Abstract
The potential of nontoxic recombinant B subunits of cholera toxin (rCtxB) and its close relative Escherichia coli heat-labile enterotoxin (rEtxB) to act as mucosal adjuvants for intranasal immunization with herpes simplex virus type 1 (HSV-1) glycoproteins was assessed. Doses of 10 μg of rEtxB or above with 10 μg of HSV-1 glycoproteins elicited high serum and mucosal anti-HSV-1 titers comparable with that obtained using CtxB (10 μg) with a trace (0.5 μg) of whole toxin (Ctx-CtxB). By contrast, doses of rCtxB up to 100 μg elicited only meager anti-HSV-1 responses. As for Ctx-CtxB, rEtxB resulted in a Th2-biased immune response with high immunoglobulin G1 (IgG1)/IgG2a antibody ratios and production of interleukin 4 (IL-4) and IL-10 as well as gamma interferon by proliferating T cells. The protective efficacy of the immune response induced using rEtxB as an adjuvant was assessed following ocular challenge of immunized and mock-immunized mice. Epithelial disease was observed in both groups, but the immunized mice recovered by day 6 whereas mock-immunized mice developed more severe corneal disease leading to stromal keratitis. In addition, a significant reduction in the incidence of lid disease and zosteriform spread was observed in immunized animals and there was no encephalitis compared with 95% encephalitis in mock-immunized mice. The potential of such mucosal adjuvants for use in human vaccines against pathogens such as HSV-1 is discussed.
Herpes simplex virus type 1 (HSV-1) is an alphaherpesvirus which invades primarily mucosal and epithelial surfaces of the eye and mouth, causing ulcerative lesions at these sites. The initial response to infection is the influx of neutrophils and macrophages, followed by CD4+ T cells, CD8+ T cells, and B cells (21, 22, 25, 30). Upon infection, however, the virus replicates, enters sensory nerve endings, travels along the axon, and becomes latent in the nerve ganglia, where it evades detection by immune cells. The precise mechanism for protection from infection is still unclear. Although CD4+ T cells and neutralizing antibodies have been shown to have roles, a number of mechanisms may be involved (29). In the eye, the immune mechanisms involved in protection against HSV-1 may be further complicated by immunopathological responses, whereby immune cells infiltrate the stroma, causing opacity and edema of the cornea. In certain cases, the cornea may become highly vascularized and thickened, particularly after recurrent infections, leading to severe stromal keratitis, a major cause of nontraumatic blindness in many countries. Ocular disease is currently controlled by administration of antiviral drugs and corticosteroids. Attempts to develop a vaccine against recurrent infection with HSV-1 have been unsuccessful, possibly due to the lack of specific immunity at the mucosal surfaces and induction of an inflammatory-type (Th1) response (5, 33). We previously investigated the potential of mucosal vaccination following intranasal administration of viral antigens mixed with the mucosal adjuvant comprising both cholera toxin (Ctx) and its B subunit (CtxB) (27). A strong systemic and mucosal immune response to HSV-1 was induced with high levels of serum immunoglobulin G1 (IgG1) and secretory IgA. In addition, specific T cells from lymph nodes local and distal to the site of immunization were shown to secrete cytokines, interleukin 4 (IL-4), IL-5, and gamma interferon (IFN-γ). Taken together, these results were consistent with the ability of Ctx to act as a potent adjuvant inducing a Th2-dominated response (20, 38). Vaccination thus protected animals from corneal disease as well as preventing the spread of virus in the nervous system, as measured by reduction in zosteriform lesions. In addition, vaccination significantly reduced the incidence of latent virus in the ophthalmic region of the trigeminal ganglion (TG). These results indicated the potential of this method of vaccination for providing protection against HSV-1 infection. However, the inclusion of whole Ctx, with its potent toxic effects, renders such a vaccine undesirable.
In attempting to move away from the use of whole Ctx as an adjuvant, we have sought to determine the relative roles played by the toxin subunits. Ctx, like its close relative the heat-labile toxin of Escherichia coli (Etx), consists of a single A subunit located in a central pore formed by interactions among five identical B-subunit monomers (24, 37). The B-subunit pentamer is a highly stable complex which binds to cell surface receptors, principally GM1 ganglioside, and mediates uptake and trafficking of the A subunit into the cell. As a result of this process, an N-terminal A1 fragment gains access to the cytosol and ADP ribosylates the cellular GTP-binding protein, Gsα, initiating a signaling cascade responsible for the toxicity of the proteins. The relative roles of the A and B subunits in mediating the adjuvant activity of Ctx and Etx have been controversial. Early reports which apparently demonstrated an adjuvant activity of CtxB have been discounted because the commercially purified preparations used were routinely contaminated with small but significant quantities (up to 0.5%) of the A subunit. Studies using recombinant preparations of CtxB, devoid of contaminating A subunit, or a nontoxic point mutant of Etx concluded that ADP ribosylation by the A subunits was an absolute requirement (18). Recently, however, other mutants of Etx and Ctx have been generated which exhibit reduced toxicity or which completely lack ribosyltransferase activity and yet function as effective adjuvants (7–9, 12, 39, 40). However, the potencies of even these new mutants as adjuvants correlate with levels of residual toxicity, and stability is often reduced (26). As a result of the high degree of sequence and structural homology, the properties of Ctx and Etx have been broadly assumed to be the same in this regard.
In this study we investigate the potential of nontoxic recombinant preparations of the B subunits of Ctx and Etx (rCtxB and rEtxB, respectively) to act as adjuvants for HSV-1 glycoproteins when administered intranasally. We show that while rCtxB is indeed unable to act as an intranasal adjuvant, rEtxB in contrast is a potent adjuvant, capable of eliciting strong protective immunity against HSV-1. Analysis of the profile of the immune response elicited using rEtxB compared to that with the combined Ctx-CtxB adjuvant reveals a more marked Th2 dominance with the B-subunit preparation. The reasons EtxB but not CtxB acts as an adjuvant are discussed.
MATERIALS AND METHODS
Mice.
Female NIH mice (originally from Harlan Olac, Bicester, United Kingdom, and then bred within the School of Medical Sciences Animal House) were immunized at 8 weeks of age.
Virus.
HSV-1 strain SC16 (14) was used throughout these studies. Virus-infected and mock-infected Vero cells were employed for the preparation of UV-inactivated virus for use in lymphocyte cultures (using serum-free media) or for preparation of surface antigens for use in enzyme-linked immunosorbent assays (ELISA). Surface antigens were prepared by lysis of infected cells with a zwitterionic detergent, followed by sonication at full power for 30 s and ultracentrifugation at 90,000 × g in a TV865 rotor (Sorvall, Wilmington, Del.) (16). The supernatant was collected, aliquoted, and stored at −80°C. Virus for use in infection or preparation of surface glycoproteins for immunization was propagated in Hep-2a cells. Viral glycoproteins were prepared as above for ELISA antigen, followed by sucrose density gradient centrifugation at 100,000 × g in a TV645 rotor (Sorvall). Fractions that contained HSV-1 specific activity, as judged by ELISA, were pooled, aliquoted, and stored at −80°C (16). The final glycoprotein preparation was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting with rabbit anti-HSV-1–horseradish peroxidase (HRP) conjugate and detection by chemiluminescence using the ECL kit (Amersham). The presence of at least four HSV-1 glycoproteins, namely, gB, gC, gD, and gE, in such preparations was shown using glycoprotein-specific monoclonal antibodies in ELISA and by radioimmunoprecipitation (17). Mock-infected preparations were made from cell cultures in the same way described above but without the addition of virus.
Purification of rEtxB and rCtxB.
Purification of rEtxB from Vibrio sp. strain 60 (pMMB68) was carried out as described previously (2, 28). Briefly, rEtxB expression was induced by IPTG (isopropyl-β-d-thiogalactopyranoside) addition to Vibrio sp. strain 60 (pMMB68) grown in Luria-Bertiani medium supplemented with 1% (wt/vol) NaCl. After 16 h, the culture medium was recovered by dia-ultrafiltration and then subjected to ammonium sulfate precipitation (30% saturation) and hydrophobic and anion-exchange chromatography. The single peak eluting from the Resource Q anion-exchange column was desalted by dialysis against phosphate-buffered saline (PBS) and stored at −80°C prior to use. Purification of rCtxB from Vibrio sp. strain 60 (pATA13) (1) was carried out using a modification of the above method. After induction and dia-ultrafiltration, the retentate fraction containing rCtxB was mixed with ammonium sulfate to a final concentration of 35% (saturated) and then subjected to hydrophobic interaction chromatography as before on a 25-ml phenyl-Superose HR5/5 column (Pharmacia). The protein was eluted with a decreasing gradient of 1.5 to 0.0 M ammonium sulfate in 20 mM Tris-HCl buffer, pH 7.5. The eluate fractions containing CtxB were pooled and dialyzed against 20 mM Tris-HCl–20 mM NaCl, pH 7.5, at 4°C overnight and then applied to a 6-ml Resource S cation-exchange column (Pharmacia) in 20 mM Tris-HCl–20 mM NaCl, pH 7.5. Protein was eluted with an increasing gradient of 20 mM to 1 M NaCl in 20 mM Tris-HCl, pH 7.5, the fractions containing CtxB were pooled and desalted using a NAP-10 column equilibrated in PBS, pH 7.4; and the purified protein was stored at −80°C prior to use.
Inoculation and immunization.
Mice anesthetized with 100 mg of ketamine (Parke-Davis, Pontypool, United Kingdom)/kg of body weight mixed with 10 mg of xylazine (Bayer, Bury St. Edmunds, United Kingdom)/kg were challenged by ocular scarification (35). Using a 26-gauge needle, 10 strokes were made across the surface of the right cornea through a 5-μl drop containing 103 or 104 PFU of HSV-1 SC16. For the production of positive control sera, mice were scarified on the skin of one side of the neck through a 10-μl drop containing 105 PFU of HSV-1.
Intranasal immunization was carried out on anesthetized mice by inhalation of a drop of immunogen containing 10 μg of HSV-1 glycoproteins mixed with either 10 μg of CtxB and 0.5 μg of Ctx (Sigma, Poole, United Kingdom) or various amounts of rCtxB or rEtxB as an adjuvant and placed on the tip of the nose. Three doses were given at 10-day intervals. In most cases, the volume of the drop was kept to a minimum (20 to 30 μl), but where different concentrations of adjuvant were compared, PBS was added to make the final volumes equal.
Measurement of antibody responses.
Samples of mouse sera, taken 1 week after the final immunization or 3 weeks after cutaneous scarification, were obtained by bleeding from the tail vein. Sera collected from infected mice were pooled, aliquoted, and stored at −20°C for use as positive controls. Sera from immunized mice were stored individually at −20°C. Eye and vaginal washings (EW and VW) were collected from lightly anesthetized mice (50 mg of ketamine/kg with 5 mg of xylazine/kg) 1 week following the final immunization by pipetting 20 μl of PBS up and down on the surface of each eye 10 times or 50 μl of PBS pipetted into the vagina 20 times. Samples of mucosal washings were pooled within each group and stored at −20°C.
Sera were analyzed for the presence of HSV-1-specific antibodies, as described previously (10), by use of HSV-1 antigen-coated assay plates, a rabbit anti-mouse Ig-HRP conjugate (Dako Ltd. High Wycombe, United Kingdom), and O-phenylenediamine (Sigma) as a substrate. The results obtained were assessed by comparison with a standard positive control serum collected from mice 4 weeks after systemic infection (cutaneous) with HSV-1 SC16, using weighted Probit analysis (3). In order to measure levels of virus-specific IgA or IgG in mucosal fluids, the conjugate was replaced with an HRP-conjugated goat anti-mouse IgA or IgG (Sigma), and endpoint titers were determined by regression analysis. The levels of IgG1 and IgG2a in the sera were measured using HRP-conjugated rat anti-mouse IgG1 or IgG2a, respectively, compared with a myeloma as a standard (Serotec).
Assessment of T-cell responses.
Single cell suspensions of lymphocytes in Hanks balanced salt solution (Gibco, Paisley, United Kingdom) containing 20 mM HEPES buffer (Gibco) were obtained by agitation of lymph nodes through wire mesh using a glass rod. The lymphocytes were washed and then cultured at 106/ml in α minimal essential medium supplemented with 20 mM HEPES, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 4 mM l-glutamine (Gibco), 50 μM 2-mecaptoethanol (Sigma), and 0.5% normal mouse serum in 25-cm2 flasks. The cells were cultured in the presence of UV-inactivated virus (prepared from serum-free supernatant of infected Vero cells) at a predetermined optimal concentration of 1.5 × 105 PFU/ml, an equivalent dilution of mock virus for assessment of nonviral responses, or medium alone (data not shown). Cultures were incubated at 37°C and 5% CO2 with humidity. Aliquots of 100 μl were removed on the desired days after initiation of the cultures and placed in triplicate into wells of a 96-well plate for assessment of [3H]thymidine incorporation using standard assay techniques (13).
Additional aliquots of cells were removed for assessment of cytokine levels by a previously described method (4). Briefly, cell samples were cultured overnight at 37°C in a humidified atmosphere of 5% CO2 in capture antibody-coated (rat anti-mouse cytokines) ELISA plates, before detection with biotinylated rat anti-mouse cytokines (Pharminogen, San Diego, Calif.). Thus, cytokine production by cells over a defined period of culture could be assessed under conditions where the effect of cytokine lability was minimized. Cytokine levels were calculated by regression analysis against standard curves produced with the appropriate recombinant cytokine (Pharminogen).
Analysis of protection from ocular challenge with HSV-1 in immunized and mock-immunized mice.
The eyes of the animals were examined using a 105L slit lamp microscope (Zeiss, Welwyn Garden City, United Kingdom), and disease scored on a scale of 0 to 4, where 0 is no disease, 1 is epithelial disease, 2 is mild opacity, 3 is moderate opacity, and 4 is necrotizing stromal keratitis. The spread of virus to other areas, resulting in ulceration and edema of the eyelid, as well as zosteriform lesions, indicated by the presence of viral lesions of the skin at sites served by the TG (the snout and lower jaw), were also recorded. Encephalitis was implicated where animals showed a significant defect in righting reflex, piloerection, loss of weight, and hunched posture; such animals were killed by cervical dislocation. Virus shedding from the eye was determined by washing the infected eyes with 20 μl of culture medium and transferring the washing fluid to Vero cells prior to a standard plaque assay. Assessment of viral latency was carried out on the three divisions of the TG dissected from the inoculated side of each mouse (34). Excised ganglion divisions were cultured separately for 5 days at 37°C and 5% CO2 with humidity before being homogenized individually and transferred to Vero cells for standard plaque assays.
Statistical analysis.
Significant differences in immune response between groups of mice were determined using Student's t test. Differences in level of protection against primary infection between HSV-1- and mock-immunized groups were assessed by chi-square analysis.
RESULTS
Induction of HSV-1-specific antibody response.
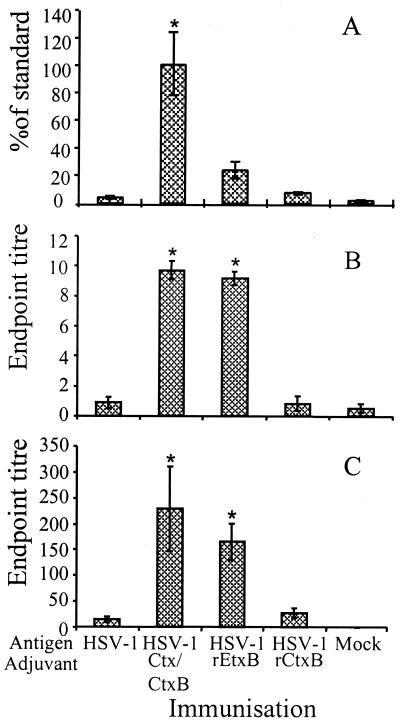
Previous studies indicated that optimum serum and mucosal antibody responses were elicited using a combination adjuvant composed of 10 μg of CtxB with 0.5 μg of Ctx. In order to test the requirement for the holotoxin in mediating the adjuvant effect, equivalent doses of rCtxB and rEtxB, devoid of any contaminating A subunit, were tested. Intranasal immunization with HSV-1 glycoproteins in the absence of adjuvant failed to induce specific antibodies in the serum; the level observed was equivalent to that in the mock-immunized control (Fig. 1A). The level of specific serum antibody in mice immunized in the presence of 10 μg of rCtxB was approximately twofold higher than in controls given glycoprotein alone. In contrast to rCtxB, 10 μg of rEtxB was able to stimulate a strong serum antibody response to HSV-1, increasing the response to approximately sixfold. The serum antibody response elicited using rEtxB as an adjuvant was nevertheless lower than that stimulated in the presence of whole Ctx, which triggered significant levels of antibody (P = 0.01; Student's t test), equivalent to that of cutaneous HSV-1 infection (Fig. 1A).
FIG. 1.
ELISA data showing HSV-1-specific Ig in sera (A) and secretory IgA in EW (B) and VW (C) in mice immunized intranasally with HSV-1 or mock glycoproteins alone or in the presence of Ctx-CtxB, rEtxB, or rCtxB as an adjuvant. The ELISA data for each serum sample were analyzed by Probit to give the percentage of the standard (positive control sera from mice infected with live virus by a systemic route). Mean values from groups of six mice plus the standard error of the mean (SEM) are given. Washings from each group of mice were pooled, and the endpoint titers were calculated by regression analysis. The mean values were calculated from the results of three similar experiments plus SEM, where each group consisted of 6 to 10 mice. ∗, Significantly higher response compared with glycoprotein only as judged by Student's t test.
Despite eliciting a lower serum antibody response to HSV-1, 10 μg of rEtxB was able to induce levels of secretory IgA comparable to those induced by the combination Ctx-CtxB adjuvant in mucosal washings of the eye (Fig. 1B) and the reproductive tract (Fig. 1C). Intranasal immunization with HSV-1 glycoproteins alone or in the presence of rCtxB failed to trigger significant mucosal antibody production. In contrast, addition of Ctx-CtxB or 10 μg of rEtxB was able to potentiate significant endpoint titers (P = 0.01) of approximately 1/10 in EW and 1/229 (P = 0.03) or 1/166 (P = 0.02) in VW.
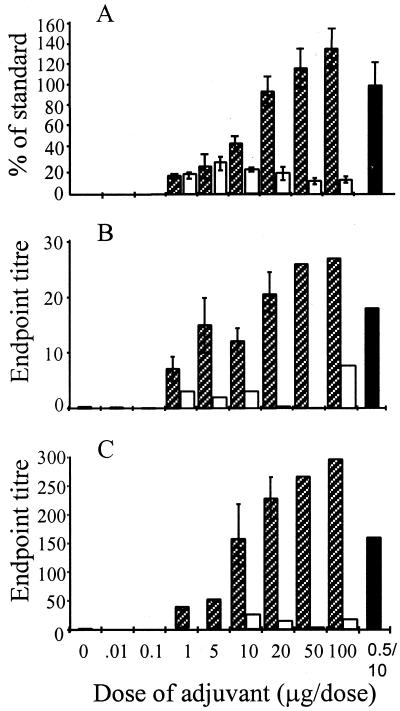
In order to assess whether an enhanced serum Ig response equivalent to that obtained using Ctx-CtxB as an adjuvant could be induced using B subunits alone, a range of doses of adjuvant were administered together with a constant amount of HSV-1 glycoprotein (10 μg). The serum antibody response to HSV-1 increased with higher quantities of rEtxB such that at 20 μg, the levels of specific Ig had reached approximately 100% of the standard control. Thus, a doubling of the rEtxB dose enhanced the response to that seen when using the combined Ctx-CtxB adjuvant. Further increases in the dose of rEtxB resulted in slightly higher levels of HSV-1-specific serum antibody, reaching approximately 140% of the standard control following administration of 100 μg of rEtxB (Fig. 2A). No such increase in the immune response to HSV-1 glycoproteins was observed using rCtxB, even at doses of 100 μg. Levels of secretory IgA in both EW (Fig. 2B) and VW (Fig. 2C) were also shown to increase with higher doses of rEtxB, with doses as low as 1 μg stimulating some response and doses of 10 μg and above inducing IgA levels equal to or greater than that obtained using Ctx-CtxB. CtxB induced only low levels of mucosal antibodies even at the higher doses tested. In order to investigate the nature of the immune responses induced using either the combined Ctx-CtxB adjuvant or rEtxB, 20 μg of rEtxB was used in subsequent immunizations, as this triggered a level of anti-HSV-1 response comparable to that induced with Ctx-CtxB.
FIG. 2.
Effect of increasing doses of rEtxB ( ) and rCtxB (□) mixed with HSV-1 glycoproteins on the level of virus-specific Ig in serum (A), as well as IgA in mucosal washings of the eye (B) and vagina (C), compared with Ctx-CtxB (■) as an adjuvant. Individual serum samples were analyzed by ELISA, and antibody responses were determined by Probit as a percent of the standard (the response following systemic infection), while mean values ± Standard errors of the mean (SEM) were determined from groups of 6 to 10 mice. Mucosal washings were pooled for each group of 6 to 10 mice, and endpoint titers were determined by regression analysis. Mean values ± SEM are given where the dose was repeated in three or more similar experiments.
) and rCtxB (□) mixed with HSV-1 glycoproteins on the level of virus-specific Ig in serum (A), as well as IgA in mucosal washings of the eye (B) and vagina (C), compared with Ctx-CtxB (■) as an adjuvant. Individual serum samples were analyzed by ELISA, and antibody responses were determined by Probit as a percent of the standard (the response following systemic infection), while mean values ± Standard errors of the mean (SEM) were determined from groups of 6 to 10 mice. Mucosal washings were pooled for each group of 6 to 10 mice, and endpoint titers were determined by regression analysis. Mean values ± SEM are given where the dose was repeated in three or more similar experiments.
Comparison of the nature of the immune response to HSV-1 using Ctx-CtxB and rEtxB as adjuvants.
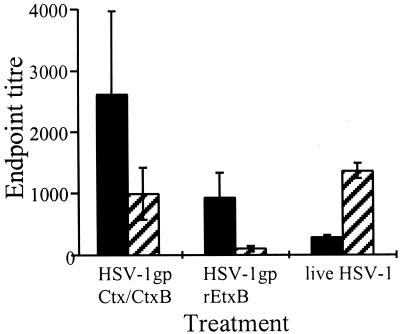
The nature of the anti-HSV-1 immune response evoked using rEtxB and Ctx-CtxB as adjuvants was determined by analysis of the IgG subclass distribution of the serum antibody response in comparison with that in HSV-1-infected mice (Fig. 3). Live-virus infection triggered an anti-HSV-1 response dominated by the presence of the Th1-associated antibody subclass, IgG2a, and low levels of IgG1. The virus-specific IgG1/IgG2a ratio in sera from infected mice was 0.2. In contrast, the use of either rEtxB or Ctx-CtxB as an adjuvant given intranasally together with HSV-1 glycoproteins induced IgG1-dominated anti-HSV-1 antibodies, which may reflect a Th2-like response (Fig. 3). Both adjuvants also gave rise to some IgG2a; however, in the case of EtxB, the levels of IgG2a were extremely low. This was reflected in an increased IgG1/IgG2a ratio with rEtxB as opposed to Ctx-CtxB (9.0 and 2.6, respectively). Analysis of the mucosal washings of immunized mice indicated the presence of HSV-1-specific antibodies, mainly of the IgA isotype, with endpoint titers of approximately 1:20 in EW and greater than 1:200 in VW. No IgG was detected in EW, and only low levels (endpoint titer, 1:35 [data not shown]) in VW.
FIG. 3.
Comparison of ELISA endpoint titers showing HSV-1-specific IgG1 (■) and IgG2a ( ) subclasses in sera of mice following infection with live virus or intranasal immunization with glycoproteins in the presence of either Ctx-CtxB or rEtxB as an adjuvant. Mean values ± standard errors of the mean were determined from groups of six mice and are representative of five similar experiments.
) subclasses in sera of mice following infection with live virus or intranasal immunization with glycoproteins in the presence of either Ctx-CtxB or rEtxB as an adjuvant. Mean values ± standard errors of the mean were determined from groups of six mice and are representative of five similar experiments.
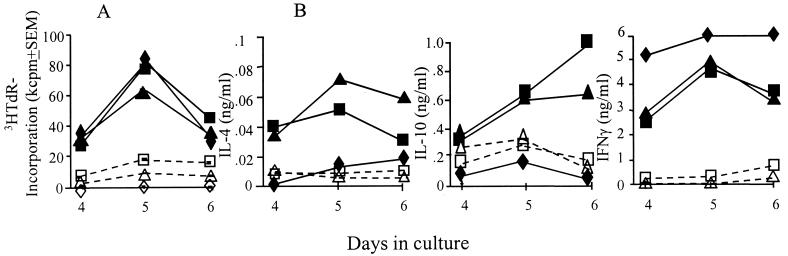
Lymph node cells from mice immunized in the presence of either Ctx-CtxB or rEtxB or infected by ocular scarification with 104 PFU of live HSV-1 all showed similar virus-specific proliferative responses when cultured in vitro in the presence of inactivated HSV-1 as an antigen (Fig. 4A). Peak proliferation occurred on day 5 for all groups, declining to background levels by day 7. The specificity of the proliferative responses to viral antigens was evidenced by the much lower [3H]thymidine incorporation observed in cultures of lymph node cells with a mock virus preparation. Analysis of the cytokines secreted in lymph node cell cultures showed that following immunization, in the presence of either adjuvant, both Th1 (IFN-γ)- and Th2 (IL-4 and IL-10)-type cytokines were produced (Fig. 4B). The kinetics were similar for both adjuvants used, with both IL-4 and IFN-γ peaking on day 5 and concentrations of IL-10 increasing gradually up to day 6. In contrast, following infection, such cultures only produced the Th1-associated cytokine, IFN-γ. The levels of this cytokine were high and were maintained throughout the culture period. IL-4 and IL-10 secretion in cultures from infected mice were comparable to those seen in mock-antigen control cultures.
FIG. 4.
Lymph node cells from mice infected with HSV-1 (◊) or immunized intranasally with HSV-1 glycoproteins in the presence of either Ctx-CtxB (□) or rEtxB (▵) as an adjuvant and cultured in vitro with HSV-1 (solid symbols) or mock antigen (open symbols) were analyzed for proliferative response (A) by [3H]thymidine (3HTdR) incorporation (mean values ± standard errors of the mean [SEM] of triplicate cultures) and cytokine secretion (B) (concentrations in duplicate cultures determined from a standard curve). The data are representative of three similar experiments.
Ocular challenge with live HSV-1 of immunized and mock-immunized mice using rEtxB as adjuvant.
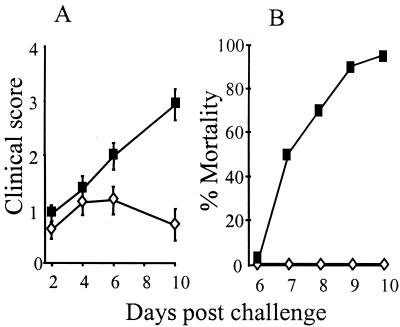
Mice immunized intranasally with either 10 μg of HSV-1 or mock glycoprotein preparations in the presence of 20 μg of rEtxB were challenged by ocular scarification for analysis of the level of disease protection. Eye disease was observed on days 2, 4, 6, and 10 following infection with 103 PFU of HSV-1 SC16. Although mice immunized with HSV-1 glycoproteins or the mock preparation showed signs of epithelial disease by day 2 after infection, the incidence of more severe clinical symptoms was significantly reduced in HSV-1-immunized mice, with only 35% developing uveitis and 10% developing stromal disease compared with 85 and 65%, respectively, for mock-immunized animals. Additionally, lid disease occurred in only 35% of immunized mice compared with 85% in mock-immunized mice, and zosteriform spread occurred in 25 and 80%, respectively (Table 1). Thus, the reduction in the incidence of clinical disease in immunized mice compared with mock-immunized mice was highly significant (P < 0.001). The severity of ocular disease in mock-immunized mice increased to give a mean score of approximately 3 by day 10, whereas the severity of disease in immunized mice peaked at 1 on days 4 and 6, declining to less than 1 by day 10 (Fig. 5A). Complete protection against the onset of encephalitis and death was observed in immunized mice compared with 25% mortality in mock-immunized mice (Table 1). Similar levels of protection were also observed when immunized mice were challenged with doses of HSV-1 as high as 104 PFU; this dose gave 95% mortality in mock-immunized animals (Fig. 5B).
TABLE 1.
Clinical disease following primary infection with 103 PFU of HSV-1 SC16 in mice previously Immunized with HSV-1 or mock glycoproteins in the presence of 20 μg of rEtxB as adjuvant
| Immunization | Clinical diseasea
|
Latencya
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Epithelial disease | Uveitis | Stromal disease | Lid disease | Zosteriform lesions | Encephalitis | TGI | TGIIb | TGIIIb | |
| HSV-1 | 17/20 (85) | 7/20 (35) | 2/20 (10) | 7/20 (35) | 5/20 (25) | 0/20 (0) | 14/19 (74) | 3/19 (16) | 1/19 (5) |
| Mock | 20/20 (100) | 20/20 (85) | 13/20 (65) | 17/20 (85) | 16/20 (80) | 5/20 (25) | 13/15 (87) | 7/15 (47) | 5/15 (33) |
Number positive/total (percent).
χ2 value, P < 0.001 between HSV-1 and mock-immunized groups of mice.
FIG. 5.
Analysis of the level of protection in mice immunized with 10 μg of HSV-1 (◊) or mock (■) glycoproteins mixed with 20 μg of rEtxB against eye disease (A) following ocular infection with 103 PFU of HSV-1 SC16 (score: 0, no disease; 1, epithelial disease; 2, mild opacity; 3, moderate opacity; 4, severe stromal keratitis) and mortality in mice given 104 PFU of HSV-1 SC16 (B). Each group included 20 mice; mean clinical scores ± standard errors of the mean were calculated.
Latent virus could be detected in the ophthalmic section of the TG (TGI) in 74% of immunized mice compared with 87% of mock-immunized mice (P = 0.03). The spread of latent virus to the maxillary (TGII) and mandibular (TGIII) sections of the TG was only detected in 16 and 5% of immunized mice compared with 47 and 33%, respectively, of mock-immunized mice (P < 0.002) (Table 1).
DISCUSSION
The precise mechanism by which Ctx and Etx act as adjuvants to coadministered antigens, including the role of the GM1-binding property of the B subunit and the necessity for ADP-ribosylating activity, is still poorly understood. In this study, we have shown conclusively that when administered intranasally, rEtxB mixed with HSV-1 glycoproteins results in an antigen-specific immune response to HSV-1. While a twofold-higher dose of rEtxB was required to stimulate a serum antibody response equivalent to that triggered by Ctx-CtxB, the results highlight the fact that the presence of the A subunit is not an absolute requirement for nasal adjuvanticity. The failure of rCtxB to act as an adjuvant points toward a marked difference in the immunological properties of these two very closely related proteins. The ability of rEtxB to stimulate protective immunity to HSV-1 points to its use as a completely nontoxic mucosal adjuvant for the prevention of ocular HSV-1 infection.
The addition of rEtxB to HSV-1 glycoproteins potentiated a strong antiviral serum antibody response. The level of this response was comparable to that triggered by a live-virus infection, provided that 20 μg or more of rEtxB was used, but with a stronger bias toward the IgG1 subclass of antibodies. EtxB was effective at triggering mucosal as well as serum antibody production, with high titers of anti-HSV-1 IgA antibodies detected in the eye secretions as well as in the reproductive tract. As viral glycoproteins were not themselves immunogenic in this system, these findings indicate that the B subunit of Etx can act as an adjuvant for HSV antigens without a requirement for the ADP ribosylation activity of the A subunit. This finding is consistent with other recent studies, where EtxB has been shown to act as an intranasal adjuvant for influenza virus hemagglutinin and ovalbumin (8, 36). Interestingly, rCtxB was not capable of acting as an adjuvant for the HSV-1 glycoproteins. Doses as high as 100 μg of rCtxB failed to trigger significant anti-HSV-1 antibody responses above those present in negative controls. This observation reveals a very marked difference in the immunological properties of EtxB and CtxB. The different abilities of CtxB and EtxB to act as adjuvants likely results from differences in either receptor specificity or stability (24). EtxB is known to bind to a wider range of receptors than CtxB. CtxB interacts with cells through binding to the surface gangliosides GM1 and GD1b (19). While these molecules are also the principal receptors for EtxB, additional galactose-containing molecules, including asialo-GM1, lactosylceramide, and certain galactoproteins, can bind EtxB (11, 15). The role of these alternative receptors for the B subunits in mediating their effects on the immune system has yet to be explored. EtxB is also much more stable than CtxB at low pH. The pentameric structure of CtxB disassociates when the pH is taken below 3.9, whereas EtxB remains stable down to pH 2.0 (28). While the importance of this difference following oral delivery is clear, since resistance to stomach acid would be severely compromised for CtxB, its role after intranasal administration is less obvious. Nevertheless, the greater stability of EtxB at low pH may reflect a greater capacity to maintain receptor binding activity following trafficking across the nasal epithelium, a property which is considered crucial to modulating the immune response at that site (37).
Despite the clear capacity of EtxB to act as an adjuvant, the apparently higher potency of Ctx-CtxB, the combined adjuvant, in triggering an anti-HSV response at low doses, together with the failure of CtxB alone to act as an adjuvant, suggests that activities of the A subunit can augment immune modulation. Thus, the A and B subunits appear to have separate properties which contribute to adjuvant activity. The role of the A subunit may be mediated through its capacity to trigger ADP ribosylation. However, given the effectiveness of at least some mutants lacking this activity to act as adjuvants, other properties are likely to be involved. The ability of the A subunit to stabilize the conformation of the B subunit and modulate vesicular trafficking through binding to K(R)DEL receptors may also contribute to its adjuvanticity (24). Stabilization of the B-subunit pentamer by the A subunit may be more critical for CtxB, since it is itself less stable than EtxB. In addition, intracellular targeting to relevant vesicular compartments may simply be more efficient in the presence of the A subunit and hence would not be an absolute requirement where other factors are optimal (37).
Characterization of the immune response evoked using rEtxB as an adjuvant was carried out to determine the ability of the B subunit alone to modulate the immune response to HSV-1 antigens toward a Th2-dominated response rather than the Th1-dominated response associated with the immunopathology of ocular disease (33). As with Ctx-CtxB, the serum antibody response was dominated by the presence of IgG1, with relatively low levels of IgG2a. This apparent bias toward a Th2 response was in contrast to the strong Th1 response observed following virus infection, where the majority of the antibodies were of the IgG2a isotype. Confirmation of the different nature of the responses to infection and intranasal immunization was provided by analysis of cytokine release in cultures of lymph node cells and IgG subclasses in serum. Lymph node cells from infected mice produced high levels of IFN-γ in response to virus in vitro but failed to secrete any detectable IL-4 or IL-10 above background, indicating a strong Th1-type response. In contrast, lymph node cells from mice immunized with either rEtxB or Ctx-CtxB produced IL-4 and IL-10 in addition to IFN-γ. As has been previously reported (27), the presence of detectable Th2 cytokines correlates with a Th2-dominated antibody response despite the fact that absolute quantities of IFN-γ were higher than those of IL-4 or IL-10 even in immunized animals. Analysis of the IgG1/IgG2a ratios in immunized mice suggested that the bias toward Th2 responsiveness was stronger when rEtxB, rather than Ctx-CtxB, was used as an adjuvant. The extremely high IgG1/IgG2a ratio elicited with rEtxB was consistent and has been observed using other model antigens, such as ovalbumin (N. A. Williams, unpublished data). This observation is interesting, since data using the whole toxins have indicated that whereas Ctx stimulates predominantly Th2 responses (20), Etx gives rise to a more balanced Th1 and Th2 response (32). Work carried out using toxic mutants of Etx with reduced (LTR72) or no (LTK63) enzyme activity also indicated higher levels of IgG1 with decreased toxicity (12). Although not directly tested here, this implies that the presence of the A subunit of Etx can affect the nature of the immune response stimulated as well as its magnitude. It is noteworthy that despite the dominance of Th2-associated IgG antibodies to HSV following the use of EtxB as an adjuvant, no virus-specific IgE was observed (data not shown). This apparent separation between IgG1 and IgE probably results from the use of a mucosal route of administration, which would predispose to IgA rather than IgE.
Mice immunized using EtxB as an adjuvant were found to be protected against severe ocular disease as well as having reduced spread of virus to the eyelid and face, so-called zosteriform spread. This reduction in zosteriform disease is indicative of a reduced spread of virus in the nervous system. In this respect also, it is noteworthy that mice were completely protected against encephalitis, even at the higher challenge dose of virus, which resulted in 95% of mock-immunized mice developing such disease. Despite this clear protection from HSV infection in the central nervous system, the establishment of latency within the ophthalmic division of the TG was not significantly reduced, although a significant reduction in the spread of the virus within the TG was observed. This is in contrast to the very high degree of protection from latency in mice immunized with Ctx-CtxB as an adjuvant (27). The ability of virus to establish latency in mice immunized in the presence of rEtxB may be the result of the Th2 dominance of the immune response and the likely low level of cytotoxic T lymphocytes induced. It is noteworthy that cytotoxic T cells are known to play an important role in clearing virus from the nervous system (23, 31). However, the involvement of Th1 immune responses in potentiating damage to the eye through the induction of immunopathological processes may be one reason for the high degree of effectiveness of EtxB-mediated immunization in preventing disease in the eye itself. Thus, while infections by virus, including HSV-1, tend to trigger predominantly Th1-associated immunity, a strong protective response following vaccination may be best achieved by inducing Th2 responsiveness. In particular, the induction of a strong mucosal antibody response, as reported here, is likely to play a critical role in reducing initial access of the virus to the tissues, and the relative lack of Th1 activity resulting from prior Th2-dominated immunity may prevent damaging immune pathology. In addition to the relative lack of immunopathology arising from the activities of cytoxic T lymphocytes, the presence of IL-10 may contribute to remission of the lesions. In this regard, it has been reported that administration of DNA encoding IL-10 suppresses ocular inflammatory disease following HSV-1 infection (6).
These studies have highlighted the potential of the B subunit of Etx as a safe and effective adjuvant capable of triggering mucosal and systemic immunity. The findings should allow the development of mucosal vaccination strategies in the absence of residual toxicity associated with the use of holotoxins or their mutants. However, the finding that the immune response triggered by EtxB is Th2 dominated indicates that careful consideration is necessary in determining whether it is suitable for use in vaccines against individual infectious agents.
ACKNOWLEDGMENTS
We thank The Wellcome Trust for providing the financial support for this work and the Department of International Development (UK-Indonesia Biodiversity for Biotechnology Project) for funding a scholarship to support the work of A. T. Aman.
We also thank Martin Kenny for the production of the rEtxB used in this study.
REFERENCES
- 1.Aman A T. Mutagenesis of a conserved loop in the B-subunit of cholera toxin: identification of residues essential for toxicity and immunomodulation. Ph.D. thesis. Bristol, United Kingdom: University of Bristol; 2000. [Google Scholar]
- 2.Amin T, Hirst T R. Purification of the B-subunit oligomer of Escherichia coli heat-labile enterotoxin by heterologous expression and secretion in a marine Vibrio. Protein Expr. Purif. . 1998;5:198–204. doi: 10.1006/prep.1994.1031. [DOI] [PubMed] [Google Scholar]
- 3.Bailey M, Williams N A, Wilson A D, Stokes C R. Probit: weighted probit regression analysis. J Immunol Methods. 1992;153:261–262. doi: 10.1016/0022-1759(92)90329-r. [DOI] [PubMed] [Google Scholar]
- 4.Beech J T, Bainbridge T, Thompson S J. Incorporation of cells into an ELISA system enhances antigen-driven lymphokine detection. J Immunol Methods. 1997;205:163–168. doi: 10.1016/s0022-1759(97)00072-0. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein D I, Stanberry L R. Herpes simplex virus vaccines. Vaccine. 1999;17:1681–1689. doi: 10.1016/s0264-410x(98)00434-4. [DOI] [PubMed] [Google Scholar]
- 6.Daheshia M, Kuklin N, Kanangat S, Manickan E, Rouse B T. Suppression of on-going inflammatory disease by topical administration of plasmid DNA encoding IL-10. J Immunol. 1997;159:1945–1952. [PubMed] [Google Scholar]
- 7.de Haan L, Feil I K, Verweij W R, Holtrop M, Hol W J, Agsteribbe E, Wischut J. Mutational analysis of the role of ADP-ribosylation activity and G(M1)-binding activity in the adjuvant properties of the Escherichia coli heat-labile enterotoxin towards intranasally administered keyhole limpet hemocyanin. Eur J Immunol. 1998;28:1243–1250. doi: 10.1002/(SICI)1521-4141(199804)28:04<1243::AID-IMMU1243>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 8.Douce G, Fontana M, Pizza M, Rappuoli R, Dougan G. Intranasal immunogenicity and adjuvanticity of site-directed mutant derivatives of cholera toxin. Infect Immun. 1997;65:2821–2828. doi: 10.1128/iai.65.7.2821-2828.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douce G, Turcotte C, Cropley I, Roberts M, Pizza M, Domenghini M, Rappuoli R, Dougan G. Mutants of Escherichia coli heat-labile toxin lacking ADP-ribosyltransferase activity act as nontoxic, mucosal adjuvants. Proc Natl Acad Sci USA. 1995;92:1644–1648. doi: 10.1073/pnas.92.5.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erturk M, Hill T J, Shimeld C, Jennings R. Acute and latent infection of mice immunised with HSV-1 ISCOM vaccine. Arch Virol. 1992;125:87–101. doi: 10.1007/BF01309630. [DOI] [PubMed] [Google Scholar]
- 11.Fukata S, Magnani J L, Twiddy E M, Holmes R K, Ginsburg V. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LTh-I, LT-IIa and LT-IIb. Infect Immun. 1988;56:1748–1753. doi: 10.1128/iai.56.7.1748-1753.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giuliani M M, Del Giudice G, Giannelli V, Dougan G, Douce G, Rappuoli R, Pizza M. Mucosal adjuvanticity and immunogenicity of LTR72, a novel mutant of Escherichia coli heat-labile enterotoxin with partial knockout of ADP-ribosyltransferase activity. J Exp Med. 1998;187:1123–1132. doi: 10.1084/jem.187.7.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harper H M, Cochrane L, Williams N A. The role of small intestinal antigen presenting cells in the induction of T-cell reactivity to soluble protein antigens: association between aberrant presentation in the lamina propria and oral tolerance. Immunology. 1996;89:449–456. doi: 10.1046/j.1365-2567.1996.d01-760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill T J, Field H J, Blyth W A. Acute and recurrent infection with herpes simplex virus in the mouse: a model for studying latency and recurrent disease. J Gen Virol. 1975;28:341–353. doi: 10.1099/0022-1317-28-3-341. [DOI] [PubMed] [Google Scholar]
- 15.Holmgren J, Freedman P, Lindblad M, Svennerholm A M, Svennerholm L. Rabbit intestinal glycoprotein receptor for Escherichia coli heat-labile enterotoxin lacking affinity for cholera toxin. Infect Immun. 1982;38:424–433. doi: 10.1128/iai.38.2.424-433.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jennings R, Quasim T, Sharrard R M, Hockley D, Potter C W. Zwitterionic detergent solubilisation of HSV-1 surface antigens. Arch Virol. 1988;98:137–153. doi: 10.1007/BF01322164. [DOI] [PubMed] [Google Scholar]
- 17.Jennings R, Erturk M. Comparative studies of HSV-1 antigens solubilised from infected cells by using non-ionic or zwitterionic detergents. J Med Virol. 1990;31:98–108. doi: 10.1002/jmv.1890310206. [DOI] [PubMed] [Google Scholar]
- 18.Lycke N, Tsuji Y, Holmgren J. The adjuvant effect of Vibrio cholerae and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosylation activity. Eur J Immunol. 1992;22:2277–2281. doi: 10.1002/eji.1830220915. [DOI] [PubMed] [Google Scholar]
- 19.MacKenzie C R, Hirama T, Lee K K, Altman E, Young N M. Quantitative analysis of bacterial toxin affinity and specificity for glycolipid receptors by surface plasmon resonance. J Biol Chem. 1997;272:5333–5338. doi: 10.1074/jbc.272.9.5533. [DOI] [PubMed] [Google Scholar]
- 20.Marinaro M, Staats H F, Hiroi T. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J Immunol. 1995;155:4621–4629. [PubMed] [Google Scholar]
- 21.Morrison L A, Knipe D M. Contributions of antibody and T cell subsets to protection elicited by immunisation with a replication-defective mutant of herpes simplex virus type 1. Virology. 1997;239:315–326. doi: 10.1006/viro.1997.8884. [DOI] [PubMed] [Google Scholar]
- 22.Nash A A, Cambouropoulos P. The immune response to herpes simplex virus. Semin Virol. 1993;4:181–186. [Google Scholar]
- 23.Nash A A, Jayasuriya A, Phelan J, Cobbold S P, Waldmann H, Prospero T. Different roles for L3T4+ and Lyt2+ T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J Gen Virol. 1987;68:825–833. doi: 10.1099/0022-1317-68-3-825. [DOI] [PubMed] [Google Scholar]
- 24.Nashar T O, Williams N A, Hirst T R. Importance of receptor binding in the immunogenicity, adjuvanticity and therapeutic properties of cholera toxin and Escherichia coli heat-labile enterotoxin. Med Microbiol Immunol. 1998;187:3–10. doi: 10.1007/s004300050068. [DOI] [PubMed] [Google Scholar]
- 25.Nesburn A B, Burke R L, Ghiasi H, Slanina S M, Wechsler S L. Therapeutic periocular vaccination with a subunit vaccine induces higher levels of herpes simplex virus-specific tear secretory immunoglobulin A than systemic vaccination and provides protection against recurrent spontaneous ocular shedding of virus in latently infected rabbits. Virology. 1998;252:200–209. doi: 10.1006/viro.1998.9454. [DOI] [PubMed] [Google Scholar]
- 26.Pizza M, Domenghini M, Hol W, Gianelli V, Fontana M R, Giuliani M M, Magagnoli C, Peppoloni S, Manetti R, Rappuoli R. Probing the structure-activity relationship of Escherichia coli LT-A by site-directed mutagenesis. Mol Microbiol. 1994;14:51–60. doi: 10.1111/j.1365-2958.1994.tb01266.x. [DOI] [PubMed] [Google Scholar]
- 27.Richards C M, Shimeld C, Williams N A, Hill T J. Induction of mucosal immunity against herpes simplex virus type 1 in the mouse protects against ocular infection and establishment of latency. J Infect Dis. 1998;177:1451–1457. doi: 10.1086/515302. [DOI] [PubMed] [Google Scholar]
- 28.Ruddock L W, Ruston S P, Kelly S M, Price N C, Freedman R B, Hirst T R. Kinetics of acid-mediated disassembly of the B subunit pentamer of Escherichia coli heat-labile enterotoxin. J Biol Chem. 1995;270:29953–29958. doi: 10.1074/jbc.270.50.29953. [DOI] [PubMed] [Google Scholar]
- 29.Shimeld C, Whiteland J L, Williams N A, Easty D L, Hill T J. Reactivation of herpes simplex virus type 1 in the mouse trigeminal ganglion: an in vivo study of virus antigen and immune cell infiltration. J Gen Virol. 1996;77:2583–2590. doi: 10.1099/0022-1317-77-10-2583. [DOI] [PubMed] [Google Scholar]
- 30.Shimeld C, Whiteland J L, Williams N A, Easty D L, Hill T J. Cytokine production in the nervous system of mice during acute and latent infection with herpes simplex virus type 1. J Gen Virol. 1997;78:3317–3325. doi: 10.1099/0022-1317-78-12-3317. [DOI] [PubMed] [Google Scholar]
- 31.Simmons A, Tscharke D C. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J Exp Med. 1992;175:1337–1344. doi: 10.1084/jem.175.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi I, Marinaro M, Kiyono H, Jackson R J, Nakagawa I, Fujihashi K, Hamada S, Clements J D, Bost K L, McGhee J R. Mechanisms for mucosal immunogenicity and adjuvanticity of Escherichia coli heat labile enterotoxin. J Infect Dis. 1996;173:627–635. doi: 10.1093/infdis/173.3.627. [DOI] [PubMed] [Google Scholar]
- 33.Thomas J, Rouse B T. Immunopathogenesis of herpetic ocular disease. Immunol Res. 1997;16:375–386. doi: 10.1007/BF02786400. [DOI] [PubMed] [Google Scholar]
- 34.Tullo A B, Shimeld C, Blyth W A, Hill T J, Easty D L. Spread of virus and distribution of latent infection following ocular herpes simplex in the non-immune and immune mouse. J Gen Virol. 1982;63:95–101. doi: 10.1099/0022-1317-63-1-95. [DOI] [PubMed] [Google Scholar]
- 35.Tullo A B, Shimeld C, Blyth W A, Hill T J, Easty D L. Ocular infection with herpes simplex virus in nonimmune and immune mice. Arch Ophthalmol. 1983;101:961–964. doi: 10.1001/archopht.1983.01040010961023. [DOI] [PubMed] [Google Scholar]
- 36.Verweij W R, de Haan L, Holtrop M, Agsteribbe E, Brands R, van Scharrenburg G J, Wilschut J. Mucosal immunoadjuvant activity of recombinant Escherichia coli heat-labile enterotoxin and its B subunit: induction of systemic IgG and secretory IgA responses in mice by intranasal immunisation with influenza virus surface antigen. Vaccine. 1998;16:2069–2076. doi: 10.1016/s0264-410x(98)00076-0. [DOI] [PubMed] [Google Scholar]
- 37.Williams N A, Hirst T R, Nashar T O. Immune modulation by the cholera-like enterotoxins: from adjuvant to therapeutic. Immunol Today. 1999;20:95–101. doi: 10.1016/s0167-5699(98)01397-8. [DOI] [PubMed] [Google Scholar]
- 38.Wilson A D, Bailey M, Williams N A, Stokes C R. The in vitro production of cytokines by mucosal lymphocytes immunised by oral administration of keyhole limpet haemocyanin using cholera toxin as an adjuvant. Eur J Immunol. 1991;21:2333–2339. doi: 10.1002/eji.1830211007. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto S, Kiyono H, Yamamoto M, Imaoka K, Fujihashi K, VanGinkel F W, Noda M, Takeda Y, McGhee J R. A nontoxic mutant of cholera toxin elicits Th2-type responses for enhanced mucosal immunity. Proc Natl Acad Sci USA. 1997;94:5267–5272. doi: 10.1073/pnas.94.10.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto S, Takeda Y, Yamamoto M, Kurazono H, Imaoka K, Fujihashi K, Noda M, Kiyono H, McGhee J R. Mutants in the ADP-ribosyltransferase cleft of cholera toxin lack diarrheagenicity but retain adjuvanticity. J Exp Med. 1997;185:1203–1210. doi: 10.1084/jem.185.7.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]