Abstract
In immunology, the role of macrophages extends far beyond their traditional classification as mere phagocytes; they emerge as pivotal architects of the immune response, with their function being significantly influenced by multidimensional environmental stimuli. This review investigates the nuanced mechanisms by which diverse external signals ranging from chemical cues to physical stress orchestrate macrophage polarization, a process that is crucial for the modulation of immune responses. By transitioning between pro-inflammatory (M1) and anti-inflammatory (M2) states, macrophages exhibit remarkable plasticity, enabling them to adapt to and influence their surroundings effectively. The exploration of macrophage polarization provides a compelling narrative on how these cells can be manipulated to foster an immune environment conducive to tissue repair and regeneration. Highlighting cutting-edge research, this review presents innovative strategies that leverage the dynamic interplay between macrophages and their environment, proposing novel therapeutic avenues that harness the potential of macrophages in regenerative medicine. Moreover, this review critically evaluates the current challenges and future prospects of translating macrophage-centered strategies from the laboratory to clinical applications.
Keywords: Macrophage polarization, Immune response, Environmental stimuli, Tissue regeneration
1. Introduction
Within the vast expanse of the immune system, macrophages stand at the helm, orchestrating a diverse array of physiological processes that span from immune surveillance to tissue regeneration [1,2]. The phenomenon of macrophage polarization, where these cells adapt their functional states in response to environmental cues, highlights their unique plasticity and critical role in modulating adaptive immune regulation. This polarization transcends the simplistic binary classification of M1 (classically activated) versus M2 (alternatively activated), unfolding into a spectrum of activation states each characterized by distinctive functional and phenotypic attributes [3]. Investigating the multifaceted factors that drive macrophage polarization and their profound implications on immune responses is essential for unraveling the complex interactions between innate and adaptive immunity. An endeavor in exploring macrophage polarization involves delineating the myriad factors that dictate the transformation of macrophage identity. These range from physical signals to chemical signals (with/without biological activity), collectively influencing macrophages towards specific polarization states. The integration of these signals not only shapes immediate immune reactions but also decisively impacts inflammation resolution, tissue repair, and remodeling [4,5].
The remarkable adaptability of macrophages in responding to and modulating their microenvironment positions them as focal points in the investigation of therapeutic strategies aimed at enhancing immune responses, alleviating pathological inflammation, and fostering tissue regeneration. Particularly in tissue reconstruction and repair, the application of macrophage polarization presents expansive prospects, paving new therapeutic pathways that leverage the inherent plasticity of these cells to promote tissue healing and regeneration [6]. This includes the development of innovative biomaterials, pharmaceuticals, and cell therapy approaches that, grounded in a profound understanding of macrophage biology, are designed to modulate macrophage function in situ, guiding the effective repair and regeneration of damaged tissues [7].
In recent years, an expanding body of research has llustrated the mechanisms of macrophage polarization and its role in modulating immune responses, the factors influencing this dynamic process, and its application in tissue reconstruction and repair constitute a vibrant research direction in the fields of immunology, regenerative medicine, and therapeutic innovation [5,8,9]. This review systematically explores macrophage polarization and its immune responses to diverse stimuli, investigates regulatory mechanisms and intervention strategies, and aims to identify potential pathways for clinical translation in tissue repair and regeneration.
2. Macrophage polarization overview
The study of macrophage activation and polarization dates back to the 1960s. The concept of macrophage activation (classical activation) was first introduced by Mackaness in the 1960s, in the context of describing the antigen-dependent yet non-specific enhancement of macrophage bactericidal activity following secondary exposure to Bacille Calmette-Guérin (BCG) and Listeria, an enhancement later confirmed to be associated with the T helper (Th)1 response and interferon (IFN)-γ produced by antigen-activated immune cells [10]. At that time, the relationship between Th2 cells and macrophages, in the context of immunoglobulin (Ig)E production, protection against extracellular parasites, and allergic reactions, remained unelucidated. It was not until the 1990s that Stein and Doyle et al. proposed another activation phenotype induced by interleukin (IL)-4 and IL-13, distinct from the IFN-γ activated state yet far from being deactivated [11,12]. They discovered selective enhancement of mannose receptor in murine macrophages under the stimulation of IL-4 and IL-13 secreted by Th2, inducing heightened endocytosis for the clearance of mannosylated ligands, increased expression of major histocompatibility complex (MHC) class II antigens, and reduced pro-inflammatory cytokine secretion. In the 2000s, Mills et al. observed qualitative differences in the response of macrophages activated in Th1 and Th2 contexts to classical stimuli such as IFN-γ or lipopolysaccharide (LPS) or both, in studies investigating factors regulating arginine metabolism in macrophages, defining a significant metabolic distinction in the pathway: M1 macrophages produce toxic nitric oxide (NO), while M2 macrophages produce nourishing polyamines [13]. Against this backdrop, they proposed naming these responses as M1 and M2 macrophage responses. To integrate similarities and differences in phenotypes, Mantovani et al. began grouping stimuli into a continuum between two functional polarization states based on macrophage markers and types of stimuli, naming them M1 (IFN-γ, LPS, tumor necrosis factor [TNF]) and M2 (M2a: IL-4, M2c: IL-10 and glucocorticoids) [14,15]. Following closely, Mosser et al. further refined the M2 group, defining the activation pathway induced by Fc receptors and immune complexes as M2b [16]. Duluc et al. reported the identification of a novel subset within tumor-associated macrophages (TAMs), named M2d [17]. Discoveries regarding the influence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on macrophages led to these being independently included as M1 and M2 stimuli [18].
The differentiation of macrophages into distinct functional phenotypes is governed by a myriad of environmental cues, driving their evolution towards either the M1 phenotype, characterized by a pro-inflammatory response initiated in a classical manner, or the M2 phenotype, indicative of a traditionally elicited immunoregulatory response [19]. The transition to the M1 or M2 states encompasses a complex regulatory framework, entailing an array of signaling cascades, transcriptional and epigenetic mechanisms, alongside post-transcriptional regulatory dynamics [20]. The classic activation of macrophages and their subsequent immune responses are precipitated by diverse pathogen-associated molecular patterns (PAMPs) such as LPS, damage-associated molecular patterns (DAMPs), and the Th1 cytokine IFN-γ, which is pivotal in fostering pro-inflammatory (M1) outcomes [21,22]. Conversely, the Th2 cytokines, including IL-4 and IL-13, are instrumental in orchestrating the alternative, M2 immune responses. Furthermore, the reprogramming and polarization processes of macrophages are modulated by a broad spectrum of stimuli, highlighting the intricate interplay of factors influencing macrophage functionality [23].
2.1. M1/2 polarized macrophage
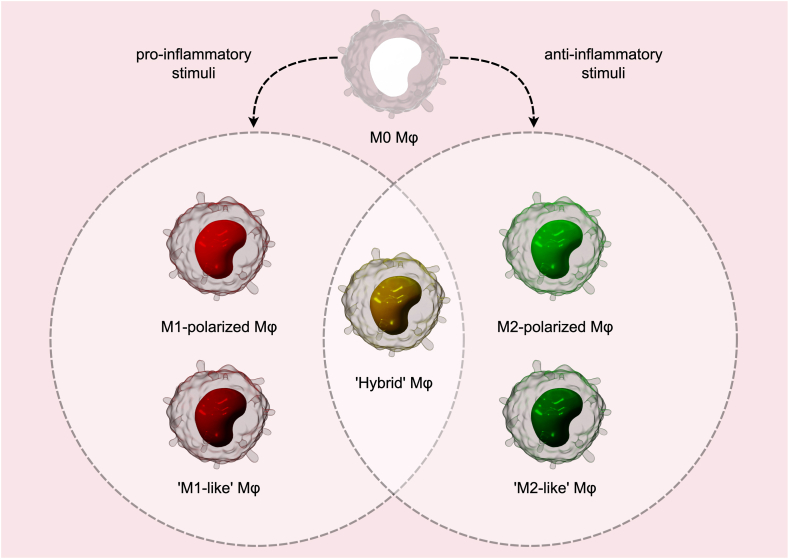
M1 and M2 macrophages epitomize divergent polarization states within the spectrum of mononuclear phagocyte differentiation, each characterized by distinct phenotypic configurations and functional capacities. The expression profile of salient polarization markers in these macrophage subsets is intricately modulated by both the qualitative nature and temporal dynamics of inciting stimuli [24]. Within the in vivo milieu, the delineation between M1 and M2 phenotypes becomes increasingly nuanced, influenced by a complex interplay of local environmental cues and intrinsic signaling mechanisms (Fig. 1) [25].
Fig. 1.
Macrophage Polarization and Hybrid Phenotypes. Left cycle depicts macrophages polarized to the M1 phenotype in response to pro-inflammatory signals. This phenotype is induced by pro-inflammatory cytokines, such as TNF-α and IFN-γ, or by pathogen-associated molecular patterns (PAMPs), such as bacterial LPS. Right cycle shows macrophages polarized to the M2 phenotype under anti-inflammatory signals. This polarization is driven by anti-inflammatory cytokines such as IL-10 and TGF-β, and is associated with tissue repair, immune response attenuation, and anti-inflammatory activities. ‘M1-like’ and ‘M2-like’ macrophages denote a broader spectrum of macrophage states that may overlap or transition between these traditional M1 and M2 categories. Central section displays Hybrid macrophages, which combine features of both M1 and M2 phenotypes. For instance, the M1M2d hybrid phenotype is optimized for targeting neoplastic cells while minimizing collateral tissue damage, whereas phenotypes such as M2aM2d and M2cM2d are associated with tumor progression. (drawn by FigDraw software).
M1 macrophages, also known as classically activated macrophages, are induced by pro-inflammatory cytokines, notably TNF-α and IFN-γ, or through engagement with PAMPs, such as bacterial LPS [26]. The integral role of M1 macrophages in orchestrating antibody-dependent cellular phagocytosis (ADCP) is well-documented, particularly within the context of neoplastic and infectious disease states. This pro-inflammatory lineage is further characterized by its dependence on growth-promoting factors, including GM-CSF and M-CSF, and is distinguished by a prodigious output of inflammatory mediators—IL-1β, IL-6, TNF-α, and IFN-γ [27]. A hallmark of M1 macrophage functionality is the secretion of IL-12, pivotal for the induction of Th1 lineage differentiation, augmented by the generation of NO via inducible nitric oxide synthase (iNOS), which, in concert with reactive oxygen species (ROS), underpins their antimicrobial effector functions [28].
In contrast, M2 macrophages, often referred to as alternatively activated macrophages, are hallmarked by their production of anti-inflammatory cytokines, IL-10 and transforming growth factor (TGF)-β, playing instrumental roles in tissue repair, remodeling, and attenuation of immune responses [29]. The M2 macrophage population is inherently heterogeneous, encompassing various subsets delineated by specific functional roles and expression of surface markers. Despite the identification of distinct M2 subsets, there exists a continuum of activation states, reflecting a degree of functional and phenotypic overlap. The M2a subset, elicited by IL-4 and IL-13, is primarily involved in tissue reparative processes and wound healing, facilitated by enhanced expression of the mannose receptor (Cluster of differentiation (CD)206), which mediates the phagocytic clearance of apoptotic cells and cellular debris [30]. M2b macrophages, induced in response to immune complex engagement and Toll-like receptors (TLR) activation, are characterized by elevated production of anti-inflammatory cytokines IL-10 and C–C motif ligand (CCL)-1, with a notable capacity for antigen presentation, as evidenced by increased CD86 expression [31]. M2c macrophages, differentiated in response to IL-10 and glucocorticoids, are implicated in the resolution phase of immune responses and the de-escalation of inflammatory processes, marked by high expression levels of the scavenger receptor CD163, critical for the clearance of hemoglobin-haptoglobin complexes [32]. The M2d subset, differentiated through adenosine receptor activation and independent of Interleukin 4 receptor (IL-4R) signaling, has been associated with both reparative functions and the modulation of neoplastic cell invasiveness, underscoring the complex and multifaceted roles of macrophages in the regulation of physiological and pathological processes [33,34].
2.2. M1/2-like macrophage
While the traditional dichotomy of M1/M2 classification offers a foundational framework for understanding macrophage polarization, its application is increasingly recognized as somewhat reductive, particularly in light of the nuanced and complex nature of macrophage function within diverse immunopathological contexts [35]. This dichotomy, however, retains a degree of relevance, particularly in delineating macrophage responses within specific settings such as tumor-immune interplay and the dynamics of host-pathogen interactions during microbial pathogenesis [36]. In these environments, the distinct signaling pathways activated by M1 and M2 macrophages substantiate the utility of the M1/M2 paradigm to a certain extent [37].
Advancements in immunological research have unveiled the remarkable plasticity and heterogeneity characterizing macrophage polarization, especially in the face of complex pathophysiological challenges such as cancer, type 2 diabetes, atherosclerosis and liver diseases [38]. These insights have led to an evolving understanding that macrophage polarization extends along a continuum rather than residing within fixed states [39]. In response to this evolving understanding, the scientific community is increasingly adopting the 'M1-like' and 'M2-like' nomenclature (Fig. 1) [40,41]. This refined classification acknowledges the dynamic spectrum of macrophage polarization and accommodates the transitional and mixed phenotypes observed, particularly in vivo, where macrophages are subject to a multitude of converging signals [42].
The introduction of 'M1-like' and 'M2-like' descriptors represents a significant conceptual shift, moving away from a binary classification towards a more fluid and nuanced characterization of macrophage states [43]. This shift not only reflects a deeper appreciation for macrophage plasticity but also aligns with the contemporary understanding of their roles in various pathologies. The 'M1-like' and 'M2-like' terms serve to capture the essence of macrophage behavior that straddles traditional classifications, embodying a spectrum of functional states influenced by the complex interplay of environmental cues and intrinsic cellular mechanisms [44]. This lexicon, therefore, provides a more accurate and flexible framework for articulating the diverse roles of macrophages in health and disease, embracing the complexity inherent in their biological functions and interactions [45].
2.3. Hybrid phenotype macrophage
The actual phenotypic landscape of macrophages is characterized by a continuum of states that exhibit considerable overlap, contingent upon the specific microenvironmental context and the nature of the signaling cues encountered [46]. Empirical evidence, particularly from studies employing Boolean network models, has elucidated the existence of several hybrid macrophage phenotypes (Fig. 1). These phenotypes assume critical functional significance in nuanced biological scenarios [47]. Notably, within the oncological milieu, the M1M2d hybrid phenotype has emerged as an optimally balanced effector profile, capable of effectively targeting neoplastic cells whilst minimizing ancillary tissue damage. In contrast, phenotypic amalgamations such as M2bM2d, M2aM2d, and M2cM2d have been associated with a propensity to foster tumor progression, with the M2aM2d configuration identified as particularly deleterious in this regard, underscoring the complex interplay between macrophage plasticity and disease pathogenesis [48]. Elena Mitsi et al. elucidated that the manifestation of a hybrid phenotype in alveolar macrophages may endow these cells with the capacity for rapid functional transitions between M1-and M2-associated modalities [49]. This phenotypic flexibility facilitates tailored responses to diverse stimuli and the specific requisites of the surrounding tissue microenvironment, thereby optimizing the immunological efficacy and adaptability of macrophages [50].
3. Macrophage polarization and its immune responses to diverse stimuli
3.1. Chemical stimuli with or without biological activity
Chemical substances can modify the physical and chemical conditions of the cellular microenvironment, which, in turn, influences macrophage behavior. Changes in biologically active substances and non biologically active substances, pH or the presence of reactive oxygen species can shift macrophage polarization towards either an M1 or M2 phenotype [51,52]. These substances directly or indirectly interact with biological molecules, they can cause cellular stress or damage that indirectly activates signaling pathways relevant to macrophage polarization. Chemical substances can also affect the metabolic pathways of macrophages, which is closely linked to their polarization. Chemicals affecting mitochondrial function, for instance, could tip this balance by affecting the energy supply of the cells [53]. Substances might alter the expression of surface molecules on macrophages or other immune cells, affecting how these cells interact with each other. This can lead to a change in cytokine production profiles, favoring the development of either M1 or M2 macrophages. Some chemicals can cause epigenetic changes that affect gene expression in macrophages without directly interacting with the genome. These changes can influence the polarization of macrophages by upregulating or downregulating genes associated with either the M1 or M2 phenotype [54]. This definition can be somewhat broad, and these typically include PAMPs and DAMPs, cytokines and chemokines, growth factors, hormones, nutrients and metabolites, complement proteins, and bioactive irons, gases in the environment (Fig. 2).
Fig. 2.
Various factors influencing the polarization of macrophages into M1 and M2 phenotypes. The diagram distinguishes between Direct Stimuli and Associated Stimuli that drive macrophage polarization. Direct Stimuli include factors such as pro-inflammatory cytokines (e.g., TNF-α, IFN-γ, LPS). These stimuli directly induce M1 polarization by enhanced inflammatory pathways (e.g., AP-1, STAT1, NF-κB). Direct Stimuli for M2 polarization involve anti-inflammatory cytokines such as IL-4, IL-13, IL-10, and LPS. These stimuli promote M2 polarization through the activation of specific signaling pathways (e.g., STAT6, NF-κB, and CREB). Associated Stimuli encompass a broader range of signaling molecules and environmental cues that can influence M1 or M2 polarization. This includes pro-inflammatory factors (e.g., PAMP, DAMP, IL-1β, IL-6, IL-12, IL-18, IL-23, omega-3 fatty acids, C3a, C5a, DHT, CO2, high-glucose, high-succinate, high-Na2+) and anti-inflammatory factors (e.g., adrenaline, oxLDLs, TGF-β, omega-3 fatty acids, EGF, FGF2, O2, Mg2+), which promote M1/M2 polarization associated with inflammation, tissue repair and immune modulation. DHT, dihydrotestosterone; IL, interleukin; PAMP, pathogen-associated molecular pattern; DAMPs, damage-associated molecular pattern; LPS, lipopolysaccharide; TNF, tumor necrosis factor; IFN, interferon; C3a, complement 3a; C5a, complement 5a; TLR, Toll-like receptor; TNFR, tumor necrosis factor receptor; IFNR, interferon receptor; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; AP-1, Activator protein 1; STAT, signal transducer and activator of transcription; EGF,epidermal growth factor; FGF, fibroblast growth factor; oxLDLs, oxidized low-density lipoproteins; TGF-β, transforming growth factor; CREB, cAMP-response element binding protein. (drawn by FigDraw software).
3.1.1. PAMPs & DAMPs
PAMPs are molecular signatures associated with pathogens, including LPS in Gram-negative bacteria, peptidoglycans in both Gram-positive and Gram-negative bacteria, flagellin in bacterial flagella, unmethylated CpG motifs in bacterial and viral DNA, double-stranded RNA (dsRNA) associated with viral infections [21]. DAMPs are endogenous molecules that normally are sequestered and become released in response to cell injury or endogenous stress signals, such as proteins (high-mobility group box [HMGB]-1, heat shock protein [HSP]s), nucleic Acids (mitochondrial DNA [mtDNA], cell-free DNA [cfDNA]), lipids (oxidized phospholipids, Sphingolipids), metabolites (Uric Acid, Adenosine triphosphate [ATP]) contributing to either pro-inflammatory responses or initiating repair and resolution processes [22]. PAMPs and DAMPs are recognized by a number of Pattern recognition receptor (PRRs) expressed on macrophage, which can trigger inflammatory and adaptive immune reactions. PRRs such as TLRs are specialized in recognizing bacterial components [55]. Specifically, TLR4 is activated by microbial ligands like LPS. Upon activation, TLR4 initiates two distinct signaling pathways mediated by the TIR-domain-containing adapter-inducing interferon-β (TRIF) and the Myeloid differentiation primary response 88 (MyD88) [56,57]. The TRIF-regulated pathway triggers kinase cascades leading to the activation of interferon regulatory factor (IRF)-3, which induces the secretion of IFNs such as IFN-α and IFN-β. In the TLR4 pathway mediated by MyD88, another adapter molecule, Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway, including its subunits p65 and p50, is activated. NF-κB is a crucial transcription factor involved in the polarization of M1 macrophages, promoting inflammation. Activator protein 1 (AP-1) is also activated by MyD88 via mitogen-activated protein kinase (MAPK) pathway [58,59]. These signaling cascades facilitate the expression of various inflammatory genes, including proinflammatory cytokines like TNF, IL-1β, and IL-12, as well as chemokines hemokine (C-X-C motif) ligand (CXCL)-10, CXCL11, co-stimulating proteins, and antigen-processing proteins [60].
3.1.2. Cytokines and chemokines
Proinflammatory cytokines like TNF-α, IL-1β, IL-6, IL-12, IL-18 and IL-23 promote M1 polarization, while anti-inflammatory cytokines such as IL-4/IL-13, low IL-12, IL-23, high Interleukin 1 receptor antagonist (IL-1Ra), IL-10 drive M2 polarization (chemokines in Table 1) [5]. Proinflammatory cytokines bind to their respective receptors on the surface of macrophages, lead to conformational changes in the receptor, activate transcription factors such as NF-κB and Signal Transducer and Activator of Transcription (STAT)-1, which drive the expression of genes associated with the M1 phenotype [61]. While, anti-inflammatory cytokines activate transcription factors such as STAT6 and peroxisome proliferator-activated receptor gamma (PPAR-γ), which promote the expression of genes associated with the M2 phenotype [62]. Typically, signaling pathways mediated by Janus Kinase (JAK)-1 and JAK3 culminate in the activation of STAT6. Upon activation, STAT6 undergoes nuclear translocation, thereby influencing the modulation of antiviral innate immune responses through the regulation of interferon gene expression [63]. Further, the transcriptional landscape is complexified by the involvement of additional transcription factors such as IRF4 and PPAR-γ. These transcriptional regulators orchestrate the expression of a diverse array of proteins, including Arginase 1 (Arg1), Ym1 (also known as Chitinase 3-like 3 Protein [Chi3l3]), Resistin-like molecule alpha (Retnla) (also known as found in inflammatory zone 1 [Fizz1]), CCL17, and CD206 [64,65]. The regulatory purview of STAT6, IRF4, and PPAR-γ encompasses a broad spectrum of immunomodulatory and effector proteins. Furthermore, the IL-10 signaling cascade plays a pivotal role in immune regulation, wherein IL-10 interacts with its receptor complex, comprising IL-10 receptor (IL-10R)1 and IL-10R2 subunits [66]. This interaction precipitates receptor autophosphorylation and subsequent activation of the STAT3. Engagement of STAT3 with its target promoters modulates gene expression profiles, notably inducing the expression of suppressor of cytokine signaling (SOCS)3, which serves as a critical inhibitor within proinflammatory cytokine signaling pathways, thereby contributing to the attenuation of inflammatory responses [67].
Table 1.
Cytokines and chemokines influencing macrophage polarity.
| M1 macrophage | M2 macrophage | |
|---|---|---|
| Cytokines | IL-1β, IL-6, IL-12, IL-18, IL-23, TNFα | IL-1RA, IL-4/IL-13, IL-10, IL-12, IL-23 |
| Chemokines | CXCL1, CXCL3, CXCL5, CXCL8, CXCL9, CXCL10, CXCL11, CXCL13, CXCL16, CX3CL1, CXCR3, CCL2, CCL3, CCL4, CCL5, CCL8, CCL11, CCL15, CCL19, CCL20; NOS2, CD64, IDO, SOCS1 | CCL1, CCL2, CCL5, CxCL10, CCL13, CCL14, CCL17, CCL18, CCL22, CCL23, CCL24, CCL26, CXCL16, CCR2, CCR3, CCR4 |
3.1.3. Growth factors
Growth factors are a diverse group of proteins capable of stimulating cellular growth, proliferation, healing, and cellular differentiation [68]. Epidermal growth factor (EGF) signaling pathways can intersect with those activated by other growth factors and cytokines involved in macrophage polarization via activation of epidermal growth factor receptor (EGFR)/protein kinase B (Akt)/cyclic adenosine monophosphate (cAMP)-responsive element binding [69,70]. This crosstalk could potentially influence the balance between M1 and M2 polarization in response to different stimuli [71]. Studies have demonstrated that either pharmacological inhibition or genetic ablation of EGFR results in diminished activation of M1 and regulatory macrophages (Mreg) during bacterial infections, both in vitro and in vivo contexts [72]. Such interventions lead to a substantial reduction in the signaling via the NF-κB pathway, affecting both upstream components such as MyD88, phosphorylated inhibitor of nuclear factor kappa B (IKB) kinase (p-IKBK), and phosphorylated NF-κB inhibitor alpha (p-NFKBIA), and downstream processes including the nuclear translocation of RELA Proto-Oncogene and subsequent NF-κB transcriptional activity. It is pertinent to mention that mice deficient in MyD88 (MyD88−/−) exhibit reduced gastritis and an increased bacterial load during Helicobacter pylori infections, suggesting that MyD88 may serve as a critical intermediary linking EGFR signaling to the NF-κB pathway in macrophages. Mechanistically, activation of EGFR is found to initiate the NF-κB and MAPK1/3 pathways, culminating in enhanced cytokine production and macrophage activation [73]. In cancer, EGF is often overexpressed, contributing to tumor growth and progression. TAMs, which are predominantly of the M2-like phenotype, play critical roles in tumor progression by promoting angiogenesis, immunosuppression, and metastasis [74]. Conditioned medium derived from colon cancer cells with a knockout of the EGFR was observed to suppress the expression of markers associated with M2 macrophages with upregulation of markers pertinent to M1 macrophages, including inducible iNOS, IL-12, TNF-α, and C-C chemokine receptor (CCR)-7 [75].
Fibroblast growth factors (FGFs) comprise a family of growth factors involved in angiogenesis, wound healing, and embryonic development. FGF2 plays a pivotal role in shifting TAMs towards a pro-tumourigenic, M2-like phenotype in the tumour microenvironment [76]. Li et al. demonstrated that in colorectal cancer, FGF2, as macrophage-regulating factor, drived M2 macrophage polarization. They showed that FGFR2 activates plasminogen activator inhibitor-1 (PAI-1) via the JAK2/STAT3 signaling pathway, enhancing immune activity and tumor suppression [77]. In gastric cancer, M2 macrophage/TAM was activated through the FGF2/fibroblast growth factor receptor (FGFR)1/phosphoinositide 3-kinase (PI3K)/AKT axis [78]. While, macrophages treated with FGF20 exhibited a reduced pro-inflammatory phenotype upon stimulation with LPS and IFN-γ [79]. This was evidenced by reduced expression of M1 macrophage markers and decreased secretion of pro-inflammatory cytokines. Mechanistic investigations indicated that FGF20 binds to the FGF receptor 1 isoform on macrophages, leading to enhanced stability of β-catenin through the phosphorylation of glycogen synthase kinase (GSK)3β. This process ultimately attenuates the polarization of macrophages towards the M1 phenotype [79]. Meanwhile, Mechanistic studies have shown that FGF12 stimulated the pro-inflammatory activation of macrophages, leading to the activation of hepatic stellate cells via the monocyte chemoattractant protein-1 (MCP-1)/CCR2 signaling axis, and additional experiments revealed that FGF12-mediated regulation of macrophage activation predominantly occurred through the Janus kinase–signal transducer of activators of transcription pathway [80].
TGF-β primarily can drive macrophage polarization toward M1 or M2, sometimes referred to as "Mreg" or "M2-like" macrophages by binding to and activating the TGF-β/Smads signaling pathway depending on different situation [25,81]. TGF-β acts through the type II receptor, which then complexes with the type I receptor to form a receptor complex, leading to the phosphorylation of the type I receptor domain. This, in turn, activates downstream signaling molecules to regulate the expression of Smad2 and Smad3 genes. Furthermore, the influence of Smad-dependent pathways on M2 polarization is modulated by Smad7, which interferes with TGF-β receptor type I (TGF-βRI) and obstructs the formation of the Smad2/3/4 complex, thus curbing the synthesis of anti-inflammatory M2 cytokines [82]. In contrast, through Smad-independent pathways, TGF-β stimulates the MAPK, c-Jun N-terminal kinase (JNK), p38, and NF-κB pathways via the TGF-β-activated kinase 1 protein, leading to macrophage reprogramming toward the M1 phenotype, particularly when the Smad-dependent pathway is inhibited [83]. Additionally, TGF-β is reported to promote an M2-like phenotype by enhancing SNAIL expression and disruption of TGF-β/SNAIL signaling, resumed the generation of pro-inflammatory cytokines [84]. TGF-β secreted by Mesenchymal stem cells (MSCs) could skew LPS-stimulated macrophage polarization towards the M2-like phenotype, reduce inflammatory reactions, and improve the phagocytic ability via the Akt/forkhead box protein O1 (FOXO1) pathway, providing potential therapeutic strategies for sepsis [85].
3.1.4. Hormones
Steroid hormones, including the vast majority of sex hormones are derived from cholesterol. The mechanism through which hormones exert their influences on macrophage polarization is multifaceted, involving intricate intracellular signaling cascades that ultimately lead to altered gene expression profiles conducive to the M1/M2 phenotype depending on the external environmental conditions, receptor expressions, and concentration of hormones. Taking dihydrotestosterone (DHT) as an example, androgen binding to classical and non-classical androgen receptors (ARs) mediates genomic and non-genomic androgen effects, respectively. Androgens play a pivotal role in regulating the expression of proinflammatory molecules, such as TNF-α, within mouse monocytes and macrophages [86]. Lai et al. demonstrated that LPS-induced TNF-α production was diminished in bone-marrow-derived macrophages (BMDMs) lacking classical ARs, while observing enhanced TNF-α promoter activity upon DHT stimulation [87]. Nonetheless, divergent findings have been reported, indicating contrasting effects of DHT on TNF-α output in various contexts. For instance, DHT was found to mitigate TNF-α secretion in LPS-stimulated splenic macrophages from hemorrhaged mice and to inhibit TNF-α and nitric oxide production in macrophage cell lines, concurrently elevating IL-10 expression levels. Moreover, DHT exhibited the capacity to augment markers associated with M1 macrophage polarization and to induce apoptosis through the fas ligand (FasL) pathway and caspase activation [88]. Recent investigations have revealed that pre-exposure to DHT amplifies markers indicative of M2 macrophage polarization, such as Chi3l3, Arg1, and Ym1, subsequent to IL-4 stimulation in BMDMs, with analogous results observed in RAW264.7 cell lines. This effect was linked to diminished TLR4 expression and reduced sensitivity to its ligands [89]. Cumulatively, these studies delineate the intricate and context-dependent impact of androgens and ARs on macrophage inflammatory responses, with an overarching tendency towards the suppression of M1 macrophage polarization. This underscores the pivotal role of androgens in modulating inflammatory pathways, particularly in male individuals. The variability in outcomes underscores the necessity for comprehensive exploration of androgen/AR-mediated immune modulation, particularly in light of their significance in immune response dynamics and cancer progression. Additionally, discrepant results may stem from differential activation of classical versus non-classical AR pathways [90].
Peptide Hormones, composed of amino acids, include hormones such as insulin, growth hormone, and prolactin. Insulin, a central regulatory hormone in glucose metabolism, also exerts significant effects on the immune system, including the modulation of macrophage polarization. Macrophages express insulin receptors (IRs) that are integral to downstream signaling networks, which exhibit considerable overlap with the signaling pathways of pattern recognition receptors and cytokine receptors. These shared signaling nodes are pivotal in shaping the polarity of macrophages. The primary action of insulin on cells, including macrophages, is mediated through the IR, a transmembrane tyrosine kinase receptor [91]. The IR, once activated, functions as a crucial hub for downstream signaling molecules such as insulin receptor substrates (IRS) and key kinases including MAPK, PI3K, and protein kinase B (PKB)/Akt [92]. These molecules influence the regulation of significant pathways such as the target of rapamycin kinase complex (TORC)1 and GSK3. Notably, the insulin signaling pathway shares common elements with the signaling pathways initiated by TLRs. TLRs are critical in recognizing PAMPs and DAMPs. Upon ligand engagement, TLRs engage various adaptor proteins, including MyD88, TIR domain containing adaptor protein (TIRAP), TRIF, and TRIF-related adaptor molecule (TRAM). These proteins subsequently activate a cascade involving interleukin-1 receptor-associated kinases (IRAKs), TNF Receptor Associated Factor 6 (TRAF6), ubiquitin E3 ligases, and a complex consisting of TGF-beta activated kinase (TAK) and TAK binding protein (TAB). This pathway culminates in the activation of the inhibitor of nuclear factor-κB kinase (IKK) complex, leading to the stimulation of the transcription factor NF-κB, which then upregulates the transcription of pro-inflammatory cytokines and IFN-γ. Furthermore, the signaling mechanisms utilized by TLRs overlap with those of the insulin receptor, particularly in the activation pathways involving NF-κB, MAPK, and Akt2, which are pivotal in promoting the transcription of pro-inflammatory mediators. The IFN-γ signaling pathway, mediated through the activation of the interferon receptor γ, also engages JAKs and STAT1, leading to MAPK activation and the pro-inflammatory polarization of macrophages. Moreover, the interaction between MAPK pathways and insulin signaling may represent a significant point of synergistic crosstalk. In addition to these pathways, IL-4-mediated signaling through STAT6 plays a crucial role in the differentiation of M2 macrophages. The synergy between insulin and IL-4 is further evidenced by the Akt1-dependent activation of TORC1 and the inhibition of GSK3, which collectively deliver anti-apoptotic and anti-inflammatory signals. IL-4 also triggers the activation of IRS2, suggesting a potential crosstalk between IL-4 and insulin signaling pathways that could drive M2 macrophage differentiation, highlighting the interconnected nature of these signaling networks in cellular responses [93].
Amino Acid-Derived Hormones are derived from amino acids, and Catecholamines, specifically noradrenaline and adrenaline, engage with α and β adrenergic receptors present on macrophages, thereby influencing the functional responses of these cells across various tissues including the small intestine, lung, and adipose tissue. Specifically, α adrenergic receptors are predominantly linked to pro-inflammatory activities, whereas β adrenergic receptors, particularly the β2 subtype, are associated with anti-inflammatory responses and facilitation of tissue remodeling [94]. The mechanism by which adrenaline promotes macrophage polarization involves the modulation of adrenergic receptors expressed on these cells. Studies have shown that beta-2 adrenergic receptor (β2AR) activation by adrenaline or synthetic agonists can stimulated macrophages firmly to some M2 side categories of the M1-M2 spectrum [95]. M2-promoting effects were mediated specifically through β2-adrenergic receptors and were associated with cyclic AMP response element binding protein (CREB), CCAAT-enhancer-binding protein (C/EBP)β, and activating transcription factor (ATF) transcription factor pathways but not with established M1-M2 STAT pathways [96]. Examination of global transcriptional profiles revealed the identification of twenty supplementary M2 genes exhibiting regulation patterns consistent with β-adrenergic and C/EBPβ signaling pathways [97]. Subsequent promoter-centric bioinformatics assessments substantiated the prevalence of C/EBPβ transcription factor binding motifs within these M2 genes [97].
3.1.5. Fats, lipids, glucose, amino acid and their metabolites
Fatty acids and lipids play an indispensable role in the functionality of immune cells, crucially maintaining membrane integrity, fluidity, and enabling biochemical processes. In the dynamic milieu of the immune system, environmental lipids orchestrate macrophage responses through direct receptor interactions and the modulation of signaling cascades, thereby exerting profound influence over their functional activities [98,99]. Specifically, saturated fatty acids (SFAs), prevalent in high-fat diets and certain microbial profiles, prompt macrophages to adopt a pro-inflammatory M1 phenotype. For instance, palmitic acid, a common SFA, activates pattern recognition receptors such as TLR4 and TLR2, which engage the NF-κB signaling pathway to upregulate pro-inflammatory gene expression. This activation spearheads the release of pro-inflammatory cytokines and chemokines, hallmarks of M1 macrophages, also facilitated by the MAPK pathways triggered by specific environmental lipids. Conversely, unsaturated fatty acids, particularly vv like eicosapentaenoic acid and docosahexaenoic acid, generally promote an anti-inflammatory M2 macrophage phenotype conducive to tumor suppression [100]. This is achieved through their interaction with G-protein coupled receptors and subsequent activation of anti-inflammatory pathways mediated by STAT3 and PPAR-γ. Among the lipids that influence M2 polarization, oxidized low-density lipoproteins (oxLDLs) are notably potent, alongside the metabolites of arachidonic acid—an omega-6 essential fatty acid—such as prostaglandin E2, which also fosters an M2 phenotype [101]. Furthermore, other lipids can trigger activation of STAT6, PPARs, and Liver X receptors (LXRs), enhancing gene expression associated with M2 macrophages and promoting processes such as tissue repair, fibrosis, and the resolution of inflammation [102]. In the metabolic activities of macrophages, while M1 macrophages primarily accumulate lipids and engage in phagocytosis via lipogenesis, M2 macrophages predominantly rely on fatty acid β-oxidation as their chief energy source. Additionally, cholesterol metabolism, regulated by sterol regulatory element-binding proteins (SREBPs), PPARs, and LXRs, plays a critical role in cholesterol efflux capacity and the development of foam cells, which are reminiscent of M2-like macrophages [102]. The dynamics of lipolysis and fatty acid uptake, marked by proteins such as CD36, further contribute to the cytokine production landscape within macrophage populations [103].
Glucose availability and the concentration levels of glucose in the environment, epecially glucose metabolism plays a crucial role in dictating macrophage polarization through various biochemical pathways and signaling mechanisms [[104], [105], [106]]. A high-glucose environment not only directly induces M1 polarization via overproducing ROS but also promotes this process by increasing the sensitivity of macrophages to LPS, commonly observed in vivo diabetic conditions [107,108]. Mechanistically, M1 macrophages are distinguished by a primarily glycolytic metabolic process [109]. Glycolysis involves the decomposition of a six-carbon glucose molecule—visually represented by blue circles, turning white upon phosphorylation—into three-carbon sugars, which are subsequently converted into pyruvate, ATP, nicotinamide adenine dinucleotide hydrogen (NADH), and hydrogen ions (H+). The transcriptional framework facilitating glycolysis is regulated by hypoxia-inducible factor (HIF)-1 and, to some extent, by IRF5. This process is supported by the augmented expression of the glucose transporter 1 (GLUT1), providing an increased glucose substrate. Additionally, various glycolytic enzymes perform non-traditional roles to bolster M1 effector functions. Disruption of the mitochondrial tricarboxylic acid (TCA) cycle in M1 macrophages leads to the accumulation of citrate and succinate, further augmenting M1 effector functions. Conversely, M2 macrophages exhibit a fully functional TCA cycle, enhanced oxidative phosphorylation (OXPHOS), and increased mitochondrial biogenesis [110]. Activation of ATP citrate lyase (ACLY), triggered by IL-4 signaling, augments M2 effector functions through epigenetic modifications and the generation of substrates for lipogenesis. The enzyme sedoheptulose kinase inhibits the pentose phosphate pathway, and the transcriptional regulation of M2 macrophage metabolism is mediated by PPAR-γ and LXR [111].
Amino acids and their metabolic pathways critically influence the activation of M1 macrophages [112,113]. The electron transport chain and mitochondrial complexes undergo remodeling in response to LPS and IFN-γ stimulation, which enhances mitochondrial ROS production and reduces respiration. This mitochondrial ROS is pivotal in driving the inflammatory response of M1-polarized macrophages. Arginine metabolism through inducible iNOS results in the production of citrulline and NO, which inhibit mitochondrial complexes I and II and facilitate the loss of these complexes during the later stages of M1 polarization [114]. Concurrently, the TCA cycle experiences rapid modifications during M1 polarization. Initially, itaconate production leads to succinate accumulation via succinate dehydrogenase inhibition, stabilizing HIF-1α and enhancing the inflammatory response. NO further inhibits aconitase 2 and isocitrate dehydrogenase, reducing carbon flux from citrate to α-ketoglutarate (α-KG), which in turn triggers increased carbon input from glutamine into the TCA cycle, supporting α-KG and succinate anaplerosis. The balance between succinate and α-KG regulates M1 polarization through prolyl prolyl hydroxylase domain (PHD)-dependent proline hydroxylation of IKK-β, crucial for the activation of NF-κB signaling [114]. High levels of succinate early in M1 polarization promote a robust inflammatory response. The later phase is marked by inhibition of the pyruvate dehydrogenase complex (PDHC) and oxoglutarate dehydrogenase complex (OGDC), drastically reducing citrate, itaconate, and succinate levels. PDHC inhibition has been observed through NO-mediated nitrosation of cysteine residues in dihydrolipoamide dehydrogenase, which is also associated with OGDC. This suggests that NO likely mediates the reduced flux from α-KG to succinate observed in the late phase [115]. Furthermore, glutamine-derived α-KG is crucial for inducing endotoxin tolerance, and inhibition of glutaminase increases mortality following repeated LPS exposures. The serine synthesis pathway (SSP) also contributes to M1 polarization by generating glycine, which drives the folate cycle essential for glutathione and s-adenosyl methionine (SAM) production. Glutathione is vital for optimal IL-1β expression, and SAM enhances the expression of inflammatory genes through H3 lysine 36 (H3K36) trimethylation [116].
3.1.6. Complement proteins
The complement system encompasses a repertoire of more than 35 cell-associated and soluble molecules. Among these, macrophages express various receptors that recognize complement components. Notably, globular C1q receptor (gC1qR) and C1q collagen-like domain binding calreticulin (cC1qR) interact with the globular heads and collagen-like tail domains of C1q, respectively. However, it is postulated that additional C1q receptors exist, some of which may also recognize the collagen-like domains of related defense collagen family members such as mannan-binding lectin and ficolins. Moreover, macrophages express receptors complement receptor (CR)1, CR3, CR4, and CRIg, which recognize C3b-opsonized targets either as intact C3b or its degradation fragment iC3b. Additionally, receptors for complement activation fragments C3a (C3aR) and C5a (C5aR1 and C5aR2) are also present on macrophages. Consequently, macrophages serve as pivotal effectors in executing the functions of complement activation. Complement components such as C3a, C5a, and C5b-9 influence cytokine production in macrophages, promoting an inflammatory (M1-like) phenotype [117]. Conversely, apoptotic cells and targets opsonized with complement components C1q or C3b enhance clearance processes and modulate cytokine production towards an anti-inflammatory, resolving (M2-like) phenotype in macrophages, potentially inhibiting pro-inflammatory signaling mediated by PAMPs [118]. C3a and C5a, known for their pro-inflammatory properties, activate macrophages through diverse signaling mechanisms [119]. For instance, C3a induces NLRP3 inflammasome activation via extracellular signal-regulated kinase 1/2 (ERK1/2)-mediated ATP release upon LPS stimulation in human monocytes. Samstad et al. observed that C5a generated during complement activation by cholesterol crystals contributes to inflammation by activating the NLRP3 inflammasome and promoting the release of IL-1β, TNFα, and ROS [117]. In contrast to other phagocytic receptors such as Fcγ receptors, the engulfment of iC3b-coated particles via CR3 has traditionally been regarded as anti-inflammatory. CR3-dependent engulfment does not trigger the arachidonic acid cascade or the release of toxic oxygen products. However, regardless of macrophage phenotype, significant levels of TNFα secretion were not observed following CR3 ligation, unlike ligation of Fcγ receptors, which induced TNFα release across all macrophage subsets [119].
3.1.7. Bioactive ions
Cumulatively, early investigations suggest that heightened extracellular concentrations of salt, induced either in vivo or in vitro through NaCl-induced hyperosmolarity, create an environment conducive to the differentiation of pro-inflammatory macrophages. Specifically, elevated salt levels (sodium chloride (NaCl)) promote the activation of pro-inflammatory macrophages via the p38 MAPK-dependent activation of the transcription factor nuclear factor of activated T cell 5 (NFAT5), subsequently leading to NO production by inducible nitric oxide synthase 2 [120]. Furthermore, elevated salt levels induce p38-dependent activation of the heterodimeric transcription factor AP-1, resulting in the expression of pro-inflammatory target genes [119]. Additionally, high salt triggers the activation of the NLR family pyrin domain containing 3 (NLRP3) and NLR family caspase activation and recruitment domain-containing 4 (NLRC4) inflammasomes, leading to caspase 1 activation and subsequent secretion of IL-1β [121]. The augmentation of pro-inflammatory macrophage activation facilitated by high salt is advantageous for pathogen clearance and promotes Th17 cell differentiation through IL-1β secretion. Conversely, heightened salt concentrations diminish alternative macrophage activation and suppressive function by downregulating the expression of STAT6 and inhibiting AKT–mammalian target of rapamycin (mTOR) signaling. These effects result in decreased expression of anti-inflammatory macrophage marker genes [122]. The compromised function of alternatively activated macrophages under conditions of elevated salt has been associated with delayed wound healing [120].
Iron plays a pivotal role in modulating the fate and function of macrophages [123]. Specifically, acute iron deprivation significantly diminishes the expression of ATF4 in quiescent human macrophages and suppresses the expression of interleukin IL-1β and TNF-α in human macrophages treated with LPS. Conversely, iron supplementation distinctly influences macrophage phenotypes. The impact of iron on pro-inflammatory macrophages exhibits variability. Increased endogenous iron levels stimulate TNF production in macrophages [124]. Additionally, exogenous iron supplementation augments the expression of M1 markers (iNOS, TNF-α, and IL-1β) while reducing the expression of M2 markers (IL-10 and CD206) in RAW264.7 cells, BMDMs, and THP-1 cells [125]. Iron-induced mechanisms influencing macrophage polarization include the suppression of NF-κB activation due to iron deprivation, potentially promoting M1-like polarization by enhancing the activity of nicotinamide adenine dinucleotide phosphate oxidase (Nox), which facilitates p65 nuclear translocation and NF-κB activity. Iron treatment may activate the MAPK-phosphorylated mitogen-activated protein kinase-activated protein kinase 2 (MK2) pathway, enhancing TNF-α production and the generation of ROS and peroxynitrite, thereby promoting M1-like polarization. Acute iron deprivation can also inhibit ATF4 expression, suppressing M2-like polarization in tumorous tissues. Moreover, iron may facilitate M1-like polarization by activating MAPK and NF-κB pathways, enhancing intracellular ROS production. In cases of iron overload, increased hepcidin expression can suppress STAT6 activation while enhancing IRF3 expression, leading to increased iNOS expression in M1-like macrophages. Iron can also activate the NLRP3 inflammasome, promoting M1-like macrophage polarization through ROS generation [123]. Conversely, iron may promote M2-like macrophage polarization by moderately activating mitochondrial ROS cascades in conjunction with the NF-κB pathway, mediated by the downregulation of glia maturation factor gamma [126].
Magnesium (Mg) emerges as a prominent divalent cation in cellular systems, occupying the second position in abundance. Its indispensability stems from its multifaceted involvement in biological processes, encompassing the regulation of energy metabolism, enzyme kinetics, signal transduction, and the synthesis of nucleic acids and proteins [127]. Over 300 enzymes crucially rely on Mg ions for catalytic activities, notably those engaged in ATP utilization or synthesis, as well as in DNA and RNA biosynthesis. Furthermore, Mg2+ exerts a regulatory influence on calcium (Ca2+) and potassium (K+) channels, profoundly influencing intracellular ion dynamics and signaling modulation. In macrophages, magnesium orchestrates the modulation of ion channels and transporters, including calcium channels, potassium channels, and sodium-potassium pumps, thereby impacting intracellular signaling cascades, membrane potential, and cytokine production, pivotal in macrophage polarization processes [128]. Notably, the observed upregulation of CD206 alongside the downregulation of CCR7 and IL-1β in response to Mg2+ stimulation hints at the promotion of a M2-like response [129]. Nevertheless, variations in intracellular Mg2+ concentrations significantly dictate macrophage differentiation outcomes; elevated intracellular Mg2+ levels favor M2-type polarization, whereas reduced levels tend to skew differentiation towards the M1 phenotype [130]. Moreover, Mg-dependent enzymes, such as protein kinase C (PKC) and MAPKs, exert regulatory control over macrophage activation and cytokine production, thereby modulating M1 or M2 polarization dynamics [131]. In the context of tendon–bone healing processes, Mg influences M2 polarization and facilitates fibrocartilage interface regeneration via the selective activation of AKT1 and the PI3K/AKT pathway [132].
3.1.8. Oxygen (O2)
Oxygen plays a crucial role in macrophage polarization through its involvement in various metabolic and signaling pathways. The level of oxygen in the tissue environment can significantly influence the behavior and function of macrophages, leading to different polarization states. In hypoxic conditions, commonly found in inflammatory or tumoral tissues, HIF-1α is stabilized [133]. HIF-1α, a transcription factor pivotal in cellular response to hypoxia, promotes the polarization of macrophages towards a pro-inflammatory phenotype [134]. Mechanistically, HIF-1α facilitates the release of pro-inflammatory cytokines and antimicrobial peptides, enhances phagocytosis and NO production, inhibits apoptosis, and promotes intracellular oxygen redistribution and suppression of PHD activity, thus stabilizing HIF-1α protein and enhancing phagocytic activity to modulate macrophage immune response. HIF-1α significantly contributes to macrophage plasticity, during Th1 cytokine-induced macrophage polarization, HIF-1α binds to inducible iNOS, promoting the M1 phenotype conversion to maintain NO homeostasis during inflammation. Under hypoxic and inflammatory conditions, the association between HIF-1α and NF-κB is notably tight, as they share stimuli, regulatory factors, and target genes. Hypoxia activates HIF-1α and NF-κB through an inhibition of IKKβ and TAK1 dependent pathways [135]. Additionally, given NF-κB's role in regulating HIF-1α levels and activity under both normoxic and hypoxic conditions, the related pathways are evolutionarily conserved [134]. Comparative to hypoxic states, hyperoxic states are less extensively studied; however, they appear to also induce macrophage polarization towards the M1 phenotype. In a cohort study on hyperoxic exposure, researchers activated macrophages with high oxygen levels (O2 = 65 %) followed by LPS (100 ng/ml) to investigate lung-related exposure factors. Post-activation with 65 % O2, preterm macrophages exhibited a significant pro-inflammatory response (increased major histocompatibility complex, class II, DR [HLA-DR] expression and cytokine release) compared to LPS stimulation alone, suggesting their involvement in exacerbated inflammation, potentially mediated by downregulation of early growth response 2 (EGR2) and growth factor independent 1 (Gfi1) in developing lungs [136].
3.1.9. Carbon dioxide (CO2)
The mechanisms by which CO2 affects immune cell function remain under investigation, and it is unclear whether these effects are due to changes in cellular and tissue pH or if they follow a pathway akin to cellular oxygen sensing via hypoxia-inducible factors, with no definitive evidence yet established for CO2 sensing [137]. Using monocytes and macrophages as examples, which play roles in inflammation and promoting wound healing, circulating monocytes are exposed to approximately 5 % CO2 in the bloodstream, while macrophages recruited to inflamed sites may encounter environments with up to 10 % CO2 concentrations, or even higher. Elevated CO2 levels can lead to acidification of the local environment. This acidification may affect macrophage polarization. In certain contexts, acidic pH can enhance the production of pro-inflammatory cytokines, whereas in others, it may promote anti-inflammatory or tissue regeneration phenotypes. CO2 is converted by carbonic anhydrase into bicarbonate (HCO3−) and protons (H+), where enzyme activity is crucial for maintaining tissue acid-base balance and can influence macrophage function [138]. The availability of bicarbonate and changes in intracellular pH may affect macrophage polarization by regulating the activity of transcription factors and signaling pathways involved in inflammation and tissue repair [139]. Additionally, CO2 levels may also indicate hypoxic conditions, particularly in tumor microenvironments. Hypoxia and CO2 accumulation can synergistically affect macrophage behavior, promoting phenotypes that support tumor growth and suppress immune responses. Recent studies have shown that CO2 reduces autocrine inflammatory gene expression, thereby inhibiting macrophage activation in a manner dependent on the reduction of intracellular pH [140].
3.2. Physical stimuli
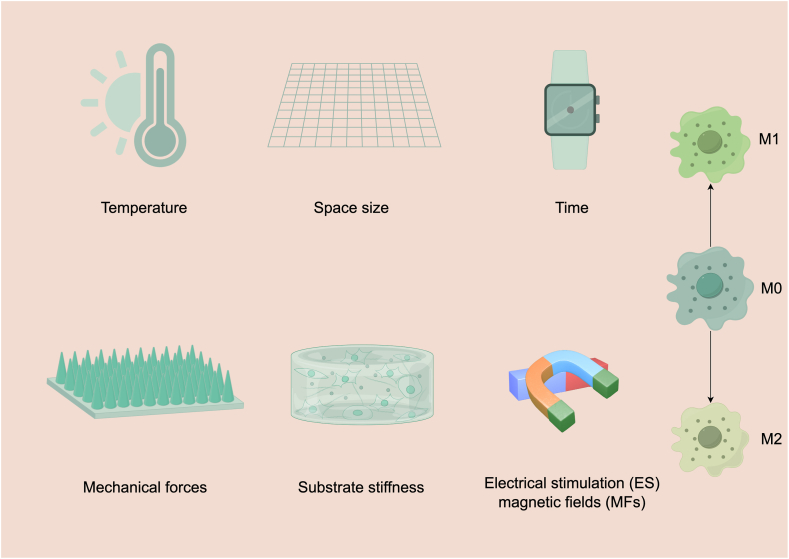
The influence of physical cues on the modulation of macrophage functionality, encompassing their polarization towards inflammatory and reparative phenotypes, constitutes a subject of considerable interest [141]. Intriguingly, the underlying mechanisms governing these responses appear distinct from those elucidated in the context of conventional mechanobiological investigations, owing primarily to disparities in the molecular apparatus implicated in mechanotransduction, notably adhesive and cytoskeletal components. In this part,we will delineate investigations highlighting the impact of temperature, space size, time, mechanical forces, electrical fields and magnetic fields, substrate stiffness on macrophage behavior (Fig. 3).
Fig. 3.
The influence of various physical stimuli on macrophage polarization into M1 and M2 phenotypes. The depicted stimuli include temperature, space size, time, mechanical forces, electrical fields, magnetic fields, and substrate stiffness. Elevated temperatures can induce heat shock proteins (HSPs), influencing cytokine production through pathways such as NF-κB and MAPK. Spatial constraints, achieved through micropatterning or confinement, inhibit macrophage spreading and alter polarization. The temporal dynamics of macrophage polarization are illustrated by the time-dependent expression of M1 and M2 markers. Mechanical forces such as shear stress and substrate stiffness modulate macrophage behavior. For instance, Piezo1 mechanosensitive channels influence macrophage polarization and function. Stiff substrates promote M2 polarization, while softer substrates encourage M1 polarization through pathways involving ROS and NF-κB. Electrical Fields (ES) can polarize macrophages towards M2 phenotypes, with specific frequencies and intensities impacting polarization. Exposure to magnetic fields affects macrophage polarization by altering ion currents and receptor aggregation. The aggregation of specific receptors under magnetic fields can lead to macrophage elongation and M2 polarization, influencing immune responses. Substrate Stiffness affects macrophage morphology and polarization. Rigid substrates generally promote M2 polarization, while softer substrates favor M1 polarization through mechanisms involving ROS production and integrin signaling. (drawn by FigDraw software).
3.2.1. Temperature
Temperature, as one of the crucial physical parameters governing cellular life cycles, plays a pivotal role in maintaining cellular homeostasis and regulating cellular functions. At the cellular level, temperature appears to play a significant role in modulating the immune response of macrophages. To investigate the impact of temperature on LPS-activated macrophages, RAW 264.7 cells were cultured with LPS under different temperature conditions, and the secretion of three cytokines was measured. Compared to 37 °C, incubation at 31 °C increased the secretion of TNF, while exposure at 39 °C resulted in decreased TNF secretion. Secretion of IL-6 was lower at 31 °C compared to 37 °C, remaining unchanged at 39 °C. Secretion of white cell protein-10 was inhibited on both sides of 37 °C. Only IL-6 secretion was sensitive to the pre-culture temperature effect [142]. Dynamics of cytokine secretion and steady-state messenger RNA analysis suggest that the temperature regulation mechanisms of TNF and IL-6 may differ. Mouse-derived RAW 264.7 macrophages stimulated with LPS exhibit distinct functional characteristics at different temperature conditions. Secretion of HMGB1, IL-1β, IL-6, and TNF-α, as well as NF-κB dimer (p50 and p65) levels, were higher when cultured in a 37 °C environment compared to 34 °C and 40 °C. However, levels of HSP70 and heat shock factor (HSF) increased in the 34 °C and 40 °C environments, potentially leading to differential immune responses [143]. In vivo studies suggest that low temperatures are a risk factor for stimulating alveolar macrophages to produce pro-inflammatory factors. Research findings indicate that exposure to lower temperatures increases the release of TNF-α, macrophage inflammatory protein (MIP)-1α, and IL-6 in vivo [144]. Given that temperature is elevated during fever, a long-recognized cardinal feature of inflammation, it is possible that macrophage function is responsive to thermal signals. A study suggested that elevating body temperature of mice results in an increase in LPS-induced downstream signaling including enhanced phosphorylation of IKK and IκB, NF-κB nuclear translocation and binding to the TNF-α promoter in macrophages upon secondary stimulation. Mild heat treatment also induces expression of HSP70 and use of HSP70 inhibitors largely abrogates the ability of the thermal treatment to enhance TNF-α, suggesting that the induction of HSP70 is important for mediation of thermal effects on macrophage function [145].
Elevated temperatures can induce the heat shock response in cells, leading to the production of HSPs as direct effects. HSPs are known to play roles in protecting cells from stress and in modulating the immune response. The febrile temperature range can exert both stimulatory and inhibitory effects on the production of pro-inflammatory cytokines by macrophages. The review by Lee et al. concluded that these divergent outcomes may be contingent upon the activation state of macrophages and the subcellular localization of HSPs. During the initial phase of macrophage activation, febrile temperatures enhance the synthesis of TNF-α, IL-6, and NO, which are induced by LPS. This augmented cytokine production is correlated with the upregulation of HSP70/72 expression within these cells. Subsequently, HSP70 can be secreted into the extracellular milieu, where it exerts its cytokine-modulating effects via the CD14/TLR pathway. Consequently, this cascade culminates in the activation of NF-κB and the MAPK pathway, ultimately leading to the transcription of cytokine genes. Conversely, febrile temperatures can also dampen the production of TNF-α, IL-6, and IL-1β, as well as the release of HMGB-1 induced by LPS. This suppression is attributed to the direct binding of thermally-induced HSF-1 to the heat shock response element within the cytokine promoter region, thereby acting as a transcriptional repressor. Moreover, both LPS and heat treatment can induce the expression of HSP70. Subsequently, HSP70 interacts with TRAF6 or IKK, inhibiting subsequent NF-κB activation and cytokine production [146].
3.2.2. Space size
The concept of "space size" influencing macrophage polarization refers to how the physical dimensions of the cell's environment or the geometry and spatial constraints of surrounding structures can affect macrophage behavior and function [147,148]. While the direct impact of physical factors on cellular physiology and differentiation is well-established, the precise influence of these mechanical aspects on macrophage activation and their functional outcomes remains predominantly elusive and has only recently garnered attention [149,150]. Spatial confinement, enforced through micropatterning, microporous substrates, or cell crowding, inhibits macrophage spreading and attenuates the late LPS-induced transcriptional programs, including biomarkers such as IL-6, CXCL9, IL-1β, and iNOS, through mechanomodulation of chromatin compaction and epigenetic modifications involving changes in histone deacetylase 3 (HDAC3) levels and H3K36 dimethylation. Mechanistically, confinement diminishes actin polymerization, thereby reducing the LPS-triggered nuclear translocation of myocardin-related transcription factor A-serum response factor (myocardin-related transcription factor-A, [MRTF-A]). Consequently, this diminishes the activity of the MRTF-A-serum response factor (SRF) complex, leading to the subsequent suppression of the inflammatory response [151].
In light of the impact of spatial considerations on macrophages and with the aim of better simulating in vivo structures, numerous studies have turned to employing 3D culture techniques for further investigation [152]. Under these conditions, such in vitro experiments, geared towards mimicking tumor microenvironments, infectious microenvironments, and tissue repair processes, predominantly explore the interactions between macrophages and other cells [[153], [154], [155]]. However, research specifically focusing on the influence of spatial factors on macrophage polarization remains relatively scarce. Of course, in the formation of 3D environments using experimental reagents, the inherent physical properties of the materials themselves, including hardness, configuration, connectivity, spatial dimensions, etc., may alter the polarization characteristics of macrophages, introducing a plethora of confounding factors.
3.2.3. Time
The influence of time on macrophage polarization is a nuanced aspect of immune response dynamics, reflecting the changing needs of tissue environments during processes such as infection, wound healing, and chronic inflammation [156]. Macrophages are highly versatile immune cells capable of adopting a spectrum of functional states between the pro-inflammatory (M1) and anti-inflammatory or tissue-repairing (M2) phenotypes, in response to various stimuli. The temporal dynamics of macrophage polarization are crucial for the resolution of inflammation and the promotion of tissue repair, as well as in the pathogenesis of chronic inflammatory and fibrotic diseases [157]. In the initial stages of an immune response, such as after infection or injury, there's a predominant polarization towards the M1 phenotype. These macrophages produce pro-inflammatory cytokines, chemokines, and ROS to eliminate pathogens and clear dead cells. This phase is crucial for the initial clearance of infection and debris. Following the clearance phase, there is a transition towards M2 polarization, characterized by the expression of anti-inflammatory cytokines, growth factors, and enzymes involved in extracellular matrix (ECM) remodeling. This phase supports tissue repair, resolution of inflammation, and return to homeostasis. The production of certain cytokines and growth factors by M1 and M2 macrophages serves as feedback to modulate the polarization state. IL-10 produced by M2 macrophages can act in an autocrine or paracrine manner to suppress M1 polarization and promote M2 traits, facilitating the shift towards resolution and healing over time [157].
Macrophage polarization, a process requiring external stimuli, exhibits time dependency in vitro. The temporal expression profiles of these polarization markers at the RNA and protein levels vary depending on the duration of external stimuli [158]. In an in vitro study of human monocyte-derived macrophages (MDMs), it was observed that markers for M1 (CD64, CD86, CXCL9, CXCL10, HLA-DR, indoleamine 2,3-Dioxygenase 1 (IDO1), IL1β, IL12, TNF), M2a (CD200R, CD206, CCL17, CCL22, IL-10, transglutaminase 2 [TGM2]), and M2c (CD163, IL-10, TGFβ) phenotypes appeared at different times after stimulation, ranging from 4 to 72 h. Generally, in vitro studies control stimulation durations within the range of 4–72 h, and the results overall demonstrate the time-dependent nature of macrophage polarization. The study comparing polarization states at different stimulation times found that a stimulation duration of 72 h yields better results compared to 24 h [158].
3.2.4. Mechanical forces
Mechanical forces within the body, such as shear stress, compression, and tensile strain, play crucial roles in influencing cellular behavior, including the polarization of macrophages. In response to heightened membrane tension, stretch-activated ion channels facilitate the passage of ions across the membrane. Significantly, piezo type mechanosensitive ion channel component 1 (Piezo1), a mechanically-sensitive, non-selective cation channel, plays a crucial role in various developmental processes and pathological conditions [159]. Recent investigations have unveiled the involvement of TLR4 signaling in augmenting macrophage bactericidal activity through the mechanosensitive Piezo1 sensor. Depletion of Piezo1 genetically abolishes these responses triggered by bacterial infection or LPS stimulation, which entails the formation of a Piezo1-TLR4 complex for the reorganization of F-actin architecture. Consequently, this reorganization enhances phagocytosis, mitochondrial phagosomal ROS production, and bacterial eradication. Furthermore, the investigation into the mechanically activated cation channel Piezo1 has extended to its impact on macrophage polarization and microenvironmental rigidity perception [160]. Studies indicate that Piezo1-deficient macrophages exhibit enhanced wound healing and reduced inflammation. Notably, calcium flux, regulated by Piezo1, is modulated by soluble cues and amplified on stiff substrates, as demonstrated by macrophages expressing a transgenic calcium reporter. These findings underscore Piezo1's pivotal role as a mechanosensor in macrophages, influencing responses to stiffness and polarization [161].
Another relevant mechanical signal transduction is integrin signaling mediating cell–cell and cell extracellular matrix adhesion [162]. Mechanical forces can alter integrin clustering and conformation, activating signaling pathways like focal adhesion kinase (FAK), which are implicated in macrophage polarization [163]. Structurally, integrins consist of heterodimeric α and β subunits, facilitating two primary roles: firstly, they serve as physical bridges linking the cytoskeleton and extracellular matrix, thereby preserving tissue morphology and function, subsequently they perceive external stimuli and modulate cellular responses [164]. Evidence indicates that matrix stiffness can directly influence macrophage cytoskeletal dynamics and phenotype via integrins. Notably, FAK predominantly associated with integrin signaling, has been implicated in this process. Studies have elucidated that a stiff matrix can induce macrophage M2 polarization through the integrin β5-FAK-mitogen-activated protein kinase kinase (MEK)1/2-extracellular signal-regulated kinase 1/2 (ERK1/2) pathway [165].
3.2.5. Electrical stimulation (ES) & magnetic fields (MFs)
Electric fields with intensities as modest as 5 mV/mm, along with magnetic fields, have been shown to modulate macrophage behavior and functional outcomes, primarily affecting processes such as migration, phagocytic activity, and the secretion of ROS and cytokines [166]. Electric stimulation (ES) has emerged as a viable approach for inducing macrophage polarization, offering versatility through variations in voltage, current, frequency, and waveform parameters. Particularly pertinent to wound healing, the transition from the inflammatory to the remodeling phase hinges upon achieving optimal macrophage polarization, a process amenable to controlled manipulation under ES [167]. Mccann et al. demonstrated the feasibility of polarizing macrophages towards the M2 phenotype using a direct current (DC) electric field ranging from 12.7 to 30.5 V/s for a duration of 2 h via glass microelectrodes. Notably, an incremental rise in intracellular calcium ions was noted post approximately 2 min of ES, characterized by repetitive calcium-dependent peaks, correlating with M2 polarization [168]. In a more recent study, electric fields characterized by capacitive properties were employed to modulate macrophage phenotype within a custom-designed system. Intriguingly, the frequency of the electric fields exhibited a significant impact on macrophage polarization, with frequencies of 10 and 60 Hz correlating with heightened expression of CD206 markers, thereby validating polarization [169]. Furthermore, Xu et al. introduced a noncontact electrical stimulation device utilizing capacitive coupling to investigate its effect on macrophage behavior during tissue repair. Under an electric field strength of 25 mV/mm, negligible alterations in the expression of CCR7 (M1 marker) and CD206 (M2 marker) were observed. However, an increased ratio of M2/M1 macrophages was evident, indicative of the successful modulation of macrophage polarization towards the M2 phenotype at a noncontact ES intensity of 53 mV/mm, thereby augmenting the immune response during the wound healing process [170].
Magnetic-field forces induce changes in ionic currents and/or distort macrophage membranes, resulting in the aggregation of transient receptor potential cation channel subfamily M Member 2 (TRPM2) cation channel receptors in magnet-exposed macrophages [171]. This receptor aggregation may render them dysfunctional, disrupting calcium ion (Ca2+) balance, consequently affecting actin polymerization and leading to macrophage elongation. Additionally, a similar aggregation pattern of CX3C motif chemokine receptor 1 (CX3CR1) receptors, responsible for guiding macrophages to transplanted organs, was observed in Ras homolog family member A (RhoA) knockout macrophages. Prior research has shown that, under controlled conditions, the polarization of M0 macrophages towards the M2 phenotype correlates with macrophage elongation and the activation of M2-specific gene expression. Therefore, the induction of macrophage elongation through magnetic field exposure may trigger the expression of M2-specific genes, such as Arg1 [172]. Adriana Vinhas and colleagues observed that magnetic stimulation exerted an influence on intercellular communication among tendon cells and macrophages, exhibiting immunomodulatory effects on macrophages and promoting the M2 phenotype. This phenomenon may involve the activation of FAK signaling pathways [173]. Jiang et al. reported that macrophages polarized from M0 to M2 phenotypes by blocking the Piezo1-AP-1-CCL2 signaling pathway under a rotating magnetic field [174].
3.2.6. Substrate stiffness
Substrate stiffness can influence macrophage polarization through various mechanotransduction pathways and interactions with the extracellular matrix (ECM) [175]. The stiffness of the ECM profoundly impacts various aspects of macrophage biology, including morphology, cytoskeletal organization, functionality, and metabolic activity [176]. On rigid substrates, macrophages display an elongated, spindle-like morphology characterized by increased spreading and the formation of filamentous pseudopodia. In tumor micro-environment, elevated stiffness ECM also upregulates cancer cell secretion of M-CSF, consequently promoting macrophage polarization towards an M2-like phenotype. In contrast, flexible substrates trigger reactive oxygen ROS production in macrophages, initiating polarization towards the M1 phenotype through the ROS-mediated NF-κB pathway, thereby augmenting the secretion of cytokines such as IL-1β and TNF-α [177]. Furthermore, interactions between betaig-h3 and type I collagen result in the formation of denser fibers, impacting macrophage behavior. Specifically, macrophages cultured on these denser fibers exhibit morphological alterations indicative of an enhanced M2 phenotype and heightened immunosuppressive functions [178]. According to another research, on soft substrates, BMDMs undergo a shift towards classically activated macrophages via modulation of the ROS-initiated NF-κB pathway. This transition is beneficial for anti-tumor responses, facilitated by the secretion of pro-inflammatory cytokines [179].
4. Role of macrophage polarization in immune response and intervention strategies for clinical translation in tissue repair and regeneration
The polarization of macrophages is critical in orchestrating the immune response. M1 macrophages are key players in the early stages of the immune response, initiating the destruction of pathogens and affected tissues. They also stimulate other immune cells through the secretion of cytokines. Following the initial inflammatory response, the shift towards M2 macrophages helps in resolving inflammation, clearing debris, and repairing damaged tissues. This shift is vital to prevent chronic inflammation and associated diseases. The process of tissue repair following injury encompasses inflammation and post-inflammatory restoration, during which macrophages play a pivotal role by self-regulating their polarization transformations in response to changes in the microenvironment. Current research frequently capitalizes on this characteristic of macrophages, employing interventions aimed at accelerating tissue repair through the modulation of macrophage activity. Effective tissue regeneration necessitates the precise phenotypic transition of macrophages. The presence of any macrophage phenotype at an inappropriate time or location within the tissue invariably results in deleterious consequences. Once the wound is stabilized, macrophages undergo a phenotypic transition towards an M2-like state, which is conducive to tissue repair, cellular proliferation, and structural remodeling [180]. These macrophages facilitate angiogenesis and ECM remodeling by secreting a variety of growth factors, such as platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), FGF, along with proteases including serine proteases, matrix metallopeptidase (MMP)-2, and MMP-9. Additionally, they perform immunosuppressive functions through the secretion of IL-10 and TGF-β [180].
4.1. Pharmacological agents and small for macrophage reprogram
The strategic manipulation of macrophage polarization through pharmacological agents and small molecules stands as a cutting-edge approach in the field of tissue repair and regeneration. This perspective focuses on modulating the immune response to favor healing processes, leveraging the plasticity of macrophages to transition between pro-inflammatory (M1) and pro-healing (M2) states [181]. The sustained presence of M1 macrophages contributes to a persistent inflammatory response, detrimental to effective tissue repair and regeneration. A plethora of research has elucidated the mechanisms through which macrophages manifest anti-inflammatory and anti-fibrotic activities in the context of altered tissue homeostasis. Therefore, in therapeutic approaches, controlling appropriate M1 macrophage polarization and appropriately prolonging M2 polarization are currently therapeutic targets in the field of regenerative medicine [182].
In terms of macrophage polarization, the main strategies for tissue repair focus on two aspects: Pro-Healing Promotion and Anti-Inflammatory Strategies [25]. Studies have shown that activation of the PPAR-γ pathway can encourage macrophages to adopt an M2 phenotype, promoting anti-inflammatory responses and tissue repair mechanisms [183,184]. Mirza et al. observed a phenotypic transition in macrophages throughout the process of skin wound healing, which coincided with the upregulation of PPAR-γ and its downstream effectors, concomitant with an augmentation in mitochondrial content [185]. In diabetic conditions, the heightened activity of PPAR-γ was hindered due to the persistent expression of IL-1β in both murine and human wounds. Notably, experimental studies utilizing myeloid-specific PPAR-γ knockout mice revealed that the absence of PPAR-γ in macrophages alone was adequate to prolong the inflammatory phase of wound healing and impede overall tissue repair [186]. Furthermore, the administration of PPAR-γ agonists facilitated the emergence of a macrophage phenotype associated with wound healing, both in vitro and in vivo, even within the diabetic wound milieu. Of significance, the topical application of PPAR-γ agonists significantly ameliorated the healing process in diabetic murine models, thereby suggesting a promising therapeutic avenue for mitigating inflammation and enhancing the resolution of chronic wounds. Thiazolidinediones, a class of PPAR-γ agonists, have been examined for their potential to facilitate wound healing and mitigate fibrotic responses [187]. Interleukins 4 and 13 are key cytokines that drive M2 polarization. Research is exploring the delivery of these cytokines, either directly or through engineered systems, to injury sites to enhance tissue regeneration by encouraging a pro-healing macrophage phenotype [188].
NF-κB orchestrates the upregulation of genes associated with pro-survival and proliferative processes, thereby fostering tissue repair and regeneration in vivo [189,190]. However, NF-κB primarily functions as a central mediator in the augmentation of inflammation through its induction of inflammatory cytokines and chemokines [191]. NF-κB stands as the predominant transcription factor governing M1 macrophage polarization, with STAT1 assuming a consequential role thereafter. Pertinent literature documents the existence of negative interplay between the pivotal IKKβ and STAT1 during instances of infection. This interplay serves to inhibit M1 polarization, possibly serving as a negative feedback mechanism conducive to inflammation resolution [192]. Inhibitors of the STAT1 and NF-κB pathways, which target signaling cascades critical in M1 polarization, have been demonstrated to mitigate chronic inflammation and fibrosis [193]. Such interventions may create an environment conducive to tissue repair and regeneration [194].
4.2. Drug delivery systems representing dual roles
Continuing the discussion on the aforementioned small molecules, the administration of these small molecules often requires drug delivery vehicles, which achieve repair objectives through the release of factors. Indeed, the design of these vehicles may inherently induce macrophage polarization due to their physicochemical properties, thereby representing dual roles of scaffolds [195]. The localized administration of active small molecular compounds, either through reservoirs or coating layers within scaffolds, presents a straightforward approach to modulating the behavior of monocytes/macrophages in tissue regeneration [196]. Given the mounting evidence underscoring the pivotal contributions of macrophages across various phases of tissue healing, researchers have devised diverse strategies for delivering bioactive substances aimed at regulating macrophage function during distinct stages of tissue regeneration. Biomaterials can be engineered to release cytokines, growth factors, or other bioactive molecules that directly influence macrophage polarization and function [197]. Present immunomodulatory delivery strategies can be categorized as follows: 1) augmentation of monocyte/macrophage recruitment to the site of injury; 2) induction of a pro-inflammatory phenotype during the initial phase of healing; 3) modulation of macrophage polarization toward an M2-like phenotype; and 4) sequential guidance of the M1-to-M2 macrophage phenotype transition to enhance tissue regeneration [104].
Alterations in the physical, chemical, and biological attributes of biomaterials can modulate the host's specific response to them, consequently influencing the microenvironmental cues and the reciprocal communication between macrophages and biomaterials (Table 2) [198,199]. In the pursuit of enhancing tissue regeneration, researchers frequently aim to design and fabricate biomaterials with enhanced tissue compatibility and the ability to induce M2 polarization of macrophages [[200], [201], [202]]. This objective is typically pursued through the manipulation of specific physicochemical properties of the materials. Notably, a prevalent approach involves engineering biomaterials with tailored physical and chemical attributes capable of regulating macrophage behavior. Such materials, characterized by appropriate stiffness, surface morphology, and degradation kinetics, possess the ability to modulate macrophage phenotype, promoting M2 polarization while concurrently attenuating inflammation [110]. Alterations in the architecture of biomaterials, such as size, geometry, and porosity, as well as surface conjugations and mechanical properties, can significantly influence macrophage activation [203,204]. The response of macrophages to these implanted materials is pivotal in determining the success or failure of the device. As previously noted, macrophages serve as principal phagocytes that rapidly detect and internalize foreign entities. Smaller particles are easily assimilated; however, larger particles (exceeding 10 μm) may provoke macrophages, leading to an inflammatory M1-like phenotype characterized by the secretion of pro-inflammatory cytokines, proteases, and reactive oxygen species. In the case of larger implants (greater than 100 μm), a foreign body reaction (FBR) occurs, culminating in the formation of a foreign body giant cell (FBGC), which consists of a conglomerate of multiple macrophages surrounding the implant [205,206]. Additionally, researchers are actively involved in developing bioactive compounds and nanoparticles that can be integrated into biomaterial matrices to actively direct macrophage polarization. These compounds effectively target distinct signaling pathways implicated in macrophage activation, thereby steering their functional trajectory towards a regenerative phenotype [207]. Moreover, advancements in the field of biomedical devices, including scaffolds and implants, incorporate strategic interventions to promote M2 polarization and enhance tissue regeneration [208].
Table 2.
Responses of macrophage mediated by the physicochemical properties of biomaterials in vivo and vitro.
| properties of biomaterials | macrophage responses | reference | ||
|---|---|---|---|---|
| biomaterial size | poly (lactic-co-glycolic acid) (PLGA) microparticles (MPs) (5–7 μm), nanoparticles (PLGA-NPs) (389 nm) | in vitro | MPs > NPs: stimulated TNF-α and IL-1β in J774 macrophages. | [218] |
| endotoxin-free intermediate chitin (40–70 μm), small chitin (<40 μm) | in vivo | intermediate chitin > small chitin: stimulated TNF production. Only small chitin particles stimulated IL-10 production in WT C57BL/6 mice lung macrophages. | [219] | |
| geometric construction | endotoxin-free Ch powder in two geometries: ChO (with orthogonal struts), ChD (with diagonal and orthogonal struts) | in vitro | ChO > ChD: TNF-αand IL-12/23 in human monocytes isolated from healthy donors. | [220] |
| subcutaneous implantation: 3D Poly ε-caprolactone (PCL) nanofiber, 2D nanofiber scaffolds | in vivo | 3D > 2D: macrophage infiltration, M2/M1 ratio after 4 weeks of implantation into 9-week old Sprague Dawley (SD) rats. | [221] | |
| composition | gelatin methacryloyl (GelMA) and poly (ethylene glycol)diacrylate (PEGDA) hydrogels | in vitro | GelMA > PEGDA hydrogels: CD206 expression in THP-1 cells PEGDA > GelMA hydrogels: CD86 expression in THP-1 cells |
[222] |
| coiled-coil (CC)- and Ca2+-crosslinked alginate hydrogels | in vivo | Ca2+- > CC-crosslinked gels: released IL-1β following LPS stimulation in female C57BL6/J mice peritoneal macrophage. | [223] | |
| surface modification | amines (NH2), carboxylic acids (COOH) and phosphonic acids (PO3H2) modified poly (styrene) (PS) surface | in vitro | Substrates modified with COOH resulted in an increase in IL-10 secretion and CD206 expression but did not alter either TNF-α secretion or CD80 expression in THP-1-macrophage. | [224] |
| polyetheretherketone (PEEK), sulfonated (SPEEK) and zinc-coated SPEEK scaffolds | in vivo | Zn-coated SPEEK > PEEK, SPEEK scaffolds: M2 (F4/80+CD206+)/M1 (F4/80+CCR7+) proportion subcutaneous implantation in C57BL/6 mice. | [225] | |
| topography | smooth Ti and rough Ti surface | in vitro | smooth Ti > rough Ti: pro-inflammatory/anti-inflammatory phenotype in primary murine macrophages. | [226] |
| bulk metallic glasses (BMGs) and BMGs with 55 nm nanorod arrays (BMG-55) | in vivo | BMG-55>flat BMG: the ratio of Arg-1/iNOS expression in macrophages of tissues surrounding after 2 weeks in C57BL/6 mice aged 9–12 weeks. | [227] | |
| stiffness | transglutaminase cross-linked gelatins (TG-gels): degrees of matrix stiffness | in vitro | matrix stiffness (high > mid > low): M1-associated markers (IL-1β and TNF-α) in RAW264.7 cells | [228] |
| methacrylate–gelatin (GelMA) hydrogel: low-, medium-, high-stiffness (2, 10, and 29 kPa) | in vivo | high-stiffness > medium- and low-stiffness hydrogel implants: M1/M2 proportion low-stiffness > medium- and high-stiffness hydrogel implants: M2/M1 proportion in BALB/c 6-week-old male mice. |
[229] | |
| dynamic physicochemical changes | PEG hydrogels: homogeneous surface to a dynamic Arg-Gly-Asp (RGD)-patterned surface upon UV exposure | in vitro | After exposure to UV radiation: iNOS+ IL-10lo M1 transformed to the Arg-1+ IL-10hi M2 phenotype in mouse BMDMs. | [230] |
| shape-memory PEG-modified gold nanorods (AuNRs)/PCL films (SMPs):flat surface to micro-grooved surface after near-infrared (NIR) irradiation | in vivo | upon exposure to NIR: iNOS+IL-10lo macrophages to the Arg + IL-10hi phenotype in Sprague Dawley (SD) rats. | [231] | |
| degradation products | Mg-based implants generate a range of bioactive substances, including degradation particles (DPs), 200, 400, 800 and 1600 μg/ml generated from Mg via electrochemical corrosion | in vitro | stimulated with DPs(1600 > 800>400 > 200 μg/ml): CD86+M1/CD206+M2 proportion in RAW264.7 cell line. | [232] |
| Zn-based porous scaffolds (incorporating 0.8 wt% Li): isotropic minimal surface G unit and anisotropic BCC unit | in vivo | isotropic minimal surface G unit > anisotropic BCC unit: degradation rate and promoting macrophage polarization toward an anti-inflammatory phenotype in 12-week-old male Sprague-Dawley rats weighing 350–400 g. | [233] | |
5. Future directions
Although autologous cell-based genetically engineered therapy is advancing rapidly in clinical trials, especially in the fields of cancer treatment, cell gene editing strategies like clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9 system for tissue regeneration treatment is still in very early stages of development [209,210]. The significance and intricacy of macrophages are further underscored by their involvement in the advancement of various diseases, including chronic wounds, diabetes and drug-induced liver injury (DILI) [211,212]. Emerging therapeutic approaches are being developed to modulate macrophage activity or ameliorate macrophage dysfunction [213]. In reality, the regulation of macrophage activity during tissue injury repair presents considerable challenges. The primary difficulty lies in the precise targeting and modulation of macrophages, which exhibit various phenotypes at different stages of the repair process. This often necessitates the modulation of multiple genes or pathways. Additionally, different tissue injuries involve distinct repair processes, or alternatively, variations in the repair process following medical intervention. Clinically, for infectious injuries, surgical debridement is frequently required to remove infectious agents. Subsequently, it can be posited that the repair enters a synthetically induced late phase, wherein macrophages can be maximally leveraged for their reparative functions. At this stage, the macrophages are engineered to adopt an M2-like phenotype, optimizing their healing capabilities. Theoretically speaking, the local genetically engineered macrophages contributed to accelerated tissue growth but not caused morbidity in animals. The versatility of genetically engineered macrophages is also highlighted by showing that they can be engineered to secrete proteins that either reduce excessive inflammatory response, such as IL-6, TNF, or promote tissue remodeling, by TGF, IL-10 [214]. The principal obstacle in applying genetic and epigenetic strategies to clinical contexts is the precise delivery to macrophages within the tissue microenvironment, which necessitates high specificity and minimal off-target effects [215]. Moreover, maintaining the long-term stability of these modifications and evaluating any unintended consequences are vital to ensuring their safe clinical use. Additionally, the deployment of genetic and epigenetic interventions in a clinical setting introduces a range of regulatory and ethical considerations, especially concerning modifications to the germline or the possibility of enduring alterations in gene expression [216]. These factors collectively underscore the complexity and the critical need for meticulous oversight in the clinical application of these advanced therapeutic technologies [217].
Funding statement
National Natural Science Foundation of China (No. 82070694).
Data availability statement
No data was used for the research described in the article.
CRediT authorship contribution statement
Jing-Dong Xue: Writing – original draft, Resources, Conceptualization. Jing Gao: Writing – original draft, Resources. Ai-Fang Tang: Writing – review & editing, Supervision. Chao Feng: Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Ai-Fang Tang, Email: 18930172691@qq.com.
Chao Feng, Email: fengchao9901790@aliyun.com.
Abbreviations
- BCG
Bacille Calmette-Guérin
- Th
T helper
- IFN
interferon
- Ig
immunoglobulin
- IL
interleukin
- MHC
major histocompatibility complex
- LPS
lipopolysaccharide
- NO
nitric oxide
- TNF
tumor necrosis factor
- TAMs
tumor-associated macrophages
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- M-CSF
gacrophage colony-stimulating factor
- PAMPs
pathogen-associated molecular patterns
- DAMPs
damage-associated molecular patterns
- ADCP
antibody-dependent cellular phagocytosis
- iNOS
inducible nitric oxide synthase
- ROS
reactive oxygen species
- TGF
transforming growth factor
- CD
Cluster of differentiation
- TLRs
Toll-like receptors
- CCL
C–C motif ligand
- IL-4R
interleukin 4 receptor
- dsRNA
double-stranded RNA
- HMGB
high-mobility group box
- HSP
heat shock protein
- mtDNA
mitochondrial DNA
- cfDNA
cell-free DNA
- ATP
Adenosine triphosphate
- PRRs
pattern recognition receptors
- TRIF
TIR-domain-containing adapter-inducing interferon-β
- MyD88
myeloid differentiation primary response 88
- IRF
interferon regulatory factor
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- AP-1
Activator protein 1
- MAPK
mitogen-activated protein kinase
- CXCL
chemokine (C-X-C motif) ligand
- IL-1Ra
interleukin 1 receptor antagonist
- STAT
signal transducer and activator of transcription
- PPAR-γ
peroxisome proliferator-activated receptor gamma
- JAK
Janus kinase
- Arg1
Arginase 1
- Chi3l3
chitinase 3-like 3 protein
- Retnla
Resistin-like molecule-alpha
- Fizz
found in inflammatory zone
- IL-10R
IL-10 receptor
- SOCS
suppressor of cytokine signaling
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- Akt
protein kinase
- BcAMP
cyclic adenosine monophosphate
- IKB
inhibitor of nuclear factor kappa B
- p-IKBK
phosphorylated inhibitor of nuclear factor kappa B
- p-NFKBIA
phosphorylated NF-kB inhibitor alpha
- CCR
C-C chemokine receptor
- FGF
fibroblast growth factor
- PAI
plasminogen activator inhibitor
- FGFR
fibroblast growth factor receptor
- PI3K
phosphoinositide 3-kinase
- GSK
glycogen synthase kinase
- MCP-1
monocyte chemoattractant protein-1
- TGF-βRI
transforming growth factor-β receptor type I
- JNK
c-Jun N-terminal kinase
- MSC
mesenchymal stem cell
- FOXO1
Forkhead box protein O1
- DHT
dihydrotestosterone
- AR
androgen receptor
- BMDMs
Bone marrow-derived macrophages
- FasL
fas ligand
- IR
insulin receptor
- IRS
insulin receptor substrates
- PKB
protein kinase B
- TORC
target of rapamycin complex
- TIRAP
TIR domain-containing adapter protein
- TRAM
TRIF-related adaptor molecule
- IRAKs
interleukin-1 receptor-associated kinases
- TRAF6
TNF receptor-associated factor 6
- TAK
TGF-β-activated kinase
- TAB
TAK binding protein
- IKK
inhibitor of nuclear factor-κB kinase
- β2AR
beta-2 adrenergic receptor
- CREB
cAMP-response element binding protein
- C/EBP
CCAAT-enhancer-binding protein
- ATF
activating transcription factor
- SFA
saturated fatty acid
- oxLDL
oxidized low-density lipoprotein
- LXR
liver X receptor
- SREBPs
sterol regulatory element-binding proteins
- NADH
nicotinamide adenine dinucleotide hydrogen
- HIF
hypoxia-inducible factor
- GLUT
glucose transporter
- TCA
tricarboxylic acid
- OXPHOS
oxidative phosphorylation
- ACLY
ATP citrate lyase
- α-KG
α-ketoglutarate
- PHD
prolyl hydroxylase domain
- PDHC
pyruvate dehydrogenase complex
- OGDC
oxoglutarate dehydrogenase complex
- SSP
serine synthesis pathway
- SAM
s-adenosylmethionine
- H3K36
H3 lysine 36
- gC1qR
globular C1q receptor
- cC1qR
C1q collagen-like domain binding calreticulin
- CR
complement receptor
- NFAT5
nuclear factor of activated T cells 5
- NLRP3
NLR family pyrin domain containing 3
- NLRC4
NLR family caspase activation and recruitment domain-containing 4
- mTOR
mammalian target of rapamycin
- MK2
mitogen-activated protein kinase-activated protein kinase 2
- PKC
protein kinase C
- HLA-DR
major histocompatibility complex
- class II
DR
- EGR
early growth response
- Gfi
growth factor independent
- HSF
heat shock factor
- MIP
macrophage inflammatory protein
- HDAC
histone deacetylase
- MRTF-A
myocardin-related transcription factor-A
- SRF
serum response factor
- IDO
indoleamine 2,3-Dioxygenase
- TGM
transglutaminase
- Piezo1
piezo type mechanosensitive ion channel component 1
- FAK
focal adhesion kinase
- MEK
mitogen-activated protein kinase kinase
- ERK
extracellular signal-regulated kinase
- TRPM
transient receptor potential cation channel subfamily M Member
- CX3CR1
CX3C motif chemokine receptor 1
- RhoA
Ras homolog family member A
- ECM
extracellular matrix
- PDGF
platelet-derived growth factor
- VEGF
vascular endothelial growth factor
- MMP
matrix metallopeptidase
- CRISPR
clustered regularly interspaced short palindromic repeats
References
- 1.Wynn T.A., Chawla A., Pollard J.W. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park M.D., et al. Macrophages in health and disease. Cell. 2022;185(23):4259–4279. doi: 10.1016/j.cell.2022.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lampiasi N. Macrophage polarization: Learning to manage it. Int. J. Mol. Sci. 2022;23(13) doi: 10.3390/ijms23137208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans S.S., Repasky E.A., Fisher D.T. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat. Rev. Immunol. 2015;15(6):335–349. doi: 10.1038/nri3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen S., et al. Macrophages in immunoregulation and therapeutics. Signal Transduct. Targeted Ther. 2023;8(1):207. doi: 10.1038/s41392-023-01452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho Y.K., et al. MicroRNA-10a-5p regulates macrophage polarization and promotes therapeutic adipose tissue remodeling. Mol Metab. 2019;29:86–98. doi: 10.1016/j.molmet.2019.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray P.J. Macrophage polarization. Annu. Rev. Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 8.Chen X., et al. Macrophage polarization and its role in the pathogenesis of acute lung injury/acute respiratory distress syndrome. Inflamm. Res. 2020;69(9):883–895. doi: 10.1007/s00011-020-01378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bashir S., et al. Macrophage polarization: the link between inflammation and related diseases. Inflamm. Res. 2016;65(1):1–11. doi: 10.1007/s00011-015-0874-1. [DOI] [PubMed] [Google Scholar]
- 10.Mackaness G.B. Pillars article: cellular resistance to infection. J. Exp. Med. 1962;116:381–406. J Immunol, 2014. 193(7): pp. 3185–221. [PubMed] [Google Scholar]
- 11.Stein M., et al. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J. Exp. Med. 1992;176(1):287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doyle A.G., et al. Interleukin-13 alters the activation state of murine macrophages in vitro: comparison with interleukin-4 and interferon-gamma. Eur. J. Immunol. 1994;24(6):1441–1445. doi: 10.1002/eji.1830240630. [DOI] [PubMed] [Google Scholar]
- 13.Mills C.D., et al. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000;164(12):6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 14.Mantovani A., et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 15.Stout R.D., Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J. Leukoc. Biol. 2004;76(3):509–513. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosser D.M. The many faces of macrophage activation. J. Leukoc. Biol. 2003;73(2):209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 17.Duluc D., et al. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood. 2007;110(13):4319–4330. doi: 10.1182/blood-2007-02-072587. [DOI] [PubMed] [Google Scholar]
- 18.Martinez F.O., Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee H., et al. Macrophage polarization in innate immune responses contributing to pathogenesis of chronic kidney disease. BMC Nephrol. 2020;21(1):270. doi: 10.1186/s12882-020-01921-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng H., et al. Regulating the polarization of macrophages: a promising approach to vascular Dermatosis. J Immunol Res. 2020;2020 doi: 10.1155/2020/8148272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greene C.J., et al. Macrophages disseminate pathogen associated molecular patterns through the direct extracellular release of the soluble content of their phagolysosomes. Nat. Commun. 2022;13(1):3072. doi: 10.1038/s41467-022-30654-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Y., Jiang W., Zhou R. DAMP sensing and sterile inflammation: intracellular, intercellular and inter-organ pathways. Nat. Rev. Immunol. 2024 doi: 10.1038/s41577-024-01027-3. Epub ahead of print. PMID: 38684933. [DOI] [PubMed] [Google Scholar]
- 23.Pemmari A., et al. Attenuating effects of nortrachelogenin on IL-4 and IL-13 induced alternative macrophage activation and on bleomycin-induced dermal fibrosis. J. Agric. Food Chem. 2018;66(51):13405–13413. doi: 10.1021/acs.jafc.8b03023. [DOI] [PubMed] [Google Scholar]
- 24.Wang L.L., et al. Cutting edge: the regulatory mechanisms of macrophage polarization and function during pregnancy. J. Reprod. Immunol. 2022;151 doi: 10.1016/j.jri.2022.103627. [DOI] [PubMed] [Google Scholar]
- 25.Li M., et al. Signaling pathways in macrophages: molecular mechanisms and therapeutic targets. MedComm. 2020;4(5):e349. doi: 10.1002/mco2.349. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toshchakov V., et al. TLR4, but not TLR2, mediates IFN-beta-induced STAT1alpha/beta-dependent gene expression in macrophages. Nat. Immunol. 2002;3(4):392–398. doi: 10.1038/ni774. [DOI] [PubMed] [Google Scholar]
- 27.Atri C., Guerfali F.Z., Laouini D. Role of human macrophage polarization in inflammation during infectious diseases. Int. J. Mol. Sci. 2018;19(6) doi: 10.3390/ijms19061801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmieri E.M., et al. Nitric oxide orchestrates metabolic rewiring in M1 macrophages by targeting aconitase 2 and pyruvate dehydrogenase. Nat. Commun. 2020;11(1):698. doi: 10.1038/s41467-020-14433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlundt C., et al. The multifaceted roles of macrophages in bone regeneration: a story of polarization, activation and time. Acta Biomater. 2021;133:46–57. doi: 10.1016/j.actbio.2021.04.052. [DOI] [PubMed] [Google Scholar]
- 30.Anders H.J., Ryu M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 2011;80(9):915–925. doi: 10.1038/ki.2011.217. [DOI] [PubMed] [Google Scholar]
- 31.Zhang B., et al. Age decreases macrophage IL-10 expression: implications for functional recovery and tissue repair in spinal cord injury. Exp. Neurol. 2015;273:83–91. doi: 10.1016/j.expneurol.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olmes G., et al. CD163+ M2c-like macrophages predominate in renal biopsies from patients with lupus nephritis. Arthritis Res. Ther. 2016;18:90. doi: 10.1186/s13075-016-0989-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrante C.J., et al. The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Ralpha) signaling. Inflammation. 2013;36(4):921–931. doi: 10.1007/s10753-013-9621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nikovics K., et al. Hybridization-chain-reaction is a relevant method for in situ detection of M2d-like macrophages in a mini-pig model. FASEB J. 2020;34(12):15675–15686. doi: 10.1096/fj.202001496R. [DOI] [PubMed] [Google Scholar]
- 35.Lawrence T., Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat. Rev. Immunol. 2011;11(11):750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y.H., et al. Modulators of the balance between M1 and M2 macrophages during pregnancy. Front. Immunol. 2017;8:120. doi: 10.3389/fimmu.2017.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kerneur C., Cano C.E., Olive D. Major pathways involved in macrophage polarization in cancer. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1026954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang C., et al. Macrophage polarization and its role in liver disease. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.803037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parisi L., et al. Macrophage polarization in chronic inflammatory diseases: killers or builders? J Immunol Res. 2018;2018 doi: 10.1155/2018/8917804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murray P.J., et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H., et al. The impact of the tumor microenvironment on macrophage polarization in cancer metastatic progression. Int. J. Mol. Sci. 2021;22(12) doi: 10.3390/ijms22126560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yunna C., et al. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020;877 doi: 10.1016/j.ejphar.2020.173090. [DOI] [PubMed] [Google Scholar]
- 43.Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oshi M., et al. M1 Macrophage and M1/M2 ratio defined by transcriptomic signatures resemble only part of their conventional clinical characteristics in breast cancer. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-73624-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strizova Z., et al. M1/M2 macrophages and their overlaps - myth or reality? Clin. Sci. (Lond.) 2023;137(15):1067–1093. doi: 10.1042/CS20220531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Leon U.A., et al. Boolean modeling reveals that cyclic attractors in macrophage polarization serve as reservoirs of states to balance external perturbations from the tumor microenvironment. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1012730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitsi E., et al. Human alveolar macrophages predominately express combined classical M1 and M2 surface markers in steady state. Respir. Res. 2018;19(1):66. doi: 10.1186/s12931-018-0777-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munoz-Rojas A.R., et al. Co-stimulation with opposing macrophage polarization cues leads to orthogonal secretion programs in individual cells. Nat. Commun. 2021;12(1):301. doi: 10.1038/s41467-020-20540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.El-Kenawi A., et al. Acidity promotes tumour progression by altering macrophage phenotype in prostate cancer. Br. J. Cancer. 2019;121(7):556–566. doi: 10.1038/s41416-019-0542-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen X., et al. pH sensing controls tissue inflammation by modulating cellular metabolism and endo-lysosomal function of immune cells. Nat. Immunol. 2022;23(7):1063–1075. doi: 10.1038/s41590-022-01231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y., et al. Mitochondrial metabolism regulates macrophage biology. J. Biol. Chem. 2021;297(1) doi: 10.1016/j.jbc.2021.100904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kiseleva V., et al. Biochemical and molecular inducers and modulators of M2 macrophage polarization in clinical perspective. Int Immunopharmacol. 2023;122 doi: 10.1016/j.intimp.2023.110583. [DOI] [PubMed] [Google Scholar]
- 55.Saeed A., et al. Regulation of cGAS-mediated immune responses and immunotherapy. Adv. Sci. 2020;7(6) doi: 10.1002/advs.201902599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar V. Toll-like receptors in sepsis-associated cytokine storm and their endogenous negative regulators as future immunomodulatory targets. Int Immunopharmacol. 2020;89(Pt B) doi: 10.1016/j.intimp.2020.107087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rad R., et al. Toll-like receptor-dependent activation of antigen-presenting cells affects adaptive immunity to Helicobacter pylori. Gastroenterology. 2007;133(1):150–163 e3. doi: 10.1053/j.gastro.2007.04.071. [DOI] [PubMed] [Google Scholar]
- 58.Xia P., et al. Research progress on Toll-like receptor signal transduction and its roles in antimicrobial immune responses. Appl. Microbiol. Biotechnol. 2021;105(13):5341–5355. doi: 10.1007/s00253-021-11406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Javaid N., Choi S. Toll-like receptors from the perspective of cancer treatment. Cancers. 2020;12(2) doi: 10.3390/cancers12020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Callahan V., et al. The pro-inflammatory chemokines CXCL9, CXCL10 and CXCL11 are upregulated following SARS-CoV-2 infection in an AKT-dependent manner. Viruses. 2021;13(6) doi: 10.3390/v13061062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kany S., Vollrath J.T., Relja B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019;20(23) doi: 10.3390/ijms20236008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wautier J.L., Wautier M.P. Pro- and anti-inflammatory prostaglandins and cytokines in humans: a mini review. Int. J. Mol. Sci. 2023;24(11) doi: 10.3390/ijms24119647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu X., et al. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct. Targeted Ther. 2021;6(1):402. doi: 10.1038/s41392-021-00791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kashfi K., Kannikal J., Nath N. Macrophage reprogramming and cancer therapeutics: role of iNOS-derived NO. Cells. 2021;10(11) doi: 10.3390/cells10113194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jablonski K.A., et al. Novel markers to delineate murine M1 and M2 macrophages. PLoS One. 2015;10(12) doi: 10.1371/journal.pone.0145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Filipek A., et al. Oleacein and foam cell formation in human monocyte-derived macrophages: a potential strategy against early and advanced atherosclerotic lesions. Pharmaceuticals. 2020;13(4) doi: 10.3390/ph13040064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xia T., et al. Advances in the role of STAT3 in macrophage polarization. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1160719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shi F., et al. Heat injured stromal cells-derived exosomal EGFR enhances prostatic wound healing after thulium laser resection through EMT and NF-kappaB signaling. Prostate. 2019;79(11):1238–1255. doi: 10.1002/pros.23827. [DOI] [PubMed] [Google Scholar]
- 69.Lu J., et al. Reprogramming of TAMs via the STAT3/CD47-SIRPalpha axis promotes acquired resistance to EGFR-TKIs in lung cancer. Cancer Lett. 2023;564 doi: 10.1016/j.canlet.2023.216205. [DOI] [PubMed] [Google Scholar]
- 70.Ma X., et al. The pancreatic cancer secreted REG4 promotes macrophage polarization to M2 through EGFR/AKT/CREB pathway. Oncol. Rep. 2016;35(1):189–196. doi: 10.3892/or.2015.4357. [DOI] [PubMed] [Google Scholar]
- 71.Hung C.H., et al. Altered monocyte differentiation and macrophage polarization patterns in patients with breast cancer. BMC Cancer. 2018;18(1):366. doi: 10.1186/s12885-018-4284-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Forrester S.J., et al. Epidermal growth factor receptor transactivation: mechanisms, pathophysiology, and potential therapies in the cardiovascular system. Annu. Rev. Pharmacol. Toxicol. 2016;56:627–653. doi: 10.1146/annurev-pharmtox-070115-095427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hardbower D.M., et al. EGFR regulates macrophage activation and function in bacterial infection. J. Clin. Invest. 2016;126(9):3296–3312. doi: 10.1172/JCI83585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu X., et al. Glutamine metabolic microenvironment drives M2 macrophage polarization to mediate trastuzumab resistance in HER2-positive gastric cancer. Cancer Commun. 2023;43(8):909–937. doi: 10.1002/cac2.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang W., et al. Polarization of macrophages in the tumor microenvironment is influenced by EGFR signaling within colon cancer cells. Oncotarget. 2016;7(46):75366–75378. doi: 10.18632/oncotarget.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Im J.H., et al. FGF2 alters macrophage polarization, tumour immunity and growth and can be targeted during radiotherapy. Nat. Commun. 2020;11(1):4064. doi: 10.1038/s41467-020-17914-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Y., et al. FGFR2 upregulates PAI-1 via JAK2/STAT3 signaling to induce M2 polarization of macrophages in colorectal cancer. Biochim. Biophys. Acta, Mol. Basis Dis. 2023;1869(4) doi: 10.1016/j.bbadis.2023.166665. [DOI] [PubMed] [Google Scholar]
- 78.Li D., et al. Cancer-associated fibroblast-secreted IGFBP7 promotes gastric cancer by enhancing tumor associated macrophage infiltration via FGF2/FGFR1/PI3K/AKT axis. Cell Death Dis. 2023;9(1):17. doi: 10.1038/s41420-023-01336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cai X., Tao W., Li L. Glioma cell-derived FGF20 suppresses macrophage function by activating beta-catenin. Cell. Signal. 2022;89 doi: 10.1016/j.cellsig.2021.110181. [DOI] [PubMed] [Google Scholar]
- 80.Li S., et al. Macrophage-specific FGF12 promotes liver fibrosis progression in mice. Hepatology. 2023;77(3):816–833. doi: 10.1002/hep.32640. [DOI] [PubMed] [Google Scholar]
- 81.Li X., et al. 11,12-EET regulates PPAR-gamma expression to modulate TGF-beta-mediated macrophage polarization. Cells. 2023;12(5) doi: 10.3390/cells12050700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang L., et al. GDF3 protects mice against sepsis-induced cardiac dysfunction and mortality by suppression of macrophage pro-inflammatory phenotype. Cells. 2020;9(1) doi: 10.3390/cells9010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rodriguez-Garcia A., et al. TGF-beta1 targets Smad, p38 MAPK, and PI3K/Akt signaling pathways to induce PFKFB3 gene expression and glycolysis in glioblastoma cells. FEBS J. 2017;284(20):3437–3454. doi: 10.1111/febs.14201. [DOI] [PubMed] [Google Scholar]
- 84.Zhang F., et al. TGF-beta induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget. 2016;7(32):52294–52306. doi: 10.18632/oncotarget.10561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu F., et al. MSC-secreted TGF-beta regulates lipopolysaccharide-stimulated macrophage M2-like polarization via the Akt/FoxO1 pathway. Stem Cell Res. Ther. 2019;10(1):345. doi: 10.1186/s13287-019-1447-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Becerra-Diaz M., Song M., Heller N. Androgen and androgen receptors as regulators of monocyte and macrophage biology in the healthy and diseased lung. Front. Immunol. 2020;11:1698. doi: 10.3389/fimmu.2020.01698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lai J.J., et al. Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-alpha expression. J. Clin. Invest. 2009;119(12):3739–3751. doi: 10.1172/JCI39335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jin L., et al. Testosterone induces apoptosis via Fas/FasL-dependent pathway in bone marrow-derived macrophages. Methods Find Exp Clin Pharmacol. 2006;28(5):283–293. doi: 10.1358/mf.2006.28.5.990201. [DOI] [PubMed] [Google Scholar]
- 89.Becerra-Diaz M., et al. Androgen and androgen receptor as enhancers of M2 macrophage polarization in allergic lung inflammation. J. Immunol. 2018;201(10):2923–2933. doi: 10.4049/jimmunol.1800352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sellau J., Groneberg M., Lotter H. Androgen-dependent immune modulation in parasitic infection. Semin. Immunopathol. 2019;41(2):213–224. doi: 10.1007/s00281-018-0722-9. [DOI] [PubMed] [Google Scholar]
- 91.Orliaguet L., et al. Mechanisms of macrophage polarization in insulin signaling and sensitivity. Front. Endocrinol. 2020;11:62. doi: 10.3389/fendo.2020.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li M., et al. Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal Transduct. Targeted Ther. 2022;7(1):216. doi: 10.1038/s41392-022-01073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Puschel G.P., Klauder J., Henkel J. Macrophages, low-grade inflammation, insulin resistance and hyperinsulinemia: a mutual ambiguous relationship in the development of metabolic diseases. J. Clin. Med. 2022;11(15) doi: 10.3390/jcm11154358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hou H., et al. Estrogen attenuates chronic stress-induced cardiomyopathy by adaptively regulating macrophage polarizations via beta(2)-adrenergic receptor modulation. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.737003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lamkin D.M., et al. beta-Adrenergic-stimulated macrophages: comprehensive localization in the M1-M2 spectrum. Brain Behav. Immun. 2016;57:338–346. doi: 10.1016/j.bbi.2016.07.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Freire B.M., de Melo F.M., Basso A.S. Adrenergic signaling regulation of macrophage function: do we understand it yet? Immunother Adv. 2022;2(1):ltac010. doi: 10.1093/immadv/ltac010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Harwani S.C. Macrophages under pressure: the role of macrophage polarization in hypertension. Transl. Res. 2018;191:45–63. doi: 10.1016/j.trsl.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Florance I., Ramasubbu S. Current understanding on the role of lipids in macrophages and associated diseases. Int. J. Mol. Sci. 2022;24(1) doi: 10.3390/ijms24010589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yan J., Horng T. Lipid metabolism in regulation of macrophage functions. Trends Cell Biol. 2020;30(12):979–989. doi: 10.1016/j.tcb.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 100.Videla L.A., et al. Omega-3 lipid mediators: modulation of the M1/M2 macrophage phenotype and its protective role in chronic liver diseases. Int. J. Mol. Sci. 2023;24(21) doi: 10.3390/ijms242115528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.He Y., Liu T. Oxidized low-density lipoprotein regulates macrophage polarization in atherosclerosis. Int Immunopharmacol. 2023;120 doi: 10.1016/j.intimp.2023.110338. [DOI] [PubMed] [Google Scholar]
- 102.Vassiliou E., Farias-Pereira R. Impact of lipid metabolism on macrophage polarization: implications for inflammation and tumor immunity. Int. J. Mol. Sci. 2023;24(15) doi: 10.3390/ijms241512032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nomura M., et al. Macrophage fatty acid oxidation inhibits atherosclerosis progression. J. Mol. Cell. Cardiol. 2019;127:270–276. doi: 10.1016/j.yjmcc.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Al Sadoun H. Macrophage phenotypes in normal and diabetic wound healing and therapeutic interventions. Cells. 2022;11(15) doi: 10.3390/cells11152430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Louiselle A.E., et al. Macrophage polarization and diabetic wound healing. Transl. Res. 2021;236:109–116. doi: 10.1016/j.trsl.2021.05.006. [DOI] [PubMed] [Google Scholar]
- 106.Zhang J., et al. Endothelial lactate controls muscle regeneration from ischemia by inducing M2-like macrophage polarization. Cell Metab. 2020;31(6):1136–1153 e7. doi: 10.1016/j.cmet.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ayala T.S., et al. High glucose environments interfere with bone marrow-derived macrophage inflammatory mediator release, the TLR4 pathway and glucose metabolism. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-47836-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang S.M., et al. High glucose environment induces M1 macrophage polarization that impairs keratinocyte migration via TNF-alpha: an important mechanism to delay the diabetic wound healing. J. Dermatol. Sci. 2019;96(3):159–167. doi: 10.1016/j.jdermsci.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 109.Zhang B., et al. Hyperglycemia modulates M1/M2 macrophage polarization via reactive oxygen species overproduction in ligature-induced periodontitis. J. Periodontal. Res. 2021;56(5):991–1005. doi: 10.1111/jre.12912. [DOI] [PubMed] [Google Scholar]
- 110.Wu H., et al. Electrical stimulation of piezoelectric BaTiO3 coated Ti6Al4V scaffolds promotes anti-inflammatory polarization of macrophages and bone repair via MAPK/JNK inhibition and OXPHOS activation. Biomaterials. 2023;293 doi: 10.1016/j.biomaterials.2022.121990. [DOI] [PubMed] [Google Scholar]
- 111.Kolliniati O., et al. Metabolic regulation of macrophage activation. J. Innate Immun. 2022;14(1):51–68. doi: 10.1159/000516780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mehla K., Singh P.K. Metabolic regulation of macrophage polarization in cancer. Trends Cancer. 2019;5(12):822–834. doi: 10.1016/j.trecan.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Saha S., Shalova I.N., Biswas S.K. Metabolic regulation of macrophage phenotype and function. Immunol. Rev. 2017;280(1):102–111. doi: 10.1111/imr.12603. [DOI] [PubMed] [Google Scholar]
- 114.Kieler M., Hofmann M., Schabbauer G. More than just protein building blocks: how amino acids and related metabolic pathways fuel macrophage polarization. FEBS J. 2021;288(12):3694–3714. doi: 10.1111/febs.15715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu P.S., et al. alpha-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 2017;18(9):985–994. doi: 10.1038/ni.3796. [DOI] [PubMed] [Google Scholar]
- 116.Yu W., et al. One-carbon metabolism supports S-adenosylmethionine and histone methylation to drive inflammatory macrophages. Mol Cell. 2019;75(6):1147–1160 e5. doi: 10.1016/j.molcel.2019.06.039. [DOI] [PubMed] [Google Scholar]
- 117.Samstad E.O., et al. Cholesterol crystals induce complement-dependent inflammasome activation and cytokine release. J. Immunol. 2014;192(6):2837–2845. doi: 10.4049/jimmunol.1302484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fraser D.A., Tenner A.J. Directing an appropriate immune response: the role of defense collagens and other soluble pattern recognition molecules. Curr. Drug Targets. 2008;9(2):113–122. doi: 10.2174/138945008783502476. [DOI] [PubMed] [Google Scholar]
- 119.Bohlson S.S., et al. Complement, c1q, and c1q-related molecules regulate macrophage polarization. Front. Immunol. 2014;5:402. doi: 10.3389/fimmu.2014.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wilck N., et al. The role of sodium in modulating immune cell function. Nat. Rev. Nephrol. 2019;15(9):546–558. doi: 10.1038/s41581-019-0167-y. [DOI] [PubMed] [Google Scholar]
- 121.Oh S., et al. Integrated NLRP3, AIM2, NLRC4, Pyrin inflammasome activation and assembly drive PANoptosis. Cell. Mol. Immunol. 2023;20(12):1513–1526. doi: 10.1038/s41423-023-01107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wilck N., et al. The role of sodium in modulating immune cell function. Nat. Rev. Nephrol. 2019;15(9):546–558. doi: 10.1038/s41581-019-0167-y. [DOI] [PubMed] [Google Scholar]
- 123.Xia Y., et al. Ironing out the details: how iron orchestrates macrophage polarization. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.669566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pereira M., et al. Acute iron deprivation reprograms human macrophage metabolism and reduces inflammation in vivo. Cell Rep. 2019;28(2):498–511 e5. doi: 10.1016/j.celrep.2019.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhou Y., et al. Iron overloaded polarizes macrophage to proinflammation phenotype through ROS/acetyl-p53 pathway. Cancer Med. 2018;7(8):4012–4022. doi: 10.1002/cam4.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Aerbajinai W., et al. Glia maturation factor-gamma regulates murine macrophage iron metabolism and M2 polarization through mitochondrial ROS. Blood Adv. 2019;3(8):1211–1225. doi: 10.1182/bloodadvances.2018026070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Diez-Tercero L., et al. Evaluation of the immunomodulatory effects of cobalt, copper and magnesium ions in a pro inflammatory environment. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-91070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cui C., et al. Coapplication of magnesium supplementation and vibration modulate macrophage polarization to attenuate sarcopenic muscle atrophy through PI3K/Akt/mTOR signaling pathway. Int. J. Mol. Sci. 2022;23(21) doi: 10.3390/ijms232112944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gao Y., et al. Advancing neural regeneration via adaptable hydrogels: enriched with Mg(2+) and silk fibroin to facilitate endogenous cell infiltration and macrophage polarization. Bioact. Mater. 2024;33:100–113. doi: 10.1016/j.bioactmat.2023.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Oh M.H., Jang J., Lee J.H. Polarization of THP-1-derived macrophage by magnesium and MAGT1 inhibition in wound healing. Arch Plast Surg. 2023;50(4):432–442. doi: 10.1055/s-0043-1770114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu L., et al. Magnesium promotes vascularization and osseointegration in diabetic states. Int. J. Oral Sci. 2024;16(1):10. doi: 10.1038/s41368-023-00271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cheng P., et al. High-purity magnesium screws modulate macrophage polarization during the tendon-bone healing process in the anterior cruciate ligament reconstruction rabbit model. Regen Biomater. 2022;9:rbac067. doi: 10.1093/rb/rbac067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Escribese M.M., Casas M., Corbi A.L. Influence of low oxygen tensions on macrophage polarization. Immunobiology. 2012;217(12):1233–1240. doi: 10.1016/j.imbio.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 134.Qiu B., et al. Hypoxia inducible factor-1alpha is an important regulator of macrophage biology. Heliyon. 2023;9(6) doi: 10.1016/j.heliyon.2023.e17167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tan H.Y., et al. The reactive oxygen species in macrophage polarization: reflecting its dual role in progression and treatment of human diseases. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/2795090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Twisselmann N., et al. Hyperoxia/hypoxia exposure primes a sustained pro-inflammatory profile of preterm infant macrophages upon LPS stimulation. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.762789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kuebler J.F., et al. Carbon dioxide suppresses macrophage superoxide anion production independent of extracellular pH and mitochondrial activity. J. Pediatr. Surg. 2007;42(1):244–248. doi: 10.1016/j.jpedsurg.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 138.Gan Z., et al. Carbon metabolism in the regulation of macrophage functions. Trends Endocrinol Metab. 2024;35(1):62–73. doi: 10.1016/j.tem.2023.09.003. [DOI] [PubMed] [Google Scholar]
- 139.Lee S.W., et al. Peritoneal macrophage and blood monocyte functions after open and laparoscopic-assisted cecectomy in rats. Surg. Endosc. 2003;17(12):1996–2002. doi: 10.1007/s00464-003-8154-5. [DOI] [PubMed] [Google Scholar]
- 140.Strowitzki M.J., et al. Carbon dioxide sensing by immune cells occurs through carbonic anhydrase 2-dependent changes in intracellular pH. J. Immunol. 2022;208(10):2363–2375. doi: 10.4049/jimmunol.2100665. [DOI] [PubMed] [Google Scholar]
- 141.Jain N., Moeller J., Vogel V. Mechanobiology of macrophages: how physical factors coregulate macrophage plasticity and phagocytosis. Annu. Rev. Biomed. Eng. 2019;21:267–297. doi: 10.1146/annurev-bioeng-062117-121224. [DOI] [PubMed] [Google Scholar]
- 142.Kirkley J.E., Thompson B.J., Coon J.S. Temperature alters lipopolysaccharide-induced cytokine secretion by RAW 264.7 cells. Scand. J. Immunol. 2003;58(1):51–58. doi: 10.1046/j.1365-3083.2003.01274.x. [DOI] [PubMed] [Google Scholar]
- 143.Tiemeijer B.M., et al. Probing single-cell macrophage polarization and heterogeneity using thermo-reversible hydrogels in droplet-based microfluidics. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.715408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hagiwara S., et al. Changes in cell culture temperature alter release of inflammatory mediators in murine macrophagic RAW264.7 cells. Inflamm. Res. 2007;56(7):297–303. doi: 10.1007/s00011-007-6161-z. [DOI] [PubMed] [Google Scholar]
- 145.Lee C.T., et al. Elevation in body temperature to fever range enhances and prolongs subsequent responsiveness of macrophages to endotoxin challenge. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lee C.T., Repasky E.A. Opposing roles for heat and heat shock proteins in macrophage functions during inflammation: a function of cell activation state? Front. Immunol. 2012;3:140. doi: 10.3389/fimmu.2012.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dutta S.D., et al. Unraveling the potential of 3D bioprinted immunomodulatory materials for regulating macrophage polarization: state-of-the-art in bone and associated tissue regeneration. Bioact. Mater. 2023;28:284–310. doi: 10.1016/j.bioactmat.2023.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.He F., et al. Jaw periosteum-derived mesenchymal stem cells regulate THP-1-derived macrophage polarization. Int. J. Mol. Sci. 2021;22(9) doi: 10.3390/ijms22094310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Huse M. Mechanical forces in the immune system. Nat. Rev. Immunol. 2017;17(11):679–690. doi: 10.1038/nri.2017.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.McWhorter F.Y., Davis C.T., Liu W.F. Physical and mechanical regulation of macrophage phenotype and function. Cell. Mol. Life Sci. 2015;72(7):1303–1316. doi: 10.1007/s00018-014-1796-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jain N., Vogel V. Spatial confinement downsizes the inflammatory response of macrophages. Nat. Mater. 2018;17(12):1134–1144. doi: 10.1038/s41563-018-0190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cutter S., et al. Towards using 3D cellular cultures to model the activation and diverse functions of macrophages. Biochem. Soc. Trans. 2023;51(1):387–401. doi: 10.1042/BST20221008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kusuma G.D., et al. Effect of 2D and 3D culture microenvironments on mesenchymal stem cell-derived extracellular vesicles potencies. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.819726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sapudom J., et al. 3D in vitro M2 macrophage model to mimic modulation of tissue repair. NPJ Regen Med. 2021;6(1):83. doi: 10.1038/s41536-021-00193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xiao J., et al. Changes of immune microenvironment in head and neck squamous cell carcinoma in 3D-4-culture compared to 2D-4-culture. J. Transl. Med. 2023;21(1):771. doi: 10.1186/s12967-023-04650-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.He L., et al. Global characterization of macrophage polarization mechanisms and identification of M2-type polarization inhibitors. Cell Rep. 2021;37(5) doi: 10.1016/j.celrep.2021.109955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mouton A.J., et al. Mapping macrophage polarization over the myocardial infarction time continuum. Basic Res. Cardiol. 2018;113(4):26. doi: 10.1007/s00395-018-0686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Unuvar Purcu D., et al. Effect of stimulation time on the expression of human macrophage polarization markers. PLoS One. 2022;17(3) doi: 10.1371/journal.pone.0265196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Solis A.G., et al. Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature. 2019;573(7772):69–74. doi: 10.1038/s41586-019-1485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Atcha H., et al. Mechanically activated ion channel Piezo1 modulates macrophage polarization and stiffness sensing. Nat. Commun. 2021;12(1):3256. doi: 10.1038/s41467-021-23482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tang Y., et al. Mechanosensitive Piezo1 protein as a novel regulator in macrophages and macrophage-mediated inflammatory diseases. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1149336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang Y., Wang H. Integrin signalling and function in immune cells. Immunology. 2012;135(4):268–275. doi: 10.1111/j.1365-2567.2011.03549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang Y., et al. Matrix stiffness regulates macrophage polarization in atherosclerosis. Pharmacol. Res. 2022;179 doi: 10.1016/j.phrs.2022.106236. [DOI] [PubMed] [Google Scholar]
- 164.Sun G., Guillon E., Holley S.A. Integrin intra-heterodimer affinity inversely correlates with integrin activatability. Cell Rep. 2021;35(10) doi: 10.1016/j.celrep.2021.109230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xing X., et al. Matrix stiffness-mediated effects on macrophages polarization and their LOXL2 expression. FEBS J. 2021;288(11):3465–3477. doi: 10.1111/febs.15566. [DOI] [PubMed] [Google Scholar]
- 166.Hoare J.I., et al. Electric fields are novel determinants of human macrophage functions. J. Leukoc. Biol. 2016;99(6):1141–1151. doi: 10.1189/jlb.3A0815-390R. [DOI] [PubMed] [Google Scholar]
- 167.Roy Barman S., Jhunjhunwala S. Electrical stimulation for immunomodulation. ACS Omega. 2024;9(1):52–66. doi: 10.1021/acsomega.3c06696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.McCann F.V., et al. Action potentials in macrophages derived from human monocytes. Science. 1983;219(4587):991–993. doi: 10.1126/science.6823563. [DOI] [PubMed] [Google Scholar]
- 169.Liao J., Li X., Fan Y. Prevention strategies of postoperative adhesion in soft tissues by applying biomaterials: based on the mechanisms of occurrence and development of adhesions. Bioact. Mater. 2023;26:387–412. doi: 10.1016/j.bioactmat.2023.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xu J., et al. Non-contact electrical stimulation as an effective means to promote wound healing. Bioelectrochemistry. 2022;146 doi: 10.1016/j.bioelechem.2022.108108. [DOI] [PubMed] [Google Scholar]
- 171.Wosik J., et al. Magnetic field changes macrophage phenotype. Biophys. J. 2018;114(8):2001–2013. doi: 10.1016/j.bpj.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Montoya R.D. Magnetic fields, radicals and cellular activity. Electromagn. Biol. Med. 2017;36(1):102–113. doi: 10.1080/15368378.2016.1194291. [DOI] [PubMed] [Google Scholar]
- 173.Vinhas A., et al. Magnetic stimulation drives macrophage polarization in cell to-cell communication with IL-1beta primed tendon cells. Int. J. Mol. Sci. 2020;21(15) doi: 10.3390/ijms21155441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jiang J., et al. Mobile mechanical signal generator for macrophage polarization. Exploration (Beijing) 2023;3(2) doi: 10.1002/EXP.20220147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Saraswathibhatla A., Indana D., Chaudhuri O. Cell-extracellular matrix mechanotransduction in 3D. Nat. Rev. Mol. Cell Biol. 2023;24(7):495–516. doi: 10.1038/s41580-023-00583-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhuang Z., et al. Control of matrix stiffness using methacrylate-gelatin hydrogels for a macrophage-mediated inflammatory response. ACS Biomater. Sci. Eng. 2020;6(5):3091–3102. doi: 10.1021/acsbiomaterials.0c00295. [DOI] [PubMed] [Google Scholar]
- 177.Mai Z., et al. Modulating extracellular matrix stiffness: a strategic approach to boost cancer immunotherapy. Cell Death Dis. 2024;15(5):307. doi: 10.1038/s41419-024-06697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bachy S., et al. betaig-h3-structured collagen alters macrophage phenotype and function in pancreatic cancer. iScience. 2022;25(2) doi: 10.1016/j.isci.2022.103758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chen M., et al. Substrate stiffness modulates bone marrow-derived macrophage polarization through NF-kappaB signaling pathway. Bioact. Mater. 2020;5(4):880–890. doi: 10.1016/j.bioactmat.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yu Y., et al. Macrophages play a key role in tissue repair and regeneration. PeerJ. 2022;10 doi: 10.7717/peerj.14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhao W., et al. M2 macrophage polarization: a potential target in pain relief. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1243149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang Z., et al. Macrophage in liver fibrosis: identities and mechanisms. Int Immunopharmacol. 2023;120 doi: 10.1016/j.intimp.2023.110357. [DOI] [PubMed] [Google Scholar]
- 183.Ballav S., et al. PPAR-Gamma partial agonists in disease-fate decision with special reference to cancer. Cells. 2022;11(20) doi: 10.3390/cells11203215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhou Z., et al. Metformin induces M2 polarization via AMPK/PGC-1alpha/PPAR-gamma pathway to improve peripheral nerve regeneration. Am J Transl Res. 2023;15(5):3778–3792. [PMC free article] [PubMed] [Google Scholar]
- 185.Mirza R.E., et al. Macrophage PPARgamma and impaired wound healing in type 2 diabetes. J. Pathol. 2015;236(4):433–444. doi: 10.1002/path.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yu L., et al. A glimpse of the connection between PPARgamma and macrophage. Front. Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1254317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Arnold S.V., et al. Understanding contemporary use of thiazolidinediones. Circ Heart Fail. 2019;12(6) doi: 10.1161/CIRCHEARTFAILURE.118.005855. [DOI] [PubMed] [Google Scholar]
- 188.Gupta M., et al. Peroxisome proliferator-activated receptors (PPARs) and PPAR agonists: the 'future' in dermatology therapeutics? Arch. Dermatol. Res. 2015;307(9):767–780. doi: 10.1007/s00403-015-1571-1. [DOI] [PubMed] [Google Scholar]
- 189.Yu H., et al. Targeting NF-kappaB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct. Targeted Ther. 2020;5(1):209. doi: 10.1038/s41392-020-00312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dorrington M.G., Fraser I.D.C. NF-kappaB signaling in macrophages: dynamics, crosstalk, and signal integration. Front. Immunol. 2019;10:705. doi: 10.3389/fimmu.2019.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cheng W., et al. NF-kappaB, A potential therapeutic target in cardiovascular diseases. Cardiovasc. Drugs Ther. 2023;37(3):571–584. doi: 10.1007/s10557-022-07362-8. [DOI] [PubMed] [Google Scholar]
- 192.Liu D., Zhong Z., Karin M. NF-kappaB: a double-edged sword controlling inflammation. Biomedicines. 2022;10(6) doi: 10.3390/biomedicines10061250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mussbacher M., et al. NF-kappaB in monocytes and macrophages - an inflammatory master regulator in multitalented immune cells. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1134661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Guo Q., et al. NF-kappaB in biology and targeted therapy: new insights and translational implications. Signal Transduct. Targeted Ther. 2024;9(1):53. doi: 10.1038/s41392-024-01757-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Smith T.D., et al. Harnessing macrophage plasticity for tissue regeneration. Adv. Drug Deliv. Rev. 2017;114:193–205. doi: 10.1016/j.addr.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 196.Sim S.L., et al. Macrophages in skin wounds: functions and therapeutic potential. Biomolecules. 2022;12(11) doi: 10.3390/biom12111659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sun L., et al. In vitro immunomodulation of magnesium on monocytic cell toward anti-inflammatory macrophages. Regen Biomater. 2020;7(4):391–401. doi: 10.1093/rb/rbaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sylvestre M., Crane C.A., Pun S.H. Progress on modulating tumor-associated macrophages with biomaterials. Adv Mater. 2020;32(13) doi: 10.1002/adma.201902007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Xuan Y., et al. Hierarchical intrafibrillarly mineralized collagen membrane promotes guided bone regeneration and regulates M2 macrophage polarization. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.781268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li J., et al. Tailoring materials for modulation of macrophage fate. Adv Mater. 2021;33(12) doi: 10.1002/adma.202004172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Liu X., et al. Immunopolarization-regulated 3D printed-electrospun fibrous scaffolds for bone regeneration. Biomaterials. 2021;276 doi: 10.1016/j.biomaterials.2021.121037. [DOI] [PubMed] [Google Scholar]
- 202.Liu X., et al. On-off phagocytosis and switchable macrophage activation stimulated with NIR for infected percutaneous tissue repair of polypyrrole-coated sulfonated PEEK. Adv. Sci. 2023;10(5) doi: 10.1002/advs.202205048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jiang J., et al. Expanded 3D nanofiber scaffolds: cell penetration, neovascularization, and host response. Adv Healthc Mater. 2016;5(23):2993–3003. doi: 10.1002/adhm.201600808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bygd H.C., Forsmark K.D., Bratlie K.M. Altering in vivo macrophage responses with modified polymer properties. Biomaterials. 2015;56:187–197. doi: 10.1016/j.biomaterials.2015.03.042. [DOI] [PubMed] [Google Scholar]
- 205.Klopfleisch R., Jung F. The pathology of the foreign body reaction against biomaterials. J. Biomed. Mater. Res. 2017;105(3):927–940. doi: 10.1002/jbm.a.35958. [DOI] [PubMed] [Google Scholar]
- 206.Zhu F., et al. Potential effects of biomaterials on macrophage function and their signalling pathways. Biomater. Sci. 2023;11(21):6977–7002. doi: 10.1039/d3bm01213a. [DOI] [PubMed] [Google Scholar]
- 207.Liu Y., Segura T. Biomaterials-mediated regulation of macrophage cell fate. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.609297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wang Y., et al. Biomimetic glycopeptide hydrogel coated PCL/nHA scaffold for enhanced cranial bone regeneration via macrophage M2 polarization-induced osteo-immunomodulation. Biomaterials. 2022;285 doi: 10.1016/j.biomaterials.2022.121538. [DOI] [PubMed] [Google Scholar]
- 209.Na Y.R., Kim S.W., Seok S.H. A new era of macrophage-based cell therapy. Exp. Mol. Med. 2023;55(9):1945–1954. doi: 10.1038/s12276-023-01068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Huang X., et al. Genetically engineered M2-like macrophage-derived exosomes for P. gingivalis-suppressed cementum regeneration: from mechanism to therapy. Bioact. Mater. 2024;32:473–487. doi: 10.1016/j.bioactmat.2023.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Salazar J.J., Ennis W.J., Koh T.J. Diabetes medications: impact on inflammation and wound healing. J Diabetes Complications. 2016;30(4):746–752. doi: 10.1016/j.jdiacomp.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Li M., et al. CCL5 deficiency promotes liver repair by improving inflammation resolution and liver regeneration through M2 macrophage polarization. Cell. Mol. Immunol. 2020;17(7):753–764. doi: 10.1038/s41423-019-0279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Liang C., et al. Engineered M2a macrophages for the treatment of osteoarthritis. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1054938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wang W., et al. Targeting macrophage polarization as a promising therapeutic strategy for the treatment of osteoarthritis. Int Immunopharmacol. 2023;116 doi: 10.1016/j.intimp.2023.109790. [DOI] [PubMed] [Google Scholar]
- 215.Luo Y.L., et al. Macrophage-specific in vivo gene editing using cationic lipid-assisted polymeric nanoparticles. ACS Nano. 2018;12(2):994–1005. doi: 10.1021/acsnano.7b07874. [DOI] [PubMed] [Google Scholar]
- 216.Lee Y.W., et al. In vivo editing of macrophages through systemic delivery of CRISPR-cas9-ribonucleoprotein-nanoparticle nanoassemblies. Adv. Ther. 2019;2(10) doi: 10.1002/adtp.201900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wang L., et al. The role of immunometabolism in macrophage polarization and its impact on acute lung injury/acute respiratory distress syndrome. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1117548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Nicolete R., dos Santos D.F., Faccioli L.H. The uptake of PLGA micro or nanoparticles by macrophages provokes distinct in vitro inflammatory response. Int Immunopharmacol. 2011;11(10):1557–1563. doi: 10.1016/j.intimp.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 219.Da Silva C.A., et al. Chitin is a size-dependent regulator of macrophage TNF and IL-10 production. J. Immunol. 2009;182(6):3573–3582. doi: 10.4049/jimmunol.0802113. [DOI] [PubMed] [Google Scholar]
- 220.Almeida C.R., et al. Impact of 3-D printed PLA- and chitosan-based scaffolds on human monocyte/macrophage responses: unraveling the effect of 3-D structures on inflammation. Acta Biomater. 2014;10(2):613–622. doi: 10.1016/j.actbio.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 221.Jiang J., et al. Expanded 3D nanofiber scaffolds: cell penetration, neovascularization, and host response. Adv Healthc Mater. 2016;5(23):2993–3003. doi: 10.1002/adhm.201600808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Cha B.H., et al. Integrin-mediated interactions control macrophage polarization in 3D hydrogels. Adv Healthc Mater. 2017;6(21) doi: 10.1002/adhm.201700289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Clapacs Z., et al. Coiled coil crosslinked alginate hydrogels dampen macrophage-driven inflammation. Biomacromolecules. 2022;23(3):1183–1194. doi: 10.1021/acs.biomac.1c01462. [DOI] [PubMed] [Google Scholar]
- 224.Buck E., et al. Protein adsorption on surfaces functionalized with COOH groups promotes anti-inflammatory macrophage responses. ACS Appl. Mater. Interfaces. 2021;13(6):7021–7036. doi: 10.1021/acsami.0c16509. [DOI] [PubMed] [Google Scholar]
- 225.Liu W., et al. Zinc-Modified sulfonated polyetheretherketone surface with immunomodulatory function for guiding cell fate and bone regeneration. Adv. Sci. 2018;5(10) doi: 10.1002/advs.201800749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Abaricia J.O., et al. Wnt signaling modulates macrophage polarization and is regulated by biomaterial surface properties. Biomaterials. 2020;243 doi: 10.1016/j.biomaterials.2020.119920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Shayan M., et al. Nanopatterned bulk metallic glass-based biomaterials modulate macrophage polarization. Acta Biomater. 2018;75:427–438. doi: 10.1016/j.actbio.2018.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.He X.T., et al. Macrophage involvement affects matrix stiffness-related influences on cell osteogenesis under three-dimensional culture conditions. Acta Biomater. 2018;71:132–147. doi: 10.1016/j.actbio.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 229.Zhuang Z., et al. Control of matrix stiffness using methacrylate-gelatin hydrogels for a macrophage-mediated inflammatory response. ACS Biomater. Sci. Eng. 2020;6(5):3091–3102. doi: 10.1021/acsbiomaterials.0c00295. [DOI] [PubMed] [Google Scholar]
- 230.Luo Y., et al. Light-induced dynamic RGD pattern for sequential modulation of macrophage phenotypes. Bioact. Mater. 2021;6(11):4065–4072. doi: 10.1016/j.bioactmat.2021.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zheng X., et al. Near-infrared-triggered dynamic surface topography for sequential modulation of macrophage phenotypes. ACS Appl. Mater. Interfaces. 2019;11(46):43689–43697. doi: 10.1021/acsami.9b14808. [DOI] [PubMed] [Google Scholar]
- 232.Grillo C.A., Alvarez F., de Mele M.A. Biological effects of magnesium particles degradation on UMR-106 cell line: influence of fluoride treatments. Colloids Surf. B Biointerfaces. 2011;88(1):471–476. doi: 10.1016/j.colsurfb.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 233.Li S., et al. Multiscale architecture design of 3D printed biodegradable Zn-based porous scaffolds for immunomodulatory osteogenesis. Nat. Commun. 2024;15(1):3131. doi: 10.1038/s41467-024-47189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.