Abstract
This study investigates the antioxidant properties of Chrysojasminum fruticans (L.) Banfi through a series of assays to measure the total phenolic content (TPC), total flavonoid content (TFC), and free radical scavenging activity using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, to ensure that it can be used as an antioxidant drug. The TPC, TFC and DPPH assay were performed using spectrophotometric method, and in terms of a linear standard curve for gallic acid, Quercetin, Vitamin C respectively. The.
aqueous extract of the flowers exhibited the highest concentration of phenolics, reaching 81.9 mg GAE (Gallic Acid Equivalent) per gram of (Dry Extract) DE, Conversely the ethanolic extract from the fruits demonstrated the lowest phenolic content, with a mere 0.249 (mg GAE/g DE). the aqueous flower extract demonstrated the highest flavonoid concentration, achieving an impressive 113.584 mg quercetin equivalents per gram of DE (mg QUE/g DE). In contrast, the methanolic fruit extract exhibited the lowest flavonoid concentration, measured at a mere 0.695 (mg QUE/g DE). Additionally, the methanolic flower extract demonstrated superior free radical scavenging activity, requiring only 0.348 mg/mL to inhibit 50 % of DPPH radicals, Conversely, the methanolic fruit extract exhibited the least antioxidant activity, as reflected by its highest IC50 value of 1.996 mg/mL.
These results underscore Chrysojasminum fruticans (L.) Banfi potent antioxidant capacity and its established role in traditional medicine practices globally, and encourage to be included as a drug contributing to the treatment of many chronic diseases such as diabetes and cardiovascular diseases, after conducting the necessary clinical studies.
Keywords: Chrysojasminum fruticans, Total phenolic content, Total flavonoid content, Antioxidant, DPPH
1. Background
Throughout history, medicinal herbs and plants have played a pivotal role in health and healing, with an astonishing 80 % of the global population today still relying on such traditional remedies [1]. This enduring legacy speaks to the profound connection between nature and human health. Within the vast diversity of medicinal flora, the Oleaceae family emerges prominently, especially the Jasminum genus, celebrated not only for its aromatic flowers but also for its substantial medicinal value [2].
Among the stars of this genus is Chrysojasminum fruticans (L.) Banfi, a plant that marries beauty with utility. This semi-evergreen shrub, with its bushy appearance and height ranging from 50 to 150 cm, stands out for its distinctive, dark green leaves and yellow, lightly scented flowers as show in Fig. 1. Native to the Mediterranean and West Irano-Turkish regions, it marks the seasons with its flowering in spring and fruiting in early summer [3,4].
Fig (1).
Morphology and site of the Chrysojasminum fruticans species.
The true value of Chrysojasminum fruticans (L.) Banfi, however, extends beyond its visual appeal. Traditionally, it has been harnessed for its soothing properties, to treat eye infections, and to combat allergies and weakness [5], showcasing the plant's integral role in traditional medicinal practices Its use in Turkey as a natural remedy for worms and as a diuretic underlines the plant's versatile health benefits [6,7].
Central to the interest in Chrysojasminum fruticans (L.) Banfi is its potent antioxidant capacity. In an age where oxidative stress contributes to chronic conditions ranging from heart disease to neurodegenerative disorders [8,9], the antioxidant properties of plants like Chrysojasminum fruticans (L.) Banfi offer a beacon of hope. These natural compounds effectively neutralize harmful free radicals, thereby mitigating the risk of cellular and molecular damage that can lead to disease [10].
With an increasing focus on diets rich in antioxidants to combat non-communicable diseases, the importance of integrating plants rich in vitamins and polyphenols into our daily intake cannot be overstated. Phenols have an important role in human health, they contribute to treatment of metabolic disorders, diabetes and cardiovascular diseases. flavonoids also have an antioxidant, anti-inflammatory and antibacterial properties, and have a positive effect on the function of blood vessels by reducing the permeability of the vessels. Chrysojasminum fruticans (L.) Banfi, with its rich array of health-promoting properties, exemplifies the untapped potential of traditional plants in contributing to modern dietary and health practices.
As we continue to explore the synergies between traditional knowledge and contemporary science, plants like Chrysojasminum fruticans (L.) Banfi stand at the crossroads, offering
Both a link to our past and a key to future health innovations. Their study not only enriches our understanding of botanical medicine but also underscores the critical role of biodiversity in health and wellness.
2. Experimental
2.1. Materials and methods
2.1.1. Plant material collection
Leaves, flowers, and fruits of Chrysojasminum fruticans (L.) Banfi were collected from diverse locations within Syria, including the Abu Qubais Reserve, Beit Yashout in Hama, and Nubbul in the northern countryside of Aleppo. The collection period spanned from December 15, 2022, to December 15, 2023. Post-collection, the leaves were air-dried in a shaded and well-ventilated area before being finely ground into powder. This powder was then stored in a cool, dry place until further use. Additionally, herbarium specimens incorporating all parts of the plant were prepared for documentation purposes. A plant taxonomy professor from the Faculty of Science at the University of Aleppo, Syria, conducted species identification.
2.1.2. Chemicals and equipments used
2.1.2.1. Chemicals used
Vitamin C purity 98 % (Merck, Germany), Querecetin (Merck, Germany), Gallic acid Purity 99.95 % (Merck, Germany), 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Purity 95 % (Sigma Aldrich, USA), Distilled water, Methanol Purity 99.8 % and Ethanol Purity 99.9 % (Eurolab, UK), Folin- ciocaltueae reagent 10 % (Merck, Germany), Sodium carbonate (Panreac quimica sau medien, Spain), Aluminium chloride Purity 95–101 (Syria, Sham Lab), Sodium nitrite (Panreac quimica sau medien, Spain).
2.1.2.2. Equipment used
Water distillation system (Lotum Co. Ltd, Taiwan), Sensitive balance (Sartorius, Germany), Rotary evaporator (Heidolph Instruments, Germany), Spectrophotometer (Shimadzu, Japan), Water bath (Jrad, Syria), Volumetric flasks and laboratory glassware.
2.1.3. Preparation of plant extracts
The dried powder of the leaves and flowers (10.0 g) were extracted, by using 100 mL of three different solvents, distilled water, methanol and ethanol. The fruits were pulverized using a manual grinder before the extraction. These extracts were then left at room temperature for 48 h, then the extracts were filtered and kept in opaque bottles. The solvents were evaporated at 40 °C using a rotary evaporator, the produce dry extracts were calculated as following equation [11].
| (1) |
2.1.4. Determination of total phenolic contents (TPC)
The contents of total phenolic content in the plant extracts were performed using spectrophotometric method, a standard solution of gallic acid (GA) were prepared at concentrations ranging from 0.01 to 0.10 mg/mL in distilled water. The standard solution 0.10 mL was transferred into a 10.0 mL volumetric flask. After that 0.5 mL of Folin-Ciocalteu reagent (10.0 % w/v) and 4.0 mL of a 7.5 % sodium carbonate solution were sequentially added after a resting period of 3 min. The mixture was then diluted to the final volume of 10.0 mL with distilled water.
The sample was maintained at 40 °C for 30 min in a water bath, and absorbance measurements were recorded at 765 nm. This procedure was similarly applied to the plant extracts, which were tested at a concentration of 5.0 mg/mL. After reviewing numerous literature sources on determining total phenolic content using spectrophotometric methods, we selected the wavelength of 765 nm for our current experiment due to its optimal absorption properties.
The total phenolic content (TPC) was calculated as following:
| (2) |
where: C represents the concentration of GA (mg/mL) as determined from the standard curve, V is the volume of the sample used in the analysis (mL), and M is the mass of the DE sample (g) [[12], [13], [14]].
This methodological approach ensures a precise determination of the phenolic content, expressed as milligrams of GAE per gram of dry extract, providing valuable insights into the antioxidant potential of the studied plant materials.
2.1.5. Determination of total flavonoids contents TFC
The contents of TFC in the DE was performed through spectrophotometric analysis, this method exploits the formation of a yellow-colored complex upon interaction between flavonoids and aluminum chloride, indicative of the flavonoid content.
Quercetin, serving as the standard for this analysis, was prepared at concentrations ranging from (0.01–0.10 mg/mL) in methanol to construct the calibration curve. The standard solution 0.5 mL was introduced into a 10.0 mL volumetric flask, followed by the addition of 1.0 mL of aluminum chloride 20.0 % and 0.3 mL of 5.0 % sodium nitrite. The mixture was then diluted to the final volume with distilled water. The resulting solution was subjected to spectrophotometric measurement at 430.0 nm.
This procedure was similarly applied to the plant extracts, with a sample concentration of 5.0 mg/mL. The wavelength of 430 nm was selected for testing total flavonoid content in the current experiment, based on the optimization for the highest absorption of the complex formed.
The TFC was subsequently calculated and expressed as milligrams of quercetin equivalent per gram of DE weight, utilizing the equation:
| (3) |
where.
-
•
C is the quercetin concentration (mg/mL) as determined from the calibration curve,
-
•
V represents the volume of the plant extract utilized in the analysis (mL), and
- •
This refined methodology ensures a rigorous and accurate determination of the flavonoid content within the plant extracts, providing a critical insight into the phytochemical profile and potential antioxidant capacity of the studied samples.
2.1.6. Determination of free radical scavenging activity using DPPH
The assessment of free radical scavenging capacity of plant extracts was conducted through the DPPH assay. Vitamin C, known for its potent natural antioxidant properties and with a purity of 98 %, was employed as the reference standard for this assay.
A series of standard solutions of Vitamin C were prepared from a mother solution (1 mg/mL) at concentrations of 0.001, 0.003, 0.005, 0.007, 0.009, 0.01, and 0.015 mg/mL in distilled water. Subsequently, 1.0 mL of a methanolic DPPH solution (0.135 mM) was combined with 1.0 mL of each Vitamin C concentration. This mixture was then incubated in the dark for 30 min to allow the reaction to proceed, after which the absorbance was measured at a wavelength of 517.0 nm.
The plant extracts were prepared in concentrations of 0.01, 0.03, and 0.09 mg/mL, and subjected to the same procedure as the standard solutions. Methanol served as the control solution for comparison in this assay. The absorbances in this test were measured at a wavelength of 517 nm, as it provides the optimal absorbance values. The percentage inhibition of DPPH radical by both the plant extracts and the Vitamin C standards was calculated using the formula:
| (4) |
where.
•Abs control: is the absorbance of the methanol control solution,
-
•
Abs sample: is the absorbance of the tested sample [17].
From the observed data, the IC50 value, which signifies the concentration of the extract necessary to inhibit 50 % of the DPPH free radical, was determined through the linear regression analysis of the dose-response curve.
This methodological approach not only quantifies the antioxidant capacity of the plant extracts in neutralizing DPPH radicals but also provides a comparative measure against the known antioxidant standard, Vitamin C, offering insights into the potential efficacy of the extracts as natural antioxidants.
3. Results
3.1. Extraction yield
The efficiency of extraction across different solvents and plant parts was quantitatively assessed through the determination of extraction yield percentages. Notably, the extraction yields varied significantly depending on the solvent and plant part used. Table 1 below summarizes the extraction yield percentages for all tested plant extracts, categorized by plant part (Leaves, Flowers, Fruits) and solvent type (Aqueous, Methanol, Ethanol).
Table 1.
The Extraction Yields of the plant parts.
| Extract | Extraction Yield (%) |
|---|---|
| L.AU.A | 30.80 |
| L.AU.M | 8.95 |
| L.AU.E | 5.00 |
| L.S.A | 14.85 |
| L.S.M | 6.90 |
| L.S.E | 4.50 |
| FL.A | 1.19 |
| FL.M | 13.10 |
| FL.E | 6.53 |
| FR.A | 33.80 |
| FR.M | 19.84 |
| FR.E | 17.43 |
Note: “L", “FL”, and “FR” denote Leaves, Flowers, and Fruits, respectively, while “A", “M" and “E" represent the solvents used: Aqueous, Methanol, and Ethanol. “AU” means Autumn Leaves, “S" means Spring Leaves.
3.2. Determination of total phenolic contents (TPC)
The investigation into the TPC of the plant extracts revealed a significant variation across the different types of extracts and parts of the plant used. Notably, the aqueous extract of the flowers exhibited the highest concentration of phenolics, reaching 81.9 mg GAE per gram of DE, underscoring the potent phenolic profile of the floral components. Conversely, the ethanolic extract from the fruits demonstrated the lowest phenolic content, with a mere 0.249 mg GAE/g DE, highlighting a considerable disparity in phenolic yield dependent on both the solvent used and the plant part extracted.
The ethanolic extract from spring leaves also showed a commendable phenolic content of 49.649 mg GAE/g DE, positioning it as the second-highest among the extracts studied. This indicates that the seasonal variation in leaf harvest (spring versus autumn) and the choice of extraction solvent (aqueous versus ethanolic) play crucial roles in determining the phenolic content yield.
The results are summarized in Table 2, which delineates the TPC measured across the diverse extracts studied, providing a comprehensive overview of the phenolic distribution:
Table 2.
The TPC of the extracts studied.
| Extract | TPC (mg GAE/g DE) |
|---|---|
| L.AU.A | 2.694 |
| L.AU.M | 38.000 |
| L.AU.E | 22.000 |
| L.S.A | 4.208 |
| L.S.M | 35.942 |
| L.S.E | 49.649 |
| FL.A | 81.900 |
| FL.M | 10.400 |
| FL.E | 16.100 |
| FR.A | 6.917 |
| FR.M | 0.781 |
| FR.E | 0.249 |
Phenol ions were formed upon the addition of sodium carbonate, which reduced the Folin-Ciocalteu reagent, resulting in a color change from blue to yellow. It is noteworthy that the blue color remained stable throughout the incubation period and during the measurements for approximately 2 h.
The elucidation of TPC across the different extracts affirms the critical influence of solvent selection and plant part on the extraction efficacy of phenolic compounds. These findings underscore the complexity of phytochemical extraction and highlight the need for strategic selection of extraction parameters to maximize phenolic yield, which is fundamental for leveraging the antioxidant potential of plant materials.
3.3. Determination of total flavonoids contents (TFC)
The assessment of TFC within the plant extracts was meticulously conducted, revealing significant variability across different extracts. Notably, the aqueous flower extract demonstrated the highest flavonoid concentration, achieving an impressive 113.584 mg quercetin equivalents per gram of DE (mg QUE/g DE). This was closely followed by the ethanolic spring leaf extract, which registered a content of 103.508 mg QUE/g DE. In contrast, the methanolic fruit extract exhibited the lowest flavonoid concentration, measured at a mere 0.695 mg QUE/g DE.
A standard curve for quercetin was established to facilitate the quantification of flavonoid content, providing a critical reference for the analysis Flavonoid Content Across Extracts.
The results of the flavonoid content analysis are succinctly summarized in Table 3, which delineates the TFC of the studied extracts, offering a comprehensive overview of the flavonoid profile across different plant parts and solvent extracts.
Table 3.
The TFC of the extracts studied.
| Extract | TFC (mg QUE/g DE) |
|---|---|
| L.AU.A | 4.042 |
| L.AU.M | 44.660 |
| L.AU.E | 25.600 |
| L.S.A | 4.780 |
| L.S.M | 30.144 |
| L.S.E | 103.508 |
| FL.A | 113.584 |
| FL.M | 11.456 |
| FL.E | 15.530 |
| FR.A | 2.337 |
| FR.M | 0.695 |
| FR.E | 2.380 |
Note: “L", “FL”, and “FR” denote Leaves, Flowers, and Fruits, respectively, while “A", “M" and “E" represent the solvents used: Aqueous, Methanol, and Ethanol. “AU” means Autumn Leaves, “S" means Spring Leaves.
A yellow complex was formed upon the addition of aluminum chloride. Using quercetin standards, the color of this yellow complex remained stable for 1 h during the measurements, which was sufficient for accurate analysis.
These findings underscore the substantial variation in flavonoid content dependent on both the part of the plant being extracted and the solvent used for extraction. The aqueous flower extract, in particular, stands out for its exceptionally high flavonoid content, suggesting a promising potential for applications requiring high antioxidant capacities.
3.4. Determination of free radical scavenging activity using DPPH assay
The antioxidant efficacy of plant extracts was quantified through their ability to scavenge DPPH free radicals, a method that hinges on the discoloration of the DPPH solution. Vitamin C, recognized for its potent antioxidant properties, served as a standard, with its IC50 value (the concentration necessary to inhibit 50 % of the free radical activity) determined to be 0.006 mg/mL. This value was established by plotting the free radical scavenging percentage against the concentration, following the derived equation from the curve. Table 4 shows Free Radical Scavenging Percentages and IC50 Value for Vitamin C.
Table 4.
Free radical scavenging percentages and IC50 value for vitamin C.
| Concentration (mg/g) | Free Radical Scavenging Percentage (%) | aIC50 Value (mg/ml) |
|---|---|---|
| 0.001 | 24.84 | 0.006 |
| 0.003 | 28.57 | 0.006 |
| 0.005 | 38.63 | 0.006 |
| 0.007 | 61.36 | 0.006 |
| 0.009 | 72.04 | 0.006 |
| 0.010 | 77.63 | 0.006 |
| 0.015 | 88.96 | 0.006 |
IC50: Half-maximal Inhibitory concentration.
3.5. Comparative analysis of plant extracts
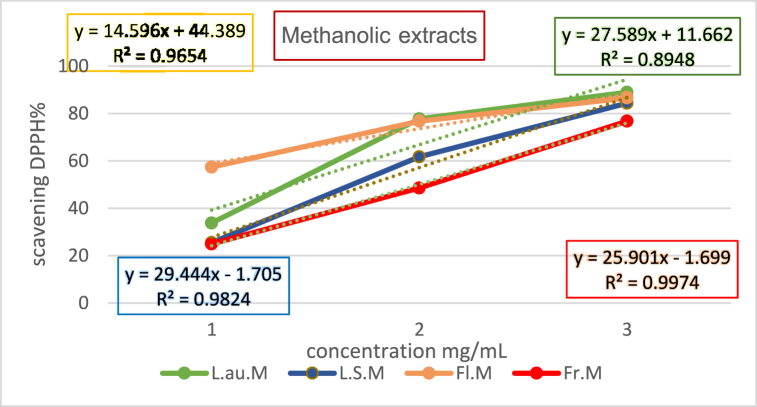
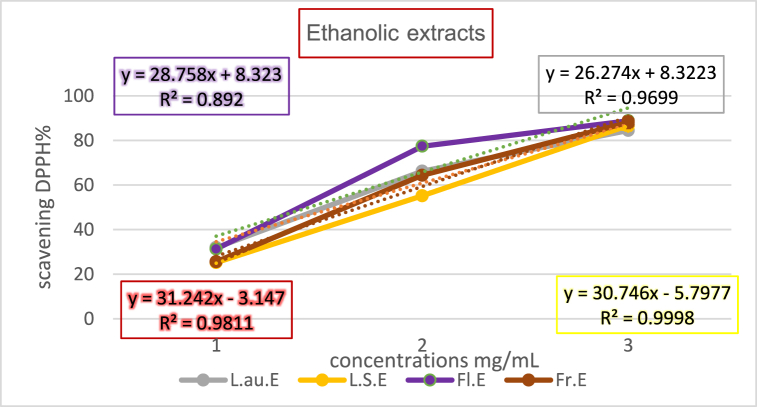
The antioxidant potential of methanolic and ethanolic plant extracts was evaluated against the benchmark set by Vitamin C, with the IC50 values indicating the concentration at which each extract achieves 50 % free radical scavenging. Lower IC50 values denote higher antioxidant efficacy. Table 5, Table 6 shows the Free Radical Scavenging and IC50 Values for Methanolic and Ethanolic Extracts. The graphical representation of Vitamin C's standard curve elucidates the direct correlation between concentration and free radical scavenging activity as show in Fig. (2), Fig. (3), substantiating the efficiency of Vitamin C as a benchmark antioxidant.
Table 5.
Free radical scavenging and IC50 values for methanolic extracts.
| Extract | Concentration (mg/mL) | Free Radical Scavenging Percentage (%) | IC50 Value (mg/mL) |
|---|---|---|---|
| L.au.M | 0.01 | 33.788 | 1.390 |
| 0.03 | 77.763 | 1.390 | |
| 0.09 | 88.965 | 1.390 | |
| L.S.M | 0.01 | 25.460 | 1.756 |
| 0.03 | 61.739 | 1.756 | |
| 0.09 | 84.347 | 1.756 | |
| Fl.M | 0.01 | 57.391 | 0.384 |
| 0.03 | 76.770 | 0.384 | |
| 0.09 | 86.583 | 0.384 | |
| Fr.M | 0.01 | 24.968 | 1.996 |
| 0.03 | 48.571 | 1.996 | |
| 0.09 | 76.770 | 1.996 |
Table 6.
Free radical scavenging and IC50 values for ethanolic extracts.
| Extract | Concentration (mg/mL) | Free Radical Scavenging Percentage (%) | IC50 Value (mg/mL) |
|---|---|---|---|
| L.au.E | 0.01 | 31.925 | 1.586 |
| 0.03 | 66.211 | 1.586 | |
| 0.09 | 84.472 | 1.586 | |
| L.S.E | 0.01 | 25.217 | 1.814 |
| 0.03 | 55.155 | 1.814 | |
| 0.09 | 86.708 | 1.814 | |
| Fl.E | 0.01 | 31.304 | 1.449 |
| 0.03 | 77.391 | 1.449 | |
| 0.09 | 88.819 | 1.449 | |
| Fr.E | 0.01 | 25.590 | 1.701 |
| 0.03 | 64.347 | 1.701 | |
| 0.09 | 88.074 | 1.701 |
Fig. (2).
Methanolic extracts curve.
Fig. (3).
Ethanolic extracts curve.
The color of the free radical DPPH (1,1-diphenyl-2-picrylhydrazyl) changes from violet to yellow upon reflux and remains stable after incubation and throughout the 2-h test period. The change to yellow indicates the plant's significant ability to suppress free radicals.
The comparative analysis reveals the methanolic flower extract as the most potent antioxidant, with the lowest IC50 value of 0.348 mg/mL, indicative of its superior free radical scavenging capability. Conversely, the methanolic fruit extract exhibited the least antioxidant activity, as reflected by its highest IC50 value of 1.996 mg/mL.
These findings, illustrated through curves as show in Fig. (2), Fig. (3) representing the change in free radical scavenging activity with varying concentrations, underscore the differential antioxidant potential across plant extracts. Such disparities highlight the importance of selecting specific plant parts and extraction methods to optimize antioxidant yield for therapeutic applications.
4. Discussion
Numerous studies across the globe have attested to the antioxidant capabilities of Chrysojasminum fruticans (L.) Banfi, highlighting not only its potency as an antioxidant plant but also its longstanding traditional uses. Our investigation aligns with this body of research, offering empirical support for the plant's antioxidant efficacy and its historical applications in herbal medicine.
A notable observation from our experiments was the seasonal variation in the phytochemical composition of Chrysojasminum fruticans (L.) Banfi, Specifically, plant parts harvested in spring, particularly the flowers, exhibited significantly higher levels of total phenolics and flavonoids compared to those collected in autumn. This seasonal fluctuation in phytochemical content could be attributed to environmental factors such as light exposure, humidity, and temperature, which are known to influence the biosynthesis of active compounds in plants Such environmental responsiveness is indicative of the plant's adaptive mechanisms for phytochemical production, aligning with findings from previous research that suggest active component production in plants varies seasonally in response to external stimuli [18].
The aqueous fruit extract exhibited the highest yield at 33.8 %, underscoring its exceptional efficiency in extracting phytochemicals. In contrast, the aqueous flower extract yielded the lowest extraction percentage at 1.19 %, indicating a relatively low efficiency under the same conditions.
These results provide insight into the selective extraction capabilities of different solvents when applied to various plant parts, highlighting the importance of solvent choice in maximizing yield and potentially influencing the phytochemical profile of the extract. The marked difference in extraction efficiency between plant parts and solvents underscores the complexity of phytochemical extraction and the necessity for optimization depending on the desired compounds and their applications.
Compared to the Turkish study [19], the total phenolic content in the methanolic extract of the species was 97.41 mg GAE/g DE, which is slightly higher than its value in the current study. In contrast, the total flavonoid content was recorded at 19.45 mg CA/g DE, which is lower than its value in the current study. Additionally, the inhibition percentage of free radical DPPH by Chrysojasminum fruticans (L.) Banfi extracts ranged between 20.36 % and 77.44 %, which is slightly less than the inhibition percentages observed in the current study.
In another Turkish study [20], the methanolic extract of the flowering branches of Chrysojasminum fruticans (L.) Banfi achieved a total phenolic content of 82.70 mg GAE/g DE and a total flavonoid content of 70.78 mg QE/g DE, which are similar to the results of the current study. The higher value of total flavonoid content compared to total phenolic content in the current study, unlike the two Turkish studies, may be attributed to the flavonoids being in a free (unbound) state.
Comparative analysis with other studies, such as the inhibition percentages of the DPPH free radical in Chrysojasminum fruticans (L.) Banfi extracts, reveals both congruencies and variances. For instance, the range of percentage inhibition observed in our study mirrors the findings of some prior investigations, albeit with deviations that could be attributed to differences in concentration, solvents used, and plant parts examined. Moreover, a study from Turkey reporting an IC50 value of 1.904 for extracts from the aerial parts of the plant shows remarkable similarity to some of our results [21], suggesting a degree of consistency in the plant's antioxidant activity across different geographical and methodological contexts.
However, discrepancies between our findings and those of other international studies underscore the significant impact of collection location, harvest timing, and processing methods on the phytochemical profile and antioxidant efficacy of plant samples [20]. As indicated in the literature, variations in phenolic and flavonoid content, as well as free radical scavenging capacity, can result from these factors, highlighting the complexity of botanical research and the need for standardized methodologies to facilitate comparison and replication of results.
5. Limitations and future directions
One notable limitation of our study is the restricted range of techniques available in our laboratory. This constraint has affected our ability to fully explore and characterize the potential of Chrysojasminum fruticans (L.) Banfi.
To build on our findings and address these limitations, we suggest several future research directions.
1. Isolation and Identification of Active Compounds: We recommend isolating specific active compounds from Chrysojasminum fruticans (L.) Banfi and identifying their chemical structures. This will help in understanding the components responsible for its bioactivity.
2. In Vitro Enzyme Inhibition Studies: Future studies should include an evaluation of the inhibitory activity of Chrysojasminum fruticans (L.) Banfi extracts on the α-amylase enzyme in vitro. This will provide insights into the potential therapeutic benefits of the plant extracts.
3. Biological Activity Evaluation: It would be beneficial to assess the biological activities of Chrysojasminum fruticans (L.) Banfi extracts in experimental animal models. This will offer a more comprehensive understanding of the plant's efficacy and safety in a living system.
Addressing these aspects will not only overcome the limitations of our current study but also contribute valuable insights into the pharmacological potential of Chrysojasminum fruticans (L.) Banfi.
6. Conclusion
The comprehensive analysis conducted in our study reinforces the recognition of Chrysojasminum fruticans (L.) Banfi as a potent antioxidant resource, corroborating its historical and traditional uses. The observed seasonal variation in phenolic and flavonoid content underscores the influence of environmental factors on the plant’s phytochemical composition, offering valuable insights for optimizing harvest and extraction processes. While our findings align with existing research, they also highlight the challenges of variability and the importance of context in botanical studies. Moving forward, further research should aim to standardize methods and conditions to better understand the full potential of Chrysojasminum fruticans (L.) Banfi and similar plants in both traditional and modern pharmacological applications.
Data availability statement
All data used in this study are mentioned in the manuscript. There are no additional data repositories.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Abdel Aleem Bello: Supervision, Project administration, Formal analysis, Conceptualization. Abdullah Katta: Project administration, Methodology, Conceptualization. Reem Hasan Obaydo: Writing – review & editing, Visualization, Methodology, Formal analysis, Data curation, Conceptualization. Alaa Jazmati: Writing – original draft, Validation, Methodology, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e37322.
Abbreviations
- GA
Gallic Acide
- TPC
total phenolic content
- C
concentration of gallic acid and quercetin
- V
Volume
- M
Mass
- TFC
total flavonoid content
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- Abs
Absorbance
- IC50
Half-maximal Inhibitory concentration
- L.Au.A
Aqua Extract for Autumn Leaves
- L.Au.M
Methanolic Extract for Autumn Leaves
- L.Au.E
Ethanolic Extract for Autumn Leaves
- L.S.A
Aqua Extract for Spring Leaves
- L.S.M
Methanolic Extract for Spring Leaves
- L.S.E
Ethanolic Extract for Spring Leaves
- Fl.A
Aqua Extract for Flowers
- Fl.M
Methanolic Extract for Flowers
- Fl.E
Ethanolic Extract for Flowers
- Fr.A
Aqua Extract for Fruits
- Fr.M
Methanolic Extract for Fruits
- Fr.E
Ethanolic Extract for Fruits
- L
Leaves
- FL
Flowers
- FR
Fruits
- A
Aqueous
- M
Methanol
- E
Ethanol
- AU
Autumn
- S
Spring
- GAE
Gallic Acid Equivalents
- DE
Dry extract
- QUE
Quercetin Equivalents
- CA
Coumaric Acid
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Bello A.A., Tahan Z.S., Kitaz A., Tiba B. Phytochemical screening and anti-multidrug resistant Pseudomonas aeruginosa of some fabaceae medicinal plants growing in Aleppo-Syria. International Journal of Pharmacognosy and Phytochemical Research (IJPPR) 2019;11(3):91–97. http://www.ijppr.com/ [Google Scholar]
- 2.Priya Joy, Raja D Patric. Anti-bacterial activity studies of Jasminum grandiflorum and Jasminum sambac. Ethnobotanical Leaflets. 2008;2008 https://opensiuc.lib.siu.edu/ebl/vol2008/iss1/59 Article 59. [Google Scholar]
- 3.Mouterde P. Beyrouth; 1983. Nouvelle flore du Liban et de la Syrie. 3 tomes (textes) et 3 tomes (atlas) Dar-el-Machreq. [Google Scholar]
- 4.Post G. Palestine and Sinai. 2. Librairie du Liban Pub. & AUB Beirut; 2007. Flora of Syria. [Google Scholar]
- 5.Arnold N., Baydoun S., Chalak L., Raus T.H. A contribution to the flora of ethnobotanical knowledge of mount hermon, Lebanon. Lebanon: fl. Mediterraneo. 2015;25:13–55. doi: 10.7320/FlMedit25.013. [DOI] [Google Scholar]
- 6.Kozan E., Küpeli E., Yesilada E. Evaluation of some plants used in Turkish folk medicine against parasitic infections for their in vivo anthelmintic activity. J. Ethnopharmacol. 2006;108(2):211–216. doi: 10.1016/j.jep.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Gűler B., Manav E., Urğurlu E. Medicinal plants used by traditional healers in bozuyuk (bilecik – Turkey) J. Ethnopharmacol. 2015;173:39–47. doi: 10.1016/j.jep.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Valko M., Leibfritz D., Moncol J., Cronin M.T.D., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Kotha R.R., Tareq F.S., Yildiz E., Luthria D.L. Oxidative stress and antioxidants - a critical review on in vitro antioxidant assays. Antioxidants. 2022;11(12):2388. doi: 10.3390/antiox11122388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romulo A. vol. 426. IOP Publishing; 2020. The principle of some in vitro antioxidant activity methods. (IOP Conference Series: Earth and Environmental Science). No 1. [DOI] [Google Scholar]
- 11.Ouis N., Hariri A. Phytochemical analysis and antioxidant activity of the flavonoids extracts from pods of Ceratonia siliqua L. Banat's Journal of Biotechnology. 2017;8(16):93–104. doi: 10.7904/2068–4738–VIII(16)–93. [DOI] [Google Scholar]
- 12.Armoskaite V., Ramanauskiene K., Maruska A., Razukas A., Dagilyte A., Baranauskas A., Briedis V. The analysis of quality and antioxidant activity of green tea extracts. J. Med. Plants Res. 2011;5(5):811–816. http://www.academicjournals.org/JMPR [Google Scholar]
- 13.Blainski A., Lopes G.C., De Mello J.C.P. Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules. 2013;18(6):6852–6865. doi: 10.3390/molecules18066852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waterhouse A.L. Determination of total phenolics. Current protocols in food analytical chemistry. 2002;6(1) doi: 10.1002/0471142913.fai0101s06. [DOI] [Google Scholar]
- 15.Molole G.J., Gure A., Abdissa N. Determination of total phenolic content and antioxidant activity of Commiphora mollis (Oliv.) Engl. resin. BMC chemistry. 2022;16(1):48. doi: 10.1186/s13065-022-00841-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Häkkinen S. vol. 221. Kuopio University Publications D. Medical Sciences; 2000. pp. 30–31.https://api.core.ac.uk/oai/oai:epublications.uef.fi:2067 (Flavonols and Phenolic Acids Berries and Berry Products Medical Sciences). [Google Scholar]
- 17.Olajuyigbe O.O., Afolayan A.J. Phenolic content and Antioxidants property of the bark extracts of ziziphus muucronata Willd. Subsp. Mucronata Willd. BMC complementary and alternative medicine. 2011;11(130):1–8. doi: 10.1186/1472-6882-11-130. http://www.biomedcentral.com/1472-6882/11/130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ncube B., Finnie J.F., Van Staden J. Seasonal variation in antimicrobial and phytochemical properties of frequently used medicinal bulbous from South Africa. S.A. J of Botany. 2011;77:387–396. doi: 10.1016/j.sajb.2010.10.004. [DOI] [Google Scholar]
- 19.Polat D.Ç., İlgün S., Karatoprak G.Ş., Akkol E.K., Capasso R. Phytochemical profiles, antioxidant, cytotoxic, and anti-inflammatory activities of traditional medicinal plants: Centaurea pichleri subsp. pichleri, Conyza canadensis, and Jasminum fruticans. Molecules. 2022;27(23):8249. doi: 10.3390/molecules27238249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akkol E.K., Kozan E., Bardakci H., Barak T.H., Khalilpour S. Potential anthelmintic and antioxidant activities of Jasminum fruticans L. and its phytochemical analysis. Pharmaceut. Sci. 2021;28(3):481–495. doi: 10.34172/PS.2021.69. [DOI] [Google Scholar]
- 21.Serteser A., Kargıoğlu M., Gök V., Bağci Y., Özcan M.M., Arslan D. Antioxidant properties of some plants growing wild in Turkey. Grasas Aceites. 2009;60(2):147–154. doi: 10.3989/gya.086208. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this study are mentioned in the manuscript. There are no additional data repositories.