Highlights
-
•
Oncovascular surgery is a rare but important component of radical surgery in gynecologic cancer.
-
•
Adult-type granulosa cell tumor of the ovary often recurs, and medical therapy has limited efficacy.
-
•
Our patient had multiple recurrences of granulosa cell tumor ultimately requiring extensive oncovascular reconstruction.
-
•
Multidisciplinary collaboration is essential in providing optimal care in this setting.
Keywords: Granulosa cell tumor, Ovarian cancer, Oncovascular surgery
Abstract
Introduction
Oncovascular surgery is a rare but important component of radical surgery in gynecologic cancer, requiring interdisciplinary collaboration and coordination. In this case report, we review the case of a patient with recurrent granulosa cell tumor who underwent extensive oncovascular resection and reconstruction.
Case presentation
Our patient was initially diagnosed with a stage IC granulosa cell tumor in 1989 following a left salpingo—oophorectomy secondary to ovarian cyst rupture. She subsequently had multiple recurrences requiring 8 surgical procedures from 1989 to 2022. Her most recent recurrence was notable for a 6 x 8 cm left pelvic tumor invading into the inferior vena cava (IVC), encasing the aorta, left common and external iliac vessels, and involving the left ureter. In a combined case with gynecologic surgery, vascular surgery, and urology, extensive oncovascular resection was performed, including an en bloc resection of the recurrent granulosa cell tumor, aorta, bilateral common and left external iliac arteries and veins, with aortal and IVC reconstruction. Despite a complicated postoperative course, she recovered well, received no further oncologic treatment, and remains on surveillance without evidence of disease 26 months later.
Conclusion
To our knowledge, this is the first reported case of oncovascular surgery involving aortic and IVC resection and reconstruction for recurrent granulosa cell tumor.
1. Introduction
Adult-type granulosa cell tumor (GCT) of the ovary is the most common ovarian sex cord stromal tumor (Schumer and Cannistra, 2003). While GCT comprises 70 % of ovarian sex cord stromal tumors, it is nevertheless a rare malignancy, constituting only 2 % to 5 % of all ovarian cancers (Schumer and Cannistra, 2003). Surgery is the mainstay of treatment for GCT, and disease stage is the most important prognostic factor for survival (Fox et al., 1975, Bjorkholm and Silfversward, 1981). Patients with stage I disease have a 5-year survival rate > 90 %; however, relapse occurs in approximately one-third of patients (Lauszus, 2001, Farkkila, 2017). Treatment recommendations for patients with stage II-IV disease include adjuvant platinum-based chemotherapy (National Comprehensive Cancer Network, 2024) (Evans, 1980).
The median time to relapse following initial diagnosis is 4 to 6 years, with some reports demonstrating relapse as many as 20 years following diagnosis (Evans, 1980, Malmstrom, 1994, Diddle, 1952, Hines, 1996). Pelvic recurrence is common, and medical therapies can be considered in this setting. Options include cytotoxic treatments such as docetaxel, paclitaxel, paclitaxel/ifosfamide, paclitaxel/carboplatin, and vincristine, actinomycin D and cyclophosphamide, and hormonal therapies such as aromatase inhibitors, leuprolide, and tamoxifen (Alhilli, 2012, Fishman, 1996, Gurumurthy et al., 2014, National Comprehensive Cancer Network, 2024). Bevacizumab or leuprolide can also be used as single-agent therapy in the recurrent setting (Tao, 2009). Small molecule cyclin-dependent kinase (CDK) inhibitors selective for CDK4 and CDK6 in combination with hormonal therapy have also shown encouraging results (Albright, 2023). Secondary cytoreductive surgery can also be an effective option for management of recurrence (National Comprehensive Cancer Network, 2024). Palliative radiation can be beneficial for certain patients (National Comprehensive Cancer Network, 2024). The median time to each subsequent recurrence is generally reduced by half, increasing the morbidity of multiple short-interval surgical procedures (Bryk, 2016).
Here, we present a case of recurrent GCT with great vessel involvement and a review of the available literature.
2. Case description
In 1989 at 38 years of age, the patient was initially diagnosed with stage IC GCT following a left salpingo—oophorectomy that was performed secondary to severe pain from a large pelvic mass. She subsequently underwent a total abdominal hysterectomy and right salpingo—oophorectomy without adjuvant treatment. Her inhibin and CA125 levels were not elevated, and she was followed clinically. She experienced her first pelvic recurrence 7 years later in 1996, noted initially as a mass on pelvic examination and confirmed with computed tomography (CT) imaging. She was then treated with 6 cycles of carboplatin and paclitaxel at an outside hospital. In 2000, 4 years after her first recurrence, she had a second pelvic recurrence and underwent a tumor resection with a low anterior rectal resection and reconstruction, followed by pelvic radiotherapy. In 2007, she underwent her fourth surgery for a 4 cm mass inferior to the bifurcation of the aorta. The tumor was resected in addition to 15 cm of small bowel. Following this surgery, the decision was made to forgo postoperative medical therapy in favor of close observation and CT imaging every 3 months, as the patient had not tolerated chemotherapy well after her first recurrence. In 2012, she presented with a small bowel obstruction and underwent her fifth surgery, which included resection of recurrent disease and another small bowel resection with side-to-side anastomosis.
During surveillance from 2012 through 2014, she took daily oral letrozole and remained without evidence of disease. In 2016, a surveillance CT scan showed progression of disease, for which she started anastrozole and experienced good disease response. A surveillance CT scan in 2017 showed progression of disease, with a 7.4 x 5.8 cm nodal mass in the gastrohepatic ligament. She then had her sixth abdominal surgery, with resection of the gastrohepatic mass. Genomic testing performed on the resected mass demonstrated somatic genetic alterations in TMPRSS2, FOXL2, MLL, MLL2, RPS6KA4, SMAD3, and TERT. In 2018, she again experienced progression of disease in an enlarged pelvic lymph node; she was started on leuprolide every 3 months and experienced disease response. In 2020, a CT scan once again showed progression of disease; she was transitioned to fulvestrant, which led to treatment response (Supplementary Table S1).
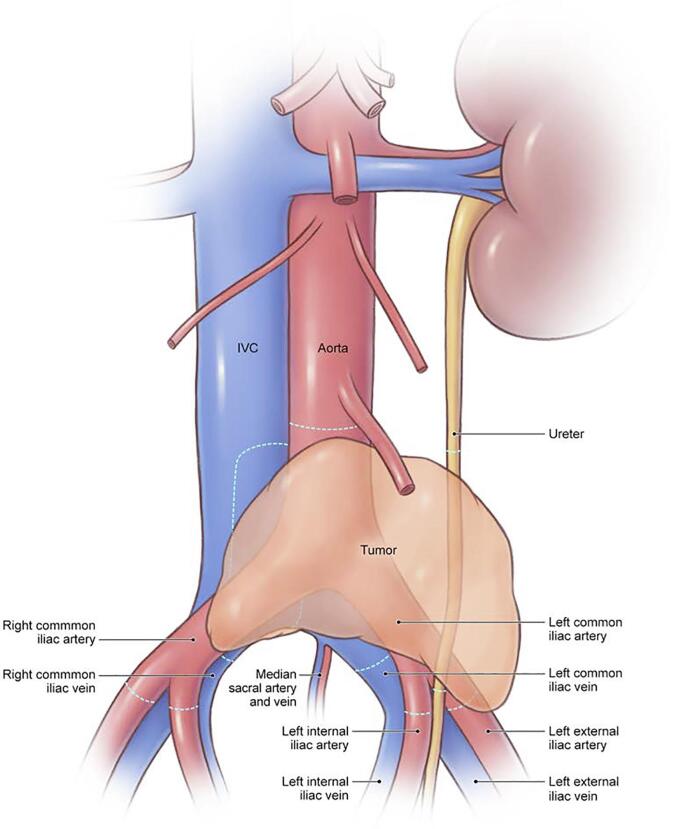
In January 2022 at 71 years of age, she developed a small bowel obstruction and worsening left lower extremity edema. She was found to have a new deep vein thrombosis (DVT) extending from her left common iliac vein to the femoral vein and a left retroperitoneal mass with central necrosis (Fig. 1, Fig. 2). She was admitted to a local hospital in another state, where she was initially managed with therapeutic enoxaparin before she was recommended to undergo left lower extremity amputation, as the vascularity to the left calf and foot appeared severely compromised.
Fig. 1.

Three-dimensional print reconstruction of granulosa cell tumor prior to removal.
Fig. 2.
Artistic rendering of granulosa cell tumor prior to removal. © 2024 Memorial Sloan-Kettering Cancer Center, Memorial Hospital for Cancer and Allied Diseases, and Sloan-Kettering Institute for Cancer Research, each in New York, NY. All rights reserved. Republished with permission.
The patient was flown to our area seeking another opinion, and had consultations with gynecologic oncology, vascular surgery, and orthopedic surgery in preparation for surgical resection of disease. Her preoperative imaging included CT angiography of the abdomen and pelvis, which showed a 6.9 x 4.9 cm left retroperitoneal mass and a left pelvic thrombus extending from the left common iliac vein to the femoral vein. Lower extremity dopplers showed a right DVT in the deep calf veins and a DVT extending along the left external iliac vein, the common femoral, femoral and popliteal vein. The orthopedic surgery team counseled the patient that she would likely need to have a femoral nerve resection, which would impact hip flexion and knee extension. She was also counseled on possible sensory deficits. She was advised by vascular surgery that the mass might be encasing the left iliac artery and vein, and that resection of the iliac vessels and reconstruction with a graft or bypass would likely be required. Given the patient’s overall good health status, including a body mass index of 23.9 kg/m2, Karnofsky Performance Status (KPS) score of 90, and limited medical comorbidities including well-controlled hypertension and osteoporosis, the patient was counseled on treatment options and strongly desired surgical management.
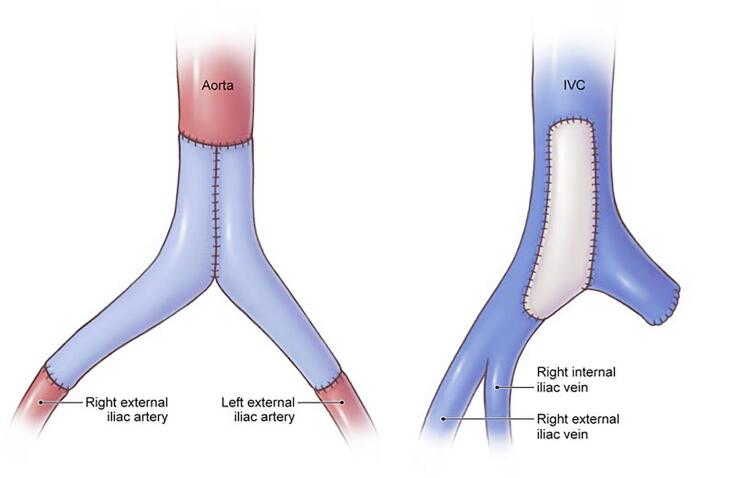
In February 2022, she underwent her seventh abdominal surgery. Intraoperatively, she was noted to have a 6 x 8 cm left pelvic tumor invading into the inferior vena cava (IVC) and encasing the aorta, left common and external iliac vessels, and involving the left ureter (Fig. 1, Fig. 2, Supplementary Figure S1). A vascular surgeon assisted with an en bloc resection of the pelvic tumor, aorta, bilateral common iliac and left external iliac arteries and veins, and left ureter (Fig. 1, Fig. 2, Supplementary Figure S1, Supplementary Figure S2), with reconstruction of the aorta using a bifurcated pantlaloon femoral vein and cryopreserved femoral graft, and repair of the IVC using a bovine pericardial patch (Fig. 3, Supplementary Figure S3). A urologic surgeon performed a ureteroureterostomy for urinary reconstruction. Estimated blood loss was 3000 ml, and the patient received 5 units of packed red blood cells, 2 units of fresh frozen plasma, and 1 unit of platelets intraoperatively. The surgical margins were negative. Postoperatively, the patient received therapeutic enoxaparin for 4 weeks prior to transitioning to rivaroxaban, before ultimately switching back to enoxaparin due to persistent vaginal bleeding.
Fig. 3.
Artistic rendering of aorta and inferior vena cava grafts after reconstruction. © 2024 Memorial Sloan-Kettering Cancer Center, Memorial Hospital for Cancer and Allied Diseases, and Sloan-Kettering Institute for Cancer Research, each in New York, NY. All rights reserved. Republished with permission.
The patient had a complicated postoperative recovery requiring multiple hospitalizations. She was initially hospitalized for sepsis from April 2022 to May 2022, secondary to a fluid collection positive for pan-sensitive klebsiella pneumoniae. She also experienced a left ureteral-arterial fistula for which a left common iliac artery stent was placed, at which time she was transitioned to dual anti-platelet therapy. She initially recovered well; however, in August 2022 she was hospitalized with pseudomonas aeruginosa bacteremia and received 2 weeks of intravenous antibiotics.
In September 2022, she noted vaginal bleeding and had a near syncopal episode. Imaging showed left hydronephrosis, and blood cultures were positive for pseudomonas aeruginosa susceptible to piperacillin and tazobactam, ceftazidime, cefepime, imipenem, meropenem, gentamicin, tobramycin, ciprofloxacin, and levofloxacin. A CT scan demonstrated a 4.6 x 3.4 x 3.5 cm left common iliac pseudoaneurysm with leak at the superior margin and left common iliac stent. Interventional radiology (IR) angiogram was performed, and the aneurysm was repaired with 2 stents placed within the left common iliac artery.
The patient was subsequently transferred from her hospital out of state to our institution for management of an infected aortic graft. She then underwent her eighth surgery, which included a left nephrectomy, colon resection, permanent colostomy, and revision of her aortic femoral bypass. The surgery was planned in order to excise a portion of the infected aortic graft and revise her bypass. However, upon entry into the abdomen, the entire retroperitoneum and abdominal cavity were adherent and there was significant scar tissue. The left ureter had a stent within it that was completely adherent to the aorta throughout its course. Based on this, it was felt that any ureteral reconstruction would not be feasible, and at that point it was deemed necessary by urology to remove the entire left kidney. During the surgery, the left colon became ischemic and general surgery was consulted. Given questionable inferior mesenteric artery perfusion and high risk for devascularization, the decision was made to proceed with permanent end colostomy. She then underwent several months of rehabilitation. The decision was made not to proceed with postoperative systemic therapy at that time until evidence of progression of disease. At her most recent follow-up, she had fully recovered, with no residual deficits and without evidence of disease 26 months after surgery (Supplementary Figure S4).
3. Discussion
GCT is a rare indolent gynecologic malignancy that is prone to late recurrences requiring multiple surgical resections due to limited response to chemotherapy (Farkkila, 2017). These highly vascular tumors have a propensity for rupture and related inflammatory damage to surrounding tissues (Kottarathil, 2013). As with cytoreductive surgery for other histologic types of ovarian cancer, survival outcomes in GCT are improved with complete gross resection (Sun, 2012).
While vascular injuries are uncommon in gynecologic oncology surgery, complicating only 0.3 % to 1 % of all surgical procedures, they are associated with significant morbidity (Jurado, 2022). Studies have demonstrated an independent relationship between oncologic surgery and need for vascular repair (Levin, 2020). This association has been attributed to the presence of invasive disease and associated anatomic distortion (Levin, 2020). A retrospective study by Woo et al. found that unplanned vascular surgery in surgical oncology cases was associated with increased blood loss, operative time, hospital stay, and postoperative vascular complications (Woo, 2020). Studies have found that patients who undergo pelvic exenterations and require vascular resection have worse survival outcomes than those with no vascular involvement (Andikyan, 2012).
Given the extent of surgery required to achieve complete gross resection in advanced ovarian cancer, it is crucial to anticipate vascular involvement and to have an interdisciplinary care team. A study by Finlay et al. reported that preoperative involvement of vascular surgery improved the frequency of clear margins from 35 % to 80 % and reduced the need for vessel repair and reconstruction (Finlay et al., 2020). There are multiple systems available to preoperatively assess vascular involvement. The Tinelli score can be used to characterize the level of vascular invasion, ranging from grade 1 (contact of tumor with vasculature) to grade 5 (complete vascular encapsulation) (Tinelli, 2017). Rajendran et al. proposed an algorithm for managing pelvic tumors involving the aortoiliac vessels, including conservative recommendations such as avoiding venous reconstruction in patients with chronic occlusion and using venous ultrasonography to identify autologous conduits (Rajendran, 2023).
Preoperative imaging including contrast CT and magnetic resonance imaging should be carefully reviewed to assess for the presence of vascular involvement, collateral vessels, and thrombi (Rajendran, 2023, Leithead, 2016). Although there are no data on the sensitivity and specificity of CT imaging for detecting vascular invasion in ovarian cancer, it has demonstrated 97 % specificity in pancreatic cancer (Jajodia, 2023, Uccella, 2023). Three-dimensional printing has been particularly useful in surgical planning for vascular procedures (Lee, 2023). While we did not use this technology preoperatively in this case, a three-dimensional reconstruction from a preoperative CT scan demonstrated extensive vascular involvement (Fig. 1).
In a small series of sarcomas and recurrent carcinomas, Cruz et al. found that while tumor encasement of an artery had prognostic significance, arterial wall involvement did not (Cruz, 2021). Given the high rate of perioperative mortality associated with arterial reconstruction in surgical oncology, ranging from 5 % to 18.5 %, it is essential to appropriately triage patients who would derive the most survival benefit from these extensive procedures (Loos, 2022, Song, 2009).
Reports of extensive oncovascular reconstruction in ovarian cancer are limited in the literature. A recent case series and systematic review by Uccella et al. included 12 cases of vascular resection during surgery for ovarian cancer, only 2 of which involved resection of the aorta, and none of which had GCT histology (Uccella, 2023). Of the 12 patients, 7 (58 %) experienced perioperative complications and 8 had no evidence of disease at last follow-up (Uccella, 2023).
To our knowledge, this case is one of just a few in which aortic and IVC resection and reconstruction were performed, and the only such case of a patient with GCT histology (Lentz, 2014, Tani, 2021). Given the limited number of cases in the literature and the significant perioperative morbidity associated with extensive vascular surgical reconstruction, more research is needed to determine the ideal candidate for this treatment approach. The high rate of complications with extensive vascular surgery must be weighed against the known survival benefit of complete gross resection. Ultimately, the decision to perform these extensive surgical procedures must be individualized based on patient goals of care and performance status.
4. Patient perspective
Investigators confirm that informed consent was obtained from the patient for creation and publication of this case report.
5. Statement of consent
Investigators confirm that informed consent was obtained from the patient for creation and publication of this case report.
Funding
This research was funded in part by the NIH/NCI Cancer Center Support Grant P30 CA008748.
CRediT authorship contribution statement
Lindsey Finch: Writing – original draft. Sharif Ellozy: Writing – review & editing, Methodology, Conceptualization. Jaspreet Sandhu: Writing – review & editing, Methodology, Conceptualization. Tulsi Patel: Writing – review & editing, Methodology. William P. Tew: Writing – review & editing, Methodology, Conceptualization. Dennis S. Chi: .
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Chi reports medical advisory board participation for Verthermia Acquio and Biom ‘Up Inc, speaker fees from AstraZeneca, and stock in BioNTech and Doximity. The remaining authors have no conflicts to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gore.2024.101496.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Albright B.B., et al. Efficacy of cyclin-dependent kinase 4/6 inhibitors in combination with hormonal therapy in patients with recurrent granulosa cell tumor of the ovary: a case series. Gynecol Oncol Rep. 2023;50 doi: 10.1016/j.gore.2023.101297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhilli M.M., et al. Aromatase inhibitors in the treatment of recurrent ovarian granulosa cell tumors: brief report and review of the literature. J Obstet Gynaecol Res. 2012;38(1):340–344. doi: 10.1111/j.1447-0756.2011.01698.x. [DOI] [PubMed] [Google Scholar]
- Andikyan V., et al. Extended pelvic resections for recurrent or persistent uterine and cervical malignancies: an update on out of the box surgery. Gynecol Oncol. 2012;125(2):404–408. doi: 10.1016/j.ygyno.2012.01.031. [DOI] [PubMed] [Google Scholar]
- Bjorkholm E., Silfversward C. Prognostic factors in granulosa-cell tumors. Gynecol Oncol. 1981;11(3):261–274. doi: 10.1016/0090-8258(81)90040-8. [DOI] [PubMed] [Google Scholar]
- Bryk S., et al. Characteristics and outcome of recurrence in molecularly defined adult-type ovarian granulosa cell tumors. Gynecol Oncol. 2016;143(3):571–577. doi: 10.1016/j.ygyno.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Cruz S.M., et al. Surgical and oncologic outcomes following arterial resection and reconstruction for advanced solid tumors. J Surg Oncol. 2021;124(8):1251–1260. doi: 10.1002/jso.26665. [DOI] [PubMed] [Google Scholar]
- Diddle A.W. Granulosa- and theca-cell ovarian tumors: prognosis. Cancer. 1952;5(2):215–228. doi: 10.1002/1097-0142(195203)5:2<215::aid-cncr2820050203>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Evans A.T., 3rd, et al. Clinicopathologic review of 118 granulosa and 82 theca cell tumors. Obstet Gynecol. 1980;55(2):231–238. [PubMed] [Google Scholar]
- Farkkila A., et al. Pathogenesis and treatment of adult-type granulosa cell tumor of the ovary. Ann Med. 2017;49(5):435–447. doi: 10.1080/07853890.2017.1294760. [DOI] [PubMed] [Google Scholar]
- Finlay B., Bednarz J., Dawson J. A multidisciplinary approach to oncological resections with vascular surgeons improves patient outcomes. Eur J Vasc Endovasc Surg. 2020;60(2):293–299. doi: 10.1016/j.ejvs.2020.04.011. [DOI] [PubMed] [Google Scholar]
- Fishman A., et al. Leuprolide acetate for treating refractory or persistent ovarian granulosa cell tumor. J Reprod Med. 1996;41(6):393–396. [PubMed] [Google Scholar]
- Fox H., Agrawal K., Langley F.A. A clinicopathologic study of 92 cases of granulosa cell tumor of the ovary with special reference to the factors influencing prognosis. Cancer. 1975;35(1):231–241. doi: 10.1002/1097-0142(197501)35:1<231::aid-cncr2820350128>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Gurumurthy M., Bryant A., Shanbhag S. Effectiveness of different treatment modalities for the management of adult-onset granulosa cell tumours of the ovary (primary and recurrent) Cochrane Database Syst Rev. 2014;2014(4):CD006912. doi: 10.1002/14651858.CD006912.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines J.F., et al. Recurrent granulosa cell tumor of the ovary 37 years after initial diagnosis: a case report and review of the literature. Gynecol Oncol. 1996;60(3):484–488. doi: 10.1006/gyno.1996.0078. [DOI] [PubMed] [Google Scholar]
- Jajodia A., et al. MRI vs. CT for pancreatic adenocarcinoma vascular invasion: comparative diagnostic test accuracy systematic review and meta-analysis. Eur Radiol. 2023;33(10):6883–6891. doi: 10.1007/s00330-023-09659-0. [DOI] [PubMed] [Google Scholar]
- Jurado M., et al. The role of oncovascular surgery in gynecologic oncology surgery. Int J Gynecol Cancer. 2022;32(4):553–559. doi: 10.1136/ijgc-2021-003129. [DOI] [PubMed] [Google Scholar]
- Kottarathil V.D., et al. Recent advances in granulosa cell tumor ovary: a review. Indian J Surg Oncol. 2013;4(1):37–47. doi: 10.1007/s13193-012-0201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauszus F.F., et al. Granulosa cell tumor of the ovary: a population-based study of 37 women with stage I disease. Gynecol Oncol. 2001;81(3):456–460. doi: 10.1006/gyno.2001.6183. [DOI] [PubMed] [Google Scholar]
- Lee J., et al. Clinical situations for which 3D Printing is considered an appropriate representation or extension of data contained in a medical imaging examination: vascular conditions. 3D Print Med. 2023;9(1):34. doi: 10.1186/s41205-023-00196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leithead C.C., et al. Analysis of emergency vascular surgery consults within a tertiary health care system. J Vasc Surg. 2016;63(1):177–181. doi: 10.1016/j.jvs.2015.08.057. [DOI] [PubMed] [Google Scholar]
- Lentz S.E., et al. Abdominal aortic resection and Y-graft placement to achieve complete cytoreduction in stage IIIc ovarian carcinoma. Obstet Gynecol. 2014;123(2 Pt 2 Suppl 2):486–488. doi: 10.1097/AOG.0000000000000039. [DOI] [PubMed] [Google Scholar]
- Levin S.R., et al. Vascular repairs in gynecologic operations are uncommon but predict major morbidity and mortality. J Vasc Surg. 2020;72(3):1059–1066 e2. doi: 10.1016/j.jvs.2019.11.036. [DOI] [PubMed] [Google Scholar]
- Loos M., et al. Arterial resection in pancreatic cancer surgery: effective after a learning curve. Ann Surg. 2022;275(4):759–768. doi: 10.1097/SLA.0000000000004054. [DOI] [PubMed] [Google Scholar]
- Malmstrom H., et al. Granulosa cell tumors of the ovary: prognostic factors and outcome. Gynecol Oncol. 1994;52(1):50–55. doi: 10.1006/gyno.1994.1010. [DOI] [PubMed] [Google Scholar]
- Rajendran S., et al. Clinical algorithm for the management of advanced pelvic tumours involving the aortoiliac axis. Eur J Surg Oncol. 2023;49(7):1317–1319. doi: 10.1016/j.ejso.2023.03.207. [DOI] [PubMed] [Google Scholar]
- Schumer S.T., Cannistra S.A. Granulosa cell tumor of the ovary. J Clin Oncol. 2003;21(6):1180–1189. doi: 10.1200/JCO.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Song T.K., et al. Major blood vessel reconstruction during sarcoma surgery. Arch Surg. 2009;144(9):817–822. doi: 10.1001/archsurg.2009.149. [DOI] [PubMed] [Google Scholar]
- Sun H.D., et al. A long-term follow-up study of 176 cases with adult-type ovarian granulosa cell tumors. Gynecol Oncol. 2012;124(2):244–249. doi: 10.1016/j.ygyno.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Tani R., et al. Aggressive resection of malignant paraaortic and pelvic tumors accompanied by arterial reconstruction with synthetic arterial graft. Am J Case Rep. 2021;22:e931569. doi: 10.12659/AJCR.931569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X., et al. Anti-angiogenesis therapy with bevacizumab for patients with ovarian granulosa cell tumors. Gynecol Oncol. 2009;114(3):431–436. doi: 10.1016/j.ygyno.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinelli G., et al. Resectability and vascular management of retroperitoneal gynecological malignancies: a large single-institution case-series. Anticancer Res. 2017;37(12):6899–6906. doi: 10.21873/anticanres.12153. [DOI] [PubMed] [Google Scholar]
- Uccella S., et al. Major vessel resection for complete cytoreduction in primary advanced and recurrent ovarian malignancies: a case series and systematic review of the literature - pushing the boundaries in oncovascular surgery. Gynecol Oncol. 2023;179:42–51. doi: 10.1016/j.ygyno.2023.10.021. [DOI] [PubMed] [Google Scholar]
- Woo H.Y., et al. Crucial roles of vascular surgeons in oncovascular and non-vascular surgery. Eur J Vasc Endovasc Surg. 2020;60(5):764–771. doi: 10.1016/j.ejvs.2020.08.026. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network, 2024. NCCN Clinical Practice Guidelines in Oncology: Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer, Version 3.2024.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.