Abstract
Background
There are currently no validated clinical biomarkers of post-acute sequelae of SARS-CoV-2 infection (PASC).
Objective
Investigate clinical laboratory markers of SARS-CoV-2 and PASC.
Design
Propensity score-weighted linear regression models were fitted to evaluate differences in mean laboratory measures by prior infection and PASC index (≥12 vs 0).
Setting
83 enrolling sites
Participants
RECOVER Adult Cohort participants with or without SARS-CoV-2 infection, with a study visit and laboratory measures 6 months after index date (or at enrollment if >6 months). Participants were excluded if the 6-month visit occurred within 30 days of reinfection.
Measurements
Participants completed questionnaires and standard clinical laboratory tests.
Results
Of 10,094 participants, 8,746 had prior SARS-CoV-2 infection, 1,348 were uninfected; 1880 had a PASC index ≥12, and 3351 had a PASC index of zero. After propensity score adjustment, participants with prior infection had lower mean platelets (265.9 x109/L, 95% CI = [264.5, 267.4]) compared to participants without known prior infection (275.2 x109/L [268.5, 282.0]), and higher mean HbA1c (5.58% [5.56, 5.60] vs. 5.46% [5.40, 5.51]) and urinary albumin/creatinine ratio (81.9 mg/g [67.5, 96.2] vs. 43.0 mg/g [25.4, 60.6]), though differences were of modest clinical significance. The HbA1c difference was attenuated after excluding participants with pre-existing diabetes. Among participants with prior infection, we found no meaningful differences in mean laboratory values between those with PASC index ≥12 versus PASC index of zero.
Limitations
We could not determine whether laboratory differences represent consequences of or risk factors for acquisition of SARS-CoV-2.
Conclusions
Overall, we found no evidence that any of the 25 routine clinical laboratory values assessed in this study could serve as a clinically useful biomarker of PASC.
Primary Funding Source
National Institutes of Health
Introduction
Post-acute sequelae of SARS-CoV-2 infection (PASC), also known as Long COVID, has been reported in millions of people worldwide and is a major public health burden (1-3). PASC is generally accepted as an umbrella term encompassing a wide spectrum of symptoms and health conditions that persist after acute COVID-19 infection, resulting in a major impact on quality of life (4-12). While potential models of pathogenesis have been postulated to include immune dysregulation, viral persistence, organ injury, endothelial dysfunction, and gut dysbiosis (13-17), there are currently no validated clinical biomarkers of PASC.
As part of the National Institutes of Health's Researching COVID to Enhance Recovery (RECOVER) Initiative (https://recovercovid.org/), we previously examined prospectively collected data from nearly 10,000 individuals in the RECOVER-Adult cohort with and without SARS-CoV-2 infection and identified 12 symptoms that most distinguish those with prior infection from those uninfected (8) (Table S1). We developed a PASC index based on these 12 symptoms, with 23% of the cohort with prior SARS-CoV-2-infection meeting this research threshold. We further identified multiple clusters or subphenotypes of PASC. This framework does not encompass all individuals experiencing PASC, but permits exploration of clinical laboratory features among those meeting the PASC threshold.
Routine clinical laboratory tests that accurately distinguish individuals with PASC from those without PASC might be useful in the diagnosis, prognosis, prevention, and treatment of PASC or PASC subtypes. Laboratory tests might also identify those who have persistent organ damage but minimal to no symptoms. Studies have found potential biomarkers associated with PASC using mostly research-focused assays, but results have been inconsistent, perhaps due to different PASC study definitions, use of only selected biomarkers, choice of comparison groups if any (PASC recovered or healthy controls), duration of symptoms, types of symptoms or phenotypes, and patient population features (e.g., sex, age, race, vaccination status, comorbidities, severity of initial infection, etc.) (18-20). Systematic reviews have identified candidate biomarker categories including inflammatory (e.g., higher levels of C-reactive protein [CRP], leukocytes), coagulation (e.g., international normalized ratio [INR]), and hematologic (e.g., lower levels of hemoglobin) which likely reflect multifaceted pathophysiology and phenotypes of PASC (18, 19, 21). Autoimmune (22, 23), hormonal (24), viral (25, 26), and other (20) biomarkers associated with PASC phenotypes have also been described, though many studies have been limited by small sample sizes, limited follow-up duration, and/or lack of appropriate controls. Early small cohort studies failed to find routine clinical biomarkers (27). There is a paucity of large studies examining the utility of standardized laboratory tests obtained in routine clinical care.
Accordingly, we conducted a study to determine whether SARS-CoV-2 infection led to persistent changes in common clinical laboratory tests, regardless of symptoms, in individuals with prior infection compared to those without prior infection. If so, laboratory studies could be used to augment symptom-based definitions of PASC. Second, we aimed to determine whether those with PASC have persistent laboratory changes compared to those unlikely to have PASC. If so, we might be able to identify specific physiologic abnormalities driving symptomatic PASC. Therefore, we analyzed 25 routinely used and standardized laboratory tests, selected based on availability across different institutions, prior literature, and clinical experience (28). These tests were prospectively measured in Clinical Laboratory Improvement Amendments (CLIA)-certified laboratories in samples from 10,094 RECOVER Adult participants, representing a diverse cohort from across the U.S. We compared results 1) between participants with and without prior SARS-CoV-2 infection at ≥6 months after index infection; 2) between participants with and without PASC, as defined by an index of ≥12 or zero, respectively; and 3) between participants with each of four previously characterized PASC symptom phenotypes and those unlikely to have PASC.
Methods
Study design.
The RECOVER-Adult study design has previously been described (28). Briefly, participants were recruited at 83 enrolling sites across 33 U.S. states plus Washington, DC and Puerto Rico. Adults aged 18 years or older were eligible to enroll regardless of prior infection with SARS-CoV-2. All participants completed a baseline set of surveys, a minimal physical examination, and standard laboratory test collection at enrollment. Participants were followed prospectively with survey collection every 3 months and laboratory collection at enrollment, 6-, 12-, 24-, 36-, and 48-months post-infection or negative test date (index date).
Participants.
Participants were enrolled between 0 days and 3 years after index date and followed a visit schedule based on time from index. All participants had laboratory tests at the 6-month visit or at the enrollment visit (if enrollment was more than 6 months after index), Table S2; however, after this 6-month or enrollment visit, laboratory tests were only repeated if the prior result was abnormal. Analysis was therefore based on the 6-month visit (or the enrollment visit if more than 6 months from index) and subsequent visits were not considered, to minimize bias. Index date was defined as date of first infection (suspected, probable, or confirmed SARS-CoV-2 infection as defined by World Health Organization criteria or positive SARS-CoV-2 infection-specific antibody testing), or date of a negative SARS-CoV-2 test for participants who were never infected. Participants were excluded if the 6-month visit was within 30 days of reinfection. Participants who did not start the protocol or had no symptom survey data were excluded. Participants who had no prior SARS-CoV-2 at entry but reported infection within 6 months were reclassified as having SARS-CoV-2 infection, with a new index date defined as date of infection. Uninfected participants reporting an on-study infection >6 months after index were considered uninfected and the 6-month visit was used in the analysis. Participants pregnant at the time of infection were included in the analysis.
Exposures and outcomes.
The primary outcome was laboratory measurement at 6 months after index date, or at enrollment if >6 months. Two primary exposures included history of SARS-CoV-2 infection and PASC classification at the same time point as laboratory measurements.
All laboratory measures were completed locally at each site’s CLIA-certified clinical laboratory. Laboratory measures were drawn as part of the study visit if not obtained through routine clinical care within 30 days of index date or 90 days prior to the 6 month follow-up. Laboratory studies collected were complete blood count with differential, complete metabolic panel, INR, d-dimer, lipid panel, 25-hydroxy vitamin D, thyroid stimulating hormone (TSH), free T4, hemoglobin A1c (HbA1c), high sensitivity (hs)-CRP, cystatin C, N-terminal pro b-type natriuretic peptide (NT-proBNP), troponin, urinalysis, and urine albumin to creatinine ratio (uACR). These tests were selected based on their routine availability and standardized use across CLIA-certified laboratories, prior literature, and clinical expertise of RECOVER investigators.
PASC was defined based on our prior work (8). Briefly, we identified 12 symptoms that best distinguished between individuals with and without prior infection, assigned each one points based on degree of difference, and summed all points corresponding to the symptoms present in an individual to construct an index (Table S1). A total of 12 or more was considered the optimal threshold above which participants were likely to have PASC. Comparisons were made to participants with a PASC index of zero. A PASC index of zero does not necessarily mean that the participant had no symptoms or no PASC; however, participants with an index of zero would be unlikely to have PASC. PASC sub-phenotypes were previously defined as cluster 1 representing high frequency of impairments in smell and taste; cluster 2 as high frequency of post-exertional malaise (PEM), defined as symptoms worse after even minor physical or mental effort, and fatigue; cluster 3 as high frequency of brain fog, PEM, and fatigue; and cluster 4 as high frequency of fatigue, PEM, dizziness, brain fog, gastrointestinal symptoms, and palpitations(8).
Statistical analysis.
Demographic characteristics of the cohort were reported overall, by history of prior SARS-CoV-2 infection, and by PASC status. In the primary analysis, participants with and without a history of SARS-CoV-2 infection were compared to determine whether SARS-CoV-2 infection led to persistent laboratory abnormalities, regardless of symptoms. In the secondary analysis, participants with prior infection with PASC index ≥12 were compared to participants with a PASC index of zero. In all analyses, separate propensity score-weighted linear regression models were fitted for each laboratory measurement to evaluate between-group differences in means. Model-based estimated means and corresponding 95% confidence intervals were reported.
Propensity scores were calculated based on age, sex, race/ethnicity, SARS-CoV-2 variant era, referral source, vaccination status at index, comorbidities prior to index, homelessness, employment status, insurance status, income, level of difficulty in covering expenses, last doctor’s visit, and food insecurity to balance on these factors between exposure groups. A complete list of adjustment variables is provided in Table 1. Multiple imputation was used to handle missing data. Propensity score-weighted means, 95% confidence intervals, medians, 10th and 90th percentiles were reported for each laboratory measurement. The target populations for estimated model based mean differences in the propensity score model were participants with prior infection in the primary analysis and PASC-positive in secondary analyses. Stabilized weights were applied in all analyses and trimming of top 1% of weights was applied in secondary analyses to address extreme values in the weights.
Table 1a.
Demographics overall and by prior SARS-CoV-2 infection status
| Characteristic | Prior Infection (n = 8,746) |
No prior infection (n = 1,348) |
Overall (n=10,094) |
|---|---|---|---|
| Age at infection [years] | |||
| Mean (SD) | 46 (15) | 52 (15) | 47 (15) |
| Median [IQR] | 45 (34, 59) | 54 (39, 64) | 47 (34, 60) |
| Missing | 5 | 1 | 6 |
| Race/Ethnicity | |||
| Non-Hispanic White | 5080 (58%) | 847 (63%) | 5927 (59%) |
| Non-Hispanic Black | 1214 (14%) | 204 (15%) | 1418 (14%) |
| Non-Hispanic Asian | 462 (5%) | 88 (7%) | 550 (5%) |
| Hispanic | 1523 (17%) | 156 (12%) | 1679 (17%) |
| Mixed race/Other | 400 (5%) | 43 (3%) | 443 (4%) |
| Missing | 67 | 10 | 77 |
| Sex assigned at birth | |||
| Female | 6329 (73%) | 911 (68%) | 7240 (72%) |
| Male | 2392 (27%) | 432 (32%) | 2824 (28%) |
| Intersex | 4 (0%) | 0 (0%) | 4 (0%) |
| Missing | 21 | 5 | 26 |
| Enrollment group and era | |||
| Pre-Omicron | 3362 (38%) | 317 (24%) | 3679 (36%) |
| Omicron acute | 2963 (34%) | 548 (41%) | 3511 (35%) |
| Omicron post-acute | 2421 (28%) | 483 (36%) | 2904 (29%) |
| Referral type | |||
| Self-referral/Community outreach/Long COVID | 4377 (50%) | 960 (71%) | 5337 (53%) |
| Another referral | 4364 (50%) | 388 (29%) | 4752 (47%) |
| Missing | 5 | 0 | 5 |
| Hospitalized during acute phase of first infection | |||
| Not hospitalized during acute phase | 7738 (91%) | n/a | 7738 (91%) |
| Hospitalized during acute phase | 723 (9%) | n/a | 723 (9%) |
| Missing | 285 | n/a | 285 |
| Vaccinated at first infection | |||
| Unvaccinated | 3042 (35%) | 182 (14%) | 3224 (32%) |
| Partially vaccinated or date of last dose unknown | 516 (6%) | 85 (6%) | 601 (6%) |
| Fully vaccinated | 5144 (59%) | 1068 (80%) | 6212 (62%) |
| Missing | 44 | 13 | 57 |
| Visit month | |||
| 6 months | 5000 (57%) | 952 (71%) | 5952 (59%) |
| 9+ | 3746 (43%) | 396 (29%) | 4142 (41%) |
| Marital status at enrollment | |||
| Divorced, widowed, separated, or never married | 3177 (37%) | 531 (40%) | 3708 (38%) |
| Married or living with partner | 5376 (63%) | 783 (60%) | 6159 (62%) |
| Missing | 193 | 34 | 227 |
| Homelessness at enrollment | |||
| Homeless | 197 (2%) | 30 (2%) | 227 (2%) |
| Not homeless | 8422 (98%) | 1294 (98%) | 9716 (98%) |
| Missing | 127 | 24 | 151 |
| Disability at enrollment | |||
| Disabled | 319 (4%) | 80 (6%) | 399 (4%) |
| Not disabled | 8271 (96%) | 1233 (94%) | 9504 (96%) |
| Missing | 156 | 35 | 191 |
| Unemployment at enrollment | |||
| Unemployed | 245 (3%) | 47 (4%) | 292 (3%) |
| Not unemployed | 8345 (97%) | 1266 (96%) | 9611 (97%) |
| Missing | 156 | 35 | 191 |
| Medicaid at enrollment | |||
| Medicaid | 1303 (15%) | 176 (13%) | 1479 (15%) |
| Not Medicaid | 7196 (85%) | 1132 (87%) | 8328 (85%) |
| Missing | 247 | 40 | 287 |
| Uninsured at enrollment | |||
| Uninsured | 273 (3%) | 38 (3%) | 311 (3%) |
| Not uninsured | 8226 (97%) | 1270 (97%) | 9496 (97%) |
| Missing | 247 | 40 | 287 |
| Lost insurance due to pandemic | |||
| Lost insurance | 274 (3%) | 23 (2%) | 297 (3%) |
| Did not lose insurance | 8256 (97%) | 1279 (98%) | 9535 (97%) |
| Missing | 216 | 46 | 262 |
| Difficulty covering expenses in the month before enrollment | |||
| Not at all difficult to cover expenses | 5105 (62%) | 933 (73%) | 6038 (63%) |
| Somewhat difficult to cover expenses | 2288 (28%) | 258 (20%) | 2546 (27%) |
| Very difficult to cover expenses | 889 (11%) | 92 (7%) | 981 (10%) |
| Missing | 464 | 65 | 529 |
| Most recent doctor’s visit before index | |||
| Within the last 5 years | 8373 (99%) | 1290 (99%) | 9663 (99%) |
| Greater than 5 years | 82 (1%) | 15 (1%) | 97 (1%) |
| Missing | 291 | 43 | 334 |
| Skipped care in 12 months before index | |||
| Skipped care | 480 (6%) | 58 (4%) | 538 (5%) |
| Did not skip care | 8035 (94%) | 1258 (96%) | 9293 (95%) |
| Missing | 231 | 32 | 263 |
| 2019 Household Income | |||
| <$25,000 | 1319 (16%) | 218 (18%) | 1537 (17%) |
| $25,000-$49,999 | 1279 (16%) | 149 (12%) | 1428 (15%) |
| ≥$50,000 | 5403 (68%) | 856 (70%) | 6259 (68%) |
| Missing | 745 | 125 | 870 |
| Food insecurity in 12 months before index | |||
| Food insecure | 1233 (14%) | 156 (12%) | 1389 (14%) |
| Not food insecure | 7310 (86%) | 1161 (88%) | 8471 (86%) |
| Missing | 203 | 31 | 234 |
Sensitivity analyses were performed excluding participants with diabetes (for HgbA1c) and an immunocompromised condition (for platelets). Additional exploratory between group comparisons were participants with prior infection within each PASC cluster vs. those with PASC index of zero.
Results
Participants.
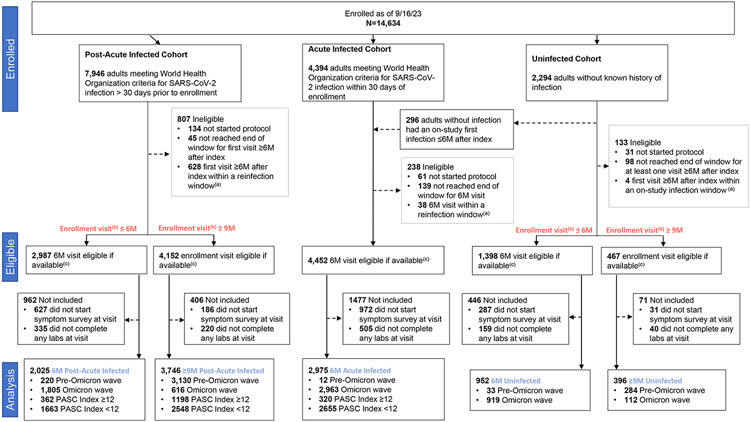
A total of 10,094 participants (8,746 with prior infection; 1,348 uninfected) met study criteria (Figure 1, 72% female [7,240/10,094]; 17% Hispanic/Latino [1,679/10,094]; 14% non-Hispanic Black [1,418/10,094]; 62% fully vaccinated at the index date [6,212/10,037]; median age, 47 years [IQR, 34-60]) (Table 1a). Of participants with prior SARS-CoV-2 infection, 21.5% (1,880/8,746) had PASC index ≥12 and 38.3% (3,351/8,746) as a PASC index of zero (Table 1b). There were no significant differences in demographics and clinical characteristics between participants included and those not included in the analysis cohort (Table S2).
Figure 1:
Consort Diagram
a ) Reinfection window for exclusion is 30 days prior and 7 days after visit
b) Enrollment visit defined as first visit if available, or enrollment date if visit date not available
c) Participants who completed visit without reach end of visit window
Table 1b.
Demographics overall and by PASC Score among individuals with a prior SARS-CoV-2 infection
| Characteristic | PASC Score ≥ 12 (n=1880) |
PASC Score 0 (n=3351) |
Overall (n=5231) |
|---|---|---|---|
| Age at infection [years] | |||
| Mean (SD) | 48 (14) | 47 (16) | 47 (15) |
| Median [IQR] | 47 (37, 58) | 45 (33, 60) | 47 (34, 59) |
| Missing | 1 | 2 | 3 |
| Race/Ethnicity | |||
| Non-Hispanic White | 1209 (65%) | 1813 (54%) | 3022 (58%) |
| Non-Hispanic Black | 198 (11%) | 541 (16%) | 739 (14%) |
| Non-Hispanic Asian | 58 (3%) | 219 (7%) | 277 (5%) |
| Hispanic | 296 (16%) | 615 (18%) | 911 (18%) |
| Mixed race/Other | 104 (6%) | 141 (4%) | 245 (5%) |
| Missing | 15 | 22 | 37 |
| Sex assigned at birth | |||
| Female | 1456 (78%) | 2242 (67%) | 3698 (71%) |
| Male | 419 (22%) | 1099 (33%) | 1518 (29%) |
| Intersex | 1 (0%) | 2 (0%) | 3 (0%) |
| Missing | 4 | 8 | 12 |
| Enrollment group and era | |||
| Pre-Omicron | 1203 (64%) | 909 (27%) | 2112 (40%) |
| Omicron Acute | 317 (17%) | 1398 (42%) | 1715 (33%) |
| Omicron Post-Acute | 360 (19%) | 1044 (31%) | 1404 (27%) |
| Referral type | |||
| Self-referral/Community outreach/Long COVID | 1180 (63%) | 1509 (45%) | 2689 (51%) |
| Another referral | 700 (37%) | 1840 (55%) | 2540 (49%) |
| Missing | 0 | 2 | 2 |
| Hospitalized during acute phase of first infection | |||
| Not hospitalized during acute phase | 1577 (85%) | 3011 (94%) | 4588 (91%) |
| Hospitalized during acute phase | 272 (15%) | 189 (6%) | 461 (9%) |
| Missing | 31 | 151 | 182 |
| Vaccinated at first infection | |||
| Unvaccinated | 1058 (56%) | 856 (26%) | 1914 (37%) |
| Partially vaccinated or date of last dose unknown | 105 (6%) | 220 (7%) | 325 (6%) |
| Fully vaccinated | 711 (38%) | 2254 (68%) | 2965 (57%) |
| Missing | 6 | 21 | 27 |
| Visit month | |||
| 6 months | 682 (36%) | 2218 (66%) | 2900 (55%) |
| 9+ | 1198 (64%) | 1133 (34%) | 2331 (45%) |
| Marital status at enrollment | |||
| Divorced, widowed, separated, or never married | 732 (40%) | 1161 (36%) | 1893 (37%) |
| Married or living with partner | 1111 (60%) | 2103 (64%) | 3214 (63%) |
| Missing | 37 | 87 | 124 |
| Homelessness at enrollment | |||
| Homeless | 49 (3%) | 69 (2%) | 118 (2%) |
| Not homeless | 1813 (97%) | 3223 (98%) | 5036 (98%) |
| Missing | 18 | 59 | 77 |
| Disability at enrollment | |||
| Disabled | 130 (7%) | 80 (2%) | 210 (4%) |
| Not disabled | 1729 (93%) | 3192 (98%) | 4921 (96%) |
| Missing | 21 | 79 | 100 |
| Unemployment at enrollment | |||
| Unemployed | 61 (3%) | 85 (3%) | 146 (3%) |
| Not unemployed | 1798 (97%) | 3187 (97%) | 4985 (97%) |
| Missing | 21 | 79 | 100 |
| Medicaid at enrollment | |||
| Medicaid | 341 (18%) | 436 (13%) | 777 (15%) |
| Not Medicaid | 1505 (82%) | 2796 (87%) | 4301 (85%) |
| Missing | 34 | 119 | 153 |
| Uninsured at enrollment | |||
| Uninsured | 61 (3%) | 105 (3%) | 166 (3%) |
| Not uninsured | 1785 (97%) | 3127 (97%) | 4912 (97%) |
| Missing | 34 | 119 | 153 |
| Lost insurance due to pandemic | |||
| Lost insurance | 136 (7%) | 55 (2%) | 191 (4%) |
| Did not lose insurance | 1706 (93%) | 3206 (98%) | 4912 (96%) |
| Missing | 38 | 90 | 128 |
| Difficulty covering expenses in the month before enrollment | |||
| Not at all difficult to cover expenses | 824 (46%) | 2211 (70%) | 3035 (61%) |
| Somewhat difficult to cover expenses | 603 (34%) | 745 (24%) | 1348 (27%) |
| Very difficult to cover expenses | 355 (20%) | 213 (7%) | 568 (11%) |
| Missing | 98 | 182 | 280 |
| Most recent doctor's visit before index | |||
| Within the last 5 years | 1826 (99%) | 3174 (99%) | 5000 (99%) |
| Greater than 5 years | 16 (1%) | 35 (1%) | 51 (1%) |
| Missing | 38 | 142 | 180 |
| Skipped care in 12 months before index | |||
| Skipped care | 180 (10%) | 110 (3%) | 290 (6%) |
| Did not skip care | 1660 (90%) | 3149 (97%) | 4809 (94%) |
| Missing | 40 | 92 | 132 |
| 2019 Household Income | |||
| <$25,000 | 299 (17%) | 494 (16%) | 793 (17%) |
| $25,000-$49,999 | 313 (18%) | 455 (15%) | 768 (16%) |
| ≥$50,000 | 1130 (65%) | 2086 (69%) | 3216 (67%) |
| Missing | 138 | 316 | 454 |
| Food insecurity in 12 months before index | |||
| Food insecure | 348 (19%) | 409 (13%) | 757 (15%) |
| Not food insecure | 1501 (81%) | 2854 (87%) | 4355 (85%) |
| Missing | 31 | 88 | 119 |
Laboratory measurements associated with prior SARS-CoV-2 infection.
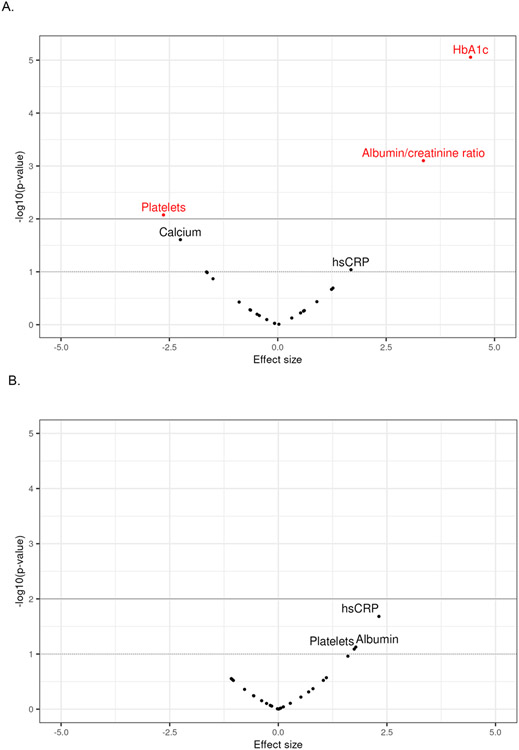
Differences in mean laboratory values between participants with and without prior infection were observed in platelets, HbA1c, and uACR (Table 2, Figure 2) based on propensity score weighted models. Covariate balance was achieved after propensity score weighting (Figure S1). Participants with prior infection had lower mean platelets (265.9 x109cells/L 95% CI = [264.5, 267.4]) compared to participants without known prior infection (275.2 x109cells/L [268.5, 282.0]) and higher mean HbA1c (5.58% [5.56, 5.60] vs. 5.46% [5.40, 5.51]) and uACR (81.9 mg/g [67.5, 96.2]) vs 43.0 mg/g [25.4, 60.6]) compared to participants without known prior infection. Boxplots and empirical cumulative distribution functions of laboratory measurements for HbA1c, platelets and uACR by infection status are provided in Figure S2.
Table 2.
Estimated mean laboratory measurements by group after propensity score weighting
| Prior SARS-CoV-2 Infection |
No Prior Infection |
PASC score ≥12 | PASC score=0 | |
|---|---|---|---|---|
| Lab Measurement | Est (95% CI) | Est (95% CI) | Est (95% CI) | Est (95% CI) |
| Sodium (mmol/L) | 139.1 (139.1, 139.2) | 139.2 (139.0, 139.4) | 139.2 (139.1, 139.3) | 139.2 (139.0, 139.4) |
| Potassium (mmol/L) | 4.16 (4.15, 4.16) | 4.16 (4.12, 4.19) | 4.16 (4.14, 4.18) | 4.16 (4.13, 4.19) |
| Chloride (mmol/L) | 103.4 (103.3, 103.5) | 103.6 (103.3, 103.9) | 103.2 (103.0, 103.3) | 103.3 (103.1, 103.6) |
| Bicarbonate (mmol/L) | 25.2 (25.2, 25.3) | 25.1 (24.9, 25.4) | 25.2 (25.1, 25.3) | 25.0 (24.7, 25.2) |
| Creatinine (serum) (umol/L) | 77.5 (76.2, 78.7) | 75.4 (72.5, 78.3) | 78.4 (75.3, 81.5) | 76.7 (73.8, 79.6) |
| Creatinine (serum) (mg/dL) | 0.876 (0.862, 0.890) | 0.853 (0.820, 0.886) | 0.887 (0.852, 0.922) | 0.868 (0.835, 0.900) |
| Calcium (mmol/L) | 2.35 (2.35, 2.36) | 2.36 (2.35, 2.37) | 2.36 (2.35, 2.36) | 2.35 (2.34, 2.36) |
| Calcium (mg/dL) | 9.43 (9.42, 9.44) | 9.47 (9.44, 9.51) | 9.45 (9.43, 9.47) | 9.43 (9.39, 9.46) |
| ALT (U/L) | 22.6 (22.2, 22.9) | 22.1 (20.6, 23.6) | 23.3 (22.5, 24.2) | 22.8 (21.4, 24.1) |
| AST (U/L) | 21.8 (21.5, 22.1) | 21.9 (19.9, 23.8) | 22.0 (21.3, 22.6) | 22.2 (21.1, 23.3) |
| Total bilirubin (umol/L) | 9.56 (9.44, 9.67) | 9.45 (9.06, 9.84) | 9.09 (8.87, 9.32) | 9.13 (8.76, 9.51) |
| Total bilirubin (mg/dL) | 0.559 (0.552, 0.565) | 0.553 (0.530, 0.575) | 0.532 (0.519, 0.545) | 0.534 (0.512, 0.556) |
| Albumin (g/L) | 43.9 (43.8, 44.0) | 44.2 (43.8, 44.5) | 44.0 (43.9, 44.2) | 43.7 (43.4, 44.0) |
| WBC (x10^9 cells/L) | 6.58 (6.53, 6.63) | 6.63 (6.42, 6.84) | 6.90 (6.80, 7.00) | 6.88 (6.62, 7.15) |
| ALC (x10^9 cells/L) | 195.2 (193.3, 197.1) | 198.1 (189.3, 207.0) | 198.3 (194.9, 201.8) | 198.3 (189.7, 206.9) |
| ANC (x10^9 cells/L) | 392.5 (388.7, 396.2) | 394.5 (379.1, 410.0) | 421.0 (412.1, 429.9) | 410.1 (392.8, 427.3) |
| Hgb (g/L) | 136.1 (135.8, 136.4) | 137.2 (135.9, 138.5) | 136.4 (135.7, 137.1) | 136.3 (135.1, 137.6) |
| Platelets (x10^9 cells/L) | 265.9 (264.5, 267.4) (a) | 275.2 (268.5, 282.0) (a) | 272.7 (269.4, 276.0) | 266.3 (259.9, 272.7) |
| INR | 1.02 (1.02, 1.03) | 1.02 (1.01, 1.03) | 1.02 (1.01, 1.03) | 1.02 (0.99, 1.05) |
| D-dimer (ug/L) | 517.6 (497.8, 537.4) | 536.8 (481.6, 591.9) | 518.6 (476.2, 561.1) | 580.0 (471.8, 688.3) |
| hsCRP (mg/L) | 4.08 (3.93, 4.23) | 3.65 (3.18, 4.12) | 5.01 (4.62, 5.39) (a) | 4.23 (3.70, 4.76) (a) |
| Cystatin-C (mg/L) | 0.895 (0.886, 0.904) | 0.887 (0.863, 0.911) | 0.918 (0.899, 0.937) | 0.933 (0.884, 0.981) |
| HbA1c (%) | 5.58 (5.56, 5.60) (a) | 5.46 (5.40, 5.51) (a) | 5.63 (5.59, 5.67) | 5.67 (5.59, 5.74) |
| HDL cholesterol (mmol/L) | 1.50 (1.49, 1.51) | 1.49 (1.45, 1.52) | 1.47 (1.45, 1.49) | 1.47 (1.43, 1.50) |
| HDL cholesterol (mg/dL) | 57.9 (57.5, 58.2) | 57.4 (56.0, 58.8) | 56.9 (56.1, 57.6) | 56.7 (55.4, 57.9) |
| Non-HDL cholesterol (mmol/L) | 3.38 (3.36, 3.40) | 3.33 (3.24, 3.41) | 3.52 (3.47, 3.57) | 3.49 (3.40, 3.59) |
| Non-HDL cholesterol (mg/dL) | 130.7 (129.8, 131.5) | 128.6 (125.4, 131.8) | 136.2 (134.3, 138.0) | 135.0 (131.3, 138.8) |
| TSH (mcIU/mL) | 1.95 (1.91, 1.99) | 2.04 (1.85, 2.23) | 2.00 (1.90, 2.10) | 2.00 (1.86, 2.15) |
| NT-Pro BNP (pg/mL) | 83.7 (76.4, 91.1) | 80.8 (64.3, 97.2) | 83.1 (67.1, 99.0) | 124.6 (51.1, 198.1) |
| Albumin/creatinine ratio (mg/g) | 81.9 (67.5, 96.2) (a) | 43.0 (25.4, 60.6) (a) | 67.0 (43.5, 90.5) | 72.2 (42.7, 101.7) |
Non-overlapping 95% CI between groups.
ALT, alanine transaminase; AST, aspartate aminotransferase; WBC, white blood cell count; ALC, absolute lymphocyte count; ANC, absolute neutrophil count, Hgb, hemoglobin; INR, international normalized ratio; hsCRP, high-sensitivity C-reactive protein; HbA1c, hemoglobin A1c; HDL, high density lipoprotein; TSH, thyroid stimulating hormone; NT-Pro BNP, N-terminal pro b-type natriuretic peptide
Figure 2:
Differences in laboratory measurements between groups after propensity score weighting.
Figure 2A. Prior SARS-CoV-2 infection vs no prior infection
Figure 2B. PASC index ≥12 vs PASC index of zero
Quantiles of propensity score-weighted laboratory values (Table S3) suggest that the right tails of the distributions of HbA1c and uACR were higher in participants with versus without prior infection, while the entirety of the distribution of platelets was shifted lower in participants with versus without prior infection (Table S4).
The difference in mean HbA1c was attenuated after excluding 867 participants with pre-existing diabetes (with prior infection: 5.40% [5.39, 5.42] vs without known infection: 5.37% [5.33, 5.41]). The small difference in mean platelets remained after excluding 640 participants with a pre-existing immunocompromising condition (with prior infection 267.0 x109 cells/L [265.6, 268.5] vs without prior infection: 277.4 x109 cells/L [270.3, 284.6]).
Laboratory measurements associated with a PASC index ≥12 versus zero.
Restricting analysis to participants with prior infection, in propensity score-weighted models balancing on all covariates (Figure S3), we found no clinically meaningful differences (Table 2) in mean laboratory values between those with a PASC index ≥12 versus zero.
Laboratory measurements associated with PASC sub-phenotypes versus PASC index of zero.
In exploratory propensity score-weighted models (Figure S3) restricted to participants with prior infection (Tables S5, S6), we observed: 1) higher mean hsCRP in cluster 1 (smell or taste impairments); 2) lower mean sodium and increase in mean calcium in cluster 2 (high frequency of PEM and fatigue); 3) no differences in cluster 3 (high frequency of brain fog, PEM, and fatigue); and higher hsCRP in cluster 4 (high frequency of fatigue, PEM, dizziness, brain fog, gastrointestinal symptoms, and palpitations), compared to participants with a PASC index of zero. Boxplots and empirical cumulative distribution functions of laboratory measurements by cluster are provided in Figure S4.
Discussion
In a cohort study of over 10,000 participants with and without prior SARS-CoV-2 infection, we found no evidence that any of 25 routine clinical laboratory values provide a reliable biomarker of prior infection, PASC, or specific type of PASC cluster. We did identify small differences in some laboratory tests between those with and without prior infection: specifically, we found that prior infection was associated with modest increases in HbA1c and uACR, and small decreases in platelets. Within PASC symptom clusters, hsCRP was slightly higher in cluster 1 (impairments in smell and taste) and cluster 4 (high frequency of fatigue, PEM, dizziness, brain fog, gastrointestinal symptoms, and palpitations), while sodium was lower and calcium higher in cluster 2 (high frequency of PEM and fatigue), compared to participants with a PASC index of zero. These results may have been due to chance given multiple comparisons, and were largely not clinically meaningful. Thus, while clinicians should rule out treatable causes of PASC symptoms with appropriate diagnostic testing, routine laboratory tests are not useful biomarkers for PASC. Earlier, smaller studies have found similar results (27). While these small differences are not useful for PASC diagnosis, they may suggest potential pathways in the pathogenesis of PASC and PASC clusters.
First, an association between severity of acute COVID-19 with diabetes or glucose intolerance has been recognized since early in the COVID-19 pandemic. SARS-CoV-2 itself may contribute to the development of diabetes and previously undiagnosed diabetes or receipt of high-doses of corticosteroids during the hospital stay may play a role in the severity of initial disease (29). The association between diabetes and PASC has been less well studied, and often limited to electronic health record data of patients with variably-defined PASC and shorter follow-up duration after initial infection. In a cohort of over 5 million patients with and without prior SARS-CoV-2 infection, prior infection was associated with increased risk of subsequent diabetes (30). A study of nearly 10 million veterans found that veterans with a SARS-CoV-2 diagnosis had an increased risk of incident diabetes and antihyperglycemic use in the 12 months after diagnosis; however, 15% of the cohort were missing HbA1c and there may have been underdiagnosis of antecedent diabetes, particularly in the control groups (31). Our results are consistent with both of these large cohort studies, with the advantage of systematic HbA1c collection and comprehensive, standardized symptom collection. Of note, we did not detect a difference in HbA1c when those with pre-existing diabetes were excluded in the sensitivity analysis. Furthermore, we compared HbA1c by PASC index severity. While in theory, SARS-CoV-2 infection may increase risk of diabetes, diabetes may also worsen PASC symptomatology, including microvascular complications (32). Despite our large cohort size, we did not detect a difference in HbA1c between those with PASC index of ≥12 and those with PASC index of zero, suggesting that SARS-CoV-2 may contribute to glucose dysregulation independent of symptoms.
A second finding distinguishing participants with and without prior SARS-CoV-2 infection was the shift towards lower platelets, although our detectable platelet differences were likely of minimal clinical relevance, and most participants were well within the clinically normal range. In contrast, among participants with PASC index ≥12, both platelets and hsCRP tended to be higher compared to those with PASC index of zero, suggesting an ongoing inflammatory state, consistent with prior literature (33-36). Platelet and clotting abnormalities are more complex than simply the total number of platelets, and abnormalities have been recognized widely during acute COVID-19, including in vitro studies demonstrating internalization of SARS-CoV-2 virion by platelets and rapid cell death (37). Some evidence supports a role of clotting and platelet abnormalities in PASC (38), though other studies have not found convincing evidence of microclots (39, 40). Low platelets seen in association with prior infection may be multifactorial, reflecting platelet destruction, marrow suppression, or consumption, as seen with other viral infections (e.g. HIV, Epstein-Barr virus). Indeed, one study found downregulation of platelet genes among patients with Long COVID (41). Other studies have demonstrated markers of platelet activation in PASC (42). Among a subset of 80 symptomatic participants in a Long COVID/PASC registry, microthrombi and platelet abnormalities (hyperactivity) were seen in nearly all participants (38, 43). Platelet abnormalities have also been described among patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) or postural orthostatic tachycardia syndrome (POTS) (44, 45). Clinical quantitation of platelet numbers is not a reliable biomarker for PASC and more specific markers of platelet biology will likely be needed to detect platelet dysfunction related to PASC.
The other difference among participants with and without prior SARS-CoV-2 was a higher uACR, without differences in cystatin C or creatinine (46). Smaller case-control studies have found increased uACR either acutely or several months following SARS-CoV-2, compared to those without infection (47, 48). Other larger observational studies have shown declines in renal function in the year following SARS-CoV-2 infection, with a slightly greater proportion of individuals with moderately or severely increased uACR from 3 to 12 months, though no statistical comparisons were provided (46, 49, 50). These findings may reflect greater comorbidity among those with prior infection or consequences of severe initial infection; other studies have suggested a direct effect of SARS-CoV-2 on renal tubules and parenchyma, resulting in renal dysfunction (51, 52). Beyond the direct association with renal function, elevated uACR (even in the microalbuminuria range) has been associated with increased cardiovascular disease risk (53, 54). In HIV, albuminuria is associated with increased mortality, cardiovascular disease, and heart failure (55) through endothelial dysfunction (56, 57), a known contributor to PASC-related cardiovascular risk (58) that will be assessed in future RECOVER analyses.
The association with hsCRP and cluster 1 (smell/taste disturbances) is consistent with a recent study that looked at the ultrastructural changes of olfactory bulbs and tracts in SARS-CoV-2 infection (59). The authors suggested that anosmia is likely due to inflammation from the virus, rather than direct viral invasion. The association with hsCRP and smell/taste disturbance conflicts with a recent systematic review of 11 different studies in which patients with chemosensory disturbances tended to have lower overall inflammation, including hs-CRP, compared to those without chemosensory disturbances (60). This discrepancy may reflect the newer variants captured in our cohort in comparison to many studies with earlier variants where chemosensory disturbances were often associated with lower disease severity (61).
Our study has many strengths as the first large study that prospectively assessed a broad battery of clinical laboratory biomarkers with standardized systematic evaluation for symptoms, reducing ascertainment bias. We had large and robust control groups, and our participants are diverse in terms of demographics, geographic distribution, variant of infection and vaccination status. We use a rigorously derived, concrete and reproducible research definition of PASC, enabling this cohort to be well characterized. However, our study also has important limitations. Laboratory studies were run at local CLIA-certified labs whose assays may differ slightly from each other. Laboratory measures were not always drawn fasting. We did not have pre-infection laboratory results on most participants, impeding ability to study pre/post infection changes and limiting the distinction between abnormalities predisposing to infection and those resulting from infection. We considered each measure independently and did not evaluate multivariate laboratory profiles. The analysis combined laboratory values and PASC status assessed between a 6-month visit (180 +/− 45 days) and up to 3 years after index date. Finally, other clinical laboratory studies not captured here (e.g., cortisol, fibrinogen) warrant further study.
Conclusions
While clinicians should obtain routine clinical tests to rule out other treatable causes of PASC symptoms, we found no evidence that 25 routine clinical laboratory values offer clinical utility as biomarkers of PASC, and are therefore not useful as part of a definition. We did find slightly lower platelets, higher HbA1c, and higher uACR among participants with prior SARS-CoV-2 infection compared to those without. Whether these differences represent consequences of or risk factors for initial acquisition or severity of SARS-CoV-2 infection cannot be determined without pre-infection evaluations. Furthermore, differences were quite small, likely of minimal clinical relevance, and may have been due to chance. Laboratory differences among those within PASC subphenotypes suggest ongoing inflammation (hsCRP) as a potential mechanism underlying PASC. Future work within the RECOVER cohort will evaluate whether clusters of biomarkers can help refine a research-based or clinically relevant PASC diagnosis.
In summary, our findings suggest that even highly symptomatic PASC may have no clinically observable objective findings on routine laboratory testing. Understanding of the basic biologic underpinnings of persistent symptoms after SARS-CoV-2 infection will likely require a rigorous focus on investigations beyond routine clinical laboratory studies (e.g., transcriptomics, proteomics, metabolomics) to identify novel biomarkers.
Supplementary Material
Acknowledgements:
Authorship has been determined according to ICMJE recommendations. This study is part of the NIH Researching COVID to Enhance Recovery (RECOVER) Initiative, which seeks to understand, treat, and prevent the post-acute sequelae of SARS-CoV-2 infection (PASC). For more information on RECOVER, visit https://recovercovid.org/. We would like to thank the National Community Engagement Group (NCEG), all patients, caregivers and community representatives, and all the participants enrolled in the RECOVER initiative.
Funding:
This research was funded by the National Institutes of Health (NIH) Agreements OTA OT2HL161847, OT2HL161841 and OT2HL156812 as part of the Researching COVID to Enhance Recovery (RECOVER) Research Initiative and R01 HL162373. No funding source had any role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Registration: NCT05172024
Disclosures:
Dr. Erlandson has served as a consultant for Gilead, Merck, and ViiV, and obtained research funding from Gilead (outside of this work, all paid to the University of Colorado). Dr. McComsey served as consultant for Gilead, Merck, Janssen and ViiV/Glaxosmithkline, and obtained research funding from Cognivue, Roche, Astellas, Pfizer, Redhill, Genentech. Dr. Krishnan reports research funding from the Patient Centered Outcomes Research Institute, payment for expert testimony from Goodwin Proctor LLC / TEVA pharmaceuticals, and personal consulting fees from AstraZeneca, CereVu Medical, BData, Inc., and American Board of Internal Medicine. Dr. Wood had an investigator-initiated grant through Pfizer, Inc. Dr. Wisnivesky has received honoraria from Banook, PPD, and Sanofi and grants from Sanofi, Regeneron and Axella.
Dr. Hess serves on a data safety monitoring board for Astellas Pharmaceuticals, outside of this work.
Dr. Singh has received research support from NIH, AHRQ, and Pfizer, Inc.; advisor to Regeneron and Gilead. Other authors report no conflict of interest.
Reproducible research statement: Study protocol: Available at https://studies.recovercovid.org/pdf/RECOVER-Adult-Protocol-v10.0.pdf. Statistical code: Not available. Data set: Available at https://biodatacatalyst.nhlbi.nih.gov/recover/.
References
- 1.Collaborators GBoDLC, Wulf Hanson S, Abbafati C, Aerts JG, Al-Aly Z, Ashbaugh C, et al. Estimated Global Proportions of Individuals With Persistent Fatigue, Cognitive, and Respiratory Symptom Clusters Following Symptomatic COVID-19 in 2020 and 2021. JAMA. 2022;328(16):1604–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie Y, Bowe B, Al-Aly Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat Commun. 2021;12(1):6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.;PagesGenerated interactively: from https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm on 1/February/2024.
- 4.Reese JT, Blau H, Casiraghi E, Bergquist T, Loomba JJ, Callahan TJ, et al. Generalisable long COVID subtypes: findings from the NIH N3C and RECOVER programmes. EBioMedicine. 2023;87:104413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang L, Yao Q, Gu X, Wang Q, Ren L, Wang Y, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398(10302):747–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thaweethai T, Jolley SE, Karlson EW, Levitan EB, Levy B, McComsey GA, et al. Development of a Definition of Postacute Sequelae of SARS-CoV-2 Infection. Jama. 2023;329(22):1934–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11(1):16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey J, Lavelle B, Miller J, Jimenez M, Lim PH, Orban ZS, et al. Multidisciplinary Center Care for Long COVID Syndrome-A Retrospective Cohort Study. Am J Med. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogungbe O, Slone S, Alharthi A, Tomiwa T, Kumbe B, Bergman A, et al. "Living like an empty gas tank with a leak": Mixed methods study on post-acute sequelae of COVID-19. PLoS One. 2022;17(12):e0279684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peluso MJ, Kelly JD, Lu S, Goldberg SA, Davidson MC, Mathur S, et al. Persistence, Magnitude, and Patterns of Postacute Symptoms and Quality of Life Following Onset of SARS-CoV-2 Infection: Cohort Description and Approaches for Measurement. Open Forum Infect Dis. 2022;9(2):ofab640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sherif ZA, Gomez CR, Connors TJ, Henrich TJ, Reeves WB. Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC). Elife. 2023;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Proal AD, VanElzakker MB. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front Microbiol. 2021;12:698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohandas S, Jagannathan P, Henrich TJ, Sherif ZA, Bime C, Quinlan E, et al. Immune mechanisms underlying COVID-19 pathology and post-acute sequelae of SARS-CoV-2 infection (PASC). eLife. 2023;12:e86014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen B, Julg B, Mohandas S, Bradfute SB, Force RMPT. Viral persistence, reactivation, and mechanisms of long COVID. eLife. 2023;12:e86015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yong SJ, Halim A, Halim M, Liu S, Aljeldah M, Al Shammari BR, et al. Inflammatory and vascular biomarkers in post-COVID-19 syndrome: A systematic review and meta-analysis of over 20 biomarkers. Rev Med Virol. 2023;33(2):e2424. [DOI] [PubMed] [Google Scholar]
- 19.Lai YJ, Liu SH, Manachevakul S, Lee TA, Kuo CT, Bello D. Biomarkers in long COVID-19: A systematic review. Front Med (Lausanne). 2023;10:1085988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsilingiris D, Vallianou NG, Karampela I, Christodoulatos GS, Papavasileiou G, Petropoulou D, et al. Laboratory Findings and Biomarkers in Long COVID: What Do We Know So Far? Insights into Epidemiology, Pathogenesis, Therapeutic Perspectives and Challenges. Int J Mol Sci. 2023;24(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin JX, Agbana YL, Sun ZS, Fei SW, Zhao HQ, Zhou XN, et al. Increased interleukin-6 is associated with long COVID-19: a systematic review and meta-analysis. Infect Dis Poverty. 2023;12(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Son K, Jamil R, Chowdhury A, Mukherjee M, Venegas C, Miyasaki K, et al. Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long COVID symptoms. Eur Respir J. 2023;61(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rojas M, Rodríguez Y, Acosta-Ampudia Y, Monsalve DM, Zhu C, Li QZ, et al. Autoimmunity is a hallmark of post-COVID syndrome. J Transl Med. 2022;20(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein J, Wood J, Jaycox JR, Dhodapkar RM, Lu P, Gehlhausen JR, et al. Distinguishing features of long COVID identified through immune profiling. Nature. 2023;623(7985):139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peluso MJ, Deveau TM, Munter SE, Ryder D, Buck A, Beck-Engeser G, et al. Chronic viral coinfections differentially affect the likelihood of developing long COVID. J Clin Invest. 2023;133(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zubchenko S, Kril I, Nadizhko O, Matsyura O, Chopyak V. Herpesvirus infections and post-COVID-19 manifestations: a pilot observational study. Rheumatol Int. 2022;42(9):1523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sneller MC, Marques AR, Liang CJ, Chung JY. A Longitudinal Study of COVID-19 Sequelae and Immunity. Ann Intern Med. 2022;175(12):W153. [DOI] [PubMed] [Google Scholar]
- 28.Horwitz LI, Thaweethai T, Brosnahan SB, Cicek MS, Fitzgerald ML, Goldman JD, et al. Researching COVID to Enhance Recovery (RECOVER) adult study protocol: Rationale, objectives, and design. PLoS One. 2023;18(6):e0286297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aluganti Narasimhulu C, Singla DK. Mechanisms of COVID-19 pathogenesis in diabetes. Am J Physiol Heart Circ Physiol. 2022;323(3):H403–h20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsieh TYJ, Chang R, Yong SB, Liao PL, Hung YM, Wei JC. COVID-19 Vaccination Prior to SARS-CoV-2 Infection Reduced Risk of Subsequent Diabetes Mellitus: A Real-World Investigation Using U.S. Electronic Health Records. Diabetes Care. 2023;46(12):2193–200. [DOI] [PubMed] [Google Scholar]
- 31.Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10(5):311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raveendran AV, Misra A. Post COVID-19 Syndrome ("Long COVID") and Diabetes: Challenges in Diagnosis and Management. Diabetes Metab Syndr. 2021;15(5):102235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Larragoiti N, Cano-Mendez A, Jimenez-Vega Y, Trujillo M, Guzman-Cancino P, Ambriz-Murillo Y, et al. Inflammatory and Prothrombotic Biomarkers Contribute to the Persistence of Sequelae in Recovered COVID-19 Patients. Int J Mol Sci. 2023;24(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Hakeim HK, Al-Rubaye HT, Almulla AF, Al-Hadrawi DS, Maes M. Chronic Fatigue, Depression and Anxiety Symptoms in Long COVID Are Strongly Predicted by Neuroimmune and Neuro-Oxidative Pathways Which Are Caused by the Inflammation during Acute Infection. J Clin Med. 2023;12(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanhueza S, Vidal MA, Hernandez MA, Henriquez-Beltran ME, Cabrera C, Quiroga R, et al. Clinical and pulmonary function analysis in long-COVID revealed that long-term pulmonary dysfunction is associated with vascular inflammation pathways and metabolic syndrome. Front Med (Lausanne). 2023;10:1271863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laubscher GJ, Lourens PJ, Venter C, Kell DB, Pretorius E. TEG(®), Microclot and Platelet Mapping for Guiding Early Management of Severe COVID-19 Coagulopathy. J Clin Med. 2021;10(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koupenova M, Corkrey HA, Vitseva O, Tanriverdi K, Somasundaran M, Liu P, et al. SARS-CoV-2 Initiates Programmed Cell Death in Platelets. Circ Res. 2021;129(6):631–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kell DB, Laubscher GJ, Pretorius E. A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications. Biochem J. 2022;479(4):537–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Appelman B, Charlton BT, Goulding RP, Kerkhoff TJ, Breedveld EA, Noort W, et al. Muscle abnormalities worsen after post-exertional malaise in long COVID. Nature Communications. 2024;15(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fox T, Hunt BJ, Ariens RAS, Towers GJ, Lever R, Garner P, et al. Plasmapheresis to remove amyloid fibrin(ogen) particles for treating the post-COVID-19 condition. Cochrane Database of Systematic Reviews. 2023(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryan FJ, Hope CM, Masavuli MG, Lynn MA, Mekonnen ZA, Yeow AEL, et al. Long-term perturbation of the peripheral immune system months after SARS-CoV-2 infection. BMC Med. 2022;20(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martins-Goncalves R, Campos MM, Palhinha L, Azevedo-Quintanilha IG, Abud Mendes M, Ramos Temerozo J, et al. Persisting Platelet Activation and Hyperactivity in COVID-19 Survivors. Circ Res. 2022;131(11):944–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cervia-Hasler C, Brüningk SC, Hoch T, Fan B, Muzio G, Thompson RC, et al. Persistent complement dysregulation with signs of thromboinflammation in active Long Covid. Science. 2024;383(6680):eadg7942. [DOI] [PubMed] [Google Scholar]
- 44.Nunes JM, Kruger A, Proal A, Kell DB, Pretorius E. The Occurrence of Hyperactivated Platelets and Fibrinaloid Microclots in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Pharmaceuticals (Basel). 2022;15(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gunning WT, Kramer PM, Cichocki JA, Karabin BL, Khuder SA, Grubb BP. Platelet Storage Pool Deficiency and Elevated Inflammatory Biomarkers Are Prevalent in Postural Orthostatic Tachycardia Syndrome. Cells. 2022;11(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atiquzzaman M, Thompson JR, Shao S, Djurdjev O, Bevilacqua M, Wong MMY, et al. Long-term effect of COVID-19 infection on kidney function among COVID-19 patients followed in post-COVID-19 recovery clinics in British Columbia, Canada. Nephrol Dial Transplant. 2023;38(12):2816–25. [DOI] [PubMed] [Google Scholar]
- 47.Gameil MA, Marzouk RE, Elsebaie AH, Rozaik SE. Long-term clinical and biochemical residue after COVID-19 recovery. Egypt Liver J. 2021;11(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hong XW, Chi ZP, Liu GY, Huang H, Guo SQ, Fan JR, et al. Characteristics of Renal Function in Patients Diagnosed With COVID-19: An Observational Study. Front Med (Lausanne). 2020;7:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Obrișcă B, Mocanu V, Vornicu A, Jurubiță R, Sorohan B, Dimofte G, et al. The impact of SARS-CoV-2 infection on renal function in patients with biopsy-proven kidney diseases. PLoS One. 2023;18(12):e0296168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Atiquzzaman M, Thompson JR, Shao S, Djurdjev O, Bevilacqua M, Wong MMY, et al. Long-term effect of COVID-19 infection on kidney function among COVID-19 patients followed in post-COVID-19 recovery clinics in British Columbia, Canada. Nephrology Dialysis Transplantation. 2023;38(12):2816–25. [DOI] [PubMed] [Google Scholar]
- 51.Martinez-Rojas MA, Vega-Vega O, Bobadilla NA. Is the kidney a target of SARS-CoV-2? Am J Physiol Renal Physiol. 2020;318(6):F1454–f62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moledina DG, Simonov M, Yamamoto Y, Alausa J, Arora T, Biswas A, et al. The Association of COVID-19 With Acute Kidney Injury Independent of Severity of Illness: A Multicenter Cohort Study. Am J Kidney Dis. 2021;77(4):490–9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahemuti N, Zou J, Liu C, Xiao Z, Liang F, Yang X. Urinary Albumin-to-Creatinine Ratio in Normal Range, Cardiovascular Health, and All-Cause Mortality. JAMA Netw Open. 2023;6(12):e2348333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin X, Song W, Zhou Y, Gao Y, Wang Y, Wang Y, et al. Elevated urine albumin creatinine ratio increases cardiovascular mortality in coronary artery disease patients with or without type 2 diabetes mellitus: a multicenter retrospective study. Cardiovasc Diabetol. 2023;22(1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi AI, Li Y, Deeks SG, Grunfeld C, Volberding PA, Shlipak MG. Association between kidney function and albuminuria with cardiovascular events in HIV-infected persons. Circulation. 2010;121(5):651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pirro M, Mannarino MR, Francisci D, Schiaroli E, Bianconi V, Bagaglia F, et al. Urinary albumin-to-creatinine ratio is associated with endothelial dysfunction in HIV-infected patients receiving antiretroviral therapy. Sci Rep. 2016;6:28741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bartz SK, Caldas MC, Tomsa A, Krishnamurthy R, Bacha F. Urine Albumin-to-Creatinine Ratio: A Marker of Early Endothelial Dysfunction in Youth. J Clin Endocrinol Metab. 2015;100(9):3393–9. [DOI] [PubMed] [Google Scholar]
- 58.Puntmann VO, Shchendrygina A, Bolanos CR, Madjiguène Ka M, Valbuena S, Rolf A, et al. Cardiac Involvement Due to COVID-19: Insights from Imaging and Histopathology. Eur Cardiol. 2023;18:e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mahajan S, Sen D, Sunil A, Srikanth P, Marathe SD, Shaw K, et al. Knockout of angiotensin converting enzyme-2 receptor leads to morphological aberrations in rodent olfactory centers and dysfunctions associated with sense of smell. Front Neurosci. 2023;17:1180868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Melo EGM, Andrade RM, de Abreu de Vasconcellos SJ, Dos Santos PL, Tanajura DM, Quintans-Júnior LJ, et al. Association between chemosensory dysfunctions and inflammatory biomarkers in patients with SARS-CoV-2 infection: a systematic review and meta-analysis. Inflammopharmacology. 2022;30(6):2079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Porta-Etessam J, Núñez-Gil IJ, González García N, Fernandez-Perez C, Viana-Llamas MC, Eid CM, et al. COVID-19 anosmia and gustatory symptoms as a prognosis factor: a subanalysis of the HOPE COVID-19 (Health Outcome Predictive Evaluation for COVID-19) registry. Infection. 2021;49(4):677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.