Abstract
Renewable lignocellulosic biomass is a favorable energy resource since its co-pyrolysis with hydrogen-rich plastics can produce high-yield and high-quality biofuel. In contrast to earlier co-pyrolysis research that concentrated on increasing product yield, this study comprehends the synergistic effects of two distinct feedstocks that were not considered earlier. This work focuses on co-pyrolyzing wheat straw (WS) with non-reusable polyethylene terephthalate (PET) for the production of pyrolysis oil. WS and PET were blended in different ratios (100/0, 80/20, 60/40, 40/60, 20/80, and 0/100), and pyrolysis experiments were conducted in a fixed-bed reactor under different temperatures to assess their synergistic effect on oil yield. Synergy rates of up to 7.78 % were achieved on yield for the blends of plastic and biomass at a temperature of 500 °C. In comparison to individual biomass or plastics, co-pyrolyzing PET-biomass blends demonstrated good process interaction and promoted the yields of value-added products. The heating value of the pyrolysis oils was in the range of 16.45–28.64 MJ/kg, which depends on the amount of plastic present in the feedstock. The physical analysis of the oils shows that they can be used for heat production by direct combustion in boilers or furnaces. The correlation between WS and PET was validated with the aid of Fourier transform infrared spectroscopy (FT-IR) and gas chromatography-mass spectrometry (GC-MS) analysis. The GC-MS result demonstrated the presence of different compounds such as O-H compounds, esters, carbonyl group elements, acids, hydrocarbons, aromatics, and nitrogenated compounds in the pyrolysis oil, which differed based on the proportions of PET in the feedstock. The increased hydrocarbon and reduced oxygen percentages in the pyrolysis oil were implicitly caused by enhanced hydrocarbon pool mechanisms, in which the breakdown of PET may be supplied as a hydrogen donor. Overall, waste lignocellulosic biomass and plastics can be used to produce biofuels, which helps reduce the amount of solid waste that ends up in landfills. This study also revealed that future research should be focused on the reaction mechanisms of WS and PET co-pyrolysis in order to examine the synergistic interactions.
Keywords: Biomass-plastic blend, Degradation behavior, Fixed bed reactor, Synergistic effect, Characterization study, Chromatographic analysis
Graphical abstract
1. Introduction
The worldwide energy scenario has changed dramatically over the last 30 years. The rapid growth and advancement of human activities are attributed to energy, which has been used for power generation, industrial development, and transportation [1]. Fossil fuels, including coal, oil, and natural gas, have played a major role in meeting this increase in energy needs [2]. Significant changes in energy production have resulted in increased road traffic and, consequently, a change in the focus on the causes and effects of NOx and volatile matter emissions [3]. Renewable energy sources—also referred to as alternative energy sources—are crucial for the development of a future world to produce energy. They could potentially offer energy services with nearly zero emissions [4]. The development of renewable energy projects in rural areas can reduce migration by generating rural-based work opportunities [5]. Biomass for power production is increasing in popularity as it produces more energy with increased efficiency. It also provides a solution for waste disposal, since solid waste management is one of the major issues for developing and underdeveloped countries [6,7]. Several cities in developing Asian countries have reported existing landfill site issues [8]. Though waste separation is mandated in several cities, only about 30 % of waste is sorted properly, meaning plastic ends up in landfills instead of being recycled. The quantity and composition of the waste are considered key factors in developing new waste management technology [6]. The estimations of these variables are imperative for the development of resource recuperation and recycling. Without these data, the successful implementation of recycling technology is very difficult. It is also helpful for the prediction and estimation of a landfill's life. The variation in composition is common and differs according to day, season, and collecting center [9]. In order to overcome these issues, a common waste treatment technique is essential to minimize domestic and industrial waste. Pyrolysis is considered a successful waste conversion technique to recover energy from lignocellulosic and plastic waste [10]. The process of pyrolysis involves heating wastes without the presence of air to produce recyclable components such as char, oil, and combustible gases.
The process of biomass pyrolysis has become increasingly popular in recent years as the primary and essential phase of thermochemical conversion [11]. The pyrolysis process has a major impact on the physicochemical characteristics and yield of the products [12]. For instance, Park et al. [13] employed approximately 100 g of rice straw to perform slow pyrolysis experiments. The authors conducted the experiments by varying the process temperature up to 700 °C. At 500 °C, the yield of pyrolysis oil and char reached up to 39 wt%. When the reactor was kept at 700 °C, the gas production was enhanced, but the oil production somewhat declined. Balagurumurthy et al. [14] performed a rice straw pyrolysis experiment at 400 °C using rice straw under hydrogen (H2) and nitrogen (N2) atmospheres and produced a maximum of 31.0 % and 12.8 % of pyrolysis oil under N2 and H2 atmospheres, respectively. Rice straw in the N2 environment produced more pyrolysis oil than in the H2 environment. After pyrolyzing corn cobs, Cao et al. [15] showed pyrolysis oil, char, and gas yields ranged from 34.0 wt% to 40.9 wt%, 23.6 wt% to 31.6 wt%, and 27.0 wt% to 40.9 wt%, respectively. The authors identified less variation in their yields above 600 °C. Alper et al. [16] conducted a pyrolysis experiment on two different agricultural residues, such as cherry stones and grape seeds, at 300–700 °C. The authors reported that the pyrolysis temperatures had a major impact on their product distribution. For cherry stones and grape seeds, higher amounts of pyrolysis oil yields were attained at 500 °C and 700 °C, respectively. The derived pyrolysis oil is primarily composed of oxygenated hydrocarbons. Sánchez et al. [17] explored the characterization study of pyrolysis oil produced from rape and sunflower wastes. The resulting pyrolysis oil was assessed as a green biofuel option that is friendly to the environment. The char products were rich in carbon and projected as a solid fuel. The produced pyrolysis gas was also identified as a biofuel due to its composition and higher heating value. Numerous studies have demonstrated that co-pyrolysis, in addition to individual pyrolysis, can improve the yield and characteristics of biofuel [[18], [19], [20]]. Co-pyrolysis of biomass and plastics aims to improve the yield and quality of biofuel products while utilizing flexible material resources and developing sustainable fuels. It was also found that co-pyrolysis had a major impact on the product's composition and quality. According to one study, when woody biomass is co-pyrolyzed with plastic waste, the formation of hydrocarbons, more stable alcohols, and esters is promoted while the formation of reactive oxygenated compounds, such as ketones, aldehydes, and acids, is suppressed [21].
Plastic can be found almost anywhere on Earth. The environment and public health are suffering greatly as a result of the accumulated plastic waste. According to World Bank estimates, the world's yearly plastic waste production is predicted to rise from 2.01 billion tons to 3.40 billion tons by 2050 [22]. Plastic may be pyrolyzed directly and can produce liquid fuel; however, liquid products are challenging to use directly as fuel oil [23]. Thus, the co-pyrolysis of waste plastics and biomass is a viable and attractive technique that will reduce environmental pollution caused by the disposal of solid wastes and produce high-value compounds. During co-conversion, chemical interactions between co-reactants may result in an increase in pyrolysis oil output. Plastic can facilitate biomass decomposition by providing hydrogen to stabilize the oxygen radicals in the biomass [24]. Xue and Bai [25] observed that co-pyrolysis of polyethylene (PE) and corn stover increased PE cracking because the free radicals formed from lignin promoted the radical-initiated breakdown of PE, demonstrating that lignin was highly interacting with plastics. Qi et al. [26] found that when a microalgae biomass was co-fed with PP, positive and negative synergy was observed in oil and gas yields, respectively. Jin et al. [27] and Van Nguyen et al. [28] reported comparable results from co-pyrolysis studies. The maximum synergy promoting gas/char output was found at 50 % plastic content, with values ranging from 25 wt% to 75 wt%. When the plastic percentage was added to the feedstock from 5 wt% to 75 wt%, the maximum variance in gas yield was observed at 40 %. When biomass and plastic were co-pyrolyzed, the results demonstrated improved conversion efficiency than individual component pyrolysis due to increased synergistic effects [29,30]. Increased gas content has been noted by certain researchers when co-pyrolyzing plastic with higher lignin [31] and cellulose biomass [32]. The output of nitrogen components in liquid products dropped significantly during the co-pyrolysis of high-density polyethylene with straw [33], and the liquid yield somewhat improved [34]. In addition to that, the outcomes of the study conducted by Ryu et al. [35], Wang et al. [36], Esso et al. [37], and Cai et al. [38] showed that the quality of pyrolysis oil can be greatly increased by co-pyrolyzing solid biomass with waste plastic. Among various plastic materials, about 7–9% of the plastic consumed worldwide is made of PET, one of the most significant commercial polyesters [39]. It is a non-biodegradable plastic material yet has a little amount of oxygen content comparable to solid biomass and a comparable H/Ceff. The recyclability rate of PET wastes is sometimes lowered to less than 10 % when combined with other polymers, despite the fact that some wastes have been recycled to generate engineering components [36]. Compared to other types of polyolefins, the calorific value of PET is low, and the addition of PET for the co-pyrolysis process may not significantly enhance the oil quality due to its inherent oxygen content. On the other side, the usage and availability of waste PET are abundant. It is most commonly used as a single-serving container for water and carbonated soft beverages. According to Recycling International, in 2021, the consumption of PET reached 20 million tonnes. The widespread use of PET generates a significant volume of PET waste. However, its low degradation and thermal stability generate major environmental and human health issues. The current PET disposal methods, such as recycling, incineration, and land filling, have limitations due to environmental degradation. Therefore, co-pyrolysis of PET with biomass was identified as a more effective and reliable method to dispose of PET waste. From these results, it can be understood that the research on co-pyrolysis of biomass and plastics typically focuses on improving the oil's characteristics and producing a higher yield of oil, but the synergistic mechanism that underlies the results is discussed very little.
The yield of pyrolysis and its chemical compositions may be influenced by a number of factors, such as types of feedstock, temperature of the reactor, reaction duration, and type of catalysts [23]. Among these, temperature had a major effect on the species generated and the content of the products [40]. Furthermore, the complex organic compounds present in the pyrolysis oil are analyzed qualitatively and quantitatively using advanced FT-IR and GC-MS diagnostics. In order to manage environmental pollution, co-pyrolysis of different types of woody and agricultural biomass with waste plastics can be utilized to produce liquid fuels and chemical compounds. To our knowledge, the co-pyrolysis of lignocellulosic biomass with different types of waste polymers has rarely been reported in detail. However, extensive information on synergistic interaction during co-pyrolysis of WS with PET in various compositions and product distribution is not available. This research aims to fill this gap. This study investigated the correlation between the co-pyrolysis product yield obtained at different blends of WS and PET (100/0, 80/20, 60/40, 40/60, 20/80, and 0/100). The synergistic effect on co-pyrolysis process yield was ascertained between biomass and plastics by comparing individual component pyrolysis. Further experimental work has been extended to conduct the catalytic pyrolysis process using the HZSM-5 catalyst. In order to examine the impact of the catalyst on the co-pyrolysis process, the yield obtained from catalytic co-pyrolysis was compared to the yield obtained from the non-catalytic process with maximum synergy.
2. Materials and methods
2.1. Materials
The wheat straw (WS) used for this study was collected from a local agricultural field in Coimbatore, India. The samples were ground initially to a grain size of <1.0 mm. After that, the collected WS was air heated for 2 days. The dried samples were further dried in a closed oven maintained at 100 ± 5 °C. The waste PET plastics used for food, water, and beverage packing having a thickness between 0.25 mm and 0.9 mm were collected from a local plastic waste vendor in Coimbatore, India. The plastic waste was collected in a pellet form of size ⁓ 1.0 mm.
2.2. Feedstock analysis
The proximate and elemental analysis of WS and PET plastics was carried out using ASTM standards (moisture - D3173; volatile matter- D3175; ash- D3174; fixed carbon-by difference; carbon, hydrogen, nitrogen, sulfur- D5373; oxygen-by difference). The proximate analysis was done in a muffle furnace at a controlled temperature. The weights of the feedstocks and pyrolysis products are measured by a balance to calculate the yields. The following equations (1), (2), (3), (4) are used for the analysis of moisture content, volatile matter, ash content and fixed carbon respectively.
| (1) |
| (2) |
| (3) |
where
| (4) |
The ultimate analysis to identify the value of carbon, hydrogen, nitrogen, sulfur, and oxygen in the samples was done using an elemental analyzer (EA 2400 Series II). The analysis was carried out using 10 mg of pure samples under nitrogen atmosphere. Before analysis, both the samples were removed from dust or moisture since it leads to deviations in the results. The moisture and dust particles were removed by initial heating and screening process. The solid biomass and plastics were converted into powdered form and the analysis is accomplished by combustion analysis. The traditional wet chemistry approach is used for the determination of lignocellulosic content of WS. The analysis was carried out using 0.50 g of biomass sample. This is a very accurate approach for determining composition analysis. In this method, an acid chlorination treatment was initially applied to the sample in a water bath for more than 1 h at a temperature of 75 °C. Then a required quality of NaClO2 and CH3COOH were added for chlorination. Finally, the solution is filtered and then rinsed with cold water. The leftover residue is classified as cellulose and hemicellulose, as determined by gravimetric analysis. A two-step sulfuric acid hydrolysis procedure is used to assess acid-insoluble lignin content.
2.3. TGA analysis
The thermal decomposition behavior of the WS and PET was investigated using the TGA701 thermogravimetric analyzer. The analysis was conducted using 3–5 mg of sample under a nitrogen atmosphere. For this, the sample was kept in a closed furnace and heated from atmospheric temperature to 1073 K at a heating rate of 10 K/min. For the entire analysis, the flow rate of the nitrogen was adjusted to 20 mL/min. The following formula is used to compute the conversion of the feedstock: The derivative of conversion in relation to time was used to generate derivative thermogravimetric (DTG) curves, which were then plotted against temperature.
2.4. Test stand
A fixed-bed cylindrical reactor with an internal diameter of 50 mm and a length of 100 mm, heated by an electric furnace, was used to carry out the pyrolysis experiments. Fig. 1 represents the schematic view of the reactor set up. Heat was applied to individual and different blends of WS/PET at an average rate of 10 °C/min to reach the desired temperature. The reaction was maintained until no vapor was detected from the condenser. For this purpose, a minimum of 30 min is given to one complete reaction. The evolved gases coming out of the reactor were cooled in a condenser. For that, the condenser system is supplied with adequate ice water maintained at 0 °C. The evolved gases are condensed using a horizontal type cylindrical condenser made of high-grade stainless steel with a diameter of 50 mm and a length of 470 mm. When the pyrolysis gas hits 50 °C within the condenser, the condensable pyrolysis oil and water typically begin to condense, while the phenolic chemicals begin to condense at 80 °C. It should be pointed out that the partial pressure of vapor compounds varies with composition and is affected by feedstock type and reactor operating conditions. The heat applied to the reactor is fully utilized for heating the sample since the reactor is well insulated with insulating material. About 100 g of feed were loaded for each test run. A combination of WS and PET particles was tested with different mass ratios. Once the material was filled inside the reactor vessel, it was closed tightly to ensure anaerobic conditions. In order to measure the temperature of the reactor, two K-type thermocouples were fixed at two different points. The internal temperature was monitored through a digital meter and was controlled by an auto transformer. At the end of each run, the reactor was allowed to cool to room temperature. Following each experimental run, the residual solid residue—also known as char—inside the reactor was weighed using a digital weighing machine and stored. The weight of pyrolysis oil is also found by direct measurement. The weight of the pyrolysis oil product and the char were subtracted from the weight of the loaded feedstock to determine the weight of the gas yield. The calculation revealed that the mean yield values of a minimum of three trials conducted with identical experimental setups fell within the experimental error range of less than ±1 %. The following equation (5) was used to measure the yield of individual products.
| (5) |
Fig. 1.
Schematic view of the pyrolysis reactor.
2.5. Method
The pyrolysis experiments were carried out on individual components as well as their different blends. The blends were obtained by mixing WS and PET at different weight ratios. Both the individual and co-pyrolysis experiments were conducted at different reaction temperatures. In order to analyze the maximum yield point, the temperature of the reactor was varied from 350 °C to 650 °C at a heating rate of 10 °C/min. The temperature range for this study was designed based on the TGA analysis of the individual components. The product yield and its variation during the co-pyrolysis process were assessed with the addition of PET with WS at four different ratios of 80/20, 60/40, 40/60, and 20/80. The blended components were pyrolyzed at a temperature of 500 °C, and the temperature of the reactor was raised at a rate of 10 °C/min. The catalytic co-pyrolysis process was carried out on the feedstocks having 60 % PET using HSZM-5 catalysts. For catalytic process, 10 % of the catalyst was loaded with the feedstock. For each run, 10 g of HSZM-5 catalysts were blended with the feedstocks. The catalyst was supplied by Nikunj Chemicals, India Ltd. Table 1 shows the characteristics of zeolite used in the catalytic degradation process. When biomass and plastic are combined in binary, ternary, or multiple combinations, a synergistic impact is produced, which may be greater or less than the result obtained from the weighted value of each individual component during co-pyrolysis. The combination of feedstocks is one of the key factors that impacts the synergistic effects [41]. To ascertain the existence and degree of a synergistic impact, the experimental and estimated product content from the co-pyrolysis of WS and PET plastic was assessed and compared. Equation (6) was utilized to assess theoretical product outcomes [19]. A synergistic impact is one that occurs when two substances/materials interact to produce an effect greater than the sum of their individual effects. This may result from the interaction of molecules of both substances, particularly carbon and hydrogen. When the values are compared, the noticeable increase is known as the positive synergy impact, while the obvious reduction is known as the negative cooperativity effect.
| (6) |
Where,
Table 1.
Characteristics of HZSM-5 catalyst.
| Characteristics | HZSM-5 | Unit |
|---|---|---|
| SiO2/Al2O3 | 28 | w/w |
| Pore size (nm) | 0.5 | nm |
| BET surface area | 341 | m2/g |
| External surface areaa | 38 | m2/g |
| Micropore volumeb | 0.17 | cm3/g |
| Particle size | 50–70 | μm |
| Total acidity | 2.03 | mmolNH3/g |
Obtained by application of the t-plot method.
Measured at p/p0: 0.995.
2.6. Individual and co-pyrolysis mechanism
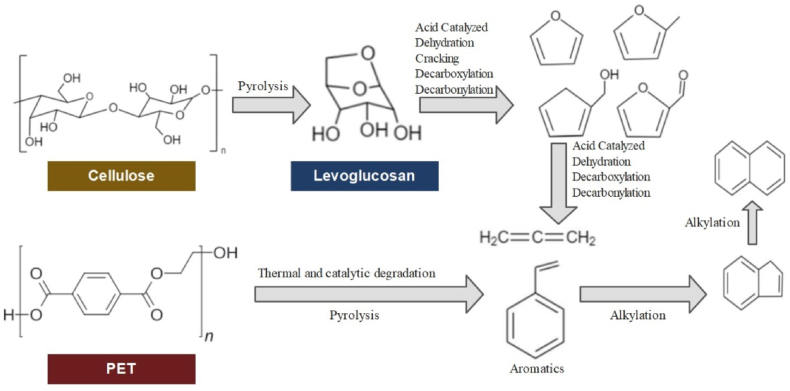
The composition of the biomass has a considerable impact on its pyrolysis behavior. Lignocellulose consists primarily of cellulose, hemicellulose, and lignin. Cellulose is the primary component of the plant's skeletal structure; hemicellulose twists over the cellulose fiber, and lignin wraps over the above two. It has been claimed that the pyrolysis of biomass is mostly composed of hemicellulose-dominated, cellulose-dominated, and lignin-dominated [42]. As a result, understanding the biomass pyrolysis mechanisms is crucial. Cellulose has a simple linear polymer chain structure that can be easily decomposed. It has been established that cellulose pyrolysis is initiated at temperatures below 250 °C, and cellulose polymers tend to be carbonized through dehydration and cross-linking processes. Similar to cellulose, hemicellulose contains sugars. However, they have been demonstrated to have lower thermal stability and quickly break down at temperatures ranging from 200 to 300 °C. The hemicellulose structure has a significant impact on product qualities. The presence of xylan in the structure affects the feedstock's pyrolysis activity. Xylan polymers with more side chains tend to form smaller molecules through ring-breaking reactions than xylose and xylan disaccharides. However, extracting hemicellulose from biomass is quite challenging. It is difficult to define the precise hemicellulose structure and the relationships between the pyrolysis products. Lignin has a more complicated structure. It consists of three basic phenyl units linked by C-C and C-O bonds. The pyrolysis reaction of lignin differs with respect to lignin structure and biomass type. For non-woody samples, biomass types have a significant impact on lignin conversion and the formation of gaseous products. PET, which contains oxygen and aromatic groups, degrades primarily through decarboxylation. Combining radicals results in the production of higher molecular weight gas products. The primary degradation of the PET structure could be identified as the random scission of ester links in the main chain.
The mechanism for co-pyrolysis is more complex than that for biomass and plastic pyrolysis since it produces a large number of chemical products. The co-pyrolysis process consists of the initiation, development of secondary radicals, and termination by radical recombination or dispersion. For the co-pyrolysis of biomass and plastics, the process started with the breakdown of biomass at a lower temperature (<350 °C) than the plastics. At that temperature, the degraded solids served as radical donors. The released radicals initiated the plastic chain scission process, which served as hydrogen donors for the biomass particles. The radicals also reacted with the solids, producing large amounts of 2-alkenes and 3n carbon atoms. These interactions increased the breakdown of biomass, resulting in reduced solid residue and a higher liquid yield, indicating a synergistic impact. The simplified mechanism of the individual and co-pyrolysis processes is provided in Fig. 2.
Fig. 2.
Mechanism of individual and co-pyrolysis of wheat straw and PET.
2.7. Pyrolysis oil analysis
The physicochemical parameters of the produced oil were tested to assess its potential as a fuel. The liquid products that were obtained included both an aqueous phase and an oil phase. The formation of these phases in the oil product is mainly recognized by the considerable amount of water content found in the WS. Prior to the physical analysis, the aqueous phase from the oil was separated by centrifuging the oil at 2000 rpm for 15 min. The pyrolysis oils obtained at maximum yield conditions were aged and subjected to several physical characterizations. As per ASTM D4052, D4287, D1293, and D240, the density, viscosity, pH value, and calorific value of the pyrolysis oil were assessed. The density of the oil was measured by weighing a known volume of oil. The viscosity and pH values were measured by a digital Redwood viscometer (Model: SICBRV-01, Shambhavi Impex, Thane, India), which can measure liquids having a Red Wood flow of 20 s–2000 s, and a digital pH meter (Model: LMPH10, LABMAN), which can measure the value between −2 and 16. The calorific value was measured using Parr-6772 calorimetric thermometer.
The pyrolysis oil obtained from different feedstocks was quantitatively analyzed using a FT-IR (BRUKER Optik GmbH TENSOR 27) and GC-MS (THERMO GC-TRACE ULTRA VER: 5.0, THERMO MS DSQ-II) detector. The FTIR spectra of the individual and co-pyrolysis oils were collected in the range of 400–4000 cm−1 region with 4 cm−1 resolution. The GC-MS analysis was carried out using an internal standard. An internal standard is a chemical substance that is added in the same concentration to all samples during a quantitative analysis. Hexadecane was chosen as the internal standard for this analysis. It is a widely used and successful method for chromatography and spectroscopy. Using an internal standard is a useful tool for minimizing the impacts of random and systematic mistakes during analysis, which helps to enhance the precision of results. For the analysis, helium was used as the carrier gas and set to flow at a rate of 1 ml/min. The analysis was carried out with the support of a capillary column (DB-5) with a thickness of 0.25 μm, a length of 30 m, and a diameter of 0.25 mm. The analysis was started by preheating the oven to 70 °C for 2 min, and then it was raised to 270 °C at a rate of 10 °C/min. This investigation allowed for the identification of every single chemical compound in the pyrolysis oils [43]. Prior to the analysis, the oil samples were filtered using a nylon microfilter. The filtered sample was further diluted using a 1:5 v/v methanol solvent solution (20 ml of methanol in a total volume of 100 ml). For the analysis, 1 μL of the sample was introduced into the GC-MS apparatus. Each sample spectrum contained a large number of peaks, and the NIST database having similarity index percentage of 89.1 % (Data Version: NIST23, Software Version 3.0) was used to compare the peaks with the compounds.
3. Results and discussion
3.1. Characterization of biomass and plastic
The ideal feedstock component for energy generation is not well understood, despite the fact that the feedstock utilized for pyrolysis is more crucial. This is due to the fact that most pyrolysis facilities are focused on processing particular types of waste and pay little attention to optimizing the components. In contrast, a number of recent studies [44,45] have concentrated on a variety of biomass feedstocks for pyrolysis applications. Proximate analysis was performed on dried samples of the raw materials to determine the relative concentrations of volatile matter, fixed carbon, moisture, and ash. In addition to that, the CHNS analyzer was also used to examine the material characteristics. Table 2 lists the results of the characterization study. The moisture content of WS is 6.43 wt%, which is favorable for the thermal conversion process. In general, a moisture level under 10 % enhances heat transfer during pyrolysis [46]. The presence of ash in pyrolysis feedstock is another element that influences energy, calorific value, and heat transfer [47]. It is the byproduct of the oxidation of inorganic materials. The lower ash present in feedstock encourages lower residual levels after pyrolysis. The ash in WS and PET was observed as 8.95 wt% and 0.1 wt%, respectively. The energy content of the feedstock can also be determined by the elemental analysis. Higher carbon content has a larger energy potential than lower carbon and oxygen feedstocks. According to the analysis, the carbon content of WS and PET was 47.41 wt% and 64.58 wt%, respectively. Table 2 indicates that the organic WS has a higher ash and oxygen content but a smaller amount of carbon, hydrogen, and volatile matter compared to waste PET. A biomass that has more hydrogen has a higher calorific value, which makes it a better fuel option [48]. In comparison to carbon and hydrogen, WS has a comparatively low nitrogen level. Lower nitrogen concentrations are typically preferred for clean combustion since they are attributed to the creation of nitrogen oxides (NOx), which can have negative environmental effects. Similarly, lower sulfur content also favors environmental sustainability. The values of celluloses, hemicelluloses, and lignin in WS in relation to the structural components were 22.80 %, 32.50 %, and 44.70 %, respectively. The quantities of these elements have an impact on the process's energy efficiency [49].
Table 2.
Properties of WS and PET used in the present study.
| Property | WS | PET |
|---|---|---|
| Proximate analysis (wt%) | ||
| Moisture | 6.43 | – |
| Volatile matter | 75.32 | 85.36 |
| Ash | 8.95 | <0.10 |
| Fixed carbona | 9.30 | 14.54 |
| Ultimate analysisb (wt%) | ||
| Carbon | 47.41 | 64.58 |
| Hydrogen | 6.32 | 4.73 |
| Nitrogen | 0.59 | 0.44 |
| Sulfur | 0.02 | 0.04 |
| Oxygena | 45.66 | 30.21 |
| HHV (MJ/kg) | 17.01 | 23.54 |
| Component analysis (wt%) | ||
| Lignin | 22.80 | – |
| Cellulose | 32.50 | – |
| Hemicellulose | 44.70 | – |
ND-Not detected.
by difference.
Ash free basis.
3.2. TGA analysis of the biomass and plastic
TGA analysis gives a better understanding of the beginning and end temperatures for thermal degradation. The TGA and DTG curves of the WS and PET samples are displayed in Fig. 3. According to the data, the percentage of weight loss increased as the temperature rose for both WS and PET materials; however, the breakdown of WS proceeded at a lower temperature than that of PET. The biomass materials are naturally organic materials that are mostly made of lignocellulosic components. They also contain significant quantities of carbon and hydrogen, which are weakly bonded. In contrast, PET is made of long-chain polymers of hydrocarbons, which are generally resilient and need higher temperatures to break their bonds. This makes them more prone to thermal disintegration at lower temperatures [50]. In this study, the complete decomposition of the selected WS occurred in three phases. The first phase represented the removal of moisture up to 140 °C, followed by active decomposition between 150 °C and 550 °C, with extreme degradation at 483 °C. In the last stage, solid elements decompose and residual char is formed at temperatures between 550 °C and 700 °C. The initial stage represented 11.2 % mass loss, and in the second stage, the maximum mass loss occurred at up to 62 %. The second stage of decomposition is known as the active pyrolysis zone, where cellulose and hemicelluloses decompose rapidly [51]. As shown in figure, which refuted the existence of moisture in the used plastics, this first stage of moisture removal was lacking in the case of PET materials. The same findings were also validated by Çepelioğullar & Pütün [52] and Chattopadhyay et al. [53]. In general, plastics showed the highest rate of breakdown in all circumstances, while biomass showed the lowest rate of mass loss and only partial disintegration. The thermal mass losses of waste plastics such as low-density polyethylene (LDPE), high-density polyethylene (HDPE), polypropylene (PP), and PET have comparable pyrolysis behaviour. During TGA analysis, only one quick narrow temperature range appears between 450 and 500 °C, and the maximum mass loss occurred at approximately 460–480 °C with little residues (approximately 1 %) [54]. In contrast to WS, waste PET degraded at higher temperatures, as shown in Fig. 3, with approximately 96.4 % of the breakdown occurring at short temperatures due to its homogeneous nature. It was noted that the TGA curve of PET resembled the earlier findings [55,56]. Further, the addition of PET with WS accelerates thermal degradation behavior during co-pyrolysis.
Fig. 3.
TGA and DTG curve of WS and PET.
3.3. Pyrolysis of the single component
3.3.1. Effect of temperature on WS pyrolysis
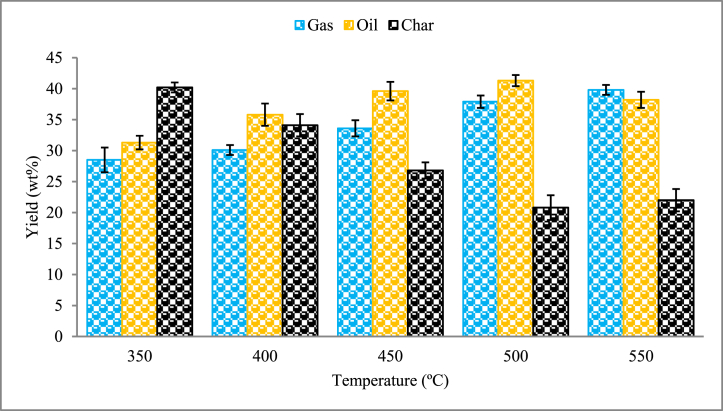
Fig. 4 represents the yield of pyrolysis products as a function of reactor temperature. The oil contains liquid oil fractions and water particles. The yield of pyrolysis oil, char, and gas is in the range of 31–41 wt%, 20–40 wt%, and 28–40 wt%, respectively, within the considered temperatures. The maximum pyrolysis oil yield of 41.3 wt% occurred at a temperature of 500 °C. At lower temperatures, the production of pyrolysis soil is lower and favours the formation of char. At 350 °C the yields of char, oil and gas were 40.2 wt%, 31.3 wt%, and 28.5 wt%, respectively. As the pyrolysis temperature was raised from the initial 350 °C to the final 550 °C, the yield of char decreased. The primary breakdown of WS residues occurs more quickly at elevated temperatures, or the residual char undergoes secondary decomposition, which could explain the drop in char yield [57]. On the other side, the yield of pyrolysis gas increased steadily from 28.5 wt% to 39.8 wt%. Higher temperatures may cause the char to secondary decompose into non-condensable gaseous compounds, which would further boost gas production as the pyrolysis temperature rose. Increasing the temperatures may cause the char to secondary decompose into non-condensable gas compounds, which would further boost gas production [58]. The yield of pyrolysis oil is observed to have an increasing and decreasing pattern. The maximum pyrolysis oil yield was observed at a temperature of 500 °C. According to Kader et al. [59], this particular temperature was sufficient for the complete pyrolysis reaction to take place, but instantaneously, it was not sufficiently high for the occurrence of secondary decomposition. Thus, 500 °C was observed as an optimum temperature for yielding maximum pyrolysis oil during WS pyrolysis. The decreasing pyrolysis oil yield beyond the optimum temperature was also noted in the literature [60].
Fig. 4.
Product distribution of WS pyrolysis.
3.3.2. Effect of temperature on PET pyrolysis
Batch-type pyrolysis of PET has been performed at five different temperatures, and their variations in yields are displayed in Fig. 5. These yields depend on the internal heat transfer behavior of the polymeric material [61]. The yields of pyrolysis oil, char, and gas at lower temperatures were 42.3 wt%, 20.5 wt%, and 37.2 wt%, respectively. The yield of oil and gas increased as the temperature increased. Severe thermal cracking at increased temperatures leads to the formation of more gas fractions [62]. The production of char is hardly affected by process temperature, showing a decreased trend with increasing temperature. It is noted that the amount of char residue left in the reactor decreases, going from 20.5 wt% at 350 °C to 2.8 wt% at 550 °C. The yield of pyrolysis oil increased from 42.3 wt% at 350 °C to 48.5 wt% at 550 °C, whereas the maximum yield was obtained at the temperature of 500 °C. The variation in stability of PET is the main reason for increased pyrolysis oil production. According to Sharypov et al. [63], at a suitable pyrolysis temperature, the C=C linkage in polymer materials breaks more frequently, leading to the production of more condensable volatiles. Other than pyrolysis oil, char, and gas products, the formation of wax during the plastic pyrolysis process is common, which can cause equipment blockage, reduced production efficiency, and lower product quality. The amount of wax produced during PET pyrolysis was considered, but a negligible amount of wax was identified inside the reactor outlet. The formation of wax was immediately cleaned up for further experimental study.
Fig. 5.
Product distribution of PET pyrolysis.
3.4. Co-pyrolysis characteristics
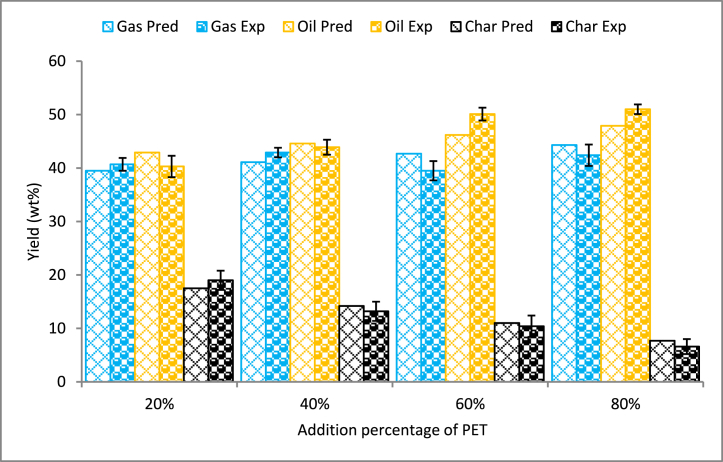
Co-pyrolysis of biomass and plastics has been investigated by various authors due to their simple operation and efficiency in producing liquid fuels with lower char formation [64]. The products derived from the pyrolysis of WS/PET blends with varying mixing weight ratios are displayed in Fig. 6. When the blend increased from 20 % to 80 %, the oil yield during experimentation varied from 40.3 wt% to 51.0 wt%. On the other side, the yield of char decreased gradually with the addition of polymers to biomass. The reduced char yield is directly proportional to the ash present in the blends. The increased oil yield is also due to the presence of additional volatile matters with the addition of polymers [65]. In general, polymers can act as hydrogen donors during biomass and plastic co-pyrolysis, which improves the conversion of biomass into oil [66]. The higher gas yield may be ascribed to the strong cracking of the liquid element into short-carbon-chain elements that enhance the yield of non-condensable gases [67].
Fig. 6.
Identification of synergistic effect.
Table 3 compares the yield during co-pyrolysis of different biomass and plastics under different ratios. Furthermore, by comparing the yield of oil with other types of biomass and plastics, we discovered that the oil yield is minimum when the biomass is blended with PET. Combining biomass with other types of polyolefins, such as polystyrene (PS), PP, and LDPE, produced the maximum oil yield due to the higher volatile matter present in the plastics. The lower volatile matter and higher oxygen content of PET produced a lower oil yield. However, the heating value of the oil produced in this study (at 2:3 ratio) is higher than that of the co-pyrolysis oil produced from Ficus benghalensis/PET under the same biomass plastic ratio. In order to boost the yield of the oil and heating value further, WS can be combined with PP, PE, or PS.
Table 3.
Comparison of yield during co-pyrolysis of different biomass and plastics.
| Biomass/plastic | Biomass-to-plastic Ratioa | Oil yield in wt% | Calorific value of the oil | Reference |
|---|---|---|---|---|
| Wheat straw/PET | 2:3 | 50.1 | 24.2 | This study |
| Neem bark/LDPE | 2:3 | 64.8 | 33.5 | [19] |
| Bamboo/PP | 4:1 | 50.9 | 27.2 | [68] |
| Bamboo/PS | 4:1 | 50.1 | 28.2 | [68] |
| Oakwood/PP | 4:1 | 55.1 | 25.0 | [68] |
| Oakwood/PS | 4:1 | 56.16 | 32.4 | [68] |
| Ficus benghalensis/PET | 2:3 | 54.2 | 23.9 | [69] |
| Karanja seeds/PS | 2:1 | 57.81 | a | [70] |
| Niger seeds/PS | 2:1 | 57.94 | a | [70] |
| Palm shell/PS | 2:3 | 68.3 %, | 40.3 | [71] |
| Poplar wood/PVC | 1:1 | 78.6 | a | [72] |
Not reported.
3.5. Analysis of synergistic effect
The synergistic effect on yields can be perceived by the comparison of theoretical and experimental yields. The additivity rule was used to find the theoretical value from individual component yield. Fig. 6 shows that, in comparison to theoretical data, co-pyrolysis of WS with PET clearly reduces the formation of gas while clearly increasing the oil phase. At a lower proportion (addition of 20 % PET), the yield of pyrolysis oil decreased and recorded a negative synergistic effect compared to char and gas. The yield of pyrolysis oil was reduced up to 6.45 %. The output of char was higher than expected, while the production of oil was lower than expected with a 20 % addition of PET with WS. Further, the addition of 40 % PET with WS showed lower pyrolysis oil and char yields and higher gas yields. The positive synergistic effect on pyrolysis oil yield was observed when more than 40 % PET was added to WS. The interactions between WS and PET can be attributed to the transference reaction of hydrogen atoms and the formation of free radicals [64]. This phenomenon could potentially impede the breakdown of functional groups bound to the cellulose structure of biomass, hence impeding the release of lower-molecular-weight gas components and promoting the synthesis of higher-molecular-weight organic compounds [73]. At 60 % addition of PET, the maximum positive synergistic effect was identified with pyrolysis oil yield of 50.1 wt%, showing an increase of 7.78 % compared to the theoretical value. Regarding char yield, the co-pyrolysis experiments produced lower char compared to individual pyrolysis, which is related to lower ash content [74]. Previously, Anandaram et al. [69] reported co-pyrolysis of forest wood (Ficus benghalensis) and PET and showed positive synergy on pyrolysis oil yield. During the individual pyrolysis process, Ficus benghalensis produced a maximum of 38.1 wt% oil at a temperature of 500 °C. On the other side, individual pyrolysis of PET at 500 °C produced 58.0 wt% oil. But during co-pyrolysis, 60 % addition of PET with biomass improved the oil yield to 54.2 wt%, which is 6.5 % more than the predicted value (50.9 wt%). Çepelioğullar and Pütün [56] showed lower gas production and higher pyrolysis oil and char yields than the total yields from individual pyrolysis of three distinct biomass and PET. Ansah et al. [75] found a higher positive synergy on oil yield while co-pyrolyzing municipal solid waste (MSW) with PET. The investigation also found a substantial interaction at a 70/30 blend between MSW and PET to provide maximum oil yield.
3.6. Effect of yield on catalytic co-pyrolysis
The results of pyrolysis experiments obtained from the non-catalytic and catalytic co-pyrolysis processes on feedstocks with 60 % PET are shown in Fig. 7. Both experiments were conducted at 500 °C, and the effect of the catalyst on the product yields was analyzed. The result shows that the catalytic co-pyrolysis process produces more oil and gas products than the conventional co-pyrolysis process. At co-pyrolysis, the highest oil and gas yields were 50.1 wt% and 39.5 wt%, respectively. However, catalytic co-pyrolysis increased oil and gas production to 51.9 wt% and 39.8 wt%, respectively. There was a slight increase in oil yield with the catalytic process. The catalytic method enhanced yield by up to 3.6 %. The increased oil and gas production and decreased char residue demonstrated a positive synergy between WS and PET. The reaction between the catalyst and the feed materials is complicated by the materials' complex structure [59]. However, the fundamental explanation for the good synergy effect on oil yields is the interaction of biomass with plastic-derived olefins [76]. In the presence of zeolites, it became clear that oxygenated elements could interact with olefins. Furthermore, it has been demonstrated that plastic-derived hydrocarbons can act as hydrogen donors for biomass-derived oxygenates, minimizing the generation of char [77]. Fig. 8 provides a simple reaction between the catalyst and the feedstock during co-pyrolysis process. During the catalytic pyrolysis process, the formation of polycyclic aromatic hydrocarbons (PAHs) can be increased by decreasing single aromatic and other oxygenated compounds. The process depicted in Fig. 8 can be used to support this increase in PAH. The reaction path was proposed between WS (cellulose component) and PET in the presence of a zeolite catalyst.
Fig. 7.
Comparison of oil yield at catalytic and non-catalytic process under maximum synergy conditions.
Fig. 8.
Reaction between the catalyst and the feedstock during co-pyrolysis process.
3.7. Characterization study
The primary and co-pyrolysis oils obtained at their optimum conditions under non-catalytic process were examined to determine their physical and chemical characteristics. Various physical properties, such as density, viscosity, flash point, and heating value, were analyzed and reported.
3.7.1. Physical analysis of the pyrolysis oil
Table 4 represents some of the common physical characteristics of the produced pyrolysis oils. The colour of pyrolysis oil is identified as dark brown, which is being influenced by the existence of micro level carbon particles. Compared to triglyceride derivatives, the lignocellulose molecules found in pyrolysis oil have larger amounts of oxygen. The oxygen content of the derived biomass pyrolysis oil varied from 30.41 to 46.02 wt%. The presence of higher oxygen content is the main reason for the reduced energy value of the pyrolysis oil. The densities of pyrolysis oil derived from different feedstocks are displayed in Table 4. Upon observation, the PET pyrolysis oil exhibits the lowest density of 915 kg/m3, and a maximum density was recorded for the WS pyrolysis oil. These findings are consistent with Faisal et al. [78] and Stedile et al. [79], who discovered that the densities of pyrolysis liquids derived from plastics and biomass generally fall within the range of 850–950 kg/m3 and 1000–1200 kg/m3, respectively. The viscosity of the pyrolysis oil represents its flow ability. The viscosity of the oils is in the range of 4.20–11.40 cSt. The viscosity of the oil is reduced with an increased PET ratio in the feedstock. The heating values of the pyrolysis oil derived from the individual components of WS and PET are 16.45 MJ/kg and 28.64 MJ/kg, respectively. Compared to PET pyrolysis oil, the heating value of WS pyrolysis oil is low due to the existence of higher oxygen molecules. In order to prevent adverse effects on the quality of the WS pyrolysis oil, it is always necessary to limit the amount of oxygen. These oils can be used for heat production by direct combustion in boilers or furnaces. Further, the lower heating value of pyrolysis oils can be upgraded utilizing a number of techniques, including esterification, hydrogenation, steam reforming, emulsification, and membrane separation.
Table 4.
Physical characteristics of the produced pyrolysis oils.
| Properties | Unit | WS pyrolysis oil | PET pyrolysis oil | WS:PET ratio |
|||
|---|---|---|---|---|---|---|---|
| 80/20 | 60/40 | 40/60 | 20/80 | ||||
| Density | kg/m3 | 1090 | 915 | 1060 | 1015 | 965 | 930 |
| Viscosity at 30 °C | cSt | 11.40 | 4.20 | 10.60 | 9.60 | 7.90 | 6.40 |
| Flash point | °C | 145 | 60 | 135 | 101 | 85 | 72 |
| Carbon | wt% | 47.25 | 64.5 | 50.31 | 53.35 | 59.40 | 61.52 |
| Hydrogen | wt% | 5.90 | 7.92 | 6.10 | 6.54 | 6.95 | 7.44 |
| Nitrogen | wt% | 0.80 | 0.55 | 0.74 | 0.70 | 0.67 | 0.61 |
| Sulfur | wt% | 0.03 | 0.02 | 0.03 | 0.03 | 0.03 | 0.02 |
| Oxygena | wt% | 46.02 | 27.00 | 42.82 | 39.38 | 32.95 | 30.41 |
| Heating value | MJ/kg | 16.45 | 28.64 | 18.13 | 20.78 | 24.20 | 26.30 |
Value calculated by difference.
3.7.2. Chemical characterization of the pyrolysis oil
3.7.2.1. FT-IR analysis
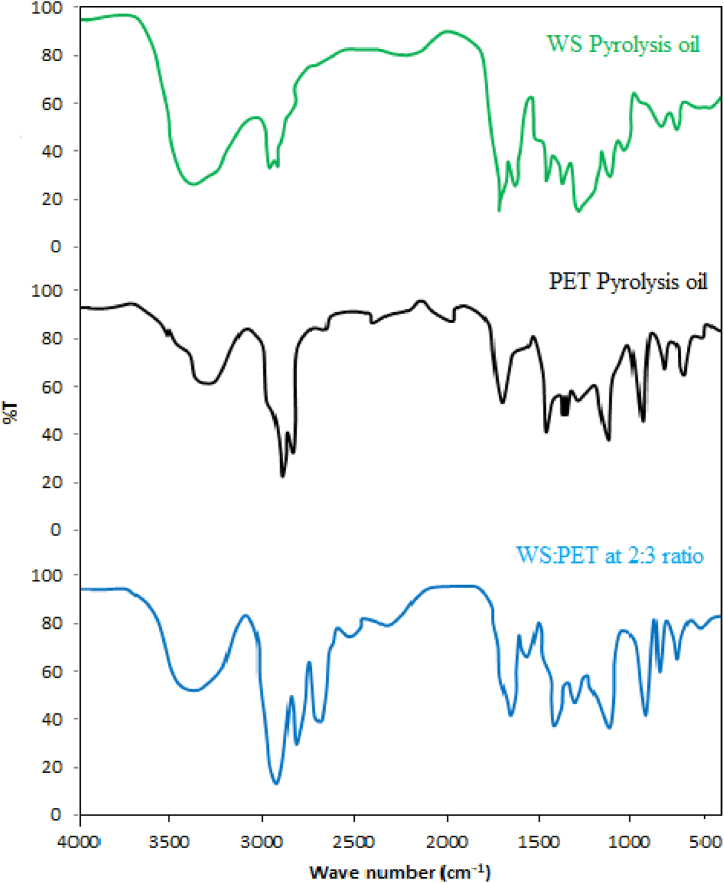
The FT-IR spectra show valuable information about the functional groups present in the oils. The FTIR spectra of the WS pyrolysis oil, PET pyrolysis oil, and co-pyrolysis oil produced from 2:3 ratio of WS and PET is shown in Fig. 9. For WS pyrolysis oil, the identification of O-H stretching vibrations, at 3386.7 cm−1, reveal the existence of alcohols and phenols. The absorbance peak of C-H vibrations between 2925.6 cm−1 and the bands between 1448.9 cm−1 show the presence of alkanes. The presence of ketones, aldehydes, and carboxylic acids is indicated by the carbohydrate content of the biomass, which has a high C=O stretching absorbance of 1717.2 cm−1 [80]. Alkenes and aromatics are present in the oil, as indicated by the absorbance peaks at 1620.4 cm−1. The presence of primary, secondary, and tertiary alcohols and phenols representing C-O stretching and O-H bending are also identified by the peaks between 1043.0 cm−1. For PET pyrolysis oil, the peak identified at the wave length of 3390.1 cm−1 indicates O–H stretching. The peaks identified at 2888.3 cm−1 indicate that PET pyrolysis oil may contain aliphatic C–H groups. The peak that appears at 1715.3 cm−1 is indicative of the -C=C- stretch, which is associated with alkenes and aromatic chemicals. The differences in peak positions and intensities provide information on the molecular relationships and compatibility of PET. The alcohol, phenols, and carboxylic acids found in the co-pyrolysis oil are depicted in the figures. The hydroxyl groups in the intra- and intermolecular hydrogen bonds are primarily responsible for the increased viscosity of the pyrolysis oils [81]. Apart from that, the pyrolysis oils are made up of a wide range of chemical substances, such as furans, ketones, acids, and aldehydes [82].
Fig. 9.
FTIR spectra of WS, PET and co-pyrolysis oil.
3.7.2.2. GC-MS analysis
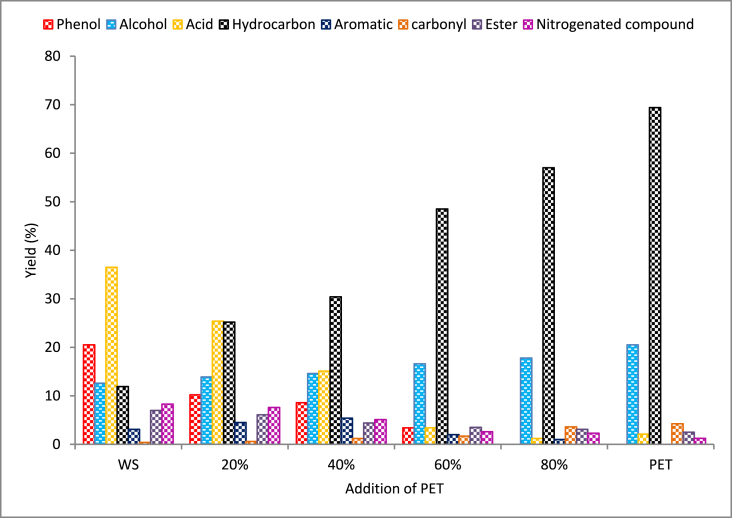
The chemical compounds of the obtained pyrolysis oil were analyzed through GC-MS. This analysis offers structural details of the substances present in the oil sample. The pyrolysis oil contains O-H compounds (phenol and alcohol), esters, carbonyl group elements, acids, hydrocarbons, aromatics, and nitrogenated compounds. The composition was significantly influenced by the WS:PET ratio. The existence of O-H compounds, hydrocarbons, and aromatic compounds is used for the production of biofuel. But the presence of oxygenated compounds is considered an undesirable byproduct [83]. The corrosiveness of pyrolysis oil is mostly caused by acids, while the lower energy value is associated with the presence of ether and esters. When pyrolysis oil is transported and stored, it becomes unstable due to the presence of ketones and aldehydes, while nitrogen-based compounds create adverse effects on the environment. Fig. 10 represents the effect of blend ratio on the chemical compositions of pyrolysis oil, and Fig. 11 represents the variation in chemical compositions with reference to the individual component analysis of WS. Pyrolysis of WS produced 20.5 % phenolic compounds, 12.6 % alcohol, 36.5 % acid, and 11.9 % hydrocarbon. The majority of acids present in the pyrolysis oil are the cause of the depolymerization of lignocellulosic components of the biomass. On the other side, the pyrolysis of PET generated maximum hydrocarbon and alcohol-based compounds. The improved hydrocarbon percentage with respect to the addition of PET represented the pool mechanisms, whereas the alkene produced by PET may have acted as a hydrogen donor [84]. An understanding of the hydrocarbon pool mechanism from a model biomass is shown in Fig. 12. The yield of the co-pyrolysis process changed dramatically at different H/C ratios. Adding 20 % PET to WS resulted in a significant increase in hydrocarbons. The study also concluded that the addition of PET is likely to have a greater impact on product distribution. The reduction of carbonyls and improvement in hydrocarbon production may be attributed to the improved hydrogenation reactions. Although numerous studies have observed synergetic effects during the co-pyrolysis procedure [85,86], the exact mechanism is still being investigated due to the complex interactions between different feedstocks. Fortunately, many experimental findings may still be explained using the widely recognized radical mechanism [87].
Fig. 10.
Effect of blend ratio on chemical compositions of pyrolysis oil.
Fig. 11.
Variation in chemical compositions with reference to the chemical compounds identified from individual component analysis of WS.
Fig. 12.
Hydrocarbon pool mechanism.
4. Conclusion
To study the yield distributions and synergistic effects at direct reactor temperature and different blending ratios, WS and PET were co-pyrolyzed in a fixed-bed reactor. The individual pyrolysis of WS and PET yielded maximum pyrolysis oil of 41.3 wt% and 49.6 wt% at a temperature of 500 °C. During the co-pyrolysis process, a higher oil yield was obtained than the predicted value due to the synergistic action between biomass and plastics. With a maximum difference of 7.78 %, the synergistic effect was greatest at 500 °C at a 40/60 WS:PET ratio. The physical and chemical characteristics of the produced pyrolysis oils can be refined into hydrocarbon fuel and utilized as a raw material for the chemical industry. The heating value of the pyrolysis oils is directly correlated with the amount of plastic present in the feedstock. The reduced phenolic content with co-pyrolysis oil increased its stability to use as a fuel for furnace. The interaction of plastics produced carbon and hydrogen biofuels. The elements identified in the co-pyrolysis oil varied considerably with the H/C ratio of the feedstocks. The addition of 20 % PET to WS produced a considerable amount of hydrocarbons. The improved hydrogenation process with a higher PET ratio produced more hydrocarbon products than carbonyls. It can be concluded from the study that the utilization of WS and land-polluted non-reusable PET wastes for biofuel conversion is advantageous from both an economic and environmental point of view. The presence of zeolite based catalytic enhanced the yield by up to 3.6 %. The findings also showed that this biomass, as well as other comparable biomass, may be blended with PET and other waste polymers to produce more sustainable fuels. Further research is needed to understand the viability and reaction mechanisms of biomass plastic pyrolysis systems in order to provide further environmental benefits. In order to get a higher yield and higher oil quality, it is critical to monitor the feedstock ratio in the co-pyrolysis process. Since the catalyst was added to the process to improve product quality and quantity, this study recommends using different types of catalysts for the co-pyrolysis process, such as aluminosilicates and metal oxides. While considering the lower heating value of the pyrolysis oil, the study also recommends upgrading the fuel using various upgrading techniques such as esterification, hydrogenation, steam reforming, emulsification, and membrane separation.
Data availability statement
Data will be made available on request.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
CRediT authorship contribution statement
Anis Kumar M: Writing – review & editing, Methodology, Data curation, Conceptualization. Swarnalatha A.P: Writing – review & editing, Methodology, Formal analysis. Shwetha J: Writing – review & editing, Methodology. Sowmya Dhanalakshmi C: Writing – review & editing, Conceptualization. Saravanan P: Writing – review & editing, Methodology, Conceptualization. Ashraf Atef Hatamleh: Writing – review & editing, Software, Project administration. Munirah Abdullah Al-Dosary: Writing – review & editing, Project administration. Ravishankar Ram Mani: Writing – review & editing, Project administration. Woo Jin Chung: Writing – review & editing. Soon Woong Chang: Writing – review & editing, Methodology. Balasubramani Ravindran: Writing – review & editing, Writing – original draft, Methodology, Investigation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors extend their appreciation to the Researchers supporting project number (RSPD2024R1095) King Saud University, Riyadh, Saudi Arabia. We are thankful for the support provided by UCSI University for the support provided by the Research Excellence and Innovation Grant (REIG) with code REIG-FPS-2022/036.
Contributor Information
Ravishankar Ram Mani, Email: ravishankar@ucsiuniversity.edu.my.
Balasubramani Ravindran, Email: kalamravi@gmail.com.
References
- 1.Sorrell S. Reducing energy demand: a review of issues, challenges and approaches. Renewable Sustainable Energy Rev. 2015;47:74–82. doi: 10.1016/j.rser.2015.03.002. [DOI] [Google Scholar]
- 2.Bakhchin D., Ravi R., Faqir M., Essadiqi E. A technical review on low temperature combustion alternatives for ultra-low emission vehicles. J. Energy Inst. 2023;111 doi: 10.1016/j.joei.2023.101410. [DOI] [Google Scholar]
- 3.Ravi R., Douadi O., Ezhilchandran M., Faqir M., Essadiqi E., Belkasmi M., Vijayalakshmi S.K. A practical approach-based technical review on effective utilization of exhaust waste heat from combustion engines. Energy Sources Part A. 2023;45(4):10010–10033. doi: 10.1080/15567036.2023.2242321. [DOI] [Google Scholar]
- 4.Panwar N.L., Kaushik S.C., Kothari S. Role of renewable energy sources in environmental protection: a review. Renewable Sustainable Energy Rev. 2011;15(3):1513–1524. doi: 10.1016/j.rser.2010.11.037. [DOI] [Google Scholar]
- 5.Fathoni H.S., Setyowati A.B., Prest J. Is community renewable energy always just? Examining energy injustices and inequalities in rural Indonesia. Energy Res. Social Sci. 2021;71 doi: 10.1016/j.erss.2020.101825. [DOI] [Google Scholar]
- 6.Patwa A., Parde D., Dohare D., Vijay R., Kumar R. Solid waste characterization and treatment technologies in rural areas: an Indian and international review. Environ. Technol. Innovat. 2020;20 doi: 10.1016/j.eti.2020.101066. [DOI] [Google Scholar]
- 7.Muneeswari M., Periyanayagi G., Ramasubramanian V. Evaluation of promotary effect of penergetics-K® on abelmocschus esculentus (L.) moench. Am.-Eurasian J. Agric. Environ. Sci. 2018;18(1):23–26. doi: 10.5829/idosi.aejaes.2018.23.26. [DOI] [Google Scholar]
- 8.Agamuthu P., Babel S. Waste management developments in the last five decades: Asian perspective. Waste Manag. Res. 2023;41(12):1699–1716. doi: 10.1177/0734242X231199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi M., Papetti A., Germani M.A. Comparison of different waste collection methods: environmental impacts and occupational risks. J. Clean. Prod. 2022;368 doi: 10.1016/j.jclepro.2022.133145. [DOI] [Google Scholar]
- 10.Arularasan A.N., Mathew M., Sudhakar M., Sivakumar K., Bhagavathi Perumal S., Madhu P.… Isaac JoshuaRamesh Lalvani, J.: a holistic framework for environment conscious based material selection and experimental assessment using digraph-based expert system. Sci. Program. 2022 doi: 10.1155/2022/2112683. [DOI] [Google Scholar]
- 11.Janardhana K., Sowmya Dhanalakshmi C., Thilagham K.T., Chinnaiyan S.K., Jai Shanker Pillai H.P., Sathish T., De Poures M.V. Experimental investigation on utilization of Sesbania grandiflora residues through thermochemical conversion process for the production of value added chemicals and biofuels. Sci. Rep. 2024;14(1):7283. doi: 10.1038/s41598-024-57040-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhanalakshmi C.S., Ahalya N., Vidhyalakshmi P., Krishnaraj C., Selvam N., Patil P.P., Prabhakar S. Individual and catalytic co-pyrolysis of agricultural outcomes and polymeric materials over nano-HZSM-5 zeolite: synergistic effects and yield analysis for heating applications. J. Nanomater. 2022;3743299 doi: 10.1155/2022/3743299. [DOI] [Google Scholar]
- 13.Park J., Lee Y., Ryu C., Park Y.K. Slow pyrolysis of rice straw: analysis of products properties, carbon and energy yields. Bioresour. Technol. 2014;155:63–70. doi: 10.1016/j.biortech.2013.12.084. [DOI] [PubMed] [Google Scholar]
- 14.Balagurumurthy B., Srivastava V., Kumar J., Biswas B., Singh R., Gupta P., Bhaskar T. Value addition to rice straw through pyrolysis in hydrogen and nitrogen environments. Bioresour. Technol. 2015;188:273–279. doi: 10.1016/j.biortech.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 15.Cao Q., Xie K.C., Bao W.R., Shen S.G. Pyrolytic behavior of waste corn cob. Bioresour. Technol. 2004;94(1):83–89. doi: 10.1016/j.biortech.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 16.Alper K., Tekin K., Karagöz S. Pyrolysis of agricultural residues for bio-oil production. Clean Technol. Environ. Policy. 2015;17:211–223. doi: 10.1007/s10098-014-0778-8. [DOI] [Google Scholar]
- 17.Sánchez M.E., Lindao E., Margaleff D., Martínez O., Morán A. Pyrolysis of agricultural residues from rape and sunflowers: production and characterization of bio-fuels and biochar soil management. J. Anal. Appl. Pyrolysis. 2009;85(1–2):142–144. doi: 10.1016/j.jaap.2008.11.001. [DOI] [Google Scholar]
- 18.Ahmed M.J., Hameed B.H. Insight into the co-pyrolysis of different blended feedstocks to biochar for the adsorption of organic and inorganic pollutants: a review. J. Clean. Prod. 2020;265 doi: 10.1016/j.jclepro.2020.121762. [DOI] [Google Scholar]
- 19.Kaushik V.S., Dhanalakshmi C.S., Madhu P., Tamilselvam P. Co-pyrolysis of neem wood bark and low-density polyethylene: influence of plastic on pyrolysis product distribution and bio-oil characterization. Environ. Sci. Pollut. Res. 2022;29(58):88213–88223. doi: 10.1007/s11356-022-21746-1. [DOI] [PubMed] [Google Scholar]
- 20.Mo F., Ullah H., Zada N., Shahab A. A review on catalytic co-pyrolysis of biomass and plastics waste as a thermochemical conversion to produce valuable products. Energies. 2023;16(14):5403. doi: 10.3390/en16145403. [DOI] [Google Scholar]
- 21.Johansson A.C., Sandström L., Öhrman O.G., Jilvero H. Co-pyrolysis of woody biomass and plastic waste in both analytical and pilot scale. J. Anal. Appl. Pyrolysis. 2018;134:102–113. doi: 10.1016/j.jaap.2018.05.015. [DOI] [Google Scholar]
- 22.Kaza S., Yao L., Bhada-Tata P., Van Woerden F. World Bank Publications; 2018. What a Waste 2.0: a Global Snapshot of Solid Waste Management to 2050. [Google Scholar]
- 23.Wu M., Wang Z., Chen G., Zhang M., Sun T., Wang Q., Gupta A.K. Synergistic effects and products distribution during Co-pyrolysis of biomass and plastics. J. Energy Inst. 2023;111 doi: 10.1016/j.joei.2023.101392. [DOI] [Google Scholar]
- 24.Likun P.K.W., Zhang H., Fan Y. Improving hydrocarbons production via catalytic co-pyrolysis of torrefied-biomass with plastics and dual catalytic pyrolysis. Chin. J. Chem. Eng. 2022;42:196–209. doi: 10.1016/j.cjche.2020.09.074. [DOI] [Google Scholar]
- 25.Xue Y., Bai X. Synergistic enhancement of product quality through fast co-pyrolysis of acid pretreated biomass and waste plastic. Energy Convers. Manag. 2018;164:629–638. doi: 10.1016/j.enconman.2018.03.036. [DOI] [Google Scholar]
- 26.Qi P., Chang G., Wang H., Zhang X., Guo Q. Production of aromatic hydrocarbons by catalytic co-pyrolysis of microalgae and polypropylene using HZSM-5. J. Anal. Appl. Pyrolysis. 2018;136:178–185. doi: 10.1016/j.jaap.2018.10.007. [DOI] [Google Scholar]
- 27.R. Jin Q., Wang X., Li S., Mikulčić H., Bešenić T., Deng S., Kumfer B.M. Synergistic effects during co-pyrolysis of biomass and plastic: gas, tar, soot, char products and thermogravimetric study. J. Energy Inst. 2019;92(1):108–117. doi: 10.1016/j.joei.2017.11.001. [DOI] [Google Scholar]
- 28.Van Nguyen Q., Choi Y.S., Choi S.K., Jeong Y.W., Kwon Y.S. Improvement of bio-crude oil properties via co-pyrolysis of pine sawdust and waste polystyrene foam. J. Environ. Manag. 2019;237:24–29. doi: 10.1016/j.jenvman.2019.02.039. [DOI] [PubMed] [Google Scholar]
- 29.Burra K.G., Gupta A.K. Kinetics of synergistic effects in co-pyrolysis of biomass with plastic wastes. Appl. Energy. 2018;220:408–418. doi: 10.1016/j.apenergy.2018.03.117. [DOI] [Google Scholar]
- 30.Burra K.G., Gupta A.K. Synergistic effects in steam gasification of combined biomass and plastic waste mixtures. Appl. Energy. 2018;211:230–236. doi: 10.1016/j.apenergy.2017.10.130. [DOI] [Google Scholar]
- 31.Sajdak M. Impact of plastic blends on the product yield from co-pyrolysis of lignin-rich materials. J. Anal. Appl. Pyrolysis. 2017;124:415–425. doi: 10.1016/j.jaap.2017.03.002. [DOI] [Google Scholar]
- 32.Zhao C., Jiang E., Chen A. Volatile production from pyrolysis of cellulose, hemicellulose and lignin. J. Energy Inst. 2017;90(6):902–913. doi: 10.1016/j.joei.2016.08.004. [DOI] [Google Scholar]
- 33.Fekhar B., Miskolczi N., Bhaskar T., Kumar J., Dhyani V. Co-pyrolysis of biomass and plastic wastes: investigation of apparent kinetic parameters and stability of pyrolysis oils. IOP Conf. Ser. Earth Environ. Sci. 2018;154 doi: 10.1088/1755-1315/154/1/012022. [DOI] [Google Scholar]
- 34.Dyer A.C., Nahil M.A., Williams P.T. Catalytic co-pyrolysis of biomass and waste plastics as a route to upgraded bio-oil. J. Energy Inst. 2021;97:27–36. doi: 10.1016/j.joei.2021.03.022. [DOI] [Google Scholar]
- 35.Ryu H.W., Kim D.H., Jae J., Lam S.S., Park E.D., Park Y.K. Recent advances in catalytic co-pyrolysis of biomass and plastic waste for the production of petroleum-like hydrocarbons. Bioresour. Technol. 2020;310 doi: 10.1016/j.biortech.2020.123473. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z., Burra K.G., Lei T., Gupta A.K. Co-pyrolysis of waste plastic and solid biomass for synergistic production of biofuels and chemicals-A review. Prog. Energy Combust. Sci. 2021;84 doi: 10.1016/j.pecs.2020.100899. [DOI] [Google Scholar]
- 37.Esso S.B.E., Xiong Z., Chaiwat W., Kamara M.F., Longfei X., Xu J., Xiang J. Review on synergistic effects during co-pyrolysis of biomass and plastic waste: significance of operating conditions and interaction mechanism. Biomass Bioenergy. 2022;159 doi: 10.1016/j.biombioe.2022.106415. [DOI] [Google Scholar]
- 38.Cai W., Wang X., Zhu Z., Kumar R., Amaniampong P.N., Zhao J., Hu Z.T. Synergetic effects in the co-pyrolysis of lignocellulosic biomass and plastic waste for renewable fuels and chemicals. Fuel. 2023;353 doi: 10.1016/j.fuel.2023.129210. [DOI] [Google Scholar]
- 39.Koshti R., Mehta L., Samarth N. Biological recycling of polyethylene terephthalate: a mini-review. J. Polym. Environ. 2018;26:3520–3529. doi: 10.1007/s10924-018-1214-7. [DOI] [Google Scholar]
- 40.Sun T., Li Z., Zhang Z., Wang Z., Yang S., Yang Y., Lei T. Fast corn stalk pyrolysis and the influence of catalysts on product distribution. Bioresour. Technol. 2020;301 doi: 10.1016/j.biortech.2020.122739. [DOI] [PubMed] [Google Scholar]
- 41.Uzoejinwa B.B., He X., Wang S., Abomohra A.E.F., Hu Y., Wang Q. Co-pyrolysis of biomass and waste plastics as a thermochemical conversion technology for high-grade biofuel production: recent progress and future directions elsewhere worldwide. Energy Convers. Manag. 2018;163:468–492. doi: 10.1016/j.enconman.2018.02.004. [DOI] [Google Scholar]
- 42.Rutkowski P. Pyrolysis of cellulose, xylan and lignin with the K2CO3 and ZnCl2 addition for bio-oil production. Fuel Process. Technol. 2011;92(3):517–522. doi: 10.1016/j.fuproc.2010.11.006. [DOI] [Google Scholar]
- 43.Sowmya Dhanalakshmi C., Madhu P. Utilization possibilities of Albizia amara as a source of biomass energy for bio-oil in pyrolysis process. Energy Sources, Part A. 2019;41(15):1908–1919. doi: 10.1080/15567036.2018.1549168. [DOI] [Google Scholar]
- 44.Gholizadeh M., Hu X., Liu Q. A mini review of the specialties of the bio-oils produced from pyrolysis of 20 different biomasses. Renewable Sustainable Energy Rev. 2019;114 doi: 10.1016/j.rser.2019.109313. [DOI] [Google Scholar]
- 45.Singh D., Sharma D., Soni S.L., Sharma S., Sharma P.K., Jhalani A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel. 2020;262 doi: 10.1016/j.fuel.2019.116553. [DOI] [Google Scholar]
- 46.Lakshmi B.M., Mathew M., Kinol A.M.J., Vedagiri B., Perumal S.B., Madhu P., Dhanalakshmi C.S. An integrated CRITIC-TOPSIS-and Entropy-TOPSIS-based informative weighting and ranking approach for evaluating green energy sources and its experimental analysis on pyrolysis. Environ. Sci. Pollut. Res. 2022;29(40):61370–61382. doi: 10.1007/s11356-022-20219-9. [DOI] [PubMed] [Google Scholar]
- 47.Gómez N., Banks S.W., Nowakowski D.J., Rosas J.G., Cara J., Sánchez M.E., Bridgwater A.V. Effect of temperature on product performance of a high ash biomass during fast pyrolysis and its bio-oil storage evaluation. Fuel Process. Technol. 2018;172:97–105. doi: 10.1016/j.fuproc.2017.11.021. [DOI] [Google Scholar]
- 48.Arif M., Li Y., El-Dalatony M.M., Zhang C., Li X., Salama E.S. A complete characterization of microalgal biomass through FTIR/TGA/CHNS analysis: an approach for biofuel generation and nutrients removal. Renew. Energy. 2021;163:1973–1982. doi: 10.1016/j.renene.2020.10.066. [DOI] [Google Scholar]
- 49.Yang H., Yan R., Chen H., Zheng C., Lee D.H., Liang D.T. In-depth investigation of biomass pyrolysis based on three major components: hemicellulose, cellulose and lignin. Energy Fuel. 2006;20(1):388–393. doi: 10.1021/ef0580117. [DOI] [Google Scholar]
- 50.Song Y., Tahmasebi A., Yu J. Co-pyrolysis of pine sawdust and lignite in a thermogravimetric analyzer and a fixed-bed reactor. Bioresour. Technol. 2014;174:204–211. doi: 10.1016/j.biortech.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 51.Sridevi V., Surya D.V., Reddy B.R., Shah M., Gautam R., Kumar T.H., Basak T. Challenges and opportunities in the production of sustainable hydrogen from lignocellulosic biomass using microwave-assisted pyrolysis: a review. Int. J. Hydrogen Energy. 2024;52:507–531. doi: 10.1016/j.ijhydene.2023.06.186. [DOI] [Google Scholar]
- 52.Çepelioğullar Ö., Pütün A.E. Products characterization study of a slow pyrolysis of biomass-plastic mixtures in a fixed-bed reactor. J. Anal. Appl. Pyrolysis. 2014;110:363–374. doi: 10.1016/j.jaap.2014.10.002. [DOI] [Google Scholar]
- 53.Chattopadhyay J., Pathak T.S., Srivastava R., Singh A.C. Catalytic co-pyrolysis of paper biomass and plastic mixtures (HDPE (high density polyethylene), PP (polypropylene) and PET (polyethylene terephthalate)) and product analysis. Energy. 2016;103:513–521. doi: 10.1016/j.energy.2016.03.015. [DOI] [Google Scholar]
- 54.Han B., Chen Y., Wu Y., Hua D., Chen Z., Feng W., Xie Q. Co-pyrolysis behaviors and kinetics of plastics–biomass blends through thermogravimetric analysis. J. Therm. Anal. Calorim. 2014;115:227–235. doi: 10.1007/s10973-013-3228-7. [DOI] [Google Scholar]
- 55.Al-Maari M.A., Ahmad M.A., Din A.T.M., Hassan H., Alsobaai A.M. Co-pyrolysis of oil palm empty fruit bunch and oil palm frond with low-density polyethylene and polypropylene for bio-oil production. Arab. J. Chem. 2021;14(8) doi: 10.1016/j.arabjc.2021.103282. [DOI] [Google Scholar]
- 56.Chaturvedi E., Roy P., Upadhyay R., Chowdhury P. Enhanced yield and production of aromatics rich fractions in bio-oil through co-pyrolysis of waste biomass and plastics. J. Anal. Appl. Pyrolysis. 2024;178 doi: 10.1016/j.jaap.2024.106379. [DOI] [Google Scholar]
- 57.Wu Y., Tao S., Wang Z., Cui Y., Jing Q., He W., Pan Z. Effect of pyrolysis atmospheres on gaseous products evolution of coal pyrolysis at high temperature. Fuel. 2024;366 doi: 10.1016/j.fuel.2024.131336. [DOI] [Google Scholar]
- 58.Madhu P., Neethi M.I., Kanagasabapathy H. Parametric analysis of cotton shell and palmyra palm fruit bunch for bio oil in fixed bed pyrolysis system. Int. J. Appl. Environ. Sci. 2014;9(5):2427–2436. [Google Scholar]
- 59.Kader M.A., Islam M.R., Parveen M., Haniu H., Takai K. Pyrolysis decomposition of tamarind seed for alternative fuel. Bioresour. Technol. 2013;149:1–7. doi: 10.1016/j.biortech.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 60.Thirugnanam S., Srinivasan R., Anand K., Bhardwaj A., Puthilibai G., Madhu P., Karthick A. Utilisation possibilities of waste medium-density fiberboard: a material recycling process. Mater. Today Proc. 2022;59:1362–1366. doi: 10.1016/j.matpr.2021.12.025. [DOI] [Google Scholar]
- 61.Anand L.V., Dhanalakshmi C.S., Mahendran M., Hepsiba D., Perumal S.B., Madhu P. Utilization possibilities of infectious waste plastic materials toward environmental sustainability. Pollut. Res. 2022;41(2):649–653. doi: 10.53550/PR.2022.v41i02.037. [DOI] [Google Scholar]
- 62.Artetxe M., Lopez G., Amutio M., Elordi G., Olazar M., Bilbao J. Operating conditions for the pyrolysis of poly-(ethylene terephthalate) in a conical spouted-bed reactor. Ind. Eng. Chem. Res. 2010;49(5):2064–2069. doi: 10.1021/ie900557c. [DOI] [Google Scholar]
- 63.Sharypov V.I., Marin N., Beregovtsova N.G., Baryshnikov S.V., Kuznetsov B.N., Cebolla V.L., Weber J.V. Co-pyrolysis of wood biomass and synthetic polymer mixtures. Part I: influence of experimental conditions on the evolution of solids, liquids and gases. J. Anal. Appl. Pyrolysis. 2002;64(1):15–28. doi: 10.1016/S0165-2370(01)00167-X. [DOI] [Google Scholar]
- 64.Chen W., Shi S., Zhang J., Chen M., Zhou X. Co-pyrolysis of waste newspaper with high-density polyethylene: synergistic effect and oil characterization. Energy Convers. Manag. 2016;112:41–48. doi: 10.1016/j.enconman.2016.01.005. [DOI] [Google Scholar]
- 65.Zhou L., Wang Y., Huang Q., Cai J. Thermogravimetric characteristics and kinetic of plastic and biomass blends co-pyrolysis. Fuel Process. Technol. 2006;87(11):963–969. doi: 10.1016/j.fuproc.2006.07.002. [DOI] [Google Scholar]
- 66.Guo J., Lua A.C. Kinetic study on pyrolytic process of oil-palm solid waste using two-step consecutive reaction model. Biomass Bioenergy. 2001;20(3):223–233. doi: 10.1016/S0961-9534(00)00080-5. [DOI] [Google Scholar]
- 67.López A., De Marco I., Caballero B.M., Laresgoiti M.F., Adrados A. Influence of time and temperature on pyrolysis of plastic wastes in a semi-batch reactor. Chem. Eng. J. 2011;173(1):62–71. doi: 10.1016/j.cej.2011.07.037. [DOI] [Google Scholar]
- 68.Vo T.A., Tran Q.K., Ly H.V., Kwon B., Hwang H.T., Kim J., Kim S.S. Co-pyrolysis of lignocellulosic biomass and plastics: a comprehensive study on pyrolysis kinetics and characteristics. J. Anal. Appl. Pyrolysis. 2022;163 doi: 10.1016/j.jaap.2022.105464. [DOI] [Google Scholar]
- 69.Anandaram H., Srivastava B.K., Vijayakumar B., Madhu P., Depoures M.V., Patil P.P., Prabhakar S. Co-pyrolysis characteristics and synergistic interaction of waste polyethylene terephthalate and woody biomass towards bio-oil production. J. Chem. 2022;3699076 doi: 10.1155/2022/3699076. [DOI] [Google Scholar]
- 70.Paradela F., Pinto F., Gulyurtlu I., Cabrita I., Lapa N. Study of the co-pyrolysis of biomass and plastic wastes. Clean Technol. Environ. Policy. 2009;11:115–122. doi: 10.1007/s10098-008-0176-1. [DOI] [Google Scholar]
- 71.Abnisa F., Daud W.W., Ramalingam S., Azemi M.N.B.M., Sahu J.N. Co-pyrolysis of palm shell and polystyrene waste mixtures to synthesis liquid fuel. Fuel. 2013;108:311–318. doi: 10.1016/j.fuel.2013.02.013. [DOI] [Google Scholar]
- 72.Ephraim A., Minh D.P., Lebonnois D., Peregrina C., Sharrock P., Nzihou A. Co-pyrolysis of wood and plastics: influence of plastic type and content on product yield, gas composition and quality. Fuel. 2018;231:110–117. doi: 10.1016/j.fuel.2018.04.140. [DOI] [Google Scholar]
- 73.Marin N., Collura S., Sharypov V.I., Beregovtsova N.G., Baryshnikov S.V., Kutnetzov B.N., Weber A.J. Copyrolysis of wood biomass and synthetic polymers mixtures. Part II: characterisation of the liquid phases. J. Anal. Appl. Pyrolysis. 2002;65(1):41–55. doi: 10.1016/S0165-2370(01)00179-6. [DOI] [Google Scholar]
- 74.Zhang Y., Zhai M., Wang X., Sun J., Dong P., Liu P., Zhu Q. Preparation and characteristics of biomass char. Bioresources. 2015;10(2):3017–3026. [Google Scholar]
- 75.Ansah E., Wang L., Shahbazi A. Thermogravimetric and calorimetric characteristics during co-pyrolysis of municipal solid waste components. Waste Manage. (Tucson, Ariz.) 2016;56:196–206. doi: 10.1016/j.wasman.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 76.Li X., Zhang H., Li J., Su L., Zuo J., Komarneni S., Wang Y. Improving the aromatic production in catalytic fast pyrolysis of cellulose by co-feeding low-density polyethylene. Appl. Catal., A. 2013;455:114–121. doi: 10.1016/j.apcata.2013.01.038. [DOI] [Google Scholar]
- 77.Zhang H., Zheng J., Xiao R., Shen D., Jin B., Xiao G., Chen R. Co-catalytic pyrolysis of biomass and waste triglyceride seed oil in a novel fluidized bed reactor to produce olefins and aromatics integrated with self-heating and catalyst regeneration processes. RSC Adv. 2013;3(17):5769–5774. doi: 10.1039/C3RA40694F. [DOI] [Google Scholar]
- 78.Faisal F., Rasul M.G., Jahirul M.I., Schaller D. Pyrolytic conversion of waste plastics to energy products: a review on yields, properties, and production costs. Sci. Total Environ. 2023;861 doi: 10.1016/j.scitotenv.2022.160721. [DOI] [PubMed] [Google Scholar]
- 79.Stedile T., Ender L., Meier H.F., Simionatto E.L., Wiggers V.R. Comparison between physical properties and chemical composition of bio-oils derived from lignocellulose and triglyceride sources. Renewable Sustainable Energy Rev. 2015;50:92–108. doi: 10.1016/j.rser.2015.04.080. [DOI] [Google Scholar]
- 80.Biswas B., Pandey N., Bisht Y., Singh R., Kumar J., Bhaskar T. Pyrolysis of agricultural biomass residues: comparative study of corn cob, wheat straw, rice straw and rice husk. Bioresour. Technol. 2017;237:57–63. doi: 10.1016/j.biortech.2017.02.046. [DOI] [PubMed] [Google Scholar]
- 81.Mallya N., Helt J.E. Research in Thermochemical Biomass Conversion. 1998. Effects of feedstock components on municipal solid waste pyrolysis; pp. 111–126. [DOI] [Google Scholar]
- 82.Sowmya Dhanalakshmi C., Madhu P. Biofuel production of neem wood bark (Azadirachta indica) through flash pyrolysis in a fluidized bed reactor and its chromatographic characterization. Energy Sources Part A. 2021;43(4):428–443. doi: 10.1080/15567036.2019.1624893. [DOI] [Google Scholar]
- 83.Hassan H., Hameed B.H., Lim J.K. Co-pyrolysis of sugarcane bagasse and waste high-density polyethylene: synergistic effect and product distributions. Energy. 2020;191 doi: 10.1016/j.energy.2019.116545. [DOI] [PubMed] [Google Scholar]
- 84.Chi Y., Xue J., Zhuo J., Zhang D., Liu M., Yao Q. Catalytic co-pyrolysis of cellulose and polypropylene over all-silica mesoporous catalyst MCM-41 and Al-MCM-41. Sci. Total Environ. 2018;633:1105–1113. doi: 10.1016/j.scitotenv.2018.03.239. [DOI] [PubMed] [Google Scholar]
- 85.Tang Y., Huang Q., Sun K., Chi Y., Yan J. Co-pyrolysis characteristics and kinetic analysis of organic food waste and plastic. Bioresource technol. 2018;249:16–23. doi: 10.1016/j.biortech.2017.09.210. [DOI] [PubMed] [Google Scholar]
- 86.Zheng Y., Zhang Y., Xu J., Li X., Xu C.C. Co-pyrolysis behavior of fermentation residues with woody sawdust by thermogravimetric analysis and a vacuum reactor. Bioresource technol. 2017;245:449–455. doi: 10.1016/j.biortech.2017.07.168. [DOI] [PubMed] [Google Scholar]
- 87.Abnisa F., Daud W.M.A.W. A review on co-pyrolysis of biomass: an optional technique to obtain a high-grade pyrolysis oil. Energy Convers. Manag. 2014;87:71–85. doi: 10.1016/j.enconman.2014.07.007. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.