Abstract
Objective
Anxiety disorder (AD) is a common mental disorder related to cardiovascular disease morbidity. Oxidative stress plays a crucial role in the anxiety state and can lead to cardiac remodeling. Over-activation of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) in cardiomyocytes and neurons can cause oxidative stress. Additionally, the AMPAR inhibitor—2,3-dihydroxy-6-nitro-7-sulfamoyl-benzoquinoxaline-2,3-dione (NBQX) plays an important role in ameliorating oxidative stress. This study aimed to explore the anti-arrhythmic effects of NBQX in a rat model of anxiety.
Methods
The AD model was induced using empty bottle stimulation. Male Sprague Dawley rats were randomly divided into four groups: control + saline, control + NBQX, AD + saline, and AD + NBQX. Open field test was conducted to measure anxiety-like behavior. Electrophysiological experiments, histological analysis, biochemical detection and molecular biology were performed to verify the effects of NBQX on the amelioration of electrical remodeling and structural remodeling. Furthermore, the nuclear factor erythroid 2-related factor 2 (Nrf2) inhibitor (ML385) was used in vitro to demonstrate the signaling pathway.
Results
Oxidative stress levels increased with AMPAR over-activation in AD rats, leading to heightened vulnerability to ventricular fibrillation (VF). NBQX reverses anxiety and VF susceptibility. Our results showed that NBQX activated the Nrf2/heme oxygenase-1 (HO-1) pathway, leading to a decline in oxidative stress levels. Connexin 43 and ion channel expression was upregulated. NBQX treatment attenuated fibrosis and apoptosis. This effect was diminished by ML385 treatment in vitro.
Conclusion
NBQX can alleviate VF susceptibility in rat models of anxiety by alleviating electrical remodeling, structural remodeling via regulating the Nrf2/HO-1 pathway to some extent.
Keywords: Anxiety disorder, Ventricular arrhythmia, Oxidative stress, NBQX, AMPAR
Highlights
-
•
NBQX could alleviate VF susceptibility in anxiety disorder rats via regulating Nrf2-HO-1 pathway.
-
•
The pathogenesis of VF susceptibility in anxiety rats were explored.
-
•
NBQX may be a potential treatment for anxiety disorder patients with VF.
1. Introduction
Ventricular fibrillation (VF) is the most dangerous type of ventricular arrhythmia and can cause sudden cardiac death. Its prevalence is high, affecting 1 % of the global population [1]. Although VFwas formally proposed in 1874, its pathogenesis is not yet well understood [2]. Many studies have suggested that arrhythmias are related to various factors, including myocardial fibrosis [3], metabolic changes [4], inflammation [5], and oxidative stress [6].
Anxiety disorder (AD) is a pathological condition caused by excessive pressure and physical symptoms that are difficult to control [7]. There are three types of AD: generalized AD (lifetime prevalence 6.2 %), social AD (lifetime prevalence 13 %), and panic disorder (lifetime prevalence 5.2 %) [8]. Clinical symptoms of AD include recurrent worry, muscle tension, fear of social performance, accidental and/or triggered panic attacks, and avoidance behavior.
A substantial body of clinical evidence highlights the close relationship between anxiety and cardiovascular disease [9]. Arrhythmia and anxiety are interconnected, with frequent arrhythmia episodes potentially inducing anxiety and prolonged anxiety, thereby increasing the risk of arrhythmia [10]. Medications commonly used to treat psychiatric disorders may increase the risk of arrhythmia [11]. For instance, anti-anxiety medications can affect repolarization [12]. A follow-up study conducted among women with no history of cardiovascular disease revealed an elevated risk of death owing to sudden cardiac arrest among those with higher anxiety scores [13]. Notably, the level of oxidative stress is significantly elevated in patients with anxiety [14], which can lead to myocardial fibrosis and electrical remodeling [15].
The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor is an ionotropic glutamate receptor controlled by glutamate transmitter conduction. It exhibits selective permeability to ions such as Na+, K+, and Ca2+, resulting in gated current and changes in the membrane potential [16]. Sustained anxiety causes an increase in extracellular fluid glutamate, which over-activates glutamate receptors, resulting in the influx of calcium ions and causing a series of cell death signals, such as apoptosis, mitochondrial damage, and cell excitatory death [17]. In a rabbit cardiopulmonary resuscitation model of cardiac arrest, glutamate receptor inhibitors not only had a significant protective effect on CA2 in the hippocampus, but also significantly reduced the apoptosis of cardiomyocytes and the level of troponin 1 [18]. Similar to neurons, cardiomyocytes have the ability to generate action potentials in response to external stimuli. Glutamate receptors are reportedly present on cardiomyocytes, indicating glutamate's ability to regulate heart function [19]. Studies by Yi-Han have shown that 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzoquinoxaline-2,3-dione (NBQX) downregulates cardiomyocyte excitability, as well as prevents and terminates arrhythmias in rats [20].
Glutamatergic signaling plays a crucial role in various physiological and pathological processes, including inflammation, pain, and oxidative stress [21]. For example, glutamate concentrations significantly increase (approximately 54-fold) in the synovial fluid of patients with osteoarthritis, and inhibiting AMPA activity can alleviate inflammation and bone remodeling [22]. In mice with acute subarachnoid hemorrhage, AMPAR was heavily activated, and the use of AMAPR antagonists could significantly inhibit the neurological dysfunction and the destruction of the blood-brain barrier after cerebral hemorrhage, which may be related to the activation of AMPAR inducing the release of pro-inflammatory factors, elevated oxidative stress and neuronal apoptosis [23]. High consumption of alcohol during development induces ROS production by activating AMPAR, which can have harmful effects on fetal neurodevelopment [24]. NBQX, an AMPA receptor antagonist, improves mitochondrial function and decreases oxidative stress [25,26]. For example, NBQX may alleviate heart rate irregularities and inhibit sympathetic nerve activity in rats poisoned with nitrous oxide by reducing oxidative stress levels [27]. Evidence also suggests that NBQX may alleviate anxiety symptoms [28].
Nuclear factor erythroid-2 related factor 2 (Nrf2) is a vital antioxidant system that regulates the expression of cytoprotective genes, including heme oxygenase-1 (HO-1), which helps prevent oxidative damage and regulates apoptosis. Under oxidative stress, Nrf2 interacts with antioxidant response elements (ARE) to activate transcriptional pathways, promote transcription of downstream antioxidant genes, and maintain cellular REDOX homeostasis. Nrf2 downstream genes can regulate ROS detoxification, involving GPX4, heme oxygenase 1(HO1), NAD(P)H, etc. Myocardial tissue is susceptible to reactive oxygen species (ROS) -induced damage that reduces Nrf2 and important antioxidant enzymes, including superoxide dismutase 1 (SOD1), catalase (CAT), and GPX4, and increasing antioxidant protein levels through Nrf2 activation is considered one way to achieve cardiac protection [29].
These findings from previous studies led us to hypothesize that NBQX could improve VF in patients with AD. This study aimed to test this hypothesis and explore its underlying molecular mechanisms.
2. Methods
2.1. Animals
Male SD rats (200 ± 20 g) were obtained from the animal experimental administration of Wuhan University and were housed in pathogen-free conditions with a 12-h light/12-h dark cycle, with free access to food and water. The experimental rats were divided into four groups (n = 14 per group): (1) control + saline (CTL group), (2) control + NBQX (CTN group), (3) AD group, and (4) AD + NBQX (ADN group). NBQX was administered via intraperitoneal injection to the CTN and ADN groups at a dose of 20 mg/kg per day for 28 days as previously described [30]. Rats in the CTL group received saline in the same manner. All animal procedures were approved by the Ethics Committee of the Wuhan University People's Hospital.
The AD model was induced employing empty bottle stimulation, following a method described in a previous study [31]. Briefly, CTL and CTN rats had access to water throughout the day, whereas AD and ADN rats received water at specific times (8:00–8:10 and 19:00–19:10 every day) during the first week to establish a new drinking habit. Subsequently, only the AD and ADN groups received water randomly at specified times over the next 2 weeks. Except for the differences in drinking habits among all groups, other feeding environments such as light/dark cycle, temperature, humidity and noise, were identical.
2.2. Materials
NBQX (AMPA receptor antagonist) and ML385 (Nrf2 inhibitor), which were obtained from MedChemExpress (HY-15068; New Jersey, USA), were dissolved in dimethyl sulfoxide and then stored at −20 °C. The primary antibodies used for western blotting analysis included AMPAR (1:1000 dilution; #4676; Cell Signaling Technology), Connexin 43 (CX43,1:1000 dilution; #3512; Cell Signaling Technology), KV4.2 (1:1000 dilution; #74748; Cell Signaling Technology), KV4.3 (1:1000 dilution; bs-3762R; Bioss), collagen Ⅰ (1:5000 dilution; 67288; Proteintech), collagen Ⅲ (1:1000 dilution; 22734; Proteintech), Nrf2 (1:2000 dilution; 16396; Proteintech), HO-1 (1:1000 dilution; 10701; Proteintech), Bcl2 (1:1000 dilution; #4223; Cell Signaling Technology), BAX (1:1000 dilution; #5023; Cell Signaling Technology), Caspase-3 (1:1000 dilution; #14220; Cell Signaling Technology) and GAPDH (1:4000 dilution; 60004; Proteintech).
2.3. Open field test
After 4 weeks of empty bottle stimulation, all animals were subjected to an anxiety behavior test, specifically the open field test (OFT), to evaluate their autonomous behavior, exploratory behavior, and tension levels in new and different environments. The test was carried out in a quiet environment using an experimental device that consists of an open field box and an automatic data processing system (EthoVisionXT; Noldus Information Technology). The reaction box measures 30–40 cm high, with a bottom length of 100 cm. The inner walls are painted black, and the bottom surface is divided into 25 small squares, each averaging 4 cm × 4 cm. The open field uses artificial lighting. At the start of the test, rats were placed in the center of a square box and allowed to move freely for 5 min During this time, their cumulative time spent in the center, the frequency of entering the central zone, and rearing times were recorded [32].
2.4. Heart rate variability (HRV) analysis
SD rats received a surface electrocardiogram from lead II for 10 min after being anesthetized with gaseous isoflurane. Generally, the induced concentration of isoflurane was adjusted to 3%–4%. When the rats were completely anesthetized, the concentration was maintained at 2%–2.5 % to sustain narcosis. HRV was analyzed, including the average RR intervals (average RR) and the square root of the mean squared differences of successive RR intervals (RMSSD), which indicate the time domain parameters.
2.5. In vivo electrophysiological experiment
SD rats underwent a surgical procedure after being anesthetized to fully expose their hearts. Platinum-stimulation electrodes were placed in the left ventricle (LV) to provide stimulation. Recording electrodes made of Ag-AgCl were positioned approximately 1 cm away from the stimulation electrodes in the LV to capture monophasic action potentials. Briefly, the S1S1 stimulation procedure at pacing cycle lengths (PCLs) of100 ms was programed to record the action potential duration (APD), and the duration of the action potential repolarized to 50 % (APD50) and 90 % (APD90) was measured. The S1S2 procedure was performed to stimulate the effective refractory period (ERP), which included eight successive stimuli (S1) for 250 ms,followed by a preceding stimulus (S2) that captured no ventricle [33]. Burst stimulation was performed 10 times at 50 Hz to asses VF susceptibility, which refers to a rapid irregular ventricular rhythm lasting for a minimum of 2 s [34].
2.6. Histological analysis
LV tissues were fixed in 4 % paraformaldehyde and embedded in paraffin, allowing for the preparation of 5-μm-thick sections for histological analysis. Masson trichrome and Sirius red staining were used to assess the degree of fibrosis. The fibrotic areas of the LV were analyzed using Image J software (n = 3 in each group). Anti-CX43 primary antibody were used for immunohistochemistry and immunofluorescence staining. LV sections were combined with Cx43 antibody at 4 °C overnight. Subsequently, it was combined with secondary antibody at 25 °C for 1 h and stained with 3,3 ′-diaminobenzidine. Image Pro Plus software (version 6.0) was used to quantitatively analyze the average optical density of CX43 (n = 5 per group).
2.7. Immunofluorescence staining
LV tissue sections from all groups were fixed with 4 % paraformaldehyde and then incubated with anti-CX43 and anti-Nrf2 antibodies at 4 °C overnight, followed by incubation with secondary antibodies at 37 °C for 1 h. The nuclei were stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) for 5 mi at room temperature. Finally, representative images were obtained, and the positive rate of CX43 and Nrf2 expressions was analyzed using Image J software (n = 5 for each group).
2.8. Cell culture and treatment
H9C2 cells (Procell Life Technologies Inc) were cultured in Dulbecco's modified Eagle medium/F12 medium containing 10 % fetal bovine serum and 1 % penicillin/streptomycin at 37 °C in 5 % CO2. The cells were divided into six groups: (1) control; (2) control + NBQX (40 μM); (3) control + ML385 (40 μM); (4) control + H2O2 (200 μM); (5) control + H2O2 + NBQX; (6) control + H2O2 + ML385. NBQX or ML385 was added to the medium for 6 h before oxidative stress was induced by the addition of H2O2 for 3 h. The drug concentrations and processing times were determined in accordance with a previous study [35].
2.9. Biochemical detection
Serum obtained from all rats and treated H9C2 cells were measured for malondialdehyde (MDA) and superoxide dismutase (SOD) alterations, following the manufacturer's protocol (SOD assay kit, A003-1-2; MDA assay kit, A001-1-2; Nanjing Jiancheng Bioengineering Institute). Briefly, animal serums were centrifuged at 3500 RPM/min for 10 min. For cell samples, digestion was performed with 0.25 % pancreatic enzyme at room temperature for 2 min. Serum was then added to terminate digestion, and all liquids were gently blown with a micropipette. The mixture was then transferred to an EP tube and centrifuged at 1000 RPM for 10 min to pellet the cells for further use. Enzyme was added to the working solution containing samples according to the instructions; the orifice was gently shaken to mix reaction solution, and the mixture was incubated at 37 °C for 20 min. Finally, the absorbance was measured with an enzyme reader.
2.10. Western blotting
Fresh LV myocardia tissues and H9C2 cells were lysed in radioimmunoprecipitation assay lysis buffer (G2002; Servicebio) to obtain total tissue and cell protein. The protein concentrations were determined using a bicinchoninic acid protein assay kit (G2026; Servicebio). Subsequently, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (G2042; Servicebio) was performed to separate proteins which were transferred to polyvinylidene difluoride membranes (EMD Millipore). The membranes were then blocked and immunoblotted with specific primary antibodies at 4 °C overnight. Enhanced chemiluminescence solution (G2020; Servicebio) was used to visualize the protein bands. All bands were analyzed using ImageJ software, and GAPDH was used for normalization.
2.11. ROS measurement
Dihydroethidium (DHE) staining was performed on LV tissue slices and H9C2 cells. Immunofluorescence was carried out to determine the production of ROS. LV tissue sections and H9C2 cells cultured in 6-well plates were co-incubated with DHE, and fluorescence images were obtained using a fluorescence microscope (Olympus, Tokyo, Japan).
2.12. Statistical analysis
Statistical analyses were performed using GraphPad Prism 9.0 (GraphPad, CA, USA). All data were presented as mean ± standard error of the mean. The difference between the two groups was determined using the unpaired Student t-test. Differences between multiple groups were analyzed using oneway analysis of variance (ANOVA). A p-value of <0.05 was considered to indicate a significant difference.
3. Results
3.1. NBQX ameliorated anxiety in rat models of anxiety
OFT results showed that NBQX significantly reduced anxiety symptoms in AD rats (Fig. 1A–D). Fig. 1B showed the representative images of movement path. As Fig. 1C–E indicated, the model rats entered less frequently than control rats, and NBQX administration could reverse this phenomenon (CTL vs AD vs ADN: 9.17 ± 0.778 vs 1.57 ± 0.481 vs 6.57 ± 0.751, p < 0.05). AD rats spent more time in the peripheral zone than CTL rat,and the NBQX-treated group rats became more active in the central zone (CTL vs AD vs ADN: 17.64 ± 1.843 vs 2.649 ± 1.65 vs 13.45 ± 2.017, p < 0.05). The number of rearing significantly decreased in AD group compared with CTL (35.78 ± 0.703 vs 12.44 ± 0.338, p < 0.05). The aforementioned variables were significantly mitigated in the ADN group compared with the AD group (12.44 ± 0.338 vs 27.56 ± 0.445, p < 0.05).
Fig. 1.
NBQX alleviated anxiety behaviors in AD rats. (A) Schematic representation of the behavioral test (n = 9 per group). (B) Representative heat map images highlighting the motion trails of SD rats in the open field test (n = 9 in each group). (C) Frequency of rats entering the central zone (n = 9 per group). (D) Cumulative duration of rats in the center zone (n = 9 per group). (E) Number of rearing instances in 5 mi of testing (n = 9 per group). Data are expressed as mean ± SD, ****p < 0.0001, **p < 0.01.
3.2. NBQX conferred protective effects on HRV in rat models of anxiety
NBQX treatment improved time domain parameters in rat models of anxiety, including average RR (143.1 ± 3.518 vs 1.275 ± 0.049, p < 0.05) and RMSSD (0.778 + 0.024 vs 1.275 ± 0.049, p < 0.05), which were reduced employing empty bottle stimulation Fig. 2 (Fig. 2A–B). Additionally, frequency domain parameters (LF, HF, and LF/HF) were restored by NBQX (AD vs ADN:13.38 ± 1.716 vs 6.706 ± 1.226, p < 0.05; 70.38 ± 0.389 vs 77.96 ± 0.407, p < 0.05; 0.202 ± 0.019 vs 0.082 ± 0.011, p < 0.05). The HF levels were significantly higher in the AD group than in the CTL and CTN groups (Fig. 2C), while NBQX reversed this alteration. LF and HF/LF increased in AD rats but were reduced after NBQX administration (Fig. 2D–E).
Fig. 2.
NBQX treatment alleviated heart rate variability (HRV). (A–B) Statistical analysis of RR and RMSSD (n = 9). (C–E) Statistical data of HF, LF, and LF/HF in all groups (n = 9). *p < 0.05, **p < 0.01, ***p < 0.001.
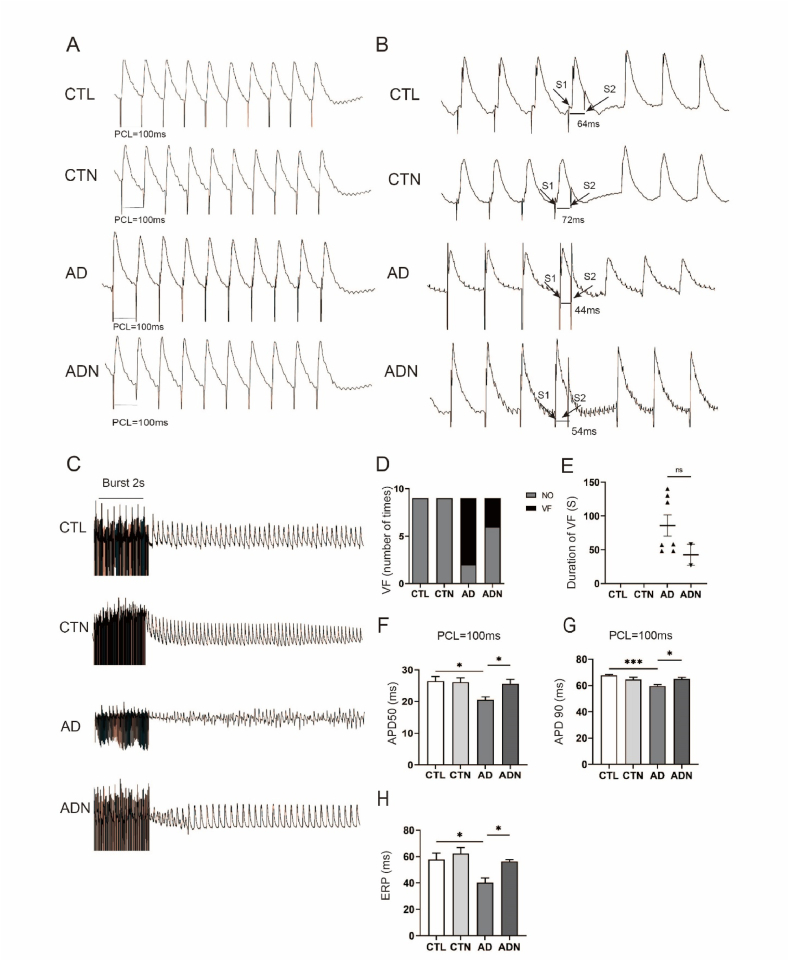
3.3. NBQX ameliorated ventricular electrical remodeling in rat models of anxiety
Fig. 3 (Fig. 3A–C) illustrates the changes in APD50 (20.58 ± 0.868 vs 25.60 ± 1.352, p < 0.05) and APD90 (59.60 ± 1.024 vs 64.99 ± 1.152, p < 0.05) at PCL = 100 ms in AD rats and the mechanism through which NBQX rescued them. A significant decrease in APDs was observed in AD group, which was reversed with NBQX treatment. The ERP of the AD group was shorter than that of CTL rats (40.2 ± 3.645 vs 57.8 ± 4.848, p < 0.05), while NBQX ameliorated this metastasis (40.2 ± 3.645 vs 56.2 ± 1.413, p < 0.05) (Fig. 3B). No significant differences were observed between the CTL and CTN groups.
Fig. 3.
Effects of NBQX on ventricular electrical remodeling. (A) Typical record of APD50/90 at PCL = 100 ms. (B) Representative images of the effective refractory period (ERP) in different groups. (C) Typical record of ventricular fibrillation (VF) in each group. (D, E) Incidence rate and duration of VF in the AD and ADN groups. (F, G) Statistical analysis of APDs at PCL = 100 ms. (H) Statistical analysis of ERP in the four groups. n = 9 per group. *p < 0.05, **p < 0.01, ***p < 0.001.
As shown in Fig. 3, NBQX significantly reduced VF susceptibility in AD group as determined using the unpaired Student's t-test to analyze the difference. After the burst pacing procedure, VF bursts were continuously induced in AD rats, with an incidence rate of approximately 77.8 % (n = 7/9). NBQX treatment reduced this incidence to 33.3 % (n = 3/9). However, no VF bursts were induced in CTL and CTN groups (Fig. 3D). Moreover, the VF duration in AD group was longer than that in ADN rats (Fig. 3E).
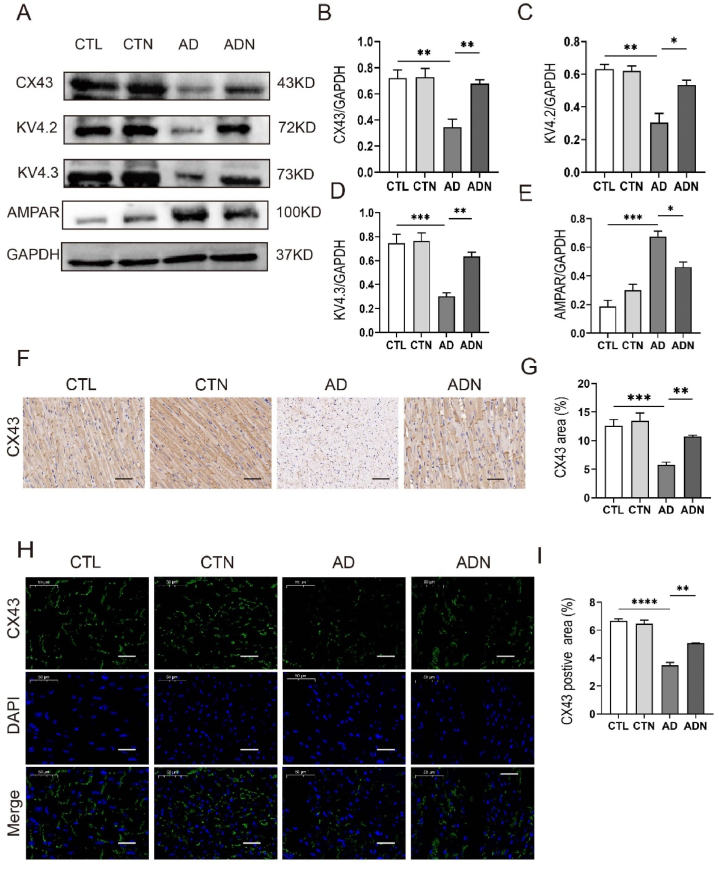
3.4. NBQX upregulated CX43 and ion channel expression
NBQX significantly improved CX43 expression in ADN rats compared with that in AD rats. However, no difference was observed between CTL rats and CTN rats (Fig. 4A–B, F-I). Additionally, western blotting results showed that after NBQX treatment, the expression of the ion channel proteins KV4.2 and KV4.3 was upregulated in AD model rats, with no significant change in CTL group rats (Fig. 4A–D). Furthermore, AMPAR was activated in AD rats, and NBQX could inhibited its expression (Fig. 4A).
Fig. 4.
NBQX enhanced the expression of CX43 and ion channel proteins. (A) Representative immunoblotting images of protein CX43, KV4.2, KV4.3, AMPAR. (B–E) Immunoblotting quantitative analysis of KV4.2, KV4.3, CX43, and AMPAR expression(n = 3 per group). (F, G) Typical images of CX43 in immunohistochemical staining and quantitative analysis of the CX43 area; scale bar = 50 μm (n = 5 in each group). (H, I) Immunofluorescence staining and quantitative analysis of CX43 expression; scale bar = 50 μm (n = 5 per group)., **p < 0.01, ***p < 0.001.
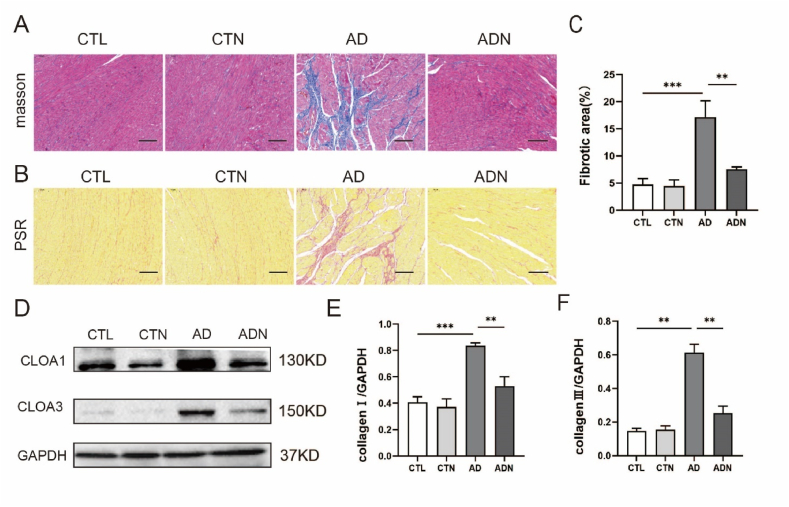
3.5. NBQX ameliorated ventricular fibrosis in rat models of anxiety
Masson's trichrome staining images (Fig. 5A) showed that the rat models of AD exhibited ventricular fibrosis compared with the CTL group, and an improvement in fibrosis was observed in ADN rats (CTL vs AD vs ADN: 4.73 ± 1.083 vs 17.16 ± 3.008 vs 7.54 ± 0.429, p < 0.05) as indicated by the quantitative analysis of fibrotic area (Fig. 5C). Moreover, the expression of collagen I and collagen III were reduced after administration of NBQX (Fig. 5D–F).
Fig. 5.
Effects of NBQX on ameliorating ventricular fibrosis. (A) Representative images of tissues stained with Masson's trichrome stain (n = 5 in each group); scale bar = 50 μm. (B) Representative images of Sirius red staining (n = 5 in each group); scale bar = 50 μm. (C) Percentage of fibrotic area. (D–F) Typical images of immunoblotting and quantitative analyses of collagen Ⅰ and collagen Ⅲ (n = 3 per group). *p < 0.05, **p < 0.01, ***p < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
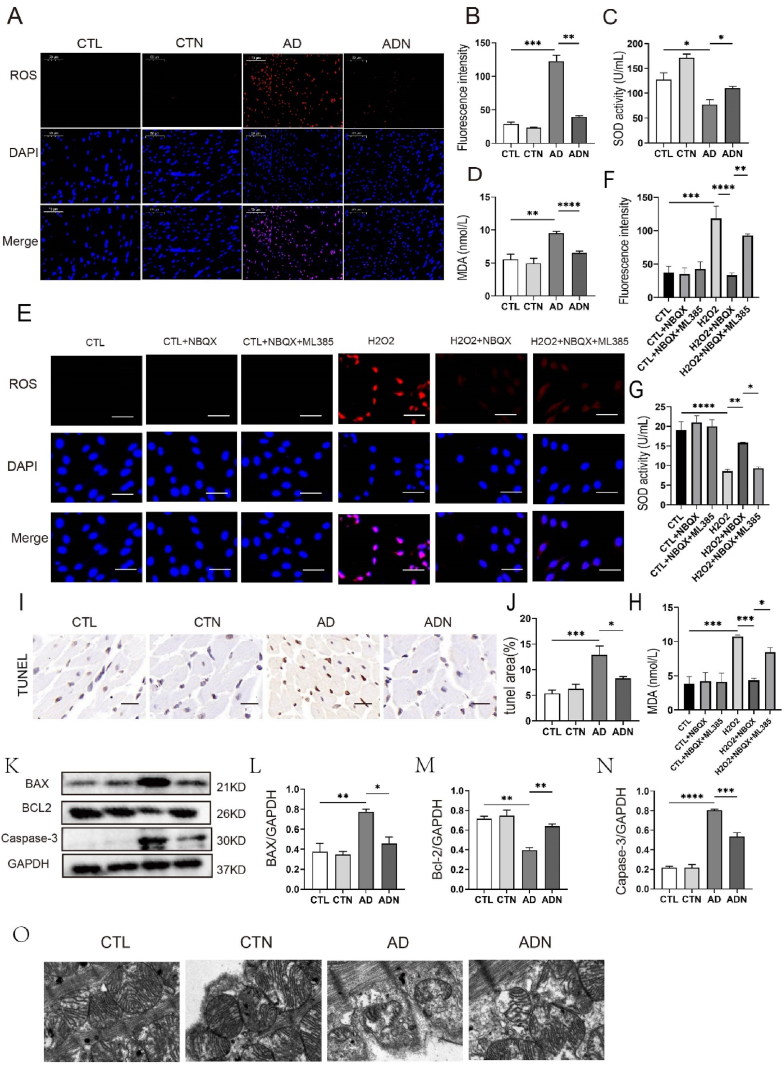
3.6. Oxidative stress level was elevated in rat models of anxiety and NBQX could ameliorate oxidative stress and apoptosis in vivo and in vitro
Fig. 6A shows the images of DHE staining performed on the LV sections of rats. The results revealed elevated ROS levels in the AD group, which were decreased following NBQX treatment (122.6 ± 8.879 vs 39.16 ± 2.031, p < 0.05). Additionally, MDA and SOD levels in the serum of SD rats indicated increased oxidative stress in AD rats, which was ameliorated by NBQX treatment (MDA: 9.506 ± 0.279 vs 6.497 ± 0.310, p < 0.05) (SOD: 77.29 ± 9.898 vs 110.0 + 3.889, p < 0.05) (Fig. 6C and D). TUNEL immunohistochemical staining and electron microscope also suggested that apoptosis occurred in AD group and NBQX mitigated apoptosis progression (Fig. 6I, J, O), with BAX, Bcl-2 and Caspase-3 expressions showing consistent results (Fig. 6K–N).
Fig. 6.
NBQX reduced oxidative stress levels and apoptosis in vivo and in vitro. (A) Representative images of reactive oxygen species (ROS) in left ventricle (LV) tissues. n = 5 per group. (B) Relative fluorescence intensity of ROS in LV. n = 5 per group, bar = 50 μm. (C, D) Concentration of malondialdehyde (MDA) and superoxide dismutase (SOD) activity in the serum. n = 12 per group. (E) Representative images of ROS in H9C2 cells. n = 5 per group; scale bar = 50 μm) (F) Relative fluorescence intensity of ROS in H9C2 cells. n = 5 per group. (G, H) Concentration of malondialdehyde (MDA) and superoxide dismutase (SOD) activity in H9C2 cell. n = 5 per group. (I, J) Typical images of TUNEL immunohistochemical staining and quantitative analysis of the positive area. n = 5; scale bar = 50 μm. (K–N) Immunoblotting and quantitative analysis of BAX, Bcl-2 and Capase-3 in LV. n = 3. (O) Representative images of electron microscopy: In the myocardium of AD rats, mitochondrial was swollen and dissolved, which was reversed following NBQX treatment. n = 5; scale bar = 500 nm *p < 0.05, **p < 0.01, ***p < 0.001.
As shown in (Fig. 6E–H), MDA and ROS levels in H9C2 cells stimulated by H2O2 were significantly enhanced, but pre-treatment with NBQX reduced these levels compared to those in the model group. SOD activity decreased in the H2O2 group but increased in the H2O2+NBQX group (Fig. 6G).
3.7. NBQX downregulated oxidative stress by regulating the Nrf2/HO-1 pathway
The Nrf2/HO-1 pathway plays a crucial role in cellular antioxidant effects [36]. We observed that the expression of Nrf2 and HO-1 significantly decreased in the AD group, but NBQX treatment increased their expression (Fig. 7A–F). These findings suggest that the Nrf2/HO-1 pathway may be linked to reducing VF susceptibility in the ADN group, as supported by Nrf2 immunofluorescence staining in LV. To confirm NBQX's antioxidant effect, we used an Nrf2 inhibitor (ML385). As previously described, SOD and MDA levels were measured in H9C2 cells after pretreatment with ML385 or NBQX and exposure to H2O2. The results indicated that the expression of Nrf2 and HO-1 significantly decreased in the H2O2 group and increased in the NBQX-treated group, but this effect was suppressed after ML385 treatment (Fig. 7G–I).
Fig. 7.
NBQX downregulated oxidative stress by regulating the Nrf2/HO-1 pathway. (A–D) Immunoblotting and quantitative analyses of Nrf2, HO-1, and NOX4 in LV, n = 3 per group. (E–F) Representative immunofluorescence staining images and positive rate of Nrf2 in LV. n = 5, bar = 50 μm. (G–I) Immunoblotting and quantitative analysis of Nrf2 and HO-1, n = 3. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
4. Discussion
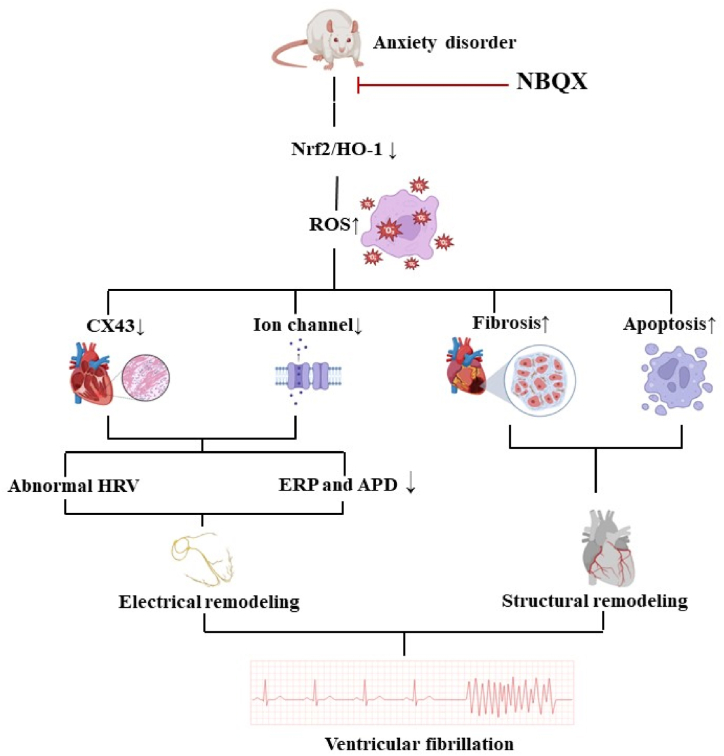
In AD rats, elevated oxidative stress levels and activated AMPA receptors led to electrical and structural remodeling, thus increasing susceptibility to VF. NBQX administration reversed this effect by activating the Nrf2/HO-1 pathway (Fig. 8).
Fig. 8.
Summary of the effects of NBQX on reducing ventricular fibrillation susceptibility in rat models of anxiety and its underlying mechanisms.
Our study demonstrated the effectiveness of NBQX in ameliorating VF vulnerability and anxiety in rats. As shown in Figure, NBQX mitigated fibrosis in the ventricles of AD rats and increased the levels of CX43 and ion channel proteins, thus improving VF sensitivity, potentially through the activation of the Nrf2/HO-1 pathway.
Electrical remodeling of the heart can affect the rhythm of contraction, leading to thromboembolism or sudden cardiac death due to arrhythmia [37]. APD represents the time from depolarization to repolarization, followed by ERP. In patients with arrhythmia, the relationship between APD and ERP changes [38]. In patients with VF, the variability of APD is more significant [39,40]. Some studies have found that the durations of APD and ERP in patients with VF is significantly shortened [41]. The results of our study are consistent with those of previous studies. ERP and APD of the LV decreased in AD rats, and were prolonged after treatment with NBQX.
Arrhythmia is primarily caused by abnormal electrical signal conduction. Electrical signals propagate between adjacent cardiomyocytes mainly through gap junction proteins such as CX43. The abnormal distribution and expression of CX43 are common in the hearts of patients with arrhythmia. As shown in Fig. 5, CX43 expression was significantly decreased in the ventricular tissue of AD rats, potentially contributing to VF sensitivity [42]. The reduction in CX43 expression was partially reversed with NBQX treatment.
The stability of cardiac electrical signals closely depends on the regulation of ion channels in the cell membrane. In the heart, more than 10 types of potassium channels play a role in forming action potentials [43]. During VF, the most significant change in potassium channels is the decrease in repolarization, mainly because of the reduced expression of the potassium channel protein subunit KV4.3 [44,45]. Decreased KV4.2 expression has been linked to arrhythmia development [46]. Our research demonstrated that empty bottle stimulation can notably inhibit KV4.2 and KV4.3 expression. Following NBQX administration, the expression of ion channel proteins expression increased.
Continuous pathological stimulation can cause structural changes in the heart, including myocardial fibrosis. In patients with arrhythmias, ventricular cardiomyocytes undergoing atrophy and apoptosis are replaced by fibrous tissue. This pathological process progresses from the epicardium to the endocardium. As the disease advances, nonfunctional fibrous tissue replaces most cardiomyocytes, increasing the susceptibility to arrhythmia [47]. As shown in Fig. 5, fibrosis worsened in AD rats, and NBQX could ameliorate fibrosis progression of.
Oxidative stress refers to an imbalance between pro-oxidants and antioxidants [48]. Elevated oxidative stress levels are associated with various neurological disorders, such as AD [49,50]. They may be linked to the excessive production of ROS and reactive nitrogen species brain microglia are overactivated [51]. Our study found that in the AD group, ROS production was accompanied by an increase in MDA levels, whereas SOD activity showed a declining trend, indicating that anxiety can induce a stressed state in the body. Abnormal oxidative stress significantly improved after NBQX treatment.
ROS, a general process involving various chemicals including hydrogen peroxide, nitric oxide, and hypochlorous acid, is primarily produced during cell metabolism [52]. Elevated ROS levels significantly increase the risk of arrhythmia [53]. Currently, many antioxidant drugs are used to treat anti-arrhythmic disorders [54,55]. Most cardiovascular diseases are associated with an abnormal mitochondrial membrane potential, affecting energy metabolism and cardiomyocyte REDOX homeostasis [56]. Mitochondria supply energy for cardiomyocyte activity, and the electron transport chain complex involved in ATP synthesis is the primary source of ROS.
When the heart undergoes pathological changes, such as cardiac hypertrophy or core muscle ischemia, ROS levels increase. ROS disorders also affect ATP levels, creating a vicious circle [57]. Oxidative stress can affect the function of ion channels in cardiomyocytes and cause impairment of CX43 distribution and expression [58,59]. This finding aligns with our results: in the AD group, ROS levels significantly increased, accompanied by decreased expression levels of KV4.2, KV4.3, and CX43 in the ventricular muscle. Therefore, AD can significantly increase oxidative stress levels, ultimately increasing VF susceptibility by inducing electrical remodeling of the heart.
Furthermore, heart oxidative stress can accelerate heart fibrosis [60]. The accumulation of OS in cardiomyocytes increases extracellular matrix deposition in the myocardium, which promotes fibroblast proliferation, cardiomyocyte destruction, and myocardial fibrosis progression. Additionally, elevated ROS levels can cause DNA strand breaks in myocardial nuclei and accelerate apoptosis [61]. ROS can also increase of COLA-Ⅰ and COLA-Ⅲ expression in the heart, further exacerbating fibrosis changes [62].
Nrf2/HO-1 is a crucial molecular pathway in the response to oxidative stress, involved in apoptosis and anti-inflammatory/antioxidant processes [63]. Our results showed that in AD rats, apoptosis worsened, leading to myocardial fibrosis [64], and NBQX treatment could rescue it. Nrf2 is a transcription factor that regulates cellular oxidative stress levels. Under normal conditions, Nrf2 and Keap1 form complexes and remain in a low-activity state. When oxidative stress levels increase, Nrf2 is phosphorylated and is released from the complex, forming heterodimers with Maf protein and Jun-bZip transcription factor in the nucleus [65]. Nrf2 plays a protective role against neuro-excitotoxicity caused by oxidative stress. HO-1 is an antioxidant enzyme directly regulated by Nrf2 and plays anti-inflammatory and antioxidant roles by converting hemoglobin into CO and Fe2+ [66]. The Nrf2-HO1 pathway plays a positive role in ameliorating anxiety symptoms [67] and suppressing VF [68]. Jin et al. reported that by upregulating the expression of Nrf2 and HO-1, the phosphorylation of calmodulin-dependent protein kinase-II (CaMKII) is enhanced, thus playing a role in alleviating heart diseases [69]. Our results are consistent with this finding. in the AD group, VF susceptibility increased with a reduction in Nrf2/HO-1 protein expression, and NBQX administration could rescue this phenomenon. Moreover, the effect of NBQX was diminished by ML385 in H9C2 cells, demonstrating that the Nrf2-HO-1 pathway contributed to the protection of NBQX in the cardiovascular system.
5. Conclusion
This study demonstrated that NBQX reduces VF susceptibility in rats with AD by addressing electrical remodeling, structural remodeling, and oxidative stress through the Nrf2-HO-1 pathway. Therefore, NBQX holds promise as a treatment for patients with AD and VF.
Data availability
The data associated with this study was not deposited into a publicly available repository for data will be made available on request.
Ethics approval and consent to participate
We ensured that this study was performed in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines. All animal experiments in our research abided by the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. All animal procedures were approved by The Ethics Committee of Wuhan University People's Hospital and the approved number was 20221003F.
Funding
This work was granted by the National Natural Science Foundation of China (No. 82170316).
CRediT authorship contribution statement
Yiqian Hu: Writing – original draft, Visualization, Validation, Data curation. Chuan Qu: Writing – review & editing. Ying Zou: Investigation. Xin Liu: Software. Cui Zhang: Supervision, Project administration. Bo Yang: Resources, Funding acquisition, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e37358.
Contributor Information
Cui Zhang, Email: cuizhang2005@126.com.
Bo Yang, Email: yybb112@whu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Reddy Y.N.V., Borlaug B.A., Gersh B.J. Management of atrial fibrillation across the spectrum of heart failure with preserved and reduced ejection fraction. Circulation. 2022;146(4):339–357. doi: 10.1161/CIRCULATIONAHA.122.057444. [DOI] [PubMed] [Google Scholar]
- 2.Fye W.B. Ventricular fibrillation and defibrillation: historical perspectives with emphasis on the contributions of John MacWilliam, Carl Wiggers, and William Kouwenhoven. Circulation. 1985;71(5):858–865. doi: 10.1161/01.cir.71.5.858. [DOI] [PubMed] [Google Scholar]
- 3.Handa B.S., Li X., Baxan N., Roney C.H., Shchendrygina A., Mansfield C.A., Jabbour R.J., Pitcher D.S., Chowdhury R.A., Peters N.S., Ng F.S. Ventricular fibrillation mechanism and global fibrillatory organization are determined by gap junction coupling and fibrosis pattern. Cardiovasc. Res. 2021;117(4):1078–1090. doi: 10.1093/cvr/cvaa141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliver M.F. Metabolic causes and prevention of ventricular fibrillation during acute coronary syndromes. Am. J. Med. 2002;112(4):305–311. doi: 10.1016/s0002-9343(01)01104-4. [DOI] [PubMed] [Google Scholar]
- 5.Peretto G., Sala S., Rizzo S., Palmisano A., Esposito A., De Cobelli F., Campochiaro C., De Luca G., Foppoli L., Dagna L., Thiene G., Basso C., Della Bella P. Ventricular arrhythmias in myocarditis. J. Am. Coll. Cardiol. 2020;75(9):1046–1057. doi: 10.1016/j.jacc.2020.01.036. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh A., Shcherbik N. Effects of oxidative stress on protein translation: implications for cardiovascular diseases. Int. J. Mol. Sci. 2020;21(8) doi: 10.3390/ijms21082661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoge E.A., Ivkovic A., Fricchione G.L. Generalized anxiety disorder: diagnosis and treatment. Bmj. 2012;345(nov27 2) doi: 10.1136/bmj.e7500. [DOI] [PubMed] [Google Scholar]
- 8.Penninx B.W.J.H., Pine D.S., Holmes E.A., Reif A. Anxiety disorders. Lancet. 2021;397(10277):914–927. doi: 10.1016/S0140-6736(21)00359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams K.L., Edwards A., Peart C., Ellett L., Mendes I., Bird G., Murphy J. The association between anxiety and cardiac interoceptive accuracy: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2022;140 doi: 10.1016/j.neubiorev.2022.104754. [DOI] [PubMed] [Google Scholar]
- 10.Peacock J., Whang W. Psychological distress and arrhythmia: risk prediction and potential modifiers. Prog. Cardiovasc. Dis. 2013;55(6):582–589. doi: 10.1016/j.pcad.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glassman A.H., Bigger J.T. Antipsychotic drugs: prolonged QTc interval, torsade de pointes, and sudden death. Am.j.psychiatry. 2001;158(11):1774–1782. doi: 10.1176/appi.ajp.158.11.1774. [DOI] [PubMed] [Google Scholar]
- 12.Vieweg W.V.R., Hasnain M., Howland R.H., Hettema J.M., Kogut C., Wood M.A., Pandurangi A.K. Citalopram, QTc interval prolongation, and torsade de pointes. How should we apply the recent FDA ruling? Am. J. Med. 2012;125(9):859–868. doi: 10.1016/j.amjmed.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Albert C.M., Chae C.U., Rexrode K.M. Phobic anxiety and risk of coronary heart disease and sudden cardiac death among women. Circulation. 2005;14(6):5–6. doi: 10.1161/01.CIR.0000153813.64165.5D. [DOI] [PubMed] [Google Scholar]
- 14.Moylan S., Eyre H.A., Maes M., Baune B.T., Jacka F.N., Berk M. Exercising the worry away: how inflammation, oxidative and nitrogen stress mediates the beneficial effect of physical activity on anxiety disorder symptoms and behaviours. Neurosci. Biobehav. Rev. 2013;37(4):573–584. doi: 10.1016/j.neubiorev.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Deng Y., Liu F., Yang X., Xia Y. The key role of uric acid in oxidative stress, inflammation, fibrosis, apoptosis, and immunity in the pathogenesis of atrial fibrillation. Frontiers in Cardiovascular Medicine. 2021;8 doi: 10.3389/fcvm.2021.641136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu S., Gouaux E.J.N. Structure and symmetry inform gating principles of ionotropic glutamate receptors. 2017;112(Pt A):11–15. doi: 10.1016/j.neuropharm.2016.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu Y.Y., Li L., Xian X.H., Zhang M., Sun X.C., Li S.Q., Cui X., Qi J., Li W.B.J.C.P.D. GLT-1 upregulation as a potential therapeutic target for ischemic brain injury. 2017;23(33) doi: 10.2174/1381612823666170622103852. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z.Y., Zhong Q.W., Tian C.N., Ma H.M., Yu J.J., Hu S.J.J.o.C.B. 2019. NMDA Receptor‐driven Calcium Influx Promotes Ischemic Human Cardiomyocyte Apoptosis through a P38 MAPK‐mediated Mechanism. [DOI] [PubMed] [Google Scholar]
- 19.Tseng M.T.J.A.H., Physiology C. Mitochondrial matrix metalloproteinase activation decreases myocyte contractility in hyperhomocysteinemia. 2008;295(2):H890–H897. doi: 10.1152/ajpheart.00099.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie D., Xiong K., Su X., Wang G., Ji Q., Zou Q., Wang L., Liu Y., Liang D., Xue J., Wang L., Gao X., Gu X., Liu H., He X., Li L., Yang J., Lu Y., Peng L., Chen Y.-H. Identification of an endogenous glutamatergic transmitter system controlling excitability and conductivity of atrial cardiomyocytes. Cell Res. 2021;31(9):951–964. doi: 10.1038/s41422-021-00499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonnet C.S., Williams A.S., Gilbert S.J., Harvey A.K., Evans B.A., Mason D.J.J.A.o.t.R.D. AMPA/kainate glutamate receptors contribute to inflammation, degeneration and pain related behaviour in inflammatory stages of arthritis. 2015;74(1):242. doi: 10.1136/annrheumdis-2013-203670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonnet C.S., Williams A.S., Gilbert S.J., Harvey A.K., Evans B.A., Mason D.J. AMPA/kainate glutamate receptors contribute to inflammation, degeneration and pain related behaviour in inflammatory stages of arthritis. Ann. Rheum. Dis. 2015;74(1):242. doi: 10.1136/annrheumdis-2013-203670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawakita F., Kanamaru H., Asada R., Imanaka-Yoshida K., Yoshida T., Suzuki H. 2021. Inhibition of AMPA (α-Amino-3-Hydroxy-5-Methyl-4-Isoxazole Propionate) Receptor Reduces Acute Blood–Brain Barrier Disruption after Subarachnoid Hemorrhage in Mice. [DOI] [PubMed] [Google Scholar]
- 24.Shah S.A., Yoon G.H., Kim M.O.J.M.N. Protection of the Developing Brain with Anthocyanins Against Ethanol-Induced Oxidative Stress and Neurodegeneration. 2015;51(3):1278–1291. doi: 10.1007/s12035-014-8805-7. [DOI] [PubMed] [Google Scholar]
- 25.Lees G.J. Pharmacology of AMPA/kainate receptor ligands and their therapeutic potential in neurological and psychiatric disorders. Drugs. 2000;59(1):33–78. doi: 10.2165/00003495-200059010-00004. [DOI] [PubMed] [Google Scholar]
- 26.Mu X., Azbill R.D., Springer J.E. NBQX treatment improves mitochondrial function and reduces oxidative events after spinal cord injury. J. Neurotrauma. 2002;19(8):917–927. doi: 10.1089/089771502320317078. [DOI] [PubMed] [Google Scholar]
- 27.Teng Y.D., Wrathall J.R. Evaluation of cardiorespiratory parameters in rats after spinal cord trauma and treatment with NBQX, an antagonist of excitatory amino acid receptors. Neurosci. Lett. 1996;209(1):5–8. doi: 10.1016/0304-3940(96)12601-x. [DOI] [PubMed] [Google Scholar]
- 28.Kapus G.L., Gacsályi I., Vegh M., Kompagne H., Hegedűs E., Leveleki C., Hársing L.G., Barkóczy J., Bilkei-Gorzó A., Lévay G. Antagonism of AMPA receptors produces anxiolytic-like behavior in rodents: effects of GYKI 52466 and its novel analogues. Psychopharmacology. 2008;198(2):231–241. doi: 10.1007/s00213-008-1121-z. [DOI] [PubMed] [Google Scholar]
- 29.Deshmukh P., Unni S., Krishnappa G., Padmanabhan B. The Keap1–Nrf2 pathway: promising therapeutic target to counteract ROS-mediated damage in cancers and neurodegenerative diseases. Biophysical Reviews. 2017;9(1):41–56. doi: 10.1007/s12551-016-0244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koh S., Tibayan F.D., Simpson J.N., Jensen F.E. NBQX or topiramate treatment after perinatal hypoxia-induced seizures prevents later increases in seizure-induced neuronal injury. Epilepsia. 2004;45(6):569–575. doi: 10.1111/j.0013-9580.2004.69103.x. [DOI] [PubMed] [Google Scholar]
- 31.Liu C., Ying Z., Li Z., Zhang L., Li X., Gong W., Sun J., Fan X., Yang K., Wang X., Wei S., Dong N. Danzhi xiaoyao powder promotes neuronal regeneration by downregulating notch signaling pathway in the treatment of generalized anxiety disorder. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.772576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshizaki K., Asai M., Hara T.J.N. High-fat diet enhances working memory in the Y-maze test in male C57bl/6J mice with less anxiety in the elevated Plus maze test. 2020;12(7):2036. doi: 10.3390/nu12072036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ng G.A., Brack K.E., Patel V.H., Coote, John H. Autonomic modulation of electrical restitution, alternans and ventricular fibrillation initiation in the isolated heart. Cardiovasc. Res. 2007;73(4):750–760. doi: 10.1016/j.cardiores.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Wang D., Liu T., Shi S., Li R., Shan Y., Huang Y., Hu D., Huang C. Chronic administration of catestatin improves autonomic function and exerts cardioprotective effects in myocardial infarction rats. J. Cardiovasc. Pharmacol. Therapeut. 2016:526. doi: 10.1177/1074248416628676. [DOI] [PubMed] [Google Scholar]
- 35.Wang H., Jiang Z., Pang Z., Qi G., Hua B., Yan Z., Yuan H. Engeletin protects against TNF-α-induced apoptosis and reactive oxygen species generation in chondrocytes and alleviates osteoarthritis in vivo. J. Inflamm. Res. 2021;14:745–760. doi: 10.2147/JIR.S297166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiang Q., Zhao Y., Lin J., Jiang S., Li W. The Nrf2 antioxidant defense system in intervertebral disc degeneration: molecular insights. Exp. Mol. Med. 2022;54(8):1067–1075. doi: 10.1038/s12276-022-00829-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mason F.E., Pronto J.R.D., Alhussini K., Maack C., Voigt N. Cellular and mitochondrial mechanisms of atrial fibrillation. Basic Res. Cardiol. 2020;115(6) doi: 10.1007/s00395-020-00827-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaw R.M., Rudy Y. Electrophysiologic effects of acute myocardial ischemia: a theoretical study of altered cell excitability and action potential duration. Circ. Res. 1997;35(1):124–138. doi: 10.1016/s0008-6363(97)00093-x. [DOI] [PubMed] [Google Scholar]
- 39.Huang J., Zhou X., Smith W.M., Ideker R.E. Restitution properties during ventricular fibrillation in the in situ swine heart. Circulation. 2004;110(20):3161–3167. doi: 10.1161/01.CIR.0000147618.93579.56. [DOI] [PubMed] [Google Scholar]
- 40.Xie F., Qu Z., Yang J., Baher A., Weiss J.N., Garfinkel A. A simulation study of the effects of cardiac anatomy in ventricular fibrillation. J. Clin. Invest. 2004;113(5):686–693. doi: 10.1172/JCI17341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiong L., Liu Y., Zhou M., Wang G., Quan D., Shen C., Shuai W., Kong B., Huang C., Huang H. Targeted ablation of cardiac sympathetic neurons improves ventricular electrical remodelling in a canine model of chronic myocardial infarction. EP Europace. 2018;20(12):2036–2044. doi: 10.1093/europace/euy090. [DOI] [PubMed] [Google Scholar]
- 42.Bezzerides V.J., Pu W.T. Two sides of the same coin: new insights into mechanisms of ventricular fibrillation. Cardiovasc. Res. 2021;117(4):983–984. doi: 10.1093/cvr/cvaa246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmitt N., Grunnet M., Olesen S.-P. Cardiac potassium channel subtypes: new roles in repolarization and arrhythmia. Physiol. Rev. 2014;94(2):609–653. doi: 10.1152/physrev.00022.2013. [DOI] [PubMed] [Google Scholar]
- 44.Varró A., Tomek J., Nagy N., Virág L., Passini E., Rodriguez B., Baczkó I. Cardiac transmembrane ion channels and action potentials: cellular physiology and arrhythmogenic behavior. Physiol. Rev. 2021;101(3):1083–1176. doi: 10.1152/physrev.00024.2019. [DOI] [PubMed] [Google Scholar]
- 45.Soltysinska E., Olesen S.-P., Christ T., Wettwer E., Varró A., Grunnet M., Jespersen T. Transmural expression of ion channels and transporters in human nondiseased and end-stage failing hearts. Pflueg. Arch. Eur. J. Physiol. 2009;459(1):11–23. doi: 10.1007/s00424-009-0718-3. [DOI] [PubMed] [Google Scholar]
- 46.Blandin C.E., Gravez B.J., Stéphane N.H., Balse E. Remodeling of ion channel trafficking and cardiac arrhythmias. Cells. 2021;10(9):2417. doi: 10.3390/cells10092417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Derynck R., Zhang Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425(6958):577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 48.Lichtenberg D., Pinchuk I. Oxidative stress, the term and the concept. Biochem. Biophys. Res. Commun. 2015;461(3):441–444. doi: 10.1016/j.bbrc.2015.04.062. [DOI] [PubMed] [Google Scholar]
- 49.Salim S. Oxidative stress and the central nervous system. J. Pharmacol. Exp. Therapeut. 2017;360(1):201–205. doi: 10.1124/jpet.116.237503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hovatta I., Juhila J., Donner J. Oxidative stress in anxiety and comorbid disorders. Neurosci. Res. 2010;68(4):261–275. doi: 10.1016/j.neures.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 51.Wilkinson B.L., Landreth G.E. J. Neuroinflammation. 2006;3(1) doi: 10.1186/1742-2094-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murphy Michael P., Holmgren A., Larsson N.-G., Halliwell B., Chang Christopher J., Kalyanaraman B., Rhee Sue G., Thornalley Paul J., Partridge L., Gems D., Nyström T., Belousov V., Schumacker Paul T., Winterbourn Christine C. Unraveling the biological roles of reactive oxygen species. Cell Metabol. 2011;13(4):361–366. doi: 10.1016/j.cmet.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zakkar M., Ascione R., James A.F., Angelini G.D., Suleiman M.S. Inflammation, oxidative stress and postoperative atrial fibrillation in cardiac surgery. Pharmacol. Therapeut. 2015;154:13–20. doi: 10.1016/j.pharmthera.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 54.Kondo H., Akoumianakis I., Badi I., Akawi N., Kotanidis C.P., Polkinghorne M., Stadiotti I., Sommariva E., Antonopoulos A.S., Carena M.C., Oikonomou E.K., Reus E.M., Sayeed R., Krasopoulos G., Srivastava V., Farid S., Chuaiphichai S., Shirodaria C., Channon K.M., Casadei B., Antoniades C. Effects of canagliflozin on human myocardial redox signalling: clinical implications. Eur. Heart J. 2021;42(48):4947–4960. doi: 10.1093/eurheartj/ehab420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song Y., Huang C., Sin J., Germano J.d.F., Taylor D.J.R., Thakur R., Gottlieb R.A., Mentzer R.M., Andres A.M. Attenuation of adverse postinfarction left ventricular remodeling with empagliflozin enhances mitochondria-linked cellular energetics and mitochondrial biogenesis. Int. J. Mol. Sci. 2021;23(1) doi: 10.3390/ijms23010437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meng J., Lv Z., Zhang Y., Wang Y., Qiao X., Sun C., Chen Y., Guo M., Han W., Ye A., Xie T., Chu B., Shi C., Yang S., Chen C. Precision redox: the key for antioxidant pharmacology. Antioxidants Redox Signal. 2021;34(14):1069–1082. doi: 10.1089/ars.2020.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arkadeep Mitra, Ritwik Datta, Santanu Rana, Sagartirtha Sarkar. Modulation of NFKB1/p50 by ROS leads to impaired ATP production during MI compared to cardiac hypertrophy. J. Cell. Biochem. 2018;119(2):1235–2449. doi: 10.1002/jcb.26318. i-i. [DOI] [PubMed] [Google Scholar]
- 58.Lissoni A., Hulpiau P., Martins-Marques T., Wang N., Bultynck G., Schulz R., Witschas K., Girao H., De Smet M., Leybaert L. RyR2 regulates Cx43 hemichannel intracellular Ca2+-dependent activation in cardiomyocytes. Cardiovasc. Res. 2021;117(1):123–136. doi: 10.1093/cvr/cvz340. [DOI] [PubMed] [Google Scholar]
- 59.García I.E., Sánchez H.A., Martínez A.D., Retamal M.A. Redox-mediated regulation of connexin proteins; focus on nitric oxide. Biochim. Biophys. Acta Biomembr. 2018;1860(1):91–95. doi: 10.1016/j.bbamem.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 60.Liu Z.-Y., Song K., Tu B., Lin L.-C., Sun H., Zhou Y., Li R., Shi Y., Yang J.-J., Zhang Y., Zhao J.-Y., Tao H. Crosstalk between oxidative stress and epigenetic marks: new roles and therapeutic implications in cardiac fibrosis. Redox Biol. 2023;65 doi: 10.1016/j.redox.2023.102820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Pol A., van Gilst W.H., Voors A.A., van der Meer P. Treating oxidative stress in heart failure: past, present and future. Eur. J. Heart Fail. 2018;21(4):425–435. doi: 10.1002/ejhf.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang X., Lin X., Wang L., Xie Y., Que Y., Li S., Hu P., Tong X. Substitution of SERCA2 Cys674 aggravates cardiac fibrosis by promoting the transformation of cardiac fibroblasts to cardiac myofibroblasts. Biochem. Pharmacol. 2022;203 doi: 10.1016/j.bcp.2022.115164. [DOI] [PubMed] [Google Scholar]
- 63.Fiorelli Porro, Cosentino M. Di, Manega Fabbiocchi, Niccoli Fracassi, Barbieri Marenzi, Crea Cavalca, Tremoli Eligini. Activation of Nrf2/HO-1 pathway and human atherosclerotic plaque vulnerability:an in vitro and in vivo study. Cells. 2019;8(4) doi: 10.3390/cells8040356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Christidi E., Brunham L.R. Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2021;12(4) doi: 10.1038/s41419-021-03614-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu L., Xie H., Liu Q., Ma F., Wu H. Klotho inhibits H(2) O(2) -induced oxidative stress and apoptosis in periodontal ligament stem cells by regulating UCP2 expression. Clin. Exp. Pharmacol. Physiol. 2021;48(10):1412–1420. doi: 10.1111/1440-1681.13547. [DOI] [PubMed] [Google Scholar]
- 66.Qiao H., Sai X., Gai L., Huang G., Chen X., Tu X., Ding Z. Association between heme oxygenase 1 gene promoter polymorphisms and susceptibility to coronary artery disease: a HuGE review and meta-analysis. Am. J. Epidemiol. 2014;179(9):1039–1048. doi: 10.1093/aje/kwu024. [DOI] [PubMed] [Google Scholar]
- 67.Xie P., Chen L., Wang J., Wang X., Yang S., Zhu G. Polysaccharides from Polygonatum cyrtonema Hua prevent post-traumatic stress disorder behaviors in mice: mechanisms from the perspective of synaptic injury, oxidative stress, and neuroinflammation. J. Ethnopharmacol. 2024;319 doi: 10.1016/j.jep.2023.117165. [DOI] [PubMed] [Google Scholar]
- 68.Zhang X., Yu Y., Lei H., Cai Y., Shen J., Zhu P., He Q., Zhao M. The nrf-2/HO-1 signaling Axis: a ray of hope in cardiovascular diseases. Cardiol. Res. Pract. 2020;2020:1–9. doi: 10.1155/2020/5695723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jin S.W., Hwang Y.P., Choi C.Y., Kim H.G., Kim S.J., Kim Y., Chung Y.C., Lee K.J., Jeong T.C., Jeong H.G. Protective effect of rutaecarpine against t-BHP-induced hepatotoxicity by upregulating antioxidant enzymes via the CaMKII-Akt and Nrf2/ARE pathways. Food Chem. Toxicol. 2017;100:138–148. doi: 10.1016/j.fct.2016.12.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data associated with this study was not deposited into a publicly available repository for data will be made available on request.