Abstract
Real-time continuous monitoring of non-cognitive markers is crucial for the early detection and management of chronic conditions. However, current diagnostic methods are often invasive and primarily available in clinical settings, limiting their use for at-home monitoring. To bridge this gap, soft and flexible hydrogels have emerged while facing challenges such as low electrical conductivity, poor mechanical properties and adhesion, limited durability, and low biocompatibility. To address these challenges, we have developed an elastic and biodegradable hydrogel-based wearable sensor with unparalleled accuracy, mechanical properties, and durability for intelligent gait sensing, capable of adhering to skin while monitoring real-time human health. Employing an innovative supramolecular engineering strategy, we synthesized a pseudo-slide-ring hydrogel, named β-CD-g-(pAAm/pAETAc), which combines polyacrylamide (pAAm), β-cyclodextrin (β-CD), and poly 2-(acryloyloxy)ethyltrimethylammonium chloride (AETAc) bio ionic liquid (Bio-IL). This novel approach decouples conflicting mechano-chemical effects arising from different molecular building blocks and provides a balance of mechanical toughness (1.1×106 Jm−3), flexibility, conductivity (~0.29 S m−1), and tissue adhesion (~27 kPa), along with rapid self-healing and remarkable stretchability (~3000%). Unlike traditional hydrogels, our one-pot synthetic strategy avoids chemical crosslinkers and metallic nanofillers, reducing cytotoxicity and immunogenicity risks. While the pAAm building blocks provide mechanical strength and toughness, the formation of the pseudo-slide-ring structure endows it with high stretchability and flexibility suitable for real-world applications. Additionally, the rational combination of pAAm backbone with naturally derived polymers, such as β-CD and pAETAc, ensures biocompatibility and biodegradability, as reflected in our cellular and in vivo biocompatibility studies. Furthermore, the hydrogel offers transparency, passive-cooling, UV-shielding, and 3D printability, enhancing its practicality for everyday use. Most importantly, the hydrogel-based sensor demonstrated improved efficiency, stability, and sensitivity in motion/haptic sensing. This multifunctional sensor represents a significant advancement in minimally invasive real-time healthcare monitoring, particularly in human movement and motor dysfunction applications.
Keywords: Bioadhesive, Self-healing conductive hydrogel, Wearable sensor, Stretchable electronics, Health monitoring, Diagnostics
Graphical Abstract

Development of an elastic, skin-adhesive, biocompatible, and biodegradable hydrogel-based wearable sensor for human health monitoring, inspired by pseudo-slide-ring hydrogels. The novel hydrogel-based sensor offers high accuracy, mechanical strength, 3D printability, passive cooling, UV resistance, self-healing, and durability. With tunable properties, biocompatibility, and biodegradability, the hydrogel-based sensor shows significant potential for real-time healthcare monitoring in human movement and motor dysfunction applications.
1. Introduction
Wearable flexible electronic sensors capable of detecting physical indicators (e.g., strain, motion)[1] offer an efficient way to monitor various health conditions and applications, including identity verification,[2] assistive technologies,[3] sports analytics,[4] human-computer interaction,[5] fall detection,[6] and real-time healthcare monitoring.[1b, 7] These sensors have been utilized in intelligent gait recognition systems, enabling the detection of coordinated limb movements and overall footstep patterns.[8] However, for effective real-world applications, these wearable sensors must possess three key properties: (i) optimal sensitivity with a low signal-to-noise ratio to ensure precise detection of minute movement changes; (ii) sufficient mechanical stability and adhesion to minimize stress on the bio-electronic interface (i.e., skin-electronics); and (iii) prolonged durability and repeatability.[1c, 9] Despite significant advancements in diagnostic methods such as neuropsychological assessments,[10] imaging,[11] and molecular biomarker analysis,[12] these techniques remain costly, requiring specialized equipment and expertise in clinical settings. The integration of novel materials in healthcare technologies has also shown promising results. Recently, diverse types of materials such as cellulose nanofibrils,[13] polyaniline (PANI),[14] polypyrrole (PPy),[13, 15] PAAm loaded with various metal ions,[16] polyvinyl alcohol (PVA),[15] graphene,[17] and MXene,[18] have been utilized for fabricating ultra-sensitive and mechanically robust sensors for continuous health monitoring. Similarly, advances in nanomaterials have led to the development of biocompatible and durable nanocomposite sensor components, paving the way for non-invasive monitoring solutions in diverse healthcare settings.[19] These examples underscore the pivotal role of novel materials in enhancing the performance and applicability of healthcare technologies, ultimately improving patient outcomes and healthcare delivery efficiency.
A feasible alternative to current diagnostic methods is the real-time monitoring of non-cognitive markers, such as mobility dysfunctions, which frequently precede cognitive impairments.[20] Real-time monitoring of non-cognitive markers provides continuous, cost-effective, and accessible health tracking, which can be particularly beneficial for early detection and ongoing management of chronic conditions. This monitoring boasts several advantages. Firstly, continuous data collection provides a comprehensive view of a patient’s condition for diseases with fluctuating symptoms like Parkinson’s.[21] Secondly, accessibility and convenience allow monitoring in everyday environments, enhancing patient consistency.[22] Thirdly, cost-effectiveness minimizes expenses associated with diagnostics and hospital visits, which is especially beneficial in resource-limited situations. Lastly, early detection of symptoms such as changes in gait, tremors, and other physical activities, can often be detected earlier than cognitive impairments in diseases like Parkinson’s, significantly improving outcomes.[23] Wearable sensors have shown promise in detecting these changes accurately, offering non-invasive early diagnosis. This technology has transformed chronic disease management, such as diabetes,[24] by providing real-time data for better control. For instance, continuous glucose monitors have revolutionized diabetes management by providing real-time blood sugar levels, enabling better glycemic control than traditional methods.[25] Real-time monitoring systems for fall detection use accelerometers and gyroscopes to detect falls in elderly patients. These systems have been shown to significantly reduce the time to emergency response, thereby reducing morbidity and mortality associated with falls.[26] Wearable cardiac monitors can also detect arrhythmias and other cardiac events in real-time, providing critical data that can prevent serious outcomes like stroke or heart attack.[27] These devices have proven to be more effective than periodic ECGs in detecting intermittent arrhythmias.[28]
Moreover, wearable flexible electronic sensors are preferred over implantable sensors for detecting physical indicators due to several key advantages. Their non-invasive nature allows them to be easily attached to the skin without requiring surgical procedures, reducing the risk of complications, infections, and recovery time.[29] They are designed for convenience and comfort, being lightweight, flexible, and easy to remove or replace, unlike implantable sensors that need medical intervention for installation and removal.[30] Their ease of use allows integration into daily life without disrupting normal activities, leading to more accurate and representative real-time data collection.[31] Users also have more control over wearable sensors, being able to start and stop monitoring as needed, which is not possible with implantable sensors managed by healthcare professionals.[1c] Although implantable sensors often have longer operational lifespans and stable power supplies, wearable flexible sensors are preferred for their non-invasive nature, ease of use, cost-effectiveness, and ability to provide continuous, accessible health monitoring.[32]
To meet these criteria, soft and flexible hydrogels with tunable mechano-physical and electrochemical properties have emerged as a substrate to fabricate wearable sensors. However, they face challenges due to their low electrical conductivity (ranging from 10−5 to 10−1 S cm−1), which is essential for digital and power electronics in sensing applications.[33] To overcome this issue, hydrogel matrices are often filled with conductive fillers such as carbon nanotubes (CNTs),[34] graphene,[7c, 35] MXenes,[1a, 36] metallic micro/nanoparticles,[37] and intrinsically conductive polymers such as PANI,[38] (PPy),[39] and poly(ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS).[40] However, these nanocomposite hydrogels require a high concentration of conductive fillers, leading to inconsistent dispersion and agglomeration, which adversely affects sensor reliability as well as biocompatibility. Moreover, there is a trade-off between electrical conductivity and mechanical properties. For instance, pure PEDOT:PSS hydrogel showed excellent electrical conductivity (40 S cm−1) but due to inherently rigid conjugated π bonds, it lacked adequate mechanical properties for biomedical applications (i.e., high Young’s modulus ~2 MPa and limited stretchability <35%).[33]
In addition, wearable hydrogel-based sensors must adhere to skin or tissues to ensure secure attachment, patient comfort, functionality, and durability. However, certain bodily movements and physiological conditions like sweating can reduce the adhesive strength of the engineered hydrogels, resulting in motion artifacts and arbitrary displacement of the sensor.[41] Incorporating catechol-based structures like dopamine,[42] tannin,[43] and gallic acid[44] into the 3D hydrogel networks has recently been explored to improve the adhesiveness of the hydrogel-based sensors. While this strategy leverages molecular interactions to crosslink polymeric backbones, it often decreases mechanical strength, resulting in cohesive failure. Besides, catechol groups in these moieties can undergo auto-oxidation, which reduces their adhesiveness over time.[45] Moreover, these methods necessitate additional synthesis steps, often employing sensitive crosslinking agents, which may pose issues such as cytotoxicity, irritation, and immunogenic responses.
Furthermore, the biodegradability of a biosensor needs careful consideration as it has two major benefits. First, biodegradable sensors can be used as implantable devices for sensing and are safe for human use, as they can be metabolized in the body without triggering harmful physiological reactions. Second, these biosensors represent the most feasible and optimal solution to tackle the problem of unmanageable electronic waste (i.e., e-waste).[46] Thus biodegradability, combined with optimal sensitivity for accurate detection, mechanical stability to withstand daily activities, and durability for long-term use, is essential for the effectiveness of wearable sensors in real-world healthcare settings.[47] By integrating biocompatible materials and designs, we can create sensors that are not only functional and reliable but also safe and comfortable for continuous monitoring of various health conditions. Therefore, there is an unmet need for the development of hydrogel-based sensors that provide unparalleled accuracy, mechanical resilience, and durability, while also minimizing cytotoxicity.
A novel type of supramolecular polymeric network (SPN)-based hydrogels, known as slide-ring or polyrotaxane gels, has recently gained considerable attention. In this hydrogel network, topological crosslinking occurs through ring structures such as α-cyclodextrin (α-CD), forming figure-eight crosslinked polymer chains.[48] The primary distinction between traditional chemical crosslinked gels and slide-ring gels lies in their crosslinking mechanisms. Traditional chemical crosslinking involves fixed crosslinking points in the polymer, leading to non-uniform stress distribution within the gel network when subjected to external forces, potentially disrupting the network structure. In contrast, slide-ring gels feature ring structures that act as mobile crosslinking points, allowing them to slide along the polymer chain akin to a “pulley” when external forces are applied. This “pulley effect” ensures even distribution of internal stress across chain segments and the entire network, imparting slide-ring hydrogels with exceptional mechanical properties such as extreme softness, relatively low initial modulus, and enhanced ductility. Recent advancements have demonstrated that high conductivity and stretchability can be simultaneously achieved through engineering a slide-ring hydrogel composed of polyrotaxanes (consisting of methacrylated polyethylene glycol (PEGMA) and α-CD) and PEDOT:PSS.[48] Despite these exciting features, the design and fabrication of slide-ring hydrogels for hydrogel-based sensors, such as strain sensors, remain largely unexplored.[49] This could be attributed to the relatively low aqueous solubility of polypseudorotaxane and the complex end-capping process of rotaxane.[50] Moreover, the design of slide-ring hydrogels is typically limited to the use of CDs as the rings and poly(ethylene glycol) (PEG) as an axial polymer.
To address the need for a multifunctional adhesive, flexible, and custom-designed hydrogel-based wearable sensor for intelligent gait sensing, inspired by slide-ring hydrogels, we aimed to develop an elastic and adhesive biodegradable wearable sensor capable of adhering to skin while monitoring real-time human health. This novel supramolecular pseudo-slide-ring conductive hydrogel consisted of polyacrylamide (pAAm), β-cyclodextrin (β-CD), and 2-(acryloyloxy)ethyltrimethylammonium chloride (pAETAc) bio ionic liquid (Bio-IL) and was synthesized via a single-pot reaction with a supramolecular crosslinking strategy, eliminating the need for a chemical crosslinker. Through precise control of component ratio, we demonstrated tunability in mechanical, adhesive, and conductive properties. While the pAAm building blocks can provide mechanical strength and toughness, the formation of the SPN-based pseudo-slide-ring structure endows it with high stretchability and flexibility suitable for real-world applications. In this pseudo-slide-ring hydrogel structure, polymer chains with side functional groups can be interlocked via supramolecular complexation, allowing supramolecular crosslinks to traverse along the chains, evenly distributing tension and neutralizing internal stress. This can result in high stretchability, elasticity, flexibility, and self-healing capability. Additionally, the engineering of pAAm backbone with other naturally derived polymers (β-CD and Bio-IL) reduces the cytotoxicity risk and increases the degradability of the hydrogel, thereby ensuring desirable properties while preserving conductivity. The presence of β-CD not only helps to form the pseudo side ring structure but also ensures desirable biocompatibility and biodegradability of the engineered hydrogel network. The use of pAETAc also enables the hydrogel to be conductive and eliminates the requirement of external conducting fillers like metal nanoparticles, graphene, CNT, etc. Therefore, this “metal-free” sensing platform makes the hydrogel biocompatible by omitting the chance of metal toxicity and adverse metabolic events related to the elimination of the conductive nanofillers from the body. To demonstrate the clinical applicability of the engineered hydrogel, in vitro cytotoxicity, and in vivo biocompatibility and biodegradability are evaluated using NIH 3T3 cells, and subcutaneous implantation into the dorsal skin of rats, respectively. As a crucial aspect of designing a personalized wearable sensor, we also assess the 3D printability, passive-cooling effectiveness, UV-resistant capability, and self-healing ability of the hydrogel. Then, we explore the potential application of this adhesive hydrogel as a flexible wearable sensor in intelligent gait sensing, including monitoring of PD progression through chrono amperometry analysis. Overall, our engineered SPN-based sensor has the potential to be expanded for minimally invasive real-time healthcare monitoring, particularly in human movement and motor dysfunction applications.
2. Results and Discussion
2.1. Rational Design and Synthesis of β-CD-g-(pAAm/pAETAc) Hydrogel
To engineer an adhesive and conductive SPN-based pseudo-slide ring hydrogel with desired mechanical robustness, strong adhesion, self-healing ability, and proper electrical performance, we utilized three key components: AAm, AETAc, and β-CD. AAm was selected as the elastic component of the hydrogel matrix due to its tunable mechanical properties, capacity to facilitate strain recovery, and low immunogenicity.[51] AETAc, composed of a quaternary ammonium group and an unsaturated vinyl group (easily polymerizable), was employed to enhance the conductivity of the hydrogel through the introduction of positively charged moieties (i.e., quaternary ammonium groups) into the hydrogel network, making it suitable for various sensing and electro-responsive applications. Additionally, the charged moieties could enhance the adhesiveness of the hydrogel through dipole-dipole interaction between the quaternary ammonium group of AETAc and the carboxyl groups present on the tissue surfaces.[52] Integration of β-CD in the hydrogel matrix could facilitate supramolecular host-guest interactions induced by crosslinking, improve biocompatibility, and enable self-healing capabilities.[53] Finally, free radical polymerization among the three components was employed to facilitate rapid monomer grafting.[54] By controlling multiple interactions among the functionalities of semi-interpenetrating polymer networks, based on flexible AAm, conductive AETAc, and macrocyclic β-CD, we were able to fabricate β-CD-g-(pAAm/pAETAc) hydrogels with excellent mechanical properties, appropriate adhesiveness, sufficient conductivity, self-healing properties, and biocompatibility. This SPN-based β-CD-g-(pAAm/pAETAc) pseudo slide-ring hydrogel was synthesized through one-pot free-radical mediated graft co-polymerization of two monomers (i.e., AAm and AETAc) on the β-CD skeleton, using potassium persulfate (KPS) as a free-radical initiator (Figure 1a). Initially, KPS dissociated under heating (60 ± 5°C) in an inert atmosphere to generate anionic sulfate (SO4•−) radicals that extracted hydrogen from the hydroxyl groups present in β-CD, leading to the formation of intermediate alkoxy radicals.[55] These highly reactive alkoxy radicals then attacked the vinylic double bonds, resulting in the formation of covalent bonds (Figure 1b-i) between the monomers (i.e., AAm and AETAc) that are in proximity, initiating chain elongation. Subsequently, these monomers became free radical donors (i.e., macroradicals) for neighboring moieties, and acted as reaction centers for sustaining the growth of grafted chains and the formation of a co-polymeric gel network.[56] Simultaneously, potential physical crosslinking mechanisms, including H-bonding among the copolymers (Figure 1b-ii), macrocyclic host-guest complexation (Figure 1b-iii) involving the hydrophobic cavity of β-CDs and quaternary amine groups of AETAc, and electrostatic interactions (Figure 1b-iv), contribute to the overall network formation. This macrocyclic host-guest complexation was further confirmed by the observed increase in the intensity of the UV absorbance peak at ~290 nm without any red or blue shift in the peak position (Figure 1c), contributing additional order and directionality to the emerging network, ultimately facilitating the formation of the SPN-based pseudo-slide-ring β-CD-g-(pAAm/pAETAc) hydrogel.[57]
Figure 1. Synthesis and chemical characterization of β-CD-g-(pAAm/pAETAc) hydrogel and its precursors.
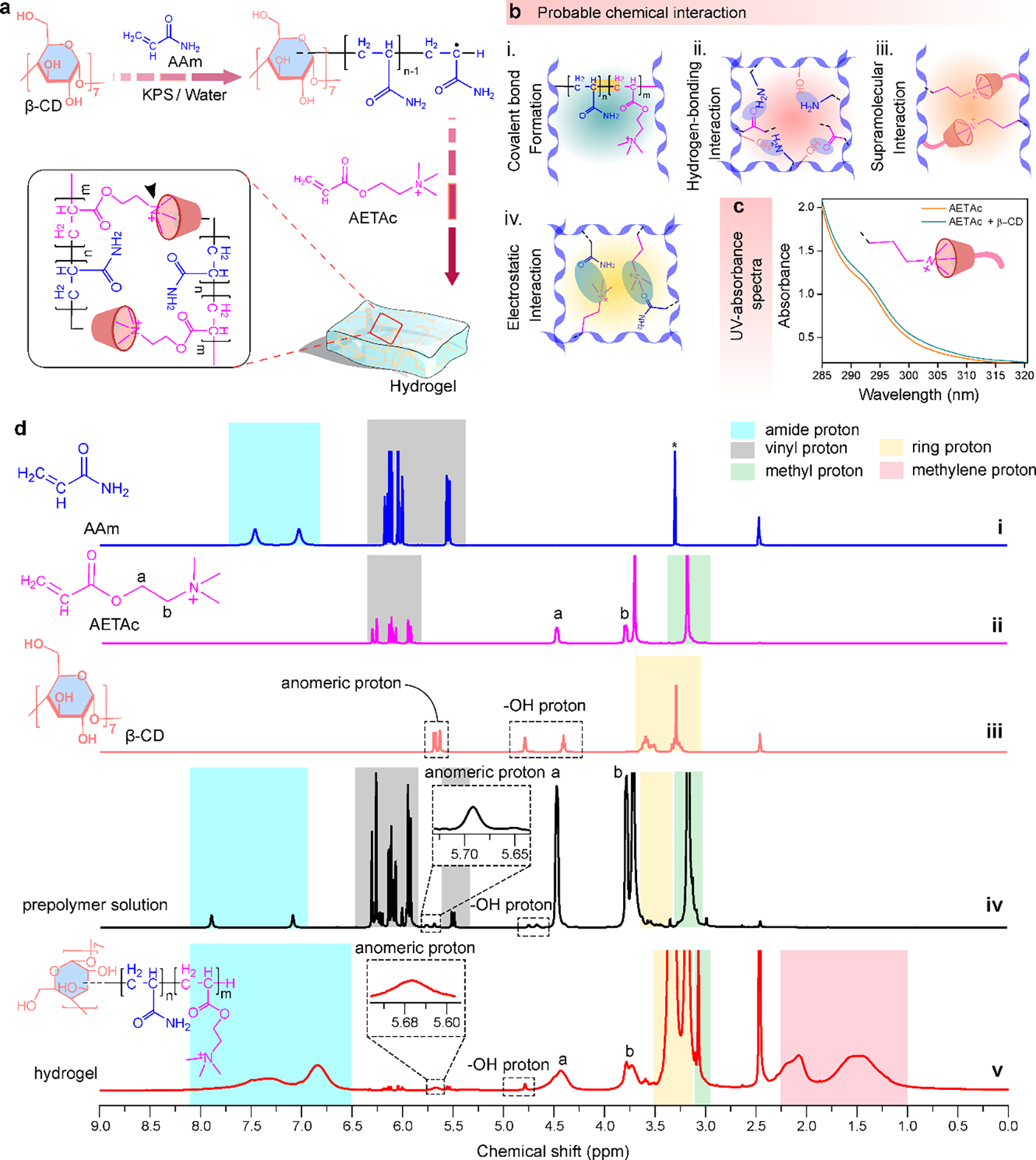
(a) Synthetic steps for the synthesis of β-CD-g-(pAAm/pAETAc) hydrogel. (b) Plausible interactions responsible for the formation of the hydrogel. (c) UV spectra of only AETAc and AETAc combined with β-CD. (d) 1H NMR spectrum of (i) AAm, (ii) AETAc, (iii) β-CD, (iv) prepolymer solution, and (v) crosslinked β-CD-g-(pAAm/pAETAc) hydrogel.
The synthesis of the hydrogel was also confirmed using proton nuclear magnetic resonance (1H NMR) spectroscopy. The 1H NMR spectrum of AAm revealed peaks at δ = 7.4 and 7.0 ppm, arising from amide protons (highlighted in blue) (Figure 1d-i). On the other hand, the peaks at δ = 5.5 and 6.1 ppm corresponded to the vinylic protons of AAm moiety (highlighted in gray). Besides, the 1H NMR spectrum of AETAc showed peaks at δ = 4.4 and 3.8 ppm representing Ha (-CH2) and Hb (-CH2) protons, respectively (Figure 1d-ii). Also, the peak at δ = 3.1 ppm revealed the presence of three methyl groups at the end of this moiety (highlighted in green). The other peaks that existed at 5.9 and 6.3 ppm were ascribed to the vinylic protons of the AETAc molecule (highlighted in gray). In the 1H NMR spectrum of β-CD, the peaks at δ = 5.6 ppm and 3.2 – 4.7 ppm were due to anomeric protons and other protons [ring protons (highlighted in yellow) and -OH protons] of the β-CD molecule, respectively (Figure 1d-iii). Likewise, the 1H NMR spectrum of the prepolymer solution also displayed all the proton signals of β-CD, AETAc, and AAm moieties, including the vinylic protons without any displacements (Figure 1d-iv). In contrast, in the 1H NMR spectrum of the crosslinked β-CD-g-(pAAm/pAETAc) hydrogel (Figure 1d-v), the vinylic proton peaks of the corresponding monomers (AETAc and Aam; which were initially highlighted in gray color for both monomers) vanished after polymerization, confirming successful synthesis of the hydrogel. The native 1H NMR signals at δ = 5.6 ppm (anomeric) and 3.1 to 4.7 ppm (ring and -OH protons) of β-CD were also present in the 1H NMR spectrum of the hydrogel, confirming the β-CD stability during the reaction. Additionally, the characteristic peaks of AETAc and AAm monomers at δ = 3.0, 3.7, 4.4, 6.4, and 7.4 ppm were all present in the spectrum of the crosslinked hydrogel. Despite some slight shift and broadening of these peaks, which could be due to the interactions between the moieties during the polymerization process, these peaks indicated the presence of β-CD, pAETAc, and pAAm in the 3D hydrogel network. The appearance of new peaks at δ = 1.5 to 2.0 ppm (highlighted in pink) represented the conversion of vinylic protons into aliphatic methylene protons, further confirming the successful synthesis of the hydrogel.
2.2. Mechanical Properties and Self-Healing Ability of the Hydrogel
To form the hydrogels, different ratios (i.e. 1:3, 1:1, and 3:1) of AAm and AETAc were used. The AAm/AETAC ratios were carefully selected based on our preliminary experiments. Higher concentrations of AETAc resulted in the formation of unstable hydrogels with poor mechanical properties. On the other hand, a further increase in the amount of AAm produced rigid gels lacking flexibility. Any other tested formulations between the selected ratios of 1:3, 1:1, and 3:1 showed no significant differences in mechanical and electrochemical properties. In all hydrogel formulations, the storage moduli (G’), determined by amplitude sweep experiments at a constant frequency of 5 Hz, were significantly higher than the corresponding loss moduli (G”) in the initial stress region (1–103 Pa) (Figure 2a), confirming the elastic nature of the hydrogels. With incremental shear stress, both moduli ran in parallel, maintaining nearly constant values until reaching a specific stress threshold (i.e., yield stress), where they abruptly crossed over with a sudden drop in values. These points indicated a transition from the hydrogel state to a quasi-liquid state (i.e., localized viscous behavior) attributed to enhanced energy dissipation resulting from the breakdown of the H-bonding network within the hydrogels.[58] In addition, the yield stress decreased with changing the AAm:AETAc ratio from 3:1 to 1:1 and 1:3 with the highest yield stress of 6.47 ± 0.38 kPa observed for the 3:1 ratio (Figure 2b). This reinforces our hypothesis that mechanical stability in the hydrogel was due to the addition of AAm and could be finely tuned by varying the amount of AAm units. In the dynamic frequency sweep experiment (1–20 Hz), G’ values were an order of magnitude higher than G”, and both moduli showed little correlation to the experimental frequency range (Figure 2c), indicative of a stable gel state.[59] The absence of any elastic–viscous crossover point in Figure 2c demonstrated the presence of an entangled fibrous network within the hydrogel.[60] Interestingly, the behavior of the SPN-based β-CD-g-(pAAm/pAETAc) hydrogel was more similar to the chemically crosslinked ones than the physically crosslinked ones due to the formation of a pseudo-slide ring structure, which typically exhibits a linear dependence of both storage and loss moduli on frequency.[61] As the β-CD-g-(pAAm/pAETAc) hydrogel lacks covalent crosslinks, extensive fibril aggregation is unlikely to take place under the synthetic scheme. Thus, the differences in moduli were probably caused by the strength and frequency of interfibrillar crosslink points, which were primarily established through macrocyclic host-guest interactions, electrostatic interactions, and H-bonding.[62] In addition, the calculated G’/G” ratio (tanδ) (from Figure 2c) for the hydrogels was ~10, which resembled the characteristics of naturally occurring tissues.[63] The viscosity of all formulations of β-CD-g-(pAAm/pAETAc) hydrogels gradually decreased with increasing shear rate (“shear-thinning”) (Figure 2d), a typical non-Newtonian pseudoplastic fluid behavior previously observed in polysaccharide containing hydrogels.[64] Such behaviour probably originated from the disentanglement of intermolecular chains due to applied shear pressure.[65] As the polymer chains start to align in the flow direction, a reduction in both intra- and inter-chain interactions can take place.
Figure 2. Rheological and mechanical characteristics of β-CD-g-(pAAm/pAETAc) hydrogels.

Rheological behavior of the hydrogel: (a) amplitude sweep, (b) yield stress, (c) frequency sweep, and (d) viscometry analysis. (e) Digital images of hydrogel exhibiting self-healing behavior. (f) Thixotropic plot confirming the self-healing ability of the hydrogel. (g) Change in viscosity under strain sweep from 1 to 1000%. (h) Schematic demonstration of self-healing mechanisms. Mechanical characterization of the hydrogels: (i) experimental setup for the tensile testing and representative tensile stress-strain curves, (j) ultimate strength, (k) Young’s modulus, and (l) stretchability, (m) toughness of the hydrogels formed by using various AAm:AETAc ratios. (n) A digital images of the flexibility of the hydrogel (bending and knotting). (o) Digital image of high stretchability along with mechanistic aspects. (p) Representative compressive stress-strain curves and (q) compressive modulus of the hydrogels formed by using various AAm:AETAc ratios. Error bars specify the standard error of the means (SEM) and asterisks mark significance levels of p < 0.05 (*), p < 0.001 (***), p < 0.0001 (****), and n = 3.
The synthesized β-CD-g-(pAAm/pAETAc) hydrogels exhibited self-healing properties, which could be due to the reversible host-guest inclusion complexation between the hydrophobic groups of pAETAc moiety and the hydrophobic core of β-CD. We performed a ‘cut and heal’ test to visualize the self-healing property of AAm/pAETAc hydrogel made of a 1:1 ratio (Supplementary Movie 1). As shown in Figure 2e, the hydrogel could heal after cutting and re-joining. To further confirm the self-healing properties, rheological strain sweep experiments were conducted. As shown in Figure 2f, under lower strain (1%), G′ was higher than G″ indicating the development of a self-standing hydrogel. Conversely, the G′ and G″ values were reversed (i.e., G″ became higher than G′) under higher strain (1000%), indicating that the β-CD-g-(pAAm/pAETAc) hydrogel was transformed into the sol state, demonstrating a flowing tendency. The cyclic strain sweep experiments also showed that the hydrogel was consecutively converted from gel to sol and sol to gel in each cycle. Besides, the β-CD-g-(pAAm/pAETAc) hydrogel exhibited very rapid recovery with no damage and ~100% recovery (Figure 2g). In all experiments, the hydrogel demonstrated instantaneous self-healing and recovery, which could be due to two types of interactions: i) supramolecular host-guest interaction, and ii) H-bonding (Figure 2h). During cutting, the hydrogel networks were ruptured. However, when they re-joined, the self-healing took place due to the dynamic reversible crosslinking between the hydrophobic cores of β-CD and hydrophobic methyl groups of pAETAc, which were present in the polymer chain. In addition, the dynamic H-bonding between the hydroxyl, carbonyl, and amide groups, present in the hydrogel network also played an important role in developing the self-reparability of the synthesized hydrogel.
We also studied the effect of hydrogel compositions on the tensile properties. The tensile stress-strain curves of the hydrogel consisted of three distinct zones (Figure 2i). After reaching the yield point (YP) in the elastic zone, the hydrogel displayed a typical J-shaped strain-stiffening behavior (i.e., the slope of the stress-strain curve increased with strain) until the breaking stress (BS) point, followed by the so-called strain-softening behavior (i.e., the slope of the stress-strain curve decreased with strain) until the fracture point (FP) was reached (Figure 2i). The ultimate tensile strength of hydrogel was 109.7 ±1 3.3 kPa for 3:1 ratio of AAm/pAETAc, followed by 53.4 ± 4.7 kPa and 26.4 ± 2.1 kPa for 1:1 and 1:3 ratios, respectively (Figure 2j). A similar trend was observed for the Young’s moduli with a slight decrease from 21.2 ± 4.1 kPa for 3:1 ratio to 14.1 ± 3.3 kPa and 9.9 ± 0.5 kPa for 1:1 and 1:3 ratios, respectively, but this change was not significant (Figure 2k). The maximum tensile strain (denoted by the fracture point) also showed a similar trend, increased with increasing AAm content in the hydrogel, and reached a maximum value of 5,443 ± 610 % at AAm:AETAc ratio of 3:1 (Figure 2l). The observed extensibility of the engineered hydrogel surpassed that of many polymeric hydrogels, even those derived from poly(N-isopropylacrylamide),[66] acrylated polyethylene glycol,[67] poly(3,4-ethylenedioxythiophene)-polyvinyl alcohol (PEDOT-PVA),[68] alginate–acrylamide,[69] and polyampholytes.[70] Therefore, the β-CD-g-(pAAm/pAETAc) hydrogel with increased extensibility and ultimate strength possessed a unique combination of elasticity and strength. It could also endure significant deformation while maintaining its structural integrity, making it suitable for applications requiring both flexibility and robustness, such as tissue engineering and wearable medical devices.[71] Further, the highest toughness of 3.5×106 J m−3 was achieved for the 3:1 AAm:AETAc ratio, followed by 1.1×106 J m−3 for 1:1 ratio, and 0.42×106 J m−3 for 1:3 ratio (Figure 2m). The digital images of the β-CD-g-(pAAm/pAETAc) hydrogel formed by using a 1:1 ratio after bending, knotting, and knotting with stretching are visually represented in Figure 2n, demonstrating its macroscale flexible nature. It is worthwhile to note that, the flexibility of a hydrogel is the prime factor that imparts its usability in wearable device fabrication to be comfortable and functional during various activities. The digital image in Figure 2o visually represents the ultra-high stretchability of the hydrogel. The numerous H-bonding interactions within the hydrogel network could readily undergo breakage, serving as a means to dissipate energy. Simultaneously, these multiple H-bonding interactions could offer sufficient stability, thereby conferring substantial elasticity to the hydrogel (Figure 2o). Figure 2p depicts the representative compression curves for all three hydrogel formulations, with the inset schematic illustrating the experimental setup. The hydrogel was found to be soft and sustained compression forces with a compressive modulus of 19.7 ± 3.1, 6.1 ± 2.9, and 1.1 ± 4.7 kPa for hydrogels formed by using 3:1, 1:1, and 1:3 AAm/AETAc ratios, respectively (Figure 2q).
2.3. Tissue Adhesive Properties of the Hydrogel
For bioelectronic communication and sensing, it is crucial to establish stable contact between the sensing device and the target tissue.[72] In addition, the establishment of robust adhesion between the engineered hydrogels and biological tissues holds profound significance for biomedical applications such as biomarker monitoring, electrophysiological sensing, tissue repair, and drug delivery.[73] Attaining adequate tissue adhesiveness in flexible materials is one of the significant challenges to addressing the hurdles associated with human-machine interfaces in the future.[74] For example, for wearable flexible electronic sensors, adhesion to the skin is particularly critical as it ensures secure attachment, consistent contact with the skin, and comfort for users, minimizing motion artifacts and ensuring accurate and reliable data collection. Therefore, we investigated the adhesive performance of the β-CD-g-(pAAm/pAETAc) hydrogel towards freshly isolated porcine skin using a lap shear test following the methods prescribed by the American Society for Testing and Materials (ASTM-F2255) (Figure 3a). The representative force/displacement curves for all hydrogel formulations, adhered to the skin tissue, are shown in Figure 3b. The adhesion strength for hydrogels increased from 15.9 ± 0.9 kPa for 3:1 AAm/AETAc ratio to 26.6 ± 1.3 and 38.4 ± 0.6 kPa for 1:1 and 1:3 ratios, respectively (Figure 3c). Further, the adhesion energy for hydrogels made with a 3:1 AAm/AETAc ratio increased from 15.8 ± 2.0 to 20.0 ± 2.3 and 33.6 ± 6.1 J m−2; for 1:1 and 1:3 ratios, respectively (Figure 3d). In addition, the adhesion strength of the hydrogel with a 1:1 AAm/AETAc ratio was comparable to that of commercially available tissue adhesives, including Evicel®, Coseal®, and Histoacryl®. (Figure 3e).[75]
Figure 3. Adhesive characteristics of β-CD-g-(pAAm/pAETAc) hydrogels.

(a) Schematic demonstration for the experimental setup used for the lap-shear test. (b) Representative force/displacement curves based on lap-shear test. (c) Adhesion strength, and (d) adhesion energy for the β-CD-g-(pAAm/pAETAc) hydrogels formed by using various AAm:AETAc ratios (n = 3). (e) Comparison of adhesion strengths of β-CD-g-(pAAm/pAETAc) (1:1 AAm/AETAc ratio) commercially available bioadhesives. (n=3 for 1:1 AAm/AETAc hydrogel and Coseal®, n=5 for Evicel® and Histoacryl®). (f) Schematic demonstration of the adhesion mechanism. Digital images of adhesion of the hydrogel on (g) gloves, (h) pig skin, rat liver (n=5), and rat heart (n=5). (i) Adhesion strength of β-CD-g-(pAAm/pAETAc) hydrogel (1:1 ratio) on skin, liver, and heart tissues based on lap-shear test. The hematoxylin and eosin (H&E) stained tissue sections from the hydrogel/tissue interfaces, demonstrating adhesion to (j) heart and (k) liver. Error bars specify the SEM and asterisks mark significance levels of p < 0.05 (*), p < 0.001 (***), p < 0.0001 (****).
The strong tissue adhesive properties of the engineered β-CD-g-(pAAm/pAETAc) hydrogel were due to different physical interactions at the hydrogel/tissue interfaces, including H-bonding and dipole-dipole interactions. When β-CD-g-(pAAm/pAETAc) hydrogel contacted the tissue surfaces, the AAm formed multivalent H-bonding arrays and created stable physical crosslinks with the tissue surface. In addition, the dipole-dipole interactions between the quaternary ammonium groups present in the AETAc moieties of the developed hydrogel and the carboxyl groups of the tissue further strengthened the interfacial attachment. The increased adhesion strength and adhesion energy with an increasing amount of AETAc in the hydrogel further supported the predominant role of these electrostatic dipole-dipole interactions (Figure 3f).[52]
Digital photographs in Figure 3g visually illustrates the glue-like nature of the β-CD-g-(pAAm/pAETAc) hydrogel when adhered to nitrile gloves. Next, we evaluated the adhesiveness of the developed hydrogel to different freshly isolated internal organs (i.e., heart and liver) to explore its potential future applications as implantable adhesive devices. Figure 3h represents digital photographs of the attachment of the hydrogel to various tissues, including skin, heart, and liver. Quantitative analysis of adhesion strength (Figure 3i) showed that the hydrogel exhibited strong adhesion to a wide range of tissue surfaces, including skin (26.6 ± 1.3 kPa), murine heart (35.4 ± 3.4 kPa), and liver (32.0 ± 3.5 kPa). The differences observed in the adhesive strength of the hydrogel to various tissues were presumably due to the differences in texture, wetness, density of functional groups present on the tissue surfaces, and the stiffness of the tested tissues.[76] The observed differences were similar to the adhesive strengths reported in the literature for other bioadhesives on the same tested tissues.[76–77] The hematoxylin and eosin (H&E) stained section from the hydrogel/tissue interface showed physical interlocking and strong bonding between the hydrogel and the tissue, while the architectures of the heart and liver tissues remained normal (Figure 3j, 3k).
2.4. Electro-Responsive Characteristics of the Hydrogel
2.4.1. Electro-Responsive Features
Conducting hydrogels became an important class of soft materials for their potential biomedical applications ranging from therapeutics and drug delivery to diagnosis and disease monitoring. Ionic conductivity of the developed β-CD-g-(pAAm/pAETAc) hydrogel ranged from 0.15 ± 0.01 S m−1 to 0.44 ± 0.02 S m−1 (Figure 4a), which was ~5 orders of magnitude higher than graphene oxide (GO)/ pAAm (GO/pAAm) hydrogels,[35a, 78] and higher than other previously reported composites including polyaniline/poly(vinyl alcohol) hydrogels.[79] The conductivity of the hydrogel was enhanced significantly by increasing the amount of positively charged pAETAc moiety in the hydrogel network (Figure 4a). To further demonstrate the conductivity of the hydrogel, we used β-CD-g-(pAAm/pAETAc) hydrogel, formed by using 1:1 AAm/AETAc, as a conductor to lighten an LED bulb (Figure 4b, Supplementary Movie 2). The mechanism for ion conduction, provided by the hydrogel, in this circuit is schematically shown in Figure 4c. The hydrogel contained a large amount of positively charged N-containing side chains and negatively charged chloride counter ions. In the presence of an electric field, the 3D conductive hydrogel network provided a platform for ion conduction, which lighten the LED (Figure 4c).
Figure 4. Electro- and pressure-responsive features of β-CD-g-(pAAm/pAETAc) hydrogels.

Electro-responsive features of β-CD-g-(pAAm/pAETAc) hydrogels formed by using various AAm:AETAc ratios: (a) conductivity, and (b) digital images of an illuminating blue LED. (c) Schematic demonstration for an experimental setup with a plausible conduction mechanism. (d) Schematic representation of the experimental setup for the ex vivo rabbit leg muscle beating experiment, and (e) quantification of the threshold voltage for rabbit leg muscle stimulation. (f) Comparative radar plot comparing the properties of β-CD-g-(pAAm/pAETAc) hydrogels formed by using various AAm/AETAc ratios. (g) Cyclic voltammetry (CV) curves within the potential range of −1 to +1 V at a scan rate 600mV/s for the hydrogels formed by using various AAm:AETAc ratios, (h) CV curves of the hydrogel (1:1 ratio) within the potential range of −1 to +1 V at different scan rates. (i, j) Cyclic stability of the 1:1 ratio hydrogel-based device up to 200 cycles. Pressure-responsive features of β-CD-g-(pAAm/pAETAc) hydrogel (1:1 ratio): (k) CV profiles at a constant scan rate of 600 mV/s with different % of compression. (l) Determination of the gauze factor and (m) response time of the hydrogel-based sensor. Signals from the hydrogel-based sensor are due to (n) low, medium, and high stretching, (o) twisting, and (p) compressing. (q) Electrical signals demonstrating the self-reparability of the hydrogel-based device. (r) Schematic representation and digital images of change in the illumination of blue LED blue upon stretching. Error bars specify the SEM and asterisks mark significance levels of p < 0.05 (*), p < 0.001 (***), p < 0.0001 (****) and n = 3.
Our engineered conductive hydrogels could also be used to restore electrical communication between the excitable cells to preserve the functionality of the tissue in an ex vivo experimental setup.[80] The capability of β-CD-g-(pAAm/pAETAc) hydrogels to revive impulse propagation across striated muscle was evaluated ex vivo using fresh rabbit thigh muscle immediately after euthanasia. The skin layer was first surgically removed to expose the thigh muscle and a rectangular hydrogel patch was then placed (Figure 4d). A direct current (DC) potential was applied (75 m sec square pulses at increasing frequencies) using electrical wires connected to the hydrogels. As a result, the muscles started beating in a synchronous rhythm. The beating of the muscle was assessed visually, and the minimum voltage required for synchronized beating of the muscle tissue (threshold voltage) was noted (Supplementary Movie 3). Our data showed that the hydrogels containing the highest amount of pAETAc moiety (1:3 ratio) exhibited the lowest threshold voltage. It was also observed that the threshold voltage decreased as the amount of pAETAc units increased (Figure 4e). Thus, the engineered β-CD-g-(pAAm/pAETAc) hydrogel can potentially revive the propagation of electrical impulses and preserve the functionality of excitable tissues damaged by trauma or disease.
To demonstrate the versatility of our engineered hydrogel across diverse biomedical applications, we employed a comparative radar plot (Figure 4f) to analyse the impact of the AAm/AETAc ratio on various properties of the hydrogel. Higher pAAm content notably enhanced mechanical properties such as stretchability, toughness, ultimate strength, Young’s modulus, and compression modulus, while increased pAETAc content improved adhesion and electrical performance. Aiming for an optimal balance among mechanical, adhesion, and electrical characteristics, we selected a 1:1 AAm/AETAc ratio as the ideal formulation for utilizing our hydrogel as a sensing device.
To further explore the electro-responsive properties of β-CD-g-(pAAm/pAETAc) hydrogels, cyclic voltammetry (CV) analyses of the various formulations of hydrogels were performed within the potential range of −1 to +1 V at a contestant scan rate of 600 mV/s. The area enclosed by the curve as well as the CV response increased with an increasing amount of pAETAc (Figure 4g). The CV results of β-CD-g-(pAAm/pAETAc) hydrogel, formed by using a 1:1 ratio, at different scan rates showed that the area and current response of the CV curves were amplified with an increase in the scan rate, further confirming the electro-responsiveness of the hydrogel (Figure 4h). The increase in the area enclosed by the CV curves was probably due to the faster diffusion kinetics over the electrode surface.[7d, 81] Further, the cyclic electro-stability of a conductive hydrogel is an important parameter for real-world applications. The β-CD-g-(pAAm/pAETAc) hydrogel was capable of retaining ∼96% stability even after 200 cycles of CV (Figure 4i–j) indicative of its high stability and reusability.
2.4.2. Pressure-Responsiveness of the Engineered Hydrogel
Pressure-responsive hydrogels can sense the externally applied pressure and respond accordingly. We examined the capability of our engineered hydrogels for the detection of various kinds of human motion to expand their potential application as a platform for disease monitoring. To fabricate a pressure sensing device based on β-CD-g-(pAAm/pAETAc) hydrogel, two electrodes were connected at each end of the hydrogel patch and connected to an electrochemical workstation. The pressure response of the engineered hydrogel was then assessed using CV analysis under different compressions at a scan rate of 600 mV/s (Figure 4k). It was found that increased compression, applied by increasing pressure, enhanced the area enclosed by the CV curves and the current response. Initially, without any compression (0%), the hydrogel network had a higher thickness, which was responsible for the generation of more resistance. In contrast, at higher compression, the distortion of the hydrogel network, due to the applied pressure, produced superior electron transportation paths. The sensitivity of the engineered hydrogel-based pressure sensor was assessed by calculating the gauge factor of the sensor. As shown in Figure 4l, the gauge factor of the β-CD-g-(pAAm/pAETAc) hydrogel-based sensor had three variation series. Under a strain range of 0–172 %, the gauge factor was ~ 0.52 (R2 = 0.99). With an increase in % strain, the gauge factor also concurrently increased and reached ~ 2.29 (R2 = 0.95) and ~ 3.74 (R2 = 0.96) at the strain ranges of 172 % - 502 % and 502 % - 834 %, respectively.
The strain sensing and self-healing performance (electrically) of β-CD-g-(pAAm/pAETAc) hydrogel-based device was detected through the change in electrical signals using chrono-amperometry measurement (Figure 4m–r). The response time of the engineered sensing device was found to be around 0.2 sec (Figure 4m). In addition, the changes in the electrical signals with respect to time were evaluated at different deformations, including stretching (Figure 4n), twisting (Figure 4o), and compression (Figure 4p). Different signals were detected for stretched and twisted hydrogels as compared to the normal ones, demonstrating the responsiveness of the hydrogel to the applied forces. For the stretched hydrogels, as the strain increased from low to moderate to high, the current response dropped simultaneously from lower to higher upon stretching. Similarly, for the twisted hydrogel, there was a decrease in the current signal by changing from low twist to high. In addition, the stretched and twisted hydrogels exhibited lower current signals as compared to the normal state (Figure 4n and 4o). In contrast, for compression, the current response was found to be amplified (Figure 4p). For lower compression, the signals were lower as compared to the higher compression with higher signals. Thus, signals due to stretching and twisting (downward) are different from compression (upward). Therefore, these distinct types of deformations could be readily detected using the engineered hydrogel-based sensor. In addition, in the absence of any deformations (stretching, twisting, or compression), the electrical signal returned almost to its initial value (~ 91 %). This proved the reliability and sensitivity of the engineered β-CD-g-(pAAm/pAETAc) hydrogel-based sensor. Additionally, it is possible to establish a relationship between the force of deformations (stretching, twisting, compression) and the trend of electrical signals to distinguish between these types of motion. Our observations indicated that compression signals could be easily differentiated from stretch and twist signals. Specifically, when the hydrogel was stretched or twisted, there was a decrease in the current signal. In contrast, compression resulted in an amplified current response. Moreover, as the strain increased from low to moderate and high levels in stretched hydrogels, the current response decreased accordingly. Similarly, lower compression yielded lower signals compared to higher compression, which generated higher signals. Future research could explore signal processing algorithms using AI for more precise distinction.[82]
We also checked the influence of pH and humidity on the sensing performance of the hydrogel-based sensor. We submerged the hydrogel, formed with a 1:1 AAm/AETAc ratio, in aqueous solutions with different pH levels (2, 3, 5, 7, and 9) at 37°C and 60% humidity to evaluate the effect of pH. After exposure to different pH solutions, the hydrogel-based sensors could accurately detect both compression (Figure S1a) and stretching signals (Figure S1b). Additionally, the response time of the sensor remained approximately the same (Figure S1c). We also evaluated the effect of humidity on sensing performance by incubating the hydrogel-based sensors at different relative humidity (50%, 60%, 70%, and 80%). Similar to the pH effect, no changes were observed in sensing performance after exposure to different humidity levels (Figure S2). These results demonstrate the excellent performance of the engineered β-CD-g-(pAAm/pAETAc) hydrogel-based sensor under various pH and humid conditions, making them suitable for real-world applications.
The effect of degradation of the engineered hydrogel on sensing performance was assessed by immersing the hydrogel in Dulbecco’s phosphate-buffered saline (DPBS) at 37°C and monitoring the sensing performance at specific time points (day 0, 1, 5, 7, and 14). As depicted in Figure S3, the hydrogel-based sensor remained stable throughout the 14 days and effectively generated different singles for various deformations, including compression (Figure S3a) and stretching (Figure S3b).
We further tested the self-healing ability of our sensor by cutting and healing the hydrogel and measuring the electrical signal initially and post-healing (Figure 4q). It was observed that the self-healed hydrogel remained stable and demonstrated similar electrical signals as a response to different deformations under multiple cutting/healing cycles. We also assessed the electrical response of the synthesized β-CD-g-(pAAm/pAETAc) hydrogel toward various distortions by connecting the hydrogel-based device to an electrical circuit as a conduction medium. The setup was then connected to a DC power supply and a blue LED bulb (Figure 4r). In this system, when an external electrical potential was exerted, the hydrogel enlightened up the blue LED bulb and the illumination of the bulb was altered significantly with the deformation in the hydrogel in terms of stretching (Figure 4r, Supplementary Movie 4). Upon stretching (150 %), the illumination of the blue LED considerably decreased. The illumination was then reverted to its initial state when the force on the hydrogel was removed, and it was taken back to its initial state. These results together indicated that the hydrogel was sensitive with a high gauge factor and response time. In addition, the engineered hydrogel-based sensor showed proper strain-responsiveness and an ideal electrical self-healing capability, suggesting its potential applications in the biomedical field as a self-repairing soft electronic.
2.5. Transparency and UV-shielding Properties of the Engineered β-CD-g-(pAAm/pAETAc) Hydrogel-based Sensor
Transparency is an important property for flexible and wearable sensors because it allows the sensors to be inconspicuously integrated into clothing or directly applied to the skin without obstructing visibility or aesthetics.[83] This is particularly relevant for applications in healthcare monitoring or human-machine interfaces, where the sensors should be minimally intrusive and not interfere with the user’s daily activities or appearance.[84] Most of the currently available hydrogel-based electronic sensors are black or opaque, limited by their inherent color.[85] In contrast, the developed β-CD-g-(pAAm/pAETAc) hydrogel was found to be transparent. Digital photographs of the hydrogel covering a color wheel (0.5 mm thickness), and the UCLA logo (1.5 mm thickness) represent the transparent nature of the hydrogel (Figure 5a). Based on the UV visible spectroscopic analysis, the transmittance of the developed β-CD-g-(pAAm/pAETAc) hydrogel exceeded 80% throughout the visible region (400–700 nm) (Figure 5b). In addition, the hydrogel exhibited negligible UV transmittance within the wavelength range of 200–320 nm, corresponding to the UVB and UVC regions of the electromagnetic spectrum. Such UV shielding functionality serves a dual purpose by safeguarding both the device and the skin tissue from harmful UV radiation, ensuring prolonged sensor functionality and skin protection. This UV shielding property could be due to the UV-absorption ability of the supramolecular inclusion complex formed between AETAc and β-CD.[86] We also evaluated the microstructure of the engineered hydrogel using scanning electron microscopy (SEM) analysis. As shown in Figure 5c, the hydrogel was highly porous, with an average pore diameter of 12.5 ± 3.0 μm (Figure 5d). The high transparency, UV-shielding, and porous nature of the engineered hydrogel can expand its utility for a wide range of applications ranging from tissue engineering to drug delivery and biosensing.
Figure 5. Additional features of the β-CD-g-(pAAm/pAETAc) hydrogel including, transparency, UV-shielding, passive cooling, and antioxidant properties.

Transparency and UV-shielding behavior: (a) Digital images of hydrogel placed on a color wheel and UCLA logo exhibiting its transparent nature. (b) UV-shielding feature observed from the plot of % transmittance vs. wavelength. (c) SEM morphology of the engineered hydrogel, and (d) pore diameter distribution curve. Passive cooling and human body heat radiation features include: (e) Digital image and thermal mapping of the β-CD-g-(pAAm/pAETAc) hydrogel on a human hand wearing gloves, recorded using an infrared (IR) thermal camera, (f) sunlight exposure time vs. temperature graph, and curve for sunlight exposure time vs. difference in temperature (ΔT) between the hydrogel and gloves. (g) FTIR spectrum of the hydrogel between 2.5 and 16 μm. The mid-IR radiation of the human body is shown as light brown regions as a reference. In vitro antioxidant efficacy: (h) % scavenging kinetics of DPPH free radical using different amounts of β-CD-g-(pAAm/pAETAc) hydrogel and plausible radical scavenging mechanism (inset), (i) determination of EC50 values for β-CD-g-(pAAm/pAETAc) hydrogel, and (j) comparison of EC50 values of hydrogel with a well-known antioxidant, Trolox.
2.6. Passive Cooling Feature of the Engineered Hydrogel
Passive cooling is another essential consumer-friendly feature for flexible wearable sensors to ensure user comfort, maintain sensor accuracy, extend wearability, promote skin health, enhance energy efficiency, and encourage user adoption. It can also facilitate excess heat dissipation that is generated during sensor use (i.e., Joule heating), contributing to overall device effectiveness and user satisfaction. To assess the passive cooling capability of our hydrogel, we conducted an outdoor on-body test by applying the hydrogel to hands covered by nitrile gloves under exposure to sunlight and comparing the temperature with/without the hydrogel using a thermal camera. The thermal images (Figure 5e) showed that the temperature of the hydrogel-covered region was ~15°C lower compared to the uncovered region throughout 30 min of sunlight exposure (Figure 5f). Such a passive cooling feature of the hydrogel was probably due to its porous structure, which allowed human-body heat dissipation.[87] Although biosensors with personalized heating capacities were extensively studied,[88] developing a hydrogel-based sensor to passively cool the human body without external energy consumption remained less investigated. There are several state-of-the-art E-skins including poly(vinylidene fluoride-co-hexafluoropropene), polyethylene, methacryloyl aerogel (FGA), and polystyrene-blockpoly(ethylene-ran-butylene)-block-polystyrene, which demonstrated the passive cooling features.[87, 89] However, a lack of optimal mechanical properties (e.g., low stretchability and ultimate strength) and lower cooling ability may impede their ability to be integrated with E-skin platforms. The Fourier-transformed infrared spectrum (FTIR) of the β-CD-g-(pAAm/pAETAc) hydrogel showed a high transmittance of ~80%, which was in the spectral window of human body radiation (λ = 7–14 μm)[87] (Figure 5g).
2.7. Antioxidant Activity of the Developed Hydrogel
The antioxidant activity of the β-CD-g-(pAAm/pAETAc) hydrogel was assessed in terms of its ability to neutralize DPPH• radicals. The introduction of hydrogel to a DPPH• solution[90] led to a time-dependent decrease in the characteristic absorption peak (λ = 535 nm) of DPPH• in a stoichiometric manner. It was found that as the concentration of hydrogel in the solution increased from 0.4 mg mL−1 to 1.6 mg mL−1, the amount of DPPH• radicals (at the end of the experiment, 30 min) decreased from ~85% to ~40% (Figure 5h). The observed decrease in the amount of DPPH• radicals indicated the formation of reduced DPPH (rDPPH) in the solution. The presence of active −OH and −NH2 groups within the polymeric structure facilitated proton or electron transfer for scavenging free radicals to form reduced DPPH (Figure 5h-inset).[7d] The effective concentration of hydrogel to neutralize 50% of the DPPH• radicals (EC50) was calculated to be 1.53 mg mL−1 (Figure 5i), which was higher than the EC50 of Trolox (Figure 5j), a well-known antioxidant standard.[90]
2.8. 3D Printability of the Hydrogel
The 3D printability is an important feature of the β-CD-g-(pAAm/pAETAc) hydrogel, extending its potential applications as customizable wearable sensors to fit various body shapes and sizes, ensuring comfortable personalized devices.[91] Due to the adjustable viscosity, by changing pAAm/pAETAc ratio in our designed hydrogel, it could be extruded easily without the need for a support bath. As shown in Figure S4, the β-CD-g-(pAAm/pAETAc) bioink could be printed in different patterns while effectively holding the structure, which cannot be achieved by using a molding method. As a proof of concept, we used the β-CD-g-(pAAm/pAETAc) bioink to print the UCLA logo and a planar array of sensors with connections to electrodes. With this approach, our developed hydrogel showed the potential to efficiently print patient-customized biosensors tailored to specific body parts and requirements, offering a high degree of flexibility. Furthermore, 3D printability may facilitate the fabrication of complex geometries and the integration of multiple components into a single structure.
2.9. Motion Sensing Capability of Engineered Hydrogel
The engineered hydrogel-based sensor was attached to different body parts (on gloves or clothes) and used to sense various human motions as changes in different electrical signals and currents. When the hydrogel-based sensor was adhered to the index finger (on gloves) (Figure 6a), it detected the change in angle from 0° to 30, 60, and 90° and produced a stair-like curve (Figure 6b). It was also observed that the sensor generated a nearly identical reduction in the relative current response, ΔI/I0, which reached its initial value when the finger came to its original state (0°). Moreover, the ΔI/I0 were found to be repeatable at each bending angle (Figure 6c), showing the fast response and excellent repeatability of the sensing performance of the hydrogel. This flexible sensor could also sense the slow and fast motion of the index finger at a fixed bending angle of 60° (Figure 6d). In this case, the relative current change was found to be almost steady, and the gap between the signals varied as a function of time from slow to fast motion. In addition, when the sensor was attached to the wrist (on gloves) (Figure 6e), it was observed that the current signals were relative to the bending of the wrist, and they reduced continuously as the wrist was bent from low to medium to high (Figure 6f). When the wrist was bent upward, the relative current change signals were increased (Figure 6g), however, during the downward bending motion of the wrist, the signals just became opposite and decreased (Figure 6h), confirming the proper sensing feature of the developed β-CD-g-(pAAm/pAETAc) hydrogel-based sensor. We also detected the sensing performance of the device by holding the wrist in the same position and then releasing it back to the normal position. As shown in Figure 6h, during bending the current response reduced and remained constant throughout the holding time, and then went back to its initial values when the wrist was turned to the normal position, suggesting the high sensitivity of the engineered motion sensor. Furthermore, we evaluated the stability of the hydrogel-based sensor in the real-time monitoring of index finger bending over a duration of 45 min. As depicted in Figure S5, the sensor exhibited precise monitoring of index finger motion over the long term, indicating its reliability for real-time health monitoring.
Figure 6. Real-time human motion monitoring using β-CD-g-(pAAm/pAETAc) hydrogel-based epidermal sensor.

(a) Schematic representation of a hydrogel-based sensor adhered to the human finger to detect movement signals, signals for (b, c) index finger motion at different bending angles and (d) slow, medium, and fast movement of finger at a certain bending angle. (e) Schematic demonstration of the hydrogel-based sensor attached to the human wrist to detect its movement signals, (f-h) different types of wrist motion singles. (i) Schematic demonstration of a handwriting detection sensor, and (j) detection of changes in signal for “A, B, C, D” writing using the sensor. (k) International Morse code, and detection of (l) “SOS” and (m) “HELP” in the International Morse coded version. (n) Plot for principal component analysis comparing our work with the state of art available in the literature.
Besides, the hydrogel was also used as a writing pad and handwriting recognition sensor (Figure 6i). To find out the repeatability of writing signals three times “A”, “B”, “C” and “D” were written on the hydrogel film. Each time, the signals for “A”, “B”, “C” and “D” were found to be identical (Figure 6j). The high sensitivity of the β-CD-g-(pAAm/pAETAc) hydrogel-based sensor may also make it useful for information security. It was possible to use static and dynamic touch patterns for communication, by using the International Morse code (Figure 6k). Herein, the static touch (3 sec) is represented as a line and the dynamic touch (1 sec) is represented as a dot. We were able to send vital signals like “SOS” (Figure 6l) and “HELP” (Figure 6m) in the Morse code version by applying static and dynamic touch to the engineered hydrogel-based sensor.
Recently, several hydrogels have shown promising results in applications such as motion sensing, handwriting detection, or communication with Morse code. However, none of them was able to detect all three signals on a single platform. For example, hydrogels composed of pAAm with polyionic liquid with alkyl chains[92] and poly(acrylic acid)/lauryl methacrylate/ cetyltrimethylammonium bromide[93] showed promising results in motion sensing but were not reported to detect handwriting and Morse code. Furthermore, these hydrogel-based sensors lacked an on-body test for efficacy detection. In addition, a hydrogel made up of pAAm, sodium alginate, and polypyrrole (pAAm-ALG-PPY)[39] was found to sense underwater motion and was able to communicate via Morse code but was not shown to detect handwriting. On the other hand, graphene oxide-based hydrogel[2] was robustly used for handwriting detection, however, it was not reported to sense bodily motions or applied for communication. In contrast, our developed hydrogel-based sensor could detect all these types of signals as a single platform. In addition, none of the previously developed hydrogel-based sensing devices showed additional customer-friendly features such as UV shielding, transparency, passive cooling, and 3D printability.[2, 39, 92–93]
Finally, we conducted a principal component analysis on the state-of-the-art SPN-based hydrogels used for motion sensing [3, 7b, 35b, 39, 92–94] (Figure 6n) to gain insights into their performance and relationships between their mechanical and electrical properties in comparison to our work. Principal component 1 (PC1) accounted for 37.5% of the variance in the dataset and was positively correlated with stretchability, adhesiveness, cytocompatibility, and transparency while having a negative correlation with electrical conductivity, gauge factor, and tensile strength. Principal component 2 (PC2) accounted for 18.9% of the variance in the dataset and was positively correlated with gauge factor and self-healing while having a negative correlation with electrical conductivity, adhesiveness, and transparency. Principal component 3 (PC3) accounted for 14.1% of the variance in the dataset and was positively correlated with conductivity and tensile strength while having a negative correlation with gauge factor, self-healing, and transparency. The engineered β-CD-g-(pAAm/pAETAc) hydrogel had high positive scores in all three principal components, indicating its robust mechanical features and good electrical performance. It is important to note that all SPN-based hydrogels in this analysis, except β-CD-g-(pAAm/pAETAc), hydrogel based on pAAm, GO, and cucurbit[8]uril (CB[8]),[35b] Poly ion complex/Ag nanowires (PIC/AgNWs),[7b] and poly (N-acryloyl glycinamide)-diacrylate/MXene hydrogel (PNAGA/MXene)[94a] scored negative in PC2 indicating lower pressure sensitivity and lack of self-healing. While GO-p(AAm-G)/CB[8][35b] and PIC/AgNWs[7b] hydrogels showed better scores in PC2, they scored negative in PC1 and PC3. On the other hand, PNAGA/MXene[94a] gel showed an exceptionally good score in PC3, indicating its good electrical performance, however, it lacked mechanical strength and adhesiveness. The engineered β-CD-g-(pAAm/pAETAc) hydrogel showed balanced mechanical strength, self-healing, and adhesiveness along with appropriate electrical performance, which was absent in most of the state-of-the-art motion sensors.
2.10. The Use of Hydrogel-based Sensor for Body Detection of Movement Disorders using a Simulated Parkinson’s Disease Condition
The management of PD traditionally relies on pharmacological approaches that predominantly address the primary dopaminergic motor symptoms encompassing tremors, rigidity, and bradykinesia (i.e., slowness of movement, progressive hesitation, and halt) (Figure 7a).[95] Therefore, real-time monitoring of non-cognitive markers like mobility dysfunction (which often precedes cognitive impairment) could be a viable alternative to complicated biomolecular and neuropsychological techniques for the detection and assessment of the impact of drugs on disease progression in PD.[96] Despite significant advances in hydrogel-based flexible sensors, to the best of our knowledge, none of them could report real-time monitoring of both tremor and gait abnormalities in PD. Therefore, as a proof-of-concept study, we assessed the capability of our engineered sensor to measure tremors and abnormal gait under simulated Parkinson’s conditions.
Figure 7. On body detection of movement disorders in simulated Parkinson’s disease condition and detection of abnormal breathing and cough using β-CD-g-(pAAm/pAETAc) hydrogel-based sensors.

(a) Symptoms of PD patient and plausible position of sensors placement (marked red). Real-time sensing of shaking of hand for (b) no tremor (normal), (c) minor, (d) moderate, and (e) severe tremors by attaching the sensor on the hand (on gloves). Sensing of (f) normal gait, (g) abnormal gait, and (h) progressive hesitation and halt using the hydrogel-based sensor adhered on the shoe toe. Detection of (i) normal/ abnormal breathing and (j) cough, using the hydrogel-based sensor attached to the abdominal muscle (on clothes).
To illustrate the potential utilization of our sensor in PD patients, we integrated the hydrogel sensor onto gloves and conducted measurements under four distinct hand tremor scenarios: normal, minor, moderate, and severe, as illustrated in Figure 7b–e. Due to the highly adhesive nature of the hydrogel, no external glue, tape, or suturing was needed to fix it on the gloves. The signals were significantly different for each tremor scenario. When no tremor was present, the signal was nearly a straight line. However, with increasing tremors (from minor to severe), the frequency of the signals increased along with the peak intensity, and the full width at half maximum decreased. In addition, the abnormal gait was assessed by placing the sensor on the toe of a shoe, followed by normal walking. The normal gait showed sharp peaks when there was a step (Figure 7f). However, in the case of abnormal gait (bradykinesia) associated with delayed steps, the sharp peak in the signal vanished and the frequency of the signal decreased (Figure 7g). In cases of progressive hesitation and halt, the signal-to-noise ratio increased (Figure 7h). Overall, these results confirmed the ability of the β-CD-g-(pAAm/pAETAc) hydrogel sensor to detect motor symptoms typical of PD which are often accompanied by breathing disorders and other problems in elderly people.
To demonstrate the effectiveness of the engineered sensor in detecting other abnormalities, such as abnormal breathing and coughing, we placed the sensor on the abdominal muscle (on clothes) to detect the abnormal signals. In the case of normal breathing, sharp signals were detected, whereas for abnormal breathing (very fast and short), the singles became improper, and the signal-to-noise ratio was increased (Figure 7i). In addition, in the case of cough, sharp peaks were observed (Figure 7j), confirming real-time monitoring of different health conditions.
2.11. In vitro Biocompatibility of Engineered Hydrogel
The cytocompatibility of the developed hydrogel was assessed by 2D seeding of the NIH/3T3 cells on the hydrogel’s surface. Our data showed that cellular growth on the β-CD-g-(pAAm/pAETAc) hydrogel was comparable to the tissue culture plates (TCPs), with ~100% cell viability after 5 days of culture shown by calcein/ethidium homodimer staining (Figure 8a–b; Figure S6a). In addition, the cellular metabolic activity determined by PrestoBlue assay showed comparable metabolic activity of the cells grown on β-CD-g-(pAAm/pAETAc) hydrogel surface and conventional TCPs (Figure 8c). Figures 8d and S6b showed nearly ubiquitous and structured organization of fluorescently labeled F-actin filaments (revealed through Alexa fluor 488 phalloidin staining), surrounding cell nuclei stained with DAPI (4′,6-diamidino-2-phenylindole). These images confirmed effective cell spreading and maintenance of a typical cellular cytoskeletal structure when cultured on the surface of β-CD-g-(pAAm/pAETAc) hydrogel over 5 days.
Figure 8. In vitro and in vivo biocompatibility, and in vivo degradation of β-CD-g-(pAAm/pAETAc) hydrogel.

In vitro biocompatibility test: (a) Representative live/dead images of 3T3 cells seeded on the hydrogel 5 days post-seeding and (b) quantitative analysis of 3T3 cell viability at days 1 and 5 post-seeding, (c) quantitative analysis of metabolic activity, relative fluorescence units (RFU), using PrestoBlue assay at days 1, 3, and 5 post-seeding, (d) representative F-actin/DAPI staining images of 3T3 cells seeded on the hydrogel 5 days post-seeding, (e) intra-cellular ROS detection mechanism using 2’, 7’-dichlorofluorescein diacetate (DCFDA) assay, (f) representative DCFDA stained images of 3T3 cells seeded on the hydrogel 5 days post-seeding, (g) mean cellular fluorescence, and (h) fluorescence intensity of dicholoroflurescene (DCF) at days 1, 3, and 5 post-seeding. In vivo biocompatibility and degradation of β-CD-g-(pAAm/pAETAc) hydrogel: (i) Schematic demonstration of subcutaneous implantation of hydrogel in rat, (j) representative H&E-stained hydrogel-skin tissue interface section 7- and 28-days post-implantation, (k) representative CD68/DAPI stained hydrogel-skin tissue interface section 7- and 28-day post-implantation, (k) Biodegradation profile of the hydrogel till 28 days post-implantation. Error bars indicate the SEM and asterisks mark significance levels of p < 0.0001 (****) and n = 3.
While high cell viability is a positive indicator of cell survival, assessing intracellular reactive oxygen species (ROS) levels is also important for understanding cellular function and the potential for oxidative stress-related damages. Thus, we performed a 2’, 7’-dichlorofluorescein diacetate (DCFDA) assay to get insights about potential changes in the cellular redox environment when in contact with the developed hydrogel. The DCFDA assay involves the diffusion of H2DCFDA into cells, where it is deacetylated by esterases, forming a non-fluorescent compound that is subsequently oxidized by intracellular ROS to produce highly fluorescent dicholoroflurescene (DCF), which can be detected using fluorescence spectroscopy or microscopy at λex 485 nm and λem 535 nm, respectively (Figure 8e).[97] Our data showed that cells grown on β-CD-g-(pAAm/pAETAc) hydrogel showed similar DCF fluorescence (i.e., very low) compared to cells cultured in TCP in both microscopic (Figures 8f–g and S6c) and fluorometric (Figure 8h) measurements. These results indicated that the presence of hydrogel did not alter the cellular ROS levels of the developed β-CD-g-(pAAm/pAETAc) hydrogel.
2.12. In vivo Biocompatibility and Biodegradation of the Hydrogel
To assess the in vivo biocompatibility and biodegradation of the engineered hydrogel, subcutaneous implantation was performed in male Wister rats (Figure 8i). The H&E-stained sections from the tissue/hydrogel interface showed normal skin architecture (Figure 8j). A thin circumferential layer of fibrous tissue (FC) was formed at the tissue/hydrogel interface, particularly visible on day 28. This layer consisted of mature fibroblasts (black arrow), connective tissue (CT), and a few inflammatory cells (yellow arrow). However, there was no sign of fibrosis. The thickness of the connective tissue layer remained the same regardless of the implantation period. However, the number of inflammatory and multi-nuclear cells was high on day 7. This was probably due to the natural inflammation in response to the surgical procedure of hydrogel implantation. CD68 immune-stained sections showed a minimal number of active macrophages in the interfacial region (Figure 8k). The observed comparatively high number of macrophages on day 7 was probably due to the post-operative host tissue response. The lower number of macrophages on day 28 suggested the in vivo non-toxicity of the hydrogel. Furthermore, due to the absence of any external crosslinker, the hydrogel showed a moderate rate of degradation after 28 days (40 ± 3 %) of implantation (Figure 8l). Several hydrogel-based motion sensing devices were reported, but, in most cases, they lacked the data related to biocompatibility (both in vitro and in vivo) and biodegradability.[3, 39, 93, 94b, 94d] Thus, after evaluating the compatibility of the developed β-CD-g-(pAAm/pAETAc) hydrogel, it could be concluded that this hydrogel is safe to use for various biomedical applications, including implantable soft electronics. Due to the biodegradation and non-toxicity of the developed hydrogel, in the future, they can be used as implantable biosensor.
3. Conclusion
In summary, a novel multifunctional SPN-based pseudo-slide-ring hydrogel with high toughness and optimal electrical conductivity was prepared in a one-pot synthesis process by fast grafting and crosslinking of AAm, AETAc and β-CD. The hydrogel exhibited high stretchability (~3000%), rapid self-healing properties, optimal adhesion strength (~27 kPa), and excellent transparency. Besides, all the physicochemical properties of the engineered hydrogel could be tuned by adjusting of AAm/AETAc ratio. In addition, the hydrogel was found to possess several customer-friendly features, including UV shielding, passive cooling, and 3D printability. The hydrogel was used to fabricate a biosensor that sensed the strain and human motion very delicately. With high sensitivity (GF = 3.74) and reliable long-term stability over 200 cycles, the sensor could quickly and accurately distinguish several minute and strong human movements. Moreover, we demonstrated its efficiency in monitoring motor dysfunctions in a simulated Parkinson’s condition. The engineered hydrogel showed non-toxicity both in vitro and in vivo. Hence, our multifunctional conductive β-CD-g-(pAAm/pAETAc) hydrogel could be potentially used as a healthcare monitoring device for neurodegenerative disorders, E-skin, smart devices, and artificial intelligence.
4. Experimental Section
4.1. Materials
AETAc, AAm, and 2, 2-diphenyl-1-picrylhydrazyl (DPPH) free radicals were purchased from Sigma-Aldrich (USA). β-CD was purchased from TCI (USA). Potassium persulfate (KPS) and DPBS were obtained from Fisher Scientific (USA). Dulbecco’s modified Eagle medium (DMEM) was purchased from Cellgro (Manassas, VA). Fetal Bovine Serum (FBS) was obtained from HyClone (Logan, UT).
4.2. Synthesis of β-CD-g-(pAAm/pAETAc) Hydrogels with Different AAm/AETAc Ratios
β-CD-g-(pAAm/pAETAc) hydrogels with different AAm/AETAc ratios were synthesized in one pot (in test tube) using a free radical grafting followed by supramolecular self-crosslinking reactions in an aqueous media. In brief, 8 mL of β-CD solution (5.5 mM) was prepared using milli-Q water at room temperature. Next, 1 mg of free radical initiator KPS was mixed with the solution of β-CD under N2 purging. Then 1 g of AAm and 1 mL of AETAc (5.8 M) were added to the reaction mixture (1:1 formulation), followed by N2 purging for 5 more min. The tubes were then appropriately sealed and placed in an oil bath at 60 ± 5 °C for 4 h. Finally, the transparent hydrogels formed inside the test tubes were collected after cooling (at room temperature) and utilized for further experiments. For the synthesis of the 3:1 formulation, 1.5 g of AAm and 0.5 mL of AETAc (5.8 M) were used, while for the 1:3 ratio, 0.5 gm of AAm and 1.5 mL AETAc (5.8 M) were used.
4.3. Chemical Characterization of β-CD-g-(pAAm/pAETAc) Hydrogels
4.3.1. 1H Nuclear Magnetic Resonance (1H NMR) Analysis
The chemical structure of all the starting materials, prepolymer solution (mixture of starting materials), and the hydrogel was confirmed from 1H NMR spectroscopic analysis using a 400 MHz Bruker AV400 spectrometer. For 1H NMR analysis, the β-CD, AAm, AETAc, prepolymer solution, and β-CD-g-(pAAm/pAETAc) hydrogel (1:1 AAm/AETAc ratio) samples were prepared by dissolving 10 mg of each one in 1 mL of deuterated dimethyl sulfoxide (DMSO-d6) solvent.
4.3.2. Ultraviolet-Visible (UV-Vis) Spectroscopy
To examine the interaction between the tertiary (hydrophobic) amine groups of AETAc and the hydrophobic core of β-CD, UV-Vis spectroscopy was conducted using a TECAN M200 Pro plate reader. The analysis involved studying an aqueous solution of AETAc alone and a solution of AETAc combined with β-CD.
4.4. Rheological and Mechanical Characterization of β-CD-g-(pAAm/pAETAc) Hydrogel
The Rheological properties and self-healing ability of β-CD-g-(pAAm/pAETAc) hydrogels with different AAm/AETAc ratios were studied using dynamic amplitude sweep, frequency sweep, viscometry, and strain sweep experiments using an Anton Parr Rheometer (model MCR 302). To measure the yield stress of the developed hydrogels, amplitude sweep experiments were carried out at a constant frequency of 5 Hz. Dynamic frequency sweep experiments were accomplished at frequencies ranging from 1 to 20 Hz for all the hydrogels with different AAm/AETAc ratios to explore the gel features of the hydrogels as a function of changing frequency. The complex viscosity of the hydrogels was measured with an increase in shear rate using the viscometry method to evaluate the shear-thinning behavior of the developed hydrogels. Besides, the cyclic strain sweep experiment (thixotropy) with alternating 1% and 1000% strain as a function of time was carried out to explore the self-healing ability of β-CD-g-(pAAm/pAETAc) hydrogel (1:1 ratio of AAm/AETAc).
Mechanical properties of the β-CD-g-(pAAm/pAETAc) hydrogels with different AAm/AETAc ratios were assessed using an Instron 5943 mechanical tester. Tensile analysis was accomplished using rectangular gel samples at a speed of 2 mm/min. From the slope of the strain vs stress plot (0–20% strain), the tensile modulus was calculated for the respective samples. The ultimate strength and stretchability of the hydrogels were obtained from the tensile stress and strain curves respectively, at the point of failure. Besides, the toughness of the hydrogels was calculated from the area enclosed by the tensile stress vs strain curves. Cylindrical gel samples were used for compression tests at a speed of 1 mm/min. Compressive moduli for each of the hydrogels were measured from the slope (10–20% strain) of the compression stress vs strain plots respectively. All the data from Instron were recorded using Bluehill 4.06 software.
4.5. Characterization of Adhesion Properties of β-CD-g-(pAAm/pAETAc) Hydrogel
The adhesion properties of the hydrogels with different AAm/AETAc ratios were characterized through the lap shear test based on American Society for Testing and Materials (ASTM)-F2255 using an Instron 5943 mechanical tester.[75, 98] In brief, fresh porcine skin tissue surfaces were completely wetted with DPBS before applying the adhesive patch. The hydrogel patches were sandwiched between the skin tissues and pressed for 5 min for proper adhesion. The lap shear test was similarly conducted on other tissues, such as fresh rat heart and liver, to evaluate the hydrogel’s adhesion on internal organs. All the tests were performed with a constant tensile speed of 2 mm/min. The adhesion energy was calculated from the area under the force vs. displace curve and the adhesion strength was measured using Equation 1
| Equation 1 |
Where, Fmax = maximum force, WL= area of adhesion.
To microscopically assess the adhesiveness of the hydrogel on internal organs, the heart and liver were immediately excised from euthanized male Wistar rats, and the hydrogel (1:1 AAm/AETAc ratio) was adhered to the organ surface. Subsequently, the hydrogel/tissue interface was cryosectioned using a standard procedure. The resulting hydrogel/tissue interfaces were stained using H&E staining to examine the condition of the interface of the sectioned tissues in the presence of adhesive hydrogel.
4.6. Electro/Pressure Responsiveness of β-CD-g-(pAAm/pAETAc) Hydrogel
4.6.1. Electro-Responsive Features
Measurement of Conductivity
The conductivity of the hydrogels with different AAm/AETAc ratios was measured using the chronoamperometry method in an electrochemical workstation (PalmsSens4).[99] For the measurement, the hydrogels were cut into rectangular pieces and placed between two gold electrodes. The current was measured at a constant voltage and the conductivity of the materials was calculated using Ohm’s law.
Ex vivo Electrical Stimulation
Adult rabbits just after euthanasia were collected from the Division of Laboratory Animal Medicine at the University of California, Los Angeles (CA, USA). The experiment was conducted using our previously published procedure.[80, 100] The capability of β-CD-g-(pAAm/pAETAc) hydrogels to revive impulse propagation across striated muscle was evaluated ex vivo using fresh rabbit thigh muscle. The skin layer of a dead rabbit was surgically removed to expose the thigh muscle and a rectangular hydrogel patch (1 cm × 0.5 cm × 0.5 cm) was placed. A direct current (DC) potential was then applied (75 m sec square pulses at increasing frequencies) using electrical wires connected to the hydrogels. The beating of the muscle was checked visually, and the minimum voltage required to beat the muscle (threshold voltage) was noted.
Assessment of the Electro-responsiveness Properties.
To explore the electro-responsiveness of β-CD-g-(pAAm/pAETAc) hydrogel formed with a 1:1 AAm/AETAc ratio, cyclic voltammetry (CV) analysis was accomplished.[101] In the CV analysis, the current responses of the hydrogel were recorded as a function of applied potential. CV analysis was accomplished at different scan rates (300, 400, 500, 600 mV sec−1) within the potential range of −1 to +1 V.
Cyclic Stability
To assess the electrical stability of hydrogel formed with 1:1 AAm/AETAc ratio, successive CV analysis was carried out up to 200 cycles, and % capacity retention was calculated by using Equation 2.[7d]
| Equation 2 |
4.6.2. Pressure-Responsiveness of Hydrogel.
The pressure-responsive properties of the hydrogel formed with 1:1 AAm/AETAc ratio were assessed using different electrochemical experiments including CV and chronoamperometry. The CV analysis was executed under different amounts of applied external pressure in terms of different percentage compressions (20%, 50% and 80%).[102] The gauge factor (GF) of the β-CD-g-(pAAm/pAETAc) hydrogel-based flexible sensing device was evaluated using the following Equation 3
| Equation 3 |
Where, ΔR= change in resistance due to change in % strain, R0= initial resistance, ΔL= Change in length, L0= initial length.
For detecting various deformations (stretch, twist, and compression) in the hydrogel using the electrochemical studies, the hydrogel was cut into rectangular pieces (2.5 cm × 1.5 cm × 0.5 cm dimension) and these pieces were placed between two electrodes linked to an electrochemical interface (PalmsSens4). Using chronoamperometry the change in current response of the hydrogel under different deformations (strain) was recorded. The real-time strain sensing feature of the synthesized hydrogel was performed by detecting the change in current signals obtained from the hydrogel-based sensor in chronoamperometry. The relative changes in current response due to compression, stretching, and twisting of the hydrogel were recorded.
To verify the self-healing efficacy, the synthesized β-CD-g-(pAAm/pAETAc) hydrogel formed with 1:1 AAm/AETAc ratio was cut into two pieces using a blade, and the separated pieces were subsequently rejoined at normal conditions (room temperature) under chronoamperometry experiment. This test aimed to assess whether the hydrogel could recover to its original electrical state. For this, the relative changes in the current response of the hydrogel were recorded before and after the cut.
To assess the influence of pH and humidity on the sensing performance of the hydrogel-based sensor, we submerged the hydrogel, formed with a 1:1 AAm/AETAc ratio, in aqueous solutions with different pH levels (2, 3, 5, 7, and 9) at 37°C and 60% humidity to evaluate the effect of pH.[103] To assess the influence of relative humidity on the hydrogel-based sensor’s sensing performance, we incubated the hydrogel-based sensors at different relative humidity (50%, 60%, 70%, and 80%).[104] We assessed the hydrogel’s real-time sensing capability by detecting the changes in current signals obtained from the hydrogel-based sensor in the amperometric i-t curve (CH instruments). The relative changes in current response due to compression and stretching of the hydrogel were recorded and the response time was measured for different pH and humidity levels.
To evaluate the effect of degradation on sensing performance, hydrogels were prepared in rectangular shape using 1:1 AAm/AETAc formulation and submerged in 2 ml DPBS at 37°C for 2 weeks.[105] After specific time points (days 0, 1, 5, 7, and 14) the hydrogel was removed from DPBS, and the sensing performance was monitored using amperometric i-t curve analysis (CH instruments) to measure the current response over time (i-t) in response to a constant applied potential. To ensure that the hydrogels remained fully submerged and in consistent conditions throughout the experiment, the DPBS solution was refilled every 2 days.
4.7. Transparency and UV-Shielding behavior of β-CD-g-(pAAm/pAETAc) Hydrogel
The transparency and UV shielding efficacy of the synthesized β-CD-g-(pAAm/pAETAc) hydrogel formed with 1:1 AAm/AETAc ratio was confirmed using a UV–vis spectrophotometer (PerkinElmer UV/VIS Lambda 365). The transmittance of pure water (for reference) and β-CD-g-(pAAm/pAETAc) hydrogel was recorded by placing a 30 mm long and 10 mm thick rectangular-shaped gel inside a quartz cuvette with path length of 10 mm.
4.8. Scanning Electron Microscopy (SEM) Analysis
The hydrogel sample was lyophilized for 48 hours and mounted onto SEM stubs, coated with a 60 sec application of gold via sputtering, and then visualized using a ZEISS Supra 40VP SEM.
4.9. Passive Cooling and Human Body Heat Radiation Test
To evaluate the passive cooling feature, a small piece (rectangular, 1.5 cm × 1.5 cm × 0.5 cm) of the engineered hydrogel (1:1 AAm/AETAc ratio) was attached to one hand wearing nitrile gloves and exposed to direct sunlight for 30 min. The temperature of the hydrogel and surrounding gloves were measured at regular intervals using a pre-calibrated thermal camera (FLIR-E8-XT). To understand whether the hydrogel can transmit the heat radiated from the human body, Fourier transform infrared spectroscopy (FTIR) was performed using a Thermo Scientific Nicolet 8700 FTIR spectrometer in transmittance mode.
4.10. In vitro Antioxidant Efficacy
The free-radical scavenging activity of the samples was determined using the DPPH method.[97] The hydrogel formed with 1:1 AAm/AETAc ratio was added to 1.0 mL of freshly prepared aqueous DPPH solution (0.1 mM). The change in absorbance was monitored at 535 nm for 30 min and percent antioxidant activity was calculated using Equation 4:
| Equation 4 |
EC50 values were measured from the percentage inhibition at different concentrations. EC50 is the concentration value that scavenged 50% of the DPPH radicals. Trolox was used as the reference compound.
4.11. 3D Printing of β-CD-g-(pAAm/pAETAc) Hydrogel
After evaluating the extrudability, the β-CD-g-(pAAm/pAETAc) hydrogel with the 1:1 formulation was selected as the bioink. It was loaded in a 5 mL syringe affixed to an 18-gauge blunt end needle (BSTEANTM). The syringe was then mounted onto an Allevi 3 bioprinter. The pressure was set to 5.0 PSI to allow for a proper flow rate of the bioink and printed into different structures with a layer height of 0.5 mm with a custom standard triangle language (STL) file.
4.12. Real-Time Human Motion Detection
To detect various signals related to human activity, the fabricated β-CD-g-(pAAm/pAETAc) hydrogel-based stretchable and flexible sensors (a rectangular shape with 2.5 cm × 1.5 cm × 0.5 cm dimension) were adhered to the different areas of the human body (on gloves or clothes). The sensing device was then connected to an electrochemical workstation to measure the alternation in the relative current response under the change in human movements.[1b] We further investigated the stability of the hydrogel-based sensor for the long-term real-time monitoring[106] of physiological signals by continuously monitoring index finger motion at a specific bending angle for 45 min using the amperometric i-t curve (CH instruments). It is important to note that in our experiments, sensors were not directly attached to the skin or organs, and no health information was collected from the subjects.
4.13. Detection of Simulated Motor Neuron Dysfunction in Simulated Perkinson’s Condition
To detect the motor movement dysfunctions characteristic of Parkinson’s disease (PD), we simulated the hand tremor (at different frequencies) and gait disbalance (including delayed steps and the indecision to put a step forward) intentionally and measured the alterations in current signals as described above. For hand tremor detection, we integrated the hydrogel sensor onto gloves. For gait detection, the sensor was on the toe of a shoe. To demonstrate the effectiveness of the engineered sensor in detecting other abnormalities such as abnormal breathing and coughing, we placed the sensor on the abdominal muscle (on clothes) to detect the abnormal signals.
4.14. In vitro Cytocompatibility Studies
The cytocompatibility of the β-CD-g-(pAAm/pAETAc) hydrogel formed with 1:1 AAm/AETAc ratio was evaluated using NIH 3T3 fibroblast cell line (CRL-1658, ATCC). Cells were grown on the engineered hydrogel and maintained at 37°C in 5% CO2 in DMEM media supplemented with 10% FBS and 1% antibiotics. Commercial live/dead kits (calcein AM and ethidium homodimer-1) and Actin/DAPI staining (Invitrogen, San Diego, CA) were used to assess cell viability and proliferation, respectively. Metabolic activity was evaluated using a PrestoBlue (Thermo Fisher Scientific, USA) assay on days 1, 3, and 5 post-seeding. The fluorescence intensity of the resulting solutions was recorded at 535–560 nm excitation and 590–615 nm emission. The spreading of NIH 3T3 cells on β-CD-g-(pAAm/pAETAc) hydrogel was visualized through the fluorescent staining of F-actin and cell nuclei. At days 1 and 5 post-seeding, the cell seeded β-CD-g-(pAAm/pAETAc) hydrogels were fixed in 4% (v/v) paraformaldehyde (Sigma) for 15 min, permeabilized in 0.1% wt/v Triton X-100 (Sigma) for 5 min, and then blocked in 1% w/v bovine serum albumin (BSA, Sigma) for 30 min. Samples were then incubated with Alexa fluor 594 phalloidin for 45 min. After consecutive washing with DPBS, samples were counterstained with 1 μL/mL DAPI (4’,6-diamidino-2-phenylindole, Sigma) in DPBS for 2 min. Fluorescent images were taken using an inverted fluorescence microscope (Zeiss Axio Observer Z7).
For reactive oxygen species (ROS) detection, the cells were incubated with 2,7-dicholorodihydro-fluorescene dye acetate (DCFH-DA, 10 × 10−6 M) in DPBS for 10 min in the dark. After rinsing with DPBS three times, the cells visualized using a fluorescence microscope. Fluorescent images were taken using a Zeiss Axio Observer Z1 inverted microscope and analyzed with ImageJ software. Cell viability was calculated as the ratio of viable cells to the total number of live and dead cells.
4.15. In vivo Biocompatibility Studies
The in vivo studies were approved by the IACUC (ARC-2021–113) at the University of California Los Angeles (UCLA). The in vivo biocompatibility study and histopathological analysis were performed following the previously published method.[75b] Male Wistar rats (250–300 g) were purchased from Charles River Laboratories (Boston, MA, YA) and anesthetized by inhalation of isoflurane (∼2%). After anesthesia, eight 1 cm incisions were made on the rats’ dorsal skin, and small subcutaneous pockets were made using a blunt scissor. The β-CD-g-(pAAm/pAETAc) hydrogel formed with 1:1 AAm/AETAc ratio was lyophilized and implanted into the subcutaneous pockets and incisions were closed with 4−0 polypropylene sutures (Ad Surgical) (n = 4). At days 7 and 28 post-operation, the rats were euthanized, and the implanted hydrogels were harvested with the tissues surrounding them. Evaluations of inflammatory responses caused by the implanted hydrogels were carried out through histology of the excised hydrogels. After retrieving the hydrogels, they were fixed in 4% paraformaldehyde for 4 h and incubated at 4 °C in 15 and 30 % (w/v) sucrose solution, respectively. The samples were then embedded in Optimal Cutting Temperature (OCT) compound, frozen in liquid nitrogen, and sectioned using a Leica CM1950 cryostat machine. The 10 μm sections were then mounted on positively charged slides and then were processed for hematoxylin and eosin (H&E) (Electron Microscopy Sciences) and CD68/DAPI staining was accomplished according to manufacturer instructions. Anti-CD68 (ab125212) (Abcam) was utilized as a primary antibody, while Goat anti-Rabbit IgG (H + L) antibody conjugated to Alexa Fluor 488 (Invitrogen) was used as a detection reagent and secondary antibody and all the antibodies were validated on respective hosts by the manufacturer and used in this study without further purification.
4.16. Statistical Analysis
Quantitative data are presented as Mean ± Standard Error of Mean (SEM) unless otherwise stated. All data are presented and analyzed as collected without any preprocessing. Comparison between multiple groups was conducted using a one-way analysis of variance (ANOVA) followed by Tukey’s post hoc multiple comparison test. Each experiment included a minimum of three samples, with the exact number specified in the respective Figures legends. Statistical analysis was performed using GraphPad Prism 8.4.3, with data fitting conducted using Origin Pro 8.5 (OriginLab, MA, USA). A p-value <0.05 was considered statistically significant for all comparisons.
Supplementary Material
Acknowledgments
We acknowledge the support from the National Institutes of Health (R01-EB023052; R01-HL140618).
Footnotes
Conflict Of Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Prof Annabi holds equity in GelMEDIX Inc.
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
References
- [1].a) Li S-N, Yu Z-R, Guo B-F, Guo K-Y, Li Y, Gong L-X, Zhao L, Bae J, Tang L-C, Nano Energy 2021, 90, 106502; [Google Scholar]; b) Liu W, Xie R, Zhu J, Wu J, Hui J, Zheng X, Huo F, Fan D, npj Flexible Electronics 2022, 6, 68; [Google Scholar]; c) Yuk H, Lu B, Zhao X, Chemical Society Reviews 2019, 48, 1642. [DOI] [PubMed] [Google Scholar]
- [2].Liu Y, Zhuo F, Zhou J, Kuang L, Tan K, Lu H, Cai J, Guo Y, Cao R, Fu Y, ACS Applied Materials & Interfaces 2022, 14, 54276. [DOI] [PubMed] [Google Scholar]
- [3].Shi X, Ma L, Li Y, Shi Z, Wei Q, Ma G, Zhang W, Guo Y, Wu P, Hu Z, Advanced Functional Materials 2023, 33, 2211720. [Google Scholar]
- [4].Li T, Qi H, Dong X, Li G, Zhai W, Advanced Materials 2024, 36, 2304145. [DOI] [PubMed] [Google Scholar]
- [5].Liu R, Liu Y, Fu S, Cheng Y, Jin K, Ma J, Wan Y, Tian Y, Small 2024, 2308092. [DOI] [PubMed] [Google Scholar]
- [6].Liu R, Wang T, Li G, Fan Z, Zhou Q, Wang K, Li P, Huang W, Advanced Functional Materials 2023, 2214917. [Google Scholar]
- [7].a) Xie C, Wang X, He H, Ding Y, Lu X, Advanced Functional Materials 2020, 30, 1909954; [Google Scholar]; b) Zhu F, Zheng SY, Lin J, Wu ZL, Yin J, Qian J, Qu S, Zheng Q, Journal of Materials Chemistry C 2020, 8, 7688; [Google Scholar]; c) Wang Z, Bu M, Xiu K, Sun J, Hu N, Zhao L, Gao L, Kong F, Zhu H, Song J, Nano Energy 2022, 104, 107978; [Google Scholar]; d) Roy A, Manna K, Ray PG, Dhara S, Pal S, ACS Applied Materials & Interfaces 2022, 14, 17065; [DOI] [PubMed] [Google Scholar]; e) Liu R, Liu Y, Cheng Y, Liu H, Fu S, Jin K, Li D, Fu Z, Han Y, Wang Y, Advanced Functional Materials 2023, 33, 2308175. [Google Scholar]
- [8].de Marzo G, Mastronardi VM, Todaro MT, Blasi L, Antonaci V, Algieri L, Scaraggi M, De Vittorio M, Nano Energy 2024, 109336. [Google Scholar]
- [9].Luo C, Huang M, Liu H, Journal of Applied Polymer Science 2022, 139, 51925. [Google Scholar]
- [10].Casaletto KB, Heaton RK, Journal of the International Neuropsychological Society 2017, 23, 778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hussain S, Mubeen I, Ullah N, Shah SSUD, Khan BA, Zahoor M, Ullah R, Khan FA, Sultan MA, BioMed research international 2022, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Raghavendra P, Pullaiah T, Advances in cell and molecular diagnostics, Academic Press, 2018. [Google Scholar]
- [13].He X-F, Zeng Z-F, Ni Q-Y, Xu Z-C, Mao P-F, Jiang B, Wu Q, Wang B, Gong L-X, Tang L-C, Composites Part B: Engineering 2023, 266, 111022. [Google Scholar]
- [14].Zhou H, Wang Z, Zhao W, Tong X, Jin X, Zhang X, Yu Y, Liu H, Ma Y, Li S, Chemical Engineering Journal 2021, 403, 126307. [Google Scholar]
- [15].Liu X, Shi H, Song F, Yang W, Yang B, Ding D, Liu Z, Hui L, Zhang F, International Journal of Biological Macromolecules 2024, 257, 128800. [DOI] [PubMed] [Google Scholar]
- [16].Huang W, Ding Q, Wang H, Wu Z, Luo Y, Shi W, Yang L, Liang Y, Liu C, Wu J, Nature Communications 2023, 14, 5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lu Y, Yang G, Wang S, Zhang Y, Jian Y, He L, Yu T, Luo H, Kong D, Xianyu Y, Nature Electronics 2024, 7, 51. [Google Scholar]
- [18].Shi X, Fan X, Zhu Y, Liu Y, Wu P, Jiang R, Wu B, Wu H-A, Zheng H, Wang J, Nature Communications 2022, 13, 1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kim J, Campbell AS, de Ávila BE-F, Wang J, Nature biotechnology 2019, 37, 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].a) Maetzler W, Hausdorff JM, Movement Disorders 2012, 27, 627; [DOI] [PubMed] [Google Scholar]; b) de Lau LM, Koudstaal PJ, Hofman A, Breteler MM, Archives of neurology 2006, 63, 362. [DOI] [PubMed] [Google Scholar]
- [21].Ossig C, Antonini A, Buhmann C, Classen J, Csoti I, Falkenburger B, Schwarz M, Winkler J, Storch A, Journal of neural transmission 2016, 123, 57. [DOI] [PubMed] [Google Scholar]
- [22].Albahri OS, Albahri AS, Mohammed K, Zaidan A, Zaidan B, Hashim M, Salman OH, Journal of medical systems 2018, 42, 1. [DOI] [PubMed] [Google Scholar]
- [23].Rovini E, Maremmani C, Cavallo F, Frontiers in neuroscience 2017, 11, 288959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rodriguez-León C, Villalonga C, Munoz-Torres M, Ruiz JR, Banos O, JMIR mHealth and uHealth 2021, 9, e25138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Teymourian H, Barfidokht A, Wang J, Chemical Society Reviews 2020, 49, 7671. [DOI] [PubMed] [Google Scholar]
- [26].Anikwe CV, Nweke HF, Ikegwu AC, Egwuonwu CA, Onu FU, Alo UR, Teh YW, Expert Systems with Applications 2022, 202, 117362. [Google Scholar]
- [27].Prieto-Avalos G, Cruz-Ramos NA, Alor-Hernández G, Sánchez-Cervantes JL, Rodríguez-Mazahua L, Guarneros-Nolasco LR, Biosensors 2022, 12, 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nazarian S, Lam K, Darzi A, Ashrafian H, Journal of medical Internet research 2021, 23, e28974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ali A, Ashfaq M, Qureshi A, Muzammil U, Shaukat H, Ali S, Altabey WA, Noori M, Kouritem SA, Sensors 2023, 23, 6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shen Z, Liu F, Huang S, Wang H, Yang C, Hang T, Tao J, Xia W, Xie X, Biosensors and Bioelectronics 2022, 211, 114298. [DOI] [PubMed] [Google Scholar]
- [31].Jalal A, Quaid MAK, Hasan AS, presented at 2018 International Conference on Frontiers of Information Technology (FIT) 2018. [Google Scholar]
- [32].a) Liu Y, Yang T, Zhang Y, Qu G, Wei S, Liu Z, Kong T, Advanced Materials 2019, 31, 1902783; [DOI] [PubMed] [Google Scholar]; b) Kim S, Baek S, Sluyter R, Konstantinov K, Kim JH, Kim S, Kim YH, EcoMat 2023, 5, e12356; [Google Scholar]; c) Junaid SB, Imam AA, Abdulkarim M, Surakat YA, Balogun AO, Kumar G, Shuaibu AN, Garba A, Sahalu Y, Mohammed A, Applied Sciences 2022, 12, 10271. [Google Scholar]
- [33].Lu B, Yuk H, Lin S, Jian N, Qu K, Xu J, Zhao X, Nature communications 2019, 10, 1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].a) Wang L, Zhang M, Yang B, Ding X, Tan J, Song S, Nie J, ACS Applied Materials & Interfaces 2021, 13, 5486; [DOI] [PubMed] [Google Scholar]; b) Sun M, Li P, Qin H, Liu N, Ma H, Zhang Z, Li J, Lu B, Pan X, Wu L, Chemical Engineering Journal 2023, 454, 140459. [Google Scholar]
- [35].a) Jo H, Sim M, Kim S, Yang S, Yoo Y, Park J-H, Yoon TH, Kim M-G, Lee JY, Acta Biomaterialia 2017, 48, 100; [DOI] [PubMed] [Google Scholar]; b) Xu C, Yu X, Liu Y, Zhang X, Liu S, ACS Applied Polymer Materials 2023, 5, 7375. [Google Scholar]
- [36].Lai QT, Zhao XH, Sun QJ, Tang Z, Tang XG, Roy VA, Small 2023, 2300283. [DOI] [PubMed] [Google Scholar]
- [37].a) Ohm Y, Pan C, Ford MJ, Huang X, Liao J, Majidi C, Nature Electronics 2021, 4, 185; [Google Scholar]; b) Lim C, Shin Y, Jung J, Kim JH, Lee S, Kim D-H, APL Materials 2019, 7; [Google Scholar]; c) Alipour S, Pourjavadi A, Hosseini SH, Carbohydrate Polymers 2023, 310, 120610. [DOI] [PubMed] [Google Scholar]
- [38].a) Dispenza C, Presti CL, Belfiore C, Spadaro G, Piazza S, Polymer 2006, 47, 961; [Google Scholar]; b) Chakraborty P, Guterman T, Adadi N, Yadid M, Brosh T, Adler-Abramovich L, Dvir T, Gazit E, ACS nano 2018, 13, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sun Z, Dong C, Chen B, Li W, Hu H, Zhou J, Li C, Huang Z, Small 2023, 19, 2303612. [DOI] [PubMed] [Google Scholar]
- [40].Kayser LV, Lipomi DJ, Advanced Materials 2019, 31, 1806133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Roberts T, De Graaf JB, Nicol C, Hervé T, Fiocchi M, Sanaur S, Advanced healthcare materials 2016, 5, 1462. [DOI] [PubMed] [Google Scholar]
- [42].a) Zhang C, Zhou Y, Han H, Zheng H, Xu W, Wang Z, ACS nano 2021, 15, 1785; [DOI] [PubMed] [Google Scholar]; b) He X, Wen N, Zhang W, He S, Yang S, Li X, Chen C, Zuo F, RSC advances 2023, 13, 7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].He Z, Liu J, Fan X, Song B, Gu H, Industrial & Engineering Chemistry Research 2022, 61, 17915. [Google Scholar]
- [44].a) Shi X, Wang K, Gao S, Zhang D, Lai C, Jin C, Li M, Wang C, Yong Q, Chu F, Industrial & Engineering Chemistry Research 2023, 62, 17765; [Google Scholar]; b) Zhao M, Tang Z, Zhang X, Li Z, Xiao H, Zhang M, Liu K, Ni Y, Huang L, Chen L, Composites Science and Technology 2020, 198, 108294. [Google Scholar]
- [45].a) Zheng Y, Baidya A, Annabi N, Bioactive Materials 2023, 29, 214; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Montazerian H, Davoodi E, Baidya A, Badv M, Haghniaz R, Dalili A, Milani AS, Hoorfar M, Annabi N, Khademhosseini A, Chemical Society Reviews 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Tan MJ, Owh C, Chee PL, Kyaw AKK, Kai D, Loh XJ, Journal of Materials Chemistry C 2016, 4, 5531. [Google Scholar]
- [47].a) Ates HC, Nguyen PQ, Gonzalez-Macia L, Morales-Narváez E, Güder F, Collins JJ, Dincer C, Nature Reviews Materials 2022, 7, 887; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lim HR, Kim HS, Qazi R, Kwon YT, Jeong JW, Yeo WH, Advanced Materials 2020, 32, 1901924. [DOI] [PubMed] [Google Scholar]
- [48].Jiang Y, Zhang Z, Wang Y-X, Li D, Coen C-T, Hwaun E, Chen G, Wu H-C, Zhong D, Niu S, Science 2022, 375, 1411. [DOI] [PubMed] [Google Scholar]
- [49].Xiong X, Chen Y, Wang Z, Liu H, Le M, Lin C, Wu G, Wang L, Shi X, Jia Y-G, Nature communications 2023, 14, 1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wang S, Chen Y, Sun Y, Qin Y, Zhang H, Yu X, Liu Y, Communications Materials 2022, 3, 2. [Google Scholar]
- [51].a) Meid J, Dierkes F, Cui J, Messing R, Crosby AJ, Schmidt A, Richtering W, Soft Matter 2012, 8, 4254; [Google Scholar]; b) Voronova MI, Surov OV, Afineevskii AV, Zakharov AG, Heliyon 2020, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].a) Li J, Celiz A, Yang J, Yang Q, Wamala I, Whyte W, Seo B, Vasilyev N, Vlassak J, Suo Z, Science 2017, 357, 378; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wang L, Gao G, Zhou Y, Xu T, Chen J, Wang R, Zhang R, Fu J, ACS applied materials & interfaces 2018, 11, 3506. [DOI] [PubMed] [Google Scholar]
- [53].a) Mohamadhoseini M, Mohamadnia Z, Coordination Chemistry Reviews 2021, 432, 213711; [Google Scholar]; b) Roy A, Manna K, Pal S, Materials Chemistry Frontiers 2022; [Google Scholar]; c) Roy A, Manna K, Dey S, Pal S, Carbohydrate Polymers 2023, 120576. [DOI] [PubMed] [Google Scholar]
- [54].a) Roy A, Guha Ray P, Bose A, Dhara S, Pal S, ACS Applied Bio Materials 2022, 5, 3530; [DOI] [PubMed] [Google Scholar]; b) Roy A, Guha Ray P, Manna K, Banerjee C, Dhara S, Pal S, Biomacromolecules 2021, 22, 5256. [DOI] [PubMed] [Google Scholar]
- [55].Mahdavinia G, Pourjavadi A, Hosseinzadeh H, Zohuriaan M, European Polymer Journal 2004, 40, 1399. [Google Scholar]
- [56].Thakur S, Arotiba OA, Polymer bulletin 2018, 75, 4587. [Google Scholar]
- [57].Periasamy R, Journal of Carbohydrate Chemistry 2020, 39, 189. [Google Scholar]
- [58].a) Li L, Zhang Y, Lu H, Wang Y, Xu J, Zhu J, Zhang C, Liu T, Nature communications 2020, 11, 62; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lee JH, Park J, Park J-W, Ahn H-J, Jaworski J, Jung JH, Nature communications 2015, 6, 6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sano K, Igarashi N, Ebina Y, Sasaki T, Hikima T, Aida T, Ishida Y, Nature Communications 2020, 11, 6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Basu A, Lindh J, Ålander E, Strømme M, Ferraz N, Carbohydrate polymers 2017, 174, 299. [DOI] [PubMed] [Google Scholar]
- [61].Gu S, Cheng G, Yang T, Ren X, Gao G, Macromolecular Materials and Engineering 2017, 302, 1700402. [Google Scholar]
- [62].Dong H, Snyder JF, Williams KS, Andzelm JW, Biomacromolecules 2013, 14, 3338. [DOI] [PubMed] [Google Scholar]
- [63].Stojkov G, Niyazov Z, Picchioni F, Bose RK, Gels 2021, 7, 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Roy A, Maity PP, Bose A, Dhara S, Pal S, Materials chemistry frontiers 2019, 3, 385. [Google Scholar]
- [65].Gong J, Wang L, Wu J, Yuan Y, Mu R-J, Du Y, Wu C, Pang J, Lwt 2019, 100, 271. [Google Scholar]
- [66].a) Xia L-W, Xie R, Ju X-J, Wang W, Chen Q, Chu L-Y, Nature communications 2013, 4, 2226; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Asoh T-A, Yamamoto T, Uyama H, Scientific reports 2020, 10, 17173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Pruksawan S, Lim JWR, Lee YL, Lin Z, Chee HL, Chong YT, Chi H, Wang F, Communications Materials 2023, 4, 75. [Google Scholar]
- [68].Wei H, Lei M, Zhang P, Leng J, Zheng Z, Yu Y, Nature communications 2021, 12, 2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Bakarich SE, Beirne S, Wallace GG, Spinks GM, Journal of Materials Chemistry B 2013, 1, 4939. [DOI] [PubMed] [Google Scholar]
- [70].Sun TL, Kurokawa T, Kuroda S, Ihsan AB, Akasaki T, Sato K, Haque MA, Nakajima T, Gong JP, Nature materials 2013, 12, 932. [DOI] [PubMed] [Google Scholar]
- [71].Fuchs S, Shariati K, Ma M, Advanced healthcare materials 2020, 9, 1901396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Chong J, Sung C, Nam KS, Kang T, Kim H, Lee H, Park H, Park S, Kang J, Nature Communications 2023, 14, 2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Pan Z, Fu Q-Q, Wang M-H, Gao H-L, Dong L, Zhou P, Cheng D-D, Chen Y, Zou D-H, He J-C, Nature Communications 2023, 14, 5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Yuk H, Wu J, Zhao X, Nature Reviews Materials 2022, 7, 935. [Google Scholar]
- [75].a) Annabi N, Zhang Y-N, Assmann A, Sani ES, Cheng G, Lassaletta AD, Vegh A, Dehghani B, Ruiz-Esparza GU, Wang X, Science translational medicine 2017, 9, eaai7466; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zheng Y, Shariati K, Ghovvati M, Vo S, Origer N, Imahori T, Kaneko N, Annabi N, Biomaterials 2023, 301, 122240. [DOI] [PubMed] [Google Scholar]
- [76].Karami P, Nasrollahzadeh N, Wyss C, O’Sullivan A, Broome M, Procter P, Bourban PE, Moser C, Pioletti DP, Macromolecular rapid communications 2021, 42, 2000660. [DOI] [PubMed] [Google Scholar]
- [77].Zhang K, Chen X, Xue Y, Lin J, Liang X, Zhang J, Zhang J, Chen G, Cai C, Liu J, Advanced Functional Materials 2022, 32, 2111465. [Google Scholar]
- [78].Alam A, Kuan H-C, Zhao Z, Xu J, Ma J, Composites Part A: Applied Science and Manufacturing 2017, 93, 1. [Google Scholar]
- [79].a) Wang L, Jiang J, Hua W, Darabi A, Song X, Song C, Zhong W, Xing MM, Qiu X, Advanced Functional Materials 2016, 26, 4293; [Google Scholar]; b) Stejskal J, Bober P, Trchová M, Kovalcik A, Hodan J. i., Hromádková J. i., Prokeš J, Macromolecules 2017, 50, 972. [Google Scholar]
- [80].Walker BW, Lara RP, Yu CH, Sani ES, Kimball W, Joyce S, Annabi N, Biomaterials 2019, 207, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Maity CK, Santra DK, Verma K, Sahoo S, Cotts S, Akinwande D, Berry V, Nayak GC, Composites Part B: Engineering 2021, 212, 108728. [Google Scholar]
- [82].a) Cui F, Yue Y, Zhang Y, Zhang Z, Zhou HS, ACS sensors 2020, 5, 3346; [DOI] [PubMed] [Google Scholar]; b) Zhang Y, Hu Y, Jiang N, Yetisen AK, Biosensors and Bioelectronics 2023, 219, 114825. [DOI] [PubMed] [Google Scholar]
- [83].Liu Y, Wang L, Mi Y, Zhao S, Qi S, Sun M, Peng B, Xu Q, Niu Y, Zhou Y, Journal of Materials Chemistry C 2022, 10, 13351. [Google Scholar]
- [84].Li Z, Liu P, Chen S, Yu Y, Li T, Tang N, Wan Y, Reactive and Functional Polymers 2023, 105644. [Google Scholar]
- [85].He Z, Yuan W, ACS Applied Materials & Interfaces 2021, 13, 1474. [DOI] [PubMed] [Google Scholar]
- [86].Shokri J, Hasanzadeh D, Ghanbarzadeh S, Dizadji-Ilkhchi M, Adibkia K, Drug research 2013, 591. [DOI] [PubMed] [Google Scholar]
- [87].Zhu Y, Haghniaz R, Hartel MC, Guan S, Bahari J, Li Z, Baidya A, Cao K, Gao X, Li J, Advanced Materials 2023, 35, 2209300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].a) Ma Z, Xiang X, Shao L, Zhang Y, Gu J, Angewandte Chemie International Edition 2022, 61, e202200705; [DOI] [PubMed] [Google Scholar]; b) Yu H, Yang X, Lian Y, Wang M, Liu Y, Li Z, Jiang Y, Gou J, Sensors and Actuators A: Physical 2021, 318, 112514. [Google Scholar]
- [89].a) Hsu P-C, Song AY, Catrysse PB, Liu C, Peng Y, Xie J, Fan S, Cui Y, Science 2016, 353, 1019; [DOI] [PubMed] [Google Scholar]; b) Xu Y, Sun B, Ling Y, Fei Q, Chen Z, Li X, Guo P, Jeon N, Goswami S, Liao Y, Proceedings of the National Academy of Sciences 2020, 117, 205; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Leroy A, Bhatia B, Kelsall C, Castillejo-Cuberos A, Di Capua H, Sci. Adv 2019, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Adhikari A, Darbar S, Chatterjee T, Das M, Polley N, Bhattacharyya M, Bhattacharya S, Pal D, Pal SK, Acs Omega 2018, 3, 15975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Promphet N, Ummartyotin S, Ngeontae W, Puthongkham P, Rodthongkum N, Analytica Chimica Acta 2021, 1179, 338643. [DOI] [PubMed] [Google Scholar]
- [92].Huang Z, Chen X, O’Neill SJ, Wu G, Whitaker DJ, Li J, McCune JA, Scherman OA, Nature materials 2022, 21, 103. [DOI] [PubMed] [Google Scholar]
- [93].Qi C, Dong Z, Huang Y, Xu J, Lei C, ACS Applied Materials & Interfaces 2022, 14, 30385. [DOI] [PubMed] [Google Scholar]
- [94].a) Zeng Z, Yu S, Guo C, Lu D, Geng Z, Pei D, Macromolecular Rapid Communications 2022, 43, 2200103; [DOI] [PubMed] [Google Scholar]; b) He Z, Zhou Z, Yuan W, ACS Applied Materials & Interfaces 2022, 14, 38205; [DOI] [PubMed] [Google Scholar]; c) Ren Y, Feng J, ACS applied materials & interfaces 2020, 12, 6797; [DOI] [PubMed] [Google Scholar]; d) Hu C, Zhang Y, Wang X, Xing L, Shi L, Ran R, ACS applied materials & interfaces 2018, 10, 44000; [DOI] [PubMed] [Google Scholar]; e) Guan L, Liu H, Ren X, Wang T, Zhu W, Zhao Y, Feng Y, Shen C, Zvyagin AV, Fang L, Advanced Functional Materials 2022, 32, 2112281. [Google Scholar]
- [95].Stern MB, Siderowf A, Movement disorders 2010, 25, S89. [DOI] [PubMed] [Google Scholar]
- [96].Morgan C, Craddock I, Tonkin EL, Kinnunen KM, McNaney R, Whitehouse S, Mirmehdi M, Heidarivincheh F, McConville R, Carey J, BMJ Open 2020, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Adhikari A, Mondal S, Chatterjee T, Das M, Biswas P, Ghosh R, Darbar S, Alessa H, Althakafy JT, Sayqal A, Communications Biology 2021, 4, 1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Montazerian H, Hassani Najafabadi A, Davoodi E, Seyedmahmoud R, Haghniaz R, Baidya A, Gao W, Annabi N, Khademhosseini A, Weiss PS, Advanced Healthcare Materials 2023, 2203404. [DOI] [PubMed] [Google Scholar]
- [99].Baidya A, Ghovvati M, Lu C, Naghsh-Nilchi H, Annabi N, ACS Applied Materials & Interfaces 2022, 14, 49483. [DOI] [PubMed] [Google Scholar]
- [100].Annabi N, Shin SR, Tamayol A, Miscuglio MM, Afshar MM, Assmann A, Mostafalu P, Sun J-Y, Mithieux S, Cheung ML, Advanced materials (Deerfield Beach, Fla.) 2016, 28, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Chakraborty P, Oved H, Bychenko D, Yao Y, Tang Y, Zilberzwige‐Tal S, Wei G, Dvir T, Gazit E, Advanced Materials 2021, 33, 2008715. [DOI] [PubMed] [Google Scholar]
- [102].Ge G, Yuan W, Zhao W, Lu Y, Zhang Y, Wang W, Chen P, Huang W, Si W, Dong X, Journal of Materials Chemistry A 2019, 7, 5949. [Google Scholar]
- [103].Escobedo P, Ramos-Lorente CE, Martínez-Olmos A, Carvajal MA, Ortega-Muñoz M, de Orbe-Payá I, Hernández-Mateo F, Santoyo-González F, Capitán-Vallvey LF, Palma AJ, Sensors and Actuators B: Chemical 2021, 327, 128948. [Google Scholar]
- [104].Bian Z, Li Y, Sun H, Shi M, Zheng Y, Liu H, Liu C, Shen C, Carbohydrate Polymers 2023, 301, 120300. [DOI] [PubMed] [Google Scholar]
- [105].Fares MM, Sani ES, Lara RP, Oliveira RB, Khademhosseini A, Annabi N, Biomaterials science 2018, 6, 2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Ge G, Zhang Y, Shao J, Wang W, Si W, Huang W, Dong X, Advanced Functional Materials 2018, 28, 1802576. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


