Abstract
Previous analyses of randomly generated, temperature-sensitive vaccinia virus mutants led to the mapping of DNA synthesis negative complementation groups to the B1R, D4R, D5R, and E9L genes. Evidence from the yeast two-hybrid system that the D4R and D5R proteins can interact with the A20R protein suggested that A20R was also involved in DNA replication. We found that the A20R gene was transcribed early after infection, consistent with such a role. To investigate the function of the A20R protein, targeted mutations were made by substituting alanines for charged amino acids occurring in 11 different clusters. Four mutants were not isolated, suggesting that they were lethal, two mutants exhibited no temperature sensitivity, two mutants exhibited partial temperature sensitivity, and two mutants formed no plaques or infectious virus at 39°C. The two mutants with stringent phenotypes were further characterized. Temperature shift-up experiments indicated that the crucial period was between 6 and 12 h after infection, making it unlikely that the defect was in virus entry, early gene expression, or a late stage of virus assembly. Similar patterns of metabolically labeled viral early proteins were detected at permissive and nonpermissive temperatures, but one mutant showed an absence of late proteins under the latter conditions. Moreover, no viral DNA synthesis was detected when cells were infected with either stringent mutant at 39°C. The previous yeast two-hybrid analysis together with the present characterization of A20R temperature-sensitive mutants suggested that the A20R, D4R, and D5R proteins are components of a multiprotein DNA replication complex.
Poxviruses, of which vaccinia virus is the prototype, are unique among DNA viruses in that they reproduce exclusively in the cytoplasm of host cells and consequently encode most of the enzymes and factors for transcription, genome replication, and virion assembly (19). Biochemical and genetic approaches have provided information regarding the roles of approximately half of the 200 proteins thought to be encoded within the 192-kbp double-stranded DNA genome of vaccinia virus. Insights regarding the roles of additional viral proteins could be obtained by determining their interactions with proteins of known function. With this objective, a genome-wide yeast two-hybrid analysis of vaccinia virus open reading frames (ORFs) was performed (16). Of the approximately 70,000 pairwise interactions assayed, 37 scored positive. An intriguing finding was that the protein encoded by the A20R ORF interacted with the proteins encoded by three other ORFs: D4R, D5R, and H5R. D4R and D5R, both of which are required for DNA replication, encode a uracil DNA glycosylase (5, 18, 22, 25) and a nucleic acid-independent nucleoside triphosphatase (6, 7), respectively. The third A20R-interacting protein, encoded by the H5R ORF, has been implicated in transcription (1, 15) and virus assembly (4). Based on its interaction with two known DNA replication proteins, a related role was suggested for the A20R protein (16). In addition, Traktman (23) cited unpublished data showing that the A20R protein enhanced the processivity of DNA polymerase in vitro. Although the A20R gene is conserved among poxviruses, no homologs were found in other virus families or organisms. Furthermore, no temperature-sensitive (ts) or other mutants that map to A20R have been reported.
To gain insight into the role of the A20R gene in vivo, we determined the time of its expression and generated conditional lethal ts mutants by “clustered charge-to-alanine” mutagenesis (27). In this approach, amino acids constituting a cluster of three or four positively or negatively charged residues are changed to alanines. Because charged amino acids are likely to be located on the surfaces of proteins, their substitution could contribute to temperature-dependent instability. Originally used in yeast (27), clustered charge-to-alanine mutagenesis has been effectively applied to animal viruses, including vaccinia virus (4, 12, 13). In this report, we describe the isolation of A20R ts mutants that are blocked in viral DNA replication at the nonpermissive temperature.
MATERIALS AND METHODS
Materials.
Restriction endonucleases and polynucleotide kinase were purchased from New England Biolabs, Inc. (Beverly, Mass.). DNA blunting and ligation kits were from Panvera (Madison, Wis.). 32P-labeled nucleoside triphosphates and [35S]methionine were purchased from Dupont, New England Nuclear Corp. (Boston, Mass.). Cycloheximide, mycophenolic acid (MPA), xanthine, and hypoxanthine were purchased from Sigma (St. Louis, Mo). 5-Bromo-4-chloro-3-indolyl-β-d-glucuronic acid (X-Gluc) was from Clontech Laboratories, Inc. (Palo Alto, Calif.).
Cells and virus.
Monolayer cultures of BS-C-1 were maintained in Eagle's minimal essential medium (EMEM; Quality Biologicals, Inc. Gaithersburg, Md.) containing 10% fetal bovine serum. Wild-type (strain) (WR) and mutant vaccinia viruses were amplified in monolayer cultures of BS-C-1 cells. Titers of virus stocks were determined by plaque assay on BS-C-1 monolayers. For ts mutants, 31 and 39°C were used as the permissive and nonpermissive temperatures, respectively.
Northern blot analysis.
Confluent monolayers of BS-C-1 cells in 35-mm-diameter dishes were infected with 15 PFU of vaccinia virus per cell and incubated at 37°C. Total RNA was extracted with TRIzol (Life Technologies, Rockville, Md.), separated on a 1% agarose–formamide gel, and transferred to an Immobilon-Ny+ transfer membrane (Millipore, Bedford, Mass.) by using the NorthernMax (Ambion, Austin, Tex.) complete Northern blotting kit. The membrane was air dried, and viral RNA was detected by hybridization using a 32P-labeled nick-translated probe representing the vaccinia virus A20R gene.
Mutagenesis and cloning of the vaccinia virus A20R gene.
The Escherichia coli xanthine-guanine phosphoribosyltransferase (gpt) gene with an adjacent vaccinia virus P7.5 promoter was excised by NheI digestion of a plasmid provided by A. Garcia. After blunting of the staggered ends and EcoRI digestion, the DNA was ligated to pBluescript KS+, which had been previously digested with SmaI and EcoRI, yielding pBSgpt. The β-glucuronidase (gus) gene with a modified vaccinia virus H5R promoter was excised by BamHI and NotI digestions of the plasmid pZippy-neo/gus provided by Teri Shors and ligated to the same sites of cleaved pBSgpt, yielding pBSgptgus.
The A20R gene was amplified by PCR using the following upstream (U) and downstream (D) primers, in which the BglII sites are shown in italics. D-A20R; 5′ AGAAGATCTAACTCGCAAAATCGTTAAGAA 3′; and U-A20R, 5′ AAAGATCTAATTTGCATTAGTGCCTCCATGAAC 3′.
Overlapping PCR was used to mutate charged amino acids in the A20R ORF to alanines. A first PCR product was made with the U primer shown above and a primer that contained the mutations D-A20-x), in which x is the first charged amino acid in a cluster. A second PCR product was made with the D primer shown above and a primer that contained the complementary strand mutations (U-A20R-x). D-A20-x and U-A20-x also contained silent mutations to create restriction sites that allowed us to distinguish mutant and wild-type viruses. The primers are shown below, with the mutated nucleotides in boldface, the restriction sites in italics, and the names of the corresponding restriction endonucleases in parentheses. D-A20R-62, 5′ GACGTCAATATCTGATTATTATGCGGCCGTAGCAAATGCACCGTTTAATATTGATCCGGGC 3′ (EagI); U-A20R-62, 5′ GCCCGGATCAATATTAAACGGTGCATTTGCTACGGCCGCATAATAATCAGATATTGACGTC 3′; D-A20R-108, 5′ GGAAACTCTTTTCAAATACCAGCTGCCATGGCAAGTGCGTGTAACAAAG 3′ (NcoI); U-A20R-108, 5′ CTTTGTTACACGCACTTGCCATGGCAGCTGGTATTTGAAAAGAGTTTCC 3′; D-A20R-167, 5′ GTACGGATTAAACATACCCAAATATTTAGCAA TTGCAATAGCGGCCGCCACATTATTTGACGACGAGTTATACTCTATT ATAGAACGC 3′ (NotI); U-A20R-167, 5′ GCGTTCTATAATAGAGTATAACTCGTCGTCAAATAATGTGGCGGCCGCTATTGCAATTGCTAAATATTTGGGTATGTTTAATCCGTAC 3′; D-A20R-177, 5′ GAAGACACATTATTTGCGGCCGCGTTATACTCTATTATAG 3′ (NotI); U-A20R-177, 5′ CTATAATAGAGTATAACGCGGCCGCAAATAATGTGTCTTC 3′; D-A20R-185, 5′ GTTATACTCTATTATAGCAGCCTCTTTTGCAGCTGCATTTCCAAAAATATCC 3′ (PvuII); U-A20R-185, 5′ GGATATTTTTGGAAATGCAGCTGCAAAAGAGGCTGCTATAATAGAGTATAAC 3′; D-A20R-204, 5′ CCATATCGTATATTAAGTTGGGTGCACTTGCGGCGCAAGTTGTAGACTT T T TC 3′ (ApaLI); U-A20R-204, 5′ GAAAAAGTCTACAACTTGCGCCGCAAGTGCACCCAACTTAATATACGATATGG 3′; D-A20R-224, 5′ CTCGTTCATGTATATTGAGTCCATCGCGGTAGCTGCGATCGGAGATAATATTTTTATTCCTAGCG 3′ (PvuI); U-A20R-224, 5′ CGCTAGGAATAAAAATATTATCT CCGATCGCAGCTACCGCGATGGACTCAATATACATGAACGAG 3′; D-A20R-248, 5′ GGAAAAAAGATATTAGTAGCAGCTGTAGCCGCTTTAATACGATCTAAGG 3′ (PvuII); U-A20R-248, 5′ CCTTAGATCGTATTAAAGCGGCTACAGCTGCTACTAATATCTTTTTTCC 3′; D-A20R-255, 5′ GTA AAAGATGTAGACCATTTAATAGCATCTGCGGTTGCAGCTGCTACATT TGTAAAAGTGAAAGAGGAAAACAC 3′ (PvuII); U-A20R-255, 5′ GTG TTTTCCTCTTTCACTTTTACAAATGTAGCAGCTGCAACCGCAGATGCTATTAAATGGTCTACATCTTTTAC 3′; D-A20R-265, 5′ GAACATACATTTGTAGCAGTGGCAGCTGCAAACACATTTTCCATTTTATAC 3′ (PvuII); U-A20R-265, 5′ GTATAAAATGGAAAATGTGTTTGCAGCTGCCACTGC TACAAATGTATGTTC 3′; D-A20R-345, 5′ GTTGATGAGATTAATAAATCCGGAACTGTAGCTGCAGCAATAGCAAACCAATCAGCATTTGATTTAAG 3′ (PstI); and U-A20R-345, 5′ CTTAAATCAAATGCTGATTGGTTTGCTATTGCTGCAGCTACAGTTCCGGATTTATTAATCTCATCAAC 3′.
A mixture of the two purified PCR products served as the template for a third PCR, which was performed with the A20R U and D primers. This final product was gel purified, digested with BglII, and cloned into the BamHI site of pBSgptgus to form pBSgptgus-A20Rx, in which the A20R ORF has mutations. The plasmids were sequenced to confirm the desired mutation and the absence of spurious changes.
Transient dominant selection and isolation of mutants.
Replacement of the wild-type A20R allele with mutant alleles was performed by the strategy of transient dominant selection (9). Semiconfluent BS-C-1 cells in 35-mm-diameter dishes were infected with 0.05 PFU of vaccinia virus strain WR per cell at 37°C. After 2 h, 1.0 μg of the appropriate pBSgptgus-A20Rx plasmid was mixed with Lipofectamine (Life Technologies) and transfected into the infected cells. After 48 h, the cells were harvested, frozen and thawed three times, and sonicated twice for 30 s, and the virus was plaqued on BS-C-1 cells in the presence of MPA, xanthine, and hypoxanthine at concentrations of 25, 250, and 15 μg per ml, respectively. Expression of the gpt and gus genes, which were integrated into the viral genome by a single-crossover recombination event, provided resistance to MPA and staining with X-Gluc, respectively. After picking recombinant viruses that expressed Gpt and Gus, the virus was sequentially plaque purified in the absence of MPA, xanthine, and hypoxanthine. The loss of the gpt and gus genes was confirmed by staining plaques with X-Gluc. PCR amplification of the A20R region followed by specific restriction enzyme digestion and gel electrophoresis was performed to distinguish plaques that had the mutant A20R allele from those that had retained the wild-type A20R allele. DNA sequencing was then used to confirm the genotype. Viruses containing the mutated alleles were propagated in BS-C-1 cells at 31°C.
One-step virus growth.
Confluent BS-C-1 cells in 35-mm-diameter dishes were infected with 15 PFU of wild-type or mutant virus per cell and maintained at 31 or 39°C. The cells were harvested at 1, 4, 9, 24, 48, and 72 h after infection, collected by centrifugation, and resuspended in EMEM containing 2% fetal calf serum. The cells were disrupted by three cycles of freezing and thawing and two 30-s bursts of sonication. Virus yields were determined by titration on BS-C-1 cells at 31°C.
Pulsed-field gel electrophoresis.
Monolayers of BS-C-1 cells were infected with 15 PFU of virus per cell, harvested after 18 h, and embedded in 1% low-melting-point agarose plugs. The plugs were embedded in a 1% agarose gel in 0.5× TBE (0.0445 M Tris-borate, 0.0445 M boric acid, 1.0 mM EDTA) that was subjected to 5.5 V/cm for 18 h at 50- to 90-s intervals in an apparatus with hexagonal electrodes (Bio-Rad, Hercules, Calif.). Following electrophoresis, the DNA was transferred to an Immobilon-Ny+ transfer membrane and detected by hybridization using a radiolabeled nick-translated probe representing the vaccinia virus E9L gene.
Analysis of viral DNA synthesis by slot blot hybridization.
Confluent BS-C-1 cells in 35-mm-diameter dishes were infected with 15 PFU of wild-type or mutant virus per cell and incubated at 31 or 39°C. The cells were harvested by scraping the dishes with a rubber policeman. The cells were collected by centrifugation, washed once with phosphate-buffered saline (140 mM NaCl, 2 mM KCl, 10 mM Na2HPO4, 1 mM KH2PO4 [pH 7.4]), and resuspended in 0.2 ml of 1.5 M NaCl–0.15 M sodium citrate (pH 7.0)–1 M ammonium acetate. The cells were disrupted by three cycles of freezing and thawing, and an additional 0.2 ml of ammonium acetate buffer was added. After being vortexed, 50 μl of each sample was spotted in triplicate onto an Immobilon-Ny+ transfer membrane in a slot blot apparatus. The membrane was treated sequentially with 0.5 M NaOH–1 M Tris-HCl (pH 7.5) and 0.3 M NaCl–0.03 M sodium citrate (pH 7.0) and cross-linked by UV irradiation. The membrane was air dried, and viral DNA was detected by hybridization using a radiolabeled nick-translated probe representing the vaccinia virus E9L gene. The blot was exposed to a phosphor screen, and data were acquired on a Storm PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.), quantified with ImageQuant software (Molecular Dynamics), and plotted with SigmaPlot (SSPS, Chicago, Ill.).
Analysis of viral protein synthesis by metabolic labeling.
Confluent BS-C-1 cells in 35-mm-diameter dishes were infected with 15 PFU of wild-type or mutant vaccinia virus per cell and maintained at 31 or 39°C. Individual plates of cells were washed and incubated with prewarmed methionine-free EMEM for 30 min and then with methionine-free EMEM supplemented with 100 μCi of [35S]methionine per ml for 30 min. The cells were then dislodged by scraping, collected by centrifugation, resuspended in phosphate-buffered saline, and lysed by the addition of sodium dodecyl sulfate (SDS) sample buffer (1% SDS, 1% 2-mercaptoethanol, 10% glycerol, 25 mM Tris [pH 6.8]). Samples were heated to 100°C for 10 min and analyzed by electrophoresis on a 5 to 20% polyacrylamide SDS gel. After electrophoresis, the gel was dried and exposed to Kodak MR film for autoradiography.
RESULTS
Early transcription of the A20R gene.
The proteins known to be required for vaccinia virus DNA replication are expressed early in infection. Inspection of the DNA preceding the A20R ORF revealed a typical early promoter consensus sequence as defined by Davison and Moss (3). In addition, a TTTTTNT early transcription termination signal (28) was located approximately 200 nucleotides downstream of the A20R ORF. Using a 32P-labeled A20R probe, a discrete band consistent with the expected size of 1,600 nucleotides was detected by Northern blotting at 2 to 4 h after infection, and it decreased in intensity at later times (Fig. 1).
FIG. 1.
Transcription of the A20R gene. BS-C-1 cells were mock infected (ui) or infected with vaccinia virus and harvested at the indicated times. Total RNA was analyzed by gel electrophoresis and Northern blotting using a 32P-labeled probe derived from the A20R gene. The positions and nucleotide lengths of rRNAs, visualized by ethidium bromide staining, and the A20R transcript are indicated by arrows.
Construction of vaccinia virus A20R clustered charge-to-alanine mutants.
Recombinant vaccinia viruses are readily formed by transfecting a plasmid with homologous sequences into cells infected with vaccinia virus (26). The development of transient dominant selection, based on the formation of unstable, drug-resistant, single-crossover recombinants, provided a general way of isolating vaccinia viruses with targeted mutations (9). By combining this selection procedure with charge-to-alanine scanning mutagenesis, Hassett and Condit (12) isolated ts mutants of vaccinia virus. The following modification of that scheme was used for the present study. (i) Alleles of the A20R ORF with three to five alanines substituted for a cluster of charged amino acids were formed by overlap PCR. (ii) The mutated A20R ORFs were then cloned into pBSgptgus, a plasmid containing the gpt and gus genes under the control of vaccinia virus promoters. (iii) Cells were infected with vaccinia virus and transfected with a pBSgptgusA20Rx plasmid to form a single-crossover, MPA-resistant recombinant virus containing the gpt and gus genes flanked on either side by a wild-type and a mutant copy of A20R. (iv) Enrichment of the MPA-resistant virus was achieved by plaque purification in the presence of selection medium. (v) Stable viruses that lost the gpt and gus genes and contained a single wild-type or mutated A20R ORF formed plaques at a permissive temperature in the absence of the selection drugs. (vi) Finally, mutant viruses were identified by the absence of Gus expression, by restriction endonuclease analysis, and by DNA sequencing.
The amino acid sequence of the A20R ORF of vaccinia virus strain WR is shown in Fig. 2 with charged amino acids indicated. The charged amino acids in each of the 12 clusters, except for the one starting with amino acid 265, are conserved in the Copenhagen strain of vaccinia virus (11). In cluster 265, KK replaces EE in the Copenhagen strain. Because the last (unnumbered) cluster (Fig. 2) overlaps the A22R ORF, which encodes a Holliday junction endonuclease (10), this mutation was not included in the series.
FIG. 2.
Clusters of charged amino acids in the vaccinia virus A20R ORF. The translated sequence of the A20R ORF is presented, with charged amino acids in boldface. Groups of amino acids containing clusters of charged amino acids are underlined. The number above each group represents the first charged amino acid in the cluster.
Ten individual MPA-resistant plaques from each of the 11 transfections were replaqued three times in the presence of MPA, and the insertion of the plasmid into the viral genome was confirmed by staining the plaques with X-Gluc. Further plaque purifications were carried out at 31°C in the absence of drugs to isolate individual viruses that had lost the gpt and gus genes. For each original transfection, a total of 60 plaques were screened by X-Gluc staining and PCR amplification to determine the presence of the wild-type or mutated A20R ORF. DNA sequencing indicated that we had obtained 7 of the desired 11 mutants. For simplicity, the mutants were named according to the number of the first amino acid mutated: mut62, mut108, mut177, mut185, mut248, mut265, and mut345 (Fig. 2). Repeated efforts to isolate viruses containing the remaining four mutations failed, implying that they have lethal phenotypes.
Plaque phenotypes of A20R mutant viruses.
The seven mutant viruses were propagated at 31°C and tested for a ts phenotype by plaque formation at 31 and 39°C. The parental vaccinia virus strain WR formed similar-size round plaques at the two temperatures (Fig. 3). The plaque phenotypes of the mutant viruses fell into three classes (Fig. 3). mut62 and mut108 produced plaques that were similar to those of the wild-type virus with no discernible temperature sensitivity. mut177, mut265, and mut345 produced fewer and smaller plaques at 39 than at 31°C. mut185 and mut248 had the most stringent ts phenotypes, and neither formed plaques at 39°C. At 31°C, plaque formation by mut185 appeared to be normal whereas mut248 produced plaques that were slightly smaller than those of the wild type. Thus, five of the seven mutants exhibited some degree of temperature sensitivity, and two of these exhibited stringent phenotypes.
FIG. 3.
Plaque formation by wild-type and A20R mutant viruses at 31 and 39°C. Confluent BS-C-1 cells were infected with similar PFU of wild-type or mutant virus and maintained at the indicated temperature. After 2 days, the medium was removed and the cells were stained with crystal violet. WR refers to the wild-type WR strain of vaccinia virus. The number on the left of each set of plaque assays corresponds to the first charged amino acid of the cluster that was mutated (Fig. 2).
One-step yields of A20R mutant viruses.
Temperature sensitivity was also determined by measuring one-step virus growth at 31 and 39°C. At the indicated times, the cells were harvested, and the virus yields were determined by plaque titration at 31°C (Fig. 4). The 72-h yields of wild-type vaccinia virus, mut62, mut108, and mut265 were similar at the two temperatures. The 24-h yield from cells infected with mut177 or mut345 was about 1 log unit less at 39 than at 31°C, but this difference narrowed after longer times. Replication of mut248 and mut185 was completely suppressed at 39°C, even after 72 h. At 31°C, mut185 produced a yield comparable to that of wild-type virus, whereas the yield of mut248 was lower by a log unit (Fig. 4). Thus, the two viruses with the most stringent plaque phenotypes, mut185 and mut248, also were blocked in virus reproduction under one-step growth conditions.
FIG. 4.
One-step growth of wild type and A20R mutant viruses. Confluent BS-C-1 cells were infected with 5 PFU of wild-type or mutant virus per cell and maintained at the indicated temperature. The infected cells were harvested at various times and disrupted, and their titers were determined on BS-C-1 cells at 31°C. The virus yields are presented as PFU per cell. Wild-type (WR) and mutant virus numbers are indicated.
Temperature shift-up and shift-down and mixed infections.
Additional virus yield experiments were carried out with mut185, which displayed the lowest ratio of virus replication at 39 versus 31°C. When cells infected with mut185 were maintained at 31°C for 2 or 6 h and then shifted to 39°C for the remaining 22 or 18 h, the resulting virus yields were comparable to those obtained when the cells were at the higher temperature from the start of infection, i.e., 2 orders of magnitude below that of an infection at the permissive temperature (Fig. 5). This timing indicated that the ts defect was unlikely to involve virus entry or early gene expression, which could occur during the first 2 to 6 h at the permissive temperature. However, when the temperature was shifted up at 12 h (at which time little infectious virus had formed, as shown in Fig. 4), the virus yield increased by nearly a log unit during the next 12 h (Fig. 5). These results suggested that the period from 6 to 12 h after infection was crucial for the activity of the A20R protein.
FIG. 5.
Temperature shift-up experiment. BS-C-1 cells were infected with 2 PFU of wild-type or mut185 virus per cell and incubated at 31°C. The temperature of the cells was either maintained for 24 h or shifted from 31 to 39°C at 2, 6, or 12 h after infection. At 24 h after infection, the cells were harvested, and the virus yields were determined by plaque assay on BS-C-1 cells at 31°C. The experimental protocol and the virus yields are depicted on the left and right, respectively.
When the infected cells were shifted down from 39 to 31°C at 2, 6, or 12 h after infection, virus yields were 1 to 2 orders of magnitude greater than those obtained when the cells were maintained at the high temperature (Fig. 6). After 12 h at 39°C, an increase in virus yield was detected within 4 h after the temperature downshift (Fig. 7). This increase was dependent on protein synthesis, as it was blocked with cycloheximide (Fig. 7). Thus, the events of the first 12 h were reversibly inhibited at the nonpermissive temperature. The virus replication following the temperature downshift could be due to either reactivation of previously synthesized A20R protein or de novo synthesis of the A20R protein.
FIG. 6.
Temperature shift-down experiment. BS-C-1 cells were infected with 2 PFU of either wild-type or mut185 virus per cell and incubated at 39°C. The temperature of the cells was either maintained for 24 h or shifted from 39 to 31°C at 2, 6, or 12 h after infection. At 24 h after infection, the cells were harvested, and the virus yields were determined by plaque assay on BS-C-1 cells at 31°C. The experimental protocol and the virus yields are depicted on the left and right, respectively.
FIG. 7.
Time course of virus replication following temperature downshift. BS-C-1 cells were infected with 2 PFU of mut185 per cell and incubated at 39°C. The cells were either harvested at 12 h after infection or shifted from 39 to 31°C and harvested at 1, 3, 6, and 12 h after the temperature shift. Where indicated, the cells were treated with 100 μg of cycloheximide per ml prior to the shift in temperature. The virus yields were determined by plaque assay on BS-C-1 cells at 31°C.
To investigate whether the mut185 protein was dominant over the wild-type protein, BS-C-1 cells were coinfected with various ratios of the wild-type and mut185 viruses at permissive or nonpermissive temperatures. At 24 h after infection, the cells were harvested, and the virus titers were determined at the permissive temperature. When cells were infected with 10 PFU of each virus, mut185 had no effect on the virus yield at 39°C (Fig. 8). Even when the cells were infected with 10 PFU of mut185 and 1 PFU of wild-type virus, replication at 39°C was only partially reduced (Fig. 8). Thus, the mut185 protein did not exert a potent dominant-inhibitory effect.
FIG. 8.
Coinfection experiment with wild-type (WR) and mut185 viruses. Confluent BS-C-1 cells were infected with wild-type and mut185 viruses at the indicated PFU per cell and maintained at either 31 or 39°C. At 24 h after infection, the cells were harvested, and virus yields were determined by plaque assay on BS-C-1 cells at 31°C.
Effect of ts mutations on viral protein synthesis.
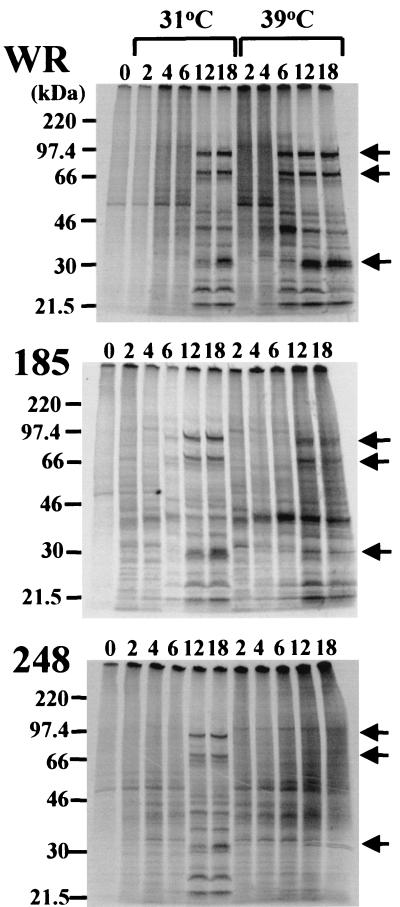
The synthesis of viral proteins is temporally regulated at the transcriptional level. The DNA packaged within the virus core is transcribed immediately after infection to produce early mRNAs. The synthesis of intermediate- and late-stage mRNAs, however, is dependent on viral DNA replication. Therefore, the types of viral proteins synthesized can provide information regarding the stage at which virus replication is blocked. Cells were infected with either wild-type or mutant viruses and metabolically labeled with [35S]methionine at various times. Cell lysates were analyzed by SDS-polyacrylamide gel electrophoresis and autoradiography. At 31°C, the patterns of protein synthesis were similar in cells infected with all three viruses: distinct early protein bands were detected at 2 to 6 h after infection, and late protein bands were prominent at 12 to 18 h (Fig. 9). Although the early patterns of viral proteins were similar in cells infected with wild-type and mutant viruses at 39°C, differences were noted at the later times. Whereas the late pattern was established at 6 h after infection with wild-type virus, the viral late pattern had not occurred by 18 h after infection with mut248 at 39°C (Fig. 9). With mut185, the late protein pattern was visible, although the intensities of the bands were less at 39 than at 31°C (Fig. 9). The diffuse “background” labeling at late times in cells infected with mut248 and mut185 at 39°C (Fig. 9) is probably due to failure to completely inhibit host protein synthesis.
FIG. 9.
Analysis of viral protein synthesis by metabolic labeling. Monolayers of BS-C-1 cells were infected with 15 PFU of wild-type (WR) or mut185 or mut248 virus per cell and maintained at 31 or 39°C. Individual plates of cells were incubated with methionine-free EMEM for 30 min and then with methionine-free EMEM plus [35S]methionine for 30 min prior to being harvested. Samples were dissociated with SDS and analyzed by electrophoresis on a 5 to 20% polyacrylamide gel. The numbers above the lanes refer to the hours after infection at which labeling was carried out. The arrows on the right point to representative viral late proteins. The electrophoretic mobilities of marker proteins of the indicated masses are shown on the left.
Protein synthesis time course experiments were also carried out by Western blotting using antiserum prepared by infecting rabbits with vaccinia virus. Again, early and late proteins were detected in lysates of cells infected by all viruses at 31°C and those of cells infected by wild-type virus and mut185 at 39°C (data not shown). However, only early viral proteins were detected in lysates of cells infected with mut248 at 39°C (data not shown).
Effect of ts mutations on viral DNA replication.
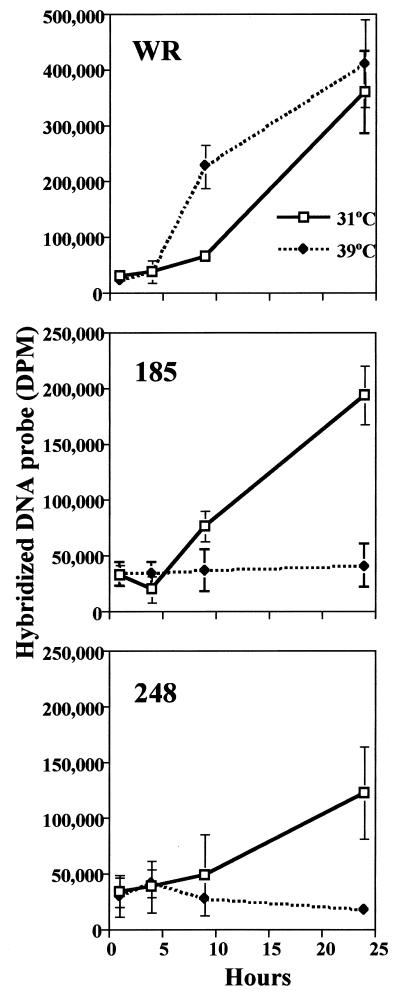
The ts effect on viral late protein synthesis suggested that DNA replication would be reduced at the nonpermissive temperature. Initial experiments, carried out by pulsed-field gel electrophoresis, indicated small amounts of viral-genome-length DNA at 24 h in cells infected with several of the ts mutants at the nonpermissive temperature compared to those in cells infected at the permissive temperature or to those in cells infected with wild-type virus (data not shown). The degree of inhibition was quantified by dot blot hybridization using 32P-labeled viral DNA as the probe. In cells infected with wild-type vaccinia virus, viral DNA synthesis was accelerated at 39°C compared to that at 31°C, but the 24-h yields of viral DNA were similar (Fig. 10). This accelerated synthesis of viral DNA at 39°C correlated with the detection of late proteins at 6 h after infection (Fig. 9). In contrast, when cells were infected with either mut185 or mut248, viral DNA synthesis was detected at 31 but not at 39°C (Fig. 10). In addition, there was some impairment of viral DNA synthesis, particularly with mut248, even at the permissive temperature (Fig. 10). These data suggested an important role for the A20R protein in viral DNA replication.
FIG. 10.
Replication of wild-type (WR) and mutant viral DNA. BS-C-1 cells were infected with 15 PFU of either wild-type, mut185, or mut248 virus per cell and maintained at 31 or 39°C. The cells were harvested at 2, 6, 9, or 24 h after infection, washed, and resuspended. Uninfected cells were harvested and used for the zero time point. Lysates were spotted onto an Immobilon-Ny+ transfer membrane. Viral DNA was detected by hybridization to 32P-labeled vaccinia virus DNA. The blot was exposed to a phosphor screen, and data were acquired on a Storm PhosphorImager and quantified using ImageQuant software. All samples were processed in triplicate and are shown as means ± standard deviations. DPM, disintegrations per minute.
DISCUSSION
Our interest in the A20R gene was stimulated by a vaccinia virus genome-wide yeast two-hybrid analysis that demonstrated interactions of the A20R protein with two other viral proteins known to be involved in DNA replication (16). Inspection of the A20R gene suggested that it has an early promoter, consistent with such a role, but no other distinguishing features were discerned. A transcriptional analysis confirmed the early expression of the A20R gene. To determine the role of the protein, we decided to make conditional lethal mutants. Three general approaches have been used to make targeted mutations in essential vaccinia virus genes. The one most extensively used employs the E. coli lac repressor-operator system to regulate expression of the viral ORF and thus create a null mutation (29). However, this method has not been successfully applied to viral genes with early promoters. One essential early gene was deleted, however, by using a complementing cell line (14). In the third approach, charged amino acids in a cluster were mutated to alanines and the mutant viruses were screened for temperature sensitivity (12). Prior to this study, charge-to-alanine mutagenesis had been used to isolate ts mutants of vaccinia virus with lesions in the G2R (12), mRNA capping enzyme (13), and H5R (4) genes.
Inspection of the A20R ORF revealed small clusters of charged amino acids throughout the sequence. We attempted to make 11 mutants for this study, but 4 (mut167, mut204, mut224, and mut255) were not isolated, suggesting that they were lethal. Of the seven mutants isolated, two (mut62 and mut108) exhibited little or no temperature sensitivity. The remaining mutants exhibited some temperature sensitivity indicated by either reduced plaque size or virus yield. Two mutants, mut185 and mut248, had stringent phenotypes and produced no plaques or infectious virus at 39°C. For mut185 and mut248, restoration of the wild-type phenotype was demonstrated by recombination with DNA containing only the A20R gene (unpublished data). Except for mut177, which exhibited a partial ts phenotype, charge-to-alanine substitutions in the central region (amino acids 167 to 255) produced stringent or presumably lethal mutants. These mutations may have weakened intrapolypeptide contacts or contacts between the A20R protein and the proteins encoded by other ORFs, such as D4R, D5R, or H5R.
Temperature shift-up experiments indicated that the crucial period for activity of the A20R protein was between 6 and 12 h, making it unlikely that the defect was in virus entry or early gene expression. A defect in viral early protein synthesis was also ruled out by metabolic labeling and Western blotting experiments that revealed similar patterns of proteins between 2 and 6 h after infection at 31 and 39°C. Since relatively little infectious virus had formed by 12 h at 31°C, the timing also made it unlikely that the defect was in a late step in virus assembly. The timing did fit either a block in DNA replication or an early stage of virion morphogenesis. Direct evidence for a block in viral DNA synthesis was obtained by quantitative dot blot hybridization analysis. With the two most stringent mutants, no viral DNA synthesis was detected when cells were infected at 39°C. Even at 31°C, there was some reduction in DNA synthesis compared to that of the wild-type virus. Temperature shift-down experiments indicated that the defect was reversible even after 12 h, a result that is compatible with the reversibility of certain drugs that block viral DNA replication. Viral late-protein synthesis, which is dependent on viral DNA replication, was blocked in one mutant (mut248) and reduced in the other (mut185) at 39°C. The synthesis of viral late proteins in cells infected with mut185 suggests that very small amounts of replicated DNA can serve as a template for intermediate and late transcription.
Vaccinia virus ts mutants that express viral early proteins but have DNA synthesis-negative phenotypes were previously identified from collections derived by random mutagenesis (2). The four DNA synthesis negative complementation groups were mapped to ORFs E9L (24), D5R (7, 8), B1R (20, 21), and D4R (18, 22), which encode the DNA polymerase, a nucleoside triphosphatase, a serine or threonine kinase, and the uracil DNA glycosylase, respectively. The A20R gene thus represents the fifth DNA negative complementation group. Traktman (23) cited unpublished data indicating that the A20R protein is a previously described 48-kDa early protein that enhances the processivity of the viral DNA polymerase in vitro (17). Based on interactions determined with the yeast two-hybrid system (16), A20R probably forms part of a multienzyme replication complex that includes the D4R and D5R proteins.
ACKNOWLEDGMENTS
We thank Christine White, Joanna Shisler, Linda Wyatt, and Tatiana Senkevich for protocols, suggestions, and discussion of the data and Norman Cooper for cells and viruses.
Koji Ishii was supported by a Japan Science and Technology Overseas Research Fellowship.
REFERENCES
- 1.Black E P, Moussatche N, Condit R C. Characterization of the interactions among vaccinia virus transcription factors G2R, A18R, and H5R. Virology. 1998;245:313–322. doi: 10.1006/viro.1998.9166. [DOI] [PubMed] [Google Scholar]
- 2.Condit R C, Niles E G. Orthopoxvirus genetics. Curr Top Microbiol Immunol. 1990;169:1–40. doi: 10.1007/978-3-642-75605-4_1. [DOI] [PubMed] [Google Scholar]
- 3.Davison A J, Moss B. The structure of vaccinia virus early promoters. J Mol Biol. 1989;210:749–769. doi: 10.1016/0022-2836(89)90107-1. [DOI] [PubMed] [Google Scholar]
- 4.DeMasi J, Traktman P. Clustered charge-to-alanine mutagenesis of the vaccinia virus H5 gene: isolation of a dominant, temperature-sensitive mutant with a profound defect in morphogenesis. J Virol. 2000;74:2393–2405. doi: 10.1128/jvi.74.5.2393-2405.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellison K S, Peng W, McFadden G. Mutations in active-site residue of the uracil-DNA glycosylase encoded by vaccinia virus are incompatable with virus viability. J Virol. 1996;70:7965–7973. doi: 10.1128/jvi.70.11.7965-7973.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans E, Klemperer N, Ghosh R, Traktman P. The vaccinia virus D5 protein, which is required for DNA replication, is a nucleic acid-independent nucleotide triphosphatase. J Virol. 1995;69:5353–5361. doi: 10.1128/jvi.69.9.5353-5361.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans E, Traktman P. Characterization of vaccinia virus DNA replication mutants with lesions in the D5 gene. Chromosoma. 1992;102:S72–S82. doi: 10.1007/BF02451789. [DOI] [PubMed] [Google Scholar]
- 8.Evans E, Traktman P. Molecular genetic analysis of a vaccinia virus gene with an essential role in DNA replication. J Virol. 1987;61:3152–3162. doi: 10.1128/jvi.61.10.3152-3162.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falkner F G, Moss B. Transient dominant selection of recombinant vaccinia viruses. J Virol. 1990;64:3108–3111. doi: 10.1128/jvi.64.6.3108-3111.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia A D, Aravind L, Koonin E V, Moss B. Bacterial-type DNA Holliday junction resolvases in eukaryotic viruses. Proc Natl Acad Sci USA. 2000;97:8926–8931. doi: 10.1073/pnas.150238697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goebel S J, Johnson G P, Perkus M E, Davis S W, Winslow J P, Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990;179:247–266. doi: 10.1016/0042-6822(90)90294-2. ; 517–563. [DOI] [PubMed] [Google Scholar]
- 12.Hassett D E, Condit D E. Targeted construction of temperature-sensitive mutations in vaccinia virus by replacing clustered charged residues with alanine. Proc Natl Acad Sci USA. 1994;91:4554–4558. doi: 10.1073/pnas.91.10.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassett D E, Lewis J I, Xing X, DeLange L, Condit R C. Analysis of a temperature-sensitive vaccinia virus mutant in the viral mRNA capping enzyme isolated by clustered charge-to-alanine mutagensis and transient dominant selection. Virology. 1997;238:391–409. doi: 10.1006/viro.1997.8820. [DOI] [PubMed] [Google Scholar]
- 14.Holzer G, Falkner F G. Construction of a vaccinia virus deficient in the essential DNA repair enzyme uracil DNA glycosylase by a complementing cell line. J Virol. 1997;71:4997–5002. doi: 10.1128/jvi.71.7.4997-5002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovacs G R, Moss B. The vaccinia virus H5R gene encodes late gene transcription factor 4: purification, cloning and overexpression. J Virol. 1996;70:6796–6802. doi: 10.1128/jvi.70.10.6796-6802.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCraith S, Holtzman T, Moss B, Fields S. Genome-wide analysis of vaccinia virus protein-protein interactions. Proc Natl Acad Sci USA. 2000;97:4879–4884. doi: 10.1073/pnas.080078197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald W F, Klemperer N, Traktman P. Characterization of a processive form of the vaccinia virus DNA polymerase. Virology. 1997;234:168–175. doi: 10.1006/viro.1997.8639. [DOI] [PubMed] [Google Scholar]
- 18.Millns A K, Carpenter M S, DeLange A M. The vaccinia virus-encoded uracil DNA glycosylase has an essential role in viral DNA replication. Virology. 1994;198:504–513. doi: 10.1006/viro.1994.1061. [DOI] [PubMed] [Google Scholar]
- 19.Moss B. Poxviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2637–2671. [Google Scholar]
- 20.Rempel R E, Anderson M K, Evans E, Traktman P. Temperature-sensitive vaccinia virus mutants identify a gene with an essential role in viral replication. J Virol. 1990;64:574–583. doi: 10.1128/jvi.64.2.574-583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rempel R E, Traktman P. Vaccinia virus B1 kinase—phenotypic analysis of temperature-sensitive mutants and enzymatic characterization of recombinant proteins. J Virol. 1992;66:4413–4426. doi: 10.1128/jvi.66.7.4413-4426.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stuart D T, Upton C, Higman M A, Niles E G, McFadden G. A poxvirus-encoded uracil DNA glycosylase is essential for virus viability. J Virol. 1993;67:2503–2512. doi: 10.1128/jvi.67.5.2503-2512.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Traktman P. Poxvirus DNA replication. In: DePamphilis M L, editor. DNA replication in eukaryotic cells. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1996. pp. 775–793. [Google Scholar]
- 24.Traktman P, Sridhar R C, Roberts B E. Transcriptional mapping of the DNA polymerase gene of vaccinia virus. J Virol. 1984;49:125–131. doi: 10.1128/jvi.49.1.125-131.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Upton C, Stuart D T, McFadden G. Identification of a poxvirus gene encoding a uracil DNA glycosylase. Proc Natl Acad Sci USA. 1993;90:4518–4522. doi: 10.1073/pnas.90.10.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weir J P, Bajszar G, Moss B. Mapping of the vaccinia virus thymidine kinase gene by marker rescue and by cell-free translation of selected mRNA. Proc Natl Acad Sci USA. 1982;79:1210–1214. doi: 10.1073/pnas.79.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wertman K F, Drubin D G, Botstein D. Systematic mutational analysis of the yeast ACT1 gene. Genetics. 1992;132:337–350. doi: 10.1093/genetics/132.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuen L, Moss B. Oligonucleotide sequence signaling transcriptional termination of vaccinia virus early genes. Proc Natl Acad Sci USA. 1987;84:6417–6421. doi: 10.1073/pnas.84.18.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Moss B. Inducer-dependent conditional-lethal mutant animal viruses. Proc Natl Acad Sci USA. 1991;88:1511–1515. doi: 10.1073/pnas.88.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]