Abstract
Objectives
Medical devices based on X-ray imaging, such as computed tomography, are considered notable sources of artificial radiation. The aim of this study was to compare the computed tomography dose volume index, the dose length product, and the effective dose of the brain non-contrast enhanced examination on two CT scanners to determine the current state in terms of radiation doses, compare doses to the reference values, and possibly optimize the examination.
Materials and methods
Data from January 2020 to the second half of 2021 were retrospectively obtained by accessing dose reports from the Picture Archiving and Communication System (PACS). Data were collected and analyzed in Microsoft Excel. The effective dose was estimated using the dose-length product parameter and the normalized conversion factor for a given anatomical region. For statistical analysis, a two-sample t-test was used.
Results
The first data set consists of 200 patients (100 and 100 for older and newer CT scanners) regardless of the scan technique; the average CTDIvol and DLP for the older CT scanner were 57.61 ± 2.89 mGy and 993.28 ± 146.18 mGy cm, and for the newer CT scanner, 43.66 ± 11.15 mGy and 828.14 ± 130.06 mGy cm. The second data set consists of 100 patients (50 for the older CT scanner and 50 for the newer CT scanner) for a sequential scan; the average CTDIvol and DLP for the older CT scanner were 58.63 ± 3.33 mGy and 949.42 ± 80.87 mGy.cm, and for the newer CT, 57.25 ± 3.4 mGy and 942.13 ± 73.05 mGy cm. The third data set consists of 40 patients (20 and 20 for older and newer CT scanners) for the helical scan - the average CTDIvol and DLP for the older CT scanner were 54.6 ± 0 mGy and 1252.2 ± 52.11 mGy.cm, and for the newer CT, 37.18 ± 2.52 mGy and 859.66 ± 72.04 mGy cm. The difference between the older and newer CT scanners in terms of dose reduction was approximately 30 % in favor of the newer scanner for noncontrast enhanced brain examinations performed using the helical scan technique.
Conclusion
A non-contrast enhanced brain examination scanned with newer CT equipment was associated with a lower radiation burden on the patient.
Keywords: Computed tomography, Radiation dose, Effective dose, Brain
1. Introduction
The introduction of computed tomography (CT) in medical practice marked a revolution in diagnostic and imaging methods. Over the years, there has been a significant increase in the use of this modality. For example, in the USA, there has been a 20-fold increase in the number of CT scans performed per year compared to the 1980s [1,2]. It is necessary to consider that CT belongs to the modalities that have a higher radiation burden on the patient. A study conducted in Ireland in 2009 found that CT accounted for 10 % of all radiological examinations but contributed up to 56 % of total radiation exposure from medical radiation [3]. The International Commission for Radiological Protection (ICRP) defines three basic principles of radiation protection, namely justification, optimization, and dose limits. Medical imaging radiation for patients has a special justification, and the dose limitation principle does not apply to medical exposures to preserve the potential benefit of exposure. Alternately, the ICRP defines diagnostic reference levels (DRLs) as a reference value to compare with examination doses. Justification and optimization of the examination should be done along with the principle of as low as reasonably achievable (ALARA), that is, X-ray examinations must be performed with adequate image quality with the lowest possible radiation dose [[4], [5], [6], [7]].
The guidelines of the European Union require the determination and use of DRLs [[8], [9], [10]]. DRLs for computed tomography are established by dose parameters that can be easily obtained and take into account the scan parameters, namely: computed tomography dose volume index (CTDIvol [mGy]) and dose length product (DLP [mGy.cm]). The CTDIvol parameter represents the dose transferred to a standard phantom (16 or 32 cm) and is independent of the size and shape of the patient. DLP is a product of scan length and CTDIvol and is a relative representation of the energy delivered during scanning, but does not consider the radiosensitivity of tissues or organs in the scan area [[10], [11], [12], [13], [14]]. Both CTDIvol and DLP are basic tools for CT optimization, although it is important to remember that these parameters only represent the CT dose output. Although DLP is not based on the real size of the patient, it is directly related to the risk of irradiation; therefore, it became an accepted value through the introduction of DRL and allows comparison of patient medical exposure [[15], [16], [17], [18], [19]].
The basic requirement of every CT image is that the image meets the minimum criteria set for image quality, i.e., that it contains enough reliable information for diagnosis. Before performing a CT scan, it is necessary that the settings of the selected CT protocol correspond to the required information and technical limitations of the X-ray tube. Analysis of how exposure parameters affect patient dose provides the first step in reducing patient dose in CT examinations [[20], [21], [22]]. In general, the relationship between tube current (mA) and patient dose is directly proportional. An increase in mA results in a proportional increase in the dose given to the patient. Although mA can be manually adjusted, many operators use automated tube current modulation (ATCM) for most applications [23]. Increases in tube current or the product of tube current and scan time (mAs) result in improved image quality, decreased image noise, and increased patient dose. The tube voltage (kVp) affects the mean energy of the spectrum, which then affects the linear attenuation coefficients. A change in tube voltage leads to a change in absorption in individual materials, which affects contrast, noise, and dose for the patient [21,22]. Reducing kVp can be an effective way of reducing the radiation dose for patients because of the reduced number of photons and the lower mean energy of the photons. On the other hand, decreases in kVp can result in increases in image noise, which usually requires an increase in mA to preserve image quality [23]. Rotation time is the time spent completing a 360° rotation of the gantry. The radiation exposure of the patient is proportional to the rotation time; faster rotation times have a lower dose effect if all other parameters are kept constant [24]. Pitch is a parameter used in helical scanning mode and is described as the ratio of table translation per rotation of the X-ray tube to the total collimation of the beam. If the tube output (in mAs) remains constant as the table moves, the radiation dose is proportionally decreased as the pitch increases [24]. Slice thickness also influences patient dose and image noise. A decreasing slice thickness leads to an increase in image noise because of the lower number of photons within each voxel. To preserve constant noise levels within an image with a smaller slice thickness, the radiation dose must consequently be increased. In general, the greater the thickness of the reconstructed slice used, the lower the dose for the patient [[20], [21], [22], [23], [24]]. Overall, adjustments to the exposure parameters should be made according to the clinical task of a CT examination.
The effective dose parameter (ED) was determined to estimate the radiation risk in quantity, which could be related to the probability of health harm due to stochastic effects from radiation exposure [25]. The potential biological effects of radiation depend not only on the radiation dose per tissue or organ, but also on the biological sensitivity of the irradiated tissue or organ [26]. ED is a sum of the equivalent doses in tissues and organs of the body that are considered to be sensitive to radiation damage, weighted according to the risk of associated harm. Since ED cannot be measured directly, for its estimation, calculations based on models and simulations are required to assess the doses of individual tissues [25,27]. It is important to note that ED describes the relative total body dose for a particular examination and scanner, not the individual's dose. Its primary use should be limited to comparing the health harm with a reference patient for different types of medical examinations [20,22]. Therefore, ED can be used to compare relative radiation harm between diagnostic radiology procedures and with other radiation sources when the effective doses calculated are for patient populations with comparable age and gender distributions [27]. Therefore, ED allows us to compare risks between different CT protocols and examinations, as well as between CT and other imaging methods. Consequently, we can use it for generic justification and proper optimization of CT examinations [25,[27], [28], [29], [30]].
2. Materials and Methods
2.1. Study design
This study was reviewed and approved by the Institutional Review Board, with approval number 158/2022. Data for analysis were collected from January 2020 to the second half of 2021. Only non-contrast enhanced brain examinations performed on patients over 18 years of age were included in the study. In this case, the weight of the patient was not taken into account. Data (DLP and CTDIvol) were retrospectively accessed from PACS dose reports, which are created at the end of each examination. The resulting data set consisted of all eligible patients within a defined period, half of whom were examined on one CT scanner and the other half on the second CT. From this dataset, we created three groups of patients based on the scan technique: Group 1 (regardless of the scanning technique), Group 2 (sequential scanning technique), and Group 3 (spiral scanning technique).
2.2. Machine specifications
Two multislice CT scanners are available at the university center. The older CT scanner, CT Philips Ingenuity Core (64 slices), was installed in 2014, and the newer GE Revolution CT (256 slices) was installed in 2020. CT scanners undergo regular maintenance and long-term stability tests with satisfactory results. Both CT scanners use an iterative reconstruction algorithm (iDose4 and ASiR-V). The principle of this algorithm is to iterate image reconstruction several times until the final image corresponds as well as possible to the measured values of total absorption coefficients from different angular projections. Through this process, image noise is suppressed more effectively. The disadvantages are longer reconstruction times and high computing power demands. However, iterative reconstruction provides a diagnostically acceptable noise level with a substantially reduced radiation dose [17,[31], [32], [33]].
2.3. Examination protocols
Table 1 summarizes the scan parameters for each scan protocol. Each vendor has its own scale of the strength of iterative reconstruction; the higher the strength, the lower the noise.
Table 1.
Dataset of CTDIvol and DLP values and estimated EDs.
|
Group 1 |
Group 2 - Sequential scan |
Group 3 - Helical scan |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| CT Philips | CT GE | T-test | CT Philips | CT GE | T-test | CT Philips | CT GE | T-test | |
| Scan parameters | |||||||||
| Tube voltage (kV) | 120 | 120 | 120 | 120 | |||||
| Tube current | 378 mAs (432 mA) | 350 mA | 423 mAs (299 mA) | 80-300 (smart mA) | |||||
| Slice thickness (mm) | 1.25 | 1.25 | 1 | 1.25 | |||||
| Rotation time (s) | 0.75 | 1 | 0.4 | 0.5 | |||||
| Pitch |
– | – | 0.399 | 0.516:1 | |||||
| Strength of IR | Level 3 | 60 % | Level 4 | 50 % | |||||
| Number of patients | 100 | 100 | 50 | 50 | 20 | 20 | |||
| ED (mSv) | |||||||||
| min | 1.76 | 1.21 | 1.76 | 1.33 | 2.46 | 1.66 | |||
| max | 2.84 | 2.14 | 2.84 | 2.14 | 2.84 | 2.06 | |||
| mean (SD) | 2.09 (0.31) | 1.74 (0.28) | p < 0.01a | 1.99 (0.17) | 1.98 (0.15) | p=0.15 | 2.63 (0.11) | 1.81 (0.15) | p < 0.01a |
| median | 1.89 | 1.76 | 2.01 | 1.95 | 2.64 | 1.79 | |||
| DLP (mGy.cm) | |||||||||
| min | 840.2 | 574.52 | 840.2 | 631.48 | 1172 | 656.61 | |||
| max | 1353 | 1017.66 | 1353 | 1017.7 | 1353 | 979.63 | |||
| mean(SD) | 993.28 (146.18) | 828.14 (130.06) | p < 0.01a | 949.42 (80.87) | 942.13 (73.05) | p=0.64 | 1252.2 (52.11) | 859.66 (72.04) | p < 0.01a |
| median | 900.55 | 839.43 | 959.1 | 928.56 | 1255 | 850.24 | |||
| third quartile | 1019.75 | 928.52 | 960 | 1017.57 | 1291.75 | 901.41 | |||
| CTDIvol(mGy) | |||||||||
| min | 54.6 | 29.27 | 56.2 | 35.08 | 54.6 | 32.91 | |||
| max | 71.9 | 59.96 | 71.9 | 59.96 | 54.6 | 41.12 | |||
| mean (SD) | 57.61 (2.89) | 43.66 (11.15) | p < 0.01a | 58.63 (3.33) | 57.25 (3.4) | p < 0.05a | 54.6 (0.0) | 37.18 (2.52) | p < 0.01a |
| median | 57.4 | 37.8 | 58 | 58.03 | 54.6 | 37.78 | |||
| third quartile | 58.6 | 56.54 | 58.93 | 58.03 | 54.6 | 39.29 | |||
Statistically significant.
2.4. Calculation of the effective dose
There are two conventional approaches to calculating the ED for a CT examination. The first option involves organ-based Monte Carlo simulations using software modeling to determine the equivalent doses of organs in the scanned region, which are then multiplied by their respective tissue weighting factors specified by the ICRP [17,34].
For a quick and rough calculation of ED for CT examination, we can use a computationally simpler method based on the dose length product (DLP) and the DLP-to-E conversion factor k, which is specific for the given anatomical area [34]. There was a linear relationship for data sets focused on the same anatomic region over a wide range of scanners between ED and ED estimated by DLP derived from organ dose calculations from the National Radiological Protection Board (NRPB) and ICRP 60 tissue weighting coefficients [17]. Based on that, the European Commission guidelines presented this fast and universal method of assessing ED [35], with updated coefficients published in 2004 and 2005 [10,36]. This method is widely used, and ED is estimated as the product of DLP and k, specific only to the scanned anatomic region. These conversion factors were later adopted by the American Association of Physicists in Medicine [29].
These conversion factors are precalculated using computational human phantoms combined with Monte Carlo radiation transport simulations of CT X-rays [37]. They are based on tissue weighting factors published in ICRP publication 60, which form the internationally accepted basis for ED [29]. This method can underestimate or overestimate real ED, but is convenient and easy to apply in practice [6,10,16]. DLP-based calculation methods are also considered to reflect more on the practical working conditions of the radiology department compared to more computationally complex Monte Carlo methods [3]. The ED values calculated by this method compared to the ED values calculated by more rigorous calculation methods are relatively consistent, with a mean deviation of approximately ±15 % [3,6,17,23]. Despite the sources of variation in the calculation of ED, it is widely used by the academic, clinical, and manufacturing communities (17).
ED in our study was estimated using DLP and the conversion factor k for a head (k = 0.0021) [29].
2.5. Statistical analysis
Data collection and statistical analysis were performed using Microsoft Office Excel. Descriptive statistics, including mean, standard deviation, minimum, maximum, median, and third quartile, were used to evaluate the collected data. The variance difference between two sets within the group was tested using the F-test. Given the comparison of two independent sets, the unpaired two-sample t-test was utilized to determine the statistical significance of the compared parameters. Differences were considered significant at p < 0.05 and 0.01. The association between scan length, CTDIvol, DLP, and ED was tested with a series of Pearson correlations.
3. Results
To compare the ED between the two CTs, we created a set of 200 patients (Group 1), regardless of the type of scan technique (sequential or spiral). 100 patients for CT Philips, of whom there were 53 men and 47 women; the average age of the group was 61.5 ± 20.51 years (from 21 to 92 years). For CT GE, it was a set of 100 patients, 50 men and 50 women, with a mean age of 60.87 ± 19.92 years (from 19 to 100 years) (Graph 1). The mean values and statistical significance of CTDIvol, DLP, and ED are summarized in Table 1.
Graph 1.
Population of group 1.
A set of 100 patients (Group 2) was created for the sequential scan technique. For Philips CT, there were 50 patients, 26 men and 24 women, with an average age of 63.18 ± 18.94 years (from 21 to 90 years). For CT-GE, there were 50 patients, 24 men and 26 women, with an average age of 58.34 ± 20.58 years (from 19 to 100 years) (Graph 2). The mean values and statistical significance of CTDIvol, DLP, and ED are summarized in Table 1.
Graph 2.
Population of group 2.
Another set of 40 patients (Group 3) was created for the spiral scan technique. For Philips CT, there were 20 patients, 14 men and 6 women, with an average age of 54.8 ± 22.91 years (from 25 to 92 years). For CT GE, there were 20 patients, 14 men and 6 women, with an average age of 62.7 ± 18.95 years (from 22 to 95 years) (Graph 3). The mean values and statistical significance of CTDIvol, DLP, and ED are summarized in Table 1.
Graph 3.
Population of group 3.
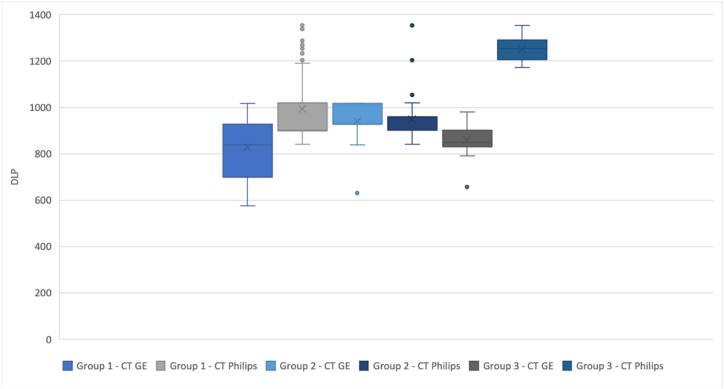
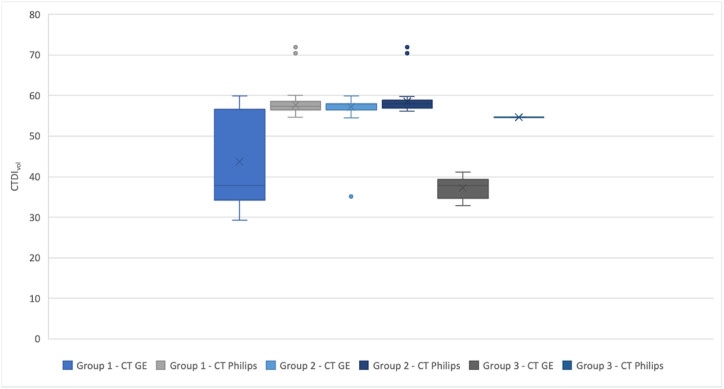
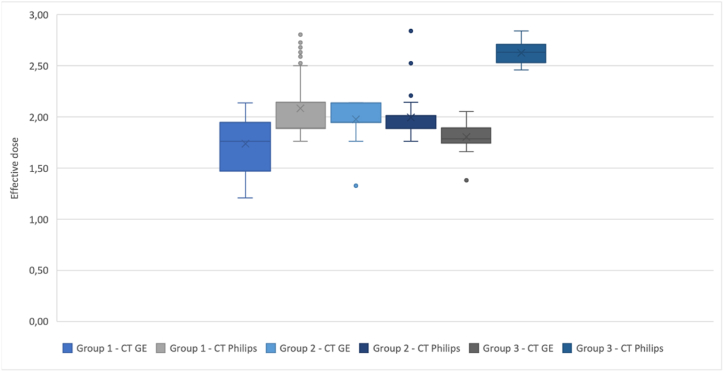
A statistically significant difference was observed between the ED, DLP, and CTDIvol averages within the two CTs in the Group 1 and Group 3 datasets of the non-contrast enhanced CT of the brain. For visual interpretation of the results, we used separate box plots for the DLP values (Graph 4), CTDIvol (Graph 5), and the ED values (Graph 6). Each box plot includes the three groups of datasets, depending on the given parameter.
Graph 4.
box plot of the DLP parameter for each group of patients.
Graph 5.
box plot of the CTDIvol parameter for each group of patients.
Graph 6.
box plot of the ED value for each group of patients.
The association between ED and various variables was calculated using a series of correlations. Differences were considered significant at p < 0.05. The scan length was additionally computed as a ratio of DLP and CTDIvol. For the sequential scan technique (Table 2) for CT GE, we found a strong positive correlation between scan length, DLP, and ED and a weak positive correlation between CTDIvol and ED. For the sequential scan technique for CT Philips, we found a strong positive correlation between scan length, CTDIvol, DLP, and ED.
Table 2.
Correlations between parameters: sequential scan technique.
|
CT GE - Sequential scan technique | |||
|---|---|---|---|
| Scan length | CTDIvol | DLP | |
| Scan length | |||
| CTDIvol | −0,483 (p < 0.05) | ||
| DLP | 0,677 (p < 0.05) | 0,318 (p < 0.05) | |
| ED | 0,677 (p < 0.05) | 0,318 (p < 0.05) | 1 (p < 0.05) |
| CT Philips - Sequential scan technique | |||
| Scan length |
CTDIvol |
DLP |
|
| Scan length | |||
| CTDIvol | 0,103 (p = 0.47) | ||
| DLP | 0,728 (p < 0.05) | 0,753 (p < 0.05) | |
| ED | 0,728 (p < 0.05) | 0,753 (p < 0.05) | 1 (p < 0.05) |
For the helical scan technique (Table 3) for CT GE, we found a strong positive correlation between scan length and DLP and ED and a very weak negative or no correlation between CTDIvol and ED. For the helical scan technique for CT Philips, we found a very strong positive correlation between scan length and DLP and ED and no correlation between scan length and CTDIvol and ED.
Table 3.
Correlations between parameters: helical scan technique.
| CT GE - Helical scan technique | |||
|---|---|---|---|
| Scan length | CTDIvol | DLP | |
| Scan length | |||
| CTDIvol | −0,647 (p < 0.05) | ||
| DLP | 0,797 (p < 0.05) | −0,059 (p = 0.81) | |
| ED | 0,797 (p < 0.05) | −0,059 (p = 0.81) | 1 (p < 0.05) |
| CT Philips - Helical scan technique | |||
| Scan length |
CTDIvol |
DLP |
|
| Scan length | |||
| CTDIvol | −3,247E-15 (p > 0.99) | ||
| DLP | 1 (p < 0.05) | 8,9541E-16 (p > 0.99) | |
| ED | 1 (p < 0.05) | −2,082E-15 (p > 0.99) | 1 (p < 0.05) |
4. Discussion
Determining the effective dose comes with many uncertainties because it involves many calculations and approximations. Depending on the chosen set of tissue weighting coefficients, the effective dose values calculated can show significant variability. Since the effective dose involves assessments of relative biological risk, which have evolved over time and lack a direct physical measurement or validation, there is no definitive "correct" standard for effective dose determination. Therefore, it is important to understand that an effective dose is just a general estimate of risk, not an exact measurement from an examination.
Our study compares radiation doses, expressed using effective doses, from non-contrast-enhanced brain examinations on two CT scanners in a single center. As expected, the newer CT scanner, CT GE, was associated with a lower radiation burden on the patient for the observed examination. When comparing radiation doses for these examinations, regardless of scan techniques (Group 1), a dose reduction of approximately 17 % can be observed for CT GE over CT Philips. Additionally, there are statistically significant differences between the CTDIvol, DLP, and ED averages.
As mentioned above, from the point of view of optimization, DRLs are used as reference values. For the anatomic area of the head, the diagnostic reference level in the Slovak Republic is estimated by CTDIvol = 60 mGy and DLP = 1000 mGy cm [38]. For sequential CT (Group 2) of non-contrast enhanced brain CT, we can conclude that there are no statistically significant differences between the averages of ED and DLP. There is statistical significance between the CTDIvol averages in this group, but the values (58.63 mGy and 57.26 mGy) do not exceed the national DRLs. For the helical scanning technique (Group 3), there are statistically significant differences between the averages of CTDIvol, DLP, and ED of two CT scanners. We can observe an approximately 30 % lower average ED for CT GE compared to the average ED for CT Philips. The scan parameters were the 120 kV tube voltage for both scanners; for CT Philips, there was a fixed tube current (Table 1), while CT GE used automatic tube current modulation. According to Refs. [3,39,40] automatic tube current modulation can lead to a dose reduction of 20–40 % while maintaining image quality. Although the quality of the images was not examined in our work, we can assume that automatic tube current modulation contributes to the reduction of a radiation dose.
Our results also suggest that the length of the scan (Table 2, Table 3) had a significant influence on the effective dose (ED), consistent with the findings published by Kumar et al. [41]. The scan length can be expressed as a product of the thickness of the slice and the number of slices. Thinner slices require a higher dose to maintain image quality, as additional X-ray photons are needed [42]. In our study, we observed differences in slice thickness between CT scanners, particularly with the Philips CT protocol for helical brain scans, which used thinner slices. Therefore, we can conclude that the thickness of the slice may have influenced the higher radiation dose for this type of examination. A similar study was conducted in 2014 by Ozdoba et al. [43], comparing a 128-slice CT scanner with older 16- and 64-slice CT scanners in terms of dose reduction. The results are very similar to those of our study, showing that the newer scanner achieved remarkable dose reductions in standard clinical spiral brain CT, specifically in terms of CTDIvol and DLP.
When CTDIvol and DLP were compared with national DRLs, the mean DLP value was exceeded for the helical scan on CT Philips. DRLs are established as the 75th percentile value of the dose distribution in a national or regional survey on the radiation doses for a given imaging procedure [42]. Although they are used as a reference to compare practices, DRLs do not serve as dose limits for specific procedures and cannot be used to judge the quality of practices. The use of DRLs for optimization frequently reduces radiation doses in numerous facilities, causing a gradual decrease in DRLs. This optimization should be an ongoing practice, performed periodically rather than once [42,44]. However, our national DRLs are currently based only on anatomical areas. Their disadvantage is that they do not take into account the clinical indication, which can affect the length of the scan and, therefore, the DLP parameter. Our study did not address the clinical indication of the examination, which may have an impact on the chosen scan technique. Furthermore, the visual quality of the images was not evaluated.
5. Conclusions
Computed tomography produces high-quality diagnostic images at the expense of an increased dose of radiation compared to other radiological examinations. Estimating the effective dose provides us with useful information and enables a direct comparison of doses with other types of radiological examination, with reference values, and with natural background values. The dose of CT can be reduced by changing certain scanning parameters. For this reason, it is important to monitor and optimize the CT scan protocol to reduce the potential harmful effects caused by radiation. Access to newer scanners and replacement of old CT equipment are also important in reducing radiation doses.
Ethics statement
This study was reviewed and approved by Ethics committee of University Hospital Martin with the approval number: 158/2022, dated February 2, 2023.
All patients provided written informed consent for the publication of their anonymized case details and images.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data are available via https://doi.org/10.17632/mzp2943cyw.1.
CRediT authorship contribution statement
Veronika Žatkuliaková: Writing – original draft, Formal analysis, Data curation. Martin Števík: Methodology, Formal analysis, Data curation. Martin Vorčák: Investigation. Ján Sýkora: Investigation. Zuzana Trabalková: Investigation. Gabriel Broocks: Writing – review & editing, Supervision. Lukas Meyer: Writing – review & editing, Supervision. Jens Fiehler: Writing – review & editing, Supervision. Kamil Zeleňák: Writing – review & editing, Supervision, Methodology, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.K-H Do General principles of radiation protection in fields of diagnostic medical exposure. J Korean Med Sci. Feb 2016;31(Suppl 1):S6–S9. doi: 10.3346/jkms.2016.31.S1.S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amis E.S., Jr., Butler P.F., Applegate K.E., et al. American College of Radiology white paper on radiation dose in medicine. J. Am. Coll. Radiol. 2007;4:272–284. doi: 10.1016/j.jacr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Curran T., Maher M., McLaughlin P., et al. Analysis of effective dose at computed tomography in a modern 64 slice multidetector CT System in an Irish tertiary care centre with local and international reference standards. 2020. https://www.medrxiv.org/content/10.1101/2020.04.08.20057059v1.full#ref-5 medRxiv [Preprint] Available via. [Google Scholar]
- 4.ICRP The 2007 recommendations of the international commission on radiological protection. ICRP Publication 103. Ann. ICRP. 2007;37(2–4) doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 5.ICRP Radiological protection and safety in medicine. ICRP publication 73. Ann. ICRP. 1996;26(2) [PubMed] [Google Scholar]
- 6.Súkupová L. vydání Grada Publishing a.s; Praha: 2018. Radiační Ochrana Při Rentgenových Výkonech – to Nejdůležitejší Pro Praxi 1. [Google Scholar]
- 7.Atli E., Uyanik S.A., Oguslu U., et al. Radiation doses from head, neck, chest and abdominal CT examinations: an institutional dose report. Diagn Interv Radiol. 2021;27(1):147–151. doi: 10.5152/dir.2020.19560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ICRP Recommendations of the ICRP. ICRP Publication 26. Ann. ICRP. 1977;1(3) [Google Scholar]
- 9.COUNCIL DIRECTIVE 2013/59/EURATOM of 5 December 2013 Laying Down Basic Safety Standards for Protection against the Dangers Arising from Exposure to Ionising Radiation, and Repealing Directives 89/618/Euratom, 90/641/Euratom, 96/29/Euratom, 97/43/Euratom and 2003/122/Euratom.
- 10.Bongartz G., Golding S.J., Jurik A.G., Leonardi M., van Persijn van Meerten E., Rodríguez R., Schneider K., Calzado A., Geleijns J., Jessen K.A., Panzer W., Shrimpton P.C., Tosi G. European Guidelines for Multislice Computed Tomography. March 2004. Funded by the European Commission. Contract number FIGM-CT2000-20078-CT-TIP. [Google Scholar]
- 11.Frush D.P. Review of radiation issues for computed tomography. Semin. Ultrasound CT MR. 2004;25(1):17–24. doi: 10.1053/j.sult.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Newman B., Ganguly A., Kim J.E., Robinson T. Comparison of different methods of calculating CT radiation effective dose in children. AJR Am. J. Roentgenol. 2012;199(2):232–239. doi: 10.2214/AJR.10.5895. [DOI] [PubMed] [Google Scholar]
- 13.Zarb F., McEntee M., Rainford L. Maltese CT doses for commonly performed examinations demonstrate alignment with published DRLs across Europe. Radiat Prot Dosimetry. 2012;150(2):198–206. doi: 10.1093/rpd/ncr393. [DOI] [PubMed] [Google Scholar]
- 14.Khoramian D., Sistani S., Firouzjah R.A. Assessment and comparison of radiation dose and image quality in multi-detector CT scanners in non-contrast head and neck examinations. Pol. J. Radiol. 2019;84:61–67. doi: 10.5114/pjr.2019.82743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pantos I., Thalassinou S., Argentos S., et al. Adult patient radiation doses from non-cardiac CT examinations: a review of published results. Br. J. Radiol. 2011;84(1000):293–303. doi: 10.1259/bjr/69070614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elmahdi A., Abuzaid M.M., Babikir E., Sulieman A. Radiation dose associated with multi-detector 64-slice computed tomography brain examinations in khartoum state, Sudan. Pol. J. Radiol. 2017;82:603–606. doi: 10.12659/PJR.902502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christner J.A., Kofler J.M., McCollough C.H. Estimating effective dose for CT using dose-length product compared with using organ doses: consequences of adopting International Commission on Radiological Protection publication 103 or dual-energy scanning. AJR Am. J. Roentgenol. 2010;194(4):881–889. doi: 10.2214/AJR.09.3462. [DOI] [PubMed] [Google Scholar]
- 18.Foley S.J., McEntee M.F., Rainford L.A. Establishment of CT diagnostic reference levels in Ireland. Br. J. Radiol. 2012;85(1018):1390–1397. doi: 10.1259/bjr/15839549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paulo G., Damilakis J., Tsapaki V., et al. European Society of Radiology. Diagnostic Reference Levels based on clinical indications in computed tomography: a literature review. Insights Imaging. 2020;11(1):96. doi: 10.1186/s13244-020-00899-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoyanov D., Vassileva J. Influence of exposure parameters on patient dose and image noise in computed tomography. Pol. J. Med. Phys. Eng. 2010;15(4):215–226. doi: 10.2478/v10013-009-0021-9. [DOI] [Google Scholar]
- 21.Wolbarst A.B., Capasso P., Godfrey D.J., et al. ume 4. Medical Physics Publishing; 2012. (Advances in Medical Physics: 2012). [Google Scholar]
- 22.Tack D., Kalra M.K., Gevenois P.A. second ed. Springer; 2012. Radiation Dose from Multidetector CT. [Google Scholar]
- 23.Raman S.P., Mahesh M., Blasko R.V., Fishman E.K. CT scan parameters and radiation dose: practical Advice for radiologists. J. Am. Coll. Radiol. 2013;10(11):840–846. doi: 10.1016/j.jacr.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 24.Mayo-Smith W.W., Hara A.K., Mahesh M., et al. How I do it: managing radiation dose in CT. Radiology. 2014;273(3):657–672. doi: 10.1148/radiol.14132328. [DOI] [PubMed] [Google Scholar]
- 25.Martin C.J. Effective dose: how should it be applied to medical exposures? Br. J. Radiol. 2007;80(956):639–647. doi: 10.1259/bjr/25922439. [DOI] [PubMed] [Google Scholar]
- 26.Huda W., Ogden K.M., Khorasani M.R. Converting dose-length product to effective dose at CT. Radiology. 2008;248(3):995–1003. doi: 10.1148/radiol.2483071964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCollough C.H., Schueler B.A. Calculation of effective dose. Med. Phys. 2000;27(5):828–837. doi: 10.1118/1.598948. [DOI] [PubMed] [Google Scholar]
- 28.Lee S.K., Kim J.S., Yoon S.W., Kim J.M. Development of CT effective dose conversion factors from clinical CT examinations in the Republic of Korea. Diagnostics. 2020;10(9):727. doi: 10.3390/diagnostics10090727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.American Association of Physicist in Medicine Report of AAPM task group 23 of the diagnostic imaging council CT comittee: the measurement, reporting, and management of radiation dose in CT. 2008. Availabe via https://www.aapm.org/pubs/reports/RPT_96.pdf.
- 30.Romanyukha A., Folio L., Lamart S., et al. Body size-specific effective dose conversion coefficients for CT scans. Radiat Prot Dosimetry. 2016;172(4):428–437. doi: 10.1093/rpd/ncv511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith-Bindman R., Lipson J., Marcus R., et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch. Intern. Med. 2009;169(22):2078–2086. doi: 10.1001/archinternmed.2009.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stiller W. Basics of iterative reconstruction methods in computed tomography: a vendor-independent overview. Eur. J. Radiol. 2018;109:147–154. doi: 10.1016/j.ejrad.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 33.Padole A., Ali Khawaja R.D., Kalra M.K., Singh S. CT radiation dose and iterative reconstruction techniques. AJR Am. J. Roentgenol. 2015;204:384–392. doi: 10.2214/AJR.14.13241. [DOI] [PubMed] [Google Scholar]
- 34.McCollough C.H., Christner J.A., Kofler J.M. How effective is effective dose as a predictor of radiation risk? AJR Am. J. Roentgenol. 2010;194(4):890–896. doi: 10.2214/AJR.09.4179. [DOI] [PubMed] [Google Scholar]
- 35.Jessen K.A., Panzer W., Shrimpton P.C., et al. European Commission; Brussels, Belgium: 2000. European Guidelines on Quality Criteria for Computed Tomography. EUR. [Google Scholar]
- 36.Shrimpton P.C., Hillier M.C., Lewis M.A., Dunn M. National Radiological Protection Board; Chilton, UK: 2005. Doses from Computed Tomography (CT) Examinations in the UK: 2003 Review. report NRPB-W67. [Google Scholar]
- 37.Lee C. How to estimate effective dose for CT patients. Eur. Radiol. 2020;30:1825–1827. doi: 10.1007/s00330-019-06625-7. [DOI] [PubMed] [Google Scholar]
- 38.Measure of the Ministry of Health of the Slovak Republic no. S02933-2018-OL . March 19, 2018. Establishing Diagnostic Reference Levels for Medical Radiation.https://www.health.gov.sk/?vestniky-mz-sr dated. [Google Scholar]
- 39.Goo H.W. CT radiation dose optimization and estimation: an update for radiologists. Korean J. Radiol. 2012;13(1):1–11. doi: 10.3348/kjr.2012.13.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCollough C.H., Primak A.N., Braun N., et al. Strategies for reducing radiation dose in CT. Radiol Clin North Am. 2009;47(1):27–40. doi: 10.1016/j.rcl.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar V., Tayal S., Ali A., Gandhi A. Assessment of effective dose received in various computed tomography protocols and factors affecting it. Indian J. Nucl. Med. 2021;36(1):32–38. doi: 10.4103/ijnm.IJNM_112_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inoue Y. Radiation dose modulation of computed tomography component in positron emission tomography/computed tomography. Semin. Nucl. Med. 2022;52(2):157–166. doi: 10.1053/j.semnuclmed.2021.11.009. [DOI] [PubMed] [Google Scholar]
- 43.Ozdoba C., Slotboom J., Schroth G., et al. Dose reduction in standard head CT: first results from a new scanner using iterative reconstruction and a new detector type in comparison with two previous generations of multi-slice CT. Clin. Neuroradiol. 2014;24(1):23–28. doi: 10.1007/s00062-013-0263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abuzaid M.M., Elshami W., El Serafi A., et al. Toward national CT diagnostic reference levels in the United Arab Emirates: a multicenter review of CT dose index and dose length product. Radiat Prot Dosimetry. 2020;190(3):243–249. doi: 10.1093/rpd/ncaa100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available via https://doi.org/10.17632/mzp2943cyw.1.