Abstract
Background:
In winter 2022/2023, a resurgence of invasive group A streptococcal (iGAS) infections in children was observed in Europe, including Germany and Switzerland. While a simultaneous increase in consultations for scarlet fever and pharyngitis was reported in England, leading to the recommendation to treat any suspected GAS disease with antibiotics, guidelines in Germany and Switzerland remained unchanged. We aimed to investigate whether this policy was appropriate.
Methods:
We conducted a retrospective multicenter study of children hospitalized for invasive GAS disease between September 2022 and March 2023 in pediatric departments in Dresden and Berlin (Germany) and Basel (Switzerland). We reviewed medical records and conducted structured telephone interviews to analyze whether suspected GAS infections with or without antibiotic treatment were reported prehospitalization.
Results:
In total, 63 patients met the inclusion criteria (median age 4.2 years, 57% males); however, clinical information was not complete for all analyzed characteristics; 32/54 (59%) had ≥1 physician visit ≤4 weeks prehospitalization. In 4/32 (13%) patients, GAS disease, that is, tonsillitis or scarlet fever, was suspected; 2/4 of them received antibiotics, and a positive rapid antigen test for GAS was documented in 1 of them.
Conclusions:
A small minority of patients had suspected GAS infection within 4 weeks before iGAS disease. These data suggest that there is little opportunity to prevent iGAS disease by antibiotic therapy, because in most patients—even if seen by a physician—there was either no evidence of GAS disease or when GAS disease was suspected and treated with antibiotics, consecutive invasive GAS disease was not prevented.
Keywords: group A streptococcus, invasive, children, antibiotics
Streptococcus pyogenes [group A streptococcus, (GAS)] is a gram-positive bacterium that causes a wide variety of diseases. In children, mild to moderate infections such as tonsillopharyngitis, scarlet fever, or impetigo are common. However, in rare cases, GAS can be invasive and cause life-threatening conditions such as sepsis, meningitis, or necrotizing fasciitis, which require immediate antibacterial treatment and sometimes surgery.1
During the COVID-19 pandemic, a decline in several infectious diseases,2 including GAS infections,3 was observed. In the 2022/2023 winter season, WHO reported a resurgence of local and invasive GAS diseases in several European countries, with children under 10 years of age being the most affected age group.4 This increase raised general concern because of the severity of many cases and related fatalities.
Recently, before the pandemic, the previous paradigm of treating all patients with a sore throat and suspected GAS infections, such as tonsillopharyngitis, with an antibiotic had been abandoned in many countries including Germany5 and Switzerland.6 With this background, the resurgence of invasive GAS infections raised the question of whether every suspected GAS tonsillopharyngitis should be treated with an antibiotic to prevent invasive GAS disease in these patients. Professional societies, however, discouraged a change of recommendations.7,8
The aim of this study was to analyze whether there is retrospective evidence that invasive GAS disease could have been prevented if the respective patients had been presented to a physician before and if those with suspected GAS disease, such as tonsillopharyngitis had been treated with antibiotics. Therefore, we set out to investigate if patients with invasive GAS disease had presented to the health care system with GAS infections shortly before developing invasive disease and if these visits were missed opportunities for antibiotic treatment.
METHODS
Study Design
We conducted a retrospective multicenter study of patients who were treated for invasive GAS disease in 3 pediatric institutions: University Children’s Hospital Basel UKBB, Switzerland (study site A); the Departments of Pediatrics, University Hospital Carl Gustav Carus, Dresden, Germany (study site B); and Sana Children’s Hospital Lichtenberg, Berlin, Germany (study site C).
Participants
Patients under 18 years of age hospitalized with invasive GAS disease in 1 of the 3 collaborating hospitals between September 01, 2022, and March 31, 2023 (Basel and Dresden) or May 31, 2023 (Berlin) were eligible for inclusion.
Invasive GAS disease was defined as the isolation of GAS from a normally sterile body site by bacterial culture and/or nucleic acid detection (polymerase chain reaction). Sterile sites included blood, cerebrospinal fluid, aspirate from pleura, joint, peritoneal or pericardial fluid, bone or muscle tissue or deep tissue from an internal body site (surgical sampling). If the treating physician’s diagnosis was necrotizing fasciitis or streptococcal toxic shock syndrome, isolation of GAS from a nonsterile site (eg, pharynx) was also considered as proof of invasive GAS disease.9
Data Collection
Relevant data were extracted from electronic medical records at each hospital and managed using the REDCap electronic data capture system.10 In the absence of information on physician visits due to potential noninvasive GAS disease in the 4 weeks before hospital admission, we conducted structured telephone interviews with the families and, if necessary, with the patient’s pediatrician (Text, Supplemental Digital Content 1, http://links.lww.com/INF/F565).
Statistical Analysis
Baseline data were summarized using descriptive statistics. Due to the limited number of patients, no analyses stratified by the study center were performed.
Ethics
In this retrospective study, informed consent for phone interviews was obtained from the parents to complete nondocumented data. Approval was granted by the ethical committees in Dresden (BO-EK-252062023) and Berlin. At the study site in Basel, after consultation with the local ethics committee, approval was granted to include patients with existing general consent (EKNZ 2023-00696).
RESULTS
Patient Characteristics and Clinical Findings on Admission
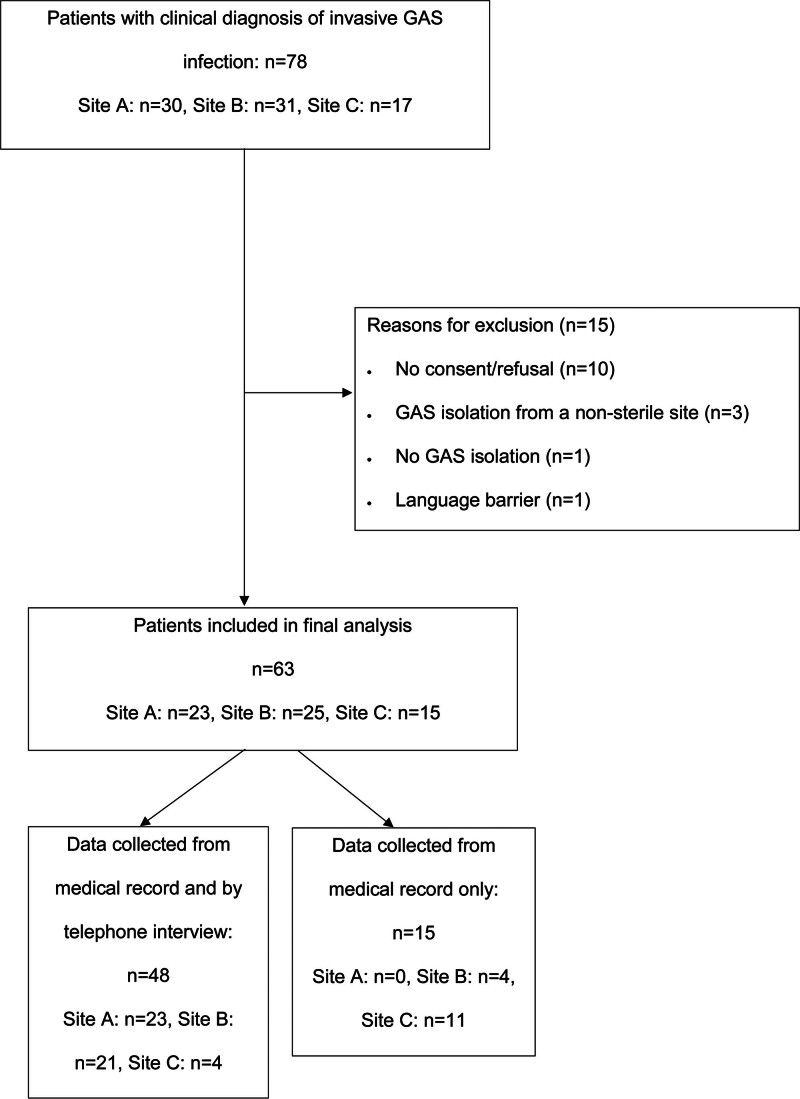
We identified a total of 78 patients hospitalized for invasive GAS, of which 63 fulfilled the inclusion criteria (Fig. 1).
FIGURE 1.
Flowchart of patient selection.
Patient characteristics are presented in Table 1. Denominators vary because the available information was not complete for all patients. Six (10%) of 62 had an underlying disease but none was immunosuppressed. The most common clinical feature of invasive GAS disease was fever (95%), with a median duration of 4 days and a range of 0 to more than 10 days before admission. Signs and symptoms suggestive of local GAS infections, such as sore throat or typical rash for scarlet fever, were less common than unspecific signs and symptoms (Table 1).
TABLE 1.
Characteristics and Clinical Presentation on Hospital Admission
| Characteristics | n/n Known (%) |
|---|---|
| Median age: 4.2 years (IQR 1.8–6.9) | 63/63 (100) |
| Age range: 3 months–14 years | 63/63 (100) |
| Male sex | 36/63 (57) |
| Chronic underlying disease | 6/62 (10) |
| Immunosuppression | 0/61 (0) |
| Fever duration: median 4 days (IQR 1–5) | 59/63 (94) |
| Signs and symptoms* | |
| Fever (≥38 °C) | 56/59 (95) |
| Cough and/or rhinitis | 29/55 (53) |
| Vomiting and/or diarrhea | 18/56 (32) |
| Limping and/or movement restrictions | 12/52 (23) |
| Sore throat | 11/48 (23) |
| Scarlet fever | 7/56 (13) |
| Exanthema (unspecific) | 6/57 (11) |
| Diagnosis of invasive GAS† | |
| Mastoiditis | 16/63 (25) |
| Bloodstream infection (sepsis, bacteremia) | 15/63 (24) |
| Pneumonia | 14/63 (22) |
| Streptococcal Toxic Shock syndrome | 12/63 (19) |
| Skin and soft tissue infection | 11/63 (17) |
| Other clinical syndromes‡ | 10/63 (16) |
| Osteoarticular infection | 9/63 (14) |
| Meningitis | 3/63 (5) |
In most patients >1 symptom was present.
21 patients had >1 diagnosis of invasive GAS.
Necrotizing fasciitis (n = 1), lymphadenitis colli (n = 3), para-/peritonsillar abscess (n = 3), pyomyositis (n = 3).
GAS, group A streptococcal infection.
Seven (16%) of 43 patients with iGAS disease had a known contact with GAS, usually in a family member, but none had contact with a case of iGAS disease.
Clinical and Microbiologic Diagnosis
The predominant presentations of invasive GAS disease were bloodstream infections, pneumonia (mostly with pleura empyema; 12/14 cases) and mastoiditis (Table 1). About 21 (33%) of 63 patients had more than 1 invasive GAS disease manifestation.
Invasive GAS disease was confirmed by the isolation of GAS from the following specimens of sterile body sites: surgical soft tissue samples, that is, abscesses (n = 25); positive cultures of blood (n = 18); pleural fluid (n = 12); bone samples (n = 6); joint fluid (n = 5); muscle tissue (n = 4); and cerebrospinal fluid (n = 1). In 10 patients GAS was isolated from 2 body sites.
In 3 patients with the clinical presentation of streptococcal toxic shock syndrome, GAS was only isolated from a nonsterile site, that is, the pharynx (n = 3).
Preadmission Physician Visits
Information on preadmission physician contact was available for 54 (86%) of 63 patients. Of these, 32 (59%) had a total of 42 physician visits (1 visit: n = 24; 2 visits: n = 6; 3 visits: n = 2) within 4 weeks before hospitalization (Table 2). However, only 4 (13%) of 32 patients with physician visits presented with a disease compatible with GAS infection: 3 with scarlet fever and 1 with tonsillopharyngitis (patients 1–4 in Table 2). Two of these 4 patients received a rapid antigen test for GAS, of which 1 was positive, and 2 of them were treated with an antibiotic.
TABLE 2.
Details on 42 Pre-admission Physician Visits in 32 Patients with Invasive GAS Disease
| Patient Number | Clinical Diagnosis | Day of Visit* | Rapid Antigen Test for GAS | Antibiotic Treatment* |
|---|---|---|---|---|
| 1 | Scarlet fever | −4 days | Unknown | Penicillin (−4 until 0) |
| 2 | 1. Scarlet fever (unknown if with or without physician contact) | −14 days | Unknown | Unknown |
| 2. Gastroenteritis | −2 days | No | No | |
| 3 | 1. Upper respiratory tract infection | −14 days | Unknown | No |
| 2. Scarlet fever, tonsillitis | −12 days | Yes (positive) | Amoxicillin clavulanate (−12 until −6) | |
| 3. Tonsillitis, otitis media | −6 days | No | Amoxicillin clavulanate (−6 until −5) |
|
| 4 | 1. Pharyngitis | −5 days | No | No |
| 2. Tonsillitis | −3 days | Yes (negative) | No | |
| 5 | Pharyngitis | −18 days | No | No |
| 6 | Upper respiratory tract infection | −27 days | No | No |
| 7 | Upper respiratory tract infection | −14 days | No | No |
| 8 | Upper respiratory tract infection | −12 days | No | No |
| 9 | Upper respiratory tract infection | −3 days | Unknown | No |
| 10 | Upper respiratory tract infection | −2 days | No | No |
| 11 | Upper respiratory tract infection | −2 days | No | No |
| 12 | Upper respiratory tract infection | −1 day | No | No |
| 13 | 1. Upper respiratory tract infection | −12 days | No | No |
| 2. Upper respiratory tract infection | −5 days | No | No | |
| 14 | 1. Upper respiratory tract infection | −17 days | No | No |
| 2. Lymphadenitis colli | −16 days | No | Amoxicillin clavulanate (−16 until −11) | |
| 3. Lymphadenitis colli | −10 days | Unknown | No | |
| 15 | 1. Other reason than infectious disease | −7 days | No | No |
| 2. Upper respiratory tract infection | −3 days | Yes (negative) | No | |
| 16 | 1. Other reason than infectious disease | −6 days | No | No |
| 2. Upper respiratory tract infection | −2 days | No | No | |
| 17 | 1. Upper respiratory tract infection, otitis media | −12 days | No | Amoxicillin clavulanate (−12 until −11) |
| 2. Otitis media | −11 days | No | Change to Cefclor (−11 until −4) |
|
| 18 | Otitis media | −28 days | No | Amoxicillin (−28 until −21) |
| 19 | Otitis media | −21 days | Unknown | Antibiotic, unknown product (−21 until −16) |
| 20 | Otitis media | −12 days | No | No |
| 21 | Otitis media | −3 days | No | No |
| 22 | Pneumonia | −14 days | No | Amoxicillin clavulanate (−14 until −6) |
| 23 | Bronchitis | −3 days | No | No |
| 24 | Bronchitis | −1 day | Yes (negative) | No |
| 25 | Gastroenteritis | −2 days | No | No |
| 26 | Varicella zoster virus infection | −3 days | No | No |
| 27 | Varicella zoster virus infection | −3 days | No | No |
| 28 | Ancle pain of unknown origin | −8 days | No | No |
| 29 | Unknown (symptomatic treatment)† | −5 days | Unknown | No |
| 30 | Unknown (symptomatic treatment)† | −2 days | Unknown | No |
| 31 | Unknown† | −14 days | No | No |
| 32 | Unknown† | −1 day | Unknown | Unknown |
In days before hospitalization for iGAS disease.
Diagnosis not documented in patient records.
DISCUSSION
There are 2 main findings in this study. First, the great majority (87%) of patients did not have any evidence for preceding local GAS disease within a 4-week window leading to a physician visit where prescription of an antibiotic treatment theoretically could have prevented their invasive GAS disease. Second, in 2 of the 4 patients with preceding suspected GAS infection, the disease was severe enough that an antibiotic was prescribed but this did not prevent consecutive invasive GAS disease. There was 1 patient with suspected GAS tonsillopharyngitis who was not treated with an antibiotic, but due to a negative rapid antigen test for GAS, this cannot truly be considered as a missed treatment opportunity.
The portal of entry for invasive GAS infections is often unclear, and invasive dissemination from the throat is still under debate.11,12 We found 3 other small pediatric studies where GAS-compatible diseases before invasive GAS infections were described. In a study from France,13 osteoarticular infections due to GAS were analyzed, and a preceding rhino-pharyngeal GAS infection was present in 7 (35%) of 20 cases. In a study from Australia,14 15 (54%) of 28 children had been seen as out-patients within 48 hours before the onset of their invasive GAS disease, but only 1 (ie, 4% of the total) of them had local GAS disease (pyoderma) within the month before. In contrast, a study on invasive GAS infections in Greece15 reported that 19 (20%) of 96 pediatric patients had tonsillopharyngitis in the 4 weeks before hospitalization. Unfortunately, no data about antibiotic treatment of preceding GAS disease is provided in any of these studies. There are 2 other pediatric studies reporting symptoms suggestive of GAS disease, such as sore throat, before invasive GAS disease in 4%16 and 18%,17 respectively, but the time interval between GAS infection and invasive GAS diseases is not mentioned.
The only other data we are aware of that also investigated whether the invasive GAS disease may have been prevented by antibiotic therapy of preceding GAS-compatible illness is reported from the pediatric children’s hospital in Bern, Switzerland. The authors found that 5 (10%) of 51 children had a physician visit because of pharyngitis and/or scarlet fever within >24 hours before invasive GAS hospitalization. Like our study, only 2 of these 5 patients (ie, 4% of the total) did not receive an antibiotic18; one could argue that these 2 instances had been missed opportunities to prevent later hospitalization for invasive GAS.
Our study has some limitations, mainly its retrospective design and the limited number of patients. Another limitation is a possible recall bias regarding the information obtained from the parents through structured telephone interviews. To reduce this bias, we first interviewed the parents and if there was uncertainty, we called the treating pediatrician for the missing information. Despite the use of several available data sources (medical records, parents and pediatricians), the completeness of the data varied, which may limit interpretation of our findings. However, with regards to the main study objective, we believe that we did not miss a meaningful number of missed treatment opportunities for local GAS disease preceding invasive GAS disease. Strengths of the study are the multidisciplinary approach with 3 different pediatric centers and the focus on the association of GAS-compatible diseases preceding invasive GAS.
CONCLUSIONS
The great majority of patients did not have a missed opportunity for antibiotic treatment preceding their iGAS disease. Therefore, based on our study and considering all limitations, withholding antibiotic treatment for suspected local GAS infections does not seem to be a risk factor for consecutive invasive GAS disease. The invasive GAS disease appears to be an independent event rather than following the preceding local GAS disease. Therefore, wisely chosen outpatient antibiotic treatment of suspected or proven noninvasive GAS disease in children remains justified and should be reinforced by current recommendations even in time periods of surging iGAS infections.
Supplementary Material
Footnotes
The authors have no funding or conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
Contributor Information
Nicole Toepfner, Email: Nicole.Toepfner@ukdd.de.
Reinhard Berner, Email: Reinhard.Berner@ukdd.de.
REFERENCES
- 1.Steer AC, Danchin MH, Carapetis JR. Group A streptococcal infections in children. J Paediatr Child Health. 2007;43:203–213. [DOI] [PubMed] [Google Scholar]
- 2.Yang MC, Su YT, Chen PH, et al. Changing patterns of infectious diseases in children during the COVID-19 pandemic. Front Cell Infect Microbiol. 2023;13:1200617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen PR, Rybak A, Werner A, et al. Trends in pediatric ambulatory community acquired infections before and during COVID-19 pandemic: a prospective multicentric surveillance study in France. Lancet Reg Health Eur. 2022;22:100497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. World Health Organization. Increased incidence of scarlet fever and invasive Group A Streptococcus infection - multi-country. December 15, 2022. Available at: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON429. Accessed March 18, 2024. [Google Scholar]
- 5.Pellegrino R, Timitilli E, Verga MC, et al. ; Other members of the Italian Panel for the Management of Acute Pharyngitis in Children. Acute pharyngitis in children and adults: descriptive comparison of current recommendations from national and international guidelines and future perspectives. Eur J Pediatr. 2023;182:5259–5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hofmann Y, Berger H, Wingeier B, et al. Behandlung der streptokokken-angina. Swiss Med Forum. 2019;19:481–488. [Google Scholar]
- 7.PIGS. Pediatric Infectious Disease Group Switzerland. Invasive group A streptococci (iGAS) infections in children. March 31, 2023. Available at: https://pigs.ch/wp-content/uploads/2023/04/PIGS-statement-iGAS31032023_def.pdf. Accessed March 18, 2024. [Google Scholar]
- 8.DGPI. Deutsche Gesellschaft Pädiatrische Infektiologie. Aktuelle DGPI-Stellungnahme zu vermehrten invasiven Infektionen durch Gruppe-A-Streptokokken (S.pyogenes). December 19. 2022. Available at: https://dgpi.de/vermehrten-invasiven-infektionen-a-streptokokken-2022-12-19/. Accessed March 18, 2024. [Google Scholar]
- 9.CDC. Centers for Disease Control and Prevention. Active Bacterial Core Surveillance (ABCs) Case Definition and Ascertainment. July 19, 2021. Available at: https://www.cdc.gov/abcs/methodology/case-def-ascertain.html. Accessed March 18, 2024. [Google Scholar]
- 10.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steer AC, Lamagni T, Curtis N, et al. Invasive group a streptococcal disease: epidemiology, pathogenesis and management. Drugs. 2012;72:1213–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kailankangas V, Vilhonen J, Gröndahl-Yli-Hannuksela K, et al. Presence of streptococcus pyogenes in the throat in invasive group A streptococcal disease: a prospective two-year study in two health districts, Finland. Infect Dis (Lond). 2023;55:405–414. [DOI] [PubMed] [Google Scholar]
- 13.Pigeolet M, Haumont E, Rubinsztajn R, et al. Increase in paediatric group A streptococcal infections. Lancet Infect Dis. 2023;23:282. [DOI] [PubMed] [Google Scholar]
- 14.Ching NS, Crawford N, McMinn A, et al. Prospective surveillance of pediatric invasive group A streptococcus infection. J. Pediatric Infect. Dis. Soc.. 2019;8:46–52. [DOI] [PubMed] [Google Scholar]
- 15.Zachariadou L, Stathi A, Tassios PT, et al. ; Hellenic Strep-Euro Study Group. Differences in the epidemiology between paediatric and adult invasive Streptococcus pyogenes infections. Epidemiol Infect. 2014;142:512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho EC, Cataldi JR, Silveira LJ, et al. Outbreak of invasive group a streptococcus in children—Colorado, October 2022–April 2023. J. Pediatric Infect. Dis. Soc.. 2023;12:540–548. [DOI] [PubMed] [Google Scholar]
- 17.Barnes M, Youngkin E, Zipprich J, et al. Increase in pediatric invasive group a streptococcus infections—Colorado and Minnesota, October–December 2022. MMWR Morb Mortal Wkly Rep. 2023;72:265–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoebi N, Duppenthaler A, Keitel K, et al. Pre-admission management of patients hospitalized with Group A streptococcal (GAS) disease in winter 2022/2023 - should antibiotics have been used more deliberately? (Abstract). Swiss Med Wkly. 2023;153(suppl 267):6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.