Abstract
Background
An association between increased aortic root dimensions (ARD) and elevated risk of cardiovascular mortality has been reported in the general population. However, evidence regarding the association between ARD and mortality in patients with acute heart failure (AHF) is limited.
Methods
In a nationwide prospective cohort of the China Patient-Centered Evaluative Assessment of Cardiac Events Prospective Heart Failure Study, ARD was measured during diastole using echocardiography and indexed to body mass index (BMI). Cox proportional hazard models were used to validate the association between BMI-indexed ARD and mortality. Additionally, the relationship between BMI-indexed ARD and mortality was presented using restricted cubic spline in all populations, and both sexes.
Results
A total of 2125 participants with ARD were included in the final analysis, among of 38.4 % were women, with a median age of 67 years. Over a median follow-up period of 54.4 (interquartile range: 30.1 to 59.7) months, 895 deaths occurred, with 750 attributed to cardiovascular causes and 145 to non-cardiovascular causes. Compared to the highest tertile group of BMI-indexed ARD, the lowest tertile group had a lower risk of cardiovascular mortality (hazard ratio [HR], 0.71; 95 % confidence interval [CI], 0.58 to 0.87; P < 0.001) and all-cause mortality (HR, 0.68; 95 % CI, 0.56 to 0.81; P < 0.001). Similarly, the middle tertile group also had a lower risk of cardiovascular mortality (HR, 0.78; 95 % CI, 0.65 to 0.93; P = 0.007) and all-cause mortality (HR, 0.75; 95 % CI, 0.63 to 0.89; P < 0.001). Considering the competing risks, the lowest BMI-indexed ARD groups showed a significant mortality risk of cardiovascular mortality in all populations, and both sexes. Moreover, the relationship between BMI-indexed ARD and mortality was linear in males, while a “J” shaped relationship was observed in females.
Conclusion
Lower BMI-indexed ARD was associated with a decreased risk of all-cause and cardiovascular mortality than those with higher BMI-indexed ARD in AHF. Additionally, a discrepancy was observed between the sexes in the relationship between BMI-indexed ARD and mortality. These findings contribute to the prompt identification of potential mortality risks in patients with AHF.
Keywords: Aortic root, Sex, Echocardiography, Mortality, Acute heart failure, Predictive
1. Introduction
The aorta is the largest artery in the human body and plays a critical role as a conduit for controlling the systemic vascular resistance and heart rate. Additionally, its elasticity additionally acts as a ‘secondary pump’ during diastole [1,2]. Aortic root dilation is a common clinical condition often influenced by comorbidities and age [1,3]. Although typically asymptomatic, studies indicate that increased aortic root dimension (ARD) not only increases the risk of aortic diseases such as dissection and aneurysm rupture but also increases the risk of cardiovascular diseases and mortality within the general population [2,[4], [5], [6], [7]]. While research on the association between ARD and mortality risk is relatively scarce, existing longitudinal studies have suggested a correlation between ARD, cardiovascular disease, and mortality risk in the general population [[8], [9], [10], [11]]. However, in populations with acute heart failure (AHF), which commonly present with comorbidities such as coronary heart disease and hypertension, there is often a higher risk of readmission and mortality. To the best of our knowledge, few studies have explored the association between ARD and cardiovascular-related outcomes in patients with AHF.
The differences in ARD between sexes were significant but unrelated to race [3,12,13]. ARD is smaller in women than men, with a lower incidence but faster growth rates of thoracic aortic aneurysms, which have a more severe impact on women [12,14]. Surgical outcomes in women are worse, with a higher surgical mortality rate than in men [6,15]. Additionally, women might experience a higher risk of cardiovascular death and stroke than men accompanying the dilation of ARD. Conversely, in AHF cohorts, the risk of death was lower in women [16]. Therefore, it remains to be verified whether discrepancies exist between the sexes in AHF.
Therefore, the objectives of our study were (1) to validate the correlation between ARD and mortality in AHF and (2) to explore whether there are sex discrepancies in the correlations between ARD and mortality.
2. Methods
2.1. Study design and population
The China Patient-Centered Evaluative Assessment of Cardiac Events Prospective Heart Failure Study (China PEACE 5p-HF Study) is a nationwide, prospective, multicenter cohort study initiated by Fuwai Hospital that focuses on AHF. The study protocol has been previously documented [17]. AHF patients were recruited from 52 hospitals across 20 provinces in China between August 2016 and May 2018. Diagnosis and treatment were conducted by local physicians according to the Chinese Heart Failure Clinical Guidelines, which are consistent with the guidelines of the European Society of Cardiology and the American College of Cardiology/American Heart Association [18]. New-onset AHF was defined as the first diagnosis of AHF with the first manifestation of HF, and acute decompensated heart failure was defined as the acute decompensation of chronic HF that had previously been diagnosed. Eligible patients aged 18 and above experiencing new onset or acute decompensated heart failure (ADHF) provided written informed consent for participation. Follow-up assessments were scheduled at 1, 6, and 12 months after discharge, with subsequent annual assessments. This study was approved by the Ethics Committee of Fuwai Hospital, with ethics approval reference 2016-770. All the participants signed an informed consent form in accordance with the principles outlined in the Declaration of Helsinki. This study was registered at www.clinicaltrials.gov (NCT02878811).
2.2. Data collection and definition
During hospitalization, demographic information, including age and sex, was gathered through face-to-face interviews utilizing standardized questionnaires. Medical history, medications upon admission, and clinical baseline characteristics were extracted from hospital electronic medical records. Heart failure phenotypes were classified according to international guidelines. The Kansas City Cardiomyopathy Questionnaire-12 (KCCQ-12) was completed within 48 h of admission to assess health status. The KCCQ-12 scores range from 0 to 100, with lower scores indicating poorer health status [19].
Transthoracic echocardiography was conducted by experienced operators at local hospitals, all of whom had undergone standardized training to ensure consistency among participants before patient enrollment. When measuring the dimension of the aortic root, the parasternal long-axis view was employed, and the dimension of the sinuses of Valsalva were measured during diastole [20,21]. Measurements were taken perpendicular to the vessel wall from the intima to the opposite intima. Aortic root dilation was defined as a dimension exceeding 40 mm [1,2].
2.3. Inclusion and exclusion criteria
The inclusion criterion was the availability of ARD. The exclusion criteria were: (1) valve replacement surgery, including surgical or transcatheter replacement, and (2) cases with undefined causes of death.
2.4. Outcome
The primary endpoint of this study was all-cause mortality, which included both cardiovascular and non-cardiovascular mortality. Cardiovascular mortality encompasses fatalities attributed to various conditions, such as AHF, sudden cardiac death, acute coronary syndromes, cerebrovascular events, peripheral arterial disease, aortic vascular diseases, pulmonary heart disease, and other cardiovascular causes. Death events were documented through death certificates, interviews with the patient's relatives, or information sourced from national mortality databases. The causes of death were adjudicated by the National Coordinating Center based on pre-established documentation criteria.
2.5. Statistical analysis
Continuous variables were expressed as mean ± standard deviation or median (interquartile range), while categorical variables were presented as counts and percentages. We used t-tests, Mann-Whitney U tests, and ANOVA tests to compare differences among continuous variables, while chi-square tests or Fisher's exact tests were used for categorical variables. Body surface area (BSA) was calculated using the formula: BSA (m2) = 0.0061 × Height (cm)+0.0128 × Weight (Kg)-0.1529. The left ventricular mass (LVM) was calculated as LVM(g) = 0.8 × 1.04 × [(interventricular septal thickness + left ventricular posterior wall thickness + left ventricular end-diastolic dimension) [3]- left ventricular end-diastolic dimension [3] +0.6, and the LVM index was calculated as LVMI (g/m2) = LVM/BSA. The Z-score was calculated as (individual data - mean value)/standard deviations to describe a value's position relative to the mean of a group of values. ARD was indexed based on body mass index (BMI) and standardized using Z-scores. BMI-indexed ARD were divided into three groups based on the tertiles. We compared the differences in all-cause and cardiovascular mortality using the log-rank test and visually presented them using Kaplan-Meier curves.
All variables were verified using the Schoenfeld residual test, which confirmed that they met the assumption of proportional hazards. Cox proportional hazard models were used to analyze the differences among the three groups in terms of all-cause and cardiovascular mortality. The model was adjusted for demographic data and clinical baseline characteristics, such as age, sex, and blood pressure; echocardiography indicators, such as left ventricular structure and function (e.g., fractional shortening); and comorbidities, such as coronary artery disease (CAD), hypertension, and diabetes. We also analyzed the correlation between ARD and mortality separately in different sexes and examined the interactions between sex and various factors in multiple subgroups. Diabetes is defined as self-reported diabetes history, discharge diagnosis of diabetes, or baseline HbA1c ≥ 6.5 %. Hypertension was defined as having been diagnosed with hypertension in the past, having hypertension at discharge, or currently using hypertension medication. CAD was previously diagnosed as CAD or as CAD upon discharge. These conditions include angina, acute coronary syndrome, and myocardial infarction.
Additionally, a competing risk model was employed to analyze the competing risks of different groups for cardiovascular mortality, considering the combination of ARD strata and sex. Furthermore, restricted cubic splines (RCS) in a multivariate Cox proportional hazards model with three knots set at 10 %, 50 %, and 90 % were used to examine the nonlinear association between ARD and subsequent all-cause mortality.
Considering the BSA and height recommended in the previous literature and guidelines [1,2,13,22], we evaluated the incremental prognostic ability to predict subsequent all-cause mortality risk and cardiovascular mortality risk using the C-statistic for ARD. Furthermore, we validated the association between ARD and mortality risk in patients without aortic dilatation (ARD less than 40 mm), given that most patients did not have concomitant aortic root dilatation.
No missing variables did not exceed over 5 %, therefore, the missing values was imputed five times using the random forest method. Statistical analyses were conducted utilizing R version 4.2.3 (R Foundation for Statistical Computing), employing a two-tailed alpha value of 0.05 to determine significance.
3. Results
3.1. Study population
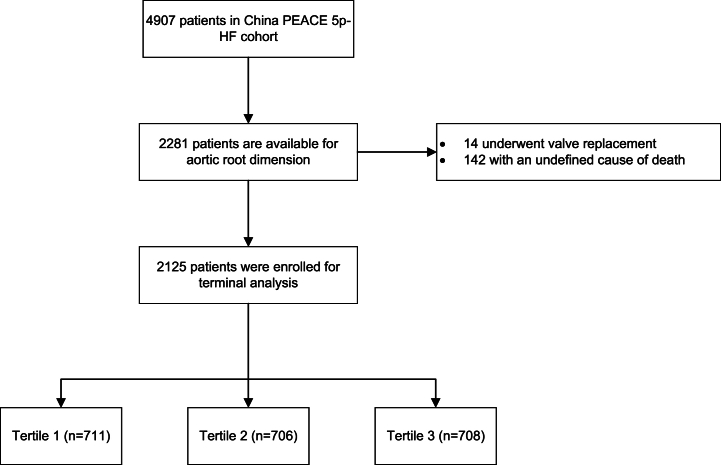
The study involved the evaluation of ARD of in 2281 participants, of which 142 had undefined causes of death and 14 underwent valve replacement. Consequently, only 2125 individuals were included in the analysis. The study flow chart is presented in Fig. 1.
Fig. 1.
Flow chart of inclusion and exclusion.
Among the evaluated participants, 38.4 % were women, with a median age of 67 years, and 80 % were diagnosed with ADHF upon admission. Table 1 shows patient characteristics according to the BMI-indexed ARD tertiles. Patients in the highest tertile group were older and had lower BMI, lower KCCQ-12 scores, shorter follow-up times, and lower diastolic blood pressure (DBP). However, no significant differences in systolic blood pressure (SBP) with the other groups. Compared to the echocardiography results, the highest tertile group showed a higher absolute ARD and higher BMI-indexed ARD, indicating more aortic root dilatation. For the left heart functional structure, the LVMI was higher, and the left atrial dimension was larger in the highest tertile group. Valvar heart disease, chronic obstructive pulmonary disease (COPD), and diabetes were more prevalent in the highest tertile group.
Table 1.
Patient characteristics according to ARD.
| Total (n = 2125) | Lowest tertile (n = 711) | Middle tertile (n = 706) | Highest tertile (n = 708) | P | |
|---|---|---|---|---|---|
| Age, years, median (Q1, Q3) | 67 (58,76) | 63 (50,72) | 67 (58,75) | 71 (64,78) | < 0.001 |
| Female, n (%) | 810 (38.1) | 272 (38.3) | 268 (38) | 270 (38.1) | 0.993 |
| Reason for hospitalization, n (%) | < 0.001 | ||||
| New onset | 424 (20) | 168 (23.6) | 147 (20.8) | 109 (15.4) | |
| ADHF | 1701 (80) | 543 (76.4) | 559 (79.2) | 599 (84.6) | |
| Heart rate, beats/min | 85 (72,100) | 88 (75,101.5) | 82 (70,97) | 84 (73,98) | < 0.001 |
| SBP, mmHg | 130 (116,148) | 131 (115,150) | 130 (117,147) | 130 (116,147) | 0.58 |
| DBP, mmHg | 80 (70,90) | 80 (70,94) | 80 (70,90) | 80 (70,90) | < 0.001 |
| KCCQ-12 | 43.8 (26.7,60.8) | 44.4 (29.2,61.2) | 45.4 (27.5,61.9) | 40.6 (24.8,57.7) | 0.005 |
| BMI, Kg/m2 | 24.3 (21.6,27) | 27.3 (25.1,30.1) | 24.5 (22.6,26.3) | 21.3 (19.4,23) | < 0.001 |
| HF phenotype, n (%) | 0.240 | ||||
| HFrEF | 842 (39.6) | 298 (41.9) | 268 (38) | 276 (39) | |
| HFmrEF | 450 (21.2) | 144 (20.3) | 142 (20.1) | 164 (23.2) | |
| HFpEF | 833 (39.2) | 269 (37.8) | 296 (41.9) | 268 (37.9) | |
| NYHA, n (%) | 0.080 | ||||
| I, II | 305 (14.4) | 116 (16.3) | 103 (14.6) | 86 (12.1) | |
| III, IV | 1820 (85.6) | 595 (83.7) | 603 (85.4) | 622 (87.9) | |
| NT pro BNP | 1454 (619.5,3157) | 1199 (559.9,2466) | 1439 (590.8,3014) | 1804.5 (751.1,3936.5) | < 0.001 |
| Follow-up, months | 54.4 (30.1,59.7) | 56 (40.4,60.2) | 55 (28.9,59.7) | 49.9 (23.9,58.7) | < 0.001 |
| Death reason, n (%) | < 0.001 | ||||
| Cardiovascular mortality | 750 (35.3) | 207 (29.1) | 242 (34.3) | 301 (42.5) | |
| Non-cardiovascular mortality | 145 (6.8) | 40 (5.6) | 43 (6.1) | 62 (8.8) | |
| Echocardiography | |||||
| ARD, mm | 33 (30,36) | 30 (28,33) | 33 (30,35) | 35 (32,38.6) | < 0.001 |
| BMI-indexed ARD, mm/(Kg/m2) | 1.3 (1.2,1.5) | 1.1 (1,1.2) | 1.3 (1.3,1.4) | 1.6 (1.5,1.8) | < 0.001 |
| LVEF, % | 45 (33.4,56) | 44 (32.9,56) | 46 (34.2,57) | 45 (34.1,56) | 0.371 |
| IVSD, mm | 10 (8.5,11) | 10 (8.3,11) | 10 (8.2,11) | 10 (8.6,11) | 0.576 |
| LVEDD, mm | 58 (50,66.5) | 58.4 (50,67) | 58 (50,66) | 58 (50,66) | 0.426 |
| LVPWD, mm | 9.7 (8,10.4) | 10 (8.3,10.8) | 9.6 (8,10.4) | 9.4 (8,10.3) | 0.218 |
| LA, mm | 44 (39,49) | 45 (40,50) | 44 (40,49) | 43 (38.9,48) | < 0.001 |
| LVEDV, mL | 167 (118,231) | 170 (118,234) | 165 (120.2234) | 166.2 (117,226) | 0.449 |
| LVESV, mL | 91.4 (51,146) | 94 (51,154.5) | 90 (51.3148.7) | 91 (51,140) | 0.389 |
| LVFS | 23 (17,30) | 23 (16,30) | 23.4 (17,30.4) | 23 (17,30) | 0.463 |
| LVMI, g/m2 | 129.7 (101.4163.3) | 123.6 (98.4154.1) | 130.1 (102.5162.2) | 135.5 (104.9170.9) | < 0.001 |
| AO dilation, n (%) | 161 (7.6) | 3 (0.4) | 26 (3.7) | 132 (18.6) | < 0.001 |
| Aortic regurgitation, n (%) | 1166 (54.9) | 290 (40.8) | 390 (55.2) | 486 (68.6) | < 0.001 |
| Comorbidities | |||||
| CAD | 1101 (51.8) | 353 (49.6) | 386 (54.7) | 362 (51.1) | 0.151 |
| VHD | 279 (13.1) | 77 (10.8) | 84 (11.9) | 118 (16.7) | 0.002 |
| CHD | 42 (2) | 11 (1.5) | 15 (2.1) | 16 (2.3) | 0.592 |
| Hypertension | 1170 (55.1) | 403 (56.7) | 395 (55.9) | 372 (52.5) | 0.247 |
| Atrial fibrillation, n (%) | 732 (34.4) | 237 (33.3) | 248 (35.1) | 247 (34.9) | 0.742 |
| Cardiomyopathy, n (%) | 650 (30.6) | 229 (32.2) | 200 (28.3) | 221 (31.2) | 0.258 |
| DM, n (%) | 678 (31.9) | 277 (39) | 233 (33) | 168 (23.7) | < 0.001 |
| COPD, n (%) | 384 (18.1) | 101 (14.2) | 116 (16.4) | 167 (23.6) | < 0.001 |
| CKD, n (%) | 240 (11.3) | 73 (10.3) | 80 (11.3) | 87 (12.3) | 0.485 |
| Medical at admission | |||||
| ACEI, n (%) | 421 (19.8) | 132 (18.6) | 150 (21.2) | 139 (19.6) | 0.444 |
| ARB, n (%) | 383 (18) | 146 (20.5) | 123 (17.4) | 114 (16.1) | 0.083 |
| β blocker, n (%) | 945 (44.5) | 321 (45.1) | 344 (48.7) | 280 (39.5) | 0.002 |
| CCB, n (%) | 313 (14.7) | 114 (16) | 110 (15.6) | 89 (12.6) | 0.136 |
| Diuretic, n (%) | 427 (20.1) | 144 (20.3) | 144 (20.4) | 139 (19.6) | 0.93 |
ADHF, acute decompensated heart failure; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; KCCQ-12, Kansas City cardiomyopathy questionnaire-12; ARD, aortic root dimensions; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; IVSD, interventricular septal diameter; RVEDD, right ventricular end-diastolic diameter; LVPWD, left ventricular posterior wall diameter; LAD, left atrial anterior-posterior diameter; RAD, right atrial diameter; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-diastolic systolic volume; LVFS, left ventricular fractional shortening; LVMI, left ventricular mass index; CAD, coronary artery disease; VHD, valvular heart disease; CHD, coronary heart disease; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CCB, calcium channel blocker.
3.2. Association between ARD and subsequent mortality
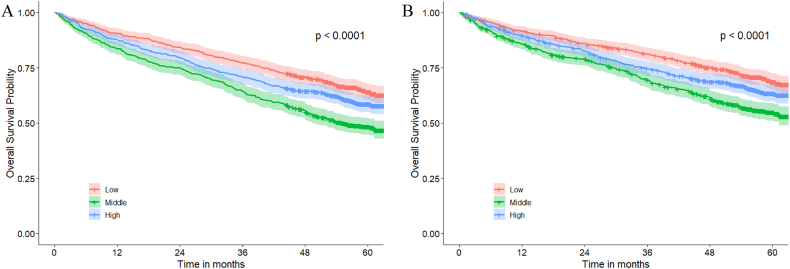
During a median follow-up of 54.4 (interquartile range:30.1 to 59.7) months, a total of 895 deaths occurred, with 750 adjudicated by cardiovascular mortality and 145 as non-cardiovascular mortality. The highest tertile group exhibited the highest incidence of all-cause death, with higher rates of cardiovascular and non-cardiovascular mortality outcomes than the other groups. The Kaplan-Meier curves showed a gradual increase in both all-cause mortality and cardiovascular mortality from lowest tertile group to highest tertile group (all-cause mortality: lowest tertile group [34.7 %], middle [40.4 %], highest [51.3 %], log-rank test P < 0.001; cardiovascular mortality: lowest tertile group [29.1 %], middle [34.3 %], highest [42.5 %], log-rank test P < 0.001, Fig. 2). Similar results were observed in different sex groups, with the highest mortality rates observed in highest tertile group (Supplementary Fig. 1).
Fig. 2.
Kaplan-Meier curve of all-cause mortality (A) and cardiovascular mortality (B). The risk of mortality gradually increased from the lowest tertile to the highest tertile of aortic root dimensions (P for trend <0.001 for all-cause and cardiovascular mortality).
Table 2 shows the relationship between BMI-indexed ARD and all-cause and cardiovascular mortality. In the all population, after multivariable adjustment, lowest tertile group and middle tertile group demonstrated a significantly lower hazard ratio (HR) for all-cause mortality and cardiovascular mortality than highest tertile group (all-cause mortality: middle tertile group [HR, 0.75; 95 % CI, 0.63 to 0.89; P < 0.001], lowest tertile group [HR, 0.68; 95 % CI, 0.56 to 0.81; P < 0.001]); cardiovascular mortality: (middle tertile group [HR, 0.78; 95 % CI, 0.65 to 0.93; P = 0.007], and lowest tertile group [HR, 0.71; 95 % CI, 0.58 to 0.87; P < 0.001]). However, notable differences were observed between the different sexes. For all-cause mortality, compared to the highest tertile group, the lowest and middle tertile group showed a significant risk reduction in men (lowest tertile group [HR, 0.67; 95 % CI, 0.53 to 0.85; P = 0.001], and middle tertile group [HR, 0.75; 95 % CI, 0.60 to 0.93; P = 0.008]). However, in women, both the lowest and middle tertile groups showed an insignificant reduction in risk. Regarding cardiovascular mortality, differences were only observed in the lowest tertile group in men compared to the highest tertile group, while no significant differences were observed in the middle tertile group compared to the highest tertile group and all groups in women.
Table 2.
Association between BMI-indexed ARD and all-cause mortality, and cardiovascular mortality in all populations, males and females.
| All | All-cause |
Cardiovascular |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusteda |
Unadjusted |
Adjusted |
|||||||
| HR,95 % CI | P | HR,95 % CI | P | P for trend | HR,95 % CI | P | HR,95 % CI | P | P for trend | |
| Lowest | 0.60 (0.51,0.70) | <0.001 | 0.68 (0.56,0.81) | <0.001 | 0.002 | 0.60 (0.50,0.72) | <0.001 | 0.71 (0.58,0.87) | <0.001 | 0.011 |
| Middle | 0.73 (0.63,0.86) | <0.001 | 0.75 (0.63,0.89) | <0.001 | 0.75 (0.63,0.89) | <0.001 | 0.78 (0.65,0.93) | 0.007 | ||
| Highest | ref | – | ref | – | ref | – | ref | – | ||
| Male | ||||||||||
| Lowest | 0.56 (0.45,0.68) | <0.001 | 0.67 (0.53,0.85) | 0.001 | 0.014 | 0.60 (0.48,0.75) | <0.001 | 0.72 (0.55,0.94) | 0.014 | 0.111 |
| Middle | 0.73 (0.60,0.88) | 0.001 | 0.75 (0.60,0.93) | 0.008 | 0.78 (0.63,0.97) | 0.023 | 0.81 (0.64,1.02) | 0.075 | ||
| Highest | ref | – | ref | – | ref | – | ref | – | ||
| Female | ||||||||||
| Lowest | 0.67 (0.51,0.87) | 0.003 | 0.73 (0.53,1.01) | 0.060 | 0.331 | 0.60 (0.45,0.81) | <0.001 | 0.78 (0.54,1.11) | 0.164 | 0.276 |
| Middle | 0.74 (0.57,0.96) | 0.025 | 0.85 (0.63,1.15) | 0.302 | 0.69 (0.52,0.92) | 0.012 | 0.83 (0.60,1.52) | 0.266 | ||
| Highest | ref | – | ref | – | ref | – | ref | – | ||
models were adjusted according to heart failure phenotypes, reason for hospitalization, age, aortic root dilation, New York Heart Association classification, systolic blood pressure, diastolic blood pressure, heart rate, Kansas city cardiomyopathy questionnaire-12 score, left ventricular end diastolic diameter, interventricular septal diameter, right ventricular end diastolic diameter, left ventricular posterior wall diameter, left atrial anterior-posterior diameter, right atrial diameter, left ventricular fractional shortening, left ventricular, aortic valve regurgitation, coronary artery disease, valvular heart disease, congenital heart disease, diabetes mellitus, chronic obstructive pulmonary disease, chronic kidney disease, hypertension, atrial fibrillation, and cardiomyopathy. HR, hazard ratio; CI, confidence interval.
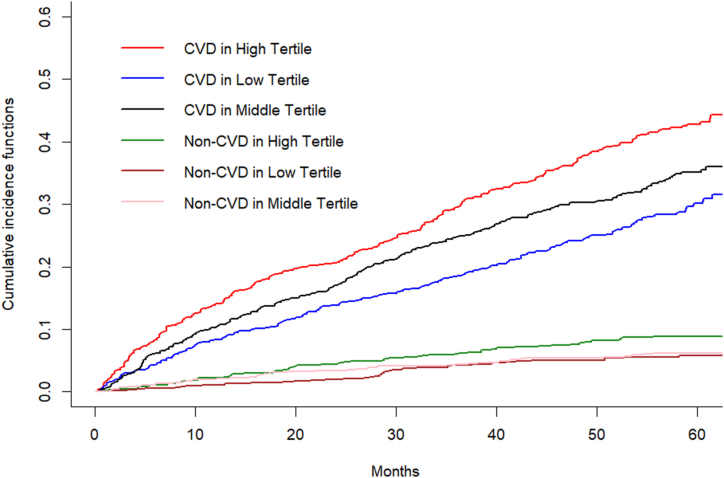
In the Fine-Gray model, we observed a gradual increase in cardiovascular mortality risk from the lowest tertile group to the highest tertile group in all populations (P for trend = 0.033; Supplementary Table 1). However, considering the competing risk, the mortality risk reduction was only observed in the lowest tertile in both male and female subgroups, while it was not significant in the middle tertile. Moreover, the competing risk persisted in the overall population (P = 0.043; Fig. 3 and Supplementary Table 1). After combining the sex and tertile groups, the risk of cardiovascular mortality was also significantly different between the sex-tertile groups with the presence of competing risks (Supplementary Table 1). After multivariate adjustment, compared to the highest tertile + male, differences were observed only in the lowest tertile + male (Supplementary Table 1).
Fig. 3.
The competing risk of aortic root dimension tertile groups. The highest Tertile showed a higher non-cardiovascular mortality of competing risk (8.9 %; P = 0.043). CVD, cardiovascular death.
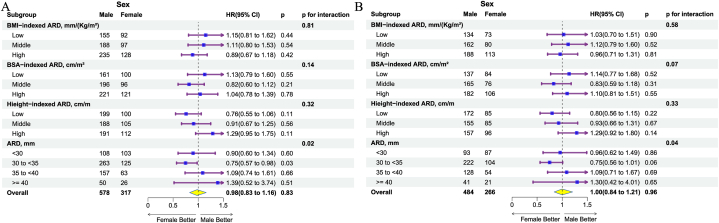
The sex-based differences in the risks of all-cause and cardiovascular mortality were examined across various ARD subgroups (Fig. 4). It was observed that women had experienced a lower risk of all-cause mortality than men in the ARD subgroups from the 30 to <35 groups. As a continuous variable, the BMI-indexed ARD was significantly associated with an increased risk of all-cause or cardiovascular mortality in the overall population and sex groups (Supplementary Table 2). Specifically, in men, for each increase of one standard deviation, the risk of all-cause mortality and cardiovascular mortality was slightly higher than that in women.
Fig. 4.
The association between sex and all-cause mortality (A), as well as cardiovascular mortality (B) in subgroups of different factors indexed ARD. Women had a lower risk of cardiovascular mortality than men in the absolute value of ARDs from 30 to <35 mm. The interactions were observed in the subgroups of ARD. BSA, body surface area; ARD, aortic root dimensions.
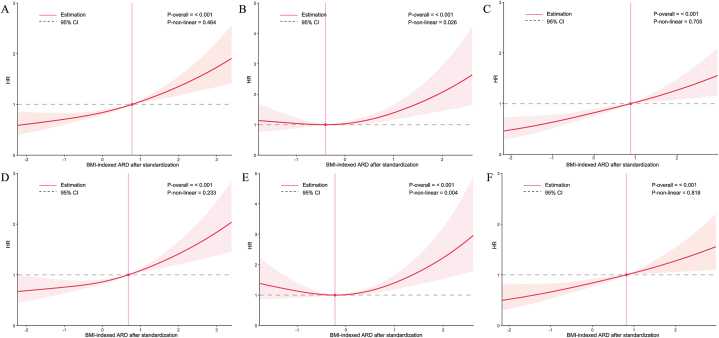
In the analysis of nonlinear patterns (Fig. 5), the association between BMI-indexed ARD and all-cause mortality or cardiovascular mortality was linear (P for nonlinearity = 0.464 and P for nonlinearity = 0.233, respectively). In the female subgroup, the relationship between BMI-indexed ARD and the risk of all-cause mortality and cardiovascular mortality were nonlinear (P for nonlinearity = 0.026 and P for nonlinearity = 0.004, respectively), exhibiting a “J” shape. However, in men, the relationship between BMI-indexed ARD and the risk of all-cause and cardiovascular mortalities was linear (P for nonlinearity = 0.705 and P for nonlinearity = 0.818, respectively).
Fig. 5.
Association between BMI-indexed ARD and subsequent all-cause mortality in all populations (A), women (B), and men (C), as well as CVD in all populations (D), women (E), and men (F). BMI, body mass index; ARD, aortic root dimensions; CVD, cardiovascular death.
3.3. Sensitivity analysis
When comparing ARD indexed by factors such as BSA and height, BMI-indexed ARD had the largest C-statistic, larger than ARD indexed by BSA with significance (P < 0.001 for all-cause mortality and P = 0.015 for CVD) but similar to ARD indexed by height or absolute ARD, regarding all-cause mortality and cardiovascular mortality (Supplementary Fig. 2). Additionally, in the ARD less than 40 mm, for each increase of one standard deviation, the risk of all-cause mortality increased by 25 % (HR, 1.25; 95 % CI 1.15 to 1.36; P < 0.001).
4. Discussion
In this AHF cohort of 2125 participants, BMI-indexed ARD was associated with an increased the risk of all-cause and cardiovascular mortality. Compared to the largest tertile group of BMI-indexed ARD, the lowest and middle tertile groups experienced a lower risk of all-cause and cardiovascular mortality, with a reduction of over 20 %. Furthermore, differences were observed between the sexes. Our study provides evidence supporting the use of BMI-indexed ARD as valuable predictors of the risk of all-cause and cardiovascular mortality.
Prior research has revealed a correlation between ARD and the risk of adverse cardiovascular events in the general population. Notably, Notably, the previous studies discovered that the increase in ARD corresponded to an increase in the risk of HF incidence and cardiovascular events regardless of race [3,6,9,[23], [24], [25]]. Nevertheless, the current research has primarily focused on the general population and has yet to analyze specific populations. In AHF populations, a combination of cardiovascular risk factors, such as hypertension and left ventricular hypertrophy, often coexist, all of which can directly or indirectly affect ARD [5,[26], [27], [28], [29], [30], [31]].
Previous studies have revealed that the risk of cardiovascular mortality is higher in women than in men [6]. Our findings concurred with prior research in that the mortality risk in males was slightly lower than in females. It is noteworthy that the relationship between the BMI index and female mortality rate presents a “J” shape. This phenomenon was described for the first time, however, there is no evidence in the literature. The study results indicated that ARD and body imbalance may be more harmful to women with AHF. The difference between men and women with ARD and a lower BMI index may be related to sex.
ARD is influenced by multiple factors and its absolute value may not directly reflect its relationship with cardiovascular events. Apart from the sex discrepancy, an increase in ARD was associated with factors such as age, height, weight, and cardiovascular risk factors [10,12,14,15,22,26,29,[32], [33], [34], [35], [36]]. Considering individual and racial differences, indexing with height or BSA can more accurately reflect individual differences in ARD [37]. In previous studies, the relationship among height, BSA, and ARD varied. Height-indexed ARD may be more accurate to aortic aneurysms compared to BSA-indexed ARD [38]. Compared with BSA, BMI simplified the calculations and reflected the inverse relationship between weight and height, which better represented individual differences from the standard body shape, avoiding situations of extreme thinness or obesity.
It is pertinent to acknowledge that ARD enlargement leading to surgical intervention is a gradual process that entails associated risks that cannot be disregarded. This study, which focuses on patients with AHF, complements the correlation between mortality risk and BMI-indexed ARD before the absolute ARD values reach the threshold for surgical intervention. The results of this study highlighted that ARD is positively correlated with an elevated risk of cardiovascular disease outcomes and mortality.
It is important to acknowledge the limitations of the present study. First, our study cohort consisted solely of patients with AHF, which precluded comparison with the general population and curtailed the generalizability of our findings to a broader population. Moreover, the study population was predominantly of Asian descent, which further restricts the generalizability of our results. Second, despite standardized training at the onset of the study, echocardiography, which we used for measurements, has inherent variability and thus requires cautious interpretation. Additionally, we did not measure the dimensions of the descending or abdominal aorta, which precludes comparisons with the aortic root. Augmenting this study with measurements of the descending and abdominal aortic dimensions in future studies could yield more meaningful results. A larger population is also needed to verify the relationship between BMI-indexed ARD and mortality in women, especially in those with a lower BMI-indexed ARD. Finally, we lacked longitudinal data, which would have been ideal for the continuous assessment of ARD over time.
5. Conclusion
Our results indicated that both male and female patients with lower BMI-indexed ARD were at a lower risk of mortality than those with higher BMI-indexed ARD. Moreover, we found a discrepancy in the relationship between BMI-indexed ARD and mortality between women and men, which requires further verification and analysis.
Funding
This research was funded by clinical research grants from central high-level hospitals (grant number 2024-GSP-GG-6).
Data availability statement
Data will be made available on request.
Ethics declarations
This study was reviewed and approved by Ethics Committee of Fuwai Hospital with the approval number: 2016-770, dated May 24, 2016.
CRediT authorship contribution statement
Zeming Zhou: Writing – original draft, Software, Methodology, Investigation, Formal analysis, Conceptualization. Wei Wang: Validation, Software, Methodology, Formal analysis, Data curation. Lili Tian: Resources, Methodology, Investigation, Data curation. Yue Peng: Validation, Software, Data curation. Lubi Lei: Validation, Data curation. Jingkuo Li: Visualization, Validation, Data curation. Boxuan Pu: Visualization, Validation, Data curation. Lihua Zhang: Supervision, Resources, Project administration, Investigation, Funding acquisition. Xin Zheng: Supervision, Resources, Project administration, Investigation, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e37026.
Contributor Information
Lihua Zhang, Email: zhanglihua@fuwai.com.
Xin Zheng, Email: zhengxin@fuwai.com.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
References
- 1.2014 ESC Guidelines on the diagnosis and treatment of aortic diseases. Eur. Heart J. 2014;35(41):2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 2.Isselbacher E.M., Preventza O., Hamilton Black J., et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American heart association/American College of Cardiology joint committee on clinical practice guidelines. Circulation. 2022;146(24) doi: 10.1161/cir.0000000000001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gardin J.M., Arnold A.M., Polak J., Jackson S., Smith V., Gottdiener J. Usefulness of aortic root dimension in persons ≥65 Years of age in predicting heart failure, stroke, cardiovascular mortality, all-cause mortality and acute myocardial infarction (from the cardiovascular health study) Am. J. Cardiol. 2006;97(2):270–275. doi: 10.1016/j.amjcard.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 4.Paruchuri V., Salhab K.F., Kuzmik G., et al. Aortic size distribution in the general population: explaining the size paradox in aortic dissection. Cardiology. 2015;131(4):265–272. doi: 10.1159/000381281. [DOI] [PubMed] [Google Scholar]
- 5.Canciello G., Mancusi C., Losi M.A., et al. Aortic root dilatation is associated with incident cardiovascular events in a population of treated hypertensive patients: the campania salute network. Am. J. Hypertens. Nov 13 2018;31(12):1317–1323. doi: 10.1093/ajh/hpy113. [DOI] [PubMed] [Google Scholar]
- 6.Rueda-Ochoa O.L., Bons L.R., Zhu F., et al. Thoracic aortic diameter and cardiovascular events and mortality among women and men. Radiology. Jul 2022;304(1):208–215. doi: 10.1148/radiol.210861. [DOI] [PubMed] [Google Scholar]
- 7.Vasan R.S., Urbina E.M., Jin L., Xanthakis V. Prognostic significance of echocardiographic measures of cardiac remodeling in the community. Curr. Cardiol. Rep. Jun 3 2021;23(7):86. doi: 10.1007/s11886-021-01512-4. [DOI] [PubMed] [Google Scholar]
- 8.Qazi S., Massaro J.M., Chuang M.L., D'Agostino R.B., Hoffmann U., O'Donnell C.J. Increased aortic diameters on multidetector computed tomographic scan are independent predictors of incident adverse cardiovascular events. Circulation: Cardiovascular Imaging. 2017;10(12) doi: 10.1161/circimaging.117.006776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam C.S.P., Gona P., Larson M.G., et al. Aortic root remodeling and risk of heart failure in the framingham heart study. JACC (J. Am. Coll. Cardiol.): Heart Fail. 2013;1(1):79–83. doi: 10.1016/j.jchf.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bons L.R., Rueda-Ochoa O.L., El Ghoul K., et al. Sex-specific distributions and determinants of thoracic aortic diameters in the elderly. Heart. Jan 2020;106(2):133–139. doi: 10.1136/heartjnl-2019-315320. [DOI] [PubMed] [Google Scholar]
- 11.Tadic M., Gherbesi E., Sala C., Carugo S., Cuspidi C. Is thoracic aortic diameter an independent predictor of cardiovascular disease and mortality? A narrative review. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.867026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thijssen C.G.E., Mutluer F.O., van der Toorn J.E., et al. Longitudinal changes of thoracic aortic diameters in the general population aged 55 years or older. Heart. Apr 28 2022 doi: 10.1136/heartjnl-2021-320574. [DOI] [PubMed] [Google Scholar]
- 13.Yu S., Guo X., Li G., Yang H., Zheng L., Sun Y. Gender discrepancy in the predictive effect of aortic root diameter on incidence of cardiovascular events among rural Northeast Chinese. BMJ Open. Sep 6 2022;12(9) doi: 10.1136/bmjopen-2020-039207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groepenhoff F., den Ruijter H.M. Sex-specific thoracic aortic dimensions and clinical implications. Heart. Jan 2020;106(2):97–98. doi: 10.1136/heartjnl-2019-315903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung K., Boodhwani M., Chan K.L., Beauchesne L., Dick A., Coutinho T. Thoracic aortic aneurysm growth: role of sex and aneurysm etiology. J. Am. Heart Assoc. Feb 3 2017;6(2) doi: 10.1161/JAHA.116.003792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motiejunaite J., Akiyama E., Cohen-Solal A., et al. The association of long-term outcome and biological sex in patients with acute heart failure from different geographic regions. Eur. Heart J. Apr 1 2020;41(13):1357–1364. doi: 10.1093/eurheartj/ehaa071. [DOI] [PubMed] [Google Scholar]
- 17.Huang X., Yu Y., Li X., et al. The China Patient-centred Evaluative Assessment of Cardiac Events (PEACE) prospective heart failure study design. BMJ Open. Feb 19 2019;9(2) doi: 10.1136/bmjopen-2018-025144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heart Failure Group of Chinese Society of Cardiology of Chinese Medical Association, Chinese Heart Failure Association of Chinese Medical Doctor Association Editorial Board of Chinese Journal of Cardiology. Chinese guidelines for the diagnosis and treatment of heart failure 2018. Zhonghua Xinxueguanbing Zazhi. 2018;46(10):760–789. doi: 10.3760/cma.j.issn.0253-3758.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Spertus J.A., Jones P.G. Development and validation of a Short version of the Kansas city cardiomyopathy questionnaire. Circ Cardiovasc Qual Outcomes. Sep 2015;8(5):469–476. doi: 10.1161/CIRCOUTCOMES.115.001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galderisi M., Cosyns B., Edvardsen T., et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. Dec 1 2017;18(12):1301–1310. doi: 10.1093/ehjci/jex244. [DOI] [PubMed] [Google Scholar]
- 21.Lang R.M., Bierig M., Devereux R.B., et al. Recommendations for chamber quantification: a report from the American society of echocardiography's guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European association of echocardiography, a branch of the European society of Cardiology. J. Am. Soc. Echocardiogr. Dec 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Milan A., Avenatti E., Tosello F., et al. Aortic root dilatation in essential hypertension: prevalence according to new reference values. J. Hypertens. Jun 2013;31(6):1189–1195. doi: 10.1097/HJH.0b013e32835f8fda. [DOI] [PubMed] [Google Scholar]
- 23.Cuspidi C., Facchetti R., Bombelli M., et al. Aortic root diameter and risk of cardiovascular events in a general population. J. Hypertens. 2014;32(9):1879–1887. doi: 10.1097/hjh.0000000000000264. [DOI] [PubMed] [Google Scholar]
- 24.Kamimura D., Suzuki T., Musani S.K., et al. Increased proximal aortic diameter is associated with risk of cardiovascular events and all-cause mortality in blacks the jackson heart study. J. Am. Heart Assoc. Jun 21 2017;6(6) doi: 10.1161/JAHA.116.005005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai C.L., Chien K.L., Hsu H.C., Su T.C., Chen M.F., Lee Y.T. Aortic root dimension as an independent predictor for all-cause death in adults <65 years of age (from the Chin-Shan Community Cardiovascular Cohort Study) Echocardiography. May 2010;27(5):487–495. doi: 10.1111/j.1540-8175.2009.01072.x. [DOI] [PubMed] [Google Scholar]
- 26.Cipolli J.A., Souza F.A., Ferreira-Sae M.C., et al. Sex-specific hemodynamic and non-hemodynamic determinants of aortic root size in hypertensive subjects with left ventricular hypertrophy. Hypertens. Res. Nov 2009;32(11):956–961. doi: 10.1038/hr.2009.134. [DOI] [PubMed] [Google Scholar]
- 27.Cuspidi C., Negri F., Salvetti M., et al. Aortic root dilatation in hypertensive patients: a multicenter survey in echocardiographic practice. Blood Press. Oct 2011;20(5):267–273. doi: 10.3109/08037051.2011.565556. [DOI] [PubMed] [Google Scholar]
- 28.Covella M., Milan A., Totaro S., et al. Echocardiographic aortic root dilatation in hypertensive patients: a systematic review and meta-analysis. J. Hypertens. Oct 2014;32(10):1928–1935. doi: 10.1097/HJH.0000000000000286. ; discussion 1935. [DOI] [PubMed] [Google Scholar]
- 29.Leone D., Airale L., Bernardi S., et al. Prognostic role of the ascending aorta dilatation in patients with arterial hypertension. J. Hypertens. Jun 1 2021;39(6):1163–1169. doi: 10.1097/hjh.0000000000002752. [DOI] [PubMed] [Google Scholar]
- 30.Lundorff I., Modin D., Mogelvang R., et al. Echocardiographic predictors of cardiovascular morbidity and mortality in women from the general population. Eur Heart J Cardiovasc Imaging. Aug 14 2021;22(9):1026–1034. doi: 10.1093/ehjci/jeaa167. [DOI] [PubMed] [Google Scholar]
- 31.Guzik B.M., McCallum L., Zmudka K., Guzik T.J., Dominiczak A.F., Padmanabhan S. Echocardiography predictors of survival in hypertensive patients with left ventricular hypertrophy. Am. J. Hypertens. Jun 22 2021;34(6):636–644. doi: 10.1093/ajh/hpaa194. [DOI] [PubMed] [Google Scholar]
- 32.Kälsch H., Lehmann N., Möhlenkamp S., et al. Body-surface adjusted aortic reference diameters for improved identification of patients with thoracic aortic aneurysms: results from the population-based Heinz Nixdorf Recall study. Int. J. Cardiol. 2013;163(1):72–78. doi: 10.1016/j.ijcard.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 33.Palmieri V., Bella J.N., Arnett D.K., et al. Aortic root dilatation at sinuses of valsalva and aortic regurgitation in hypertensive and normotensive subjects: the Hypertension Genetic Epidemiology Network Study. Hypertension. May 2001;37(5):1229–1235. doi: 10.1161/01.hyp.37.5.1229. [DOI] [PubMed] [Google Scholar]
- 34.Teixido-Tura G., Almeida A.L., Choi E.Y., et al. Determinants of aortic root dilatation and reference values among Young adults over a 20-year period: coronary artery risk development in young adults study. Hypertension. Jul 2015;66(1):23–29. doi: 10.1161/HYPERTENSIONAHA.115.05156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cuspidi C., Facchetti R., Quarti-Trevano F., et al. Incident aortic root dilatation in the general population: findings from the Pamela study. J. Hypertens. Mar 1 2022;40(3):544–552. doi: 10.1097/hjh.0000000000003047. [DOI] [PubMed] [Google Scholar]
- 36.Liu L.Y., Yun C.H., Kuo J.Y., et al. Aortic root remodeling as an indicator for diastolic dysfunction and normative ranges in asians: comparison and validation with multidetector computed tomography. Diagnostics. Sep 18 2020;10(9) doi: 10.3390/diagnostics10090712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Girardi L.N., Lau C., Gambardella I. Aortic dimensions as predictors of adverse events. J. Thorac. Cardiovasc. Surg. Apr 2021;161(4):1193–1197. doi: 10.1016/j.jtcvs.2020.06.137. [DOI] [PubMed] [Google Scholar]
- 38.Zafar M.A., Li Y., Rizzo J.A., et al. Height alone, rather than body surface area, suffices for risk estimation in ascending aortic aneurysm. J. Thorac. Cardiovasc. Surg. 2018;155(5):1938–1950. doi: 10.1016/j.jtcvs.2017.10.140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.