Abstract
Hypertrophic scarring (HS) is a complication of wound healing that causes physiological and psychological distress in patients. However, the possible mechanism underlying HS is not fully understood, and there is no gold standard for its treatment. Natural products are more effective, economical, convenient, and safe than existing drugs, and they have a wide application prospect. However, there is a lack of literature on this topic, so we reviewed in vivo, in vitro, and clinical studies and screened natural products showing beneficial effects on HS that can become potential therapeutic agents for HS to fill in the gaps in the field. In addition, we discussed the drug delivery systems related to these natural products and their mechanisms in the treatment of HS. Generally speaking, natural products inhibit inflammation, myofibroblast activation, angiogenesis, and collagen accumulation by targeting interleukins, tumor necrosis factor-α, vascular endothelial growth factors, platelet-derived growth factors, and matrix metalloproteinases, so as to play an anti-HS effects of natural products are attributed to their anti-inflammatory, anti-proliferative, anti-angiogenesis, and pro-apoptotic (enhancing apoptosis and autophagy) roles, thus treating HS. We also screened the potential therapeutic targets of these natural compounds for HS through network pharmacology and constructed a protein-protein interaction (PPI) network, which may provide clues for the pharmacological mechanism of natural products in treating this disease and the development and application of drugs.
Keywords: Hypertrophic scars, Natural products, Traditional Chinese medicine, Drug discovery, Mechanisms
1. Introduction
The skin has a protective barrier function that shields the organism from external injuries of varying intensities and depths [1]. Scar formation is an important stage in wound healing after injury, which involves changes in the skin morphology and histology [2]. Superficial skin injuries can heal effectively resulting in a flat, elastic, and slightly discolored scar. Excessive scarring is caused by excessive proliferation of myofibroblasts and increased collagen deposition and often occurs after deep skin damage caused by surgery, burns, and trauma [3,4]. The scars are classified as mature scars, immature scars, hypertrophic scars, keloids, and atrophic scars [5]. Of these, hypertrophic scars (HS), which can be classified as linear or extensive, are the most common type of scars in clinical practice [2,6].
HS resembles skin tumors but is benign in nature. In contrast to normal scars and normal skin, HS histologically exhibits abundant blood vessels, numerous mesenchymal cells, thickened epidermal cell layers, extracellular matrix (ECM) deposition, and infiltration of lymphocytes, macrophages, and mast cells [7].
The prevalence of HS varies between 15% and 63 % in Caucasians and is >70 % in non-Caucasians [8]. The development of HS is influenced by intrinsic and extrinsic factors. Some intrinsic factors include gender, age, dark skin type, and anatomical location of the burn and extrinsic factors include total burn area, burn depth, multiple surgeries, retinal transplants, healing time, bacterial colonization, skin stretching, chemotherapy, and smoking [1,9]. It is characterized by redness, swelling, and contraction of the injured area, which is accompanied by pain and itching. In addition to the physical symptoms, unaesthetic scars can also have psychological effects on patients, including lack of confidence, stigma, disruption of daily activities, anxiety, and depression. Furthermore, if the scar in the joint, eye, nose, mouth, or any other functional part continues to shrink, the pathological outcomes of scar contracture appear, which damage the normal function of the organ and even limit the daily activities of the individual [6,10,11]. The clinical and economic burden of excessive hypertrophic scarring and the resulting disabilities and revision surgeries is enormous [12]. Given the high incidence of HS and its tremendous impact on patients, its management is a major challenge for patients and healthcare providers.
Several strategies have been used for HS treatment and prevention. Surgical resection is the most commonly used option when the scar loses its function and develops severe contractures. However, surgical resection and scar repair may result in larger wounds with a recurrence rate of up to 45 %–100 % [13]. Pressure garment therapy has been used clinically for many years; however, its efficacy and adverse effects remain controversial [14,15]. Intralesional corticosteroid injections, which are simple and cheap, have been the traditional treatment for more than sixty years and are most frequently used for the treatment and prevention of pathological scars. In addition, a combination of corticosteroid injection and surgery is commonly used as a second-line therapy, which has shown synergistic effects in clinical settings with minimal side effects [5,16,17]. In addition, laser therapy, cryotherapy, radiation therapy, topical photodynamic therapy, percutaneous collagen induction with micro-needling, and anti-tumor agents such as bleomycin and 5-fluorouracil are also recommended. Several new therapeutic approaches are available as the molecular mechanisms of HS are being clarified. Some emerging therapeutic options include botulinum toxin A, tumor necrosis factor alpha antagonists, interferon, calcium antagonists, imidazoquinolines, transforming growth factor (TGF)-β, fat grafting, and mesenchymal stem cells[[18], [19], [20], [21], [22]]. However, these treatment options have variable and inconsistent benefits, limitations, and adverse effects. The management of HS is challenging and no effective “gold standard” has been accepted to date. Therefore, an efficient and reliable therapeutic agent is urgently required, which is convenient, sustainable, low in cost with fewer side effects.
2. Physiopathology of a hypertrophic scar
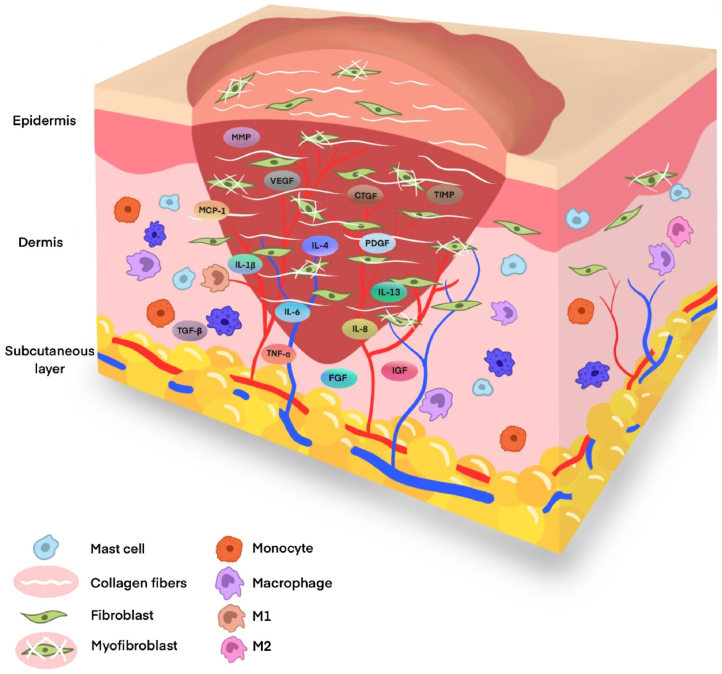
Wound healing—a complicated and dynamic process—leads to scar formation and ultimately restores skin integrity, thereby protecting the patient from infection and loss of body fluids. This process is separated into three distinct (but often superimposed) phases: inflammation, proliferation, and remodeling [10,13], which involve several cytokines, mediators, cells, and matrix molecules. The scar development may be affected by the malfunctioning of any component or pathway in any of these stages. Therefore, understanding the mechanisms of these three stages is key to the treatment or intervention of HS. Here, we summarized these mechanisms and factors that affect the formation of HS (Fig. 1).
Fig. 1.
Diagram of the histological features of hypertrophic scars. The formation process of HS involve several cytokines, mediators, cells, and matrix molecules. HS histologically exhibits abundant blood vessels, numerous mesenchymal cells, thickened epidermal cell layer, ECM deposition, and infiltration of lymphocytes, macrophages, and mast cells.
Immediately after an injury occurs, the inflammatory stage begins, which often lasts for two to three days. The clotting cascade is initiated, and a network of fibrin is formed around the platelets to form stable thrombi, while platelets secrete cytokines and growth factors [23]. The immune system and resulting inflammatory responses are then activated to fight local infections, engulf local debris and damaged tissue to form a clean cell bed, and subsequently remove fibrin [16]. After receiving signals of damage-associated molecular patterns or pathogen-associated molecular patterns, interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-α, chemokine C-C motif ligand 2, mast cells, neutrophils, and monocytes from the damaged blood vessels accumulate at the wound site, and the monocytes subsequently differentiate into macrophages [24]. These phagocytes increase the gene expression levels of various molecules, including growth factors, cytokines, and chemokines, to stimulate fibrous proliferation and angiogenesis and prepare for the next phase of creating a temporary ECM. These molecules include TGF-β, interleukins (IL-1β, IL-4, IL-6, IL-8, and IL-13), vascular endothelial growth factor (VEGF), monocyte chemoattractant protein (MCP)-1, platelet-derived growth factor (PDGF), fibroblast growth factor, and TNF-α providing early [[25], [26], [27]]. Although inflammation is an important defense for skin healing and pro-inflammatory cytokines are crucial in the initial stage of wound repair, persistent inflammation delays wound healing and is a key event for HS formation [28,29]. Further, the cells involved in the inflammatory response also influence the outcome of the wound. Macrophages have two phenotypes, the pro-inflammatory M1 phenotype is prevalent at the initial stage of scar formation and the anti-inflammatory M2 phenotype is found at subsequent stages [30]. A reduced expression of M1 cytokines in the early stages and delayed and prolonged expression of M2 and anti-inflammatory cytokines in the later stages may lead to the formation of HS [24,31]. Compared to normal skin, mast cell expression in HS is increased and positively correlates with the severity of scars. Mast cells have a strong association with skin scar formation and fibrosis; they can enhance acute inflammation and stimulate re-epithelialization and angiogenesis [28]. VEGF is a key pro-angiogenic mediator that assists wound healing; however, it also contributes to excessive angiogenesis resulting in skin scars [32].
Various anti-inflammatory mediators stimulate fibroblasts and keratinocytes to signal the transition to the proliferation stage [5]. This phase is characterized by tissue granulation and neovascularization from pre-existing blood vessels. Fibroblasts degrade the temporary matrix by producing matrix metalloproteinases (MMPs) and replace the matrix with granulation tissue [33]. Various cytokines, matrix factors, and growth factors such as TNF, TGF-β, PDGF, insulin-like growth factor, and connective tissue growth factor (CTGF) stimulate and activate fibroblasts, myofibroblasts, keratinocytes, and endothelial cells to synthesize ECM, which serves as a scaffold for tissue repair. Notably, fibroblasts and muscle fibroblasts play a major role in this process [34]. Platelets, endothelial cells, and macrophages release signaling molecules to stimulate the differentiation of fibroblasts into profibrotic cells or myofibroblasts [33]. Myofibroblast is a special contractile cell, which is activated by TGF-β1 and expresses α-smooth muscle actin (SMA). It can produce and deposit large quantities of collagen and also secrete ECM proteins, including collagen (COL)-I and COL-III [34]. The second phase restores organizational cohesion and replaces lost tissue organizations, and the accumulated collagen forms most of the final scar [35]. Further, abundant vascularization and angiogenesis begin at this stage with the upregulation of VEGF to provide the necessary nutrients, signals, and cells for the coordinating repair [36].
The critical factor in controlling scar formation is the balance between proliferation (in the proliferation stage) and degradation (in the remodeling stage) [37]. Excessive scarring in HS results from a shift to the proliferative direction [16]. Collagen synthesis in HS is approximately seven-fold higher than in normal skin. During wound healing, MMP-1 and MMP-2 degrade ECM and COL-I, respectively. Tissue inhibitor matrix metalloproteinase (TIMP)-1 is an inhibitor of MMP-1 in tissues, and the amount of COL-I is strictly regulated by the balance between MMP-1 and TIMP-1 [37]. In addition, MMP-1 reduces scar formation by accelerating epithelialization and reducing inflammation and vascularization [38]. Fibroblasts are considered critical for wound healing and HS formation [39]. They do not undergo apoptosis during abnormal or pathological wound healing. Fibroblasts and myofibroblasts persist even after wound closure, and they produce excessive ECM and induce sustained wound contraction resulting in HS[[40], [41], [42]].
Members of the B-cell lymphoma (Bcl)-2 family are essential to regulate the mitochondrial death pathway. Pro-apoptotic protein Bax and anti-apoptotic protein Bcl-2 are closely related to cell death and survival, respectively, and their ratio determines the fate of cells [43]. Apoptosis-related cysteine peptidase (caspase-3) is indirectly cleaved by Bcl-2 by releasing cytochrome c from the mitochondria, which, in turn, further induces apoptosis [44]. The Bcl-2/Bax ratio is associated with the apoptosis of hypertrophic scar fibroblasts (HSF) and participates in HS formation. The formation of HS also involves the excessive conversion of fibroblasts into myofibroblasts, which is induced by the overproduction of TGF-β [45]. α-SMA expressed by myofibroblasts is a pathological diagnostic marker of HS. Scarless healing of human fetuses is the result of TGF-β deficiency [46]. TGF-β1, TGF-β2, and TGF-β3 are the highly conserved and dominant isoforms of the TGF-β family in the human body. TGF-β1 and TGF-β2 are associated with fibrosis. In contrast, TGF-β3 reduces fibrosis and prevents scar formation. Myofibroblasts are absent from wounds in fetal skin. However, the application of TGF-β1 to human fetal fibroblasts allows them to acquire characteristics similar to myofibroblasts [47]. TGF-β receptor receives stimulation from the corresponding antigen and acts downstream to transmit the signal through the Smad signal transduction pathway.
The remodeling stage begins immediately after the wound is closed, lasting a year or longer. These changes are externally manifested as a marked softening of the scars and a change in color from pink to pale. The number of blood vessels is reduced, thereby inducing apoptosis of fibroblasts and myofibroblasts and removing some scar material. The immature COL-III becomes mature COL-I and the ECM breaks down, which is an important step in the formation of relatively cell-free scars and the end point of normal wound healing [48].
The TGF-β/Smad signaling pathway regulates collagen formation in myofibroblasts and fibroblasts, and its continuous activation leads to their long-term over-activation, consequently leading to excessive collagen formation in HS [49]. Smad7 is an inhibitory Smad that participates in the negative feedback regulation of the TGF-β/Smad signaling pathway. It blocks the phosphorylation of Smad2 and Smad3, thereby inhibiting the activation of TGF-β. In addition, Smad7 promotes wound healing by regulating cytokines and controlling cell growth, differentiation, and apoptosis [50,51]. In addition, Notch, phosphoinositide 3-kinase (PI3K)/AKT, and MAPK signaling pathways are also crucial for HS formation. The Notch signaling pathway participates in HS formation by regulating keratinocyte phenotype, collagen production, and angiogenic factors. This pathway is associated with COL-I, COL-III, VEGF, Ang-1, TGF-β1, and MMP-2, and its inhibition impairs their expression. Further, the originally upregulated angiogenic factors are downregulated, and vascular growth is inhibited followed by the relief of HS[[52], [53], [54]]. The PI3K/AKT signaling pathway can regulate the growth, movement, and differentiation of fibroblasts [31]. AKT/FoxO/p27 signal axis enhances the anti-proliferative ability of pentoxifylline in HS and the PI3K/AKT pathway is inactivated, collagen expression is decreased, and the formation of HS is inhibited [31,55]. As potential molecular targets for treating HS, micro (mi)RNAs, namely miRNA-494, miRNA-486-5p, miRNA-21, and miRNA-155, control the expression of collagen and the growth of fibroblasts through the PI3K/AKT signaling pathway, thereby affecting HS formation and indicating that the pathway is a crucial link in the disease pathogenesis [31,[55], [56], [57]]. Mitogen-activated protein kinases (MAPK), the serine/threonine kinases, include extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MAPK, which are activated by phosphorylation and play a vital role in cell proliferation, differentiation, and apoptosis [58]. The pathway is closely associated with inflammation and promotes collagen synthesis in the skin fibroblasts and ECM production. In addition, it participates in the cellular transdifferentiation process and is activated by the TGF-β1/Smad2/3 signaling pathway in HS fibrosis. The downregulation of this pathway markedly reduces the expression and deposition of collagen in HS and inhibits the transformation of fibroblasts into myofibroblasts [59].
3. Natural products for the treatment of hypertrophic scars
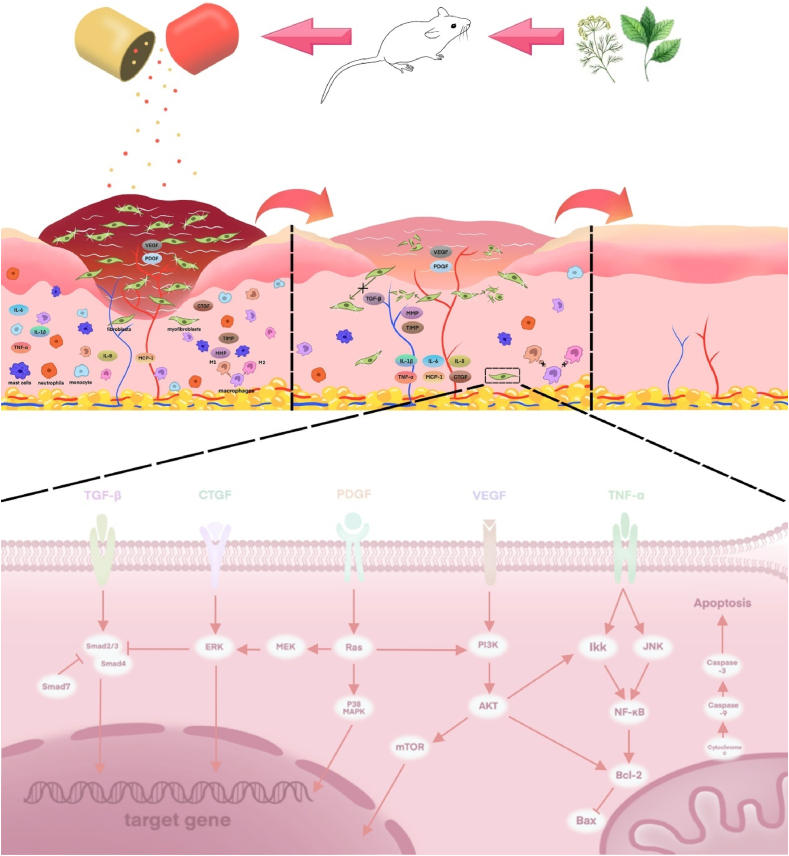
Natural products are low-molecular-weight metabolites, which are synthesized during primary and secondary metabolism in living organisms [60]. Given the rich history of herbal remedies for human diseases and the complexity and diversity of natural products, the World Health Organization has developed a strategy for traditional medicine that includes the formulation of guidelines for the evaluation of herbal medicines [61,62]. Natural products used as actual drugs or as drug leads offer the possibility of discovering new structures that could be effective agents for treating many human diseases, as well as the best sources for new compounds or active templates [63]. They have received widespread attention for their therapeutic and preventive effects in HS. Several researchers have elucidated their mechanism of action in treating HS and found that these compounds exert their therapeutic effects through various mechanisms at different stages of HS formation, but there is no article to summarize this, so we will describe it specifically below (Fig. 2, Fig. 3, Table 1).
Fig. 2.
Mechanism diagram of natural products acting on hypertrophic scars. Natural products exert various effects such as anti-inflammation, anti-proliferation, inhibition of angiogenesis and pro-apoptotic effects through multiple targets in the wound healing stage, explaining their potential for prevention and treatment of hypertrophic scars.
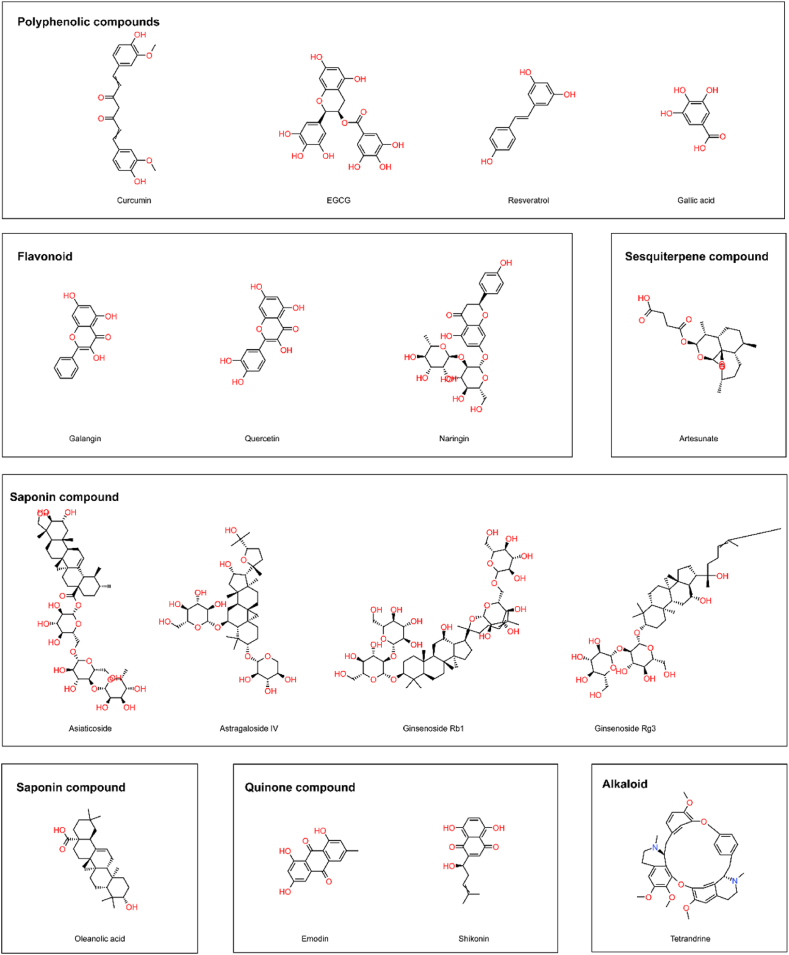
Fig. 3.
Structural formulae of natural products with HS therapeutic effects.
Table 1.
Therapeutic effects of natural products on HS. The table shows the types, origins, the experimental types, doses investigated, the major effects mediated and the targets found to be influenced of these nature products.
| Type | Compound/extacts | Origin | In vitro/In vivo/Clinical trial | Dose | Mechanism of action(s) | Reference |
|---|---|---|---|---|---|---|
| Polyphenolic compounds | Curcumin | Curcuma longa L. | In vitro (human fibroblasts, myofibroblast and epidermal keratinocytes cells) | 25 μM | ↓TGF-β1/Smad, NF-κB and CTGF signaling pathway ↓myofibroblasts, TIMP-1, IL-6, IL-8, IL-1β ↑MMP, PPARγ, ROS |
[42,64,65] |
| In vitro (mouse fibroblasts) | 5 mg/mL | |||||
| In vivo (rabbit ear hypertrophic scar model) | 30 μg/kg | |||||
| Epigallocatechin-3-gallate | Camellia sinensis (L.) Kuntze | Clinical trial (Human health volunteers with scar) | – | ↓mast cell, mast cell tryptase, mast cell chymase, fibronectin, vascular, COL-Ⅰ ↑M2 ↓TGF-β1, TNF-α, IL-1β, IL-6, CTGF, PAI-1 ↑Hemeoxygenase-1, TGF-β3 |
[[66], [67], [68], [69], [70],[71], [72], [73], [74]] | |
| Clinical trial (Preoperative human health volunteers) | – | |||||
| In vivo (Rat wound healing model) | 10 mg/kg | |||||
| In vitro (an optimised ex vivo skin organ culture model) | 21.2 mg/ml | |||||
| In vitro (human fibroblasts, hypertrophic scar-derived fibroblasts) | 50 μM | |||||
| Resveratrol | cuspidatum Sieb. et Zucc., Veratrum nigrum L. and Cassia tora L. | In vitro (normal skin-derived fibroblasts, hypertrophic scar-derived fibroblasts) | 10,50,100 μM 25, 75, 150, 300, and 400 μM 1, 10 or 100 μM |
↓α-SMA, COL-Ⅰ and COL-Ⅲ ↑fibroblasts autophagy ↓TGF-β1, Smad-2, 3, 4, AKT, mTOR, 70S6K ↑Smad7 |
[75,[76], [77], [78], [79], [80], [81], [82], [83], [84], [85]] | |
| In vivo (Rat skin injury model) | 8 and 32 μg/ml | |||||
| In vivo (Mouse wound healing model) | 4.4 mM, 0.5 mL · 100 g−1 bodyweight | |||||
| Gallic acid | Rheum palmatum L., Eucalyptus globulus Labill., Cornus officinalis Sieb. et Zucc. and Rhus coriaria L. | In vitro (Human hypertrophic scar fibroblasts) | 25,50,75,100,150,200 μM | ↓RhoA/ROCK signal cascade, α-SMA, F-actin, myosin light chains ↓TNF-α, IL-6, IL-1β, IL-8, TLR-4 ↑PPARγ |
[[86], [87], [88]] | |
| Flavonoid | Galangin | Alpinia officinarum Hance | In vitro (Human hypertrophic scar fibroblasts, human dermal fibroblasts) | 5, 10 or 25 μM | ↓ALK5/Smad2/3 signaling pathway ↑Smad 7 ↓COL-Ⅰ, COL-Ⅲ, collagen deposition |
[[89], [90], [91], [92]] |
| In vivo (Mouse hypertrophic scar model) | 10 μM | |||||
| In vivo (Rabbit ear hypertrophic scar Models) | 0.5,1,2 mg/mL | |||||
| Quercetin | Alpinia officinarum Hance, Tussilago farfara L., Taxillus sutchuenensis (Lecomte) Danser, Panax notoginseng (Burkill) F.H.Chen ex C.Y.Wu & K.M.Feng, and Euphorbia characias L. | In vitro (Hypertrophic scar fibroblasts) | 5–20 μg/mL | ↑JNK, ERK signaling pathways ↓TGF-β/Smad signaling pathway ↑TNF-α, nitroxide, IL-12, MMP-1 ↓macrophages, myofibroblasts, Smad2, Smad3, Smad4 |
[37,93,94] | |
| In vitro (Human skin fibroblasts) | 10–40 μM | |||||
| In vitro (RAW264.7 macrophage cell line) | 6.25,12.5, 25,50 μM | |||||
| In vivo (hairless mice) | – | |||||
| In vivo (New Zealand rabbits) | 100 μL of 5 % | |||||
| Naringin | Rhizoma Drynariae (Kunze) J. Sm. And citrus fruits | In vitro (Human hypertrophic scar fibroblasts,human keloid fibroblasts) | 10,20 μM | ↓TGF-β, IL-1β, IL-6, TNF-α, Aktp−Ser473/Thr308 ↓COL-Ⅰ, COL-Ⅲ, α-SMA |
[[95], [96], [97]] | |
| In vivo (hypochlorous acid (HOCl)-induced mouse model) | 50 mg/kg/BW | |||||
| In vivo (mechanical stretch-induced hypertrophic scar mouse model) | 25,50 μM | |||||
| Quinone compound | Emodin | Reynoutria japonica Houtt., Pleuropterus multiflorus (Thunb.) Turcz. ex Nakai, Rheum palmatum L., Rheum tanguticum (Maxim. ex Regel) Balf., and Rheum australe D. Don | In vitro (Rat macrophages) | 0, 25, 50, or 75 μg/mL | ↓TGF-β and Notch signaling pathways in macrophages, PI3K/AKT signaling pathway, p38 MAPK signaling pathway ↓TNF-α and MCP-1 ↓M1, M2, monocytes, HSFs, COL-Ⅰ, COL-Ⅲ and α-SMA |
[30,98,99] |
| In vitro (Human hypertrophic scar fibroblasts) | 10,20,50μΜ | |||||
| In vitro (Mouse hypertrophic scar fibroblasts and normal fibroblasts) | 0,20,40,80 or 120 μg/ml | |||||
| In vivo (Rat tail hypertrophic scar model) | 10 mg/kg | |||||
| In vivo (Mouse hypertrophic scar model induced by mechanical stress) | 10 mg/kg | |||||
| Shikonin | Lithospermum erythrorhizon Sieb. et Zucc., Onosma paniculate Bureau & Franch., and Arnebia euchroma (Royle ex Benth.) I.M.Johnst. | In vitro (human keratinocytes, Hypertrophic scar-derived fibroblasts and normal fibro blasts) | 0,0.5, 1 and 3 μg/mL | ↑NF-κB signaling pathway ↓ERK/Smad signaling pathway ↑p-ERK, p-JNK ↓COL1A1, COL3A1, α-SMA, p-p38, Caspase 3, Bcl-2, p63, CK10, α-SMA, TGF-β1 and COL-Ⅰ |
[44,[100], [101], [102]] | |
| In vitro (porcine full-thickness burn hypertrophic scar model) | 1 mL of 1.0 μg/mL | |||||
| Saponin compound | Asiaticoside | Centella asiatica (L.) Urb. | In vivo (rabbit ear scar model) | 12 mg/kg/d 5,10 g/L |
↓COL-Ⅰ, COL-Ⅲ, TGF-β1, IL-1β, IL-6, IL-8 ↑Smad 7, PPAR-γ |
[[103], [104], [105]] |
| In vitro (adult human dermal fibroblasts, mouse RAW 246.7 macrophages and Human umbilical vein endothelial cells) | 0, 62.5, 125, 250, 500, and 1000 M | |||||
| In vitro (Human normal skin fibroblasts and hypertrophic scar fibroblasts) | 5,25,50,100,250,500 mg/L | |||||
| Astragaloside Ⅳ | Astragalus membranaceus (Fisch.) Bge. | In vitro (rabbits wounded skin) | 0.5g | ↓COL-Ⅰ and COL-Ⅲ ↓TGF-β1 |
[106] | |
| In vivo (rat full-skin excision model) | 0.5 % 0.5 mg every 2 d |
|||||
| In vivo (rat partial-thickness burn wound model) | 0.5 mg every 2 d | |||||
| In vitro (Human skin fibroblasts) | 0,12.5,25,50,100μΜ | |||||
| Ginsenoside | P. Ginseng C.A.Meyer | In vivo (rabbits ear hypertrophic scar model) In vitro (Human hypertrophic scar fibroblasts) |
0.07,0.28,0.56 mg (Rb1) 4 mg/L 1,2,3,4 mg/mL 10, 30 and 50 μg, 50,100 μg/ml 25,50,75,100,200 μg/mL |
Rb1 ↓MMP-2, TIMP-1, α-SMA, COL-Ⅰ, TGF-β1 |
[107] | |
|
Rg3 ↓NF-IκB, TGF-β/Smad, Erk1/2 Signaling Pathways ↓neutrophils,COL-Ⅰ, COL-Ⅲ, α-SMA, VEGF, CTGF, IL-6, MCP-1, TNF-α, VEGF, PDGF |
[108,109,110,111] | |||||
| Oleanolic acid | Olea europaea L. and ligustrum lucidum Ait. | In vivo (rabbits ear hypertrophic scar model) | 2.5 %, 5 %, and 10 % | ↓TGF-β1, TIMP-1, COL-I, COL-III ↑MMP-2, Bax/Bcl-2, Cytochrome c, AIF, Caspase-9, Caspase-3 ↑p38 MAPK, JNK signaling pathways |
[43,112] | |
| In vitro (Human hypertrophic scar fibroblasts) | 10,20,40 μg/ml | |||||
| In vivo (rabbits ear hypertrophic scar model) | – | |||||
| In vitro (Mouse fibroblast NIH/3T3) | 0.001,0.01,0.1,1,10 mM | |||||
| In vivo (Murine dorsal skin wound model) | 0.001,0.1,10 mM | |||||
| Sesquiterpene compound | Artesunate | Artemisia annua L. | In vivo (rabbits ear hypertrophic scar model) | 0.48 %,0.96 %,1.92 % 20 μl/cm2 |
↓TGF-β/Smad3 signaling pathway ↑Fas, BMP-7 ↓TGF-β1, PCNA |
[[113], [114], [115], [116], [117], [118]] |
| Alkaloid | Tetrandrine | Stephania tetrandra S. Moore | In vitro (Human hypertrophic scar fibroblasts) | 20,80,320 μg/ml 2.5, 7.5, 10, 12.5 μg/ml 5 mg/mL |
↓TGF-β/Smad signaling pathway ↑Smad7 ↓Smad2, TGF-β1, COL-Ⅰ, COL-Ⅲ Affect HSF miRNA expression profile |
[119,120] |
| Others | Honey extract | Honey | Clinical trial (Patients with first and second degree burns of less than 50 % of the total body surface area) | – | Improve hyperplastic scar | 161–163 |
| Clinical trial (Plastic surgical patients with Bilateral symmetric incisions) | – | |||||
| Onion extract | Allium cepa L. (Onion) | In vitro (Fibroblast human cell lines) | 50,250,100 μg/mL | ↓fibroblasts, ECM ↑MMP-1 Improve scar color |
37,164 | |
| Clinical trial (Patients after median sternotomy incisions) | Topical application | |||||
| In vitro (Human skin fibroblasts) | 0,10,20,40 μM | |||||
| In vivo (Hairless mice SKH-1) | 0.05 and 1 % | |||||
| Ligusticum chuanxiong extract | Ligusticum chuanxiong Hort. | In vitro (Human hypertrophic scar fibroblasts) In vivo (rabbits ear hypertrophic scar model) |
12.5,25,50,100,200 μg/mL (EO) 5,10,20,40 μM(TMP) 5, 10, and 20 % (EO) |
EO ↑MMP, Caspase-3, ROS ↓TGF-β1, COL-Ⅰ, COL-Ⅲ |
[121,122] | |
|
TMP ↓fibroblasts ↓COL-Ⅰ, COL-Ⅲ and α-SMA, Bcl-2, Caspase-3, p-AKT ↑cleave Caspase-3 and Bax |
[123] | |||||
| Panax noteginseng saponins | Notoginseng Radix et Rhizoma | In vitro (Hypertrophic scar fibroblasts and normal human dermal fibroblasts) | 0, 200, 300, 400 μg/mL | ↓PI3K/AKT signaling pathway ↓TRPM7, CTGF, α-SMA ↑MMP-1, NO |
[[124], [125], [126]] |
↓: the indicator goes up↑:the indicator goes down.
3.1. Polyphenols
3.1.1. Curcumin
Curcumin is a natural polyphenol compound extracted from the rhizomes of some plants of the Araceae and Zingiberaceae families. It is a major biologically active compound used in the traditional Chinese medicine Curcuma longa L. Curcuma longa L. extract mucoadhesive formulation accelerates skin wound repair by regulating inflammatory process and stimulating epithelial regeneration [127].
Curcumin exerts anti-inflammatory effects through the downregulation of nuclear factors NF-κB, COX-2, STAT3, and Nrf2 [128]. Further, curcumin inhibits the TGF-β1/Smad signaling pathway in fibroblasts to reduce the overproduction of ECM and also inhibits NF-κB and CTGF signaling pathways, thereby reducing collagen deposition [64]. It also reduces the secretion of IL-6, IL-8, IL-1β, TIMP-1 and TGF-β and increases the expression of MMPs and the activity of peroxisome proliferator-activated receptor (PPAR)γ [64,65]. Curcumin induces apoptosis in a cell- and concentration-dependent manner in various cell lines [129]. Lundvig et al. found that curcumin induced caspase-independent apoptosis in both fibroblasts and myofibroblasts in a reactive oxygen species (ROS)-dependent manner [42]. Mehrabani reported that the systemic administration of pure curcumin significantly promoted non-ischemic wound healing and reduced HS in a rabbit model [64].
However, the bioavailability of curcumin is low because of its inherent low water solubility and stability in vivo, thereby limiting its clinical efficacy [130]. Curcumin nanoplexes can improve the clinical bioavailability of curcumin by increasing the degree of supersaturation and improving the chemical stability of curcumin [131]. Nguyen et al. developed high-payload amorphous nanocomposites of curcumin (CUR) and chitosan (CHI) to enhance the solubility of CUR through polyelectrolyte complexes. The colloidal stability of CUR–CHI nanocomposites can also be improved by replacing CHI with its oligomer. The application of CUR–OCH nanoplexes and oligochitosan-coated curcumin-loaded-liposome (OCH-Lip-CUR) on wounds resulted in faster healing and a better scar treatment effect compared with the application of natural curcumin [132,133].
3.1.2. Epigallocatechin-3-gallate
Green tea, picked from the tea plant Camellia sinensis (L.) Kuntze, is characterized by a high polyphenol content [134]. Green tea extract may significantly inhibit the production of COL-I by interfering with PI-3K/Akt/mTOR signaling pathway, indicating that it has therapeutic potential in the intervention and prevention of keloids and other fibrous diseases [7]. Epigallocatechin-3-gallate (EGCG) is a catechin monomer extracted from tea leaves and one of the main components responsible for the biological activity of tea polyphenols [66].
Several recent studies have elaborated on the role of EGCG in fibrosis. EGCG plays a potential role in preventing fibrosis in many organs by inhibiting the expression of VEGF, CTGF and TGF-β1[[67], [68], [69], [70]]. EGCG is thought to promote wound healing and prevent scar formation during the inflammatory and proliferative phases [135]. Klass et al. stimulated TGF-β1-related excessive scar formation in wound closure and observed that EGCG downregulated the fibrotic reaction in cell culture and reduced the production of COL-I [71]. Two clinical trials by Ud-Din et al. have shown that EGCG may be used to treat skin scars by reducing blood flow, skin thickness, mast cell activity, angiogenesis, increasing heme oxygenase-1 levels, and increasing M2 macrophage count, elastin, antioxidant activity, and hydration [66]. The preventive use of EGCG before the wound was induced by surgery had a beneficial effect on scar formation in human skin [32]. Li et al. found that EGCG had a synergistic effect on wound healing induced by mesenchymal stem cells (MSC) in vivo. EGCG specifically altered the expression of fibrosis-related genes in bone marrow MSCs to endow them with anti-scar properties, which may be associated with the decreased expression of TGF-β1 and increased expression of TGF-β3. In addition, EGCG reduced the expression and secretion levels of pro-inflammatory cytokine genes TNF-α, IL-1β, and IL-6 [72]. In an in vitro study, EGCG downregulated the expressions of α-SMA, fibronectin, mast cell trypsin and chymotrypsin, TGF-β1, CTGF, and PAI-1 [73]. However, the clinical use of EGCG is limited by its low bioavailability because of its instability under alkaline conditions of the intestinal and circulatory systems. Xu et al. suggested that local application may be an ideal strategy to fully realize the function of EGCG [135].
3.1.3. Resveratrol
Resveratrol (Res), synthesized in grape leaves and skins, is a polyphenol compound existing in many Chinese herbs such as Polygonum cuspidatum Sieb. et Zucc., Veratrum nigrum L., and Cassia tora L. Polygonum cuspidatum has long been used as a traditional medicine for burns and skin wounds, and an animal experiment had shown that topical application of its extract significantly possesses wound healing activity [74]. Res has anti-oxidant, anti-inflammatory, anti-cancer, and cardiovascular-protective effects. It also supports skin wound healing, offsets excessive scar formation, and prevents skin photoaging [136].
Res inhibits cell proliferation and induces apoptosis in a variety of cells, thereby playing a therapeutic role in proliferative diseases [75]. Zhai et al. demonstrated that resveratrol treatment of human HSF inhibited their proliferation by inhibiting Smad-2,3,4 and enhancing Smad7 mRNA and protein expression [137]. Moreover, Zeng et al. also demonstrated that Res caused cell cycle arrest and induced apoptosis in a dose- and time-dependent manner and down-regulated the mRNA expression of type I and type III procollagen [75]. Bai et al. found that the expression of Sirtuin1 in HS tissue was inhibited, and Res reduced the production of α-SMA, COL-I, and COL-III in HSF by upregulating SIRT1 and also blocked the activation of normal skin fibroblasts induced by TGF-β1. In addition, in a mouse healing model, ordered and thin collagen fibers similar to those observed during normal wound healing were obtained after the use of Res [76]. The mTOR signaling pathway regulates cell proliferation and differentiation, and the activated mTOR acts on a downstream target protein, p70 ribosomal protein S6 kinase (70S6K), which is a key regulator of protein translation [77]. Tang et al. found that after the intervention of Res on fibroblasts from pathological scars, the mRNA and protein expressions of mTOR and 70S6K were decreased, and they were negatively correlated to the Res concentration [78]. Subsequently, the authors found that the mechanism by which Res inhibits HSF proliferation may involve decreasing the expression levels of AKT and mTOR [79]. Starvation stress can induce the expression of autophagy-associated mRNA and proteins in HS [80]. The proliferation and survival of HSFBs may be related to autophagy, and a reduction in autophagy may be involved in the pathogenesis of HS[[81], [82], [83]]. Pang et al. found that Res may promote autophagy by up-regulating the expression of miRNA-4654, which is involved in cell proliferation, cell differentiation, apoptosis and autophagy regulation, while miRNA-4654 down-regulates the expression of Rheb, a key regulator of autophagy in disease states [84]. The wound dressing comprising Res-loaded peptide-hydrogels can accelerate wound healing, inhibit macrophages, reduce pro-inflammatory cytokines, and ultimately prevent scar formation [85].
3.1.4. Gallic acid
Gallic acid (GA) is a polyphenolic compound found in many natural foods and plants, such as gallnuts, sumac, witch hazel, watercress, oak bark, tealeaves, areca nut, bearberry (Arctostaphylos), blackberry, and Caesalpinia Mimosoideae [87]. Rhus coriaria extract has antimicrobial, anti-inflammatory, and antioxidant activity, which support its traditional uses as a wound healing agent, particularly in infected wound [138]. GA exerts anti-oxidant, anti-cancer, anti-viral, anti-inflammatory, and anti-fibrotic effects [139].
Hsieh et al. found that GA significantly inhibited the contraction of HSF stimulated by TGF-β1 in a dose- and time-dependent manner. Further, it also inhibited the expression of α-SMA, the formation of F-actin, and phosphorylation of myosin light chain by downregulating the RhoA/ROCK signal cascade [88]. The growth inhibition, apoptosis, and necrosis of HSF were dependent on the dose of GA. Apoptosis was induced by the Bcl2/Bax mitochondrial-dependent pathway, and necrosis was caused by the calcium elevation/calpain I activation/lysosomal membrane rupture cascade [86]. The expressions of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β, and IL-8) were decreased in the HSF, which may be related to TLR-4/NF-κB/PPARγ pathway [87].
3.2. Flavonoids
3.2.1. Galangin
Galangin, also called 3,5,7-trihydroxyflavone, is extracted from the root of Alpinia officinarum Hance of the ginger family. Topical Alpinia officinarum treatment has an acutely beneficial effect on experimental contact type burns [140].
Galangin significantly inhibits the proliferation and activation of HSF and induces the anti-contraction of HSF in the collagen gel. It significantly inhibited mRNA and protein levels of collagen type-I alpha 2 (Col1A2) and collagen type-III alpha 1 (Col3A1) in the HSF and reduced collagen deposition in a mouse model of HS. Galangin inhibits the ALK5/Smad2/3 signaling pathway, and the overactivation of this pathway is related to many fibroproliferative diseases [89,90]. Galangin directly downregulates the phosphorylated Smad2/3 levels by targeting ALK5-selective inhibition of the Smad2/3 signaling pathway, while total Smad2, Smad3, and Smad4 levels are unaffected [93,94]. Ru et al. found that galangin negatively regulated the TGF-β/Smad signaling pathway by upregulating Smad7, thereby inhibiting the stimulatory effect of TGF-β1 on collagen deposition in the ECM [92].
3.2.2. Quercetin
Quercetin is a flavonoid widely found in food, and its daily intake is approximately 4–68 mg in humans [141]. It is also an active component of many medicinal plants, such as Alpinia officinarum Hance, Tussilago farfara L., Taxillus sutchuenensis (Lecomte) Danser, Panax notoginseng (Burkill) F.H.Chen ex C.Y.Wu & K.M.Feng, and Euphorbia characias L., which have been used as medicinal plants to treat wounds in traditional medicine and showe significant wound healing activity [142].
Cho et al. found that quercetin reduced the proliferation rate of fibroblasts and significantly increased the expression of MMP-1 in a dose-dependent manner. In addition, quercetin may exert anti-scarring effects by upregulating MMP-1 through the JNK and ERK signaling pathways (but not through the p38 signaling pathway) [37]. Phan et al. demonstrated that quercetin can inhibit the proliferation of fibroblasts in the earlobe keloids and HS from burns by arresting cell growth and fibroblast contraction [93]. They further found that quercetin inhibits TGF-β/Smad signaling and its downstream signaling molecules in fibroblasts at the receptor level. Similar results were obtained by Song et al., and quercetin reduced the number of macrophages and myofibroblasts and inhibited cellular signals that activate macrophages, such as TNF-α, nitroxide, and IL-12, as well as cellular signaling that is activated by dermal fibroblasts in response to the collagen and TGF-β1 treatmentz [94]. Jin et al. combined silicone gel sheets with quercetin with different action mechanisms to produce a synergistic effect of downregulating TGF-β1 expression and reducing collagen formation [143].
3.2.3. Naringin
Three citrus peel extracts; Lemon (Citrus limon (L.) Burm.f.), Grapes fruits (Citrus paradisi Macfad.) and Orange (Citrus sinensis (L.) Osbeck) have been proven to promote wound healing in experimental animals [144]. Naringin is a natural citrus flavonoid, which mainly exists in citrus fruits and is also one of the main active components in Chinese herbal medicines such as Rhizoma Drynariae (Kunze) J. Sm.
Mohammadi et al. found that naringin prevented the complications in an HOCI-induced skin fibrosis model by reducing collagen production and serum TGF-β levels and downregulating COL-I, COL-III, and α-SMA [95]. Shan et al. found that the retention of CD4+ T lymphocytes and CD68+ cells (monocytes/macrophages) and the levels of IL-1β, IL-6, and TNF-α were inhibited by naringin in an animal model. The local application of naringin in a mouse model with HS significantly inhibited the formation of HS, fibroblast activation, and local inflammation [96]. Song et al. found that naringin specifically inhibited the formation of HS by inhibiting Aktp−Ser473/Thr308 in an in vitro experiment and inhibited the proliferation and migration of fibroblasts through the Akt signaling pathway and promoted the apoptosis of fibroblasts [97].
3.3. Quinones
3.3.1. Emodin
Emodin, a natural anthraquinone derivative, has been used for more than 2000 years as a component of some Chinese herbal medicines such as Reynoutria japonica Houtt., Pleuropterus multiflorus (Thunb.) Turcz. ex Nakai, and Radix et Rhizoma Rhei (Dahuang), including Rheum palmatum L., Rheum tanguticum (Maxim. ex Regel) Balf., etc. Rheum australe D. Don was used for cure traditionally [145].
Macrophage polarization is involved in regulating phagocytosis and tissue fibrosis in wound healing, which is mediated by the Notch signaling pathway. Xia et al. found that emodin suppresses HS formation and fibrosis, possibly by suppressing M1/M2 macrophage polarization and TGF-β and Notch signaling. Emodin regulated the polarization of macrophages; the M1 phenotype reduced the early inflammatory response to wound healing and the M2 phenotype inhibited late TGF-β expression [30]. Based on animal studies, Liu et al. demonstrated that emodin may inhibit mechanical stress-induced HS inflammation by attenuating inflammatory cell recruitment and adhesion and suppressing the secretion of inflammatory cytokines by inhibiting the PI3K/Akt signaling pathway [99]. He et al. showed that emodin inhibited HS proliferation and induced apoptosis by suppressing collagen synthesis and transdifferentiation of fibroblasts to myofibroblasts. In addition, emodin exerted anti-fibrotic effects by interfering with the p38 MAPK signaling pathway [98].
3.3.2. Shikonin
Shikonin (SHI) is a major active component extracted from the dried roots of traditional Chinese herbal medicine Zicao (Lithospermum erythrorhizon Sieb. et Zucc., Onosma paniculate Bureau & Franch., and Arnebia euchroma (Royle ex Benth.) I.M.Johnst.), which has been clinically used for the treatment of burns and scars for thousands of years. Ether and water extracts of Lithospermum erythrorhizon all can enhance wound healing [146].
Xie et al. found that SHI downregulates the expression of the collagen, type I, alpha 1 (COL1A1), collagen, type III, alpha 1 (COL3A1) and α-SMA [147]. SHI inhibited cell proliferation and induced apoptosis in HSF and human skin keratinocytes (Kc), whose delayed function in re-epithelialization is associated with the formation of HS. Further, SHI upregulated phosphorylated ERK and phosphorylated JNK in Kc and HSF and downregulated phosphorylated p38, caspase-3, and Bcl-2 expressions. Different concentrations of SHI can induce the inhibition of collagen production by fibroblasts without affecting cell proliferation and inhibition of HSF proliferation and apoptosis induction without affecting the function of Kc. In addition, SHI also activated the inflammatory NF-κB signaling pathway in the HSF and reduced TGF-β1-induced collagen production through downregulated ERK/Smad signaling pathway. Further, SHI weakened the TGF-β1-induced cellular contraction by downregulating the expression of α-SMA in the HSF [44,100]. Fan et al. studied the effects of SHI and its analogs on HS-associated cells and found that SHI and naphthazarin were most effective in inhibiting the activity of HSF and collagen synthesis [101]. In a porcine full-thickness burn hypertrophic scar model, SHI treatment resulted in reduced skin thickness (except for the subcutaneous layer), a thin and homogeneous epithelial layer, reduced keratinocytes, a regular distribution of fibroblasts, and uniform and loosely arranged collagen fibers in the dermis. Immunohistochemistry findings revealed that the expression of p63, cytokeratin 10, α-SMA, TGF-β1, and COL-I was suppressed [102].
3.4. Saponins
3.4.1. Asiaticoside
Centella asiatica (L.) Urb. is a perennial herb of the Umbelliferae family and has been historically used for treating skin injuries and diseases, such as burns, ulcers, eczema, psoriasis, and HS. Centella asiatica extracts act in multiple phases of the cutaneous repair process [148]. Its active ingredients include asiaticoside (AS; highest activity), asiatic acid, madecassic acid, and other unidentified compounds [99,149].
The research on the mechanism of AS in HS treatment mainly focuses on the TGF-β/Smad signaling pathway. Xie et al. found that the animals treated with a high concentration of AS ameliorated the histological characteristics of scars, significantly reduced the expression of TGF-β1, and enhanced the expression of Smad7 mRNA and protein [103]. Huang et al. observed reduced scar thickness, suppressed gene expression of COL-I, COL-III, TGF-β1, IL-1β, IL-6, and IL-8, and increased expression of Smad7 and PPARγ genes in an animal model after oral administration of AS. Researchers suggested that the low bioavailability of AS could be overcome by administering sufficient doses of it because the side effects were almost undetectable [104]. Qi et al. reported that AS upregulates the expression of Smad7 to inhibit TGF-β signaling through a negative feedback loop [105].
AS does not easily pass through the stratum corneum after local administration because of its high molecular weight, low water solubility, and low lipophilicity. AS-loaded nanoemulsion-based gels and AS-laden silk nanofiber hydrogels designed by Liu et al. effectively and safely allow penetration of the drug into the skin without impairing the biological activity of AS [51,150].
3.4.2. Astragaloside IV
Astragalus membranaceus (Fisch.) Bge., having a long history of medicinal use and is often used for the repair and regeneration of damaged organs and tissues, contains several active substances, including saponins, polysaccharides, and flavonoids. Radix Astragali, the dried root of Astragalus membranaceus (Fisch.) Bge., and Radix Rehmanniae significantly enhanced the viability of fibroblasts isolated from foot ulcers in diabetic patients [151].
Chen et al. found that astragaloside IV treatment effectively inhibited scar complications of wound healing. It reduced the proportion of COL-I and COL-III in a dose-dependent manner, which were secreted by fibroblasts in the wound remodeling phase, and also decreased the level of TGF-β1 without inducing any cytotoxicity to fibroblasts [106]. However, astragaloside IV is a sparingly soluble substance with low viscosity. Chen et al. used solid lipid nanoparticles to develop the astragaloside IV -loaded nanoparticle-enriched hydrogel, which prolonged the residence time of the drug on the skin and had good compatibility and moisturizing effects [152]. Shan et al. and Zhang et al. found that silk fibroin/gelatin (SF/GT)-nanofiber dressings significantly enhanced cell adhesion and proliferation in vitro. Compared with the astragaloside IV solution, SF/GT electrospun nanofiber dressing loaded with astragaloside IV demonstrated better inhibition of the expression of α-SMA [153,154].
3.4.3. Ginsenoside
The use of ginseng has a long history, and seven major species of it have been identified. Panax ginseng C. A. Mey. is the most commonly used species and has various biological activities helpful for wound healing and stimulating human dermal fibroblast proliferation and collagen synthesis [155]. Ginsenosides, the main biologically active compounds of ginseng [156]. The effects of Rb1 and Rg3 on HS have been extensively studied.
Tark et al. found that Rb1 levels were negatively correlated with mRNA expression and immunohistochemical reactivity of MMP-2, TIMP-1, α-SMA, and TGF-β1 in an animal model. In addition, Rb1 inhibits the expression of COL-I [107]. IκB participates in the inflammatory response through phosphorylation, leading to nuclear translocation of NF-κB and upregulation of the HS-related genes [108]. Ma et al. found that Rg3 inhibited the IκB phosphorylation, thereby reducing NF-κB-mediated inflammation in HS. In addition, Rg3 also inhibited the synthesis of COL-I and COL-III and induced fibroblast apoptosis [108]. Cheng et al. found that Rg3 inhibited HSF proliferation and induced HSF apoptosis, thereby reducing the accumulation of collagen fibers. Moreover, Rg3 downregulated the expression of VEGF in the HS tissue [109]. An optimal drug delivery system is critical for Rg3 because of its low solubility for maximizing its effects. Cui et al. developed an Rg3/PLA electrospun fiber scaffold that could rapidly reduce fibroblast growth and recover the structural and functional characteristics of injured skin [157]. Cheng et al. successfully prepared implantable biodegradable GS-Rg3-loaded PLA fibrous membranes using co-electrospinning technology to control drug release and improve drug utilization. Their results showed that the VEGF protein level in the Rg3/PLA electrospun membrane treatment group was much lower than that in other control groups [110]. In addition, they also prepared poly (lactic-co-glycolic acid) (PLGA) electrospun fibers carrying Rg3 by co-solvent electrospinning and coated the surface of the drug-loaded electrospun fibers with hyaluronic acid using pressure-driven permeation coating. This system exerted the combined effect of promoting the healing of rabbit ear trauma and inhibiting the formation of HS [111]. Further, they explored the specific functional mechanism of Rg3, and the results showed that the Rg3 treatment downregulated the mRNA and protein levels of COL-I, COL-III, fibronectin, and pro-fibrosis molecules, such as CTGF, IL-6, MCP-1, TNF-α, and α-SMA. In addition, MMP-2/TIMP-1, MMP-9/TIMP-2, and MMP13/TIMP-1 ratios were increased, indicating either inhibition or reversal of HS fibrosis. Rg3 inhibited cell proliferation and angiogenesis and reduced ECM deposition of HSF through TGF-β/Smad and Erk1/2 signaling pathways without delaying the wound healing process [158].
3.4.4. Oleanolic acid
Oleanolic acid (OA) is a type of natural pentacyclic triterpene that is mainly extracted from the leaves of Olea europaea L., fruits of ligustrum lucidum Ait. The whole grass Swertia mileensis T. N. Ho et W. L. Shi. Olive leaf extract demonstrated strong in vivo wound healing activity due to its antioxidant and antimicrobial features [159].
The expression levels of P311 mRNA and protein were significantly upregulated in human HS tissue [160]. Zhang et al. found that OA may inhibit TGF-β1 production in fibroblasts, reduce the secretion of TIMP-1, increase the activity of MMP-2, and then upregulate the degradation of COL-I and COL-III, to ameliorate the scar formation by interfering with the expression of the P311 gene [112]. Wei et al. determined the inhibitory effect of OA on HS formation in rabbit ears and observed similar results [161]. In China, Zhangshi-Ba-Hen-ZhiYang-Ruan-Hua ointment is used to treat HS by promoting blood circulation to soften the scars, remove dampness, and relieve itching. Zhang et al. found that several active ingredients, including OA, significantly inhibited cell survival and caused apoptosis. OA promoted the translocation of Bax from the cytoplasm to mitochondria, increased the Bax/Bcl-2 protein ratio, and disrupted the permeability of the mitochondrial membrane, which resulted in the loss of MMP and increased the release of mitochondrial cytochrome c and apoptosis-inducing factor, activated caspase-9 and -3. The activated caspases induced the apoptosis of HSF, which was associated with the activation of p38 MAPK and JNK signaling pathways but not the ERK pathway. Notably, caspase-8 was not activated during this process, suggesting that OA does not induce HSF apoptosis through the death receptor pathway. In addition, the acute toxicity test showed that OA was not toxic to normal cells [43].
3.5. Sesquiterpenes
3.5.1. Artesunate
Artesunate (ART) is an artemisinin derivative isolated from the Chinese medicinal plant Artemisia annua L., which is extensively used in malaria treatment and also used for the fabrication of wound dressing [162].
Nong et al. found that ART promoted wound healing and reduced fibroblast proliferation and collagen production by inhibiting the TGF-β/Smad3 signaling pathway in a dose-dependent manner. In addition, ART also weakened the TGF-β1/Smad3-induced contraction of HSF-containing gel [113]. ART is often used in combination with other treatments in clinical practice. Zhang et al. reported that ART combined with 595-nm pulsed dye laser (PDL) or fractional CO2 laser (FCO2L) was associated with the expression of bone morphogenetic protein-7 (BMP-7) and Fas. BMP-7, a member of the TGF-β superfamily, reduces fiber production in HS and other tissues. The Fas gene is an important factor in triggering the Fas-associated death domain and activating-induced cell death to mediate apoptosis. These proteins inhibit the formation of HS by inducing fibroblast apoptosis in the scar tissue [114,115]. According to the results of the two experiments conducted by Zhang et al., the HS in PDL, ART, FCO2L, PDL + ART, and FCO2L + ART groups was inhibited, and the expression of Fas and BMP-7 proteins was increased, specifically in the combined treatment group [116,117]. In addition, the authors also found that the therapeutic effect of ART combined with FCO2L was related to the inhibition of TGF-β1 and proliferating cell nuclear antigen, which is a nuclear protein closely related to cell cycle, DNA synthesis, fibroblast proliferation, and HS formation [118].
3.6. Alkaloids
3.6.1. Tetrandrine
Tetrandrine, a dibenzylisoquinoline alkaloid, is derived from Stephania tetrandra S. Moore and other related species of the family Menispermaceae, which has been used in China as traditional Chinese medicine “Fang Ji” for eczema and inflamed sores over a thousand years [163].
Liu et al. found that tetrandrine significantly inhibited the proliferation of HS fibroblasts in a dose-dependent manner [164]. Tetrandrine inhibited TGF-β1 transcription and its intracellular signaling, at least in part by inducing Smad7 and inhibiting Smad2 expressions, and reduced the production of COL-I and COL-III and inhibited cell regeneration activity by blocking the TGF-β/Smad signaling pathway [119]. Subsequently, the authors explored the effect of tetrandrine on the expression profile of HSF microRNA in vitro. The results showed that tetrandrine-treated HSFs were less in number and had smaller and rounder cell shapes with shorter or absent spindles compared with the control cells. In addition, the expression levels of 186 microRNAs in the experimental group were decreased, whereas the expression levels of 7 microRNAs were increased. Many of these microRNA targets were associated with important signaling pathways associated with scar wound healing, including VEGF, apoptosis, and cell cycle [119,120].
3.7. Other natural products
3.7.1. Honey extract
Honey has been used for thousands of years for its medicinal properties worldwide, especially as the oldest wound dressing. Modern pharmacological studies have shown that honey promotes wound healing through anti-bacterial, anti-inflammatory, and anti-oxidant effects. It also boosts the immune system and cleanses and regenerates wounds [165].
In a randomized clinical trial, Goharshenasan et al. found that the use of honey dressing improved the healing process of surgical wounds and its final aesthetic effect was superior to that of the traditional dressing [166]. Gupta et al. conducted a retrospective study, and the results showed that surface honey dressings were more effective than sulfadiazine dressings in treating HS [167]. In addition, honey has no significant allergic reactions or side effects in clinical applications, and it is considered a safe, economical, convenient, and beneficial wound treatment dressing. However, scar scores were not significantly different between patients using honey and those not using honey (controls) [168].
3.7.2. Onion extract
Contractubex gel is used for the desalination of old and new scars and even pockmarks. It contains onion (Allium cepa L.) extract, heparin, and allantoin and is thought to have anti-HS and anti-keloid effects. Onion extracts have an inhibitory effect on fibroblasts, which, in turn, reduces their proliferative activity and the production of ECM [169]. Pikuła et al. found that both onion extract and enoxaparin induced apoptosis of dermal fibroblasts and inhibited the expression of β1 integrin expressed by fibroblasts at selected concentrations. Notably, β1 integrin is necessary for fiber formation [170]. In a clinical trial, Jenwitheesuk et al. showed that patients with HS who were treated with the silicone derivatives plus onion extract gel had significantly lighter pain, itching, and pigmentation than those who received placebo gel treatment; however, there was no significant improvement in terms of vascularity, pliability, or height [171]. Similarly, Hosnuter et al. investigated the therapeutic effects of onion extract alone and in combination with silica gel tablets. The results showed that onion extract was statistically more effective in improving scar color and ineffective in ameliorating scar height and itching, whereas silica gel tablets were more effective in reducing scar height. Therefore, the authors suggested that the best therapeutic effect can be achieved only by combining different treatments [172].
Although the preventive and therapeutic effects of onion extract on HS have been reported in many clinical trials, its specific mechanism of action has not been clarified yet. Cho et al. found that onion extract upregulated the expression of MMP-1 in cellular and animal experiments, thereby modifying ECM; however, it did not significantly affect the expression of COL-I [37]. Chung et al. found that onion extract gel did not improve the cosmetic appearance or ameliorate the symptoms of scars [173]. Yuan et al. concluded that the onion extract gel did not have the advantages of common local treatments and might increase the incidence of adverse reactions in scar management [174].
3.7.3. Rhizoma chuanxiong extract
Rhizoma chuanxiong (RCX), the dry rhizome of Ligusticum chuanxiong Hort. (LC), is a perennial herb with developed rhizomes. The compounds present in LC are divided into five categories, namely, volatile oils, alkaloids, phenolic acids, phthalate lactones, and other components [175].
Wu et al. demonstrated that essential oil (EO) from rhizomes of Ligusticum Chuanxiong was effective in the treatment of HS. EO can significantly inhibit the cell viability of HSF and induce apoptosis, which is partially achieved by inducing MMP loss, causing excessive ROS accumulation, and triggering caspase-3 activation. EO was applied to HS in rabbit ears and significant inhibition of HS and reduction of scar elevation index were observed. Histological examination also revealed a marked decrease in TGF-β1, COL-I, and COL-III levels and a significant increase in MMP-1 levels [121,122].
Tetramethylpyrazine (TMP), an alkaloid extracted from RCX, has remarkable therapeutic efficacy in fibrotic diseases of various parts, such as the myocardium, lung, and liver. Wu et al. found that TMP inhibited the proliferation of scar fibroblasts and activated apoptosis. Protein expression levels of COL-I, COL-III, α-SMA, Bcl-2, and caspase-3 were significantly decreased, whereas those of cleaved caspase-3 and the pro-apoptotic protein Bax were increased. In addition, PI3K/AKT signaling pathway also participates in the protection of HS by TMP [123].
3.7.4. Panax noteginseng saponins
Panax noteginseng saponins (PNS) are extracted from the root and rhizome of Panax notoginseng (Burkill) F.H.Chen ex C.Y.Wu & K.M.Feng. PNS can promote wound healing, anti-oxidant, and anti-thromboembolism [176].
Zhi et al. found that PNS inhibited collagen accumulation by reducing CTGF expression while increasing MMP-1 expression, thereby inhibiting HS formation [124]. PNS inhibited scar formation by regulating the PI3K/AKT signaling pathway, inhibiting ECM, and stimulating apoptosis. The channel-kinase transient receptor potential ion channel subfamily M member 7 (TRPM7) is a potential pharmacological target in PNS that promotes excessive ECM deposition and is associated with proliferative diseases [125]. The authors suggested that PNS plays a role by inhibiting TRPM7 to modulate PI3K/AKT signaling pathway; therefore, TRPM7 may be a new therapeutic molecular target for HS [125]. In addition, Men et al. found that PNS increases nitric oxide levels in fibroblasts (3T3 cell line), inhibits cell proliferation, and accelerates wound healing in mouse skin. PNS reduced the accumulation of fibroblasts and subsequently the expression of α-SMA in wounds [126].
4. Network target prediction
Traditional Chinese medicine (TCM) is characterized by its multi-component, multi-target, and holistic nature. Network pharmacology allows for systematic prediction, offering valuable insights into the synergistic mechanisms between active components of TCM and facilitating the identification of potential drug targets [177]. This review employs network pharmacology to elucidate the action mechanisms of natural products in the context of HS. The application of network pharmacology to understanding HS provides a methodological and theoretical foundation for exploring the pharmacological mechanisms of natural products associated with HS and for the development and utilization of therapeutic agents.
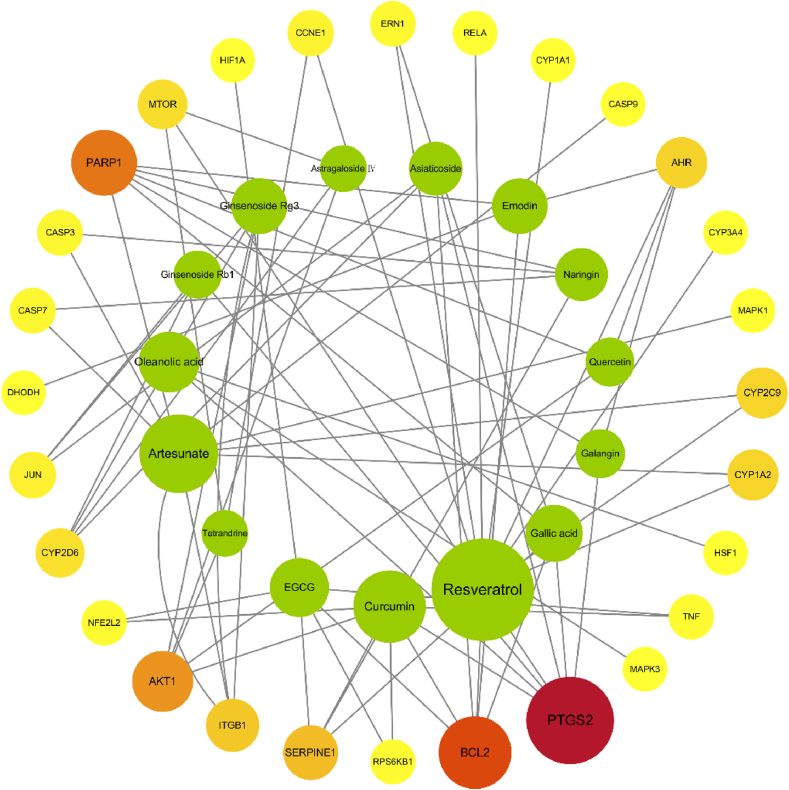
We conducted a validation of 28 HS targets using the SwissTargetPrediction, MalaCards, and ctdbase databases, focusing on fifteen specific pure natural products (Curcumin, Resveratrol, EGCG, Gallic acid, Galangin, Quercetin, Naringin, Emodin, Asiaticoside, Astragaloside Ⅳ, Ginsenoside Rg3, Ginsenoside Rb1, Oleanolic acid, Artesunate, and Tetrandrine) mentioned earlier. To visualize the interactions, we constructed a compound-target network using Cytoscape 3.6.1 software. This compound-target network was predicted using network pharmacology and demonstrates the relationships between the natural products and their respective targets. After that, we calculated and analyzed the topological properties of the following three data for each node in the PPI network using the Cytoscape software plug-in CytoNCA (degree centrality, betweenness centrality, and closeness centrality. And the size of the circle representing each node is determined based on the value of betweenness centrality. Fig. 4 depicts this network, where green circles represent the natural products and the different colored circles represent the targets. The color transition from yellow to red indicates the increasing value of the betweenness centrality of the targets in the process of HS treatment. Among the natural products, Resveratrol has the highest number of candidate targets, followed by Artesunate, Curcumin, Oleanolic acid, EGCG, Gallic acid, Ginsenoside Rg3, Emodin, Asiaticoside, Naringin, Galangin, Quercetin, Ginsenoside Rb1, Astragaloside Ⅳ, and Tetrandrine. The candidate targets identified in the progression of HS mainly involve inflammation-related proteins (TNF, PTGS2, RELA, etc.), apoptosis-related proteins (CASP7, CASP3, BAX, BCL2, AKT1, PARP1, ITGB1, etc.), oxidation-reduction-related proteins (CYP1A2, CYP2C9, CYP3A4, CYP1A1, CYP2D6, etc.), as well as angiogenesis-related proteins (SERPINE1). Notably, inflammation and apoptosis-related targets (e.g., PTGS2, BCL2, PARP1, AKT1) exhibit a higher correlation, indicating the potential therapeutic effect of these natural products on HS by modulating these specific proteins. as well as angiogenesis-related proteins (SERPINE1). Notably, inflammation and apoptosis-related targets (e.g., PTGS2, BCL2, PARP1, AKT1) exhibit a higher correlation, indicating the potential therapeutic effect of these natural products on HS by modulating these specific proteins (Fig. 4).
Fig. 4.
The natural product-target network predicted by network pharmacology. The figure was constructed by Cytoscape 3.6.1. Green circles represent the natural products and the different colored circles represent the targets. The size of the circle representing each node is determined based on the value of betweenness centrality. The color transition from yellow to red indicates the increasing value of betweenness centrality of the targets in the process of HS treatment.
Curcumin, Resveratrol, EGCG, Gallic acid, Galangin, Quercetin, Naringin, Emodin, Asiaticoside, Astragaloside Ⅳ, Ginsenoside Rg3, Ginsenoside Rb1, Oleanolic acid, Artesunate, and Tetrandrine were subjected to target prediction using the SwissTargetPrediction database. The obtained protein targets were then used to construct a compound-target network using Cytoscape 3.6.1. On the other hand, HS targets were identified from the MalaCards and ctdbase databases. The intersection of compound targets and disease targets was determined to identify the overlapping targets. These coinciding targets were further analyzed using the STRING database to construct a protein-protein interaction (PPI) network (Fig. 5).
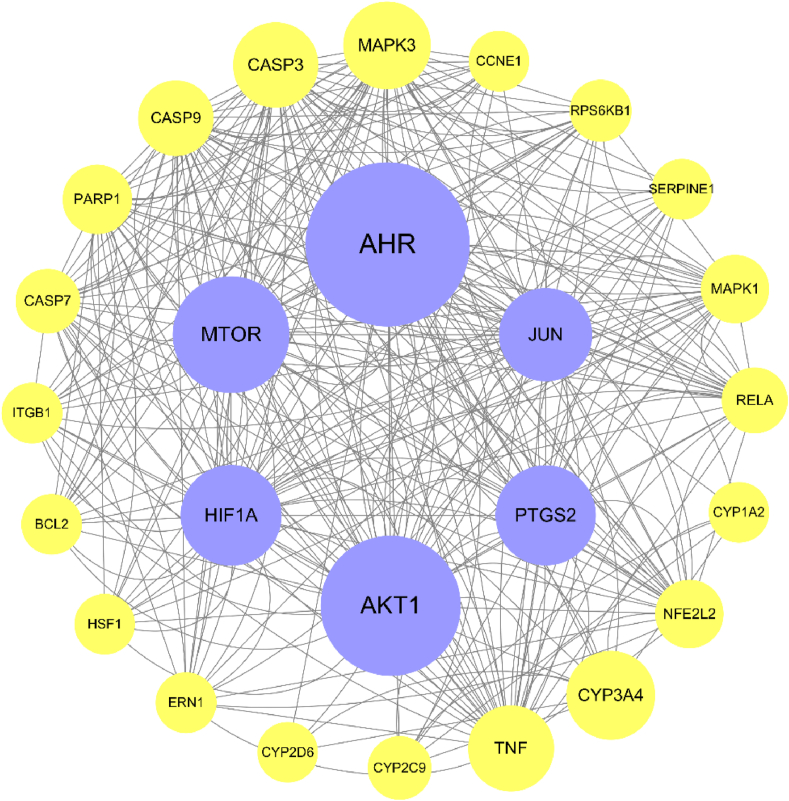
Fig. 5.
The PPI network. The figure was constructed by Cytoscape 3.6.1. The size of the circle representing each node is determined based on the value of betweenness centrality. AHR, AKT1, MTOR, PTGS2, HIF1A, and JUN emerge as the pivotal targets within the network.
As depicted in Fig. 5, AHR, AKT1, MTOR, PTGS2, HIF1A, and JUN emerge as the pivotal targets within the network. AHR is found in mast cells and upon activation, it stimulates the production of IL-17 and ROS, thereby influencing the inflammatory response [178]. AKT1 and MTOR regulate cell growth via the PI3K/AKT/mTOR pathway, which plays a crucial role in inflammation and apoptosis. Inhibition of this pathway can impede HSF growth, migration, and ECM accumulation through autophagy activation [179]. PTGS2, also known as cyclooxygenase-2 (COX-2), is involved in the inflammatory response and modulates cell proliferation and apoptosis. Its overexpression exerts significant regulatory effects on aberrant fibrogenic responses and proinflammatory mediators [180]. HIF1A contributes to scar formation during skin wound healing and may contribute to the excessive fibrosis characteristic of hypertrophic scars [181]. JUN is also implicated in the regulation of cell proliferation, apoptosis, and various biological processes. It exhibits high expression levels in HS and serves as a key regulator of pathological skin scar formation [182].
5. Critical considerations
5.1. Mechanism of actions of natural products against hypertrophic scars
Here, we discussed the effects and mechanisms of medicinal plants in treating HS. Our systematic analysis revealed that several natural compounds show anti-HS effects through anti-inflammatory, anti-proliferative, anti-angiogenesis, and pro-apoptoticproperties [64,65,86,93,97,98].
Furthermore, the components of natural products and herbal medicines are complex and act through multiple molecular mechanisms to improve the prognosis of HS. Some of them can also promote wound healing during the healing stage.
Natural products and other treatments for HS have different action mechanisms. Therefore, combined treatments, like quercetin combined with silica gel tablets, artesunate combined with 595-NM PDL, and onion extract combined with silica gel tablets, have better efficacy in alleviating pain, pruritus, and scar thickness [183,169,170,180]. However, most of the researchers have only evaluated the active ingredients of a single natural product. Therefore, combination therapies with natural products should be the focus of research on HS treatment.
5.2. Potential toxicity and side effects
The toxicity and side effects of natural products should be extensively evaluated. The therapeutic effects of natural products involve identifying their pharmacologically active ingredients and evaluating their effects and properties. However, natural products are commonly used in the form of crude extracts; therefore, their safety is questionable. Although most of the natural products summarized in this review have low toxicity and are safe within a certain dose range, a few of them still have certain toxicity and side effects that need further evaluation. For example, adverse reactions, such as allergy and hepatorenal toxicity, related to PNS have been reported in some clinical trials [176]. Emodin may cause hepatotoxicity, nephrotoxicity, and reproductive toxicity, and ginsenoside may cause central nervous excitement [107,184]. Therefore, future studies should focus on developing strategies for the safe administration of drugs within the prescribed dose range. Moreover, novel methods should be developed to extract the maximum amount of active components from crude natural products rather than toxic ones.
5.3. Clinical application and transformation
Natural products are a potential source of new drugs, and some of them have strong preventive and therapeutic effects on HS. However, the discovery and identification of these natural products are generally based on historical experience. Although some natural products have shown efficacy in the treatment of HS, detailed studies on the mechanisms by which they exert their therapeutic effect have not been conducted. Several molecules, such as curcumin, resveratrol, gallic acid, galangin, quercetin, naringin, emodin, shikonin, asiaticoside, astragaloside Ⅳ, ginsenoside, oleanolic acid, artesunate, tetrandrine, ligusticum chuanxiong extract, and panax noteginseng saponins have only been tested in cell lines or animals. Although multiple in vivo and in vitro studies have reported the safety and efficacy of medicinal plants in treating HS, experimental data obtained from animal models are insufficient to confirm their safety and efficacy in humans. Therefore, extensive clinical validation of natural products using approved strategies is required before the final clinical application.
5.4. Improved pharmaceutical preparations of natural products
The therapeutic effect of most natural products on HS is limited because of their low bioavailability. The topical application of drugs is usually preferred for HS treatment. However, the barrier effect of the skin limits transdermal penetration, which hinders the optimal application of the drugs. Therefore, a suitable excipient is required that can enhance the use of natural products with synergistic effects such as promoting wound healing and relieving pain. Many natural products have been designed with carriers that help them penetrate and constantly release into the skin. Some examples of such carrier-based natural products are AS-loaded nano-emulsions, astragaloside IV-loaded nanoparticle-enriched hydrogels, and ginsenoside RG3-loaded electrospun PLGA fiber membranes.
6. Conclusions and perspectives
The molecular mechanisms involved in fibrosis are similar in different tissues. Several natural products have inhibitory effects on fibrosis in multiple organs. Some compounds that are effective in the treatment of HS can also be used for treating myocardial fibrosis, renal fibrosis, liver fibrosis, peritoneal adhesion, and tendon adhesion. These multitargeted natural products also offer a new approach to developing drugs for HS treatment.
We have not included natural products with limited published data in this review. For example, Radix Astragali seu Hedysari and Radix Salviae Miltiorrhizae extracts inhibit HS in rabbits by regulating the TGF-β/Smad signal pathway, osthole inhibits fibroblast growth in HS and reduces TGF-β1 expression through apoptosis, oxymatrine promotes HS repair by reducing the activity of human scar fibroblasts and collagen and induces apoptosis by autophagy inhibition, dihydromyricetin and baicalein attenuate HS formation by targeting TGF-b receptor I, named activin receptor-like kinase 5, Sal-B inhibits HSFs proliferation, migration, fibrotic marker expression and attenuates HTS formation in vivo and in vitro, protocatechuic aldehyde can be used to prevent and treat HS by simultaneously regulating HSF and human umbilical vein endothelial cells, and paclitaxel has a strong inhibitory effect on fibroblast proliferation, collagen deposition, and angiogenesis of HS in rabbit ears [[185], [186], [187], [188], [189], [190], [191], [192]]. In addition, keloids share some similarities with HS in appearance and formation, and researchers have found that some natural products play a role in the treatment of keloid scars; however, there are no studies on their efficacy in treating HS. For example, dihydroartemisinin induces mitochondrial mRNA degradation and apoptosis in keloid fibroblasts, and tanshinone IIA inhibits proliferation and induces apoptosis by downregulating Survivin in the keloid fibroblasts [193,194]. The existing gaps in evaluating the therapeutic potential of these natural products should be further explored.
Although a lot of challenges still exist in terms of the routine clinical use of natural products as established drugs, their diverse biological activities and mechanisms involved in the treatment of HS have been elaborated in several studies. Natural products offer advantages in the treatment of HS, including affordability, proven efficacy, and multitarget therapy. However, several natural products still need to be studied using modern pharmacological approaches, and many new compounds with therapeutic effects on HS may be isolated and identified in the future.
Funding
This study was supported by the National Natural Science Foundation of China (No.82074443), the Scientific and Technological Research Special Program of Sichuan Provincial Administration of Traditional Chinese Medicine (No.22CP1423), and the Hundred Talents Program of Chengdu University Hospital (22-B09). State Administration of Traditional Chinese Medicine “Young Qihuang Scholars" (2022-256), and the “Basic Thickening” Action Plan of Chengdu University of Traditional Chinese Medicine (No.2023–42).
Data availability statement
The data related to this study are not stored in the publicly available repository. This study is a review and the raw data are available in references studies.
Ethical statement
Review and/or approval by an ethics committee was not needed for this study because this review article does not involve ethical issues.
CRediT authorship contribution statement
Yuxiao Zhang: Writing – original draft. E. Liu: Project administration, Methodology. Hongjin Gao: Formal analysis, Data curation. Qingying He: Writing – review & editing, Software. Anjing Chen: Supervision. Yaobing Pang: Project administration, Data curation. Xueer Zhang: Writing – review & editing. Sixian Bai: Visualization, Software. Jinhao Zeng: Supervision, Funding acquisition. Jing Guo: Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Jing Guo reports financial support was provided by National Natural Science Foundation of China. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank the Hospital of Chengdu University of traditional Chinese medicine and also the Chengdu University of traditional Chinese medicine for support of the article.
Contributor Information
Jinhao Zeng, Email: zengjinhao@cdutcm.edu.cn.
Jing Guo, Email: guojing66@cdutcm.edu.cn.
References
- 1.Nabai L., Pourghadiri A., Ghahary A. Hypertrophic scarring: current knowledge of predisposing factors, cellular and molecular mechanisms. J. Burn Care Res. Off. Publ. Am. Burn Assoc. 2020;41:48–56. doi: 10.1093/jbcr/irz158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lv K., Xia Z. Chinese expert consensus on clinical prevention and treatment of scar(+) Burns Trauma. 2018;6:27. doi: 10.1186/s41038-018-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finnerty C.C., Jeschke M.G., Branski L.K., Barret J.P., Dziewulski P., Herndon D.N. Hypertrophic scarring: the greatest unmet challenge after burn injury. Lancet. 2016;388:1427–1436. doi: 10.1016/S0140-6736(16)31406-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahdavian Delavary B., van der Veer W.M., Ferreira J.A., Niessen F.B. Formation of hypertrophic scars: evolution and susceptibility. J. Plast. Surg. Hand Surg. 2012;46:95–101. doi: 10.3109/2000656X.2012.669184. [DOI] [PubMed] [Google Scholar]
- 5.Poetschke J., Gauglitz G.G. Current options for the treatment of pathological scarring. J. Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. JDDG. 2016;14:467–477. doi: 10.1111/ddg.13027. [DOI] [PubMed] [Google Scholar]
- 6.Monstrey S., Middelkoop E., Vranckx J.J., Bassetto F., Ziegler U.E., Meaume S., Téot L. Updated scar management practical guidelines: non-invasive and invasive measures. J. Plast. Reconstr. Aesthetic Surg. JPRAS. 2014;67:1017–1025. doi: 10.1016/j.bjps.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q., Kelly A.P., Wang L., French S.W., Tang X., Duong H.S., Messadi D.V., Le A.D. Green tea extract and (-)-epigallocatechin-3-gallate inhibit mast cell-stimulated type I collagen expression in keloid fibroblasts via blocking PI-3K/AkT signaling pathways. J. Invest. Dermatol. 2006;126:2607–2613. doi: 10.1038/sj.jid.5700472. [DOI] [PubMed] [Google Scholar]
- 8.Chen J., Zhou R., Liang Y., Fu X., Wang D., Wang C. Blockade of lncRNA-ASLNCS5088-enriched exosome generation in M2 macrophages by GW4869 dampens the effect of M2 macrophages on orchestrating fibroblast activation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019;33:12200–12212. doi: 10.1096/fj.201901610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butzelaar L., Ulrich M.M.W., Mink van der Molen A.B., Niessen F.B., Beelen R.H.J. Currently known risk factors for hypertrophic skin scarring: a review. J. Plast. Reconstr. Aesthetic Surg. JPRAS. 2016;69:163–169. doi: 10.1016/j.bjps.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J., Li Y., Bai X., Li Y., Shi J., Hu D. Recent advances in hypertrophic scar. Histol. Histopathol. 2018;33:27–39. doi: 10.14670/HH-11-908. [DOI] [PubMed] [Google Scholar]
- 11.Oosterwijk A.M., Mouton L.J., Schouten H., Disseldorp L.M., van der Schans C.P., Nieuwenhuis M.K. Prevalence of scar contractures after burn: a systematic review. Burns J. Int. Soc. Burn Inj. 2017;43:41–49. doi: 10.1016/j.burns.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Barnes L.A., Marshall C.D., Leavitt T., Hu M.S., Moore A.L., Gonzalez J.G., Longaker M.T., Gurtner G.C. Mechanical forces in cutaneous wound healing: emerging therapies to minimize scar formation. Adv. Wound Care. 2018;7:47–56. doi: 10.1089/wound.2016.0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barone N., Safran T., Vorstenbosch J., Davison P.G., Cugno S., Murphy A.M. Current advances in hypertrophic scar and keloid management. Semin. Plast. Surg. 2021;35:145–152. doi: 10.1055/s-0041-1731461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J.Y., Willard J.J., Supp D.M., Roy S., Gordillo G.M., Sen C.K., Powell H.M. Burn scar biomechanics after pressure garment therapy. Plast. Reconstr. Surg. 2015;136:572–581. doi: 10.1097/PRS.0000000000001507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ai J.-W., Liu J.-T., Pei S.-D., Liu Y., Li D.-S., Lin H.-M., Pei B. The effectiveness of pressure therapy (15-25 mmHg) for hypertrophic burn scars: a systematic review and meta-analysis. Sci. Rep. 2017;7 doi: 10.1038/srep40185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coentro J.Q., Pugliese E., Hanley G., Raghunath M., Zeugolis D.I. Current and upcoming therapies to modulate skin scarring and fibrosis. Adv. Drug Deliv. Rev. 2019;146:37–59. doi: 10.1016/j.addr.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Kelemen O., Kollár L., Menyhei G. [A comparative clinical study of the treatment of hypertrophic scars with either intralaesional steroid or silicone gel sheeting] Magy. Sebeszet. 2007;60:297–300. doi: 10.1556/MaSeb.60.2007.6.5. [DOI] [PubMed] [Google Scholar]
- 18.Rosenthal A., Kolli H., Israilevich R., Moy R. Lasers for the prevention and treatment of hypertrophic scars: a review of the literature. J. Cosmet. Laser Ther. Off. Publ. Eur. Soc. Laser Dermatol. 2020;22:115–125. doi: 10.1080/14764172.2020.1783451. [DOI] [PubMed] [Google Scholar]
- 19.Putri K.T., Prasetyono T.O.H. A critical review on the potential role of adipose-derived stem cells for future treatment of hypertrophic scars. J. Cosmet. Dermatol. 2022;21:1913–1919. doi: 10.1111/jocd.14385. [DOI] [PubMed] [Google Scholar]
- 20.Al Qurashi A.A., Siddiqi A.K., Alghamdi A.A., Aljalfan A.A.N., Almenhali A.A., Al Jabr F.A., Rashid A.M., Almas T., Menezes R.G. Effectiveness of autologous fat transfer in the treatment of scar-related conditions: a systematic review and meta-analysis. Aesthetic Plast. Surg. 2022 doi: 10.1007/s00266-022-02869-9. [DOI] [PubMed] [Google Scholar]
- 21.Verhiel S., Piatkowski de Grzymala A., van der Hulst R. Mechanism of action, efficacy, and adverse events of calcium antagonists in hypertrophic scars and keloids: a systematic review. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. Al. 2015;41:1343–1350. doi: 10.1097/DSS.0000000000000506. [DOI] [PubMed] [Google Scholar]
- 22.Berman B. Biological agents for controlling excessive scarring. Am. J. Clin. Dermatol. 2010;11(Suppl 1):31–34. doi: 10.2165/1153419-S0-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.a Yazarlu O., b Iranshahi M., c Kashani H.R.K., d Reshadat S., e Habtemariam S., f Iranshahy M., b Hasanpour M. Perspective on the application of medicinal plants and natural products in wound healing: A mechanistic review. Pharmacol. Res. 2021;174 doi: 10.1016/j.phrs.2021.105841. [Review] [DOI] [PubMed] [Google Scholar]
- 24.Xu X., Gu S., Huang X., Ren J., Gu Y., Wei C., Lian X., Li H., Gao Y., Jin R., Gu B., Zan T., Wang Z. The role of macrophages in the formation of hypertrophic scars and keloids. Burns Trauma. 2020;8 doi: 10.1093/burnst/tkaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Theilgaard-Mönch K., Knudsen S., Follin P., Borregaard N. The transcriptional activation program of human neutrophils in skin lesions supports their important role in wound healing. J. Immunol. Baltim. Md. 2004;172:7684–7693. doi: 10.4049/jimmunol.172.12.7684. 1950. [DOI] [PubMed] [Google Scholar]
- 26.Ellis S., Lin E.J., Tartar D. Immunology of wound healing. Curr. Dermatol. Rep. 2018;7:350–358. doi: 10.1007/s13671-018-0234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cañedo-Dorantes L., Cañedo-Ayala M. Skin acute wound healing: a comprehensive review. Int. J. Inflamm. 2019;2019 doi: 10.1155/2019/3706315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilgus T.A., Wulff B.C. The importance of mast cells in dermal scarring. Adv. Wound Care. 2014;3:356–365. doi: 10.1089/wound.2013.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang C., Murphy G.F., Akaishi S., Ogawa R. Keloids and hypertrophic scars: update and future directions. Plast. Reconstr. Surg. Glob. Open. 2013;1:e25. doi: 10.1097/GOX.0b013e31829c4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia Z., Wang J., Yang S., Liu C., Qin S., Li W., Cheng Y., Hu H., Qian J., Liu Y., Deng C. Emodin alleviates hypertrophic scar formation by suppressing macrophage polarization and inhibiting the Notch and TGF-β pathways in macrophages. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Medicas E Biol. 2021;54 doi: 10.1590/1414-431X2021e11184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao Y. MiR-486-5p inhibits the hyperproliferation and production of collagen in hypertrophic scar fibroblasts via IGF1/PI3K/AKT pathway. J. Dermatol. Treat. 2021;32:973–982. doi: 10.1080/09546634.2020.1728210. [DOI] [PubMed] [Google Scholar]
- 32.Ud-Din S., Wilgus T.A., McGeorge D.D., Bayat A. Pre-emptive priming of human skin improves cutaneous scarring and is superior to immediate and delayed topical anti-scarring treatment post-wounding: a double-blind randomised placebo-controlled clinical trial. Pharmaceutics. 2021;13:510. doi: 10.3390/pharmaceutics13040510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xue M., Jackson C.J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care. 2015;4:119–136. doi: 10.1089/wound.2013.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu Z., Hou Q., Li M., Fu X. Molecular mechanism of myofibroblast formation and strategies for clinical drugs treatments in hypertrophic scars. J. Cell. Physiol. 2020;235:4109–4119. doi: 10.1002/jcp.29302. [DOI] [PubMed] [Google Scholar]
- 35.Werner S., Krieg T., Smola H. Keratinocyte-fibroblast interactions in wound healing. J. Invest. Dermatol. 2007;127:998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- 36.Berman B., Maderal A., Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. Al. 2017;43(Suppl 1):S3–S18. doi: 10.1097/DSS.0000000000000819. [DOI] [PubMed] [Google Scholar]
- 37.Cho J.-W., Cho S.-Y., Lee S.-R., Lee K.-S. Onion extract and quercetin induce matrix metalloproteinase-1 in vitro and in vivo. Int. J. Mol. Med. 2010;25:347–352. [PubMed] [Google Scholar]
- 38.Keskin E.S., Keskin E.R., Öztürk M.B., Çakan D. The effect of MMP-1 on wound healing and scar formation. Aesthetic Plast. Surg. 2021;45:2973–2979. doi: 10.1007/s00266-021-02369-2. [DOI] [PubMed] [Google Scholar]
- 39.Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 40.Scharstuhl A., Mutsaers H.a.M., Pennings S.W.C., Szarek W.A., Russel F.G.M., Wagener F.a.D.T.G. Curcumin-induced fibroblast apoptosis and in vitro wound contraction are regulated by antioxidants and heme oxygenase: implications for scar formation. J. Cell Mol. Med. 2009;13:712–725. doi: 10.1111/j.1582-4934.2008.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nedelec B., Shankowsky H., Scott P.G., Ghahary A., Tredget E.E. Myofibroblasts and apoptosis in human hypertrophic scars: the effect of interferon-α2b. Surgery. 2001;130:798–808. doi: 10.1067/msy.2001.116453. [DOI] [PubMed] [Google Scholar]
- 42.Lundvig D.M.S., Pennings S.W.C., Brouwer K.M., Mtaya-Mlangwa M., Mugonzibwa E.A., Kuijpers-Jagtman A.M., Von den Hoff J.W., Wagener F.A.D.T.G. Curcumin induces differential expression of cytoprotective enzymes but similar apoptotic responses in fibroblasts and myofibroblasts. Exp. Cell Res. 2015;330:429–441. doi: 10.1016/j.yexcr.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 43.Chen J.-Y., Zhang L., Zhang H., Su L., Qin L.-P. Triggering of p38 MAPK and JNK signaling is important for oleanolic acid-induced apoptosis via the mitochondrial death pathway in hypertrophic scar fibroblasts. Phytother. Res. PTR. 2014;28:1468–1478. doi: 10.1002/ptr.5150. [DOI] [PubMed] [Google Scholar]
- 44.Fan C., Xie Y., Dong Y., Su Y., Upton Z. Investigating the potential of Shikonin as a novel hypertrophic scar treatment. J. Biomed. Sci. 2015;22:70. doi: 10.1186/s12929-015-0172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heng M.C.Y. Signaling pathways targeted by curcumin in acute and chronic injury: burns and photo-damaged skin. Int. J. Dermatol. 2013;52:531–543. doi: 10.1111/j.1365-4632.2012.05703.x. [DOI] [PubMed] [Google Scholar]
- 46.Kishi K., Okabe K., Shimizu R., Kubota Y. Fetal skin possesses the ability to regenerate completely: complete regeneration of skin. Keio J. Med. 2012;61:101–108. doi: 10.2302/kjm.2011-0002-ir. [DOI] [PubMed] [Google Scholar]
- 47.Yin J.-L., Wu Y., Yuan Z.-W., Gao X.-H., Chen H.-D. Advances in scarless foetal wound healing and prospects for scar reduction in adults. Cell Prolif. 2020;53 doi: 10.1111/cpr.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee H.J., Jang Y.J. Recent understandings of biology, prophylaxis and treatment strategies for hypertrophic scars and keloids. Int. J. Mol. Sci. 2018;19:E711. doi: 10.3390/ijms19030711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang T., Wang X.-F., Wang Z.-C., Lou D., Fang Q.-Q., Hu Y.-Y., Zhao W.-Y., Zhang L.-Y., Wu L.-H., Tan W.-Q. Current potential therapeutic strategies targeting the TGF-β/Smad signaling pathway to attenuate keloid and hypertrophic scar formation. Biomed. Pharmacother. Biomedecine Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110287. [DOI] [PubMed] [Google Scholar]
- 50.Wu M.-F., Zeng Q.-Y., Huang J.-H., Wang H.-W. The role of Smad7 in cutaneous wound healing. Ital. J. Dermatol. Venereol. 2021;156:13–19. doi: 10.23736/S2784-8671.20.06514-1. [DOI] [PubMed] [Google Scholar]
- 51.Liu L., Ding Z., Yang Y., Zhang Z., Lu Q., Kaplan D.L. Asiaticoside-laden silk nanofiber hydrogels to regulate inflammation and angiogenesis for scarless skin regeneration. Biomater. Sci. 2021;9:5227–5236. doi: 10.1039/d1bm00904d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li B., Gao C., Diao J.-S., Wang D.-L., Chu F.-F., Li Y., Wang G., Guo S.-Z., Xia W. Aberrant Notch signalling contributes to hypertrophic scar formation by modulating the phenotype of keratinocytes. Exp. Dermatol. 2016;25:137–142. doi: 10.1111/exd.12897. [DOI] [PubMed] [Google Scholar]
- 53.He T., Bai X., Jing J., Liu Y., Wang H., Zhang W., Li X., Li Y., Wang L., Xie S., Hu D. Notch signal deficiency alleviates hypertrophic scar formation after wound healing through the inhibition of inflammation. Arch. Biochem. Biophys. 2020;682 doi: 10.1016/j.abb.2020.108286. [DOI] [PubMed] [Google Scholar]
- 54.Li S., Fan H., Liu L., Ling J., Wu Y. Inhibition of Notch signaling pathway reduces angiogenesis in hypertrophic scar. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2021;46:1195–1202. doi: 10.11817/j.issn.1672-7347.2021.210234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He T., Zhang Y., Liu Y., Wang H., Zhang W., Liu J., Li N., Li Y., Wang L., Xie S., Hu D. MicroRNA-494 targets PTEN and suppresses PI3K/AKT pathway to alleviate hypertrophic scar formation. J. Mol. Histol. 2019;50:315–323. doi: 10.1007/s10735-019-09828-w. [DOI] [PubMed] [Google Scholar]
- 56.Zhu H.-Y., Li C., Bai W.-D., Su L.-L., Liu J.-Q., Li Y., Shi J.-H., Cai W.-X., Bai X.-Z., Jia Y.-H., Zhao B., Wu X., Li J., Hu D.-H. MicroRNA-21 regulates hTERT via PTEN in hypertrophic scar fibroblasts. PLoS One. 2014;9 doi: 10.1371/journal.pone.0097114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu X., Li J., Yang X., Bai X., Shi J., Gao J., Li Y., Han S., Zhang Y., Han F., Liu Y., Li X., Wang K., Zhang J., Wang Z., Tao K., Hu D. miR-155 inhibits the formation of hypertrophic scar fibroblasts by targeting HIF-1α via PI3K/AKT pathway. J. Mol. Histol. 2018;49:377–387. doi: 10.1007/s10735-018-9778-z. [DOI] [PubMed] [Google Scholar]
- 58.He T., Bai X., Yang L., Fan L., Li Y., Su L., Gao J., Han S., Hu D. Loureirin B inhibits hypertrophic scar formation via inhibition of the TGF-β1-ERK/JNK pathway. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015;37:666–676. doi: 10.1159/000430385. [DOI] [PubMed] [Google Scholar]
- 59.Li Y., Zhang W., Gao J., Liu J., Wang H., Li J., Yang X., He T., Guan H., Zheng Z., Han S., Dong M., Han J., Shi J., Hu D. Adipose tissue-derived stem cells suppress hypertrophic scar fibrosis via the p38/MAPK signaling pathway. Stem Cell Res. Ther. 2016;7:102. doi: 10.1186/s13287-016-0356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shen Y., Hao X. Natural product sciences: an integrative approach to the innovations of plant natural products. Sci. China Life Sci. 2020;63:1634–1650. doi: 10.1007/s11427-020-1799-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang C., Zhang W., Dong X., Fu C., Yuan J., Xu M., Liang Z., Qiu C., Xu C. A natural product solution to aging and aging-associated diseases. Pharmacol. Ther. 2020;216 doi: 10.1016/j.pharmthera.2020.107673. [DOI] [PubMed] [Google Scholar]
- 62.Adegboye O., Field M.A., Kupz A., Pai S., Sharma D., Smout M.J., Wangchuk P., Wong Y., Loiseau C. Natural-product-based solutions for tropical infectious diseases. Clin. Microbiol. Rev. 2021;34 doi: 10.1128/CMR.00348-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020;83:770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 64.Jia S., Xie P., Hong S.J., Galiano R., Singer A., Clark R.A.F., Mustoe T.A. Intravenous curcumin efficacy on healing and scar formation in rabbit ear wounds under nonischemic, ischemic, and ischemia-reperfusion conditions. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2014;22:730–739. doi: 10.1111/wrr.12231. [DOI] [PubMed] [Google Scholar]
- 65.Hsu Y.-C., Chen M.-J., Yu Y.-M., Ko S.-Y., Chang C.-C. Suppression of TGF-β1/SMAD pathway and extracellular matrix production in primary keloid fibroblasts by curcuminoids: its potential therapeutic use in the chemoprevention of keloid. Arch. Dermatol. Res. 2010;302:717–724. doi: 10.1007/s00403-010-1075-y. [DOI] [PubMed] [Google Scholar]
- 66.Ud-Din S., Foden P., Mazhari M., Al-Habba S., Baguneid M., Bulfone-Paus S., McGeorge D., Bayat A. A double-blind, randomized trial shows the role of zonal priming and direct topical application of epigallocatechin-3-gallate in the modulation of cutaneous scarring in human skin. J. Invest. Dermatol. 2019;139:1680–1690.e16. doi: 10.1016/j.jid.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 67.Moyle C.W.A., Cerezo A.B., Winterbone M.S., Hollands W.J., Alexeev Y., Needs P.W., Kroon P.A. Potent inhibition of VEGFR-2 activation by tight binding of green tea epigallocatechin gallate and apple procyanidins to VEGF: relevance to angiogenesis. Mol. Nutr. Food Res. 2015;59:401–412. doi: 10.1002/mnfr.201400478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cai Y., Yu S.-S., Chen T.-T., Gao S., Geng B., Yu Y., Ye J.-T., Liu P.-Q. EGCG inhibits CTGF expression via blocking NF-κB activation in cardiac fibroblast. Phytomedicine Int. J. Phytother. Phytopharm. 2013;20:106–113. doi: 10.1016/j.phymed.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 69.Sriram N., Kalayarasan S., Manikandan R., Arumugam M., Sudhandiran G. Epigallocatechin gallate attenuates fibroblast proliferation and excessive collagen production by effectively intervening TGF-β1 signalling. Clin. Exp. Pharmacol. Physiol. 2015;42:849–859. doi: 10.1111/1440-1681.12428. [DOI] [PubMed] [Google Scholar]
- 70.Lin H.-L., Qin Y.-J., Zhang Y.-L., Zhang Y.-Q., Chen Y.-L., Niu Y.-Y., Pang C.-P., Chu W.-K., Zhang H.-Y. Epigallocatechin-3-gallate (EGCG) inhibits myofibroblast transformation of human Tenon's fibroblasts. Exp. Eye Res. 2020;197 doi: 10.1016/j.exer.2020.108119. [DOI] [PubMed] [Google Scholar]
- 71.Klass B.R., Branford O.A., Grobbelaar A.O., Rolfe K.J. The effect of epigallocatechin-3-gallate, a constituent of green tea, on transforming growth factor-beta1-stimulated wound contraction. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2010;18:80–88. doi: 10.1111/j.1524-475X.2009.00552.x. [DOI] [PubMed] [Google Scholar]
- 72.Li M., Xu J., Shi T., Yu H., Bi J., Chen G. Epigallocatechin-3-gallate augments therapeutic effects of mesenchymal stem cells in skin wound healing. Clin. Exp. Pharmacol. Physiol. 2016;43:1115–1124. doi: 10.1111/1440-1681.12652. [DOI] [PubMed] [Google Scholar]
- 73.Sidgwick G.P., McGeorge D., Bayat A. Functional testing of topical skin formulations using an optimised ex vivo skin organ culture model. Arch. Dermatol. Res. 2016;308:297–308. doi: 10.1007/s00403-016-1645-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu X., Luo X., Gu S., Xu J. The effects of Polygonum cuspidatum extract on wound healing in rats. J. Ethnopharmacol. 2012;141:934–937. doi: 10.1016/j.jep.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 75.Zeng G., Zhong F., Li J., Luo S., Zhang P. Resveratrol-mediated reduction of collagen by inhibiting proliferation and producing apoptosis in human hypertrophic scar fibroblasts. Biosci. Biotechnol. Biochem. 2013;77:2389–2396. doi: 10.1271/bbb.130502. [DOI] [PubMed] [Google Scholar]
- 76.Bai X.-Z., Liu J.-Q., Yang L.-L., Fan L., He T., Su L.-L., Shi J.-H., Tang C.-W., Zheng Z., Hu D.-H. Identification of sirtuin 1 as a promising therapeutic target for hypertrophic scars. Br. J. Pharmacol. 2016;173:1589–1601. doi: 10.1111/bph.13460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shinojima N., Yokoyama T., Kondo Y., Kondo S. Roles of the Akt/mTOR/p70S6K and ERK1/2 signaling pathways in curcumin-induced autophagy. Autophagy. 2007;3:635–637. doi: 10.4161/auto.4916. [DOI] [PubMed] [Google Scholar]
- 78.Tang Z.-M., Zhai X.-X., Ding J.-C. Expression of mTOR/70S6K signaling pathway in pathological scar fibroblasts and the effects of resveratrol intervention. Mol. Med. Rep. 2017;15:2546–2550. doi: 10.3892/mmr.2017.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tang Z., Ding J.-C., Zhai X.-X. Effects of resveratrol on the expression of molecules related to the mTOR signaling pathway in pathological scar fibroblasts, G. Ital. Dermatol. E Venereol. Organo Uff. Soc. Ital. Dermatol. E Sifilogr. 2020;155:161–167. doi: 10.23736/S0392-0488.17.05556-0. [DOI] [PubMed] [Google Scholar]
- 80.Shi J., Wang H., Guan H., Shi S., Li Y., Wu X., Li N., Yang C., Bai X., Cai W., Yang F., Wang X., Su L., Zheng Z., Hu D. IL10 inhibits starvation-induced autophagy in hypertrophic scar fibroblasts via cross talk between the IL10-IL10R-STAT3 and IL10-AKT-mTOR pathways. Cell Death Dis. 2016;7 doi: 10.1038/cddis.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Felice B., Garbi C., Santoriello M., Santillo A., Wilson R.R. Differential apoptosis markers in human keloids and hypertrophic scars fibroblasts. Mol. Cell. Biochem. 2009;327:191–201. doi: 10.1007/s11010-009-0057-x. [DOI] [PubMed] [Google Scholar]
- 82.Shi J., Xiao H., Li J., Zhang J., Li Y., Zhang J., Wang X., Bai X., Tao K., Hu D., Guan H. Wild-type p53-modulated autophagy and autophagic fibroblast apoptosis inhibit hypertrophic scar formation. Lab. Investig. J. Tech. Methods Pathol. 2018;98:1423–1437. doi: 10.1038/s41374-018-0099-3. [DOI] [PubMed] [Google Scholar]
- 83.Shi J.-H., Hu D.-H., Zhang Z.-F., Bai X.-Z., Wang H.-T., Zhu X.-X., Su Y.-J., Tang C.-W. Reduced expression of microtubule-associated protein 1 light chain 3 in hypertrophic scars. Arch. Dermatol. Res. 2012;304:209–215. doi: 10.1007/s00403-012-1204-x. [DOI] [PubMed] [Google Scholar]
- 84.Pang K., Li B., Tang Z., Yang W., Hao L., Shi Z., Zhang J., Cai L., Li R., Liu Y., Lv Q., Ding J., Han C. Resveratrol inhibits hypertrophic scars formation by activating autophagy via the miR-4654/Rheb axis. Mol. Med. Rep. 2020;22:3440–3452. doi: 10.3892/mmr.2020.11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao C.-C., Zhu L., Wu Z., Yang R., Xu N., Liang L. Resveratrol-loaded peptide-hydrogels inhibit scar formation in wound healing through suppressing inflammation. Regen. Biomater. 2020;7:99–107. doi: 10.1093/rb/rbz041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hsieh S.-C., Wu C.-C., Hsu S.-L., Yen J.-H. Molecular mechanisms of gallic acid-induced growth inhibition, apoptosis, and necrosis in hypertrophic scar fibroblasts. Life Sci. 2017;179:130–138. doi: 10.1016/j.lfs.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 87.P. Fan, S. Chen, Z. Li, X. Yang, S. Lei, W. Tan, Gallic Acid Inhibits LPS Induced Hypertrophic Scar, (n.d.) 9.
- 88.Hsieh S.-C., Wu C.-C., Hsu S.-L., Feng C.-H., Yen J.-H. Gallic acid attenuates TGF-β1-stimulated collagen gel contraction via suppression of RhoA/Rho-kinase pathway in hypertrophic scar fibroblasts. Life Sci. 2016;161:19–26. doi: 10.1016/j.lfs.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 89.Park J., Ryu S.-H., Choi E.K., Ahn S.D., Park E., Choi K.-C., Lee S. SKI2162, an inhibitor of the TGF-β type I receptor (ALK5), inhibits radiation-induced fibrosis in mice. Oncotarget. 2015;6:4171–4179. doi: 10.18632/oncotarget.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Higashiyama H., Yoshimoto D., Kaise T., Matsubara S., Fujiwara M., Kikkawa H., Asano S., Kinoshita M. Inhibition of activin receptor-like kinase 5 attenuates bleomycin-induced pulmonary fibrosis. Exp. Mol. Pathol. 2007;83:39–46. doi: 10.1016/j.yexmp.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Y., Shan S., Wang J., Cheng X., Yi B., Zhou J., Li Q. Galangin inhibits hypertrophic scar formation via ALK5/Smad2/3 signaling pathway. Mol. Cell. Biochem. 2016;413:109–118. doi: 10.1007/s11010-015-2644-3. [DOI] [PubMed] [Google Scholar]
- 92.Ru Z., Hu Y., Huang S., Bai L., Zhang K., Li Y. Bioflavonoid galangin suppresses hypertrophic scar formation by the TGF-β/smad signaling pathway. Evid.-Based Complement. Altern. Med. ECAM. 2021;2021 doi: 10.1155/2021/2444839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Phan T.-T., Sun L., Bay B.-H., Chan S.-Y., Lee S.-T. Dietary compounds inhibit proliferation and contraction of keloid and hypertrophic scar-derived fibroblasts in vitro: therapeutic implication for excessive scarring. J. Trauma. 2003;54:1212–1224. doi: 10.1097/01.TA.0000030630.72836.32. [DOI] [PubMed] [Google Scholar]
- 94.Song J.-Y., Truong D.V., Yang B.-S. Quercetin shows the pharmacological activity to simultaneously downregulate the inflammatory and fibrotic responses to tissue injury in association with its ability to target multi-kinases. Pharmacology. 2018;102:142–153. doi: 10.1159/000490417. [DOI] [PubMed] [Google Scholar]
- 95.Mohammadi M., Kohan L., Saeidi M., Saghaeian Jazi M., Mohammadi S. The antifibrotic effects of naringin in a hypochlorous acid (HOCl)-induced mouse model of skin fibrosis. Immunopharmacol. Immunotoxicol. 2022:1–8. doi: 10.1080/08923973.2022.2077217. [DOI] [PubMed] [Google Scholar]
- 96.Shan S., Zhang Y., Wu M., Yi B., Wang J., Li Q. Naringenin attenuates fibroblast activation and inflammatory response in a mechanical stretch-induced hypertrophic scar mouse model. Mol. Med. Rep. 2017;16:4643–4649. doi: 10.3892/mmr.2017.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Song Y., Guo B., Ma S., Chang P., Tao K. Naringin suppresses the growth and motility of hypertrophic scar fibroblasts by inhibiting the kinase activity of Akt. Biomed. Pharmacother. 2018;105:1291–1298. doi: 10.1016/j.biopha.2018.06.103. [DOI] [PubMed] [Google Scholar]
- 98.He B., Chen J., Liu L., Wang H., Wang S., Li P., Zhou J. Emodin inhibits formation of hypertrophic scars through regulating the p38/MAPK signaling pathway. Int. J. Clin. Exp. Med. 2018;11:12310–12317. [Google Scholar]
- 99.Lee J.-H., Kim H.-L., Lee M.H., You K.E., Kwon B.-J., Seo H.J., Park J.-C. Asiaticoside enhances normal human skin cell migration, attachment and growth in vitro wound healing model. Phytomedicine Int. J. Phytother. Phytopharm. 2012;19:1223–1227. doi: 10.1016/j.phymed.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 100.Fan C., Dong Y., Xie Y., Su Y., Zhang X., Leavesley D., Upton Z. Shikonin reduces TGF-β1-induced collagen production and contraction in hypertrophic scar-derived human skin fibroblasts. Int. J. Mol. Med. 2015;36:985–991. doi: 10.3892/ijmm.2015.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fan C., Lim L.K.P., Loh S.Q., Ying Lim K.Y., Upton Z., Leavesley D. Application of “macromolecular crowding” in vitro to investigate the naphthoquinones shikonin, naphthazarin and related analogues for the treatment of dermal scars. Chem. Biol. Interact. 2019;310 doi: 10.1016/j.cbi.2019.108747. [DOI] [PubMed] [Google Scholar]
- 102.Deng X., Chen Q., Qiang L., Chi M., Xie N., Wu Y., Yao M., Zhao D., Ma J., Zhang N., Xie Y. Development of a porcine full-thickness burn hypertrophic scar model and investigation of the effects of shikonin on hypertrophic scar remediation. Front. Pharmacol. 2018;9:590. doi: 10.3389/fphar.2018.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ju-Lin, Shao-Hai Q., Tian-Zeng L., Bin H., Jing-Ming T., Ying-Bin X., Xu-Sheng L., Bin S., Hui-Zhen L., Yong H. Effect of asiaticoside on hypertrophic scar in the rabbit ear model. J. Cutan. Pathol. 2009;36:234–239. doi: 10.1111/j.1600-0560.2008.01015.x. [DOI] [PubMed] [Google Scholar]
- 104.Huang J., Zhou X., Xia L., Liu W., Guo F., Liu J., Liu W. Inhibition of hypertrophic scar formation with oral asiaticoside treatment in a rabbit ear scar model. Int. Wound J. 2021;18:598–607. doi: 10.1111/iwj.13561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qi S.H., Xie J.-L., Pan S., Xu Y.-B., Li T.-Z., Tang J.-M., Liu X.-S., Shu B., Liu P. Effects of asiaticoside on the expression of Smad protein by normal skin fibroblasts and hypertrophic scar fibroblasts. Clin. Exp. Dermatol. 2008;33:171–175. doi: 10.1111/j.1365-2230.2007.02636.x. [DOI] [PubMed] [Google Scholar]
- 106.Chen X., Peng L.-H., Li N., Li Q.-M., Li P., Fung K.-P., Leung P.-C., Gao J.-Q. The healing and anti-scar effects of astragaloside IV on the wound repair in vitro and in vivo. J. Ethnopharmacol. 2012;139:721–727. doi: 10.1016/j.jep.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 107.Tark K.C., Lee D.W., Lew D.H., Kang E.H., Roh H., Lee M.C. Effects of ginsenoside Rb1 on hypertrophic scar remodeling in rabbit model. Eur. J. Pharmacol. 2015;750:151–159. doi: 10.1016/j.ejphar.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 108.Ma L., Li L.Y., Zhao T.L. Anti-inflammatory effects of ginsenoside Rg3 on the hypertrophic scar formation via the NF-κB/IκB signaling pathway in rabbit ears. Pharm. Times. 2020;75:102–106. doi: 10.1691/ph.2020.9852. [DOI] [PubMed] [Google Scholar]
- 109.Cheng L., Sun X., Hu C., Jin R., Sun B., Shi Y., Cui W., Zhang Y. In vivo early intervention and the therapeutic effects of 20(s)-ginsenoside rg3 on hypertrophic scar formation. PLoS One. 2014;9 doi: 10.1371/journal.pone.0113640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cheng L., Sun X., Hu C., Jin R., Sun B., Shi Y., Zhang L., Cui W., Zhang Y. In vivo inhibition of hypertrophic scars by implantable ginsenoside-Rg3-loaded electrospun fibrous membranes. Acta Biomater. 2013;9:9461–9473. doi: 10.1016/j.actbio.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 111.Cheng L., Sun X., Li B., Hu C., Yang H., Zhang Y., Cui W. Electrospun Ginsenoside Rg3/poly(lactic-co-glycolic acid) fibers coated with hyaluronic acid for repairing and inhibiting hypertrophic scars. J. Mater. Chem. B. 2013;1:4428–4437. doi: 10.1039/c3tb20441c. [DOI] [PubMed] [Google Scholar]
- 112.Zhang H., Zhang Y., Jiang Y.-P., Zhang L.-K., Peng C., He K., Rahman K., Qin L.-P. Curative effects of oleanolic Acid on formed hypertrophic scars in the rabbit ear model, Evid.-Based Complement. Altern. Med. ECAM. 2012;2012 doi: 10.1155/2012/837581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nong X., Rajbanshi G., Chen L., Li J., Li Z., Liu T., Chen S., Wei G., Li J. Effect of artesunate and relation with TGF-β1 and SMAD3 signaling on experimental hypertrophic scar model in rabbit ear. Arch. Dermatol. Res. 2019;311:761–772. doi: 10.1007/s00403-019-01960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guo J., Lin Q., Shao Y., Rong L., Zhang D. BMP-7 suppresses excessive scar formation by activating the BMP-7/Smad1/5/8 signaling pathway. Mol. Med. Rep. 2017;16:1957–1963. doi: 10.3892/mmr.2017.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Le X., Wu W.-W. The therapeutic effect of Interleukin-18 on hypertrophic scar through inducing Fas ligand expression. Burns J. Int. Soc. Burn Inj. 2021;47:430–438. doi: 10.1016/j.burns.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 116.Zhang J., Luo W., Han M., Wu L., Peng Z., Xia Z., Yang R. Verifying the outcomes of artesunate plus 595-nm PDL in hypertrophic scars via determining BMP-7 and Fas level in model rabbits. Lasers Surg. Med. 2022;54:716–724. doi: 10.1002/lsm.23518. [DOI] [PubMed] [Google Scholar]
- 117.Zhang J., Zhou S., Xia Z., Peng Z., Luo W., Cheng X., Yang R. Effectiveness of artesunate combined with fractional CO2 laser in a hypertrophic scar model with underlying mechanism. Burns J. Int. Soc. Burn Inj. 2022;48:662–671. doi: 10.1016/j.burns.2021.05.013. [DOI] [PubMed] [Google Scholar]
- 118.Zhang J., Xia Z., Zhou S., Luo W., Peng Z., Yang R. Effect of artesunate combined with fractional CO2 laser on the hypertrophic scar in a rabbit model. Lasers Surg. Med. 2021 doi: 10.1002/lsm.23384. [DOI] [PubMed] [Google Scholar]
- 119.Zunwen L., Shizhen Z., Dewu L., Yungui M., Pu N. Effect of tetrandrine on the TGF-β-induced smad signal transduction pathway in human hypertrophic scar fibroblasts in vitro. Burns J. Int. Soc. Burn Inj. 2012;38:404–413. doi: 10.1016/j.burns.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 120.Ning P., Peng Y., Liu D.W., Hu Y.H., Liu Y., Liu D.M. Tetrandrine induces microRNA differential expression in human hypertrophic scar fibroblasts in vitro. Genet. Mol. Res. GMR. 2016;15 doi: 10.4238/gmr.15017288. [DOI] [PubMed] [Google Scholar]
- 121.Wu J.-G., Ma L., Zhang S.-Y., Zhu Z.-Z., Zhang H., Qin L.-P., Wei Y.-J. Essential oil from rhizomes of Ligusticum chuanxiong induces apoptosis in hypertrophic scar fibroblasts. Pharm. Biol. 2011;49:86–93. doi: 10.3109/13880209.2010.499517. [DOI] [PubMed] [Google Scholar]
- 122.Wu J.-G., Wei Y.-J., Ran X., Zhang H., Nian H., Qin L.-P. Inhibitory effects of essential oil from rhizomes of Ligusticum chuanxiong on hypertrophic scarring in the rabbit ear model. Pharm. Biol. 2011;49:764–769. doi: 10.3109/13880209.2010.542761. [DOI] [PubMed] [Google Scholar]
- 123.Wu X., Wang Z., Wu G., Xu X., Zhang J., Li Y., Zhang H., Guo S. Tetramethylpyrazine induces apoptosis and inhibits proliferation of hypertrophic scar-derived fibroblasts via inhibiting the phosphorylation of AKT. Front. Pharmacol. 2020;11:602. doi: 10.3389/fphar.2020.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhi Y., Zeng M., Wang H., Li W., Yan L., Zhang W. Panax notoginseng saponins attenuates hypertrophic scar formation by inhibiting collagen synthesisin a rabbit ear model. Int. J. Clin. Exp. Med. 2017;10:15221–15228. [Google Scholar]
- 125.Zhi Y., Wang H., Huang B., Yan G., Yan L.-Z., Zhang W., Zhang J. Panax Notoginseng Saponins suppresses TRPM7 via the PI3K/AKT pathway to inhibit hypertrophic scar formation in vitro. Burns J. Int. Soc. Burn Inj. 2021;47:894–905. doi: 10.1016/j.burns.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 126.Men S.-Y., Huo Q.-L., Shi L., Yan Y., Yang C.-C., Yu W., Liu B.-Q. Panax notoginseng saponins promotes cutaneous wound healing and suppresses scar formation in mice. J. Cosmet. Dermatol. 2020;19:529–534. doi: 10.1111/jocd.13042. [DOI] [PubMed] [Google Scholar]
- 127.Danilevicz C.K., Wagner V.P., Ferreira N., Bock H., Salles Pilar E.F., Webber L.P., Schmidt T.R., Alonso E.C.P., de Mendonça E.F., Valadares M.C., Marreto R.N., Martins M.D. Curcuma longa L. Effects on akt/mTOR pathway and NF-κB expression during skin wound healing: an immunohistochemical study. Appl. Immunohistochem. Mol. Morphol. AIMM. 2021;29:e92–e100. doi: 10.1097/PAI.0000000000000961. [DOI] [PubMed] [Google Scholar]
- 128.Kunnumakkara A.B., Bordoloi D., Padmavathi G., Monisha J., Roy N.K., Prasad S., Aggarwal B.B. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017;174:1325–1348. doi: 10.1111/bph.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gupta S.C., Patchva S., Aggarwal B.B. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mohammadi Z., Sharif Zak M., Majdi H., Mostafavi E., Barati M., Lotfimehr H., Ghaseminasab K., Pazoki-Toroudi H., Webster T.J., Akbarzadeh A. The effect of chrysin-curcumin-loaded nanofibres on the wound-healing process in male rats. Artif. Cell Nanomed. Biotechnol. 2019;47:1642–1652. doi: 10.1080/21691401.2019.1594855. [DOI] [PubMed] [Google Scholar]
- 131.Nguyen M.H., Yu H., Kiew T.Y., Hadinoto K. Cost-effective alternative to nano-encapsulation: amorphous curcumin-chitosan nanoparticle complex exhibiting high payload and supersaturation generation. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik EV. 2015;96:1–10. doi: 10.1016/j.ejpb.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 132.Nguyen M.-H., Vu N.-B.-D., Nguyen T.-H.-N., Le H.-S., Le H.-T., Tran T.-T., Le X.-C., Le V.-T., Nguyen T.-T., Bui C.-B., Park H.-J. In vivo comparison of wound healing and scar treatment effect between curcumin-oligochitosan nanoparticle complex and oligochitosan-coated curcumin-loaded-liposome. J. Microencapsul. 2019;36:156–168. doi: 10.1080/02652048.2019.1612476. [DOI] [PubMed] [Google Scholar]
- 133.Nguyen M.-H., Lee S.E., Tran T.-T., Bui C.-B., Nguyen T.-H.-N., Vu N.-B.-D., Tran T.-T., Nguyen T.-H.-P., Nguyen T.-T., Hadinoto K. A simple strategy to enhance the in vivo wound-healing activity of curcumin in the form of self-assembled nanoparticle complex of curcumin and oligochitosan. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;98:54–64. doi: 10.1016/j.msec.2018.12.091. [DOI] [PubMed] [Google Scholar]
- 134.Pinto G., Illiano A., Carpentieri A., Spinelli M., Melchiorre C., Fontanarosa C., di Serio M., Amoresano A. Quantification of polyphenols and metals in Chinese tea infusions by mass spectrometry. Foods. 2020;9 doi: 10.3390/foods9060835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xu F.-W., Lv Y.-L., Zhong Y.-F., Xue Y.-N., Wang Y., Zhang L.-Y., Hu X., Tan W.-Q. Beneficial effects of green tea EGCG on skin wound healing: a comprehensive review. Mol. Basel Switz. 2021;26:6123. doi: 10.3390/molecules26206123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hecker A., Schellnegger M., Hofmann E., Luze H., Nischwitz S.P., Kamolz L.-P., Kotzbeck P. The impact of resveratrol on skin wound healing, scarring, and aging. Int. Wound J. 2022;19:9–28. doi: 10.1111/iwj.13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhai X., Ding J., Tang Z. Resveratrol inhibits proliferation and induces apoptosis of pathological scar fibroblasts through the mechanism involving TGF-β1/smads signaling pathway. Cell Biochem. Biophys. 2015;71:1267–1272. doi: 10.1007/s12013-014-0317-6. [DOI] [PubMed] [Google Scholar]
- 138.Gabr S.A., Alghadir A.H. Evaluation of the biological effects of lyophilized hydrophilic extract of rhus coriaria on myeloperoxidase (MPO) activity, wound healing, and microbial infections of skin wound tissues, evid.-based complement. Altern. Med. ECAM. 2019;2019 doi: 10.1155/2019/5861537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Al Zahrani N.A., El-Shishtawy R.M., Asiri A.M. Recent developments of gallic acid derivatives and their hybrids in medicinal chemistry: a review. Eur. J. Med. Chem. 2020;204 doi: 10.1016/j.ejmech.2020.112609. [DOI] [PubMed] [Google Scholar]
- 140.Kadam K., Kıyan S., Uyanıkgil Y., Karabey F., Çetin E.Ö. Investigation of acute effects of topical Alpinia officinarum (galangal) treatment in experimental contact type burns and comparison with topical silver sulfadiazine treatment, Ulus. Travma Ve Acil Cerrahi Derg. Turk. J. Trauma Emerg. Surg. TJTES. 2022;28:15–26. doi: 10.14744/tjtes.2020.69002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Phan T.-T., Lim I.J., Chan S.-Y., Tan E.-K., Lee S.-T., Longaker M.T. Suppression of transforming growth factor beta/smad signaling in keloid-derived fibroblasts by quercetin: implications for the treatment of excessive scars. J. Trauma. 2004;57:1032–1037. doi: 10.1097/01.ta.0000114087.46566.eb. [DOI] [PubMed] [Google Scholar]
- 142.Özbilgin S., Acıkara Ö.B., Akkol E.K., Süntar I., Keleş H., İşcan G.S. In vivo wound-healing activity of Euphorbia characias subsp. wulfenii: isolation and quantification of quercetin glycosides as bioactive compounds. J. Ethnopharmacol. 2018;224:400–408. doi: 10.1016/j.jep.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 143.Jin J., Tang T., Zhou H., Hong X.-D., Fan H., Zhang X.-D., Chen Z.-L., Ma B., Zhu S.-H., Wang G.-Y., Xia Z.-F. Synergistic effects of quercetin-modified silicone gel sheet in scar treatment. J. Burn Care Res. Off. Publ. Am. Burn Assoc. 2022;43:445–452. doi: 10.1093/jbcr/irab100. [DOI] [PubMed] [Google Scholar]
- 144.Ahmad M., Ansari M.N., Alam A., Khan T.H. Oral dose of citrus peel extracts promotes wound repair in diabetic rats. Pak. J. Biol. Sci. PJBS. 2013;16:1086–1094. doi: 10.3923/pjbs.2013.1086.1094. [DOI] [PubMed] [Google Scholar]
- 145.Garbyal S.S., Aggarwal K.K., Babu C.R. Traditionally used medicinal plants in Dharchula Himalayas of Pithoragarh districtUttaranchal. Vol. 4. 2005. pp. 199–207. [Google Scholar]
- 146.Kim H., Kim J., Park J., Kim S.H., Uchida Y., Holleran W.M., Cho Y. Water extract of gromwell (Lithospermum erythrorhizon) enhances migration of human keratinocytes and dermal fibroblasts with increased lipid synthesis in an in vitro wound scratch model. Skin Pharmacol. Physiol. 2012;25:57–64. doi: 10.1159/000330897. [DOI] [PubMed] [Google Scholar]
- 147.Xie Y., Fan C., Dong Y., Lynam E., Leavesley D.I., Li K., Su Y., Yang Y., Upton Z. Functional and mechanistic investigation of Shikonin in scarring. Chem. Biol. Interact. 2015;228:18–27. doi: 10.1016/j.cbi.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 148.Diniz L.R.L., Calado L.L., Duarte A.B.S., de Sousa D.P. Centella asiatica and its metabolite asiatic acid: wound healing effects and therapeutic potential. Metabolites. 2023;13:276. doi: 10.3390/metabo13020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bylka W., Znajdek-Awiżeń P., Studzińska-Sroka E., Dańczak-Pazdrowska A., Brzezińska M. Centella asiatica in dermatology: an overview. Phytother. Res. PTR. 2014;28:1117–1124. doi: 10.1002/ptr.5110. [DOI] [PubMed] [Google Scholar]
- 150.Li H., Peng Q., Guo Y., Wang X., Zhang L. Preparation and in vitro and in vivo study of asiaticoside-loaded nanoemulsions and nanoemulsions-based gels for transdermal delivery. Int. J. Nanomed. 2020;15:3123–3136. doi: 10.2147/IJN.S241923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lau T.W., Chan Y.W., Lau C.P., Lau K.M., Lau C.B.S., Fung K.P., Leung P.C., Ho Y.Y. Radix Astragali and Radix Rehmanniae, the principal components of two antidiabetic foot ulcer herbal formulae, elicit viability-promoting effects on primary fibroblasts cultured from diabetic foot ulcer tissues. Phytother. Res. PTR. 2009;23:809–815. doi: 10.1002/ptr.2649. [DOI] [PubMed] [Google Scholar]
- 152.Chen X., Peng L.-H., Shan Y.-H., Li N., Wei W., Yu L., Li Q.-M., Liang W.-Q., Gao J.-Q. Astragaloside IV-loaded nanoparticle-enriched hydrogel induces wound healing and anti-scar activity through topical delivery. Int. J. Pharm. 2013;447:171–181. doi: 10.1016/j.ijpharm.2013.02.054. [DOI] [PubMed] [Google Scholar]
- 153.Shan Y.-H., Peng L.-H., Liu X., Chen X., Xiong J., Gao J.-Q. Silk fibroin/gelatin electrospun nanofibrous dressing functionalized with astragaloside IV induces healing and anti-scar effects on burn wound. Int. J. Pharm. 2015;479:291–301. doi: 10.1016/j.ijpharm.2014.12.067. [DOI] [PubMed] [Google Scholar]
- 154.Zhang D., Li L., Shan Y., Xiong J., Hu Z., Zhang Y., Gao J. In vivo study of silk fibroin/gelatin electrospun nanofiber dressing loaded with astragaloside IV on the effect of promoting wound healing and relieving scar. J. Drug Deliv. Sci. Technol. 2019;52:272–281. doi: 10.1016/j.jddst.2019.04.021. [DOI] [Google Scholar]
- 155.Lee G.-Y., Park K.-G., Namgoong S., Han S.-K., Jeong S.-H., Dhong E.-S., Kim W.-K. Effects of Panax ginseng extract on human dermal fibroblast proliferation and collagen synthesis. Int. Wound J. 2016;13(Suppl 1):42–46. doi: 10.1111/iwj.12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhou W.-J., Li J.-L., Zhou Q.-M., Cai F.-F., Chen X.-L., Lu Y.-Y., Zhao M., Su S.-B. Ginsenoside Rb1 pretreatment attenuates myocardial ischemia by reducing calcium/calmodulin-dependent protein kinase II-medicated calcium release. World J. Tradit. Chin. Med. 2020;6:284. doi: 10.4103/wjtcm.wjtcm_24_20. [DOI] [Google Scholar]
- 157.Cui W., Cheng L., Hu C., Li H., Zhang Y., Chang J. Electrospun poly(L-lactide) fiber with ginsenoside rg3 for inhibiting scar hyperplasia of skin. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tang M., Wang W., Cheng L., Jin R., Zhang L., Bian W., Zhang Y. The inhibitory effects of 20(R)-ginsenoside Rg3 on the proliferation, angiogenesis, and collagen synthesis of hypertrophic scar derived fibroblasts in vitro, Iran. J. Basic Med. Sci. 2018;21:309–317. doi: 10.22038/ijbms.2018.19451.5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.U K., I S., E A., D Y. Wound repair potential of Olea europaea L. leaf extracts revealed by in vivo experimental models and comparative evaluation of the extracts' antioxidant activity. J. Med. Food. 2011;14 doi: 10.1089/jmf.2010.0039. [DOI] [PubMed] [Google Scholar]
- 160.Tan J., Peng X., Luo G., Ma B., Cao C., He W., Yuan S., Li S., Wilkins J.A., Wu J. Investigating the role of P311 in the hypertrophic scar. PLoS One. 2010;5 doi: 10.1371/journal.pone.0009995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wei Y.-J., Yan X.-Q., Ma L., Wu J.-G., Zhang H., Qin L.-P. Oleanolic acid inhibits hypertrophic scarring in the rabbit ear model. Clin. Exp. Dermatol. 2011;36:528–533. doi: 10.1111/j.1365-2230.2010.04012.x. [DOI] [PubMed] [Google Scholar]
- 162.Mirbehbahani F.S., Hejazi F., Najmoddin N., Asefnejad A., Artemisia annua L. As a promising medicinal plant for powerful wound healing applications. Prog. Biomater. 2020;9:139–151. doi: 10.1007/s40204-020-00138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jiang Y., Liu M., Liu H., Liu S. A critical review: traditional uses, phytochemistry, pharmacology and toxicology of Stephania tetrandra S. Moore (Fen Fang Ji) Phytochem. Rev. Proc. Phytochem. Soc. Eur. 2020;19:449–489. doi: 10.1007/s11101-020-09673-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu D.W., Hu X., Liu D.M., Zou P. Inhibitory effect of tetrandrine on the proliferation of human dermal fibroblasts derived from hypertrophic scars. Adv. Mater. Res. 2011;268–270:838–840. doi: 10.4028/www.scientific.net/AMR.268-270.838. [DOI] [Google Scholar]
- 165.Oryan A., Alemzadeh E., Moshiri A. Biological properties and therapeutic activities of honey in wound healing: a narrative review and meta-analysis. J. Tissue Viability. 2016;25:98–118. doi: 10.1016/j.jtv.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 166.Goharshenasan P., Amini S., Atria A., Abtahi H., Khorasani G. Topical application of honey on surgical wounds: a randomized clinical trial, forsch. Komplementarmedizin. 2016;23:12–15. doi: 10.1159/000441994. 2006. [DOI] [PubMed] [Google Scholar]
- 167.Gupta S.S., Singh O., Bhagel P.S., Moses S., Shukla S., Mathur R.K. Honey dressing versus silver sulfadiazene dressing for wound healing in burn patients: a retrospective study. J. Cutan. Aesthetic Surg. 2011;4:183–187. doi: 10.4103/0974-2077.91249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Thamboo A., Mulholland G., Matthews K., Ayoub N., Anderson D. Objective and subjective scar aesthetics with topical Manuka honey post-thyroidectomy: a randomized control study. World J. Otorhinolaryngol. - Head Neck Surg. 2016;2:203–207. doi: 10.1016/j.wjorl.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Atiyeh B.S. Nonsurgical management of hypertrophic scars: evidence-based therapies, standard practices, and emerging methods. Aesthetic Plast. Surg. 2020;44:1320–1344. doi: 10.1007/s00266-020-01820-0. [DOI] [PubMed] [Google Scholar]
- 170.Pikuła M., Żebrowska M.E., Pobłocka-Olech L., Krauze-Baranowska M., Sznitowska M., Trzonkowski P. Effect of enoxaparin and onion extract on human skin fibroblast cell line - therapeutic implications for the treatment of keloids. Pharm. Biol. 2014;52:262–267. doi: 10.3109/13880209.2013.826246. [DOI] [PubMed] [Google Scholar]
- 171.Jenwitheesuk K., Surakunprapha P., Jenwitheesuk K., Kuptarnond C., Prathanee S., Intanoo W. Role of silicone derivative plus onion extract gel in presternal hypertrophic scar protection: a prospective randomized, double blinded, controlled trial. Int. Wound J. 2012;9:397–402. doi: 10.1111/j.1742-481X.2011.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hosnuter M., Payasli C., Isikdemir A., Tekerekoglu B. The effects of onion extract on hypertrophic and keloid scars. J. Wound Care. 2007;16:251–254. doi: 10.12968/jowc.2007.16.6.27070. [DOI] [PubMed] [Google Scholar]
- 173.Chung V.Q., Kelley L., Marra D., Jiang S.B. Onion extract gel versus petrolatum emollient on new surgical scars: prospective double-blinded study. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. Al. 2006;32:193–197. doi: 10.1111/j.1524-4725.2006.32045.x. [DOI] [PubMed] [Google Scholar]
- 174.Yuan X., Shen J., Chen L., Wang L., Yan Q., Zhang J. Onion extract gel is not better than other topical treatments in scar management: a meta-analysis from randomised controlled trails. Int. Wound J. 2021;18:396–409. doi: 10.1111/iwj.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ran X., Ma L., Peng C., Zhang H., Qin L.-P. Ligusticum chuanxiong Hort: a review of chemistry and pharmacology. Pharm. Biol. 2011;49:1180–1189. doi: 10.3109/13880209.2011.576346. [DOI] [PubMed] [Google Scholar]
- 176.Wang T., Guo R., Zhou G., Zhou X., Kou Z., Sui F., Li C., Tang L., Wang Z. Traditional uses, botany, phytochemistry, pharmacology and toxicology of Panax notoginseng (Burk.) F.H. Chen: a review. J. Ethnopharmacol. 2016;188:234–258. doi: 10.1016/j.jep.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 177.Li X., Liu Z., Liao J., Chen Q., Lu X., Fan X. Network pharmacology approaches for research of Traditional Chinese Medicines. Chin. J. Nat. Med. 2023;21:323–332. doi: 10.1016/S1875-5364(23)60429-7. [DOI] [PubMed] [Google Scholar]
- 178.Mariuzzi L., Domenis R., Orsaria M., Marzinotto S., Londero A.P., Bulfoni M., Candotti V., Zanello A., Ballico M., Mimmi M.C., Calcagno A., Marchesoni D., Di Loreto C., Beltrami A.P., Cesselli D., Gri G. Functional expression of aryl hydrocarbon receptor on mast cells populating human endometriotic tissues. Lab. Investig. J. Tech. Methods Pathol. 2016;96:959–971. doi: 10.1038/labinvest.2016.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chen H., Xu K., Sun C., Gui S., Wu J., Wang S. Inhibition of ANGPT2 activates autophagy during hypertrophic scar formation via PI3K/AKT/mTOR pathway, an. An. Bras. Dermatol. 2023;98:26–35. doi: 10.1016/j.abd.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Z. J, Z. Y, S. V, X. Jj, L. K, W. D, S. J, Z. T, Z. L, Z. L, H. B, T. S, L. Ay, M. Aj, S. Y, L. Py, L. Q Simultaneous silencing of TGF-β1 and COX-2 reduces human skin hypertrophic scar through activation of fibroblast apoptosis. Oncotarget. 2017;8 doi: 10.18632/oncotarget.20869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Liu Y., Xu S., Zu T., Li F., Sang S., Liu C., An Y., Mi B., Orgill D.P., Murphy G.F., Lian C.G. Reversal of TET-mediated 5-hmC loss in hypoxic fibroblasts by ascorbic acid. Lab. Investig. J. Tech. Methods Pathol. 2019;99:1193–1202. doi: 10.1038/s41374-019-0235-8. [DOI] [PubMed] [Google Scholar]
- 182.Griffin M.F., Borrelli M.R., Garcia J.T., Januszyk M., King M., Lerbs T., Cui L., Moore A.L., Shen A.H., Mascharak S., Diaz Deleon N.M., Adem S., Taylor W.L., desJardins-Park H.E., Gastou M., Patel R.A., Duoto B.A., Sokol J., Wei Y., Foster D., Chen K., Wan D.C., Gurtner G.C., Lorenz H.P., Chang H.Y., Wernig G., Longaker M.T. JUN promotes hypertrophic skin scarring via CD36 in preclinical in vitro and in vivo models. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abb3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liu C. Inhibition of mechanical stress-induced hypertrophic scar inflammation by emodin. Mol. Med. Rep. 2015;11:4087–4092. doi: 10.3892/mmr.2015.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dong X., Fu J., Yin X., Cao S., Li X., Lin L., Huyiligeqi, Ni J. Emodin: a review of its pharmacology, toxicity and pharmacokinetics. Phytother. Res. PTR. 2016;30:1207–1218. doi: 10.1002/ptr.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Huang L.-P., Wang G.-Q., Jia Z.-S., Chen J.-W., Wang G., Wang X.-L. Paclitaxel reduces formation of hypertrophic scars in the rabbit ear model. Ther. Clin. Risk Manag. 2015;11:1089–1095. doi: 10.2147/TCRM.S82961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wu C., Jiang J., Boye A., Jiang Y., Yang Y. Compound Astragalus and Salvia miltiorrhiza extract suppresses rabbits' hypertrophic scar by modulating the TGF-β/Smad signal. Dermatol. Basel Switz. 2014;229:363–368. doi: 10.1159/000365784. [DOI] [PubMed] [Google Scholar]
- 187.Hou X.-H., Cao B., Liu H.-Q., Wang Y.-Z., Bai S.-F., Chen H. Effects of osthole on apoptosis and TGF-beta1 of hypertrophic scar fibroblasts. J. Asian Nat. Prod. Res. 2009;11:663–669. doi: 10.1080/10286020902975772. [DOI] [PubMed] [Google Scholar]
- 188.Deng X., Zhao F., Zhao D., Zhang Q., Zhu Y., Chen Q., Qiang L., Xie N., Ma J., Pan X., Wu Y., Guan L., Xie Y. Oxymatrine promotes hypertrophic scar repair through reduced human scar fibroblast viability, collagen and induced apoptosis via autophagy inhibition. Int. Wound J. 2022;19:1221–1231. doi: 10.1111/iwj.13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.a Ye X., b Pang Z., a Zhu N. Dihydromyricetin attenuates hypertrophic scar formation by targeting activin receptor-like kinase 5. Eur. J. Pharmacol. 2019;852:58–67. doi: 10.1016/j.ejphar.2019.02.039. [DOI] [PubMed] [Google Scholar]
- 190.Chong C.-H., Sun J.-M., Liu Y.-X., Tsai Y.-T., Zheng D.-N., Zhang Y.-F., Yu L. Salvianolic acid B attenuates hypertrophic scar formation in vivo and in vitro. Aesthetic Plast. Surg. 2023 doi: 10.1007/s00266-023-03279-1. [DOI] [PubMed] [Google Scholar]
- 191.Hao R., Wang C., Yang C., Chang J., Wang X., Yuan B., Xu H., Zhou S., Fan C., Li Z. Transdermal delivery of Protocatechuic aldehyde using hyaluronic acid/gelatin-based microneedles for the prevention and treatment of hypertrophic scars. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik EV. 2023;184:202–213. doi: 10.1016/j.ejpb.2023.02.003. [DOI] [PubMed] [Google Scholar]
- 192.Zhang Y.F., Zhou S.Z., Cheng X.Y., Yi B., Shan S.Z., Wang J., Li Q.F. Baicalein attenuates hypertrophic scar formation via inhibition of the transforming growth factor-β/Smad2/3 signalling pathway. Br. J. Dermatol. 2016;174:120–130. doi: 10.1111/bjd.14108. [DOI] [PubMed] [Google Scholar]
- 193.Chen G., Liang Y., Liang X., Li Q., Liu D. Tanshinone IIA inhibits proliferation and induces apoptosis through the downregulation of Survivin in keloid fibroblasts. Ann. Plast. Surg. 2016;76:180–186. doi: 10.1097/SAP.0000000000000544. [DOI] [PubMed] [Google Scholar]
- 194.Li Q., Wu P., Xia Y., Liao D., Zuo Y., Wu J., Xia Q. Dihydroartemisinin-induced mitochondrial mRNA degradation and apoptosis in keloid fibroblasts. Chin. Med. J. (Engl.) 2022 doi: 10.1097/CM9.0000000000001860. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data related to this study are not stored in the publicly available repository. This study is a review and the raw data are available in references studies.