from Switzerland, 2 (4.0%) from Canada and one study each from France, Germany and Japan (5.9%). The majority were classified as having a moderate risk of bias, based on the JBI critical review checklist (Appendix file S2). We found no evidence of publication bias using unweighted, non-randomized values in the Begg test (p = 0.06), for the overall prevalence.
Table 1.
Characteristics of included studies assessing the prevalence of Chagas disease in Latin American migrants living in non-endemic countries.
| Publication | Country | Period | Population (Latin American) | Study site | Age mean (SD) or Age range | Sample | Number infected (%) |
|---|---|---|---|---|---|---|---|
| Abras_2020 | Spain | 2017–2018 | Non-selected population | Hospital | 33.1 | 786 | 102 (13) |
| Angheben_2011 | Italy | 1998–2010 | Non-selected population | Tropical medicine service | 34 | 266 | 30 (11.3) |
| Antinori_2018 | Italy | 2013–2014 | Non-selected population | Community | 39 | 501 | 48 (9.5) |
| Arzanegui_2013 | Spain | 2008–2010 | Pregnant women | Hospital | 28,5 (5.3) | 158 | 19 (12) |
| Barona-Vilar_2012 | Spain | 2009–2010 | Pregnant women | Hospital | 29 | 1975 | 226 (11.4) |
| Basile_2019 | Spain | 2010–2015 | Pregnant women | Hospital/Primary care services | 33 | 33,469 | 818 (2.4) |
| Bocanegra_2014 | Spain | 2007–2010 | Non-selected population | Tropical medicine service | NI | 416 | 224 (53.8) |
| Cancino-Faure_2015 | Spain | 2011–2013 | Blood donors | Blood banks | NI | 1201 | 23 (1.91) |
| Castro-Sesquen_2021 | USA | 2016–2018 | Non-selected population | Community | NI | 1514 | 98 (6.5) |
| Chappuis_2010 | Switzerland | 2009 | Non-selected population | Primary care services | 36 | 999 | 125 (12.5) |
| Cobo_2014 | Spain | 2004–2013 | Non-selected population | Hospital | NI | 196 | 72 (36.7) |
| Escobio_2020 | Spain | 2011–2012 | Non-selected population | Primary care services | 34.6 | 251 | 48 (19.1) |
| Francisco-González_2018 | Spain | 2013–2015 | Pregnant women | Hospital | NI | 1244 | 40 (3.2) |
| Fumado_2014 | Spain | 2003–2008 | Children under 18 years old | Infectious diseases hospital | 01–14 | 202 | 22 (10.8) |
| Ghouzzi_2010 | France | 2007–2008 | Blood donors | Blood banks | NI | 972 | 3 (0.33) |
| Giménez-Mart_2006 | Spain | 2001 | Blood donors | Blood banks | NI | 432 | 16 (3.7) |
| Girolamo_2015 | Italy | 2010–2013 | Non-selected population | Hospital | 37.5 (13.1) | 151 | 12 (7.94) |
| Gómez i Prat_2022 | Spain | 2020 | General population | Community | 43 (33–53) | 299 | 55 (18.3) |
| Herrero-Martínez_2023 | Spain | 2011–2016 | Pregnant women | Hospital | NI | 11,048 | 309 (2.9) |
| Hochberg_2011 | USA | 2009 | PLHIV | Infectious diseases hospital | NI | 77 | 3 (3.9) |
| Jackson_2016 | Switzerland | 2008 | Non-selected population | Primary care services | 37.2 (11.3) | 1012 | 130 (12.8) |
| Jackson_2018 | Switzerland | 2012–2015 | Non-selected population | Hospital | NI | 903 | 244 (27) |
| Lienas-García_2021 | Spain | 2014–2018 | Pregnant women | Hospital | NI | 1178 | 23 (1.95) |
| Llenas-García_2012 | Spain | 2008–2009 | PLHIV | Infectious diseases hospital | 36.9 (8.4) | 155 | 3 (1.9) |
| Martelli_2017 | Italy | 2012–2014 | Non-selected population | Hospital | 37.8 | 180 | 7 (3.9) |
| Martinez_2009 | Spain | 2004–2006 | Non-selected population | Tropical medicine service | 35 | 216 | 46 (21.3) |
| Meymandi_2017 | USA | 2008–2014 | Non-selected population | Community | NI | 4755 | 59 (1.24) |
| Muñoz_2009 | Spain | 2004–2007 | Non-selected population | Tropical medicine service | 34 (11) | 489 | 202 (41.3) |
| Muñoz-Vilches_2012 | Spain | 2007–2011 | Pregnant women | Hospital | NI | 261 | 4 (1.53) |
| Navarro_2011 | Spain | 2008–2009 | Non-selected population | Primary care services | NI | 276 | 44 (15.9) |
| Navarro_2017 | Germany | 2013–2014 | Non-selected population | Community/Tropical medicine service | 39 (17.2) | 43 | 4 (9.33) |
| O’Brien_2013 | Canada | 2009–2010 | Blood donors | Blood banks | NI | 7255 | 13 (0.2) |
| Otero_2012 | Spain | 2008–2010 | Pregnant women | Hospital | 29.5 (6) | 633 | 22 (3.5) |
| Pane_2018 | Italy | 2014 | Non-selected population | Community | 42 | 368 | 32 (8.7) |
| Paricio-Talayero_2008 | Spain | 2005–2007 | Pregnant women | Hospital | 28.3 (5.8) | 624 | 29 (4.7) |
| Perez-Ayala_2010 | Spain | 2003–2009 | Non-selected population | Tropical medicine service | 36 | 1146 | 357 (31.1) |
| Raglio_2023 | Italy | 2013–2020 | Non-selected population | Hospital | NI | 512 | 60 (11.7) |
| Ramos_2009 | Spain | 2006–2007 | Pregnant women | Hospital | 24 (5.2) | 220 | 4 (1.82) |
| Ramos_2012A | Spain | 2009–2010 | Non-selected population | Community | 30 | 201 | 13 (6.47) |
| Ramos_2012B | Spain | 2006–2010 | Pregnant women | Hospital | 28.9 | 545 | 7 (1.28) |
| Ramos_2015 | Spain | 2012–2014 | Non-selected population | Hospital | 38 | 176 | 5 (2.8) |
| Ramos-Sesma_2021 | Spain | 2017–2018 | Non-selected population | Hospital/Primary care services | 41 | 596 | 54 (9.06) |
| Repetto_2015 | Italy | 2012–2013 | Non-selected population | Hospital | NI | 1305 | 223 (17) |
| Roca_2011 | Spain | 2007–2009 | Non-selected population | Primary care services | 39.8 (9.8) | 766 | 22 (2.9) |
| Rodari_2022 | Italy | 1997–2018 | PLHIV | Infectious diseases hospital | 33 | 389 | 5 (1.29) |
| Rodríguez_2023 | Japan | 2019–2020 | Non-selected population | Hospital | 43.5 (13.6) | 428 | 7 (1.6) |
| Salvador_2013 | Spain | 2010–2011 | PLHIV | Infectious diseases hospital | 37 | 126 | 5 (3.9) |
| Santiago_2012 | Spain | 2007–2008 | Pregnant women | Hospital | NI | 265 | 13 (4.9) |
| Simón_2020 | Spain | 2007–2018 | Children under 18 years old | Infectious diseases hospital | 0–14 | 949 | 40 (4.2) |
| Steele_2007 | Canada | NI | Non-selected population | Primary care services | NI | 102 | 1 (1) |
| Zamora_2021 | USA | 2019 | Pregnant women | Hospital | NI | 138 | 0 |
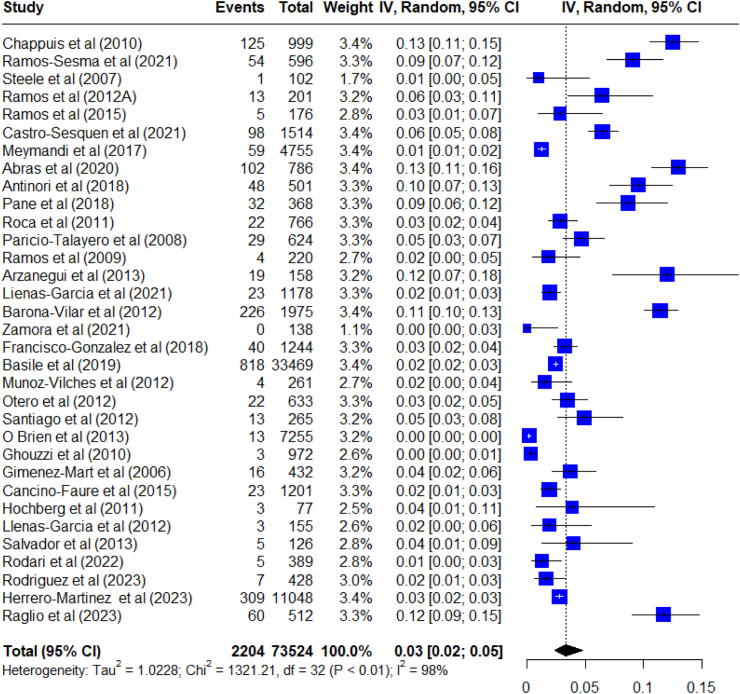
Observed prevalence among studies ranged from 0 to 53.8%, evidencing a difficulty in appraising a summary estimate. The global prevalence was estimated by considering studies in which the diagnostic test was administered solely based on the individual being Latin American, such as in protocolled screening procedures on general representative population. In this sense, the pooled prevalence estimate of CD in non-endemic countries was 3.5% (95% CI: 2.5–4.7; I2: 97.7%) among a population of 73,524 Latin American-born migrants in 33 studies, between 2006 and 2023. There was significant between-study heterogeneity (Fig. 2). A sensitivity analysis was conducted removing one study at a time. The results of the sensitivity analysis pointed out that no study removal significantly affected the prevalence of CD since the confidence intervals overlap in all cases (Appendix file S4).
Fig. 2.
Meta-analysis of the prevalence of Chagas disease in non-endemic countries with studies that used a screening protocol applied to the overall Latin American population.
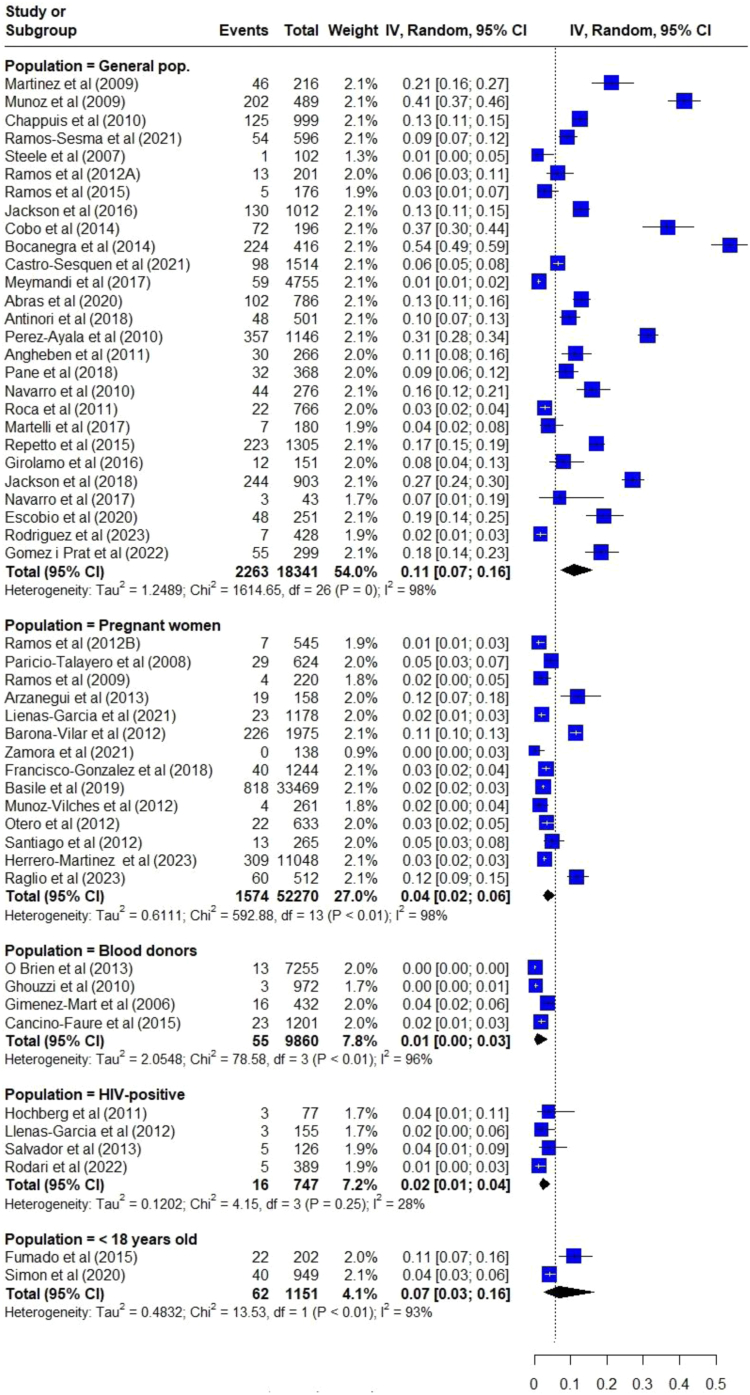
When the CD prevalence was estimated considering different sub-populations, data revealed great differences. Subpopulations were categorized by whether the screening targeted a clinical/epidemiological characteristic (blood donors, pregnant women or PLHIV) or if it was non-targeted (unselected population). The higher pooled CD prevalence was observed for unselected Latin American population (11.0% [95% CI: 7.7–15.5]; 27 studies) enrolled either in hospitals, specialized clinics, primary care centers, or in the community, followed by studies carried out with individuals under 18 years of age (6.8% [95% CI: 2.6–16.5]; 2 studies). For specific populations, the pooled prevalence was lower than the general population for blood donors (0.8% [95% CI: 0.2–3.4]; 4 studies); among PLHIV born in Latin America (2.4% [95% CI: 1.4–4.3]; 4 studies), and for Latin American pregnant and postpartum women (3.7% (95% CI: 2.4–5.6]; 14 studies) (Fig. 3). Heterogeneity for all analyses was high maintaining I2 > 90% and p < 0.001, except for the subgroup of PLHIV born in Latin America, which had low heterogeneity (I2: 27.7%, p = 0.246).
Fig. 3.
Meta-analysis of the prevalence of Chagas disease in non-endemic countries by population subgroups.
Of those studies including pregnant women, 15 studies reported data on congenital transmission. These studies analyzed 1201 pregnancies of which 45 newborns were diagnosed with CD. The pooled proportion of congenital transmission was 4.4% (95% CI: 3.3–5.8). However, these results are limited due to a very small sample size and several studies showing no cases of transmission.
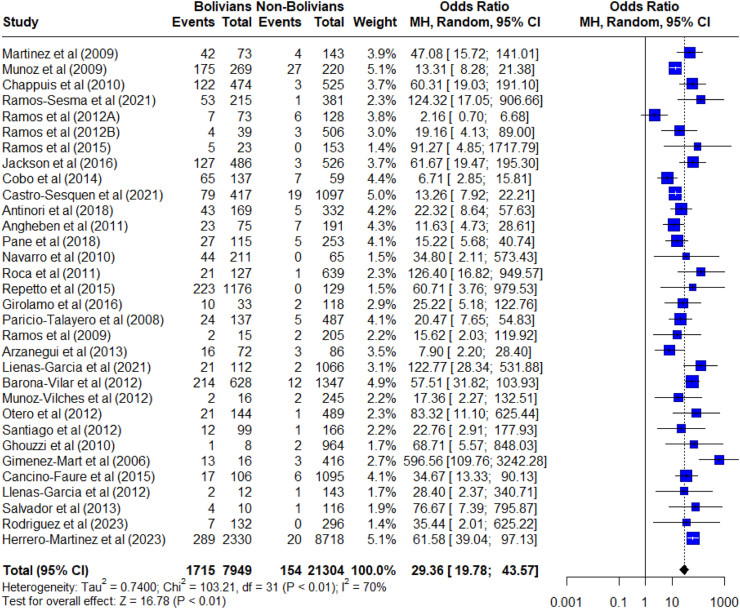
Regarding the participants’ country of origin, 32 studies provided sufficient data for analysis. These studies evaluated a total of 29,253 individuals originary from Latin America. Of these, 7964 were from Bolivia of which, 1715 were diagnosed with CD (21.5%), and 21,304 from other Latin American countries of which, 154 were diagnosed (0.72%). That is, 91.7% of those infected were from Bolivia. The chance of people from Bolivia being the most infected with T. cruzi compared to people coming from other Latin American countries was statistically much greater (OR: 29.3, CI 95%: 19.8–43.5) (Fig. 4).
Fig. 4.
Meta-analysis of the odds ratio of Chagas disease seroprevalence among Bolivian vs. Non-Bolivian Latin American individuals living in non-endemic countries.
Discussion
This systematic review with meta-analysis provides an up-to-date and comprehensive overview of the prevalence of CD in Latin American-born peoplein non-endemic countries. These results emphasize that CD is not solely a concern for endemic countries. In this era of extensive and widespread population flows, CD should instead be seen as a global public health challenge. According to our analyses, the overall pooled prevalence of CD is 3.5% among people from Latin America living in non-endemic countries, reflecting a considerable underlying burden of disease in these countries. These data come from studies in which the included population was considered to be representative of the Latin American community, thus trying to minimize selection and interpretation biases since those studies where people living with CD could be overrepresented were excluded. Despite these estimates being consistent with previously published data,12 many of these studies were not designed to estimate global prevalence. In addition, no stratified sampling procedures were consistently available. Finally, the number of individuals included in our meta-analysis allowed for greater statistical precision of the estimates, as illustrated by the more precise CIs when compared with another meta-analysis.12
In our analyses, prevalence estimates changed drastically according to the population or place of enrollment. When prevalence was estimated taking into account non targeted population, that is to say, individuals not specifically selected, prevalence increased to 11.0% (95% CI: 7.7%–15.5%). The reason for this discrepancy might be due to the fact that those studies were mainly carried out in specialized tropical medicine units, where there is a bias due to the overrepresentation of individuals already diagnosed with CD, or even people referred for pertaining to high-risk groups from endemic regions.
Interestingly, when data came from studies in which a screening protocol has been applied simply for being Latin American-born, such as those studies carried out on blood donors, pregnant women, or PLHIV, prevalence dropped to 0.8%, 3.7%, and 2.4%, respectively. These scenarios are much more in line with the official estimates for endemic regions and could be partially explained by the fact that those studies express the result of the application of protocols to the general population in a relatively unbiased way.1 However, in the specific case of blood donors, it could underestimate the prevalence, since being aware of the carrier status could be a reason for no longer donating blood, as happens for other diseases.18
Another factor to be considered, which may potentially have an impact on the pooled estimates, is the nationality of the individuals included, because the estimated prevalence in the countries of origin were not homogeneous. In this sense, we have been able to obtain information about the participants’ origin in nearly 29,000 cases. Of these, 27.2% were Bolivian, with an overall prevalence of the disease of 21.5%. This may reflect one type of selection bias, as some groups such as the Bolivian-born and the North Argentinian-born populations are known to have disproportionately higher prevalence and may be targeted or prioritized for screening in non-endemic settings.
These prevalence estimates are almost 4 times higher than official country data (6.1% according to WHO).1 This could be explained by two reasons. First, many tropical medicine or specialized care centers use the word-of-mouth strategy to identify new individuals living with the disease.19 In this way, the probability of having CD for a relative or cohabitant as a disease carrier is greater than the general population, since they can share the same epidemiological environment, similar sub-regions of origin, as well as other coexisting risk factors for seropositivity.
Another possible reason is that the Bolivian individuals attended in health centers at non-endemic countries usually come from hyper-endemic areas of the country such as the departments of Tarija, Cochabamba, or Chuquisaca, where prevalence is up to 5 times higher than in other regions of the country.20 Therefore, studies in which there is a clear overrepresentation of people from Bolivia can overestimate the global prevalence of the disease and represent a bias of interpretation.21, 22, 23, 24, 25
The proportion of congenital transmission reported among pregnant women was 4.4%. In addition to blood transfusions, transplants and laboratory accidents, mother-to-child infection is a potential transmission route in non-endemic settings. A previous meta-analysis showed a lower pooled rate of 2.7% in non-endemic countries, however, our results align more closely with the general transmission rate of 4.7% reported in those studies.26 These figures are significant as many of these transmissions are preventable if women of childbearing age receive treatment before pregnancy. Moreover, if diagnosed later, parasitological treatment can achieve cure rates of nearly 100% in children. Nevertheless, many countries still lack screening protocols despite numerous recommendations, so more efforts should be made in this sense.4,5
In this review, unlike those previously published pooled analyses, studies carried out in hospitals or specialized centers have not been excluded. Obviously, the analysis has taken into account the selection bias and therefore the over-representation of CD carriers, and for this reason, we have presented data separately for different subgroups. While this may be considered as a limitation when assessing prevalence in the general population, it reflects the situation in numerous tropical medicine centers in Europe and other non-endemic regions. This approach allows us to estimate the pre-test probability of Chagas disease for individuals seeking consultation.27, 28, 29
Our study has some limitations, especially those related to data quality. At first, it was not possible to determine, from available studies, the overall populational prevalence of CD in non-endemic countries. Second, the different settings, data collection strategies, sampling, and inclusion criteria accounted for the observed high heterogeneity. Sensitivity and subgroup analyses, however, presented results for specific subgroups and scenarios, aiming to make the estimates more specific and less prone to bias. Third, the results tend to overrepresent selected populations, in terms of study setting and population subgroups, without stratified sampling procedures, which adds to heterogeneity and tends to overestimate prevalence, limiting the extrapolation of the findings.
In this sense, it would have been interesting to be able to compare the prevalence data from studies carried out in a hospital/specialized center environment with those obtained in the community, but many of these studies considered as community studies, were carried out in places or events with an overrepresentation of the Bolivian community, so it could not be considered a cross-sectional community-based survey with an adequate sampling strategy.
Despite these limitations, this is, to our knowledge, the most detailed and up-dated meta-analysis on the prevalence of Chagas disease among Latin American immigrants living in non-endemic regions, although specific numerical rates necessitate cautious and individualized evaluation. Obtaining accurate and updated data on the prevalence of diseases is essential to understand the impact of population flows, which must be considered as dynamic events that need to be constantly monitored. The prevalence of CD in cross-sectional studies on imported pathologies in non-endemic areas prior to that period was either non-existent or appeared marginally.30, 31, 32
Likewise, having these data is crucial to update the current estimates of the CD burden of disease, associated complications, and even mortality worldwide to be able to design adequate control strategies, implement screening and clinical management protocols, and might help health authorities to optimize the existing resources.
Conclusions
Our systematic analysis estimated that the pooled prevalence of CD among Latin American-born individuals in non-endemic regions is 3.5%, keeping in mind methodological limitations and limited quality of data. These pooled data reflect that CD is no longer a problem limited to the American continent and must be considered as a global health challenge. More robust studies are required, specifically designed to estimate the prevalence of CD in different regions, which would emphasize the demand for adequate medical care, improved screening protocols which might minimize new avoidable cases in non-endemic regions, as well as better access to diagnostics and optimized treatments.
Contributors
Conception and design of the research: ALPR, MQ, GNA, BRN, IM; Acquisition of data: GNA, PBN, BRN, FRMM, IM; Analysis and interpretation of data: GNA, PBN, BRN, FRMM, IM, ALPR; Statistical analysis: GNA; Obtaining financing: YG, CD, MQ, PP; Writing of the manuscript: GNA, PBN, IM; Critical revision of the manuscript for intellectual content: All authors; Authors responsible for the overall content as guarantors: BRN, ALPR, GNA, PBN, BRN, FRMM, IM.
Data sharing statement
Data analytic methods and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure, from the corresponding author upon reasonable request.
Declaration of interests
Yvonne Geissbuhler, Caroline Demacq, and Monica Quijano are Novartis employees and declare stocks of the company. Jonathan F Mosser is supported partially from Bill and Melinda Gates and GAVI Foundation. Ewerton Cousin is supported partially from Bill and Melinda Gates Foundation. Dr. Ribeiro is supported in part by CNPq (310790/2021-2 and 465518/2014-1) and FAPEMIG (RED 00192-23). Dr. Nascimento is supported in part by CNPq (Bolsa de produtividade em pesquisa, 310749/2022-0), by the Edwards Lifesciences Foundation (Improving the Prevention and Detection of Heart Valve Disease Across the Lifespan, 2023) and by FAPEMIG (grant APQ-000627-20).
Acknowledgements
This study was funded by Novartis Pharma AG as part of a research collaboration with the World Heart Federation, project number CLCZ696D2010R. The World Heart Federation funded the Federal University of Minas Gerais to lead the study.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2024.101040.
Appendix ASupplementary data
References
- 1.Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec. 2015;90(6):33–43. English, French. PMID: 25671846. [PubMed] [Google Scholar]
- 2.Cucunubá Z.M., Nouvellet P., Peterson J.K., et al. Complementary paths to Chagas disease elimination: the impact of combining vector control with etiological treatment. Clin Infect Dis. 2018;66(suppl_4):S293–S300. doi: 10.1093/cid/ciy006. PMID: 29860294; PMCID: PMC5982731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shikanai Yasuda M.A. Emerging and reemerging forms of Trypanosoma cruzi transmission. Mem Inst Oswaldo Cruz. 2022;117 doi: 10.1590/0074-02760210033. PMID: 35584508; PMCID: PMC9113729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Requena-Méndez A., Albajar-Viñas P., Angheben A., Chiodini P., Gascón J., Muñoz J. Health policies to control Chagas disease transmission in European countries. PLoS Negl Trop Dis. 2014;8(10) doi: 10.1371/journal.pntd.0003245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soriano-Arandes A., Angheben A., Serre-Delcor N., et al. Control and management of congenital Chagas disease in Europe and other non-endemic countries: current policies and practices. Trop Med Int Health. 2016;21(5):590–596. doi: 10.1111/tmi.12687. Epub 2016 Mar 17. PMID: 26932338. [DOI] [PubMed] [Google Scholar]
- 6.Murcia L., Simón M., Carrilero B., Roig M., Segovia M. Treatment of infected women of childbearing age prevents congenital Trypanosoma cruzi infection by eliminating the parasitemia detected by PCR. J Infect Dis. 2017;215:1452–1458. doi: 10.1093/infdis/jix087. [DOI] [PubMed] [Google Scholar]
- 7.Pérez-Molina J.A., Molina I. Chagas disease. Lancet. 2018;391(10115):82–94. doi: 10.1016/S0140-6736(17)31612-4. [DOI] [PubMed] [Google Scholar]
- 8.Ramos-Rincón J.M., Mira-Solves J.J., Ramos-Sesma V., Torrús-Tendero D., Llenas-García J., Navarro M. Healthcare professionals and students’ awareness of Chagas disease: design and validation of Chagas level of knowledge scale (ChaLKS) Am J Trop Med Hyg. 2020;103(1):437–444. doi: 10.4269/ajtmh.19-0677. Epub 2020 Apr 23. PMID: 32342845; PMCID: PMC7356480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization Chagas disease non-endemic countries initiative and the technical groups. https://www.who.int/chagas/strategy/technical_groups_more/en/
- 10.Basile L., Jansa J.M., Carlier Y., et al. Working Group on Chagas Disease Chagas disease in European countries: the challenge of a surveillance system. Euro Surveill. 2011;16(37) PMID: 21944556. [PubMed] [Google Scholar]
- 11.Manne-Goehle J., Umeh C.A., Montgomery S.P., Wirtz V.J. Estimating the burden of Chagas disease in the United States. PLoS Negl Trop Dis. 2016;10(11) doi: 10.1371/journal.pntd.0005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Requena-Méndez A., Aldasoro E., de Lazzari E., et al. Prevalence of Chagas disease in Latin-American migrants living in Europe: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2015;9(2) doi: 10.1371/journal.pntd.0003540. PMID: 25680190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanaway J.D., Roth G. The burden of Chagas disease: estimates and challenges. Glob Heart. 2015;10(3):139–144. doi: 10.1016/j.gheart.2015.06.001. PMID: 26407508. [DOI] [PubMed] [Google Scholar]
- 14.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moola S., Munn Z., Tufanaru C., et al. In: JBI manual for evidence synthesis. Aromataris E., Munn Z., editors. JBI; 2020. Chapter 7: systematic reviews of etiology and risk.https://synthesismanual.jbi.global Available from. [Google Scholar]
- 16.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 17.Begg Colin B. Mazumdar Madhuchhanda Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 18.Sedyaningsih-Mamahit E., Schinaia N., Lazzari S., Walker N., Vercauteren G. The use of blood donor data for HIV surveillance purposes. AIDS. 2004;18(13):1849–1851. doi: 10.1097/00002030-200409030-00016. [DOI] [PubMed] [Google Scholar]
- 19.Gómez I., Prat J., Peremiquel-Trillas P., et al. Comparative evaluation of community interventions for the immigrant population of Latin American origin at risk for Chagas disease in the city of Barcelona. PLoS One. 2020;15(7) doi: 10.1371/journal.pone.0235466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alonso-Vega C., Billot C., Torrico F. Achievements and challenges upon the implementation of a program for national control of congenital Chagas in Bolivia: results 2004–2009. PLoS Negl Trop Dis. 2013;7(7) doi: 10.1371/journal.pntd.0002304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navarro M., Perez-Ayala A., Guionnet A., et al. Targeted screening and health education for Chagas disease tailored to at-risk migrants in Spain, 2007 to 2010. Euro Surveill. 2011;16(38) doi: 10.2807/ese.16.38.19973-en. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19973 Available online: [DOI] [PubMed] [Google Scholar]
- 22.Navarro M., Berens-Riha N., Hohnerlein S., et al. Cross-sectional, descriptive study of Chagas disease among citizens of Bolivian origin living in Munich, Germany. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-013960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Favila Escobio P., Ribas J., Morillo M.G., Rodríguez-Ramírez G., Vicens-Ferrer J., Esteva M. Prevalence of Chagas disease in the Bolivian population of majorca (Spain) Gac Sanit. 2015;29(4):288–291. doi: 10.1016/j.gaceta.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Repetto E.C., Zachariah R., Kumar A., et al. Neglect of a neglected disease in Italy: the challenge of access-to-care for Chagas disease in bergamo area. PLoS Negl Trop Dis. 2015;9(9) doi: 10.1371/journal.pntd.0004103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson Y., Pula D.V.d.M., Finckh A., Chizzolini C., Chappuis F. Chagas disease and systemic autoimmune diseases among Bolivian patients in Switzerland. Mem Inst Oswaldo Cruz. 2018;113(4) doi: 10.1590/0074-02760170383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howard E.J., Xiong X., Carlier Y., Sosa-Estani S., Buekens P. Frequency of the congenital transmission of Trypanosoma cruzi: a systematic review and meta-analysis. BJOG. 2014;121(1):22–33. doi: 10.1111/1471-0528.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pérez-Molina J.A., López-Polín A., Treviño B., et al. +Redivi Study Group 6-year review of +Redivi: a prospective registry of imported infectious diseases in Spain. J Travel Med. 2017;24(5) doi: 10.1093/jtm/tax035. [DOI] [PubMed] [Google Scholar]
- 28.Bocanegra C., Salvador F., Sulleiro E., Sánchez-Montalvá A., Pahissa A., Molina I. Screening for imported diseases in an immigrant population: experience from a teaching hospital in Barcelona, Spain. Am J Trop Med Hyg. 2014;91(6):1277–1281. doi: 10.4269/ajtmh.14-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salvador F., Treviño B., Sulleiro E., et al. Trypanosoma cruzi infection in a non-endemic country: epidemiological and clinical profile. Clin Microbiol Infect. 2014;20(7):706–712. doi: 10.1111/1469-0691.12443. [DOI] [PubMed] [Google Scholar]
- 30.Monge-Maillo B., Jiménez B.C., Pérez-Molina J.A., et al. Imported infectious diseases in mobile populations, Spain. Emerg Infect Dis. 2009;15(11):1745–1752. doi: 10.3201/eid1511.090718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vilajeliu Balagué A., de Las Heras Prat P., Ortiz-Barreda G., Pinazo Delgado M.J., Gascón Brustenga J., Bardají Alonso A. Parasitosis importadas en la población inmigrante en España [Imported parasitic diseases in the immigrant population in Spain] Rev Esp Salud Publica. 2014;88(6):783–802. doi: 10.4321/S1135-57272014000600010. Spanish. [DOI] [PubMed] [Google Scholar]
- 32.Manzardo C., Treviño B., Gómez i Prat J., et al. Communicable diseases in the immigrant population attended to in a tropical medicine unit: epidemiological aspects and public health issues. Travel Med Infect Dis. 2008;6(1–2):4–11. doi: 10.1016/j.tmaid.2007.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.