Abstract
Introduction and importance
Hibernomas are benign soft tissue tumors containing prominent brown adipocytes that resemble normal brown fat, they occur in a wide age range (from 2 to 75 years) and make up for about 1 % of all adipocytic tumors <2 % of benign fatty neoplasms.
They have traditionally been regarded as benign tumors with no potential for malignancy; due to their similarity in clinical and radiographical presentation, they are often misdiagnosed as malignant tumors like liposarcomas.
While these tumors are generally considered non-malignant, their distinctive characteristics and uncommon occurrence make them an intriguing subject for medical study.
Case presentation
This article describes the clinical, radiographic, and histologic features of a young patient with a thigh hibernoma compressing the obturator nerve.
Clinical discussion
Hibernomas are more likely to develop in brown fat-enriched areas in newborns, such as the neck, scapular area, armpit, chest, and retroperitoneum.
Increased awareness among clinicians and pathologists, coupled with advances in imaging and immunohistochemistry, has enhanced our ability to accurately identify and treat these intriguing tumors.
Conclusion
The rarity of hibernomas together with the close resemblance of their radiological features to those of malignant tumors like liposarcomas or atypical lipomatous tumors, make these lesions extremely difficult to identify and often misdiagnosed.
Continued research is essential to further our understanding this neoplasm and refine diagnostic and therapeutic approaches.
Keywords: Hibernoma, Nerve compression, Adipose tissue
Highlights
-
•
A case report of radical excision of an hibernoma of the thigh causing obturator nerve compression.
-
•
The preoperative study of this uncommon lesion should include an MRI with contrast in order to assess the relationships with the vessels and nerves.
-
•
Surgical treatment of hibernomas must be radical and hemostasis must be carefully monitored as they are highly vascularized.
1. Introduction
Hibernomas are rare benign tumors of soft tissue that develop from residual brown fat cells. The brown adipose tissue (BAT) is primarily responsible for non-shivering thermogenesis in animals.
They are rare tumor, <1 % of all adipocytic tumors, and typically observed in adults aged 30–40 years, with equal gender distribution [1]. They occur sporadically without a clear genetic predisposition.
Hibernomas typically present as slow-growing, painless masses in the subcutaneous tissues or intermuscular spaces. They show a predilection for areas where brown fat is more represented, such as the underarms, shoulders, mediastinum, and retroperitoneum, and they are uncommon in the cranium and popliteal fossa [2]. Other uncommon locations include the lower and upper extremities and the abdomen.
Accurate diagnosis often requires a combination of clinical, radiological, and histopathological assessments.
Sometimes, they present a rapid growth, associated with axillary or inguinal pain indicating associated lymphadenopathy or the patient have an unexpected weight loss [3].
They can reach a considerable size, but are usually well circumscribed and encapsulated.
Despite their benign nature, hibernomas can cause symptoms due to compression of adjacent structures, tissues, blood vessels or nerves.
Radiological imaging, like computed tomography (CT) and magnetic resonance (MRI), play an important role in characterizing hibernomas [4]. These tumors typically have homogenous enhancement on contrast studies and can display varying degrees of fat content, contributing to recognize them. Positron emission tomography (PET) scans may show higher metabolic activity [5], to differentiate them from other soft tissue tumors [6].
The tumor may also contain fat cells resembling lipoblasts, which makes it difficult to distinguish it from atypical lipomatous tumor/well differentiated liposarcoma (ALT/WDLS). Although nuclear expressions of murine double minute 2 (MDM2) and cyclin-dependent kinase 4 (CDK4) are widely used as immunohistochemical surrogate markers for ALT/WDLS, the utility of these proteins in distinguishing between hibernoma and ALT/WDLS still remains to be elucidated [7].
Hibernomas can pose diagnostic challenges due to their rarity and histological resemblance to other adipocytic tumors. The differential diagnosis includes lipomas, liposarcomas, hemangiomas, fibromatosis, myxoid neoplasms, other primary malignancies, metastatic disease.
Complete surgical excision is the treatment of choice for these tumors.
Conservative management may be considered in asymptomatic cases or when surgery leads to significant risks.
2. Case report
A 23-year-old man was referred to our Plastic and Reconstructive Department for evaluation of a large and soft mass located in his left medial thigh (Fig. 1, Fig. 2).
Fig. 1.
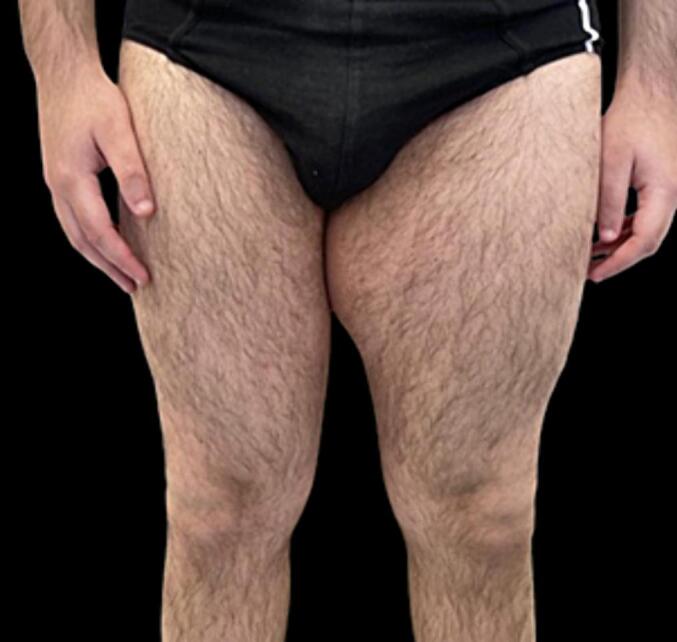
Frontal preoperative view.
Fig. 2.
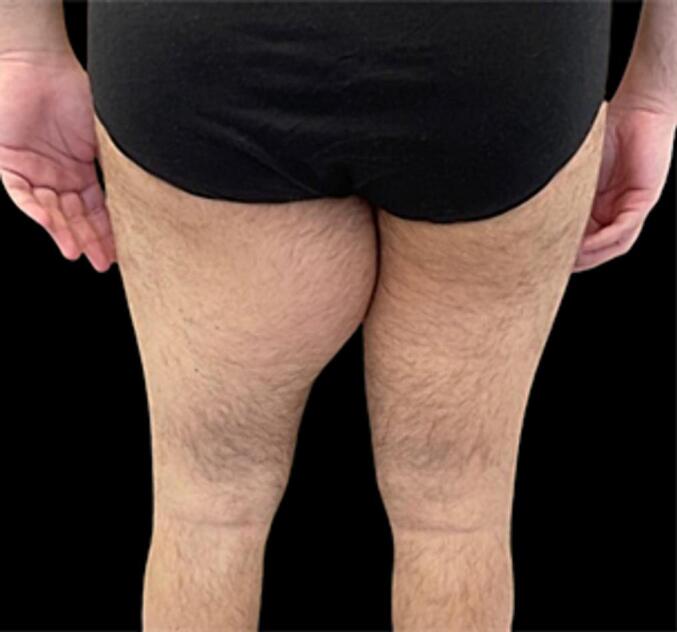
Posterior preoperative view.
He first noticed this large mass 3 years before, when he sought medical help because it caused him difficulties in wearing pants. Some medical exams were ordered then, but the patient ignored the prescription.
Later, he turned to another hospital, where he underwent instrumental examinations (ultrasound and MRI) and FNA, because the swelling was getting bigger and annoying, causing an Obturator Nerve compression syndrome with numbness, tingling, burning, or pain in the groin or inner thigh. Decreased sensation in the thigh, sometimes extending to the calf.
An ultrasound showed a nodular, inhomogeneously hyperechoic formation of about 19 × 8 cm in the subfascial area, with regular margins, apparently compressing the surrounding muscle tissues without infiltrating them. Furthermore, a mild intralesional vascularization was found with the Doppler ultrasound.
MRI scan performed shortly after confirmed the presence, in the proximal third of the adductor magnus muscle, of an oval formation of 21x13x11 cm, with regular margins, showing slightly inhomogeneous adipose signal with areas of initial colliquation, some vascular structures and internal septa of 3 mm. There was no evidence of injury to the myotendinous structures but confirmed the compression of the Obturator Nerve (Figs. Fig. 3, Fig. 4).
Fig. 3.
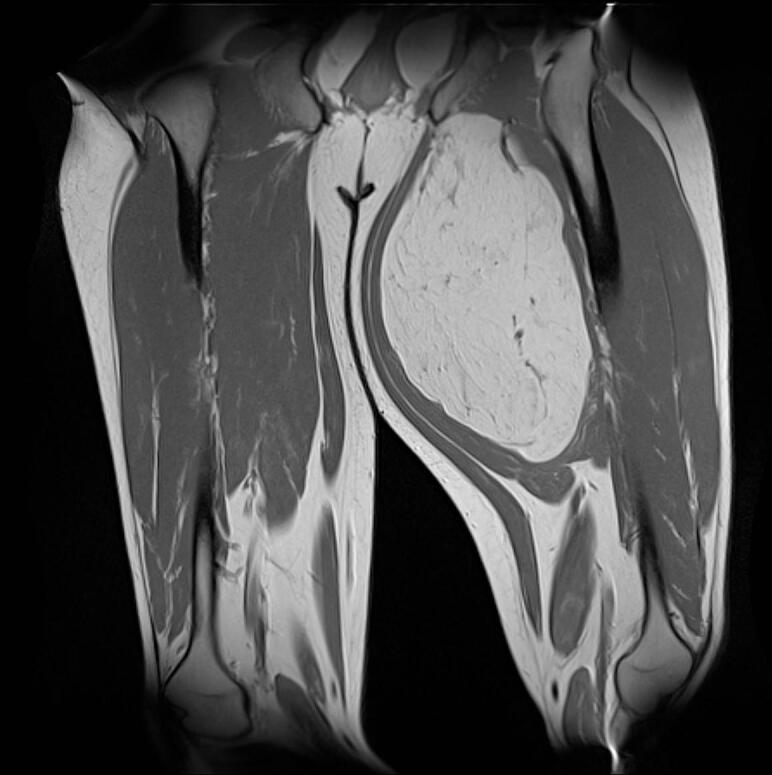
MRI coronal section of the lesion.
Fig. 4.
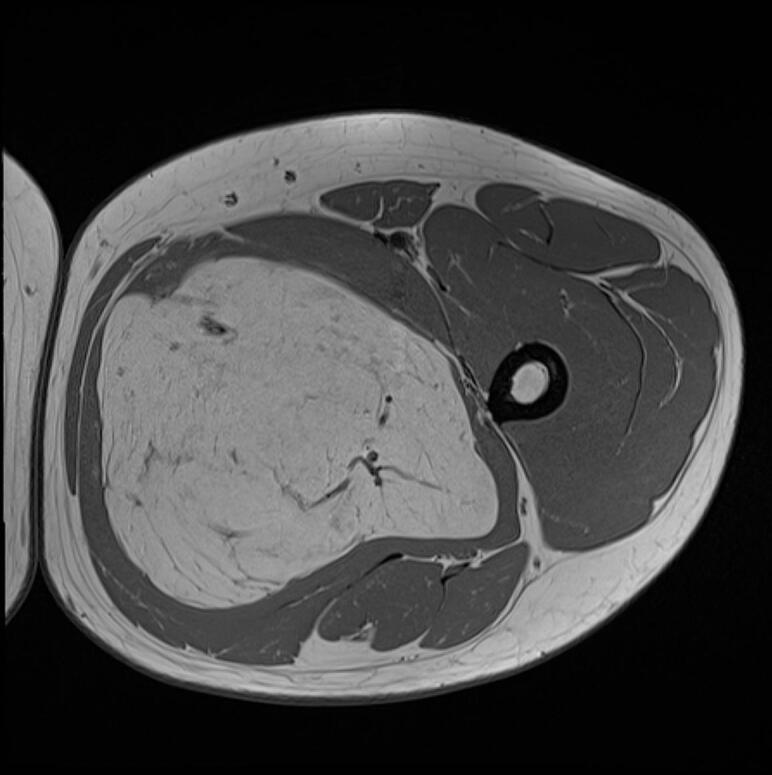
MRI transverse section of the lesion.
The pathology report on the FNA procedure revealed the presence of mature adipose tissue mixed with adipocytes with clear microvacuolated cytoplasm, devoid of stromal atypia. Also, MDM2 immunohistochemistry as well as MDM2 fluorescence in-situ hybridization (FISH) were performed on this bioptic sample, showing no expression nor amplification, respectively, of MDM2. Therefore, a diagnosis of lipomatous tumor, consistent with hibernoma was rendered and complete excision of the whole mass was advised to confirm the diagnosis.
Subsequently, the patient went to our Unit for a radical surgical excision: a large skin incision was required, all feeding vessels were ligated and the adductor magnus muscle was successfully spared. The mass was then removed and hemostasis was checked. After checking that there was no involvement of the vessels and the femoral nerve, a suction drain was put. The incision was closed in multiple layers and compressive garments were applied.
The patient was discharged from the hospital on postoperative day 3. His postoperative course was uneventful.
Excisional biopsy on the surgical sample definitely confirmed the diagnosis of Hibernoma (lipoma-like subtype). Grossly, the tumor appeared as finely-encapsulated mass measuring 22 × 17 × 8 cm, with a homogeneous yellowish and soft cut surface, resembling mature adipose tissue (Fig. 10).
Fig. 10.
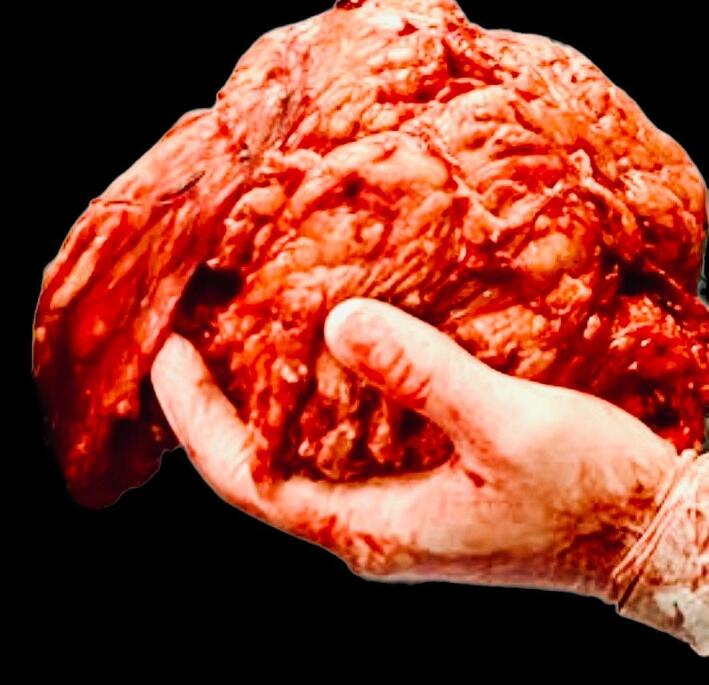
The specimen removed.
Microscopic examination showed a mixture of unilocular mature adipocytes, and cells with microvacuolated cytoplasm, central nucleus and fine chromatin, in absence of atypical stromal cells and in the presence of a fine network of capillaries. (Fig. 5).
Fig. 5.
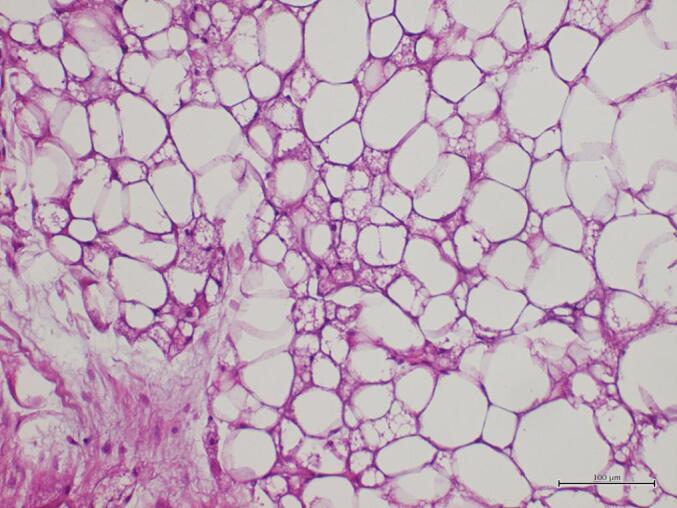
Histologic features on excisional biopsy: adipocytic tumor mainly composed of univacuolated adipocytes; a minor component of brown fat cells is noted (Hematoxylin and Eosin stain; original magnification: 200×). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Also the molecular investigation (by FISH technique) to search for alterations in the MDM2 gene confirmed the negative outcome of the previous incisional biopsy. (Fig. 6).
Fig. 6.
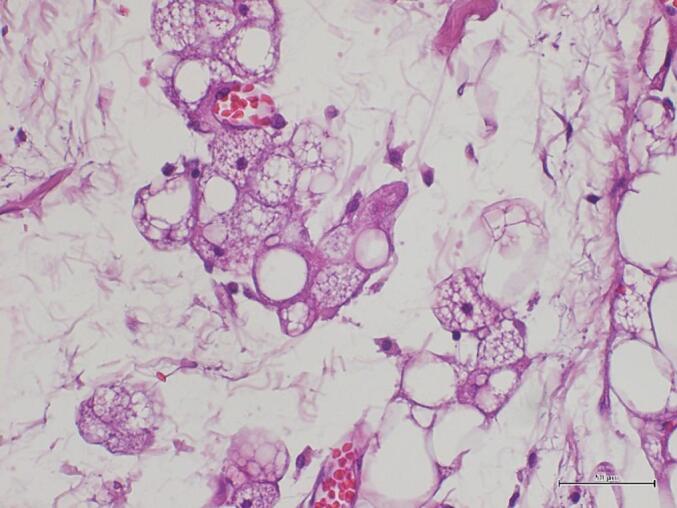
Brown fat cells show multivacuolated, granular, polygonal cytoplasms and small, round, centrally-located nuclei (Hematoxylin and Eosin stain; original magnification: 400×). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
After the operation, the patient no longer had symptoms related to compression of the obturator nerve and did not report any complication or discomfort with an significant improvement in his quality of life (Fig. 7, Fig. 8, Fig. 9). No postoperative radiation or chemotherapy was administered, according to our soft tissue cancer multidisciplinary team.
Fig. 7.
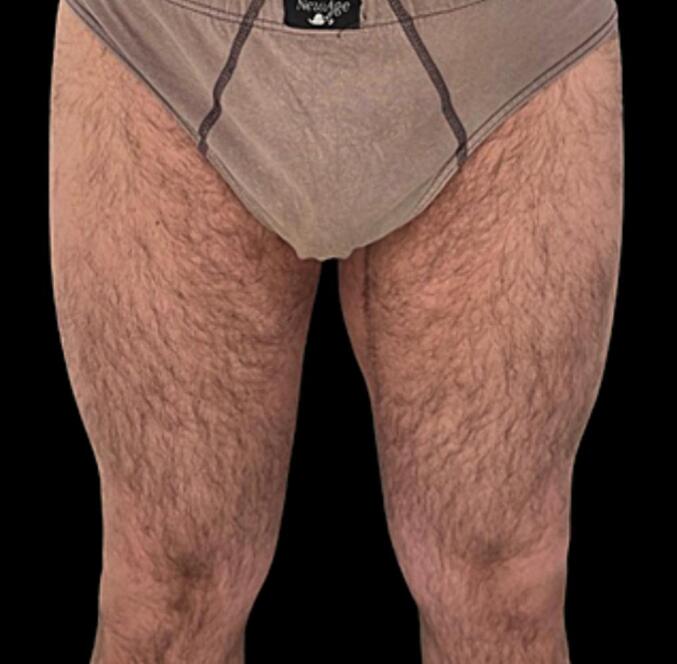
Frontal postoperative view.
Fig. 8.
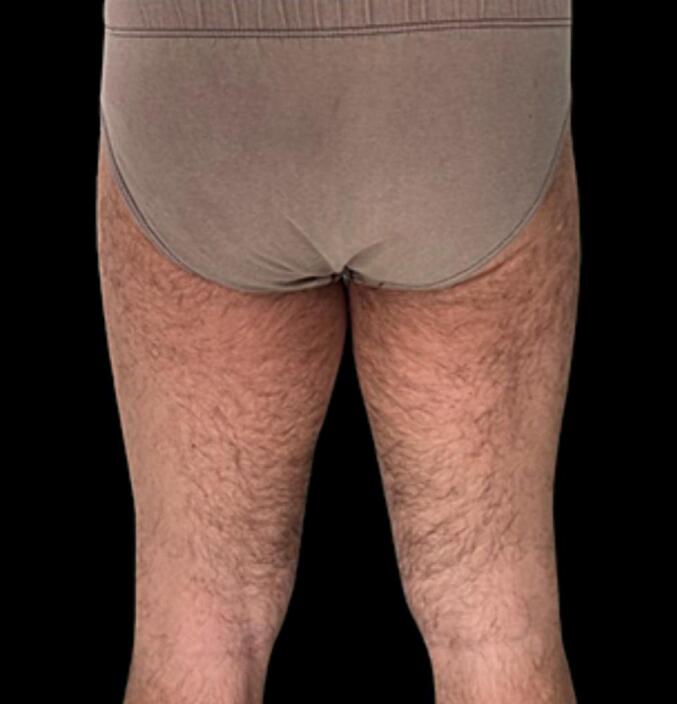
Posterior postoperative view.
Fig. 9.
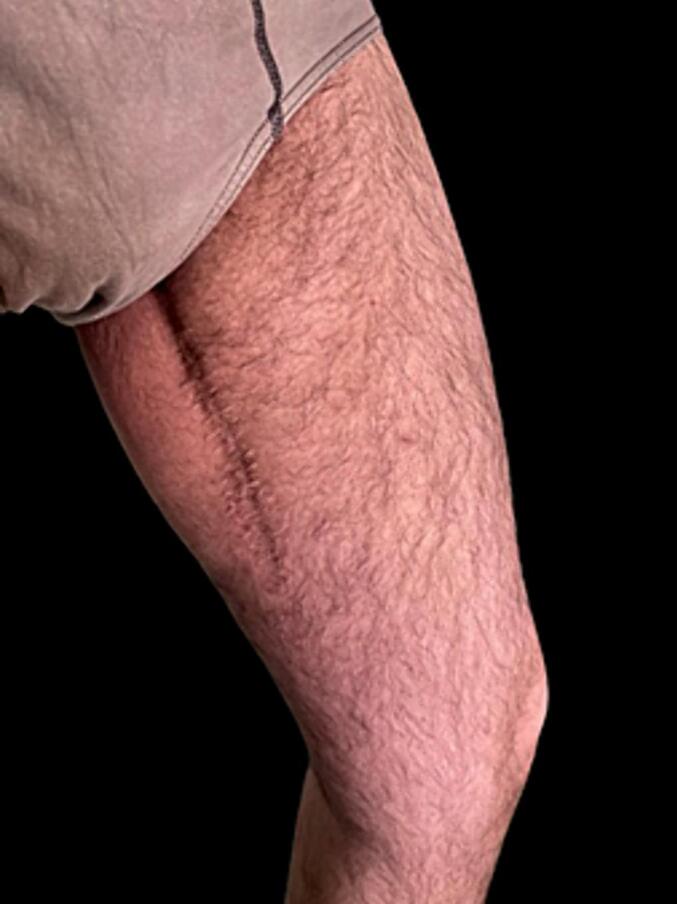
Final scar at 1 year follow-up.
3. Discussion
Hibernomas, though rare, present a unique challenge in terms of diagnosis and management.
This kind of tumor arises from brown adipose tissue, a special fat involved in thermogenesis and metabolic function, derived from adipose stem cells (ASCs).
These pluripotent cell have gained significant interest in recent times due to the promising therapeutic tools in regenerative medicine, because of the self-renewal, differentiation and immunomodulatory capacities [8].
They can be misdiagnosed as atypical lipomatous tumors or liposarcoma on clinical presentation, imaging [9] and even histologically [[10], [11], [12]].
According to literature, a brown fat tumor was first described by Merkel in 1906, and it was called “pseudolipoma”; it was not until 1914 that Gery coined the term hibernoma, due to the lesion's similarity to the tissue found in animals that undergo hibernation [13]. (Table 1).
Table 1.
Cases of hibernoma described in literature.
| Anatomical Site | Authors |
|---|---|
| Periadrenal Region | Gerasimenko PP [16] |
| Larinx | Sellari Franceschini S et al. [17], Cain RB et al. [18] |
| Scrotum | Sayrak H et al. [19] |
| Gluteal Region | Kolokythas O et al. [20] |
| Intradural Spinal Region | Chitoku S et al. [21] |
| Spermatic Chord | San Miguel P et al. [22] |
| Mediastinum | Udwadia ZF et al. [23], Baldi A et al. [24] |
| Breast | Riley MP et al. [25], Gardner-Thorpe D et al. [26], Dsouza R et al. [27] |
| Subpleural Region | Ugalde PA et al. [28] |
| Vulvar | Sheth A et al. [29], Williams EF et al. [30] |
| Heart | Di Tommaso L et al. [31] |
| Thigh | Salim B et al. [32], Della Volpe C et al. [33], Sayed W et al. [34], Huang C et al. [15] |
| Intraosseus Region | Myslicki FA et al. [35] |
| Dorsum | Niasse A et al. [36] |
This kind of tumor accounts for approximately 1.6 % of all benign lipomatous tumors and approximately 1.1 % of all tumors derived from fat tissue [14].
Hibernomas are more likely to develop in brown fat-enriched areas in newborns, such as the neck, scapular area, armpit, chest, and retroperitoneum; however, several studies have reported that the thigh is also often involved, accounting for approximately 30 % of all cases [15].
Increased awareness among clinicians and pathologists, coupled with advances in imaging and immunohistochemistry, has enhanced our ability to accurately identify and treat these intriguing tumors. Continued research is essential to further our understanding this neoplasm and refine diagnostic and therapeutic approaches, as occurs in skin metastasis of tumors of the digestive system [37] or for extranodal lymphomas [38].
4. Conclusion
The rarity of hibernomas together with the close resemblance of their radiological features to those of malignant tumors like liposarcomas or atypical lipomatous tumors, make these lesions extremely difficult to identify and often misdiagnosed.
Future perspectives in the study of this tumors are aimed at studying their gene expression and their ability to degenerate into liposarcomas.
Although hibernomas are rare tumors, whenever we are facing large masses compatible with lipoma diagnosis we believe it is useful to integrate routine ultrasound with a contrast-enhanced MRI to better study the tissue and vascular architecture of the mass.
Through magnetic resonance imaging we can first see the relationships that the tumor has with the surrounding neurovascular and muscular structures, furthermore allow us to perform a minimally invasive biopsy procedure.
As regards surgical therapy, when possible, radical excision always represents the gold standard.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
SCARE guidelines
The work has been reported in line with the SCARE criteria [39].
Ethical approval
Ethics approval is not required for case reports or case series because do not constitute research at our institution.
Funding
None.
Author contribution
Mario Faenza: Surgical procedure and writing.
Roberta Boffo: writing.
Erminia Crisci: literature review.
Gaya Franzese: photo taking.
Francesca Pagliuca: pathology report.
Chiara D'Addato: paper organisation.
Guarantor
Mario Faenza.
Research registration number
Not required.
Declaration of competing interest
No conflicts of interest.
References
- 1.Furlong M.A., Fanburg-Smith J.C., Miettinen M. The morphologic spectrum of hibernoma: a clinicopathologic study of 170 cases. Am. J. Surg. Pathol. 2001;25(6):809–814. doi: 10.1097/00000478-200106000-00014. Jun. (PMID: 11395560) [DOI] [PubMed] [Google Scholar]
- 2.Murphey M.D., Carroll J.F., Flemming D.J., Pope T.L., Gannon F.H., Kransdorf M.J. From the archives of the AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433–1466. doi: 10.1148/rg.245045120. Sep–Oct. (PMID: 15371618) [DOI] [PubMed] [Google Scholar]
- 3.Tafti D., Cecava N.D. StatPearls [Internet] StatPearls Publishing; Treasure Island (FL): 2023. Hibernoma. 2023 May 22. Jan–. PMID: 34033341. [Google Scholar]
- 4.Dursun M., Agayev A., Bakir B., Ozger H., Eralp L., Sirvanci M., Guven K., Tunaci M. CT and MR characteristics of hibernoma: six cases. Clin. Imaging. 2008;32(1):42–47. doi: 10.1016/j.clinimag.2007.07.001. Jan–Feb. (PMID: 18164394) [DOI] [PubMed] [Google Scholar]
- 5.Qi J, Kurian E, Öz OK. Omental hibernoma revealed by 18 F-FDG PET/CT. Clin. Nucl. Med. 2023 Sep 1;48(9):796–798. doi: 10.1097/RLU.0000000000004753. Epub 2023 Jun 23. PMID: 37351901. [DOI] [PubMed]
- 6.Pothen L., D’Abadie P., Kozyreff A., Mourin A., Coubeau L. Hibernoma mimicking liposarcoma. Lancet. 2018;392(10143):244. doi: 10.1016/S0140-6736(18)31436-3. Jul 21. (PMID: 30043750) [DOI] [PubMed] [Google Scholar]
- 7.Tsuda Y., Matsuyama A., Makihara K., et al. Nuclear expression of MDM2 in hibernoma: a potential diagnostic pitfall. Virchows Arch. 2021;478:527–534. doi: 10.1007/s00428-020-02914-5. [DOI] [PubMed] [Google Scholar]
- 8.Anastasiadou E., Ceccarelli S., Messina E., Gerini G., Megiorni F., Pontecorvi P., Camero S., Onesti M.G., Trivedi P., Faenza M., Coscioni E., Nicoletti G.F., Napoli C., Marchese C. MiR-200c-3p maintains stemness and proliferative potential in adipose-derived stem cells by counteracting senescence mechanisms. PLoS One. 2021;16(9) doi: 10.1371/journal.pone.0257070. e0257070. Sep 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patil S.D., Sheik A.R., Tewari V., Mutreja D. Hibernoma: a missed diagnosis!! Indian J. Pathol. Microbiol. 2019;62(3):461–463. doi: 10.4103/IJPM.IJPM_577_18. Jul–Sep. (PMID: 31361241) [DOI] [PubMed] [Google Scholar]
- 10.Goldblum J.R. In: Rosai and Ackerman’s Surgical Pathology. 11th ed. Goldblum J.R., Lamps L.W., McKenney J.K., Myers J.L., editors. Elsevier; Philadelphia: 2018. Soft tissues; pp. 1810–1915. [Google Scholar]
- 11.Daubner D., Spieth S., Pablik J., Zöphel K., Paulus T., Laniado M. Hibernoma – two patients with a rare lipoid soft-tissue tumour. BMC Med. Imaging. 2015;15:4. doi: 10.1186/s12880-015-0046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shackelford R.E., Al Shaarani M., Ansari J., Wei E., Cotelingam J. A twenty-four-year-old woman with left flank lipoma-like hibernoma. Case Rep Oncol. 2017;10:438–441. doi: 10.1159/000475708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vassos N., Lell M., Hohenberger W., et al. Deep-seated huge hibernoma of soft tissue: a rare differential diagnosis of atypical lipomatous tumor/well differentiated liposarcoma. Int. J. Clin. Exp. Pathol. 2013;6:2178–2184. [PMC free article] [PubMed] [Google Scholar]
- 14.Fletcher C.D., Unni K.K., Mertens F. Iarc; 2002. Pathology and Genetics of Tumours of Soft Tissue and Bone. [Google Scholar]
- 15.Huang C., Zhang L., Hu X., Liu Q., Qu W., Li R. Femoral nerve compression caused by a hibernoma in the right thigh: a case report and literature review. BMC Surg. 2021;21(1):30. doi: 10.1186/s12893-020-01040-y. Jan 7. PMID: 33413245; PMCID: PMC7792216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerasimenko P.P., Smol’iannikov A.A. Okolonadpochechnikovaia gibernoma [Periadrenal hibernoma] Klin Med (Mosk). 1965 Oct;43(10):137–141. 5872474 Russian. [PubMed] [Google Scholar]
- 17.Sellari Franceschini S., Segnini G., Berrettini S., Bruschini P., Cagno M.C., Testi C. Hibernoma of the larynx. Review of the literature and a new case. Acta Otorhinolaryngol. Belg. 1993;47(1):51–53. (PMID: 8470550) [PubMed] [Google Scholar]
- 18.Cain R.B., Zarka M.A., Hinni M.L. Laryngeal hibernoma: case series of a rare tumor. Head Neck. 2014;36(4):E39–E43. doi: 10.1002/hed.23460. Apr. Epub 2013 Nov 18. PMID: 23970475. [DOI] [PubMed] [Google Scholar]
- 19.Sayrak H., Gönül E., Sayrak F. Hibernoma in the scrotum. Br. J. Urol. 1997;80(4):679–680. doi: 10.1046/j.1464-410x.1997.00321.x. Oct. (PMID: 9352718) [DOI] [PubMed] [Google Scholar]
- 20.Kolokythas O., Deli M., Keck A.M., von Baer A., Aschoff A.J. Gluteales hibernom [gluteal hibernoma] Rontgenpraxis. 1997;50(10):313–315. Oct. German. PMID: 9432730. [PubMed] [Google Scholar]
- 21.Chitoku S., Kawai S., Watabe Y., Nishitani M., Fujimoto K., Otsuka H., Fushimi H., Kotoh K., Fuji T. Intradural spinal hibernoma: case report. Surg. Neurol. 1998;49(5):509–512. doi: 10.1016/s0090-3019(97)00304-2. May. discussion 512–3. (PMID: 9586928) [DOI] [PubMed] [Google Scholar]
- 22.San Miguel P., Clemente L.M., García González R., Fernández E. Hibernoma of the spermatic cord. The second reported case and literature review. Scand. J. Urol. Nephrol. 1998 Apr;32(2):153–155. doi: 10.1080/003655998750014594. 9606794 [DOI] [PubMed] [Google Scholar]
- 23.Udwadia Z.F., Kumar N., Bhaduri A.S. Mediastinal hibernoma. Eur. J. Cardiothorac. Surg. 1999;15(4):533–535. doi: 10.1016/s1010-7940(99)00051-2. Apr. (PMID: 10371136) [DOI] [PubMed] [Google Scholar]
- 24.Baldi A., Santini M., Mellone P., Esposito V., Groeger A.M., Caputi M., Baldi F. Mediastinal hibernoma: a case report. J. Clin. Pathol. 2004;57(9):993–994. doi: 10.1136/jcp.2004.017897. Sep. PMID: 15333666; PMCID: PMC1770406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riley M.P., Karamchandani D.M. Mammary Hibernoma: a rare entity. Arch. Pathol. Lab Med. 2015;139(12):1565–1567. doi: 10.5858/arpa.2014-0318-RS. Dec. (PMID: 26619029) [DOI] [PubMed] [Google Scholar]
- 26.Gardner-Thorpe D., Hirschowitz L., Maddox P.R. Mammary hibernoma. Eur. J. Surg. Oncol. 2000;26(4):430. doi: 10.1053/ejso.1999.0913. Jun. (PMID: 10873369) [DOI] [PubMed] [Google Scholar]
- 27.Dsouza R., Cherian A.J., Ananthakrishnan R., Menon N. Bilateral mammary hibernoma mimicking breast carcinoma: a diagnostic challenge. BMJ Case Rep. 2021;14(3) doi: 10.1136/bcr-2020-240552. e240552. Mar 22. PMID: 33753385; PMCID: PMC7986953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ugalde P.A., Guilbault F., Vaillancourt R., Couture C. Subpleural hibernoma. Ann. Thorac. Surg. 2007;84(4):1376–1378. doi: 10.1016/j.athoracsur.2007.05.044. Oct. (PMID: 17889004) [DOI] [PubMed] [Google Scholar]
- 29.Sheth A, Terzic M, Arsenovic N. Vulvar hibernoma. Indian J. Pathol. Microbiol. 2011 Oct-Dec;54(4):817-8. doi: 10.4103/0377-4929.91532. (PMID: 22234120). [DOI] [PubMed]
- 30.Williams E.E., Sullivan S.A., Sayeed S., Karjane N.W. Vulvar hibernoma: an unusual Lipomatous tumor in an adolescent. J. Pediatr. Adolesc. Gynecol. 2021;34(3):421–423. doi: 10.1016/j.jpag.2021.01.009. Jun. Epub 2021 Jan 20. PMID: 33484846. [DOI] [PubMed] [Google Scholar]
- 31.Di Tommaso L., Chiesa G., Arena V., Guanella G., Galli C., Roncalli M. Cardiac hibernoma: a case report. Histopathology. 2012;61(5):985–987. doi: 10.1111/j.1365-2559.2012.04264.x. Nov. Epub 2012 Jul 2. PMID: 22747461. [DOI] [PubMed] [Google Scholar]
- 32.Salim B., Belkacem C. Hibernoma of the thigh: a report of four cases. J. Orthop. Surg. (Hong Kong) 2014;22(1):118–121. doi: 10.1177/230949901402200129. Apr. (PMID: 24781629) [DOI] [PubMed] [Google Scholar]
- 33.Della Volpe C., Salazard B., Casanova D., Vacheret H., Bartoli J.F., Magalon G. Hibernoma of the antero-lateral thigh. Br. J. Plast. Surg. 2005;58(6):859–861. doi: 10.1016/j.bjps.2005.01.020. Sep. (PMID: 15950954) [DOI] [PubMed] [Google Scholar]
- 34.Sayed W., Mahjoub H., Dougaz H., Ben Salah M., Trabelsi M., M’Barek M. Hibernome de la cuisse. A propos d’un cas [Hibernoma of the thigh. A case report] Tunis. Med. 2015;93(4):269. 26375748 Apr. French. [PubMed] [Google Scholar]
- 35.Myslicki F.A., Rosenberg A.E., Chaitowitz I., Subhawong T.K. Intraosseous hibernoma: five cases and a review of the literature. J. Comput. Assist. Tomogr. 2019;43(5):793–798. doi: 10.1097/RCT.0000000000000912. 31453977 Sep/Oct. [DOI] [PubMed] [Google Scholar]
- 36.Niasse A, Faye PM, Ndong A, Thiam O, Gueye O, Gueye ML, Sarr ISS, Seye Y, Toure AO, Seck M, Cisse M, Dieng M. Lipome géant du dos: rapport de cas avec revue de la littérature [Giant lipoma of the back: a case report and litterature review]. Pan Afr. Med. J. 2022 Aug 18;42:292. French. doi: 10.11604/pamj.2022.42.292.21047. PMID: 36415335; PMCID: PMC9643782. [DOI] [PMC free article] [PubMed]
- 37.Faenza M., Del Torto G., Di Costanzo P., Pieretti G., Lamberti R., Franco R., Ferraro G.A., Nicoletti G.F. Large single cutaneous metastasis of colon adenocarcinoma mimicking a squamous cell carcinoma of the skin: a case report. Int. J. Surg. Case Rep. 2019;56:96–100. doi: 10.1016/j.ijscr.2019.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faenza M., Ronchi A., Santoriello A., Rubino C., Pieretti G., Guastafierro A., Ferraro G.A., Nicoletti G.F. What’s new on primary Hodgkin’s lymphoma of the breast? A case report and review of the literature. Int. J. Surg. Case Rep. 2017;38:149–153. doi: 10.1016/j.ijscr.2017.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sohrabi C., Mathew G., Maria N., Kerwan A., Franchi T., Agha R.A. The SCARE 2023 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int J Surg Lond Engl. 2023;109(5):1136. doi: 10.1097/JS9.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]


