Abstract
Background:
Dermatophytosis is a fungal infection that targets the skin and its appendages, such as the nails and hair. It affects all age groups and is estimated to affect approximately 20–25% of the population across the world. There are insufficient data on the clinic-mycological pattern of dermatophytosis in Odisha, a coastal state in eastern India. The study aims to explore the clinico-mycological pattern of prevailing superficial cutaneous fungal infections and to identify the specific species as per the site of skin involvement.
Methods:
This is a cross-sectional study conducted in the Department of Dermatology in collaboration with the Department of Microbiology at a tertiary health care centre, Odisha, for a period of 2 years, from October 2020 to September 2022. Participants aged 18–65 years with active dermatophyte infections of the skin were included in the study. Clinical examination and mycological workup were performed, and the collected samples were divided into two parts, one for direct microscopy and the other for fungal culture.
Results:
According to our study, the most common isolate was Trichophyton mentagrophytes (21.7%), followed by T. rubrum (11.7%), and 5.3% of cultures showed T. schoenleinii isolates, whereas Microsporum canis and Microsporum gypseum constituted 2.7% and 0.7%, respectively.
Conclusion:
The present study focuses on the prevalence and clinical trends of different dermatophyte species associated with dermatophytosis in eastern India. Due to the favourable climate of Odisha, superficial mycoses are prevalent here, and according to our study, Trichophyton mentagrophytes is the predominate isolate in this region.
KEY WORDS: Dermatophytosis, direct microscopy, Epidermophyton floccosum, fungal culture, tinea corporis, tinea cruris, Trichophyton mentagrophytes, Trichophyton rubrum
Introduction
The term “dermatophytosis” is used to describe a cluster of fungal infections that primarily target the horny layer of the skin and its associated appendages, such as the nails and hair.[1]
Dermatophytes have been traditionally classified into three broad genera, that is, Trichophyton, Microsporum, and Epidermophyton. They can readily infect any site on the body and have been named accordingly. A few of the manifestations include tinea corporis, tinea cruris, tinea pedis, tinea manuum, tinea unguium, and tinea capitis.[2]
Dermatophytosis has become a major public health concern that affects all age groups, including children, adolescents, adults, and the elderly population. It is estimated that approximately 20–25% of the population across the world suffers from superficial fungal infections.[3] Fungal infections are mostly prevalent in hot and humid countries like India.[4]
Numerous studies have been carried out on the clinical and species patterns of dermatophytosis across the nation, but there are insufficient data in Odisha, a coastal state in eastern India. This prompted us to conduct this cross-sectional study to explore the clinico-mycological pattern of prevailing superficial cutaneous fungal infections along with the identification of specific species as per the site of skin involvement. Moreover, Trichophyton mentagrophytes was discovered to be the most prevalent isolate in the eastern section of the country during the pre-COVID era.[2] We also hope to see if the pattern has changed since the outbreak through this investigation.
Materials and Methods
This study was conducted in the Department of Dermatology in collaboration with the Department of Microbiology for a period of 2 years, from October 2020 to September 2022, at a tertiary health care centre, Odisha. This study included participants aged 18–65 years and of both sexes with active dermatophyte infections of the skin with a clinically characteristic skin lesion with a sharply marginated red, scaly, elevated border studded with vesicles and an area of central clearing.
Patients with contact dermatitis, atopic dermatitis, psoriasis, or any other disease and those who were using antifungal agents or topical steroid agents for the disease in the past month were excluded from the study.
Informed consent was obtained from all the patients who satisfied the inclusion criteria and became a part of our study. All included participants were subjected to a clinical examination and mycological workup as per the standard protocol of the study.
Clinical examination
It included clinical assessment as per the site of involvement for classification, morphological pattern, and finally the activity of the skin lesions of dermatophytosis based on three main parameters, including erythema, scaling, and itching.
Sample collection
We selected an appropriate site for the collection of the sample, which was first cleaned with 70% ethanol and left for a few minutes so that it completely dries up. After this, an active edge of the lesion was chosen, from which skin scrapings were obtained with the help of a sterile surgical blade. We divided the collected sample into two parts, one of which was meant for direct microscopy, and the other half was further processed for fungal culture.
Direct microscopy
Skin scrapings were collected in sterile containers and sent to laboratories, where they were then treated with 10% KOH. For easy identification of fungal elements, keratin from the sample should be digested, and for that purpose, 10% KOH was added. It was then examined under a microscope with low magnification (10x) and high magnification (40x) for the determination of fungal elements. Positive scrapings were indicated by the presence of smooth, branching, long, and refractile hyphae with or without arthroconidiospores.
Fungal culture
Fungal culture was performed on Sabouraud Dextrose Agar (SDA) as well as Dermatophytes Test Medium (DTM) to see fungal growth and identify the species. We incubated the fungal culture at 280 Celsius for 3–4 weeks, and fungal growth was checked at every 3–4-day interval.
Identification of fungus
The characteristics of the colony, the microscopic morphology, and the form of the macroconidia were used to differentiate between different types of dermatophytes. The cultural features, texture, rate of growth, size of colony, and colour produced on both the reverse and obverse sides of slants were used in the identification of the dermatophyte species.
The characteristics of microconidia and macroconidia on the LPCB mount were studied to identify different genera and species.
The ethical committee approval has been obtained on 3 November 2020.
Observations and Results
A total of 300 cases of dermatophytosis were included in this study. Out of 300 cases, 58.3% were males and 41.7% were females. The predominant age group was between 31 and 40 years old (41%).
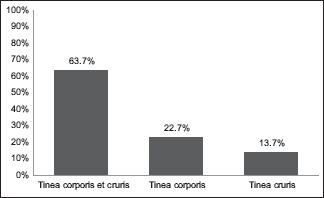
Among the different types of clinical presentations, tinea corporis et cruris (TCC) was the most prevalent (63.7%), followed by only tinea corporis (22.7%) and only tinea cruris (13.7%) [Graph 1]. The majority of the infected people were field workers (31%), followed by students (28.3%) and housewives (26.7%) [Table 1].
Graph 1.
Bar graph depicting clinical presentation wise distribution
Table 1.
Baseline characteristics of the study
| Number | Percentage | |
|---|---|---|
| Gender | ||
| Male | 175 | 58.3% |
| Female | 125 | 41.7% |
| Age groups (in years) | ||
| 18-30 | 87 | 29% |
| 31-40 | 123 | 41% |
| 41-50 | 46 | 15.3% |
| >50 | 44 | 14.7% |
| Occupation | ||
| Students | 85 | 28.3% |
| Housewives | 80 | 26.7% |
| Field workers | 93 | 31% |
| Businessmen | 9 | 3% |
| Others | 33 | 11% |
| Clinical presentation | ||
| Tinea corporis et cruris (TCC) | 191 | 63.7% |
| Tinea corporis | 68 | 22.7% |
| Tinea cruris | 41 | 13.7% |
Of 300 clinically proven cases, 288 (96%) showed fungal filaments by direct microscopy, and 162 (54%) showed fungal culture positivity. 127 (42.3%) were culture-positive for dermatophytes, and 35 (11.7%) were culture-positive for nondermatophytes [Table 2]. Out of 127 dermatophytes isolated, Trichophyton species were implicated in 116 (91.2%) cases, while Microsporum and Epidermophyton species were isolated in 10 (7.87%) and 1 (0.78%) cases, respectively.
Table 2.
KOH and fungal culture status of 300 patients
| Number | Percentage | |
|---|---|---|
| KOH wet mount | ||
| Positive | 288 | 96% |
| Negative | 12 | 4% |
| Fungal culture | ||
| Positive | 162 | 54% |
| Negative | 138 | 46% |
Trichophyton mentagrophytes was the predominant dermatophyte isolated in 65 out of 127 dermatophytes (51.1%), followed by Trichophyton rubrum, which was isolated in 35 (27.5%) cases. Among the 35 nondermatophyte isolates, Candida albicans was the most common nondermatophyte isolated in 21 cultures (60%), followed by Aspergillus in 9 isolates (25.7%) followed by a few isolates of Fusarium and a single isolate of Penicillium [Table 3].
Table 3.
Species-wise distribution
| Name of species | Total dermatophyte=127 | Name of species | Total nondermatophyte=35 | ||
|---|---|---|---|---|---|
|
|
|
||||
| Number | Percentage | Number | Percentage | ||
| T.mentagrophytes | 65 | 51.18% | C.albicans | 21 | 60% |
| T.rubrum | 35 | 27.55% | Aspergillus | 9 | 25.71% |
| T.schoenleinii | 16 | 12.59% | Fusarium | 4 | 11.42% |
| M.canis | 8 | 6.29% | Penicillium | 1 | 2.85% |
| M.gypseum | 2 | 1.57% | |||
| E.floccosum | 1 | 0.78% | |||
| Total | 127 | 100% | Total | 35 | 100% |
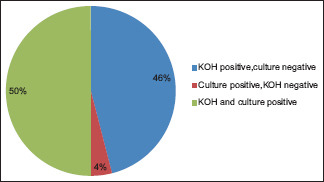
The correlation of KOH as well as culture positivity suggests that out of 300 patients, 150 patients (50%) were positive for both KOH and fungal culture, whereas 138 patients (46%) were KOH-positive but culture-negative and 12 patients (4%) were KOH-negative but found out to be culture-positive [Table 4, Graph 2].
Table 4.
Correlation between KOH and fungal culture
| Correlation | KOH and culture positive | KOH positive, culture negative | KOH negative, culture positive | Total | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| (n) | % | (n) | % | (n) | % | ||
| Total | 150 | 50% | 138 | 46% | 12 | 4% | 300 |
Graph 2.
Pie chart depicting correlation between KOH and fungal culture status
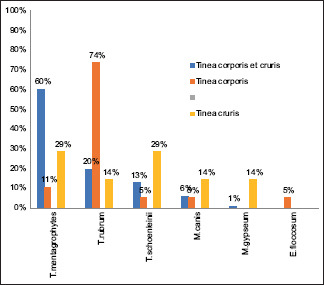
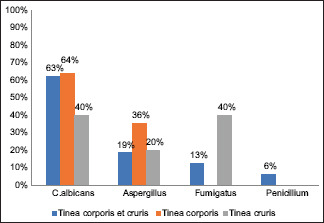
The correlation of species with clinical presentation revealed that T. mentagrophytes was the commonest species in tinea corporis et cruris presentations, whereas T. rubrum was the commonest causative organism in tinea corporis patients [Table 5, Graph 3]. Among the nondermatophytes, C. albicans remains the most prevalent isolate in all types of clinical presentations [Table 6, Graph 4].
Table 5.
Clinical presentation-wise dermatophyte species distribution
| Tinea corporis et cruris (TCC) | Tinea corporis | Tinea cruris | Total | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| n | % | n | % | n | % | ||
| T.mentagrophytes | 61 | 60.39% | 2 | 10.52% | 2 | 28.57% | 65 |
| T.rubrum | 20 | 19.80% | 14 | 73.68% | 1 | 14.28% | 35 |
| T.schoenleinii | 13 | 12.87% | 1 | 5.26% | 2 | 28.57% | 16 |
| M.canis | 6 | 5.94% | 1 | 5.26% | 1 | 14.28% | 8 |
| M.gypseum | 1 | 0.99% | 0 | 0% | 1 | 14.28% | 2 |
| E.floccosum | 0 | 0% | 1 | 5.26% | 0 | 0% | 1 |
| Total | 101 | 100% | 19 | 100% | 7 | 100% | 127 |
Graph 3.
Bar graph depicting clinical presentation wise dermatophyte species distribution
Table 6.
Clinical presentation-wise nondermatophyte species distribution
| Tinea corporis et cruris (TCC) | Tinea corporis | Tinea cruris | Total | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| n | % | n | % | n | % | ||
| C.albicans | 10 | 62.5% | 9 | 64.2% | 2 | 40% | 21 |
| Aspergillus | 3 | 18.75% | 5 | 35.71% | 1 | 20% | 9 |
| Fumigatus | 2 | 12.5% | 0 | 0% | 2 | 40% | 4 |
| Penicillium | 1 | 6.25% | 0 | 0% | 0 | 0% | 1 |
| Total | 16 | 100% | 14 | 100% | 5 | 100% | 35 |
Graph 4.
Bar graph depicting clinical presentation wise non dermatophyte species distribution
Discussion
In the present study, we included 300 cases of dermatophytosis. We observed male predominance in this study, with a male: female ratio of 1.4. Several researchers from different parts of India also found similar results.[5,6] The male preponderance might be due to increased outdoor exposure, enhanced strenuous activity, more involvement in fields, a hot and humid climate, and a greater tendency to wear tight inner dresses (vests and briefs) for prolonged periods of time in males. The lesser presentation of females in the study group may be due to lower educational status, less awareness, and less utilisation of locally available traditional medicaments.
Our study revealed that dermatophytic infection is common in the 31–40 age group (41% cases), which is well correlated with the other studies.[7,8] It has been observed that people of this age group tend to have more outdoor exposure, which results in excessive perspiration and favours the growth of fungus.
In the present study, the majority of the participants were field workers (31%), followed by students (28.3%) and housewives (26.7%). Previous studies by Hanumanthappa et al.[9] also showed similar results. Dermatophytoses are common among active workers, mostly engaged in field activities, following increased perspiration and exposure to infection. In contrast to our study, Pires et al.[10] reported housewives as the most common group presenting with dermatophytosis due to long hours of working in the kitchen in a hot and humid climate. However, Kucheria et al.[11] found that the incidence of dermatophytosis in students is the highest owing to longer periods of use of occlusive tight dresses.
We observed that tinea corporis et cruris was the commonest clinical presentation, followed by tinea corporis and then tinea cruris, which is also very well corroborated with several other studies.[12,13] However, studies done by Jain et al.[8] and Lakshmanan et al.[14] suggested tinea corporis to be the most common clinical presentation, whereas Jegadeesan et al.[5] and Hosthota et al.[15] found tinea cruris to be the most common clinical presentation. This significant change in the pattern of presentation may be due to the hesitation of patients to expose their crural areas in front of a dermatologist, thereby preventing a correct and timely diagnosis. Also, the use of OTC drugs delays diagnosis and aids in the spread of infection.
According to our study, 288 out of 300 patients (96%) were positive for KOH in the wet mount. This is similar to the findings of studies done by Singh et al.[2] and Surenderan et al.[16] However, as low as 50% KOH positivity has also been reported by Lakshmanan et al.[14] and in contrast, Tigga et al.[12] reported 98% KOH positivity in their study. This reflects the variability in KOH positivity, ranging from 50% to almost 99% in various studies.[11,17]
Figure 1.
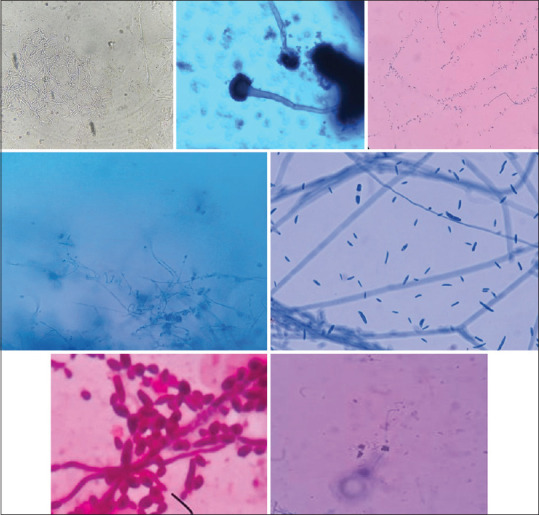
Microscopic images of: (a) KOH wet mount showing thin,long fungal hyphae (b) Aspergillus fumigatus species, LPCB (40×) (c) Tear drop shaped microconidia of T.rubrum, LBCB mount (d) Culture of microconidia and spiral hyphae of T.mentagrophytes, LPCB (40×) (e) Sickle shaped macroconidia suggestive of Fusarium species, LPCB mount (40×), (f) Round to oval budding yeast cells with pseudohyphae suggestive of C.albicans (100×) (g) Clusters of microconidia and spiral hyphae of T.mentagrophytes, LBCB mount (40×)
Figure 2.
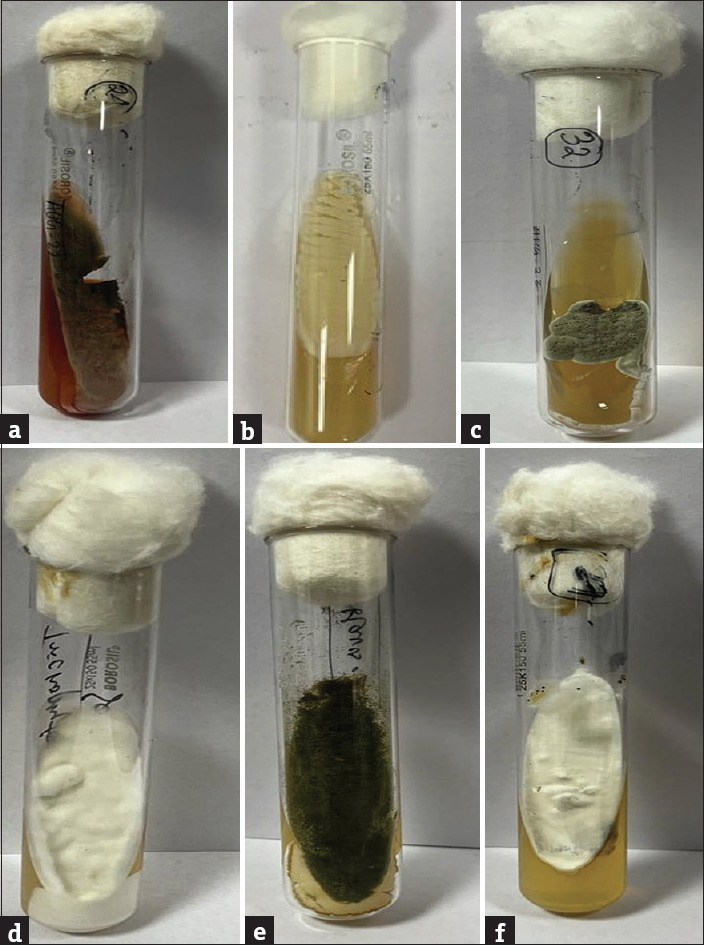
Culture on SDA media: (a) Penicillium (b) Candida albicans (c) Trichophyton schoenleini (d) Microsporum canis (e) Aspergillus fumigatus (f) Trichophyton mentagrophytes
In the present study, 162 out of 300 patients (54%) showed growth in fungal culture, which is similar to the results shown by Kucheria et al.,[11] Hosthotha et al.[15] and Pradhan et al.[17]
Out of 54% of patients who showed fungal culture positivity, 42.3% had dermatophyte growth, whereas 11.7% revealed growth of nondermatophyte species in the isolate that may be due to contamination. Fungal culture positive rates range from as low as 39% in studies done by Surenderan et al.[16] and Noronha et al.[6] to as high as 74% as suggested by studies done by Nenoff et al.[18] and Jegadeesan et al.[5] Good and efficient laboratory support could be a contributing factor to the high positivity rate in our study.
According to our study, the most common isolate was Trichophyton mentagrophytes (21.7%), followed by T. rubrum (11.7%), and 5.3% of cultures showed T. schoenleinii isolates, whereas Microsporum canis and Microsporum gypseum constituted 2.7% and 0.7%, respectively. Similar reports have been suggested by Singh et al.[2] and Tigga et al.,[12] whereas T. rubrum has been the most common isolate in studies done by Surenderan et al.[16] and Hosthota et al.[15]
It is well documented from published literature that Trichophyton is the most common dermatophyte isolate in most of the studies, out of which T. mentagrophytes and T. rubrum have been the two most common. T. mentagrophytes was the most prevalent isolate in our study, possibly due to an epidemiological switch observed in recent studies. Singh et al. reported comparable findings in this area for the first time in 2019. The epidemiological switch may be attributed to “anthropopization,” whereby the fungus shows increased survival capabilities in highly stressful environments owing to its increased keratinase enzyme levels. This property has been a major cause of the emergence of T. mentagrophytes as the most common isolate in recent years.[19,20,21] Various other factors, like topical steroid use and host immunity, have also been shown to be major contributors.[18]
Nondermatophyte growth accounted for 11.7% of our study. Candida albicans was the most commonly isolated organism, which is similar to the findings of studies done by Lakshmanan et al.[14] and Garg et al.[22]
Our study also highlights that the most prevalent isolate in tinea corporis at cruris and tinea cruris was T. mentagrophyte, whereas tinea corporis patients showed T. rubrum as the most prevalent causative isolate. Our findings are in contrast with the study done by Bhatia and Sharma,[23] which shows T. mentagrophyte to be the predominant species with both tinea cruris and tinea corporis. Another study reveals T. rubrum to be the most prevalent isolate in tinea corporis as well as tinea cruris.[24]
Conclusion
The present study focuses on the prevalence and clinical trends of different dermatophyte species associated with dermatophytosis in eastern India. Due to the favourable climate of Odisha, superficial mycoses are prevalent here.
Despite numerous therapeutic advancements and modernisations in recent decades, the frequency is rising. Although dermatophytosis is a simple clinical diagnosis for an experienced dermatologist, mistakes are possible because the skin lesions of dermatophytosis mimic many other skin disorders. As a result, antifungal medications or steroids may be used inappropriately. As these organisms may be easily identified with only the most minimal equipment, laboratory diagnosis of these fungal infections should be promoted before starting antifungal therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Agarwal U, Saran J, Agarwal P. Clinico-mycological study of dermatophytes in a tertiary care centre in northwest India. Indian J Dermatol Venereol Leprol. 2014;80:194. doi: 10.4103/0378-6323.129434. [DOI] [PubMed] [Google Scholar]
- 2.Singh BSTP, Tripathy T, Kar B, Ray A. Clinicomycological study of dermatophytosis in a tertiary care hospital in eastern India: A cross-sectional study. Indian Dermatol Online J. 2020;11:46–50. doi: 10.4103/idoj.IDOJ_62_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verma SB, Panda S, Nenoff P, Singal A, Rudramurthy SM, Uhrlass S, et al. The unprecedented epidemic-like scenario of dermatophytosis in India: I. Epidemiology, risk factors and clinical features. Indian J Dermatol Venereol Leprol. 2021;87:154–75. doi: 10.25259/IJDVL_301_20. [DOI] [PubMed] [Google Scholar]
- 4.Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;51((Suppl 4)):2–15. doi: 10.1111/j.1439-0507.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- 5.Jegadeesan M, Kuruvila S, Nair S. Clinico-etiological Study of Tinea Corporis: Emergence of Trichophyton mentagrophytes. Int J Sci Study. 2017;5:161–5. [Google Scholar]
- 6.Noronha T, Tophakhane R, Nadiger S. Clinico-microbiological study of dermatophytosis in a tertiary-care hospital in North Karnataka. Indian Dermatol Online J. 2016;7:264–71. doi: 10.4103/2229-5178.185488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deshmukh G, Vishwanath V, Londhe P, Tare D, Dhoot D. Effectiveness and safety of combination of Itraconazole and Amorolfine in management of patients with recalcitrant multi-site dermatophytosis who failed previous combination antifungal therapy. IP Indian J Clin Exp Dermatol. 2020;6:391–6. [Google Scholar]
- 8.Jain N, Sharma M, Saxena V. Clinico-mycological profile of dermatophytosis in Jaipur, Rajasthan. Indian J Dermatol Venereol Leprol. 2008;74:274–5. doi: 10.4103/0378-6323.41388. [DOI] [PubMed] [Google Scholar]
- 9.Hanumanthappa H, Sarojini K, Shilpashree P, Muddapur S. Clinicomycological study of 150 cases of dermatophytosis in a tertiary care hospital in South India. Indian J Dermatol. 2012;57:322–3. doi: 10.4103/0019-5154.97684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pires CAA, da Cruz NFS, Lobato AM, de Sousa PO, Carneiro FRO, Mendes AMD. Clinical, epidemiological, and therapeutic profile of dermatophytosis. An Bras Dermatol. 2014;89:259–64. doi: 10.1590/abd1806-4841.20142569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kucheria M, Gupta SK, Chhina DK, Gupta V, Hans D, Singh K. Clinico-mycological profile of dermatophytic infections at a tertiary care hospital in North India. Int J Community Health Med Res. 2016;2:17–22. [Google Scholar]
- 12.Tigga RA, Das S, Bhattacharya SN, Saha R, Pandhi D, Datt S, et al. Burden of chronic dermatophytosis in a tertiary care hospital: Interaction of fungal virulence and host immunity. Mycopathologia. 2018;183:951–9. doi: 10.1007/s11046-018-0303-4. [DOI] [PubMed] [Google Scholar]
- 13.Bhatia A, Kanish B, Badyal D, Kate P, Choudhary S. Efficacy of oral terbinafine versus itraconazole in treatment of dermatophytic infection of skin –A prospective, randomized comparative study. Indian J Pharmacol. 2019;51:116–9. doi: 10.4103/ijp.IJP_578_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lakshmanan A, Ganeshkumar P, Mohan SR, Hemamalini M, Madhavan R. Epidemiological and clinical pattern of dermatomycoses in rural India. Indian J Med Microbiol. 2015;33((Suppl)):S134–6. doi: 10.4103/0255-0857.150922. [DOI] [PubMed] [Google Scholar]
- 15.Hosthota A, Gowda T, Manikonda R. Clinical profile and risk factors of dermatophytoses: A hospitalbased study. Int J Res Dermatol. 2018;4:508–13. [Google Scholar]
- 16.Surendran K, Bhat R, Boloor R, Nandakishore B, Sukumar D. A clinical and mycological study of dermatophytic infections. Indian J Dermatol. 2014;59:262–7. doi: 10.4103/0019-5154.131391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pradhan MB, Paudel V. Clinico-mycological study of dermatophytosis and their antifungal susceptibility, a hospital based study. Nepal J Dermatol Venereol Leprol. 2021;19:30–6. [Google Scholar]
- 18.Nenoff P, Verma SB, Vasani R, Burmester A, Hipler U, Wittig F, et al. The current Indian epidemic of superficial dermatophytosis due to Trichophyton mentagrophytes — A molecular study. Mycoses. 2019;62:336–56. doi: 10.1111/myc.12878. [DOI] [PubMed] [Google Scholar]
- 19.de Hoog GS, Dukik K, Monod M, Packeu A, Stubbe D, Hendrickx M, et al. Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia. 2017;182:5–31. doi: 10.1007/s11046-016-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Rossi NM, Peres NTA, Rossi A. Pathogenesis of dermatophytosis: Sensing the host tissue. Mycopathologia. 2017;182:215–27. doi: 10.1007/s11046-016-0057-9. [DOI] [PubMed] [Google Scholar]
- 21.Verma SB, Panda S, Nenoff P, Singal A, Rudramurthy SM, Uhrlass S, et al. The unprecedented epidemic-like scenario of dermatophytosis in India: II. Diagnostic methods and taxonomical aspects. Indian J Dermatol Venereol Leprol. 2021;87:326–32. doi: 10.25259/IJDVL_302_20. [DOI] [PubMed] [Google Scholar]
- 22.Garg J, Tilak R, Garg A, Prakash P, Gulati AK, Nath G. Rapid detection of dermatophytes from skin and hair. BMC Res Notes. 2009;2:60. doi: 10.1186/1756-0500-2-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhatia VK, Sharma PC. Epidemiological studies on dermatophytosis in human patients in Himachal Pradesh, India. Springerplus. 2014;3:134. doi: 10.1186/2193-1801-3-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balakumar S, Rajan S, Thirunalasundari T, Jeeva S. Epidemiology of dermatophytosis in and around Tiruchirapalli, Tamilnadu, India. Asian Pac J Trop Dis. 2012;2:286–9. [Google Scholar]


