Abstract
Bovine leukemia virus (BLV), a retrovirus related to human T-cell leukemia virus types 1 and 2, can induce persistent nonneoplastic expansion of the CD5+ B-cell population, termed persistent lymphocytosis (PL). As in human CD5+ B cells, we report here that CD5 was physically associated with the B-cell receptor (BCR) in normal bovine CD5+ B cells. In contrast, in CD5+ B cells from BLV-infected PL cattle, CD5 was dissociated from the BCR. In B cells from PL cattle, apoptosis decreased when cells were stimulated with antibody to surface immunoglobulin M (sIgM), while in B cells from uninfected cattle, apoptosis increased after sIgM stimulation. The functional significance of the CD5-BCR association was suggested by experimental dissociation of the CD5-BCR interaction by cross-linking of CD5. This caused CD5+ B cells from uninfected animals to decrease apoptosis when stimulated with anti-sIgM. In contrast, in CD5+ B cells from PL animals, in which CD5 was already dissociated from the BCR, there was no statistically significant change in apoptosis when CD5 was cross-linked and the cells were stimulated with anti-sIgM. Disruption of CD5-BCR interactions and subsequent decreased apoptosis and increased survival in antigenically stimulated B cells may be a mechanism of BLV-induced PL.
Bovine leukemia virus (BLV), a member of the human T-cell leukemia virus (HTLV)-BLV group of retroviruses, causes persistent lymphocytosis (PL), a polyclonal increase in peripheral blood B-lymphocyte numbers, in approximately 30% of infected cattle (14, 20). PL is a strong risk factor for development of lymphoma and/or leukemia, and 1 to 5% of PL animals eventually develop neoplasia (14). Theoretically, either increased cell proliferation or decreased cell death can cause PL. B cells from BLV-infected sheep and cattle may have a higher proliferation rate (18, 25, 38). A decreased rate of apoptosis and therefore increased cell longevity may also contribute to PL (11, 12, 32, 33). There is an overall increase in apoptosis in ex vivo-cultured peripheral blood mononuclear cells (PBMCs) from PL cows, but infected cells that express viral proteins are less prone to undergo spontaneous apoptosis than are cells from the same animals that do not express viral proteins (11). Additionally, supernatants from cultures of PL PBMCs have antiapoptotic properties and delay apoptosis when added to uninfected cell cultures (11). These results suggest that increased cell longevity due to delayed apoptosis may be mechanism of PL, particularly in more densely packed environments such as lymph nodes, even if delayed apoptosis is not seen in ex vivo cells from peripheral blood.
Several concurrent mechanisms may result in BLV-induced cell proliferation or delayed apoptosis. The Tax protein is one critical factor in BLV-induced cell proliferation and neoplasia (42, 43). BLV and HTLV Tax activate the viral long terminal repeat promoter, and HTLV-1 Tax is also known to activate many cellular promoters, including those for interleukin-2 (IL-2), IL-2 receptor alpha, granulocyte-macrophage colony-stimulating factor, c-Fos, and vimentin (16). Several mechanisms have been investigated. Tax facilitates dimerization of CREB transcription factors and binding to Tax-responsive elements in promoters (1, 44) and activates NF-κB (4). Polymorphisms in major histocompatibility complex (MHC) class II alleles also play a major role in progression to PL (45).
An additional, potentially concurrent mechanism for BLV-induced B-cell expansion may be direct interactions of viral proteins with cellular signaling pathways originating at the cell membrane. Interaction with the phosphatase SHP-1 may be one such mechanism. In normal human and mouse B cells, SHP-1 associates with CD22 (13) and CD32b (FcγRIIB) (8) and acts as a critical negative regulator of the B-cell receptor (BCR) (8, 13). We previously showed that in bovine B cells, SHP-1 physically associates with the BLV transmembrane protein gp30 and suggested that this interaction may sequester SHP-1 and make the BCR hyperresponsive to antigenic signaling (6).
CD5 is one notable cell membrane protein in PL B cells. As in humans and mice, bovine CD5 is present in most T cells but only a small number of normal, uninfected B cells. In BLV-infected PL cattle, however, nearly all of the B cells are CD5+ (10). In human and mouse T cells, CD5 physically associates with CD2 (27, 34) and T-cell receptor components, including CD3 zeta chain (5, 26), and transduces signals to Lck (31), SHP-1 (7), and phosphatidylcholine-specific phospholipase C (35) that can modify T-cell receptor signaling. In human B cells, CD5 associates with the BCR (22). In mouse B cells, the interaction of CD5 with mouse BCR downregulates BCR-mediated signaling to undergo proliferation and to increase cell survival by delayed apoptosis (3).
Here, we show that the association of CD5 with the BCR differs in CD5+ B cells from normal, uninfected cattle and those from BLV-infected PL cattle. CD5 and BCR were associated in cells from normal animals and not associated in PL animals. BCR stimulation of B cells from PL animals resulted in decreased apoptosis, while stimulation of B cells from uninfected animals increased apoptosis. When CD5 and BCR from uninfected bovine CD5+ B cells were experimentally dissociated to simulate conditions in PL cells, the uninfected cells showed decreased apoptosis after BCR stimulation. This suggests that the dissociation of CD5 and BCR in the PL B cells is functionally significant and is a mechanism that contributes to increased longevity and PL in B cells from BLV-infected animals.
MATERIALS AND METHODS
Animals and cells.
Naturally infected adult Holstein-Friesian cows were BLV seropositive by enzyme-linked immunosorbent assay and persistently lymphocytotic based on three consecutive monthly lymphocyte counts above 8,800 lymphocytes/μl (40). The animals were negative for bovine immunodeficiency virus, based on PCR and serology. Negative control cows from the same herd were BLV seronegative. To determine how closely the animals were related, the sire, dam, and grandparents of each animal were determined. For two animals, ancestry could not be determined. Uninfected animals 1937 and 1830 are half-sisters. None of the other animals had common parents or grandparents.
PBMCs from peripheral blood were purified by gradient centrifugation through Lymphoprep cell separation medium (specific gravity, 1.077 g/ml) (Nycomed, Inc.) (37). B cells were enriched by depletion of T cells. PBMCs (1.5 × 108 in RPMI 1640 with 10% fetal bovine serum [FBS], 100 μM streptomycin, and 100 μU penicillin) were incubated with mouse monoclonal antibodies (MAbs) to bovine CD3 (MM1A; immunoglobulin G1 [IgG1]; 160 μg), CD4 (ILA11A; IgG2a; 160 μg), and CD8 (CACT80C; IgG1; 40 μg) and an MAb to the δ chain of bovine γδ T cells (GB21A; IgG2b; 40 μg). After rotating incubation for 30 min at 4°C, cells were washed twice and incubated with 2 ml of anti-mouse IgG Magna Beads (Pierce, Rockford, Ill.) for 30 min at 4°C. T cells were removed by three rounds of magnetic depletion, 5 min per application, at 4°C. In some experiments, MAbs to CD2 (B26A; IgM), CD8 (BAQ111A; IgM), CD4 (GC50A; IgM), and the δ chain of γδ T cells (CACT148A; IgM) were used. In these experiments, T cells were depleted by complement lysis, as previously described (39). T-cell depletion was verified by flow cytometric (FC) analysis. In the coimmunoprecipitation experiments, T cells from uninfected animals were reduced to 10% or less of the cell population, and T cells from PL animals were reduced to 1% or less. In the experiments using BCR stimulation to reduce apoptosis, T cells from uninfected animals constituted 2% or less and T cells from PL animals constituted 0.2% or less of the cell population.
Antibodies.
Mouse MAbs were anti-bovine CD5, CACT105A (IgG1), MUC1A (IgG1), and B29A (IgG2a); anti-bovine IgM, 1H4 (IgG1) (23) and PIG45A2 (IgG2b); anti-CD79a (synonyms, Ig-α and mb-1), HM57 (IgG1) (Dako) (24); anti-SHP-1 (IgG1) (Transduction Laboratories, Lexington, Ky.); anti-Escherichia coli (negative control MAbs) ColiS69A (IgG1) and ColiS169A (IgG2a); anti-chicken MHC class-II-like (negative control MAb) AV64A (IgG1); and the T-cell depletion MAbs as above. With the exception of 1H4, HM57, and anti-SHP-1, antibodies were from the Washington State University Monoclonal Antibody Center.
Coimmunoprecipitations.
Surface labeling of cells with biotin and coimmunoprecipitations were performed as previously described (6). In some experiments, 1% digitonin was substituted for 1% Brij 96 in the lysis buffer.
FC.
FC analyses were performed on a Becton Dickinson FACScan flow cytometer. Data were analyzed with Cellquest software (Becton Dickinson Immunocytometry, San Jose, Calif.) (11). Debris was excluded from analyses by scatter gating. In some analyses, CD5+ cells or surface IgM+ (sIgM+) cells were gated, and apoptotic analyses were restricted to the gated population.
Apoptosis assays.
Cells were cultured for 16 h in RPMI 1640 medium with 10% FBS, 100 μM streptomycin, and 100 μU penicillin. Cells were labeled using MAbs to surface proteins at 15 μg/ml for 30 min at 4°C and with fluorescein isothiocyanate or phycoerythrin-conjugated isotype-specific secondary antibodies, followed by apoptosis assays. Apoptotic cells were identified using the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) method, as described previously (11), or propidium iodide staining. Propidium iodide staining was performed after surface labeling for FC. Cells were suspended in 70% ethyl alcohol overnight at −20°C, washed, permeabilized in 0.1% Triton X-100–0.1% mM EDTA–50 μg of RNase A per ml in phosphate-buffered saline (PBS) for 30 min at 37°C and stained with propidium iodide (20 μg/ml), followed immediately by FC analysis. Cells with less than the 2N amount of DNA were classified as apoptotic, while cells with more than 2N were classified as proliferative. In the initial assays, results were verified by DNA ladder assay (11).
CD5 cross-linking.
CD5 molecules were cross-linked to dissociate from the BCR as described by Bikah et al., using biotinylated MAb to CD5 (CACT105A) and avidin (group I) (3). Controls were incubated with biotinylated MAb to CD5 without avidin (group II) or with biotinylated isotype control MAb ColiS69 and avidin (group III). MAbs were dialyzed overnight in 50 mM sodium bicarbonate buffer (pH 8.5) at 4°C, incubated for 2 h on ice with Sulfo-NHS-LC-Biotin (Pierce) at 0.045 μg of biotin per μg of MAb, and dialyzed again to remove unbound biotin. Biotinylation was quantitated by the 4-hydroxyazobenzene-2-carboxylic acid (Sigma, St. Louis, Mo.)-avidin reaction (15), and reactivity with CD5+ cells was confirmed by FC analysis. Cells (6 × 106 per group) were centrifuged and resuspended in 400 μl of RPMI with 10% FBS at 4°C as above. Biotinylated MAb to CD5 or biotinylated isotype control MAb was added (50 μg/ml) and incubated for 30 min at 4°C while rotating. Cells were washed twice in RPMI–10% FBS. The biotinylated MAb-bound CD5 molecules were cross-linked with NutrAvidin, a deglycosylated form of avidin (Pierce), 100 μg per group, for 20 min at 4°C while rotating and washed twice. Following CD5 cross-linking, cells were incubated at 37°C for 30 min and then stimulated using anti-IgM MAb 1H4 at 6 μg/ml and 37°C in 5% CO2 for 16 h. One control group (IV) was incubated with biotinylated MAb to CD5 and avidin but cultured for 16 h without anti-IgM MAb. To gate on the CD5+ cells, cells were labeled with anti-CD5 MAb B29A (IgG2a) or with isotype control MAb ColiS169A, followed by phycoerythrin-conjugated anti-mouse IgG2a, 1:150.
Statistical analyses.
We used 95% confidence intervals to determine if means were significantly different from zero. To assess change in apoptosis after BCR stimulation, the percent change in cells from PL versus uninfected animals was compared using Student's t test if data were normally distributed and the Mann-Whitney rank sum test if data were not normally distributed. In the CD5 cross-linking experiments, data were compared by analysis of variance (ANOVA) and Fisher's least significant difference (LSD) method. Significance was determined at P < 0.05.
RESULTS
CD5 is physically associated with the BCR in B cells from normal cattle but not in B cells from BLV-infected PL animals.
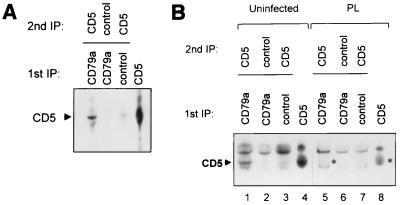
CD5 was shown previously to coimmunoprecipitate with BCR from normal human CD5+ B cells (22). Consistent with these results, we found that CD5 coimmunoprecipitated with CD79a (the Ig-alpha component of the BCR complex) of B cells from uninfected cattle (Fig. 1A). First, T-cell-depleted, biotin-labeled B cells in 1% digitonin lysis buffer (6) were immunoprecipitated using MAb to CD79a, a tightly linked BCR component, or isotype control MAb. To identify CD5 that was physically associated with CD79a, the precipitates were resolubilized in higher stringency 1% NP-40 lysis buffer and immunoprecipitated a second time (6) with either the CD5 MAb MUC1A or isotype control MAb. As a control, single immunoprecipitation was performed with MAb to CD5.
FIG. 1.
(A) CD5 coimmunoprecipitates with the BCR in uninfected animals. B cells were enriched from PBMCs by T-cell depletion. Lanes 1 to 3, biotin-labeled cells in 1% digitonin lysis buffer were immunoprecipitated (IP) with either MAb to CD79a (the Ig-alpha component of the BCR) or isotype control MAb AV64A. Precipitates were resuspended in 1% NP-40 lysis buffer, and a second immunoprecipitation was performed using MAb to CD5 (MUCIA) or isotype control MAb. As a control, CD5 was immunoprecipitated directly from the PBMC NP-40 lysate using MAb to CD5 (lane 4). (B) CD5 does not coimmunoprecipitate with BCR from BLV-infected PL animals. Methods and design were as in panel A. Despite the presence of a higher proportion of B cells in the PBMCs from the PL animal, CD5 did not coimmunoprecipitate with the BCR (lane 5), although it was seen in coimmunoprecipitations from an uninfected animal (lane 1). A light background band which migrates faster than CD5 is present in lanes 5 to 7. The position of CD5 in the single immunoprecipitation (lane 8) and the predicted position of CD5 in the double immunoprecipitation (lane 5) from PL cells are indicated with asterisks.
In contrast, in B cells from BLV-infected PL animals, CD5 did not coimmunoprecipitate with CD79a (Fig. 1B). In PL cell immunoprecipitations, a faster-migrating background band (60 kDa) was seen in all lanes (lanes 5 to 7) regardless of the primary antibody, but the slightly larger CD5 protein (63 kDa), shown in the single immunoprecipitation control lanes 4 and 8 and in the uninfected-cell coimmunoprecipitation (lane 1), is not seen in association with CD79a from PL cells (lane 5). Even overexposure of the film failed to show a distinct CD5 band in lane 5 compared with control lanes 6 and 7 (data not shown). The identity of the slower-migrating background band (70 kDa) seen in all lanes, including those from cells of uninfected and PL animals, is not known, but it may be Ig heavy chain that attached to the protien G beads in the immunoprecipitations. The experiment was repeated with two other PL and normal animals, and results were similar (data not shown).
BCR stimulation delays apoptosis in PL B cells but increases apoptosis in uninfected B cells.
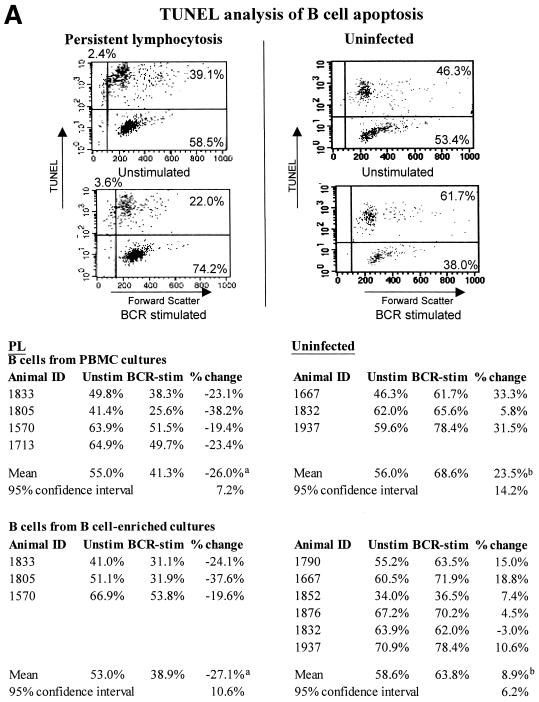
To determine the effect of antigenic stimulation of the BCR on apoptosis of B cells from PL and uninfected animals, ex vivo PBMCs and B-cell-enriched cell cultures were cultured for 16 h with or without MAb to IgM (1H4; IgG1) to stimulate the BCR. Apoptotic cells were identified using two different methods, TUNEL (Fig. 2A and B) and propidium iodide (Fig. 2B), and B cells were identified and gated using anti-IgM MAb PIG45A2 (IgG2b).
FIG. 2.
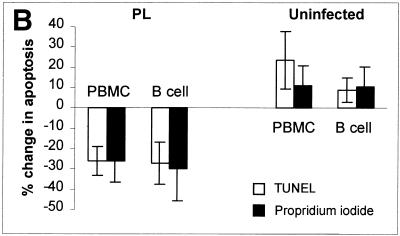
Apoptosis decreases in BCR-stimulated PL B cells but not in B cells from uninfected animals. The percentage of B cells undergoing apoptosis, as determined by TUNEL analysis, is shown, together with the percent change in apoptosis. Ex vivo PBMCs or B-cell-enriched cell cultures were incubated for 16 h with or without MAb to IgM (1H4; IgG1). Apoptosis was detected using TUNEL (A and B) or propidium iodide staining (B). During FC analyses, B cells were gated for analysis using MAb PIG45A2 (IgG2b) to IgM at 4°C. FC analyses from representative experiments are shown, and the experiments are summarized. In TUNEL analyses, the number of apoptotic cells is defined as the sum of cells in the upper right and left quadrants (A). Significant differences (Student's t test or Mann-Whitney test) are indicated by superscript letters. Means that are significantly different are indicated by different letters, while means that are not statistically different are indicated by the same letter. In panel B, results with TUNEL are compared with those using propidium iodide. The 95% CI are shown. Results with cells from PL animals are significantly different than those with cells from uninfected animals, but are not significantly different based on the technique used to detect apoptosis.
When apoptosis in PBMC cultures from the PL animals was analyzed by TUNEL, BCR stimulation reduced apoptotic cells from 55.0% of the B-cell population to 41.3%, which represents a 26.0% decrease (mean for four animals). Results were similar in TUNEL-stained B-cell-enriched cell cultures (27.1% decrease) (Fig. 2A). In Fig. 2A, FC analysis from one experiment with one PL and one uninfected animal is illustrated as a dot plot, and data for each of the animals are summarized.
In contrast, in B cells from the uninfected animals, BCR stimulation increased apoptosis. B-cell apoptosis increased 23.5% in PBMCs (mean for three animals) and 8.9% in B cell-enriched cultures (mean for six animals), as determined by TUNEL (Fig. 2A).
Regardless of whether apoptosis was detected by TUNEL or propidium iodide, similar changes were seen (Fig. 2B). When the change in apoptosis of PL cells was compared to that of uninfected cells, the difference was statistically significant in both PBMCs and B-cell enriched cultures and in both TUNEL and propidium iodide experiments (P < 0.05).
To determine if BCR stimulation also altered B-cell proliferation, the percentage of proliferating cells with more than 2N DNA content was also determined in the propidium iodide-stained cells. No significant differences were detected between the PL and uninfected cells or between unstimulated and BCR-stimulated cells (data not shown).
BCR stimulation delays apoptosis in uninfected B cells when CD5 is cross-linked.
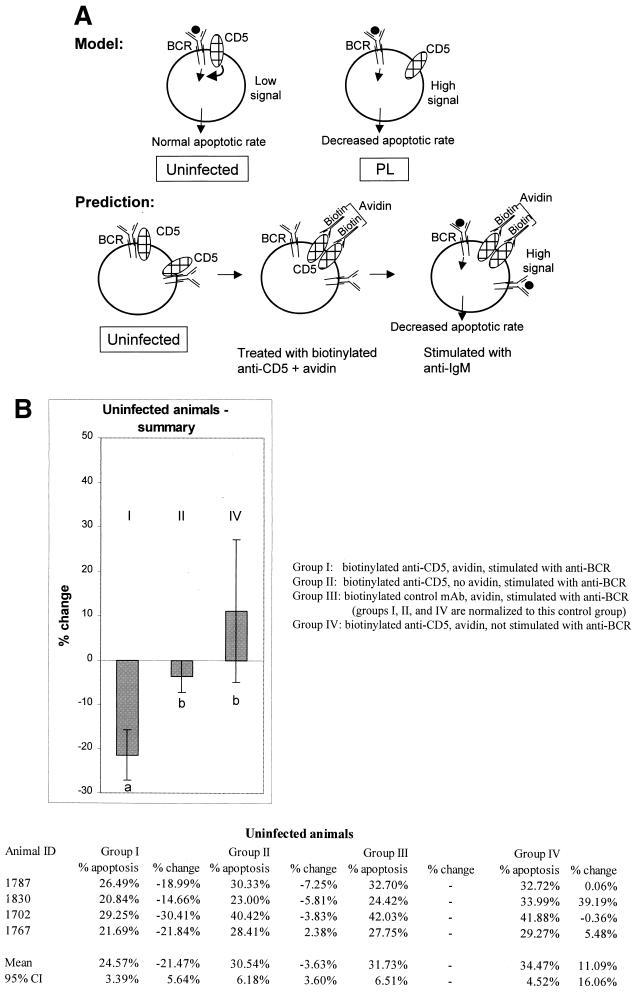
Observations that CD5 was dissociated from the BCR in PL cattle, BCR-mediated signaling in cells from PL cattle resulted in decreased apoptosis, and data from mice suggesting that CD5 downregulates BCR signaling (3, 34) made us ask if the dissociation of CD5 from the BCR in the PL cells might account for their decreased apoptosis and increased survival in response to BCR stimulation (Fig. 3A).
FIG. 3.
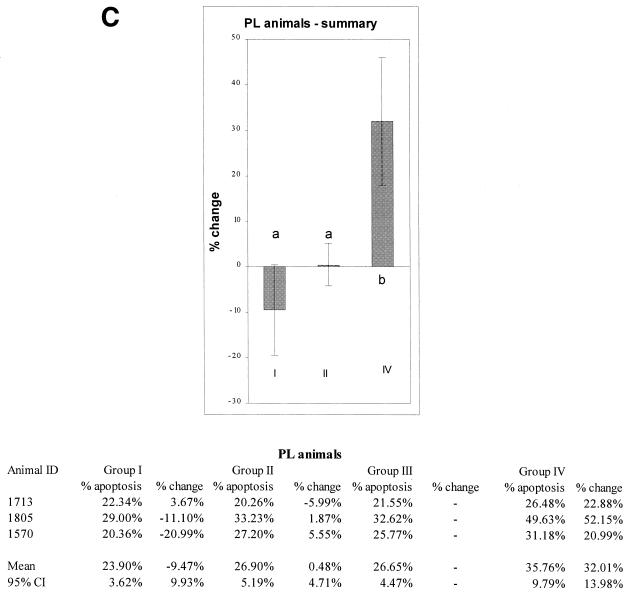
(A) Proposed model and predictions. In B cells from uninfected animals, CD5 is associated with the BCR and downregulates BCR signaling when the BCR is triggered by antigen (indicated by a solid circle). In PL animals, CD5 is dissociated from the BCR and does not downregulate the BCR. The model predicts that in PL animals, in the absence of downregulation by CD5, antigen-triggered BCR signaling will result in decreased apoptosis and increased B-cell longevity. In B cells from uninfected animals, CD5 downregulates antigen-triggered BCR signaling, and apoptosis increases. The model predicts that in B cells from uninfected animals, experimental separation of CD5 from BCR will decrease apoptosis mediated by antigen binding in the same way that apoptosis is decreased in the antigen-triggered PL B cells in which CD5 is already dissociated from the BCR. (B) Cross-linking of CD5 followed by BCR stimulation inhibits apoptosis in CD5+ B cells of uninfected animals. (C) No statistically significant inhibition of apoptosis is seen in CD5+ B cells from PL animals when CD5 is cross-linked followed by BCR stimulation. The percentage of apoptotic cells in each group was compared with group III (non-cross-linked cells stimulated with MAb to BCR) to determine the percent change. Means and 95% CI are shown; significant differences are indicated by superscript letters. In each figure, means that are significantly different are indicated by different letters, while means that are not statistically different are indicated by the same letter.
To experimentally test this possibility, CD5 was dissociated from the BCR by cross-linking (3). After T-cell depletion, cells were incubated with biotinylated MAb to CD5 (CACT105A) or with biotinylated isotype control MAb, followed by incubation with avidin to cross-link the CD5. The BCR was stimulated with MAb to IgM (1H4), as above (Fig. 3A). Analysis was restricted to CD5+ cells by gating on cells that reacted with CD5 MAb B29A (IgG2a), and apoptosis was evaluated by TUNEL. The percentage of cells in apoptosis is shown in Fig. 3B and C. To facilitate comparisons among experiments performed at different times, data were also normalized to control group III (biotinylated isotype control MAb, avidin, and MAb to IgM). The percentage of change relative to group III is shown in Fig. 3B and C.
If the observed dissociation of CD5 from the BCR in PL cells causes the BCR-induced decreased apoptosis, we predicted that experimental dissociation of CD5 from the BCR in cells from uninfected animals should likewise decrease BCR-induced apoptosis (Fig. 3A). In B cells from uninfected animals, which previously showed an increase in apoptosis when the BCR was stimulated (Fig. 2A and B), there was a decrease in apoptosis when the CD5-cross-linked cells were BCR stimulated (Fig. 3B). The mean decrease in apoptosis was 21.47% (95% confidence interval [CI], ±5.64%) (group I) compared with the cells incubated with biotinylated isotype control antibody (group III). To evaluate the possibility that the effect could be due to antibody stimulation of CD5, control group II consisted of cells treated with biotinylated antibody to CD5 but no cross-linking avidin, followed by BCR stimulation. In control group II, in which cells were treated with anti-CD5 without cross-linking, there was only a 3.63% inhibition of apoptosis (95% CI, ±3.60%). As a control for the effects of CD5 cross-linking alone without BCR stimulation (group IV), there was an 11.09% increase in apoptosis, not significantly different from zero (95% CI, ±16.06%). When groups were compared by ANOVA followed by Pearson's LSD method, group I was significantly different from control groups II and IV.
If experimentally induced CD5-BCR dissociation results in decreased apoptosis in uninfected, BCR-stimulated B cells, and if CD5 and BCR are already dissociated in B cells from PL animals, then we predicted that CD5 cross-linking should have little or no effect on the B cells from PL animals. CD5 cross-linking followed by stimulation with MAb to the BCR resulted in only a 9.47% mean decrease in apoptosis (group I) compared with the cells incubated with biotinylated negative control antibody (group III) (Fig. 3C). This decrease was not significantly different from zero (95% CI, ±9.93%). In control group II, there was a 0.48% increase in apoptosis (95% CI, ±4.71%), not significantly different from zero. Consistent with the results in Fig. 2A and B, apoptosis was increased in control group IV, in which cells from PL animals were not treated with antibody to the BCR. When the groups were compared by ANOVA followed by Pearson's LSD method, groups I and II were not significantly different and group IV was different from group III.
DISCUSSION
This study is consistent with and extends observations that CD5 normally functions as a downregulatory modulator of the BCR (3, 9). Here, we identify a naturally occurring disease in which CD5 is dissociated from the BCR and experimentally test the functional importance of this dissociation in delaying apoptosis. CD5+ B cells from uninfected animals were experimentally induced to behave like the CD5+ B cells from PL animals by dissociation of CD5 and BCR. Indeed, the functional significance of this effect is suggested by the similarity between the magnitude of the effect on uninfected cells of CD5 cross-linking followed by BCR stimulation (mean 21.5% decrease in apoptosis) and the magnitude of the decreased apoptosis in PL cells when they are BCR stimulated (mean 26.9% decrease in apoptosis). Although the magnitude of these apoptotic changes is relatively small in vitro, the biological significance could be much greater. The experiments were performed using cultured cells during a short time, but since PL takes months to years to develop after initial infection, small differences in apoptotic rates can be considerably amplified in vivo (2).
It is important to differentiate the effects of CD5 cross-linking followed by BCR stimulation from potential effects of antibody-mediated CD5 stimulation alone. In some studies, stimulation of CD5 by antibodies led to increased signaling and cellular responses (17, 19, 21). It was recently shown that ligation of both CD5 and IgM resulted in increased apoptosis in human tonsillar B cells (28). Here, the possible effect of antibody-mediated CD5 stimulation was controlled by incubating group II with MAb to CD5 but no avidin and then stimulating with MAb to the BCR. A small effect was seen (3.63% decrease in apoptosis; 95% CI, 3.60%) in cells from uninfected animals, but this effect was much less than the effect of CD5 cross-linking (21.47% decrease in apoptosis; 95% CI, 5.64%). It may also be asked if cross-linking of CD5 alone (i.e., aggregation of the CD5 molecules) without BCR-CD5 dissociation could be responsible for the delayed apoptosis seen here. This is unlikely, because cross-linking of CD5 without BCR stimulation of uninfected cells instead leads to an increase in apoptosis (group IV; Fig. 3B and C). The mechanisms of the CD5-BCR dissociation in cells from PL animals remain unknown. Physical associations, either direct or indirect, between viral proteins and either CD5 or BCR may disrupt CD5-BCR interactions. One such interaction may involve the BLV transmembrane (TM) protein gp30, CD5, and SHP-1. CD5 physically associates with SHP-1 in human T cells (27) and mouse B-1 B cells (34), and we found similar results in bovine CD5+ B cells (unpublished data). We previously showed that BLV TM is physically associated with SHP-1 (6), and a competition between BLV TM, SHP-1, and CD5 may be envisioned that results in dissociation of CD5 and BCR. However, interactions with viral proteins cannot fully explain the dissociation of CD5 and BCR, since not all infected cells express viral proteins. We previously showed that only 15 to 20% of PBMCs from PL cattle express viral p24 even after 24 h of culture (11). This experiment was done with PBMCs rather than B cells and with expression of p24 rather than TM. However, if viral proteins are only expressed in a small percentage of infected cells, then physical associations between viral proteins and CD5, BCR, or associated proteins such as SHP-1 do not fully explain the CD5-BCR dissociation in ex vivo PL B cells. Correlation of viral load and the percentage of infected cells with apoptotic rates in future experiments may be of assistance in resolving this issue.
Alternatively, the CD5-BCR dissociation may be due to altered cytokines or cellular environment or other changes in cellular signaling components. PL B cells produce increased amounts of IL-2 and IL-10 mRNA, increased IL-2 activity, and decreased amounts of IL-12 mRNA (29, 30, 41), and it is possible that this altered cytokine environment may trigger changes in CD5 or BCR interactions. Changes in levels of other B-cell surface receptors, including IL-2 receptor alpha and class II MHC, may also be involved (36).
A third alternative is that the CD5+ B cells of PL cattle may represent a different lineage or different developmental stage than CD5+ B cells of uninfected cattle. A diversity of CD5+ B cells are recognized in other species, including cells in which CD5 expression is either constitutive or induced (46). Although CD5+ B cells from the uninfected and PL cattle both express CD5, there may be enough other phenotypic differences that only the cell type from the uninfected animals has an association between CD5 and the BCR.
A puzzling conundrum of both HTLV and BLV infection is that only a subset of infected animals develop lymphocyte proliferation. The data here suggest a model to explain this. Delayed apoptosis may be a property of those PL cells that are antigenically stimulated via the BCR. Since PL is a polyclonal condition, virus-expressing cells are a broad array of B cells that are specific for many different antigens. A variety of antigens, then, may trigger delayed apoptosis in those B cells in which the downregulatory CD5 is dissociated from BCR. There may be some threshold of appropriate antigenic triggering of the BCR that results in decreased apoptosis and development of PL in some but not all animals at various times after BLV infection.
The advantage of BLV as an animal model for the closely related HTLV viruses and also for the nonviral human neoplastic disease chronic lymphocytic leukemia is that results from ex vivo or in vitro studies can be experimentally tested in vivo by infection of animals with altered infectious molecular clones of BLV. Determination of mechanisms of the disruption of CD5-BCR interactions and mapping of amino acids responsible for this disruption will allow testing of the significance of these observations in sheep or cattle.
ACKNOWLEDGMENTS
We thank D. Hoover, V. Hamilton, I. Eriks, K. Mealey, G. Palmer, D. Stone, T. Besser, and A. Burny for valuable discussions; J. Evermann for serologic analyses; E. Wagner (University of Idaho) for excellent animal care; and Hao Zhang for statistical consultation.
Grant support is from NIH K08 AI01198, USDA 94-37204-1253, American Cancer Society IRG 1190, ACS B-72451, FB Assurances (Fortis), Services de Programmation pour la politique scientifique (SSTC-P4/30), International Union Against Cancer Postdoctoral Fellowship, and the Belgian National Fund for Scientific Research. R.K. and L.W. are Research Directors and F.D. is a Senior Research Assistant of the Belgian National Fund for Scientific Research.
REFERENCES
- 1.Adam E, Kerkhofs P, Mammerickx M, Kettmann R, Burny A, Droogmans L, Willems L. Involvement of the cyclic AMP-responsive element binding protein in bovine leukemia virus expression in vivo. J Virol. 1994;68:5845–5853. doi: 10.1128/jvi.68.9.5845-5853.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berman J J, Moore G W. The role of cell death in the growth of preneoplastic lesions: a Monte Carlo simulation model. Cell Prolif. 1992;25:549–557. doi: 10.1111/j.1365-2184.1992.tb01459.x. [DOI] [PubMed] [Google Scholar]
- 3.Bikah G, Carey J, Ciallella J R, Tarakhovsky A, Bondada S. CD5-mediated negative regulation of antigen receptor-induced growth signals in B-1 B cells. Science. 1996;274:1906–1909. doi: 10.1126/science.274.5294.1906. [DOI] [PubMed] [Google Scholar]
- 4.Brooks P A, Cockerell G L, Nyborg J K. Activation of BLV transcription by NF-κB and Tax. Virology. 1998;243:94–98. doi: 10.1006/viro.1998.9035. [DOI] [PubMed] [Google Scholar]
- 5.Burgess K E, Yamamoto M, Prasad K V S, Rudd C E. CD5 acts as a tyrosine kinase substrate within a receptor complex comprising T-cell receptor zeta chain/CD3 and protein-tyrosine kinases p56lck and p59fyn. Proc Natl Acad Sci USA. 1992;89:9311–9315. doi: 10.1073/pnas.89.19.9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantor G H, Pritchard S M, Orlik O, Splitter G A, Davis W C, Reeves R. Bovine leukemia virus transmembrane protein gp30 physically associates with the down-regulatory phosphatase SHP-1. Cell Immunol. 1999;193:117–124. doi: 10.1006/cimm.1999.1475. [DOI] [PubMed] [Google Scholar]
- 7.Carmo A M, Mason D W, Beyers A D. Physical association of the cytoplasmic domain of CD2 with the tyrosine kinases p56lck and p59fyn. Eur J Immunol. 1993;23:2196–2201. doi: 10.1002/eji.1830230922. [DOI] [PubMed] [Google Scholar]
- 8.D'Ambrosio D, Hippen K L, Minskoff S A, Mellman I, Pani G, Siminovitch K A, Cambier J C. Recruitment and activation of PTP1C in negative regulation of antigen receptor signaling by FcγRIIB1. Science. 1995;268:293–297. doi: 10.1126/science.7716523. [DOI] [PubMed] [Google Scholar]
- 9.Dennehy K M, Broszeit R, Ferris W F, Beyers A D. Thymocyte activation induces the association of the proto-oncoprotein c-Cbl and Ras GTPase-activating protein with CD5. Eur J Immunol. 1998;28:1617–1625. doi: 10.1002/(SICI)1521-4141(199805)28:05<1617::AID-IMMU1617>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 10.Depelchin A, Letesson J J, Lostrie-Trussart N, Mammerickx M, Portetelle D, Burny A. Bovine leukemia virus (BLV)-infected B-cells express a marker similar to the CD5 T cell marker. Immunol Lett. 1989;20:69–76. doi: 10.1016/0165-2478(89)90071-0. [DOI] [PubMed] [Google Scholar]
- 11.Dequiedt F, Cantor G H, Hamilton V T, Pritchard S M, Davis W C, Kerkhofs P, Burny A, Kettmann R, Willems L. Bovine leukemia virus-induced persistent lymphocytosis in cattle does not correlate with increased ex vivo survival of B lymphocytes. J Virol. 1999;73:1127–1137. doi: 10.1128/jvi.73.2.1127-1137.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dequiedt F, Hanon E, Kerkhofs P, Pastoret P-P, Portetelle D, Burny A, Kettmann R, Willems L. Both wild-type and strongly attenuated bovine leukemia viruses protect peripheral blood mononuclear cells from apoptosis. J Virol. 1997;71:630–639. doi: 10.1128/jvi.71.1.630-639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doody G M, Justement L B, Delibrias C C, Matthews R J, Lin J, Thomas M L, Fearon D T. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science. 1995;269:242–244. doi: 10.1126/science.7618087. [DOI] [PubMed] [Google Scholar]
- 14.Ferrer J F, Marshak R R, Abt D A, Kenyon S J. Relationship between lymphosarcoma and persistent lymphocytosis in cattle: a review. J Am Vet Med Assoc. 1979;175:705–708. [PubMed] [Google Scholar]
- 15.Green N M. Spectrophotometric determination of avidin and biotin. Methods Enzymol. 1970;18A:418–424. [Google Scholar]
- 16.Green P L, Chen I S Y. Molecular features of the human T-cell leukemia virus: mechanisms of transformation and leukemogenicity. In: Levy J A, editor. The Retroviridae. Vol. 3. New York, N.Y: Plenum; 1994. pp. 277–311. [Google Scholar]
- 17.Gringhuis S I, de Leij L F M H, Coffer P J, Vellenga E. Signaling through CD5 activates a pathway involving phosphatidylinositol 3-kinase, Vav, and Rac1 in human mature T lymphocytes. Mol Cell Biol. 1998;18:1725–1735. doi: 10.1128/mcb.18.3.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hailata N, Johnson R, Al-Bagdadi F, Hanash S. Proliferating cell nuclear antigen expression in sheep infected with bovine leukemia virus. Vet Immunol Immunopathol. 1995;44:211–222. doi: 10.1016/0165-2427(94)05319-n. [DOI] [PubMed] [Google Scholar]
- 19.Imboden J B, June C H, McCutcheon M A, Ledbetter J A. Stimulation of CD5 enhances signal transduction by the T cell antigen receptor. J Clin Investig. 1990;85:130–134. doi: 10.1172/JCI114402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.International Committee on Bovine Leukosis. Criteria for the determination of the normal and leukotic state in cattle. JNCI. 1968;41:243–263. [PubMed] [Google Scholar]
- 21.Jamin C, Le Corre R, Lydyard P M, Youinou P. Anti-CD5 extends the proliferative response of human CD5+ B cells activated with anti-IgM and interleukin-2. Eur J Immunol. 1996;26:57. doi: 10.1002/eji.1830260109. [DOI] [PubMed] [Google Scholar]
- 22.Lankester A C, van Schijndel G M W, Cordell J L, van Noesel C J M, van Lier R A W. CD5 is associated with the human B cell antigen receptor complex. Eur J Immunol. 1994;24:812–816. doi: 10.1002/eji.1830240406. [DOI] [PubMed] [Google Scholar]
- 23.Letesson J J, Lostrie-Trussart N, Depelchin A. Production d'anticorps monoclonaux spécifiques d'isotypes d'immunoglobulines bovines. Ann Med Vet. 1985;129:131–141. [Google Scholar]
- 24.Mason D Y, Cordell J L, Tse A G D, Van Dongen J J M, van Noesel C J M, Micklem K, Pulford K A F, Valensi F, Comans-Bitter W M, Borst J, Gatter K C. The IgM-associated protein mb-1 as a marker of normal and neoplastic B cells. J Immunol. 1991;147:2474–2482. [PubMed] [Google Scholar]
- 25.Matheise J P, Delcommenne M, Mager A, Didembourg C H, Letesson J J. CD5+ B cells from bovine leukemia virus infected cows are activated cycling cells responsive to interleukin 2. Leukemia. 1992;6:304–309. [PubMed] [Google Scholar]
- 26.Osman N, Lazarovits A I, Crumpton M J. Physical association of CD5 and the T cell receptor/CD3 antigen complex on the surface of human T lymphocytes. Eur J Immunol. 1993;23:1173–1176. doi: 10.1002/eji.1830230530. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Villar J J, Whitney G S, Bowen M A, Hewgill D H, Aruffo A A, Kanner S B. CD5 negatively regulates the T-cell antigen receptor signal transduction pathway: involvement of SH2-containing phosphotyrosine phosphatase SHP-1. Mol Cell Biol. 1999;19:2903–2912. doi: 10.1128/mcb.19.4.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pers J O, Jamin C, Le Corre R, Lydyard P M, Youinou P. Ligation of CD5 on resting B cells, but not on resting T cells, results in apoptosis. Eur J Immunol. 1998;28:4170–4176. doi: 10.1002/(SICI)1521-4141(199812)28:12<4170::AID-IMMU4170>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 29.Pyeon D, O'Reilly K L, Splitter G A. Increased interleukin-10 mRNA expression in tumor-bearing or persistently lymphocytotic animals infected with bovine leukemia virus. J Virol. 1996;70:5706–5710. doi: 10.1128/jvi.70.8.5706-5710.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pyeon D, Splitter G A. Interleukin-12 p40 mRNA expression in bovine leukemia virus-infected animals: increase in alymphocytosis but decrease in persistent lymphocytosis. J Virol. 1998;72:6917–6921. doi: 10.1128/jvi.72.8.6917-6921.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raab M, Yamamoto M, Rudd C E. The T-cell antigen CD5 acts as a receptor and substrate for the protein-tyrosine kinase p56lck. Mol Cell Biol. 1994;14:2862–2870. doi: 10.1128/mcb.14.5.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reyes R A, Cockerell G L. Increased ratio of bcl-2/bax expression is associated with bovine leukemia virus-induced leukemogenesis in cattle. Virology. 1998;242:184–192. doi: 10.1006/viro.1998.9029. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz-Cornil I, Chevallier N, Belloc C, Le Rhun D, Lainé V, Berthelemy M, Mateo A, Levy D. Bovine leukaemia virus-induced lymphocytosis in sheep is associated with reduction of spontaneous B cell apoptosis. J Gen Virol. 1997;78:153–162. doi: 10.1099/0022-1317-78-1-153. [DOI] [PubMed] [Google Scholar]
- 34.Sen G, Bikah G, Venkataraman C, Bondada S. Negative regulation of antigen receptor-mediated signaling by constitutive asociation of CD5 with the SHP-1 protein tyrosine phosphatase in B-1 B cells. Eur J Immunol. 1999;29:3319–3328. doi: 10.1002/(SICI)1521-4141(199910)29:10<3319::AID-IMMU3319>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 35.Simarro M, Calvo J, Vila J M, Places L, Padilla O, Alberola-Ila J, Vives J, Lozano F. Signaling through CD5 involves acidic sphingomyelinase, protein kinase C-zeta, mitogen-activated protein kinase kinase, and c-Jun NH2-terminal kinase. J Immunol. 1999;162:5149–5155. [PubMed] [Google Scholar]
- 36.Stone D M, Hof A J, Davis W C. Up-regulation of IL-2 receptor α and MHC class II expression on lymphocyte subpopulations from bovine leukemia virus infected lymphocytotic cows. Vet Immunol Immunopathol. 1995;48:65–76. doi: 10.1016/0165-2427(95)05423-4. [DOI] [PubMed] [Google Scholar]
- 37.Stone D M, McElwain T F, Davis W C. Enhanced B-lymphocyte expression of IL-2Rα associated with T lymphocytosis in BLV-infected persistently lymphocytotic cows. Leukemia. 1994;8:1057–1061. [PubMed] [Google Scholar]
- 38.Stone D M, Norton L K, Davis W C. Modulation of bovine leukemia virus-associated spontaneous lymphocyte proliferation by monoclonal antibodies to lymphocyte surface molecules. Clin Immunol Immunopathol. 1997;83:156–164. doi: 10.1006/clin.1997.4340. [DOI] [PubMed] [Google Scholar]
- 39.Stone D M, Norton L K, Magnuson N S, Davis W C. Elevated pim-1 and c-myc proto-oncogene induction in B lymphocytes from BLV-infected cows with persistent B lymphocytosis. Leukemia. 1996;10:1629–1638. [PubMed] [Google Scholar]
- 40.Thurmond M C, Carter R L, Picanso J P, Stralka K. Upper-normal prediction limits of lymphocyte counts for cattle not infected with bovine leukemia virus. Am J Vet Res. 1990;51:466–470. [PubMed] [Google Scholar]
- 41.Trueblood E S, Brown W C, Palmer G H, Davis W C, Stone D M, McElwain T F. B-lymphocyte proliferation during bovine leukemia virus-induced persistent lymphocytosis is enhanced by T-lymphocyte-derived interleukin-2. J Virol. 1998;72:3169–3177. doi: 10.1128/jvi.72.4.3169-3177.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willems L, Grimonpont C, Heremans H, Rebeyrotte N, Chen G, Portetelle D, Burny A, Kettmann R. Mutations in the bovine leukemia virus Tax protein can abrogate the long terminal repeat-directed transactivating activity without concomitant loss of transforming potential. Proc Natl Acad Sci USA. 1992;89:3957–3961. doi: 10.1073/pnas.89.9.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willems L, Heremans H, Chen G, Portetelle D, Billiau A, Burny A, Kettmann R. Cooperation between bovine leukaemia virus transactivator protein and Ha-ras oncogene product in cellular transformation. EMBO J. 1990;9:1577–1581. doi: 10.1002/j.1460-2075.1990.tb08277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willems L, Kettmann R, Chen G, Portetelle D, Burny A, Derse D. A cyclic AMP-responsive DNA-binding protein (CREB2) is a cellular transactivator of the bovine leukemia virus long terminal repeat. J Virol. 1992;66:766–772. doi: 10.1128/jvi.66.2.766-772.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu A, Van Eijk M J T, Park C, Lewin H A. Polymorphism in BoLA-DRB3 exon 2 correlates with resistance to persistent lymphocytosis caused by bovine leukemia virus. J Immunol. 1993;151:6977–6985. [PubMed] [Google Scholar]
- 46.Youinou P, Jamin C, Lydyard P M. CD5 expression in human B-cell populations. Immunol Today. 1999;20:312–316. doi: 10.1016/s0167-5699(99)01476-0. [DOI] [PubMed] [Google Scholar]