Abstract
Background:
In the neonatal intensive care unit (NICU), infants are at risk for late-onset sepsis. When blood cultures are negative, antibiotic stewardship efforts encourage stopping antibiotics, yet the duration of therapeutic exposure after the last dose is unknown.
Methods:
This retrospective cohort study of simulated antibiotic exposures used published population pharmacokinetic models within drug-specific NICU cohorts of preterm and term infants, postnatal age 7–60 days, exposed to cefepime, piperacillin-tazobactam, or tobramycin. Monte Carlo simulations (NONMEM 7.3) were used to predict steady-state exposures after 72-hour antibiotic course per Neofax® dosing. Exposure was assessed relative to drug-specific minimum inhibitory concentration (MIC) targets between 1 and 16mcg/mL for Pseudomonas and Enterobacteriaceae species. Post-discontinuation antibiotic exposure (PDAE) was defined as time from last dose to when antibiotic concentration decreased below a specific MIC.
Results:
Piperacillin-tazobactam, cefepime, and tobramycin cohorts included infants with median gestation age 29, 32, and 32 weeks and postnatal age 17, 19, and 15 days, respectively. The mean PDAE was 19–68 hours, depending on specific antibiotic/MIC combination. PDAE was longer for infants <28 days old and preterm (vs. term) infants. Cefepime exhibited the longest mean PDAE of 68 hours for Enterobacteriaceae MIC 1. Piperacillin mean PDAE was 25 hours for Enterobacteriaceae MIC 8. Tobramycin had a short mean PDAE of 19 hours.
Conclusions:
Piperacillin and cefepime exposures remained therapeutic long after the expected 8–12 hour dosing interval. PDAE is an important consideration for antibiotic stewardship among hospitalized infants, particularly premature infants and those within one month post-birth.
Keywords: Neonate, late-onset sepsis, pharmacokinetics, antimicrobial stewardship
INTRODUCTION
In neonatal intensive care units (NICUs), infants are at risk for late-onset sepsis (LOS) due to a wide array of gram-positive and gram-negative bacteria.1,2 When signs of infection occur, broad-spectrum empiric antibiotics are indicated however, prolonged exposure to broad-spectrum antibiotics also contributes to adverse drug consequences, emerging multidrug resistance, and cost.3,4 Among infants with LOS evaluations, most blood cultures are negative and empiric antibiotics are discontinued. Within the framework of antimicrobial stewardship, major professional societies promote the optimization of antibiotic duration to maximize clinical cure while limiting unnecessary exposure.5–7
The duration of therapeutic exposure after discontinuing antibiotics is not well characterized. Post-discontinuation antibiotic exposure (PDAE) has been defined as the time from the last dose to when the antibiotic concentration decreased below a specific pathogen MIC.8,9 After discontinuing an antibiotic, clinicians may assume that therapeutic exposures are limited after one dosing interval; however, during early-onset sepsis evaluations, preterm infants have been shown to have prolonged ampicillin PDAE, an average 94 hours for group-B Streptococci.8 During LOS evaluation, the PDAE of broad-spectrum antibiotics is unknown.
To address optimal antibiotic duration during late-onset sepsis evaluations in the NICU, we conducted a simulation study to quantify the post-discontinuation antibiotic exposure (PDAE) after the last dose of antibiotics among retrospective cohorts of preterm and term infants who had been exposed to cefepime, piperacillin-tazobactam, or tobramycin. Therapeutic exposure was analyzed over a broad range of MIC associated with Enterobacteriaceae and P. aeruginosa organisms. We hypothesized that the PDAE for each antibiotic would last longer than the typical dosing interval of the specific antibiotic and be prolonged in preterm infants. By increasing awareness of PDAE duration, clinicians and pharmacists may curtail unnecessary antibiotic exposure by shortening the duration of empiric antibiotic therapy among infants with negative cultures.
MATERIALS AND METHODS
Design
This pharmacokinetic (PK)-pharmacodynamic (PD) simulation study was designed to evaluate antibiotic concentrations during and after empiric courses of antibiotic therapy for late onset sepsis and specifically to evaluate post-discontinuation antibiotic exposures (PDAE) after the last dose. All simulations were performed in NONMEM 7.3 (Icon, Dublin, Ireland) using 1) retrospective drug-specific infant cohorts (n=1000) that included infants exposed to specific antibiotics within the Pediatrix Data Warehouse10, 2) published PK models for cefepime11, piperacillin12, and tobramycin13, and 3) antibiotic dosing using NeoFax® 2019 formulary. PDAE was defined as the time between the last dose of antibiotics and the time when the antibiotic concentration was below the MIC of a potential pathogen. PDAE time was evaluated relative to a range of MIC for specific organisms. The study was approved by the Duke University Institutional Review Board as exempt research.
Subject Selection
The Pediatrix Medical Group Clinical Data Warehouse contains data obtained from admission notes, daily progress notes, and discharge summaries, including demographic data, medications, laboratory results, and diagnoses.10 Using this data warehouse, we created three retrospective cohorts, one for each antibiotic of interest including cefepime, piperacillin-tazobactam, and tobramycin. Each antibiotic cohort included hospitalized preterm and term infants who were between postnatal age (PNA) 7 and 60 days when they received their first dose of antibiotic. The minimum gestational age (GA) criteria varied for each drug cohort in order to match the GA range of infants participating in population-based PK studies selected for simulation (cefepime ≥28 weeks; piperacillin-tazobactam ≥23weeks; and tobramycin ≥30 weeks). Infants with serum creatinine (SCr) >3.5 mg/dL were excluded. We extracted demographic data with pertinent covariates including birth weight, current weight, postmenstrual age (PMA), PNA, and SCr from the Clinical Data Warehouse. The Mosteller equation was used to calculate body surface area.14 Simulation cohorts for each antibiotic included 1000 randomly selected infants from each of the antibiotic cohorts.
Model Selection
A PubMed search for population-based PK models for cefepime, piperacillin/tazobactam, or tobramycin in neonates was performed.11–13,15–18 From the search results, we selected the population-based PK models of cefepime,11 piperacillin-tazobactam,12 and tobramycin13 based on the demographic characteristics of neonates used to construct each model (i.e., birthweight, current weight, GA, and PNA), as well as model robustness and sample sizes included in the original PK studies.
Monte Carlo Simulations
Monte Carlo simulations were performed in NONMEM 7.3 (Icon, Dublin, Ireland) using the selected population-based PK models, infant characteristics, and NeoFax® 2019 dosing to determine the antibiotic exposures during a 72-hour antibiotic course and the distribution of PDAE after the last dose of antibiotic relative to a range of MIC. Individual pharmacokinetic parameters, including volume of distribution (Vd), clearance (CL), half-life (t½), and elimination rate constant (k) were calculated for each infant. Antibiotic dosing was adjusted for PMA and PNA following the recommendations of the NeoFax® 2019 formulary (Table 1). Infusion times were 30 minutes for all antibiotics. We used NONMEM to perform 10 simulations per subject, therefore 10,000 per antibiotic, to generate a range of concentration time profiles that included interindividual and residual variabilities. Simulated concentration profiles were generated during the antibiotic course and for up to 4 days after the last dose. The 72-hour simulated antibiotic courses administered the last dose at 48, 60, or 64 hours for dosing intervals of 24, 12, and 8 hours respectively. Post-discontinuation antibiotic exposure (PDAE) after a 72-hour course were considered at steady-state. PDAE was defined as the duration of time between the last dose of antibiotic and the time antibiotic concentrations fell below a specified range of MIC breakpoints for each drug-organism combination.
TABLE 1.
Demographic and Pharmacokinetic Data of Simulated Infant Populations
| Drug (Minimum GA Selected for Cohort) | Cefepimea (≥28 weeks GA) | Piperacillin/Tazobactama (≥23 weeks GA) | Tobramycina (≥30 weeks GA) |
|---|---|---|---|
| N | 988 | 999 | 951 |
| Gestational age at birth (weeks) | 32 (29, 36) | 29 (25, 33) | 33 (31, 36) |
| Birth weight (grams) | 1655 (1186, 2573) | 1068 (740, 1750) | 1781 (1417.5, 2500) |
| Postnatal age (days) | 19 (12, 31) | 17 (11, 30) | 16 (11, 25) |
| Postmenstrual age (weeks) | 35 (32, 38) | 31 (28, 35) | 35 (33, 38) |
| Weight (g) | 2060 (1449, 2890) | 1350 (910, 2021) | 2045 (1636.5, 2800) |
| Body surface area (m2) | 0.159 (0.127, 0.196) | 0.120 (0.093, 0.157) | 0.156 (0.136, 0.188) |
| Serum creatinine (mg/dL) | 0.40 (0.30, 0.60) | 0. 50 (0.37, 0.80) | 0.40 (0.30, 0.60) |
| Creatinine clearance (mL/min/1.75 m2)b | 35.5 (25.1, 52.8) | 25.1 (15.1, 41.3) | 35.1 (24.0, 52.8) |
| Volume (L/kg) | 0.65 (0.29, 0.67) | 0.62 (0.42, 0.91) | 1.1 (0.85, 1.55) |
| Clearance (L/hr/kg) | 0.18 (0.14, 0.24) | 0.11 (0.06, 0.21) | 0.09 (0.07, 0.13) |
| Neofax 2019 dosing used in simulation | Using piperacillin component | ||
| All PMA, ≤28 days PNA, 30 mg/kg/dose q12h | ≤29wk PMA, 8–28 days PNA, 100 mg/kg/dose q12h | 30–34wk PMA, 8–60 days PNA, 4 mg/kg/dose q24h | |
| All PMA, >28 days PNA, 50 mg/kg/dose q12h | ≤29wk PMA, 29–60 days PNA, 100 mg/kg/dose q8h | ≥35 wk PMA, 8–60 days old 4 mg/kg/dose q24 | |
| 30–36 wk PMA, 8–14 days PNA, 100 mg/kg/dose q12h | |||
| 30–36 wk PMA, 15–60 days PNA, 100 mg/kg q8h | |||
| 37–44 wk PMA, 8–60 days PNA, 100 mg/kg q8h | |||
| Pharmacokinetic model11,12,13 | Piperacillin component | ||
| Sample Size | 31 (Index group) 10 (Validation group) | 32 | 100 (Index group) 40 (Validation group) |
| Compartment | 1 | 1 | 1 |
| Variability | CL interindividual 36.87% Vd interindividual 40.8% Additive residual error 6.98 mg/L | CL interindividual 37% Proportional residual error 33% Additive residual error 6.90 mg/L Not determined for Vd | CL interindividual 25.8% Vd interindividual 21.9% Proportional residual error 19.2% |
| Vd | Vd = 4.12 * BSA | Vd = 0.42 * WTKG | Vd = 0.533 * WTKG |
| CL | CL = 0.457 * BSA + 0.243 * CLcr | CL = 0.08 * WTKG * (PMA/33)1.76 | CL = 0.0508 * WTKG (birthweight>2500g) CL = 0.0508 * WTKG * 0.843 (birthweight ≤2500g) |
Numbers, except for sample size and where indicated, represent median with interquartile range in parentheses.
Creatinine clearance was estimated using the Schwartz formula: CLcr = (k*height)/SCR where k=0.33 for preterm (<37wk) and low birth weight neonates (<2500g) until 1 year of age, k = 0.45 for full term neonates until 1 year old.19
BSA, body surface area my Mosteller formula; BW, birthweight; CL, clearance; CLcr, creatinine clearance GA, gestational age; N/A, not applicable or available; PMA, postmenstrual age; SCR, serum creatinine; Vd, volume of distribution; WTKG, weight in kg.
The susceptible MIC interpretative breakpoints were obtained from USCAST (http://www.uscast.org) and EUCAST (http://www.eucast.org), which were concordant, except for P. aeruginosa. Both USCAST and EUCAST breakpoints for P. aeruginosa were evaluated. For cefepime, MIC susceptible breakpoint values were evaluated from 1 to 8 mcg/mL which covered most gram-negative pathogens including the specific breakpoints for P. aeruginosa (MIC 8 mcg/mL) and Enterobacteriaceae (MIC 2 mcg/mL). For piperacillin/tazobactam, all evaluations were performed for the piperacillin component alone, and the MIC susceptible breakpoint values were evaluated from 8 to 16 mcg/mL to include the upper limit of susceptibility (MIC 16) for P. aeruginosa and Enterobacteriaceae. For tobramycin, MIC susceptible breakpoint values were evaluated from 1 to 2 mcg/mL for P. aeruginosa and Enterobacteriaceae.
Statistical Analysis
R version 3.5.0 was used for statistical computation and graphical visualization. Elimination curves were plotted using a decay equation, C=Co*e(−kt), with elimination constant k calculated for each patient from simulation-derived Vd and CL. Simulated antibiotic concentration profiles over time were summarized in visual plots using the mean and a shaded area corresponding to values between the 2.5 and 97.5 percentile of drug concentration data points every 6 hours for up to 4 days after the last dose. The distribution of PDAE after various dosing regimens of cefepime, piperacillin-tazobactam, and tobramycin were evaluated using susceptibility MIC breakpoints.
RESULTS
Each antibiotic virtual population consisted of approximately 1,000 hospitalized preterm and term infants exposed to the respective antibiotic between 7 and 60 days of age (Table 1). The minimum GA of each drug-specific cohort matched the minimum GA of infants included in development of population PK model. The piperacillin cohort had a younger GA, smaller birth weight, and lower estimated creatinine clearance. The dosing regimens and estimated PK parameters, volume of distribution (Vd), and clearance (CL) for each antibiotic cohort using the selected published population-based PK models are provided in Table 1.11–13 While PDAE after a 72-hour antibiotic course was assessed, the mean Vd and CL for our simulation population indicated similar PDAE results for a 36- to 48-hour course. Simulated antibiotic exposures for the 72-hour course and the PDAE period are provided in Figure 1.
FIGURE 1.
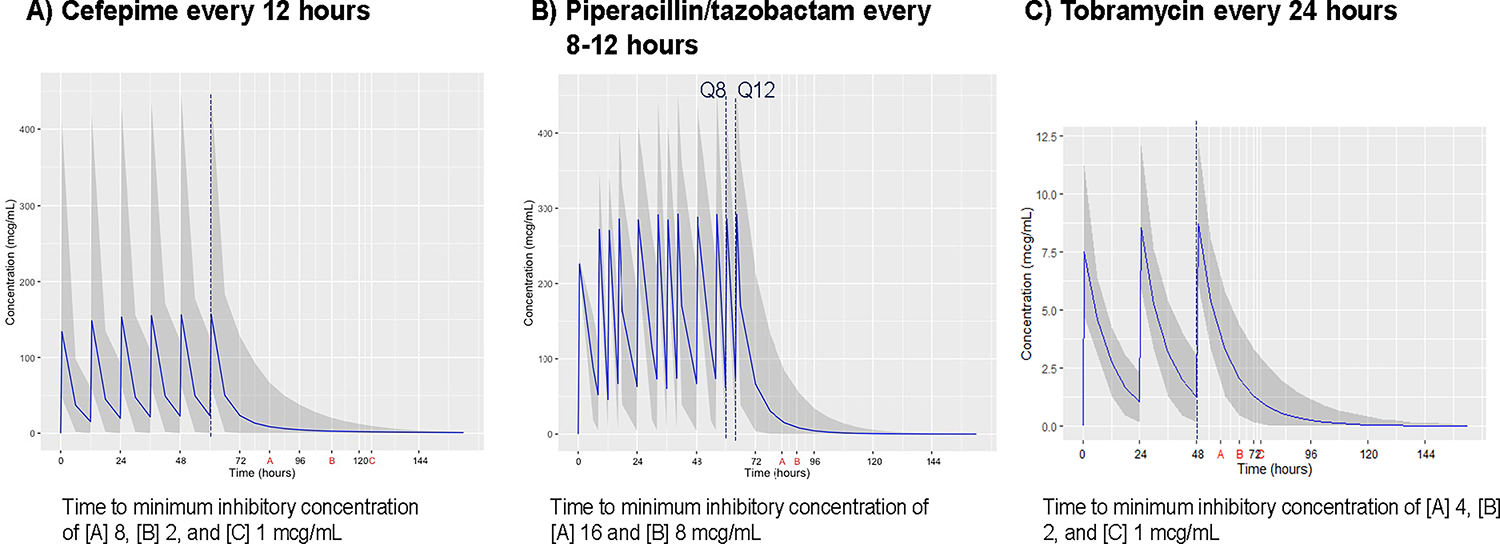
Simulated Plasma Concentration Profiles of Common Antibiotics Relative to Susceptibility Breakpoints of Common Pathogens.
Black solid line denotes mean drug concentration; shaded area, the 2.5 to 97.5 percentile of concentration data points; vertical dashed line, time at last antibiotic dose; letter symbols (A, B, C) on X-axis indicate time points at which mean antibiotic concentrations decay to susceptibility breakpoint MIC for different bacteria post-antibiotic discontinuation. PDAE represents time between red line and symbol representing MIC on X-axis. Figures represent standard antibiotic exposures during a 72-hour course. A) Cefepime was administered every 12 hours and MIC susceptibility breakpoints ranged from 1–2 mcg/mL for Enterobacteriaceae to 8 mcg/mL for P. aeruginosa. B) Piperacillin-tazobactam was administered every 8 or 12 hours depending on infant’s postmenstrual and postnatal age. Concentration curves represents piperacillin component. MIC susceptibility breakpoints ranged from 8 to 16 mcg/mL for P. aeruginosa and Enterobacteriaceae. C) Tobramycin was administered every 24 hours. For Piperacillin, MIC susceptibility breakpoints ranged from 1–2 for P. aeruginosa and Enterobacteriaceae.
CI, confidence interval; MIC, minimum inhibitory concentration; PDAE, post-discontinuation empiric antibiotic exposure.
The mean duration of PDAE varied from 19–68 hours for specific antibiotic/organism MIC combinations (Table 2). For all antibiotic/MIC combinations, PDAE was longer for the more preterm infants and those less than 28 days of age. Cefepime had the longest PDAE and highest frequency of infants with prolonged exposure beyond the dosing interval.
TABLE 2.
PDAE among Infants Exposed to Cefepime, Piperacillin/Tazobactam, or Tobramycin using MIC Susceptibility Breakpoints for Enterobacteriaceae & P. aeruginosa
| Antibiotic | Organism | MIC (mcg/mL) | PDAE (hours)a | % Patients Achieving Time Above MIC | ||
|---|---|---|---|---|---|---|
| 1x Dosing Interval after Discontinuation | 2x Dosing Interval after Discontinuation | 3x Dosing Interval after Discontinuation | ||||
| Cefepime | P. aeruginosa | 8 | 26 ± 17 (3, 69) | 58.5 | 18.2 | 8.1 |
| Enterobacteriaceae | 2 | 54 ± 23 (6, 95) | 79.9 | 38.8 | 18.4 | |
| 1 | 68 ± 25 (7, 104) | 84.7 | 49.0 | 25.2 | ||
| Piperacillin/ tazobactam | Enterobacteriaceae & P. aeruginosa | 16 | 19 ± 8 (7, 36) | 72.6 | 8.2 | 2.2 |
| Enterobacteriaceae | 8 | 25 ± 9 (9, 44) | 85.0 | 12.5 | 4.3 | |
| Tobramycin | P. aeruginosa & Enterobacteriaceae | 2 | 19 ± 7 (10, 36) | 18.0 | 0.2 | 0 |
| P. aeruginosa & Enterobacteriaceae | 1 | 28 ± 9 (15, 52) | 59.1 | 3.6 | 0.1 | |
Numbers, except for sample size, represent mean ± standard deviation with 95% confidence interval in parentheses.
MIC, minimum inhibitory concentration; PDAE, post-discontinuation antibiotic exposure.
Among infants receiving cefepime using a 12-hour dosing interval, simulated cefepime concentrations remained above the MIC 8 (Pseudomonas) or MIC 1 (Enterobacteriaceae) for an average of 26 hours or 68 hours, respectively (Table 2). While most infants maintained a cefepime concentration above the MIC for one 12-hour dosing interval after discontinuation, 49% continued to maintain cefepime concentrations above the Enterobacteriaceae MIC of 1 mcg/mL for 2 dosing intervals (24 hours) and 25% for 3 dosing intervals (36 hours).
Among infants receiving piperacillin-tazobactam, using an 8- or 12-hour dosing interval based on PMA and PNA age groups, simulated piperacillin concentrations remained above the MIC 16 (Pseudomonas) or MIC 8 (Enterobacteriaceae) for an average of 19 or 25 hours, respectively (Table 2). While most infants maintained a piperacillin concentration above the MIC for one dosing interval after discontinuation, only 12.5% continued to maintain piperacillin concentrations above the Enterobacteriaceae MIC of 8 mcg/mL for 2 dosing intervals (16–24 hours).
Among infants receiving tobramycin using the extended dosing interval of 24 hours, simulated tobramycin concentrations remained above the MIC 2 (Pseudomonas) or MIC 1 (Enterobacteriaceae) for 19 hours or 28 hours, respectively (Table 2). Some infants (18% to 59%) maintained concentrations above the MIC of 1–2 for the one dosing interval (24 hours) after the final dose.
The concentration-time curves for each antibiotic demonstrate exposure above the MIC during therapy, as well as the concentration decay after the last dose (Fig. 1). As expected, PDAE was longer for organisms with lower MIC. Overall, the duration of PDAE was longer for cefepime and piperacillin compared to tobramycin. Mean tobramycin concentrations decreased below the MIC breakpoints before 24 hours.
The piperacillin cohort included broad range of GA infants, among whom dosing interval varied with PMA and PNA groups (Table 3). Despite dose interval adjustment, PDAE was consistently longer in the younger PMA groups. Within a PMA group, the PDAE was more consistent between the different PNA groups.
TABLE 3.
Piperacillin PDAE in Different Dosing Groups Based on PMA and PNA
| Drug | Organism | MIC Breakpoint (mcg/mL) | Dosing Regimen Based on Age | PDAE (Hours)a | ||||
|---|---|---|---|---|---|---|---|---|
| PMA (Weeks) | PNA (Days) | Per Dose and Interval | ||||||
| Piperacillin | Enterobacteriaceae & P. aeruginosa | 16 | ≤29 | 8–28 | 100 mg/kg q12h | 27 ± 11 (10, 53) | ||
| ≤29 | 29–60 | 100 mg/kg q8h | 25 ± 11 (9, 50) | |||||
| 30–36 | 8–14 | 100 mg/kg q12h | 18 ± 7 (6, 34) | |||||
| 30–36 | 15–60 | 100 mg/kg q8h | 20 ± 8 (5, 38) | |||||
| 37–44 | 8–60 | 13 ± 5 (5, 26) | ||||||
| Enterobacteriaceae | 8 | ≤29 | 8–28 | 100 mg/kg q12h | 35 ± 13 (13, 64) | |||
| ≤29 | 29–60 | 100 mg/kg q8h | 32 ± 12 (11, 58) | |||||
| 30–36 | 8–14 | 100 mg/kg q12h | 24 ± 9 (8, 42) | |||||
| 30–36 | 15–60 | 100 mg/kg q8h | 25 ± 10 (8, 46) | |||||
| 37–44 | 8–60 | 18 ± 6 (7, 32) | ||||||
Numbers represent mean ± standard deviation (95% CI).
CI, confidence interval; MIC, minimum inhibitory concentration; PDAE, post-discontinuation antibiotic exposure; PMA, postmenstrual age; PNA, postnatal age.
DISCUSSION
Infants hospitalized in the NICU are at risk for LOS and therefore commonly receive empiric broad-spectrum antibiotics during sepsis evaluations. Nevertheless, many signs of sepsis overlap with signs of non-infectious neonatal conditions such as apnea, respiratory insufficiency, or deficient thermoregulation. From a stewardship perspective and to limit adverse effects of prolonged antibiotic exposure, it is important to limit duration of antibiotics when cultures are negative.20,21 In this simulation study, piperacillin-tazobactam and cefepime continue to exhibit therapeutic exposure well beyond 1 dosing interval after last dose. For more common Enterobacteriaceae species with lower MIC, the duration extends even beyond 24 hours after the last dose. Cefepime concentrations remained above typical MIC for a longer duration than piperacillin. On the contrary, the PDAE of tobramycin exposure was short relative to the 24-hour dose interval. The longer PDAE for cefepime and piperacillin supports the need for more research on the potential benefits of earlier discontinuation of antibiotics when cultures remain negative and alternatively, the potential long-term adverse effects of longer PDAE. PDAE also serves as an important PK-PD exposure metric for studies refining dose recommendations in critically ill newborns.
The difference of PDAE duration, short for tobramycin and longer for cefepime and piperacillin, can be explained by the pharmacodynamics principles supporting their respective dosing regimens. Cefepime and piperacillin are both time-dependent antibiotics with minimal bacterial killing once concentrations are below the MIC. For time-dependent antibiotics, dosing regimens aim to maintain antibiotic concentrations above the MIC for a fraction of the dosing interval time (fT>MIC). In critically ill infants and those born prematurely with incompetent immune systems, the optimal fT>MIC has not been determined.22 Dosing regimens that target longer fT>MIC or higher MIC breakpoints will promote longer PDAE. This could be particularly relevant for infants with negative cultures. The longer PDAE of cefepime and piperacillin suggest that further research is needed to optimize both the dosing recommendations and duration of empiric antibiotics when cultures are negative.
Alternatively, tobramycin is a concentration dependent antibiotic. The high-dose, extended-interval dosing is designed to achieve high, early tobramycin concentrations to improved bacterial killing, and the long dose interval takes advantage of the post-antibiotic effect that provides ongoing bacteriostatic activity, even when the concentration falls below the pathogen MIC. For concentration-dependent antibiotics with long post-antibiotic effects, the PDAE will be shorter, even less than the dosing interval, since the drug maintains some activity at concentrations below the pathogen MIC.
Among beta-lactam medications known for their time-dependent antibiotic activity, the PDAE duration was longer for cefepime compared to piperacillin. This difference is most likely attributed to the lower MIC breakpoints for cefepime compared to piperacillin. For Pseudomonas, the cefepime MIC breakpoint 8 is half the piperacillin MIC breakpoint 16 mcg/mL. For Enterobacteriaceae, the difference is even greater with cefepime MIC breakpoint of 1–2 mcg/mL compared to 8 mcg/mL for piperacillin. Cefepime and piperacillin have similar PDAE duration when using an MIC of 8 mcg/mL. The longer cefepime PDAE duration could also indicate high cefepime exposure and support the use of PDAE as an exposure target during dose-evaluation studies.
The longer PDAE duration among the most premature infants (<29 weeks PMA) is likely attributed to delays in renal clearance among very preterm infants that may not be fully accounted for with longer dosing interval. Delayed drug clearance and the subsequent higher drug exposures is expected to prolong the PDAE duration. The piperacillin infant cohort contained the most premature infants and broadest range of maturity. Among hospitalized infants in the NICU, drug clearance decreases with the degree of prematurity and younger PNA. The clearance of renally-eliminated antibiotics is highly dependent on kidney maturation and function, yet nephrogenesis is not expected to be complete before 36-weeks gestation.23, 24 Piperacillin-tazobactam dosing is also designed to maintain piperacillin concentrations above Pseudomonas and Enterobactereaciae species with higher MIC. When dosing regimen target high MIC susceptibility breakpoint for most of the dosing interval then the PDAE will be longer. PDAE is an important stewardship metric for future dose-exposure research in very preterm infants.
The use of broad-spectrum antibiotics is prudent for empiric treatment during LOS evaluations in hospitalized infants; however, when cultures remain negative, the continued exposure is less desirable. Clinicians and pharmacists can use both PDAE and time to blood culture positivity to curtail antibiotic exposure in infants with negative cultures. Extended use of broad-spectrum antibiotics has been associated with increased risk of highly-resistant bacterial colonization, late-onset infections, and mortality.25–34 Along with environmental and genetic factors, prolonged antibiotic exposure changes the intestinal microbiota and immune system of hospitalized infants.35 Early exposure to antimicrobial agents has been linked to an increased risk for unfavorable outcomes, including asthma, allergy, eczema, obesity, irritable bowel disease, and diminished microbiome diversity.35–38 The disruption in the intestinal microbiota has been associated with subsequent development of necrotizing enterocolitis.39 When the PDAE is prolonged, then shorter empiric courses could be considered. Notably, the MIC breakpoints used in this study offer the most conservative approach; the PDAE on a typical infant would likely be longer since the MICs of organisms in United States NICUs are often lower than the susceptibility breakpoint MICs, including the upper limit of susceptible range.
The primary limitation of our study was that although we employed a large national database representative of real-world subjects to conduct Monte Carlo simulations, we did not directly assess antibiotic concentrations. Another limitation is that the population PK models for piperacillin/tazobactam and tobramycin did not include subjects that were GA >32 weeks and PNA >14 days, respectively. Nonetheless, the inclusion of weight in the PK model did allow for adjustment among larger, older infants. The cefepime and tobramycin cohorts did not include very premature infants with GA <28 weeks; therefore, we could not assess the impact of PMA <29 weeks on PDAE for these medications. Prospective studies are underway to confirm the presence and extent of PDAE via PK sampling after antibiotic discontinuation.
CONCLUSION
PDAE is an innovative antimicrobial stewardship metric that allows clinicians and pharmacists to define the endpoint of therapeutic exposure, and to consider shorter empiric antibiotic courses (<48 hours) for infants with negative cultures. PDAE is particularly relevant to time-dependent antibiotics with minimal post-antibiotic effects, such as cefepime and piperacillin-tazobactam. Efforts to minimize unnecessary antibiotic exposure may mitigate the risk of adverse outcomes associated with antibiotic use.
ACKNOWLEDGMENTS
PTN Steering Committee Members: Daniel K. Benjamin Jr., Christoph Hornik, Kanecia Zimmerman, Phyllis Kennel, and Rose Beci, Duke Clinical Research Institute, Durham, NC; Chi Dang Hornik, Duke University Medical Center, Durham, NC; Gregory L. Kearns, Scottsdale, AZ; Matthew Laughon, University of North Carolina at Chapel Hill, Chapel Hill, NC; Ian M. Paul, Penn State College of Medicine, Hershey, PA; Janice Sullivan, University of Louisville, Louisville, KY; Kelly Wade, Children’s Hospital of Philadelphia, Philadelphia, PA; Paula Delmore, Wichita Medical Research and Education Foundation, Wichita, KS
The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD): Perdita Taylor-Zapata and June Lee
The Emmes Company, LLC (Data Coordinating Center): Ravinder Anand, Gaurav Sharma, Gina Simone, Kim Kaneshige, and Lawrence Taylor
PTN Publications Committee: Chaired by Thomas Green, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL
Source of Funding:
This work was funded under the National Institute of Child Health and Human Development (NICHD) contract (HHSN275201000003I) for the Pediatric Trials Network (PI Danny Benjamin). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest
All authors declare no relevant financial disclosures or conflicts of interest.
REFERENCES
- 1.Cogins SA, Glaser K. Updates in Late-onset Sepsis: Risk Assessment, therapy and outcomes. . Neoreviews. 2022;23: 738–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karlowicz MG, Buescher ES, Surka AE. Fulminant late-onset sepsis in a neonatal intensive care unit, 1988–1997, and the impact of avoiding empiric vancomycin therapy. Pediatrics. 2000;106:1387–1390. [DOI] [PubMed] [Google Scholar]
- 3.Dong Y, Speer CP. Late-onset neonatal sepsis: recent developments. Arch Dis Child Fetal Neonatal Ed. 2015;100:F257–F263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–291. [DOI] [PubMed] [Google Scholar]
- 5.United States Centers for Disease Control and Prevention (CDC). The core elements of hospital antibiotic stewardship programs: 2019. Atlanta, GA: US Department of Health and Human Services, CDC; 2019. Available at: https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf. Published 2019. Accessed May 22, 2023.
- 6.Dellit TH, Owens RC, McGowan JE, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–177. [DOI] [PubMed] [Google Scholar]
- 7.Pediatric Infectious Diseases Society. Pediatric Antibiotic Stewardship Program Toolkit. Available at: https://pids.org/pediatric-asp-toolkit/. Accessed May 22, 2023. [Google Scholar]
- 8.Le J, Greenberg RG, Benjamin DK, et al. Prolonged post-discontinuation antibiotic exposure in very low birth weight neonates at risk for early-onset sepsis. J Pediatric Infect Dis Soc. 2021;10:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le J, Greenberg RG, Yoo Y, et al. Ampicillin dosing in premature infants for early-onset sepsis: exposure-driven efficacy, safety, and stewardship. J Perinatol. 2022;42:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spitzer AR, Ellsbury DL, Handler D, Clark RH. The Pediatrix BabySteps Data Warehouse and the Pediatrix QualitySteps improvement project system--tools for “meaningful use” in continuous quality improvement. Clin Perinatol. 2010;37:49–70. [DOI] [PubMed] [Google Scholar]
- 11.Lima-Rogel V, Medina-Rojas EL, Del Carmen Milán-Segovia R, et al. Population pharmacokinetics of cefepime in neonates with severe nosocomial infections. J Clin Pharm Ther. 2008;33:295–306. [DOI] [PubMed] [Google Scholar]
- 12.Cohen-Wolkowiez M, Watt KM, Zhou C, et al. Developmental pharmacokinetics of piperacillin and tazobactam using plasma and dried blood spots from infants. Antimicrob Agents Chemother. 2014;58:2856–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falcão AC, Buelga DS, Méndez ME, García MJ, Pardo M. Population kinetics of tobramycin in neonates. Ther Drug Monit. 2001;23:202–208.14. [DOI] [PubMed] [Google Scholar]
- 14.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. [DOI] [PubMed] [Google Scholar]
- 15.Cohen-Wolkowiez M, Benjamin DK Jr, Ross A, et al. Population pharmacokinetics of piperacillin using scavenged samples from preterm infants. Ther Drug Monit. 2012;34:312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Chen Y, Li Q, et al. Population pharmacokinetics of piperacillin/tazobactam in neonates and young infants. Eur J Clin Pharmacol. 2013;69:1223–1233. 17. [DOI] [PubMed] [Google Scholar]
- 17.De Cock RF, Allegaert K, Brussee JM, et al. Simultaneous pharmacokinetic modeling of gentamicin, tobramycin and vancomycin clearance from neonates to adults: towards a semi-physiological function for maturation in glomerular filtration. Pharm Res. 2014;31:2643–2654.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nahata MC, Powell DA, Durrell DE, Miller MA. Tobramycin pharmacokinetics in very low birth weight infants. Br J Clin Pharmacol. 1986;21:325–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571–590. [DOI] [PubMed] [Google Scholar]
- 20.Cotten CM. Adverse consequences of neonatal antibiotic exposure. Curr Opin Pediatr. 2016;28:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ting JY, Synnes A, Roberts A, et al. Association between antibiotic use and neonatal mortality and morbidities in very low-birth-weight infants without culture-proven sepsis or necrotizing enterocolitis. JAMA Pediatr. 2016;170:1181–1187 [DOI] [PubMed] [Google Scholar]
- 22.Hong LT, Downes KJ, FakhriRavari A, Abdul-Mutakabbir JC, Kuti JL, Jorgensen S, Young DC, Alshaer MH, Bassetti M, Bonomo RA, Gilchrist M, Jang SM, Lodise T, Roberts JA, Tängdén T, Zuppa A, Scheetz MH. International consensus recommendations for the use of prolonged-infusion beta-lactam antibiotics: Endorsed by the American College of Clinical Pharmacy, British Society for Antimicrobial Chemotherapy, Cystic Fibrosis Foundation, European Society of Clinical Microbiology and Infectious Diseases, Infectious Diseases Society of America, Society of Critical Care Medicine, and Society of Infectious Diseases Pharmacists: An executive summary. Pharmacotherapy. 2023. Aug;43(8):740–777. [DOI] [PubMed] [Google Scholar]
- 23.Abitbol CL, DeFreitas MJ, Strauss J. Assessment of kidney function in preterm infants: lifelong implications. Pediatr Nephrol. 2016;31:2213–2222. [DOI] [PubMed] [Google Scholar]
- 24.Le J, Bradley JS. Optimizing antibiotic drug therapy in pediatrics: current state and future needs. J Clin Pharmacol. 2018;58 Suppl 10:S108–S122. [DOI] [PubMed] [Google Scholar]
- 25.Filioti J, Spiroglou K, Roilides E. Invasive candidiasis in pediatric intensive care patients: epidemiology, risk factors, management, and outcome. Intensive Care Med. 2007;33:1272–1283. [DOI] [PubMed] [Google Scholar]
- 26.Flokas ME, Karanika S, Alevizakos M, Mylonakis E. Prevalence of ESBL-producing Enterobacteriaceae in pediatric bloodstream infections: a systematic review and meta-analysis. PLoS One. 2017;12:e0171216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenberg RG, Chowdhury D, Hansen NI, et al. Prolonged duration of early antibiotic therapy in extremely premature infants. Pediatr Res. 2019;85:994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159:720–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le J, Nguyen T, Okamoto M, McKamy S, Lieberman JM. Impact of empiric antibiotic use on development of infections caused by extended-spectrum beta-lactamase bacteria in a neonatal intensive care unit. Pediatr Infect Dis J. 2008;27:314–318. [DOI] [PubMed] [Google Scholar]
- 30.Madan JC, Salari RC, Saxena D, et al. Gut microbial colonisation in premature neonates predicts neonatal sepsis. Arch Dis Child Fetal Neonatal Ed. 2012;97:F456–F462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel SJ, Saiman L. Antibiotic resistance in neonatal intensive care unit pathogens: mechanisms, clinical impact, and prevention including antibiotic stewardship. Clin Perinatol. 2010;37:547–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spiliopoulou A, Dimitriou G, Jelastopulu E, Giannakopoulos I, Anastassiou ED, Christofidou M. Neonatal intensive care unit candidemia: epidemiology, risk factors, outcome, and critical review of published case series. Mycopathologia. 2012;173:219–228. [DOI] [PubMed] [Google Scholar]
- 33.Cotten CM, McDonald S, Stoll B, et al. The association of third-generation cephalosporin use and invasive candidiasis in extremely low birth-weight infants. Pediatrics. 2006;118:717–722. [DOI] [PubMed] [Google Scholar]
- 34.Clock SA, Jia H, Patel S, et al. Infant colonization with methicillin-resistant Staphylococcus aureus or vancomycin-resistant Enterococci preceding neonatal intensive care unit discharge. J Pediatric Infect Dis Soc. 2017;6:e144–e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat Med. 2016;22:713–722. [DOI] [PubMed] [Google Scholar]
- 36.Nogacka AM, Salazar N, Arboleya S, et al. Early microbiota, antibiotics and health. Cell Mol Life Sci. 2018;75:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gasparrini AJ, Crofts TS, Gibson MK, Tarr PI, Warner BB, Dantas G. Antibiotic perturbation of the preterm infant gut microbiome and resistome. Gut Microbes. 2016;7:443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arboleya S, Sánchez B, Milani C, et al. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J Pediatr. 2015;166:538–544. [DOI] [PubMed] [Google Scholar]
- 39.Alexander VN, Northrup V, Bizzarro MJ. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr. 2011;159:392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]


