Highlights
-
•
Tiagabine use has been associated with acute encephalopathy and status epilepticus.
-
•
VNS has been used to treat status epilepticus and may disrupt cortical synchronization.
-
•
VNS may interrupt encephalopathy associated with tiagabine use.
Keywords: Case report, Encephalopathy, Tiagabine, Vagus nerve stimulator, VNS
Abstract
Tiagabine has been associated with reports of status epilepticus as well as encephalopathy, even when used within therapeutic doses. Vagus nerve stimulation (VNS) has been used successfully to reduce seizure frequency in the outpatient setting as well as in the acute setting of status epilepticus. It is also theorized to reduce cortical synchronization. We present a case of a patient on adjunctive tiagabine therapy who developed sudden onset encephalopathy and rhythmic delta activity soon after vagus nerve stimulation was turned off in preparation for magnetic resonance imaging. The bilateral rhythmic delta activity significantly reduced in burden after VNS was turned back on and encephalopathy also gradually improved to baseline. We hypothesize that vagus nerve stimulation successfully interrupted diffuse hypersynchrony, in the form of bilateral rhythmic delta activity, caused by tiagabine. To our knowledge, this is the first report of such a phenomenon.
1. Introduction
Tiagabine, a γ-aminobutyric acid (GABA) uptake inhibitor, has been associated with occurrence of non-convulsive status epilepticus and diffuse slowing in adult and pediatric epilepsy patients [1], [2]. This has been seen in both generalized epilepsies and focal epilepsies, and at therapeutic doses of tiagabine [2]. Vagus nerve stimulation (VNS) is used for adjunctive treatment of medically refractory focal epilepsy, originally approved in the United States in 1997. Since that time, there have been reports of acute implantation of a vagus nerve stimulator successfully treating refractory- and super-refractory status epilepticus [3]. A possible mechanism for antiseizure effects of vagus nerve stimulation may be related to effects on cortical desynchronization [4]. However, to our knowledge, no reports of tiagabine-induced encephalopathy, rhythmic patterns, or status epilepticus treated by VNS have been published.
2. Case report
A 27-year-old man with refractory left temporal lobe epilepsy, status post left anterior temporal lobectomy at 6 years of age and VNS placement implanted in teenage years, presented to the hospital after having four seizures without return to baseline. On EMS arrival, he had been altered and combative, which was typical post-ictal behavior for the patient; as this was not improving spontaneously, he was given midazolam and intravenous lorazepam. When examined by the admitting team, he was confused and somnolent, unable to answer orientation questions or provide additional history. In the prior two years, he had been nearly seizure-free on tiagabine 12 mg three times daily (which was originally started at age 21), levetiracetam extended-release 1500 mg three times daily, and lacosamide 200 mg two times daily, together with a VNS, set on rapid cycling, with detailed settings as per Table 1. He was compliant with his medication regimen and had no recent illnesses. Upon VNS interrogation, the device was found to have low impedance. Based on our experience and discussions with LivaNova representatives, the interrogation of the VNS was consistent with end-of-service and recommended urgent generator replacement. Long-term electroencephalography (LTM EEG) monitoring was started, showing frequent high-amplitude bilateral rhythmic delta activity, left hemispheric maximal, at 1.5–2 Hz, but remained without clear clinical correlate and were not clearly temporally associated with definitive seizures (Fig. 1). He had replacement of his generator days later; he gradually returned to baseline and was discharged with increase in tiagabine to 16 mg three times daily. EEG was not reconnected following the generator replacement.
Table 1.
Vagus nerve stimulator settings at first admission.
| LivaNova Pulse™ Model 102 | ||
|---|---|---|
| Mode | Setting | Value |
| Normal | Current (mAmp) | 2.75 |
| Signal Frequency (Hz) | 30 | |
| Pulse Width (msec) | 250 | |
| Signal On Time (sec) | 14 | |
| Signal Off Time (min) | 1.1 | |
| Magnet | Current (mAmp) | 3.0 |
| Pulse Width (msec) | 250 | |
| Signal On Time (sec) | 60 | |
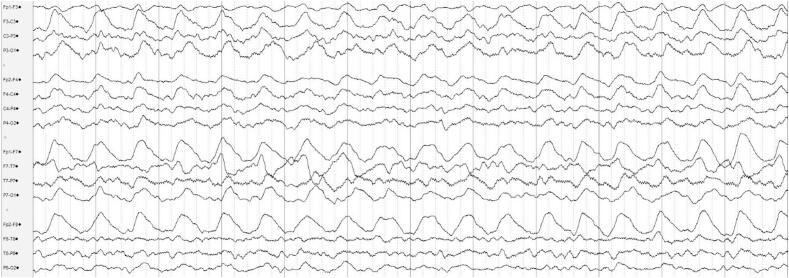
Fig. 1.
EEG prior to VNS generator replacement, showing high-amplitude bilateral rhythmic delta activity, left hemispheric maximal, at 1.5–2 Hz. Per American Clinical Neurophysiology Standardized Critical Care EEG Terminology, 2021 [5]: lateralized, bilateral asymmetric, left hemispheric rhythmic delta activity. Bipolar longitudinal montage, timebase 30 mm/s, HFF 70 Hz, LFF 1 Hz, Notch 60 Hz.
Over the next two years, his seizure control worsened, despite continued use of levetiracetam (4500 mg/day), increase in lacosamide (to 600 mg/day), increase in tiagabine (to 56 mg/day), and addition of eslicarbazepine (at 800 mg/day), as well as increase in VNS current from 2.75 to 3 mAmp. He was ultimately admitted to the Epilepsy Monitoring Unit. His baseline EEG showed bilateral temporal slowing and epileptiform discharges, but no rhythmic delta activity was seen. His medications were gradually tapered, after which he had three right temporal seizures, followed by right hemispheric slowing and attenuation. The following day, home doses of medications were resumed, he had no further definitive seizures through the morning and afternoon, and later that evening, in preparation for updated MRI brain, his VNS was turned off at approximately 16:30. The VNS settings are detailed in Table 2. At approximately 18:00, the neurologist on-call was contacted due to new agitation and confusion. EEG showed diffuse high amplitude rhythmic delta activity, distinctly different from baseline EEG and had not been seen previously post-ictally (Fig. 2). The pattern was also not clearly ictal. The VNS was turned back on at 19:20, at the same settings, and he was given a one-time dose of intravenous lacosamide and lorazepam. He gradually returned to clinical baseline and the rhythmic delta activity also resolved.
Table 2.
Vagus nerve stimulator settings during Epilepsy Monitoring Unit admission.
| LivaNova DemiPulse™ Model 103 | ||
|---|---|---|
| Mode | Setting | Value |
| Normal | Current (mAmp) | 3.00 |
| Signal Frequency (Hz) | 30 | |
| Pulse Width (msec) | 250 | |
| Signal On Time (sec) | 14 | |
| Signal Off Time (min) | 1.1 | |
| Magnet | Current (mAmp) | 3.25 |
| Pulse Width (msec) | 250 | |
| Signal On Time (sec) | 60 | |
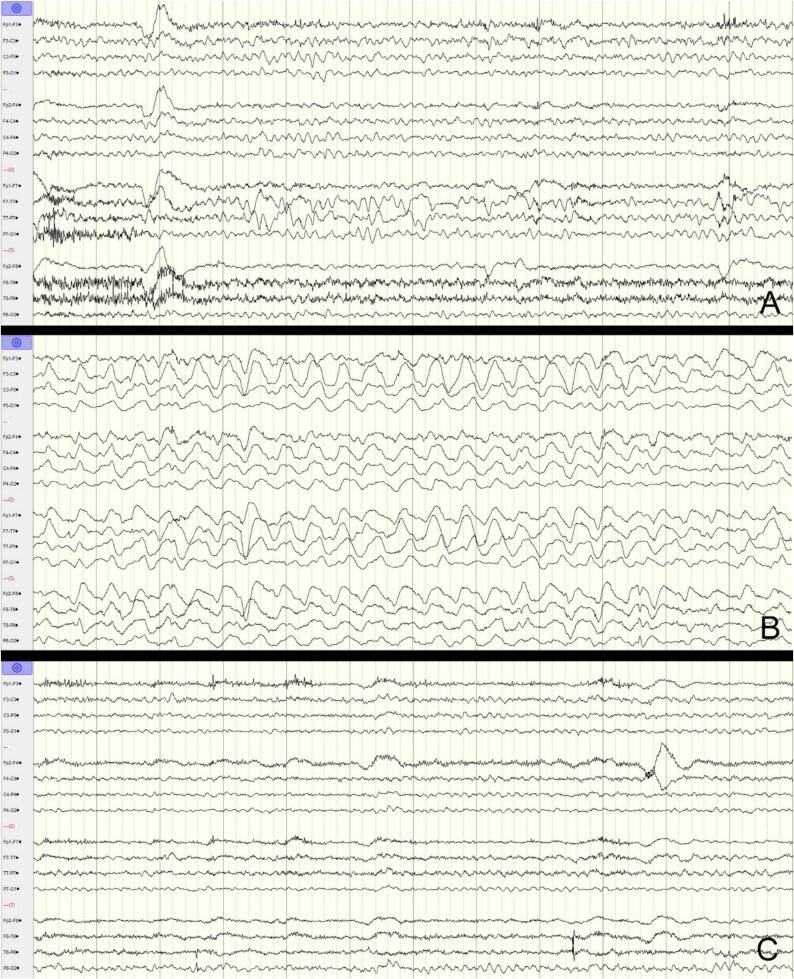
Fig. 2.
EEG before VNS turned off (A), after VNS turned off (B) and after VNS turned back on (C). Bipolar longitudinal montage, timebase 30 mm/s, HFF 70 Hz, LFF 1 Hz, Notch 60 Hz. A: Baseline EEG showing intermittent bitemporal slowing, left more than right. B: EEG with high-amplitude bilateral rhythmic delta activity, left hemispheric maximal, at 1.5–2 Hz. Per American Clinical Neurophysiology Standardized Critical Care EEG Terminology, 2021 [5]: lateralized, bilateral asymmetric, left hemispheric rhythmic delta activity. C: EEG at similar baseline.
Of note, ten years prior to the first hospital stay described above, he was admitted to the Epilepsy Monitoring Unit and had VNS turned off for MRI without developing encephalopathy or rhythmic delta activity on EEG. The VNS settings were current output of 2.0 mAmp, signal frequency of 20 Hz, pulse width of 250 msec, on-time 30 s, off-time 1.8 min. He had been prescribed levetiracetam 1500 mg three times daily, topiramate 200 mg twice daily, and oxcarbazepine 1200 mg twice daily.
Three years later, he had a VNS generator change and there were no reports of encephalopathy around the time of its replacement; the settings prior to replacement were current output of 3.0 mAmp, signal frequency of 30 Hz, pulse width of 250 msec, on-time 14 s, off-time 0.8 min. He had been prescribed levetiracetam extended-release 1500 mg three times daily, topiramate 300 mg three times daily, carbamazepine 400 mg morning/300 mg midday/400 mg nightly, and clonazepam 1 mg three times daily, and had recently started lacosamide 100 mg twice daily. Of note, he was not taking tiagabine during either of these admissions.
3. Discussion
In summary, we have presented the case of a patient who experienced two instances of encephalopathy with high-amplitude rhythmic delta activity in the setting of tiagabine use and pause of vagal nerve stimulation. We believe these changes and encephalopathy were specifically due to the use of tiagabine: in the past, prior to the initiation of tiagabine, turning the VNS off had not caused any notable change in mental status, and stimulation settings were comparable.
Tiagabine has been associated with non-convulsive status epilepticus as well as encephalopathy with diffuse slowing on EEG, within usual therapeutic doses [2]. Vagus nerve stimulation has been used successfully in the prevention of seizures in patients with focal epilepsy and has also been reported to successfully treat refractory status epilepticus in the acute setting [3]. The mechanism of vagus nerve stimulation in preventing seizures has been thought to be due to effects on cortical synchronization [4]. In the study performed by Sangare et al, among responders to VNS, VNS ON periods led to decrease in interictal synchronization, including specifically in the delta band.[4].
We hypothesize that, in our patient, the VNS was suppressing hypersynchrony caused by tiagabine, leading to resolution of associated encephalopathy and improvement in rhythmic delta activity. Though we do not believe the epochs of rhythmic delta activity reflected ictal phenomena, reports of successful use of VNS in status epilepticus imply that it may be effective in acute termination of synchronous EEG patterns.
To our knowledge, no case reports of VNS interrupting rhythmic patterns or tiagabine-related encephalopathy have been previously published.
Our case also illustrates the importance of regularly checking the status of vagus nerve stimulator battery and impedance, as even a brief lapse in stimulation may cause increase in seizure activity or acute encephalopathy.
4. Conclusion
Vagus nerve stimulation has been reported to successfully treat refractory status epilepticus in the acute setting. Tiagabine use has been implicated in the development of status epilepticus and diffuse rhythmic delta activity on EEG. It is possible that VNS may interrupt tiagabine-induced encephalopathy through cortical desynchronization. Especially in patients on tiagabine therapy, status of VNS battery should be regularly checked and patients should be educated about this potential presentation of stimulation malfunction or failure.
Credit authorship contribution statement
Christine N. Smith: Writing – review & editing, Writing – original draft, Investigation, Conceptualization. Stephan Eisenschenk: Writing – review & editing, Supervision, Investigation, Conceptualization. Yue Wang: Writing – review & editing, Supervision, Investigation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Kellinghaus C., Dziewas R., Ludemann P. Tiagabine-related non-convulsive status epilepticus in partial epilepsy: three case reports and a review of the literature. Seizure. 2002;11:243–249. doi: 10.1053/seiz.2001.0594. [DOI] [PubMed] [Google Scholar]
- 2.De Borchgrave V., Lienard F., Willemart T., van Rijckevorsel K. Clinical and EEG findings in six patients with altered mental status receiving tiagabine therapy. Epilepsy Behav. 2003;4(3):326–337. doi: 10.1016/s1525-5050(03)00107-0. [DOI] [PubMed] [Google Scholar]
- 3.Dibué-Adjei M., Brigo F., Yamamoto T., Vonck K., Trinka E. Vagus nerve stimulation in refractory and super-refractory status epilepticus – A systematic review. Brain Stimul. 2019;12(5):1101–1110. doi: 10.1016/j.brs.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Sangare A., Marchi A., Pruvost-Robieux E., Soufflet C., Crepon B., Ramdani C., et al. The Effectiveness of Vagus Nerve Stimulation in Drug-Resistant Epilepsy Correlates with Vagus Nerve Stimulation-Induced Electroencephalography Desynchronization. Brain Connect. 2020;10(10):566–577. doi: 10.1089/brain.2020.0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirsch L.J., Fong M.W.K., Leitinger M., LaRoche S.M., Beniczky S., Abend N.S., et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2021 Version. J Clin Neurophysiol. 2021;38(1):1–29. doi: 10.1097/WNP.0000000000000806. [DOI] [PMC free article] [PubMed] [Google Scholar]