Abstract
IMPORTANCE:
A time-limited trial (TLT) is a collaborative plan among clinicians, patients, and families to use life-sustaining therapy for a defined duration, after which the patient’s response informs whether to continue care directed toward recovery or shift the focus toward comfort. TLTs are a promising approach to help navigate uncertainty in critical illness, yet little is known about their current use.
OBJECTIVES:
To characterize TLT use in patients with acute respiratory failure (ARF).
DESIGN, SETTING, AND PARTICIPANTS:
Prospective 12-month observational cohort study at an U.S. academic medical center of adult ICU patients with ARF receiving invasive mechanical ventilation for greater than or equal to 48 hours.
MAIN OUTCOMES AND MEASURES:
Primary exposure was TLT participation, identified by patients’ ICU physician. Patient characteristics, care delivery elements, and hospital outcomes were extracted from the electronic medical record.
RESULTS:
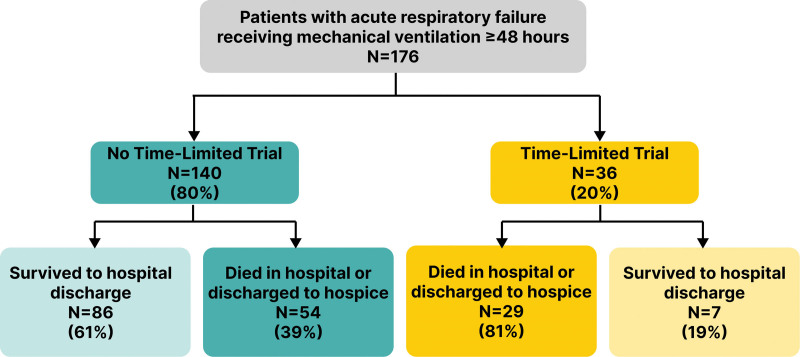
Among 176 eligible patients, 36 (20.5%) participated in a TLT. Among 18 ICU attending physicians, nine (50%) participated in greater than or equal to 1 TLT (frequency 0–39% of patients cared for). Median TLT duration was 3.0 days (interquartile range [IQR], 3.0–4.5 d). TLT patients had a higher mean age (67.4 yr [sd, 12.0 yr] vs. 60.0 yr [sd, 16.0 yr]; p < 0.01), higher Charlson Comorbidity Index (5.1 [sd, 2.2] vs. 3.8 [sd, 2.6]; p < 0.01), and similar Sequential Organ Failure Assessment score (9.6 [sd, 3.3] vs. 9.5 [sd, 3.7]; p = 0.93), compared with non-TLT patients. TLT patients were more likely to die or be discharged to hospice (80.6% vs. 42.1%; p < 0.05) and had shorter ICU length of stay (median, 5.7 d [IQR, 4.0–9.0 d] vs. 10.3 d [IQR, 5.5–14.5 d]; p < 0.01).
CONCLUSIONS AND RELEVANCE:
In this study, approximately one in five patients with ARF participated in a TLT. Our findings suggest TLTs are used primarily in patients near end of life but with substantial physician variation, highlighting a need for evidence to guide optimal use.
Keywords: acute respiratory failure, critical care, mechanical ventilation, medical decision-making, palliative care
KEY POINTS.
Question: How often and in whom are time-limited trials (TLTs) currently used, among adult ICU patients with acute respiratory failure receiving invasive mechanical ventilation?
Findings: In this single-center, prospective cohort study, we found that 20.5% of patients participated in a TLT (as defined by their ICU physician) and 50.0% of attending physicians participated in at least one TLT. TLT patients were significantly older (67.4 vs. 60.0 yr) with higher Charlson Comorbidity Index (5.1 vs. 3.8) compared with non-TLT patients.
Meaning: TLTs are a relatively common approach to care among patients with acute respiratory failure, although with substantial physician-level variation, which may reflect a lack of evidence and/or supports for their optimal use.
Prognostication for critically ill patients with acute respiratory failure (ARF) is fraught with uncertainty (1–3), which contributes to the challenge of decision-making about the use of life-sustaining therapies like invasive mechanical ventilation. Time-limited trials (TLTs) are promoted by experts in palliative and critical care as a specific approach to navigate these uncertain but consequential courses of action (4, 5). A TLT is a collaborative plan among clinicians and a patient and/or their surrogates to try life-sustaining therapy for a defined duration, after which the patient’s response to therapy informs the decision to continue care directed toward recovery, transition to care focused exclusively on comfort, or extend the trial’s duration (6). Many older adults report concurrent goals of extending life when possible and avoiding prolonged life-sustaining therapy when recovery is unlikely and end of life is near (7–11). TLTs hold promise to help patients, families, and clinicians balance these concurrent goals, as evidenced by a large quality improvement project designed to promote TLTs among critically ill patients (12).
Prior research demonstrates that TLTs are part of current practice in some ICUs within the United States (13–16), but little is known about how often and for whom this approach is currently used. A better understanding of the current landscape of TLTs is necessary to inform efforts to improve this approach and, ultimately, to better support critically ill patients, families, and clinicians. Thus, the objectives of this single-center observational study were to: 1) describe the frequency and characteristics of TLTs among patients with ARF, including physician-level variation and 2) compare demographic and hospitalization characteristics of patients who participated in a TLT with those who did not.
MATERIALS AND METHODS
Study Design and Setting
We conducted a prospective, single-center observational cohort study over 12 months (March 1, 2022, to February 28, 2023). The study took place in a medical ICU within an academic medical center located in the Midwestern United States. The University of Wisconsin-Madison Minimal Risk Research Institutional Review Board determined this study was human subjects research exempt from review (Study No. 2021-1413). This report follows the Strengthening the Reporting of Observational Studies in Epidemiology guidelines (17).
Participants
All adults (age ≥ 18) admitted to the medical ICU service with ARF who were receiving invasive mechanical ventilation were eligible and enrolled unless they were excluded, as described below. Given the heterogeneous nature of critical illnesses and the variation in how and when TLTs are applied in different clinical scenarios, we chose to scope the population for this study to patients with ARF receiving invasive mechanical ventilation, which is one of the most common reasons for ICU admission in the United States (18–20). We defined ARF as the presence of hypoxemia (a ratio of Pao2 [or imputed from oxygen saturation (21, 22)] to fractional inspired oxygen < 300) and/or hypercapnia (a Paco2 > 45 mm Hg). We excluded patients receiving chronic invasive mechanical ventilation before their hospital admission, those who died or were extubated before 48 hours of mechanical ventilation, and those previously enrolled during the same admission. We also excluded patients with missing data about whether or not a TLT was being conducted (see next section for details).
Time-Limited Trial Identification and Characterization
The primary exposure of interest was participation in a TLT, as defined and identified by an affirmative response to a systematic query sent to each attending ICU physician treating an eligible patient. This query included a consensus definition of a TLT followed by the question, “Is this patient receiving a time-limited trial? Please respond: Yes/No/Other.” Before study initiation, participating attending physicians were notified about the query procedure. We emphasized that the query purpose was to identify TLTs already initiated by the clinical team and that the query was not meant to prompt or encourage the physician to use this approach. Electronic queries were sent to attending ICU physicians via health record secure chat or professional email. Nonresponders were sent a reminder at 24 hours, followed by a query to the ICU fellow. If a negative or no response was received, the query procedure was repeated every 7 days until the patient was discharged from the ICU or no longer eligible (e.g., extubated). To align with feedback from study-site physicians that typically greater than or equal to 48 hours are required to initiate a TLT (accounting for initial stabilization, diagnostics, and discussion with patient and/or surrogate), we first queried ICU physicians 48 hours after a patient was initiated on mechanical ventilation. Thus, only patients who survived and were not extubated within 48 hours were eligible for cohort inclusion. To identify TLTs initiated between the 7-day query intervals, flyers were posted in the study unit asking any ICU staff to notify the research team of patients potentially receiving a TLT. Received notifications were followed-up with a confirmation query to the ICU physician.
We collected plan characteristics for patients participating in a TLT through a brief electronic survey sent to the identifying ICU physician at the time of their affirmative query response. The survey asked for: 1) the planned duration of the TLT (in d), 2) the criteria being used to evaluate whether or not the patient is getting better at the end of the TLT (open-ended question with free-text response box), and 3) who the attending had discussed the TLT with (categorical selection). We also reviewed the electronic health record and abstracted all verbatim sections of text referencing the TLT.
Clinical Data Collection
Research staff abstracted de-identified demographic characteristics, clinical data, and hospital outcomes from the electronic health record for all enrolled patients. Clinical data included their admission source(s), Sequential Organ Failure Assessment (SOFA) score from the 24 hours preceding the initiation of mechanical ventilation (or first 24 hr of admission if transferred from another hospital while receiving ventilation), Charlson Comorbidity Index (CCI) before ICU admission, code status order at ICU admission and any changes during their ICU stay, advance directive documentation at the time of ICU admission, and whether the patient experienced a cardiac arrest immediately preceding ICU admission. We also collected data about care delivered during their hospitalization, including additional life-sustaining therapies, tracheostomy and/or gastrostomy, and palliative care consultation. Hospital outcomes included survival to hospital discharge, discharge location for survivors, hospital and ICU length of stay, and duration of invasive mechanical ventilation.
For the subset of enrolled patients who died during hospitalization, we collected measures of end-of-life care (23–25), including: pain and delirium in the last 24 hours of life, comfort care orders at the time of death, cardiopulmonary resuscitation (CPR) within 1 hour of death, documentation of spiritual support, and family presence at time of death.
Statistical Analysis
We used descriptive statistics to evaluate the frequency of TLTs within the cohort and to summarize patients’ demographic and clinical characteristics and hospital outcomes. To examine differences in characteristics between patients who participated in a TLT and those who did not, we used t tests or Wilcoxon rank-sum tests and chi-square tests or Fisher exact tests, as appropriate. We repeated the analysis after restricting the cohort to decedents (defined as those patients who died in hospital or were discharged to hospice [inpatient or home]), to evaluate whether any observed differences in the TLT and non-TLT groups could be explained by a propensity to use this approach in patients at high risk of death. We also used this restricted cohort to examine the impact of TLTs on end-of-life care delivery. We used conventional content analysis (26) to analyze the open-ended responses from ICU physicians about the criteria used to evaluate the patient’s response to therapy during the TLT, first deriving categories from the free-text data and then assigning one or more categories to each physician response.
We organized study data using the University of Wisconsin Research Electronic Data Capture tools (14, 15). Statistical analyses were conducted using SAS software (Version 9.4, SAS Institute, Cary, NC). p values of less than 0.05 were considered statistically significant.
RESULTS
Frequency and Characteristics of Time-Limited Trials
We received ICU physician query responses for 176 of 200 eligible patients (88% response rate). A total of 36 (20.5%) of the 176 cohort patients with ARF participated in a physician-identified TLT (Fig. 1). Of the 18 ICU attending physicians who cared for enrolled patients, nine (50%) participated in at least one TLT during the study period (range, 0–7) and the frequency of TLT use by individual physician ranged from 0% to 39% of the patients they cared for (Fig. 2). eFigure 1 and eTable (http://links.lww.com/CCX/B395) illustrate the monthly rate of TLTs among cohort patients over the course of the study demonstrating no apparent trends over time.
Figure 1.
The frequency and outcomes of time-limited trials among patients with acute respiratory failure receiving invasive mechanical ventilation.
Figure 2.
Number of time-limited trials (TLTs) conducted per attending ICU physician in relationship to their total number of cohort patients cared for. Individual ICU physicians are ordered along the X-axis from the highest to lowest number of TLTs conducted.
The median TLT duration was 3.0 days (full range, 1.3–14.0; interquartile range, 3.0–4.5). We identified five major categories of criteria used by ICU physicians to evaluate a patient’s response to therapy during the TLT (Table 1). Of the 36 TLTs, 23 (63.9%) had at least one instance of health record documentation specifically referencing the trial. Almost all documentation was within ICU physician and advanced practice provider progress and family conference notes, but we additionally found occasional TLT documentation by other specialties (cardiology, infectious disease, and palliative care) and professions (dietitian, nursing, social work, and speech and language pathology). ICU physicians reported personally discussing the TLT with patients in 11% of trials (4/35, one trial with missing data), designated surrogate decision makers in 94% (33/35), and other family, friends, or caregivers in 46% (16/35). Physicians also reported discussing the TLT with nurses in 71% (25/35), non-ICU consultants/specialists in 31% (11/35), ICU pharmacists in 26% (9/35), respiratory therapists in 20% (7/35), social workers or case management in 11% (4/35), primary care providers in 11% (4/35), and chaplains in 3% (1/35) of cases.
TABLE 1.
Criteria Used to Evaluate Patients’ Response to Therapy During a Time-Limited Trial
| Major Categories of Criteria | Verbatim Examples | Frequency of Category Usea |
|---|---|---|
| % of Time-Limited Trialsb (n) | ||
| Status of a specific life-sustaining therapy | “Able to extubate” | 54% (19) |
| “Off vasopressors” | ||
| Clinical evaluation of neurologic function | “Mental status” | 37% (13) |
| “More awake” | ||
| Physiologic measures | “Improved ratio of Pao2/Fio2” | 29% (10) |
| “Hemodynamic stability” | ||
| General improvement | “Improvement in acute respiratory distress syndrome, delirium” | 17% (6) |
| “Response to lung-protective ventilation, steroids, diuresis” | ||
| Results of neurologic imaging/testing | “Electroencephalogram” | 17% (6) |
| “Brain imaging” |
The sum of percentages is > 100%, as more than one category of criteria could be used in each time-limited trial (TLT).
One TLT had no attending response to criteria question, thus percentages are calculated out of 35 TLTs.
Comparison of Patient Characteristics
We found several differences in demographic and clinical characteristics at the time of ICU admission between patients who participated in a TLT and those who did not (Table 2). TLT patients tended to be older age (67.4 yr [sd, 12.0 yr] vs. 60.0 yr [sd, 16.0 yr]; p < 0.01) and had higher CCI scores at the time of ICU admission, indicating a greater degree of comorbidity (5.1 [sd, 2.2] vs. 3.8 [sd, 2.6]; p < 0.01). However, the severity of acute illness measured by SOFA score was similar in the two groups at the initiation of invasive mechanical ventilation (9.6 [sd, 3.3] vs. 9.5 [sd, 3.7]; p = 0.93). A higher proportion of patients in the TLT group experienced a cardiac arrest immediately preceding the ICU admission (27.8% vs. 12.1%; p = 0.02) and had limitations on code status (e.g., no CPR, ok to intubate) at ICU admission (30.6% vs. 14.3%; p = 0.02).
TABLE 2.
A Comparison of Patient Characteristics at the Time of ICU Admission Between Patients Who Participated in a Time-Limited Trial and Those Who Did Not
| Characteristic | Full Cohort, n = 176 | Decedents Only, n = 86 | ||||
|---|---|---|---|---|---|---|
| No Time-Limited Trial, n = 140 | Time-Limited Trial, n = 36 | p e | No Time-Limited Trial, n = 57 | Time-Limited Trial, n = 29 | p e | |
| Demographic characteristics | ||||||
| Age, yr, mean (sd) | 60.0 (16.0) | 67.4 (12.0) | < 0.01 | 60.5 (15.9) | 65.7 (11.3) | 0.08 |
| Female sex, n (%) | 55 (39.3) | 12 (33.3) | 0.51 | 22 (38.6) | 11 (37.9) | 0.95 |
| Racea,b, n (%) | 0.73 | 0.62 | ||||
| American Indian or Alaska Native | 2 (1.4) | 0 (0.0) | 2 (3.5) | 0 (0.0) | ||
| Asian | 3 (2.4) | 0 (0.0) | 2 (3.5) | 0 (0.0) | ||
| Black or African-American | 11 (7.9) | 3 (8.3) | 6 (10.5) | 3 (10.3) | ||
| White | 121 (86.4) | 31 (86.1) | 46 (65.7) | 24 (82.8) | ||
| Otherc | 4 (2.9) | 2 (5.6) | 1 (1.8) | 2 (6.9) | ||
| Ethnicity, n (%) | 0.58 | > 0.99 | ||||
| Not Hispanic/Latinxc | 136 (97.1) | 36 (100.0) | 56 (98.3) | 29 (100.0) | ||
| Hispanic/Latinx | 4 (2.9) | 0 (0.0) | 1 (1.8) | 0 (0.0) | ||
| Clinical characteristics | ||||||
| Hospital admission source, n (%) | 0.71 | 0.61 | ||||
| Home | 83 (59.3) | 20 (55.6) | 35 (61.4) | 15 (51.7) | ||
| Outside acute care hospital | 30 (21.4) | 10 (27.8) | 14 (24.6) | 10 (34.5) | ||
| Other type of medical facility | 27 (19.3) | 6 (16.7) | 8 (14.0) | 4 (13.8) | ||
| ICU admission source, n (%) | 0.42 | 0.34 | ||||
| Emergency department | 63 (45.0) | 13 (36.1) | 24 (42.1) | 9 (31.0) | ||
| Hospital ward | 53 (37.9) | 13 (36.1) | 22 (38.6) | 16 (55.2) | ||
| Outside hospital | 24 (17.1) | 5 (13.9) | 11 (19.3) | 4 (13.8) | ||
| Charlson Comorbidity Index, mean (sd) | 3.8 (2.6) | 5.1 (2.2) | < 0.01 | 4.4 (2.8) | 5.2 (2.2) | 0.19 |
| Prior history of solid organ transplant, n (%) | 9 (6.4) | 2 (5.6) | > 0.99 | 5 (8.8) | 2 (6.9) | > 0.99 |
| Prior history of stem cell/bone marrow transplant, n (%) | 5 (3.6) | 2 (5.6) | 0.63 | 2 (3.5) | 2 (6.9) | 0.60 |
| Cardiac arrest preceding ICU stayd, n (%) | 17 (12.1) | 10 (27.8) | 0.02 | 9 (15.8) | 10 (34.5) | 0.05 |
| Sequential Organ Failure Assessment score, mean (sd) | 9.5 (3.7) | 9.6 (3.3) | 0.93 | 10.4 (3.9) | 9.8 (3.5) | 0.54 |
| Documented advance directive, n (%) | 106 (75.7) | 28 (80.0) | 0.59 | 42 (73.7) | 22 (78.6) | 0.62 |
| Limitation on code status at ICU admission, n (%) | 20 (14.3) | 11 (30.6) | 0.02 | 9 (15.8) | 7 (24.1) | 0.35 |
As recorded/classified in the electronic health record.
Totals can be > 100% as some patients had more than one recorded race.
Includes unknown, missing, or declined to answer.
Immediately preceding this ICU admission, including out of hospital immediately before emergency department arrival, in emergency department, or on hospital ward before ICU admission.
When comparing the time-limited trial (TLT) group to the non-TLT group, by χ2 or Wilcoxon rank-sum test.
Comparison of Hospital Care Delivery and Outcomes
Table 3 illustrates patterns in hospital care delivery and outcomes for TLT and non-TLT patients. The frequency of additional life-sustaining therapies was similar between both groups, with the exception of more frequent use of vasopressors in the TLT group (75.0% of patients vs. 53.6%; p = 0.02). In the TLT group, three patients (8.3%) underwent tracheostomy and 2 (5.6%) underwent gastrostomy, compared with 29 (20.7%) and 11 (8.0%) in the non-TLT group, respectively (p = 0.09 for tracheostomy; p > 0.99 for gastrostomy). The frequency of palliative care consultation was similar between the groups (16.7% in TLT patients, 13.6% in non-TLT patients; p = 0.64).
TABLE 3.
A Comparison of Hospital Care Delivery and Outcomes Between Patients Who Participated in a Time-Limited Trial and Those Who Did Not
| Characteristic or Outcome | Full Cohort, n = 176 | Decedents Only, n = 86 | ||||
|---|---|---|---|---|---|---|
| No Time-Limited Trial, n = 140 | Time-Limited Trial, n = 36 | p h | No Time-Limited Trial, n = 57 | Time-Limited Trial, n = 29 | p h | |
| Characteristics of care delivery | ||||||
| Additional life-sustaining therapy, n (%) | ||||||
| Renal replacement therapy | 26 (18.6) | 8 (22.2) | 0.62 | 14 (24.6) | 7 (24.1) | 0.97 |
| Vasopressors | 75 (53.6) | 27 (75.0) | 0.02 | 38 (66.7) | 23 (79.3) | 0.22 |
| Extracorporeal membrane oxygenationa, n (%) | 1 (0.7) | 0 (0.0) | > 0.99 | 1 (1.8) | 0 (0.0) | > 0.99 |
| Tracheostomy during hospitalization, n (%) | 29 (20.7) | 3 (8.3) | 0.09 | 7 (12.3) | 2 (6.9) | 0.71 |
| Gastrostomy during hospitalization, n (%) | 11 (8.0) | 2 (5.6) | > 0.99 | 0 (0.0) | 2 (6.9) | 0.11 |
| Change in code status during hospitalization, n (%) | 57 (40.7) | 30 (83.3) | < 0.01 | 51 (89.5) | 26 (89.7) | > 0.99 |
| Palliative care consultation during hospitalization, n (%) | 19 (13.6) | 6 (16.7) | 0.64 | 15 (26.3) | 6 (20.7) | 0.61 |
| Location of death | — | — | — | > 0.99 | ||
| ICU | — | — | — | 43 (79.6) | 24 (82.8) | |
| General care hospital unit | — | — | — | 4 (7.4) | 2 (6.9) | |
| Palliative care unit | — | — | — | 7 (13.0) | 3 (10.3) | |
| End-of-life care measures | ||||||
| Number of significant pain episodes in last 24 hr,b mean (sd) | — | — | — | 0.9 (0.2) | 1.1 (0.4) | 0.92 |
| Number of delirium episodes in last 24 hr,c mean (sd) | — | — | — | 2.2 (0.3) | 2.0 (0.4) | 0.66 |
| Comfort care orders at time of death, n (%) | — | — | — | 46 (85.2) | 27 (93.1) | 0.48 |
| Cardiopulmonary resuscitation within 1 hr of death, n (%) | — | — | — | 0 (0.0) | 0 (0.0) | — |
| Mechanically ventilated at the time of death, n (%) | — | — | — | 9 (16.7) | 3 (10.3) | 0.53 |
| Death by neurologic criteria, n (%) | — | — | — | 2 (3.7) | 0 (0.0) | 0.54 |
| Family presence at the time of death, n (%) | — | — | — | 43 (79.6) | 21 (72.4) | 0.70 |
| Documentation of at least one family conference during ICU stay, n (%) | — | — | — | 43 (79.6) | 25 (86.2) | 0.46 |
| Documentation of spiritual care/chaplain consultation during ICU stay, n (%) | — | — | — | 41 (75.9) | 23 (79.3) | 0.73 |
| Hospitalization outcomes | ||||||
| Total days of invasive mechanical ventilationd, median (IQR) | 7.8 (4.5–12.8) | 5.3 (3.5–10.3) | 0.06 | 7.0 (3.5–12.0) | 5.5 (4.0–10.0) | 0.23 |
| Total length of ICU stay, de, median (IQR) | 10.3 (5.5–14.5) | 5.7 (4.0–9.0) | < 0.01 | 8.5 (5.5–16.5) | 6.5 (4.5–9.0) | 0.02 |
| Total length of hospital stay, d, median (IQR) | 17.3 (10.5–32.5) | 14.0 (8.3–22.5) | 0.11 | 13.5 (7.0–32.5) | 14.5 (6.5–22.5) | 0.53 |
| In-hospital death, n (%) | 54 (38.6) | 29 (80.6) | < 0.01 | — | — | — |
| Discharge destination, n (%) | 0.26 | — | — | — | ||
| Hospice (home or inpatient)f, n (%) | 3 (3.5) | 0 (0.0) | — | — | — | |
| Long-term acute care hospital, n (%) | 18 (20.9) | 0 (0.0) | — | — | — | |
| Skilled nursing facility, n (%) | 17 (19.7) | 2 (28.6) | — | — | — | |
| Acute inpatient rehabilitation, n (%) | 6 (7.0) | 2 (28.6) | — | — | — | |
| Home, n (%) | 36 (41.9) | 2 (28.6) | — | — | — | |
| Otherg, n (%) | 6 (7.0) | 1 (14.3) | — | — | — | |
IQR = interquartile range.
Includes venoarterial and venovenous extracorporeal membrane oxygenation.
In the last 24 hr of life and among patients with at least one documented pain assessment. Significant pain was defined as either a Critical Care Pain Observation Tool score > 2, Numerical Rating Scale score > 3, or Checklist of Nonverbal Pain Indicators score ≥ 1.
In the last 24 hr of life and among patients who died in the ICU and had a Confusion Assessment Method for ICU (CAM-ICU) assessment in the last 24 hr of life. An episode of delirium was defined as a positive CAM-ICU rating.
Total amount of time receiving invasive mechanical ventilation during hospital stay, rounded to nearest 0.5 d. This could include more than one distinct episode of ventilation.
Total amount of time spent admitted to an ICU service (as determined by admit/transfer/discharge orders) during hospitalization, rounded to the nearest 0.5 d. This could include more than one distinct ICU admission.
Also includes patients who are discharged to other facilities with concurrent hospice care (e.g., to a skilled nursing facility with hospice).
Other includes patient-directed discharge (i.e., discharge against medical advice), discharge to another acute care hospital (e.g., back to referring hospital), and discharge to inpatient psychiatric care.
When comparing the time-limited trial (TLT) group to the non-TLT group, by χ2 or Wilcoxon rank-sum test.Dashes indicate the characteristic or outcome was not applicable to that cohort subgroup.
The median ICU length of stay was shorter in the TLT group compared with the non-TLT group (5.7 vs. 10.3 d; p < 0.01; Fig. 3) and the duration of invasive mechanical ventilation was 5.3 days in the TLT group compared with 7.8 days in the non-TLT group (p = 0.06). A higher proportion of patients in the TLT group either died during the hospital stay or were discharged to hospice (80.6% vs. 42.1%; p < 0.01).
Figure 3.
The distribution of ICU length of stay for patients who participated in a time-limited trial (TLT) compared with those who did not. A, The distribution for the full cohort. B, The distribution for the restricted cohort of decedents.
Restricted Analysis With Decedents
After restricting the analytic cohort to decedents (defined as patients who died in the hospital or were discharged to hospice), the observed differences in ICU admission characteristics between patients who participated in a TLT and those who did not were no longer statistically significant (Table 2). Most characteristics of hospital care delivery were similar between decedents who participated in a TLT and those who did not, including metrics of end-of-life care delivery (Table 3). However, the ICU length of stay for TLT decedents was shorter than non-TLT decedents (6.5 vs. 8.5 d; p = 0.02), as was the duration of mechanical ventilation, which was 5.5 days in the TLT decedents compared with 7.0 in the non-TLT decedents (p = 0.23).
DISCUSSION
In this single-center, prospective observational study in a Midwestern medical ICU, approximately one in five patients with ARF received care according to a “time-limited trial” approach, as defined by their ICU physician. Only half of ICU physicians used this approach during the study, and the frequency of TLT use by individual physicians varied substantially. Patients with ARF participating in a TLT tended to be older and have greater comorbidity before admission but had similar acute severity of illness measured by SOFA score compared with patients who did not. Our findings also suggest that TLT patients receive similar (if not slightly more) life-sustaining therapies but for a shorter duration, as indicated by the overall shorter ICU length of stay. When we focused the comparison only on those patients who ultimately died during hospitalization or were discharged to hospice, the differences in patient characteristics and care delivery between the TLT and non-TLT groups were no longer apparent. However, decedents who participated in a TLT still had a shorter ICU length of stay compared with those who did not. Overall, these findings suggest that the current use of TLTs aligns with a fundamental premise of the approach—using life-sustaining therapies to extend life when possible while avoiding their prolonged use once end of life is near (6, 10, 11).
This study adds to the growing body of evidence demonstrating this structured approach to care known as a “time-limited trial” has become part of usual care in ICUs across the United States (13, 14, 16). While the optimal frequency of TLTs remains unknown, prior qualitative studies with purposive samples identified this approach in 8–27% of studied patients (13, 14). To our knowledge, this is the first study to prospectively and systematically identify TLTs within mechanically ventilated ICU patients with ARF, and our results suggest TLTs are used fairly often—in 20% of these patients. Strikingly, our study found that more than 80% of patients participating in a TLT went on to die during their hospital stay. This finding is not surprising, as TLTs are often implicitly and explicitly framed as an approach for patients with “poor prognosis” who are near the end of life (4, 12, 27). Yet, a 2024 consensus report from the American Thoracic Society emphasizes that TLTs should be framed as an approach for patients with prognostic “uncertainty” and not only for those with “near certain” poor prognosis (6). Thus, our observation that TLTs are currently being used almost exclusively in patients who die during hospitalization suggests an underuse of this recommended approach in patients with “uncertainty” about the outcome of their critical illness.
Our results also build upon prior work suggesting important clinical practice variation in the current use of TLTs (14, 15, 28). For example, in a prior ethnographic study comparing two ICUs, Barnato et al (13) found that TLT use varied between ICUs, with higher TLT frequency in the unit that tended to provide lower-intensity end-of-life care. In this study, we demonstrate that this clinical practice variation extends beyond the unit-level and further includes variation between individual physicians within the same ICU. We observed, in clinical practice, that some ICU physicians are TLT adopters while others never use this approach. These findings are consistent with a survey study by Viglianti et al (28) in which ICU physicians reported variation in their perceptions of the TLT approach. We additionally identified variation in how these trials were conducted among the TLT adopters, with durations ranging from 1 to 14 days, criteria evaluating a patient’s response to therapy ranging from specific physiologic measurements (e.g., ratio of Pao2/Fio2) to general assessments (e.g., improvement in acute respiratory distress syndrome) and variable communication about the TLT among ICU team roles. The difficulty of changing physician behavior has been well documented in the field of implementation science (29), even for critical care practices like low tidal volume ventilator management with a strong evidence base (30). Individual critical care physicians also display marked variation in their tendency to adopt new practices (31). This understanding of physician behavior change helps explain why we found little evidence of an observer effect from our study queries and why we saw variation of TLT adoption among the study ICU physicians. Reconciling this variation in clinical practice and understanding to what extent it is unwarranted will require more and rigorous evidence about whether and how TLTs impact outcomes for patients, surrogates, and clinicians.
This study has limitations. We conducted this study in a single academic medical center, so our findings may not generalize to other settings. Our estimate of TLT frequency is within the range of estimates derived from qualitative studies in academic settings in the United States, which increases the likelihood that our estimate reflects other academic practices. Yet, it remains unknown the extent to which these findings reflect practices in nonacademic settings and those outside of the United States. This study also focused on adult patients with ARF receiving invasive mechanical ventilation, and it is unknown whether our findings will extend to other patient populations. Additionally, we identified TLTs in the study by directly querying ICU physicians. Direct observation is the only feasible approach to identify TLTs prospectively and systematically in clinical practice, given there is no electronic health record signature or other consistent artifact to establish whether or not a TLT is taking place. This direct physician query could have contributed to an observer effect whereby physicians increased their use of TLTs during the study, but examining the overall and individual attending rate of TLT use by month over the course of the study we found neither a trend of increased use over time nor a “spike” in reported use at the onset of the study. This study only captured whether a TLT was initiated for an enrolled patient and the initial plan characteristics. Ongoing work in our group is focusing on investigating the extent to which the essential elements of a TLT are actually carried out as planned. We also did not examine whether and how the patients, their surrogate decision makers, and the other members of the ICU team understood and experienced the TLT. Future work should focus on additional patient populations as well as understanding how TLTs are perceived by all those affected by the use of this approach to ICU care.
CONCLUSIONS
In this study, approximately one in five ICU patients with ARF participated in a TLT, as defined by their ICU physician. We found that TLTs are primarily used in patients near the end of life, but with substantial physician-level variation in the use of this approach. These findings highlight a need for more robust evidence to guide the optimal use of TLTs.
Supplementary Material
Footnotes
Dr. Kruser was supported, in part, by National Institutes of Health/National Heart, Lung, and Blood Institute grant K23HL146890 and R01HL168474. Dr. Kruser’s spouse receives honoraria for lectures and speakers bureaus from Astra Zeneca. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Contributor Information
Joy X. Moy, Email: jmoy@medicine.wisc.edu.
Anica C. Law, Email: anicalaw@bu.edu.
Lily N. Stalter, Email: stalter@surgery.wisc.edu.
Michael D. Peliska, Email: Michael.Peliska@hcmed.org.
Geralyn Palmer, Email: GPalmer@uwhealth.org.
Bret M. Hanlon, Email: bmhanlon@wisc.edu.
Sean Mortenson, Email: SMortenson@uwhealth.org.
Elizabeth M. Viglianti, Email: eviglian@med.umich.edu.
Douglas A. Wiegmann, Email: dawiegmann@wisc.edu.
REFERENCES
- 1.Meadow W, Pohlman A, Frain L, et al. : Power and limitations of daily prognostications of death in the medical intensive care unit. Crit Care Med 2011; 39:474–479 [DOI] [PubMed] [Google Scholar]
- 2.Meadow W, Pohlman A, Reynolds D, et al. : Power and limitations of daily prognostications of death in the medical ICU for outcomes in the following 6 months. Crit Care Med 2014; 42:2387–2392 [DOI] [PubMed] [Google Scholar]
- 3.Detsky ME, Harhay MO, Bayard DF, et al. : Discriminative accuracy of physician and nurse predictions for survival and functional outcomes 6 months after an ICU admission. JAMA 2017; 317:2187–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quill TE, Holloway R: Time-limited trials near the end of life. JAMA 2011; 306:1483–1484 [DOI] [PubMed] [Google Scholar]
- 5.Vink EE, Azoulay E, Caplan A, et al. : Time-limited trial of intensive care treatment: An overview of current literature. Intensive Care Med 2018; 44:1369–1377 [DOI] [PubMed] [Google Scholar]
- 6.Kruser JM, Ashana DC, Courtright KR, et al. : Defining the time-limited trial for patients with critical illness: An official American Thoracic Society Workshop Report. Ann Am Thorac Soc 2024; 21:187–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger JT, Majerovitz D: Stability of preferences for treatment among nursing home residents. Gerontologist 1998; 38:217–223 [DOI] [PubMed] [Google Scholar]
- 8.Givens JL, Sudore RL, Marshall GA, et al. : Advance care planning in community-dwelling patients with dementia. J Pain Symptom Manage 2018; 55:1105–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patrick DL, Pearlman RA, Starks HE, et al. : Validation of preferences for life-sustaining treatment: Implications for advance care planning. Ann Intern Med 1997; 127:509–517 [DOI] [PubMed] [Google Scholar]
- 10.Cox CE, White DB, Hough CL, et al. : Effects of a personalized web-based decision aid for surrogate decision makers of patients with prolonged mechanical ventilation: A randomized clinical trial. Ann Intern Med 2019; 170:285–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auriemma CL, Harhay MO, Haines KJ, et al. : What matters to patients and their families during and after critical illness: A qualitative study. Am J Crit Care 2021; 30:11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang DW, Neville TH, Parrish J, et al. : Evaluation of time-limited trials among critically ill patients with advanced medical illnesses and reduction of nonbeneficial ICU treatments. JAMA Intern Med 2021; 181:786–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnato AE, Tate JA, Rodriguez KL, et al. : Norms of decision making in the ICU: A case study of two academic medical centers at the extremes of end-of-life treatment intensity. Intensive Care Med 2012; 38:1886–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schenker Y, Tiver GA, Hong SY, et al. : Discussion of treatment trials in intensive care. J Crit Care 2013; 28:862–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruce CR, Liang C, Blumenthal-Barby JS, et al. : Barriers and facilitators to initiating and completing time-limited trials in critical care. Crit Care Med 2015; 43:2535–2543 [DOI] [PubMed] [Google Scholar]
- 16.Goss AL, Voumard RR, Engelberg RA, et al. : Do they have a choice? Surrogate decision-making after severe acute brain injury. Crit Care Med 2023; 51:924–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, et al. ; STROBE Initiative: The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann Intern Med 2007; 147:573–577 [DOI] [PubMed] [Google Scholar]
- 18.Rubenfeld GD, Caldwell E, Peabody E, et al. : Incidence and outcomes of acute lung injury. N Engl J Med 2005; 353:1685–1693 [DOI] [PubMed] [Google Scholar]
- 19.Bellani G, Laffey JG, Pham T, et al. ; LUNG SAFE Investigators: Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016; 315:788–800 [DOI] [PubMed] [Google Scholar]
- 20.Cartin-Ceba R, Kojicic M, Li G, et al. : Epidemiology of critical care syndromes, organ failures, and life-support interventions in a suburban US community. Chest 2011; 140:1447–1455 [DOI] [PubMed] [Google Scholar]
- 21.Zeiberg D, Prahlad T, Nallamothu BK, et al. : Machine learning for patient risk stratification for acute respiratory distress syndrome. PLoS One 2019; 14:e0214465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown SM, Duggal A, Hou PC, et al. ; National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) Prevention and Early Treatment of Acute Lung Injury (PETAL) Network: Nonlinear imputation of PaO2/FIO2 from SpO2/FIO2 among mechanically ventilated patients in the ICU: A prospective, observational study. Crit Care Med 2017; 45:1317–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kruser JM, Aaby DA, Stevenson DG, et al. : Assessment of variability in end-of-life care delivery in intensive care units in the United States. JAMA Netw Open 2019; 2:e1917344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mularski RA, Curtis JR, Billings JA, et al. : Proposed quality measures for palliative care in the critically ill: A consensus from the Robert Wood Johnson Foundation Critical Care Workgroup. Crit Care Med 2006; 34:S404–S411 [DOI] [PubMed] [Google Scholar]
- 25.Nelson JE, Puntillo KA, Pronovost PJ, et al. : In their own words: Patients and families define high-quality palliative care in the intensive care unit. Crit Care Med 2010; 38:808–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsieh HF, Shannon SE: Three approaches to qualitative content analysis. Qual Health Res 2005; 15:1277–1288 [DOI] [PubMed] [Google Scholar]
- 27.Cheung EH-L, Cheung JC-H, Yip Y-Y: Raising awareness for time-limited trial discussion upon ICU triage and admission. Intensive Care Med 2022; 48:240–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viglianti EM, Ervin JN, Newton CA, et al. : Time-limited trials in the ICU: A mixed-methods sequential explanatory study of intensivists at two academic centres. BMJ Open 2022; 12:e059325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grimshaw JM, Eccles MP, Walker AE, et al. : Changing physicians’ behavior: What works and thoughts on getting more things to work. J Contin Educ Health Prof 2002; 22:237–243 [DOI] [PubMed] [Google Scholar]
- 30.Weiss CH: Why do we fail to deliver evidence-based practice in critical care medicine? Curr Opin Crit Care 2017; 23:400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss CH, Poncela-Casasnovas J, Glaser JI, et al. : Adoption of a high-impact innovation in a homogeneous population. Phys Rev X 2014; 4:041008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.