Abstract
Molecular tumor profiling has become an important diagnostic for prostate cancer, allowing for personalized treatment regimens based on somatic and germline genetic information. We report a 67-year-old patient with metastatic castrate-resistant prostate cancer which was intermittently responsive to androgen-deprivation therapy, docetaxel, abiraterone, radium-223, Sipuleucel-T, and radiotherapy who ultimately demonstrated a remarkable and durable response to pembrolizumab. Our case report underlines the significance of early tumor molecular profiling in aggressive or atypical prostate cancer patients and exhibits the potential for a remarkable clinical response with immunotherapy in candidates with the appropriate tumor profiles.
Keywords: Metastatic castrate-resistant prostate cancer, Pembrolizumab, Mismatch repair deficiency, Germline testing
1. Introduction
Prostate cancer remains the second leading cause of cancer death in US males according to the American Cancer Society. In recent years, identifying germline and somatic mutations associated with prostate cancer has had significant implications for its staging, screening, treatment, and prognostication.1 Up to 57 % of prostate cancer cases can be attributed to genetic risk factors.2 Approximately 5 %–10 % of prostate cancer cases are related to germline mutations in genes of moderate to high penetrance.3 Specifically, males with defective mismatch repair (MMR) genes associated with Lynch Syndrome (MLH1, MSH2, and MSH6) may have up to a fourfold increased risk for prostate cancer.4
DNA repair mutations are associated with poor prognosis in prostate cancer. Most patients with metastatic prostate cancer treated with androgen deprivation therapy (ADT) will develop resistance to primary ADT leading to disease progression.5 This is a disease state known as metastatic castration-resistant prostate cancer (mCRPC). A 2015 clinical sequencing study demonstrated that of 150 mCRPC patients, nearly 90 % had a potentially actionable somatic or germline event.6 23 % of men had mutated DNA repair genes including BRCA1, ATM, and BRCA2, 8 % of which were germline mutations.6 This further underscores the role of early tumor molecular profiling in the treatment and prognostication of mCRPC.
Screening for MMR mutations has transformed the clinical landscape of personalized cancer treatment. In the absence of appropriate mechanisms to repair DNA, hypermutation causes the production of abundant neoantigens, which are paired with tumor-specific T-cell responses that serve as targets for immune checkpoint inhibitors (ICIs). ICIs have dramatically improved the treatment algorithm for numerous tumors with MMR deficiencies including melanoma, lung, kidney, and bladder cancer.7 The PD-1 inhibitor pembrolizumab has been found to improve progression-free survival and results in a PSA decline of over 50 % in patients with MMR deficiency metastatic prostate cancer.8
The criteria for germline and somatic testing for prostate cancer remains controversial. Given the aggressive nature of mCRPC and several studies showing a robust response to pembrolizumab in MMR-deficient mCRPC patients, this emphasizes the need to better define candidacy for immunotherapy in patients with high-risk mutations and disease refractory to traditional therapies. In this case report, we discuss the clinical course of a patient with mCRPC intermittently responsive to androgen-deprivation therapy, docetaxel, abiraterone, radium, Sipuleucel-T, and various courses of radiotherapy who showed a remarkable and durable response to pembrolizumab.
2. Case presentation
A 67-year-old man with no family history of prostate cancer and a brother with a history of Lynch syndrome presented to our institution with left-sided flank pain and gross hematuria in 2017. CT of the abdomen/pelvis demonstrated left hydronephrosis. Digital rectal exam revealed a nodular prostate and his initial serum PSA was 39 ng/mL. On subsequent diagnostic ureteroscopy, an approximately 3 cm left distal ureteral tumor was biopsied revealing low-grade transitional cell carcinoma (TCC) and this was laser ablated. The patient did not wish to proceed with more invasive surgical intervention for TCC at that time. Prostate needle biopsy revealed Gleason score (GS) 4 + 5 = 9 prostatic adenocarcinoma in 14/14 cores. A prostate MRI revealed right pelvic sidewall adenopathy (2.1 cm × 1.7 cm), posterior pelvic adenopathy (1.5 cm × 1 cm), and bony metastatic disease involving the right superior acetabulum, right posterior acetabulum, ischium, left acetabulum, T9 and T11 vertebrae. He was diagnosed with metastatic prostate cancer (cT3aN1M1).
The patient was started on androgen deprivation therapy (initially degarelix transitioned to leuprolide) and completed 6 cycles of docetaxel with PSA improvement to 0.3 ng/mL. Within 7 months, our patient's PSA progressed to 9.44 ng/mL. He underwent Sipuleucel-T for 3 cycles and then four months later underwent left palliative radiotherapy (XRT) for continued PSA progression. In 2020, he was initiated on abiraterone and received this for the next 15 months. Foundation One somatic testing did not reveal any abnormalities. Germline testing in 2021 revealed an MSH6 gene mutation, PTEN loss, FGFR1 amplification, and an ARH875Y amplification. Microsatellite instability (MSI) was not assessed.
His PSA did nadir to 7.15 ng/mL 3 months after pelvic XRT and abiraterone therapy. Only 7 months later, however, the PSA ultimately rose to 17 ng/mL. The patient then underwent XRT to the prostate, right pubic ramus, and T11 compression fracture. His PSA remained <2.5 ng/mL for the next 9 months before doubling to 5.4 ng/dL in a 5-month interval. A PSMA PET scan revealed multiple new osseous metastases including lesions of the left frontal bone, proximal right humerus, right transverse process of C7, T1, right acetabulum, and bilateral inferior pubic rami. Nuclear medicine bone scans from July 15, 2021 demonstrated similar findings (Fig. 1). The patient then underwent one course of XRT to the left frontal skull, an additional cycle of XRT to his C6-T1 lesion to palliate his right upper extremity weakness, and 6 cycles of radium-223 though his PSA continued to rise.
Fig. 1.
Nuclear medicine bone scans demonstrating degenerative uptake in upper and lower extremities, cervical spine, lumbar spine, pelvis, and calvarium from July 15, 2021.
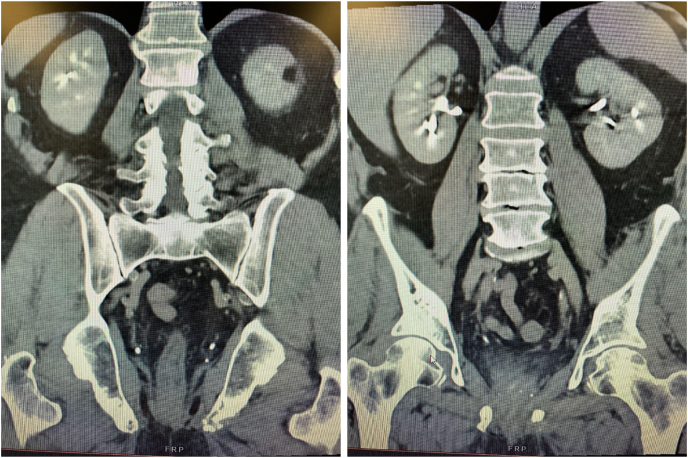
In March of 2022, the patient started pembrolizumab infusion intravenously once every 3 weeks for 8 cycles of immunotherapy. Following 8 cycles of pembrolizumab, his PSA became undetectable <0.04 ng/mL. A repeat PSMA PET scan was consistent with a complete clinical response and no evidence of active disease. A CT of the abdomen and pelvis on June 27, 2022 showed no evidence of metastasis within the abdomen or pelvis (Fig. 2). Currently, his complete PSA response appears durable approaching 2 years of follow-up. The patient has restarted pembrolizumab therapy for a recurrence of upper tract TCC.
Fig. 2.
CT Abdomen and Pelvis in two views demonstrating no evidence of metastasis within abdomen or pelvis from June 27, 2022.
3. Discussion
While genetic testing in prostate cancer is becoming more routinely recommended and various guidelines do exist, there is still an appreciable degree of controversy regarding which patients should be candidates for testing and how they should be tested. Germline testing, which is used to identify inherited detrimental DNA variants, can be used in addition to somatic testing, which identifies acquired tumor-specific variants. Various studies suggest about 12–15 % of prostate cancer cases carry an identifiable germline DNA damage repair defect.9,10 Mutations in mismatch repair genes (MLH1, MSH2, PMS2, and MSH6) are correlated with a younger age of cancer onset, a more aggressive clinical course, and increased cancer-specific mortality.1
Initially, studies investigating the potential role for ICIs in mCRPC yielded discouraging results. The KEYNOTE-921 randomized, double-blind, phase 3 study evaluated the efficacy of pembrolizumab and docetaxel compared to placebo and docetaxel in 1030 patients with mCRPC. The addition of pembrolizumab to docetaxel did not significantly improve progression-free survival or overall survival (PFS median 8.6 months with pembrolizumab and docetaxel vs 8.3 months with placebo and docetaxel; OS median 19.6 months vs 19.0 months).11 A separate phase 1 study of nivolumab in 17 patients with mCRPC demonstrated an overall response rate of 0 %.12 In contrast, more recent studies have demonstrated a positive response to pembrolizumab even in non-genomically targeted men. A 2016 study demonstrated an 18 % PSA response rate using pembrolizumab plus enzalutamide in mCRPC patients who had previously progressed after treatment with enzalutamide alone.13 In May 2017, the US Food and Drug Administration approved pembrolizumab for all MMR-deficient solid tumors that had progressed on prior therapy and had no satisfactory remaining treatment options.
Currently, the National Comprehensive Cancer Network recommends germline testing for patients with clinically low-to-intermediate localized disease with a family history of prostate cancer, high-to-very high-risk localized disease, and all patients with regional or distant metastatic disease regardless of initial risk.10 However, genetic testing and precision treatment often remain restricted to tertiary or comprehensive cancer centers limiting access to equitable care to resource-privileged patients.14 Furthermore, oncology providers have been slow to incorporate genetic testing into their practices despite the advancement of NCCN guidelines.15
In this case report, we present an interesting case of a mCRPC patient identified as having an MSH6 germline mutation heavily pre-treated with androgen-deprivation therapy, docetaxel, abiraterone, radium, Sipuleucel-T, and various courses of XRT who demonstrated a >99 % PSA reduction which appears durable approaching 2 years. With only 1 out of 5 prostate cancer cases harboring germline mutations, sporadic defects in MMR genes are much more common.16 Interestingly, our patient had no somatic mutations identified with Foundation One testing, underpinning the importance of both somatic and germline testing in patients with mCRPC cases.
Prior retrospective analyses have shown that clinical response with Pembrolizumab post-docetaxel in genomically non-targeted men with mCRPC can be exceedingly low.17 However, several multi-institutional studies have demonstrated a niche role for immunotherapy specifically in MMR-deficient mCRPC. Lenis et al. reported a decline in PSA level of more than 50 % (PSA50 response) after pembrolizumab in 65 % of patients identified with MMR deficiency metastatic prostate cancer as well as a progression-free survival of 41 months.9 Additionally, Graham et al. reported a PSA50 response in 53.3 % of MMR-deficient prostate cancer patients who were treated with pembrolizumab at median follow-up of 12 months.18
Several studies have demonstrated a role for immunotherapy not only in mCRPC with MMR deficiency but also for mCRPC with microsatellite instability (MSI), high tumor mutational burden (TMB), and other tumor molecular profile alterations. MSI was notably undetermined in our patient, which does limit our study. It is well-documented that inactivation of MMR genes can induce a high frequency of MSI and it is unclear to what extent the interplay between his germline mutation and microsatellite status molded our patient's profile into ideal candidacy for immunotherapy response. One patient reported by Tucker et al. with MSI-high disease demonstrated >90 % PSA decline with pembrolizumab.19 Additionally, a case report by Fujiwara et al. revealed a 63 % PSA response with 5 cycles of pembrolizumab as well as radiographic evidence of disease shrinkage in a patient with MSI-high mCRPC heavily pre-treated with docetaxel.20 Further studies are needed to elucidate potential sequelae of mismatch repair gene alterations on the tumor molecular profile and how exactly these changes shape candidacy for immunotherapy.
4. Conclusion
As the therapeutic landscape for mCRPC continues to evolve and novel immunotherapy agents have become more prevalent, molecular tumor profiling has become an essential diagnostic. Our case report underlines the significance of early tumor molecular profiling in aggressive or atypical prostate cancer patients and exhibits the potential for a remarkable clinical response with immunotherapy in accurately selected candidates.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Brian F. Dinerman: Conceptualization, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – review & editing. Andrew Skomra: Visualization, Writing – original draft, Writing – review & editing. Iryna Dovirak: Visualization, Writing – original draft, Writing – review & editing. John Rutkowski: Investigation, Supervision, Writing – review & editing.
Declaration of competing interest
None.
Contributor Information
Brian F. Dinerman, Email: bdinerma@buffalo.edu.
Iryna Dovirak, Email: irynadov@buffalo.edu.
References
- 1.Castro E., Goh C., Olmos D., et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31(14):1748. doi: 10.1200/JCO.2012.43.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mucci L.A., Hjelmborg J.B., Harris J.R., et al. Familial risk and heritability of cancer among twins in Nordic countries. JAMA. 2016;315(1):68–76. doi: 10.1001/jama.2015.17703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.PDQ Cancer Genetics Editorial Board. PDQ Genetics of Prostate Cancer. Bethesda, MD: National Cancer Institute https://www.cancer.gov/types/prostate/hp/prostate-genetics-pdq Updated <02/15/2024>. Available at: Accessed <6/January/2024>. [PMID: 26389227].
- 4.Haraldsdottir S., Hampel H., Wei L., et al. Prostate cancer incidence in males with Lynch syndrome. Genet Med. 2014;16(7):553–557. doi: 10.1038/gim.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beer T.M., Armstrong A.J., Rathkopf D.E., et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson D., Van Allen E.M., Wu Y.M., et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunn C.M., Li E.X., Gignac G.A., et al. Delivering genetic testing for patients with prostate cancer: moving beyond provider knowledge as a barrier to care. Cancer Control. 2023;30 doi: 10.1177/10732748221143884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenis A.T., Ravichandran V., Truong H., et al. Response to immune checkpoint blockade in patients with microsatellite instable and high tumor mutational burden prostate cancer. J Urol. 2021, September;206 doi: 10.1158/1078-0432.CCR-23-3403. E411-E411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicolosi P., Ledet E., Yang S., et al. Prevalence of germline variants in prostate cancer and implications for current genetic testing guidelines. JAMA Oncol. 2019;5(4):523–528. doi: 10.1001/jamaoncol.2018.6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giri V.N., Obeid E., Gross L., et al. vol. 1. JCO Precision Oncology; 2017. pp. 1–17. (Inherited Mutations in Men Undergoing Multigene Panel Testing for Prostate Cancer: Emerging Implications for Personalized Prostate Cancer Genetic Evaluation). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrylak D.P., Ratta R., Matsubara N., et al. 2023. Pembrolizumab Plus Docetaxel for Patients with Metastatic Castration-Resistant Prostate Cancer (mCRPC): Randomized, Double-Blind, Phase 3 KEYNOTE-921 Study. [Google Scholar]
- 12.Topalian S.L., Hodi F.S., Brahmer J.R., et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graff J.N., Alumkal J.J., Drake C.G., et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget. 2016;7(33) doi: 10.18632/oncotarget.10547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterson E.B., Chou W.Y.S., Gaysynsky A., et al. Communication of cancer-related genetic and genomic information: a landscape analysis of reviews. Translational Behavioral Medicine. 2018;8(1):59–70. doi: 10.1093/tbm/ibx063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paller C.J., Antonarakis E.S., Beer T.M., Borno H.T., Carlo M.I., George D.J.…PCCTC Germline Genetics Working Group Germline genetic testing in advanced prostate cancer; practices and barriers: survey results from the germline genetics working group of the prostate cancer clinical trials consortium. Clin Genitourin Cancer. 2019;17(4):275–282. doi: 10.1016/j.clgc.2019.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan H.M., Cheng H.H. Germline genetics of prostate cancer. Prostate. 2022;82:S3–S12. doi: 10.1002/pros.24340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonarakis E.S., Lu C., Luber B., et al. Germline DNA-repair gene mutations and outcomes in men with metastatic castration-resistant prostate cancer receiving first-line abiraterone and enzalutamide. Eur Urol. 2018;74(2):218–225. doi: 10.1016/j.eururo.2018.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham L.S., Montgomery B., Cheng H.H., et al. Mismatch repair deficiency in metastatic prostate cancer: response to PD-1 blockade and standard therapies. PLoS One. 2020;15(5) doi: 10.1371/journal.pone.0233260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tucker M.D., Zhu J., Marin D., et al. Pembrolizumab in men with heavily treated metastatic castrate‐resistant prostate cancer. Cancer Med. 2019;8(10):4644–4655. doi: 10.1002/cam4.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujiwara M., Komai Y., Yuasa T., et al. Pembrolizumab for a patient with metastatic castration‐resistant prostate cancer with microsatellite instability‐high. IJU Case Reports. 2020;3(2):62–64. doi: 10.1002/iju5.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]