Abstract
Objectives
Myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease (MOGAD) is frequently preceded by infections. The underlying pathomechanism, however, remains poorly understood. Here, we present the clinical data of two MOGAD patients with concurrent syphilis infection and investigate the reactivity of patient-derived antibodies to MOG and Treponema pallidum (T. pallidum).
Methods
Longitudinal serum samples and soluble immunoglobulins in single B cell supernatants were measured for MOG reactivity by a live cell-based assay. Reactivity against T. pallidum was assessed by enzyme-linked immunosorbent assay.
Results
The two patients presented MOGAD and concurrent latent syphilis infection, manifesting as cervical myelitis and unilateral optic neuritis, respectively. The first patient had been living with HIV on antiretroviral therapy, and the second was concomitantly diagnosed with chronic hepatitis B infection. Upon screening of B cell supernatants, we identified reactivity to MOG or T. pallidum. Notably, one B cell showed reactivity to both antigens.
Discussion
The coexistence of MOGAD diagnoses and latent syphilis, alongside the identification of antibody reactivity to MOG and T. pallidum, underscores the potential pathomechanistic link between syphilis infection and subsequent autoimmune neuroinflammation. Cross-reactivity between MOG and T. pallidum antibodies remains to be validated on a molecular level, and further characterization of infectious triggers associated with MOGAD is needed.
Keywords: myelin oligodendrocyte glycoprotein, myelin oligodendrocyte glycoprotein antibody-associated disease, syphilis, infection, case report, molecular mimicry, antibodies, B cells
Introduction
Myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease (MOGAD) is a recently classified autoimmune demyelinating disease entity of the central nervous system (CNS), characterized by the presence of specific core clinical features and antibodies against MOG (1, 2). Importantly, MOGAD is frequently preceded by infections or vaccinations and often follows a monophasic disease course (1), suggesting a parainfectious etiology. Potential mechanisms include molecular mimicry, epitope spreading, and bystander activation of autoreactive lymphocytes. Yet, molecular evidence linking infection to the generation of a MOG-specific B cell response is missing.
Methods
Imaging and (para)clinical data were retrieved from hospital records. Available serum samples were measured for MOG-IgG by a live cell-based assay (CBA), as previously described (3). In brief, sera (1:100) and cerebrospinal fluid (CSF) (1:5) were examined for IgG reactivity against full-length human MOG using a live cell-based assay. For each sample, the ratio of the geometric mean channel fluorescence intensity (geometric MFI) of the MOG-transfected cell line divided by the geometric MFI of the control cell line was calculated. Values below 2.4 are considered negative, values between 2.4 and 3 positive, and values above 3 clear-positive.
To investigate the potential cross-reactivity of patient-derived B cells, B cells reactive to MOG were enriched from the two patients with an established protocol (4). In brief, B cells were isolated from peripheral blood mononuclear cells by magnetic negative selection and cultivated with a stably transfected cell line to allow antigen uptake. Here, we used a cell line expressing full-length human MOG fused to a green fluorescence protein to allow flow cytometry cell sorting of MOG-recognizing B cells. Supernatants of single-plated B cells were screened for immunoglobulin reactivity against MOG by CBA, and reactivity against common T. pallidum antigens (TpN15+TpN17+TpN47, Alpha Diagnostic, San Antonio, USA) was measured by enzyme-linked immunosorbent assay. Both patients provided written informed consent, and CARE reporting guidelines were followed.
Results
We present two cases of patients with diagnoses of latent syphilis and MOGAD with clear-positive MOG-IgG levels according to the International MOGAD Panel proposed criteria (2), hereby expanding on the existing literature to a total of five reported cases of concurrent syphilis infection and MOGAD ( Table 1 ).
Table 1.
Case summaries of concurrent MOGAD and syphilis infection.
| Publication | Age (y), sex | Neurological diagnosis | MOG-Ig positivity at onset; serum, CSF [titer or MFI ratio] |
Syphilis stage | Co-infections |
|---|---|---|---|---|---|
| Gudenkauf et al., Neuroimmunology Reports. 2021 ( 13) | 31, male | Meningoencephalomyelitis | Positive [1:80], N/A | Latent | None |
| Jeyakumar et al., Neuroimmunology Reports. 2021 ( 14) | 45, male | Papillitis, intermediate uveitis | N/A a , positive | Neurosyphilis | None |
| Shi et al., Front Neurol. 2023 ( 15) | 37, male | Optic neuritis | Positive [1:200], positive [1:200] | Neurosyphilis | None |
| Kulsvehagen et al., Front Immunol. 2024 | 43, male | Cervical myelitis | Positive [14], N/A | Latent | HIV, latent tuberculosis |
| Kulsvehagen et al., Front Immunol. 2024 | 44, male | Optic neuritis | Positive [20], borderline positive [3] | Latent | Hepatitis B |
Summary of five reported patients with coexisting (neuro)syphilis and MOGAD. MOG-IgG titers and MFI ratios were included when available.
CSF, cerebrospinal fluid; MOG, myelin oligodendrocyte glycoprotein; N/A, not available; HIV, human immunodeficiency virus.
The serum sample at disease onset was not available (the serum sample collected 2 months after disease onset was MOG-IgG negative).
Case 1
A 43-year-old man was admitted to our service with a 2-day history of numbness in the genital area and lower limbs. He had been living with human immunodeficiency virus (HIV) for 5 years and was on antiretroviral therapy with elvitegravir, cobicistat, emtricitabine, and tenofovir. The lowest initial CD4 count was 406/µl (35.8%) and the highest viral load was 304,000 cp/ml. The patient’s history suggested that primary HIV infection occurred 3 months before diagnosis and treatment was initiated right away, leading to sustained viral suppression. Additionally, he reported previous diagnoses of untreated latent tuberculosis 1 year prior and latent syphilis 2 years prior, which was treated with benzathine penicillin. One year before neurological symptom onset, a lab test confirmed negative serology for syphilis as assessed by the venereal disease research laboratory (VDRL) test, indicating adequate treatment response.
On full neurological examination, he presented lower limb hypoesthesia with a sensory level at T12, not associated with hypalgesia, pallhypesthesia, thermhypesthesia, motor, or autonomic dysfunction; reflexes were symmetrical. Magnetic resonance imaging (MRI) of the spinal cord revealed a gadolinium-enhancing T2-signal hyperintense lesion at the level of C4 and C5 ( Figure 1 ). Serologies for syphilis with the VDRL test presented a titer of 1:16, which had previously been negative for over a year, and an increased T. pallidum particle agglutination (TPPA) titer of 1:20,480 after being stable at 1:640 for more than a year. CSF was normal, with no signs of pleocytosis, disturbed blood–CSF barrier, or intrathecal immunoglobulin production, except for a TPPA of 1:16. VDRL was also negative. The patient was diagnosed with latent syphilis reinfection or reactivation in association with short cervical myelitis. Due to the near-normal CSF analysis and atypical clinical presentation, diagnosis of neurosyphilis was considered unlikely. Additional opportunistic infections were ruled out at neurological presentation: VZV IgG was positive in serum but negative in CSF, and IgM was negative in both serum and CSF. CMV IgG had been positive prior to neurological presentation and remained unchanged. HSV 1 + 2 IgG was positive in serum and negative in CSF, and IgM was borderline positive in serum (most likely unspecific) and negative in CSF. Borrelia and FSME IgG and IgM were negative in both CSF and serum. EBV and HIV PCR were negative in CSF.
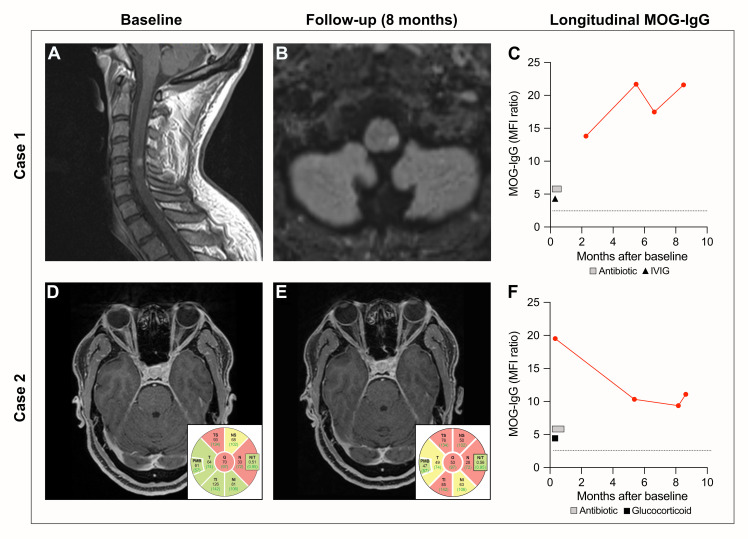
Figure 1.
Clinical features at baseline and follow-up. Case 1: (A) Spinal MRI with sagittal T1 showing contrast-enhancing lesion on the level of cervical vertebrae 4/5, presenting as numbness in the genital area and both legs. (B) Cerebral MRI with transversal FLAIR revealing a new lesion in the left brainstem 6 months after initial treatment with IVIG. (C) Longitudinal MOG-IgG values (geometric MFI ratio). The dotted line indicates the cutoff for clear-positive MOG-IgG signals (geometric MFI ratio = 3). The square indicates treatment with an antibiotic (intravenous penicillin [20 million units/day] for 14 days) and the triangle indicates IVIG treatment (150 g over 5 days). Case 2: (D) Transversal T1 showing contrast enhancement of the right optic nerve at diagnosis. OCT showing slightly (yellow) and severely (red) diminished pRNFL thickness of the right eye. (E) Transversal post-contrast T1 showing bilateral normal optic nerves; however, OCT showed a decrease of the pRNFL thickness of the right eye 8 months after initial treatment with methylprednisolone. (F) MOG-IgG values (geometric MFI ratio) at baseline and follow-up time points. Cutoff for clear-positive MOG-IgG signals (geometric MFI ratio = 3) indicated as a dotted line. The square indicates treatment with an antibiotic (benzathine penicillin [2.4 million U/week] over 3 weeks) and the triangle indicates treatment with glucocorticoids (methylprednisolone 3 g over 3 days). MRI, magnetic resonance imaging; FLAIR, fluid-attenuated inversion recovery; IVIG, intravenous immunoglobulin; MOG, myelin oligodendrocyte glycoprotein; MFI, mean fluorescence intensity; OCT, optical coherence tomography; pRNFL, peripapillary retinal nerve fiber layer.
The patient was treated with intravenous penicillin (20 million units/day) for 14 days and 150 g of intravenous immunoglobulin (IVIG) over 5 days. He responded well to treatment and presented a fast partial recovery with the presence of a residual Lhermitte’s sign. A MOG-IgG test in serum sampled at the 2-month follow-up was clear-positive (2) ( Figure 1 , Table 2 ). At the 8-month follow-up, the patient presented new asymptomatic pontine and medullary lesions on the MRI ( Figure 1 ). The clear-positive MOG-IgG serum levels increased over time, while neurofilament light-chain (sNfL) levels remained within the same range ( Table 2 ). The patient was diagnosed with MOGAD. While there may be a certain risk of a false-positive serum MOG-IgG in the 2-month measurement due to IVIG treatment, this cannot explain the positive results obtained at follow-up, especially at 9 months. The risk of attack recurrence was discussed with the patient, and we recommended maintenance treatment with intravenous immunoglobulin. While the patient was satisfied with the initial treatment response, he was opposed to chronic treatment and preferred a wait-and-see approach.
Table 2.
Demographic and (para)clinical features.
| Case 1 | Case 2 | |
|---|---|---|
| Age (y), sex | 43, male | 44, male |
| Initial symptoms | Numbness in the genital area and lower limbs | Vision loss and pain in the right eye |
| Neurological diagnosis | Cervical myelitis | Optic neuritis |
| Syphilis serology | TPPA+ (1:20,480), VDRL+ (1:16) | TPPA+ (1:1,280), VDRL− |
| Serum MOG-IgG (months after disease onset) | Month 2: geometric MFI ratio of 14 Month 5: geometric MFI ratio of 22 Month 7: geometric MFI ratio of 17 Month 9: geometric MFI ratio of 22 |
Month 0: geometric MFI ratio of 20 Month 5: geometric MFI ratio of 10 Month 8: geometric MFI ratio of 9 Month 9: geometric MFI ratio of 11 |
| Serum NfL (months after disease onset) | Month 2: 5.4 pg/ml (15 percentile) Month 5: 4.0 pg/ml (2.3 percentile) Month 7: 5.2 pg/ml (13 percentile) Month 9: 5.1 pg/ml (12 percentile) |
Month 0: 10.7 pg/ml (88 percentile) Month 5: 8.8 pg/ml (71 percentile) Month 8: 10.2 pg/ml (84 percentile) Month 9: 14.9 pg/ml (97 percentile) |
| Lumbar puncture | 2 leukocytes, protein 440mg, OCB negative, TPPA positive (1:16), VDRL negative | 2 leukocytes, protein 340 mg, OCB negative, TPPA positive (1:2), VDRL negative |
| Anti-AQP4 | Negative | Negative |
| Neurological treatment | IVIG 150 g over 5 days | Methylprednisolone 3 g over 3 days |
| Syphilis treatment | Penicillin 20 million U per day over 14 days | Benzathine penicillin 2.4 million U per week over 3 weeks |
| Comorbidities | HIV CDC A2 (bictegravir, emtricitabine, tenofovir), latent tuberculosis (untreated) | Hepatitis B (tenofovir) |
| Follow-up (8 months) | Asymptomatic new FLAIR lesions in the pons and medulla oblongata and increase in MOG-IgG serum level | Decrease in pRNFL thickness (as expected after optic neuritis) and persistent MOG-IgG seropositivity |
For serum MOG-IgG levels, values below 2.4 are considered negative, values between 2.4 and 3 positive, and values above 3 clear-positive.
TPPA, Treponema pallidum particle agglutination; VDRL, venereal disease research laboratory; OCB, oligoclonal band; IVIG, intravenous immunoglobulin; HIV, human immunodeficiency virus; CDC, Centers for Disease Control and Prevention; FLAIR, fluid-attenuated inversion recovery; MOG, myelin oligodendrocyte glycoprotein; MFI, mean fluorescence intensity; NfL, neurofilament light chain.
Case 2
A 44-year-old man was referred to our service with a 1-week history of vision loss and pain in the right eye. Ophthalmological examination revealed a right eye visual acuity of 10/50 with red color desaturation (Ishihara 5/13 on the right eye, 13/13 on the left eye), and full neurological examination was normal. The brain MRI showed optic disk edema and a gadolinium-enhancing T2-signal hyperintensity of the intraorbital and intracanalicular segment of the right optic nerve, nerve sheath, and adjacent fatty tissue suggesting a diagnosis of optic neuritis (ON) ( Figure 1 ). Serological investigation revealed chronic hepatitis B virus (HBV) infection and suspected latent syphilis infection over neurosyphilis based on VDRL being negative (TPPA 1:1,280, VDRL negative). The CSF analysis was normal, except for a TPPA of 1:2. The patient was treated with intravenous methylprednisolone (1 g/day for 3 days), tenofovir (245 mg/day), and intramuscular benzathine penicillin (2.4 million units/week over 3 weeks). He responded with full visual acuity recovery and minor color desaturation after 1 week. At follow-up, a clear-positive (2) MOG-IgG serum result sampled at hospital admission was detected, leading to the diagnosis of MOGAD ( Figure 1 , Table 2 ). At the 8-month follow-up, no new lesions were detected on the MRI. Yet, sNfL and MOG-IgG levels remained high ( Figure 1 , Table 2 ). Optical coherence tomography (OCT) showed further decrease of the peripapillary retinal nerve fiber layer thickness (pRNFL) in the right eye ( Figure 1 ). The patient was diagnosed with monophasic MOGAD, but regular follow-up examinations were appointed. The patient was satisfied with the treatment response and regular follow-ups.
Experimental results
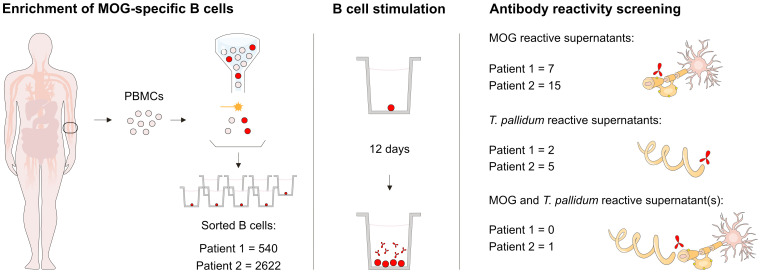
To examine whether the patients had B cells reactive to MOG and T. pallidum, we assessed the reactivity of isolated B cell supernatants from the two patients in vitro. For the first patient, from 19 million peripheral blood mononuclear cells (PBMCs), we obtained 1.3 million cells after B cell enrichment, of which 540 were gated as MOG-recognizing B cells. These were sorted and single-plated, of which a total of seven supernatants recognized MOG and two showed reactivity to T. pallidum antigens ( Figure 2 ). From the second patient, from 12 million PBMCs, we obtained 1.3 million cells after B cell enrichment, and 2,622 enriched B cells were sorted and single-plated. Of these, reactivity to MOG was identified in 15 supernatants, whereas reactivity to T. pallidum antigens was detected in five supernatants ( Figure 2 ). Notably, of these supernatants, one showed reactivity to both MOG and T. pallidum. Measurements of positive B cell supernatants were repeated and validated in independent experiments.
Figure 2.
Experimental design. Schematic illustration of the experimental setup and the number of isolated B cell supernatants with detected reactivities to MOG and T. pallidum. MOG, myelin oligodendrocyte glycoprotein; T. pallidum, Treponema pallidum; PBMCs, peripheral blood mononuclear cells.
Discussion
The current study expands on the existing literature of concurrent infections in MOGAD by reporting two MOGAD cases with latent syphilis infection among other latent infections, specifically HIV, tuberculosis, and HBV, respectively. Previously reported infections associated with MOGAD include respiratory infections (2, 5) (e.g., SARS-CoV-2) (6), Zika virus (7), mumps (8), herpes simplex virus (9), Borrelia (10), Epstein–Barr virus (11), and tuberculosis (12). Furthermore, three cases of MOGAD and coexisting syphilis infection have been described: one patient with latent syphilis and meningoencephalomyelitis associated with MOG-IgG seropositivity (13), one patient with acute neurosyphilis (presenting with papillitis and uveitis) and positive MOG-IgG results in the CSF (14), and one case of coexisting neurosyphilis and MOGAD manifesting as ON (15), a rare manifestation of neurosyphilis that shows similarities to ON in MOGAD (16). Due to infections being commonly reported in MOGAD, the hypothesis of post-infectious generation of MOG-specific antibodies has been suggested (1). The proposed underlying mechanisms include molecular mimicry, epitope spreading, bystander activation, and polyclonal B cell activation. Yet, molecular evidence linking specific infectious triggers to the generation of autoreactive MOG-specific B cells is missing.
The identification of a patient-derived B cell showing reactivity to both MOG and T. pallidum in one of the patients with MOGAD and concurrent syphilis infection suggests a potential link between this infectious antigen and the development of autoreactive B cells through molecular mimicry. However, whether cross-reactivity is indeed involved in the pathomechanism of the autoimmune responses in MOGAD remains to be validated on a molecular level. For instance, this may be assessed by investigating the cross-reactivity of MOG-specific patient-derived monoclonal antibodies. Furthermore, although we identified a B cell reactive to MOG and T. pallidum, we cannot rule out the involvement of additional coexisting infections in these patients. In fact, cross-reactivity between HBV surface antigen and MOG peptides has been reported previously (17), and it has been suggested that syphilis infection can lead to Epstein–Barr virus reactivation and hepatitis (18). To further characterize the link between infectious triggers and MOGAD, additional molecular and functional studies investigating cross-reactivity to antigens from additional infections are warranted. Moreover, to prevent diagnostic delay and ensure optimal treatment strategies, testing of MOG-Ig in serum, including MOG-IgA as recently described (3), should be considered in patients not only with suspected neurosyphilis but also in patients with syphilis infection and indications of a demyelinating event.
This study has limitations. Firstly, serum samples before disease onset to assess baseline MOG-IgG and sNfL were not available. Furthermore, for the first case, MOG-IgG was measured after IVIG administration, which may have resulted in a false-positive MOG-IgG result. Yet, this is unlikely to explain the positive results, especially at later follow-ups. Additionally, while we observed B cell reactivity against antigens specific for syphilis, the shared infection across the two patients, we cannot exclude that the other coexisting infections (e.g., HIV and HBV) may have also played a role in the underlying pathogenesis. Lastly, although our study provides evidence for a potential link between syphilis infection and the development of autoreactive MOG-specific B cells, cross-reactivity of patient-derived antibodies to MOG and T. pallidum remains to be validated molecularly.
Acknowledgments
We thank the patients in this study for their participation. We also thank Anne-Catherine Lecourt and Julia Gutzwiller for their valuable assistance in the lab.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was funded by an ECTRIMS Clinical Fellowship and a Swiss Government Excellence Scholarship (to AA), a doctoral fellowship from the Goldschmidt Jacobson Foundation (to PL), and a Swiss Young Talents in Clinical Research Fellowship (to JF). The study was further funded by the Swiss National Science Foundation (SNF Eccellenza Professorship: 194609; SNF Starting Grant: 211318); the Swiss State Secretariat for Education, Research and Innovation (Horizon 2020 ID Dark Matter Consortium 101136582); the National MS Society (FG-1708-28871); the Fondation Pierre Mercier pour la Science; the Propatient Foundation; the Goldschmidt Jacobson Foundation; and the Gottfried and Julia Bangerter Rhyner Foundation (all to A-KP).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethikkommission Nordwest- und Zentralschweiz (EKNZ 2022-00004). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LK: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Project administration, Methodology, Investigation, Data curation, Conceptualization. TW: Writing – review & editing, Writing – original draft, Validation, Methodology, Conceptualization, Data curation. AA: Writing – review & editing, Writing – original draft, Methodology. PL: Writing – review & editing, Data curation. TN: Writing – review & editing, Methodology. JF: Writing – review & editing, Data curation. KL: Writing – review & editing, Supervision. TD: Writing – review & editing, Validation. JK: Writing – review & editing, Supervision. AP: Writing – review & editing, Supervision. AP: Writing – review & editing, Resources, Project administration, Funding acquisition, Conceptualization.
Conflict of interest
AA has received a research grant from Roche and travel grants from Roche and Biogen. JK received speaker fees, research support, and travel support and/or served on advisory boards by the Swiss MS Society, Swiss National Research Foundation 320030_189140/1, University of Basel, Progressive MS Alliance, Bayer, Biogen, Bristol Myers Squibb, Celgene, Merck, Novartis, Octave Bioscience, Roche, and Sanofi. AP received financial compensation for participation in advisory boards and/or speaker fees from Eli Lilly, Lundbeck, Sanofi-Genzyme, and Teva, all used for research support; she also received travel support from Bayer AG, Eli Lilly, F. Hoffmann-La Roche, and Teva. A-KP received financial compensation for participation in advisory boards and consultations from Biogen, Roche, and Novartis, all used for research support.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Marignier R, Hacohen Y, Cobo-Calvo A, Pröbstel AK, Aktas O, Alexopoulos H, et al. Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol. (2021) 20:762–72. doi: 10.1016/S1474-4422(21)00218-0 [DOI] [PubMed] [Google Scholar]
- 2. Banwell B, Bennett JL, Marignier R, Kim HJ, Brilot F, Flanagan EP, et al. Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: International MOGAD Panel proposed criteria. Lancet Neurol. (2023) 22:268–82. doi: 10.1016/S1474-4422(22)00431-8 [DOI] [PubMed] [Google Scholar]
- 3. Ayroza Galvão Ribeiro Gomes AB, Kulsvehagen L, Lipps P, Cagol A, Cerdá-Fuertes N, Neziraj T, et al. Immunoglobulin A antibodies against myelin oligodendrocyte glycoprotein in a subgroup of patients with central nervous system demyelination. JAMA Neurol. (2023) 80:989–95. doi: 10.1001/jamaneurol.2023.2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zimmermann M, Rose N, Lindner JM, Kim H, Gonçalves AR, Callegari I, et al. Antigen extraction and B cell activation enable identification of rare membrane antigen specific human B cells. Front Immunol. (2019) 10:829. doi: 10.3389/fimmu.2019.00829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, et al. MOG-IgG in NMO and related disorders: A multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflamm. (2016) 13:280. doi: 10.1186/s12974-016-0718-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ismail II, Salama S. Association of CNS demyelination and COVID-19 infection: an updated systematic review. J Neurol. (2022) 269:541–76. doi: 10.1007/s00415-021-10752-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neri VC, Xavier MF, Barros PO, Bento CM, Marignier R, Alvarenga RP. Case report: Acute transverse myelitis after Zika virus infection. Am J Trop Med Hygiene. (2018) 99:1419–21. doi: 10.4269/ajtmh.17-0938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mbonde AA, Arca KN, Grill MF. Anti-MOG antibody associated encephalomyelitis in an HIV-infected patient. Mult Scler Relat Disord. (2021) 49:102753. doi: 10.1016/j.msard.2021.102753 [DOI] [PubMed] [Google Scholar]
- 9. Nakamura M, Iwasaki Y, Takahashi T, Kaneko K, Nakashima I, Kunieda T, et al. A case of MOG antibody-positive bilateral optic neuritis and meningoganglionitis following a genital herpes simplex virus infection. Mult Scler Relat Disord. (2017) 17:148–50. doi: 10.1016/j.msard.2017.07.023 [DOI] [PubMed] [Google Scholar]
- 10. Vieira JP, Sequeira J, Brito MJ. Postinfectious anti-myelin oligodendrocyte glycoprotein antibody positive optic neuritis and myelitis. J Child Neurol. (2017) 32:996–9. doi: 10.1177/0883073817724927 [DOI] [PubMed] [Google Scholar]
- 11. Nakamura Y, Nakajima H, Tani H, Hosokawa T, Ishida S, Kimura F, et al. Anti-MOG antibody-positive ADEM following infectious mononucleosis due to a primary EBV infection: A case report. BMC Neurol. (2017) 17:6–9. doi: 10.1186/s12883-017-0858-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miraclin TA, Sivadasan A, Nair AA, Rajakumar P, Nair AV, Joseph T, et al. Drug resistant tuberculosis, Myelitis and MOG antibody. Neuroimmunology Rep. (2022) 2:100123. doi: 10.1016/j.nerep.2022.100123 [DOI] [Google Scholar]
- 13. Gudenkauf JC, Gadani SP, McArthur JC, Sotirchos ES. Neurosyphilis, MOGAD or both? A case of meningoencephalomyelitis associated with MOG-IgG seropositivity in a patient with syphilis. Neuroimmunology Rep. (2021) 1:100003. doi: 10.1016/j.nerep.2021.100003 [DOI] [Google Scholar]
- 14. Jeyakumar N, Waller S, Mahant N, Ramanathan S. Henderson APD. A case of CSF anti-MOG antibody-positive papillitis with intermediate uveitis in the setting of acute neurosyphilis. Neuroimmunology Rep. (2021) 1:100033. doi: 10.1016/j.nerep.2021.100033 [DOI] [Google Scholar]
- 15. Shi M, Luo D, Li Z, Li M, Jin S, Yang D, et al. A case report of neurosyphilis coexisting with a positive MOG antibody manifested as optic neuritis. Front Neurol. (2023) 14:1258043. doi: 10.3389/fneur.2023.1258043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Apinyawasisuk S, Poonyathalang A, Preechawat P, Vanikieti K. Syphilitic optic neuropathy: re-emerging cases over a 2-year period. Neuro-Ophthalmology. (2016) 40:69–73. doi: 10.3109/01658107.2015.1134586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bogdanos DP, Smith H, Ma Y, Baum H, Mieli-Vergani G, Vergani D. A study of molecular mimicry and immunological cross-reactivity between hepatitis B surface antigen and myelin mimics. Clin Dev Immunol. (2005) 12:217–24. doi: 10.1080/17402520500285247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hirsiger JR, Fuchs PS, Häusermann P, Müller-Durovic B, Daikeler T, Recher M, et al. Syphilis reactivates latent Epstein-Barr virus reservoir via toll-like receptor 2 and B-cell receptor activation. Open Forum Infect Dis. (2019) 6:1–6. doi: 10.1093/ofid/ofz317 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.