Abstract
The management of chronic myeloid leukemia (CML) diagnosed during pregnancy is a rare and challenging situation. We report the treatment and outcome of 87 cases diagnosed in chronic phase from 2001–2022 derived from the largest international observational registry, supported by the European LeukemiaNet (ELN), of 400 pregnancies in 299 CML women. Normal childbirth occurred in 76% without an increased rate of birth abnormalities or life-threatening events, including in patients untreated or treated with interferon-α and/or imatinib in 2nd–3rd trimester. The low birth weight rate of 12% was comparable to that seen in the normal population. Elective and spontaneous abortions occurred in 21% and 3%, respectively. The complete hematologic response rate before labor was 95% with imatinib and 47% with interferon only. No disease progression during pregnancy was observed, 28% of the patients switched their therapy at varying times after delivery. Treatment options balance the efficacy and safety for mother and infant: interferon-α can commence in the 1st trimester and continued throughout in cases of good disease control and tolerability. Because of limited placental crossing, selected tyrosine kinase inhibitors (imatinib and nilotinib) seem to be safe and effective options in 2nd and 3rd trimester while hydroxycarbamide offers few benefits.
Subject terms: Myeloproliferative disease, Diseases
Background
Chronic myeloid leukemia (CML) presenting in pregnancy is a rare event and lacks standard recommendations for treatment. Most patients with CML present in the chronic phase (CP). The median duration of CP without cytoreductive therapy is of the order of 2.5 years [1], offering an opportunity to consider continuing the pregnancy without therapeutic intervention. This possibility is more attractive since the introduction of tyrosine kinase inhibitors (TKIs) as they can induce rapid disease control once treatment starts [2, 3]. An ever-increasing number of case reports and case series over the past 10 years have described the management of planned and unplanned pregnancies in CML patients, providing evidence for some treatments during gestation [3–6].
In managing a patient newly diagnosed in pregnancy there are two main considerations.
First, for the fetus there is the risk of potential teratogenicity of treatment [7–9]. Although there are now several reports of successful pregnancy outcomes in patients with established CML who received imatinib (IM) during pregnancy [5, 10, 11], and of limited placental transfer of IM [12–14] the drug has potential risks during pregnancy. Preclinical studies identified teratogenicity and birth malformations were reported, usually in connection with the use of IM during the 1st trimester [7].
Data regarding the use of second generation of TKIs (TKI-2G) during pregnancy are more limited [8, 15, 16]. There was no evidence of teratogenicity of nilotinib in preclinical experiments but embryo- and fetotoxicity were observed in rats and rabbits. However, information on nilotinib use during pregnancy continues to accumulate and gives cautious optimism, with around a dozen cases describing the use of nilotinib in late pregnancy with good fetal and maternal outcomes [4, 13, 15, 17]. Nilotinib also has limited placental transfer.
Dasatinib and bosutinib may have less limited placental transfer [13, 18], and the use of dasatinib was of considerable concern given the reports of hydrops fetalis in 3 of 15 exposed cases, even when dasatinib was commenced after the 1st trimester in one patient [8]. In contrast, good outcomes have been described when women conceived on dasatinib or bosutinib and stopped the drug at the time of confirmation of the pregnancy [16].
Interferon-α (IFN), previously used extensively in CML and other myeloproliferative diseases, has no known teratogenicity in humans [2, 3, 15, 19]. It acts through a complex multifactorial mechanism involving the induction of differentiation, immunomodulation, apoptosis, and anti-angiogenic effects. IFN is less effective than the TKIs in terms of inducing cytogenetic and molecular responses, and is slow in cytoreduction.
Hydroxycarbamide (HC) is still used in CML to rapidly reduce cell counts. As a cell-cycle inhibitor, it has acknowledged teratogenicity although there are a few reports of its use in pregnancy with a good outcome [20, 21].
The second risk is that of disease progression without effective treatment. Although CML is a “slowly” developing disease it is difficult to predict the consequences of a long period without treatment especially in patients at diagnosis, knowing that a high leukemic burden is a basis for disease progression. Moreover, uncontrolled increases in the white blood cell and platelet counts in untreated women may interfere with placental blood flow and result in obstetric complications [22].
Thus, the key questions in patients diagnosed with CML during pregnancy are whether to start or not treatment before delivery, and if treatment is given, which option to choose.
Given the enhanced knowledge, inducing an abortion at the onset of CML to start TKI immediately, with the prospect of a planned pregnancy at a later time after achieving a deep and durable molecular response, is no longer deemed necessary. As a matter of fact, irrespective of the psychological, social and human distress it might cause, this decision leaves the woman waiting indefinitely for the right moment to discontinue treatment for a planned pregnancy. Clearly, achieving the minimum criteria for treatment-free remission (TFR) after at least 4–5 years of TKI treatment offers the best chance of safe TKI cessation for the period of conception and pregnancy [23, 24]. Such deep responses are not always achieved in every patient, even in those who attain early sustained major molecular response (MMR) [25]. Importantly, not every woman can postpone childbirth for several years for biological (age-dependent decreased fertility) and/or social (family-based or religious) reasons.
The experience with CML diagnosed during pregnancy is scarce [2, 4, 15, 24] and we now report the largest series to date, gathered through multinational collaboration and derived from a larger database of pregnancies in patients with CML.
Patients and methods
The data were obtained through a multicenter observational study of conception/pregnancy in adult patients with CML supported by the European LeukemiaNet (ELN). The trial design was observational, both retrospective and prospective. The main objective was to describe the management and outcome of pregnancy in patients with CML. The key inclusion criteria were (1) adult women, age ≥18 years; (2) CML confirmed with the presence of Ph’ chromosome and/or BCR::ABL1 transcript; (3) pregnancy; (4) written informed consent. Considering the rarity of cases, the sample size was not defined. The trial protocol was given to participants to adapt for any local regulatory procedures according to ICH/EU/GCP and national/local laws. The data were collected by using the online “Redcap” database, a secure web application for building and managing online databases for research studies, developed by Vanderbilt University (Nashville, TN, USA), and distributed free of charge.
The information included demographic characteristics, therapy, monitoring, pregnancy outcomes, characteristics of the newborns and follow-up. For the purpose of description of the cases with CML diagnosed during pregnancy we made a targeted search for this particular subpopulation within the database.
Results
At the time of analysis (March 2023) the ELN pregnancy registry contained data from 17 countries of 400 pregnancies in 299 CML women (some patients had more than one pregnancy). CML was diagnosed during pregnancy in 87 of 299 (29%) women from 11 countries in years 2001–2022 (Supplementary Table 1).
The median age of the patients at diagnosis was 27 years (range 18–41 years). CML was diagnosed during 1st, 2nd and 3rd trimesters in 50 (57%), 21 (24%) and 16 (19%) women, respectively (Table 1). The median gestational period at the time of the diagnosis of CML was 11 weeks (range 5–38 weeks). A gestational week was calculated based on the first day of last menstrual period.
Table 1.
Clinical and demographic characteristics of 87 women with CML diagnosed during pregnancy.
| Characteristics of patients | All patients | Pregnancy outcome | |
|---|---|---|---|
| Childbirth | Abortion or miscarriage | ||
| Chronic phase, n (%) | 87 (100) | 66 (100) | 21 (100) |
| Age, median (min–max), years | 27 (18–41) | 28 (18–38) | 27 (20–41) |
| Sokal score | |||
| Low, n (%) | 62 (71) | 51 (77) | 11 (52) |
| Intermediate, n (%) | 15 (17) | 11 (17) | 4 (19) |
| High, n (%) | 5 (6) | 1 (1,5) | 4 (19) |
| No data, n (%) | 5 (6) | 3 (4,5) | 2 (10) |
| Trimester at time of diagnosis | |||
| 1st trimester, n (%) | 49 (56) | 29 (44) | 20 (95) |
| 2nd trimester, n (%) | 22 (25) | 21 (32) | 1 (5) |
| 3rd trimester, n (%) | 16 (18) | 16 (24) | – |
| Median gestational week (range) | 11 (5–38) | 14 (5–38) | 8 (5–14) |
| Previous pregnancies with a childbirth | |||
| Yes, n (%) | 38 (44) | 31 (47) | 8 (38) |
| No, n (%) | 37 (43) | 27 (41) | 9 (43) |
| No data, n (%) | 12 (13) | 8 (12) | 4 (19) |
All patients were diagnosed in CP with most being low Sokal score (71%), with intermediate plus high score accounting for 23% of patients. About 40% of the patients had a pregnancy pre-dating the diagnosis of CML.
Pregnancy outcomes
After the diagnosis of CML the pregnancy was terminated in 21 (24%) patients by elective (n = 18, 21%) or spontaneous (n = 3, 3%) abortion. Only one patient underwent elective termination in the 2nd trimester, at week 17, by a joint physician-patient decision in order to start treatment with a TKI.
Pregnancy resulted in childbirth in 66 (76%) patients, with labor to term in 59 (89%) and preterm labor in 7 (11%) patients, at 34 (n = 1), 35 (n = 3) and 36 (n = 3) weeks of gestation respectively.
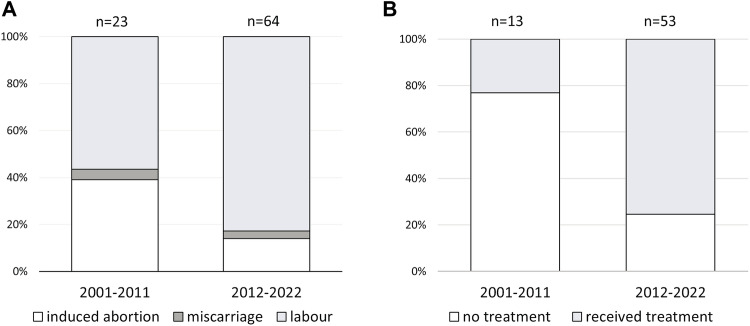
Pregnancy resulting in childbirth was more common and elective abortion less frequent in recent years with 13 (57%) children born from 23 pregnancies in 2001–2011 and 53 from 64 (83%) pregnancies in 2012–2022 (Fig. 1A) reflecting increasing knowledge from both physicians and patients. The proportion of patients who received treatment during pregnancy versus untreated cases also increased by decade from 23% in 2001–2011 to 75% in 2011–2022 (Fig. 1B).
Fig. 1. Changes in pregnancy outcome and therapy in a period between 2001 and 2022 year.
A Pregnancy outcome by decade. B Treatment in pregnancy by decade in 66 cases of childbirth.
Therapy during pregnancies resulting in childbirth (n = 66)
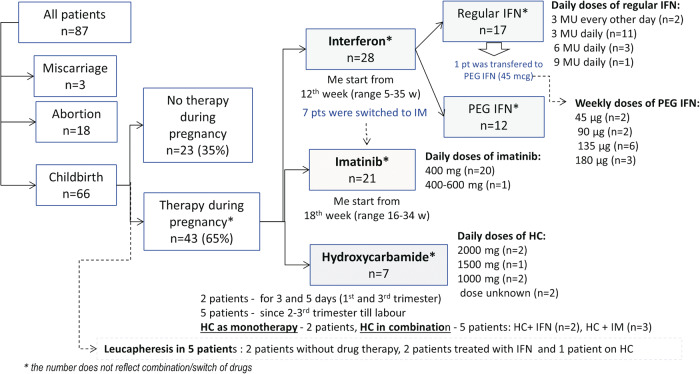
The proportion of treated and untreated patients during pregnancy was 17 (26%) and 49 (74%) in the 1st trimester, 34 (51.5%) and 32 (48.5%) in the 2nd trimester and 43 (65%) and 23 (35%) in the 3rd trimester. Of 23 patients untreated throughout their pregnancy 5, 6 and 12 were diagnosed in 1st, 2nd and 3rd trimester respectively. In contrast 43 women (65%) were treated with the specific drugs varying by trimester (Fig. 2).
Fig. 2. Therapy on 66 women with CML onset during pregnancy.
IM imatinib, IFN interferon, PEG IFN pegylated interferon, HC hydroxycarbamide, Me median, pt patient, w week.
Twenty-eight (42%) women received IFN with a median start of treatment at 12 weeks of gestation (range 5 - 35 weeks). Initially pegylated (PEG-IFN) and regular IFN were given to 12 and 16 women respectively. One patient was switched from regular IFN to PEG-IFN in the 2nd trimester.
Twenty-one (32%) patients received IM in late pregnancy: 13 patients in the 2nd trimester and 8 patients in the 3rd trimester. The median time to the start of IM therapy was 18 weeks of gestation (range 16–34 weeks).
Seven (11%) women received HC during pregnancy: 2 as monotherapy (in the 2nd trimester till therapy change and in the 3rd trimester till labor) and 5 patients got HC in combination with IFN or IM (Fig. 2).
The dosages of IFN, IM and HC were similar to those used routinely in CML (Fig. 2). Additionally, 5 (8%) patients had leucapheresis procedures (from 2 to 17 times).
The change or combinations of therapy during pregnancy were as follows. Seven patients were switched from IFN to IM: 5 in the 2nd trimester and 2 in the 3rd trimester. Two patients received IFN + HC in the 2nd - 3rd trimester. One patient received IFN + HC (HC for 3 days only) in the 1st trimester and IM from the 2nd trimester to term. Two patients received IM + HC in the 3rd trimester (one for just 5 days and the other for 5 weeks). One patient received HC monotherapy in the 2nd trimester and was then switched to IM in the 3rd trimester.
In summary, 33 of 66 (50%) patients were pre-treated with IFN, HC or both before TKI (either during pregnancy (IM) or after delivery). The median delay to start of TKI after diagnosis was 6.5 months (range 1 week – 12 months).
Outcome of patients before delivery
Information relating to the achievement of complete hematologic responses (CHR) before delivery was available in 40 of 43 patients who started therapy during pregnancy (Table 2). CHR was achieved in 19 of 20 (95%) of patients who received IM (including 1 patient with IM + HC) and in 9 of 19 (47%) patients who received IFN without subsequent IM during pregnancy (including 2 patients with IFN + HC). The treatment period before delivery lasted for 3 weeks (from 32 to 35th gestational week) in a single patient who did not achieve CHR on IM. There was no difference in the median duration of IFN therapy in patients who did or did not achieve CHR before delivery (p = 0.8).
Table 2.
Complete hematologic response on therapy achieved before delivery in CML women (n = 40).
| Therapy at the moment of delivery | CHR achieved before delivery | No CHR achieved before delivery | Time of exposure, weeks, Me (min-max) |
|---|---|---|---|
| n = 40 | n = 28 | n = 12 | |
| IFNa, n = 19 | 9 | 10 | 14 (4–31) |
| IMa, n = 20 | 19 | 1 | 19 (3–24) |
| HC, n = 1 | – | 1 | 9 |
aIncluding 2 patients with IFN + HC, 1 patient with IM + HC.
Disease progression was not seen in any patient irrespective of therapy or not during the pregnancy.
Adverse events during pregnancy in 66 pregnancies resulting in childbirth
Seventeen of the 66 (25%) patients reported AEs during pregnancy. One of the two women who did not receive any therapy had a thrombosis (details unknown) and the other experienced post-partum bleeding (a patient whose initial platelet count was >1000 × 109/L and who underwent multiple apheresis procedures during pregnancy). Adverse events related to IM occurred in 2 women (nausea and vomiting in one, thrombocytopenia grade 2 and rash grade 1 in the other). Only one patient on regular IFN described an AE (fever) lasting for few days at first injections of IFN.
The remaining 11 women described AEs not thought to be attributable to treatment with any drug, and included abdominal pain and diarrhea (n = 1), asthenia and shortness of breath (n = 1), vertigo (n = 1), anemia (n = 2), gestational diabetes (n = 1), hypoamnion and preeclampsia (n = 1), threatening abortion (n = 1), thrombosis of superficial veins (n = 2), mild COVID 19 (n = 1), undefined viral infection and infection of the urinary tract (n = 1).
Neonatal AEs were reported in 2 (3%) cases: intrauterine infection (n = 1, on IM therapy), prematurity with respiratory insufficiency and perinatal cephalohematoma as complication of a delivery (n = 1, on IFN therapy). None was considered to be therapy- or CML-related.
Follow-up of the patients after the end of pregnancy
All 87 patients received TKI therapy after delivery. The median follow-up on TKI treatment was 29 months (range 8 months to 17 years) in 66 patients who delivered children and 60 months (range 8 months – 15 years) in the 21 patients with pregnancy termination.
First line TKI was IM in 71 (82%) and TKI 2G in 16 (18%) patients (Table 3). Twenty four (28%) patients were switched to another TKI, 18 (21%) for resistance or suboptimal response. No significant differences were found in the rates of resistance/suboptimal response to 1st line therapy in patients with childbirth who received during pregnancy only IFN (±HC) without switch to IM (3 of 21), received IM ± HC (6 of 21) or were untreated (5 of 23) (p = 0.531).
Table 3.
Follow-up data after pregnancy completion in 87 CML patients.
| Follow-up data | All patients | Pregnancy outcome | |
|---|---|---|---|
| n = 87 | Childbirth | Abortion/miscarriage | |
| n = 66 | n = 21 | ||
| Living status | |||
| Alive, n (%) | 84 (97) | 64 (97) | 20 (95) |
| Dead, n (%) | 3 (3) | 2 (3) | 1 (5) |
| First line TKI | |||
| Imatinib, n(%) | 71 (82) | 51 (77) | 20 (95) |
| Dasatinib, n (%) | 9 (10) | 9 (14) | – |
| Nilotinib, n (%) | 7 (8) | 6 (9) | 1 (5) |
| TKI switch, main reason | |||
| Resistance/suboptimal, n (%) | 18 (21) | 14 (21) | 4 (19) |
| Intolerance/other, n (%) | 6 (7) | 4 (6) | 2 (10) |
| No switch, n (%) | 63 (72) | 48(73) | 15 (71) |
| Best overall response, n (%) | |||
| MMR | 28 (32) | 22 (33) | 6 (29) |
| DMR | 38 (44) | 31 (47) | 7 (33) |
| No MMR | 12 (14) | 8 (12) | 4 (19) |
| No data | 9 (10) | 5 (8) | 4 (19) |
At the last follow-up, cytogenetic response (CyR) or molecular response (MR) data were available in 78 patients treated for more than 3 months. The best overall MR on treatment was MMR (BCR::ABL1 ≤ 0.1% IS) in 28 (32%) patients and DMR (BCR::ABL1 ≤ 0.01% IS) in 38 (44%) patients. Two patients with a sustained DMR were already observed in TFR, one for 6 years now, while for the other case the TFR duration is not known.
Three of 87 (3%) patients died at 19, 24 and 119 months after completion of pregnancy. One (CML diagnosed in 2013) received IFN in doses up to 9 MU daily from 12 to 30 weeks gestation, no CHR was achieved and IM 400 mg was started at week 31. She achieved a CHR and continued IM after delivery but had no CCyR; no BCR::ABL1 mutations were detected. The patient was switched to dasatinib and then underwent allogenic stem cell transplantation (allo-SCT) complicated by graft failure and died 19 months from diagnosis from transplant complications.
The second patient (diagnosed in 2007) started IM shortly after diagnosis following an elective abortion but was resistant to IM with a T315I/F359V mutation. She progressed to blast crisis and died 24 months from diagnosis of relapse following an allo-SCT, at a time when no effective drugs against T315I were available.
The final patient (diagnosed in 2007) was untreated during pregnancy, started IM after delivery and was switched to nilotinib due to resistance. No kinase domain mutations of BCR::ABL1 were identified and the patient achieved a CCyR as the best overall response. However, this patient was non-compliant to therapy, progressed to blast crisis and died 119 months after diagnosis.
The 2 babies that were born had normal growth and development.
Characteristics of the newborns
Sixty-seven children were born (one set of twins): 38 (56.7%) boys, 28 (41.8%) girls (no information was given for 1 case).
Congenital abnormalities were reported in 2 (3%) newborns. One baby girl had an abdominal cutaneous angioma 5 cm diameter, she was exposed to PEG-IFN during 2nd and 3rd trimester and to HC in 3rd trimester. Another child (a girl, prematurely born at 35th week) had a patent foramen ovale at birth, her mother received IM from week 33.
Data of height at birth were available in 47 newborns. The median height was 51 cm (range 41–57). Data of birth weight were available in 58 newborns: The median weight was 3100 g (range 1700–4330 g). Seven of 58 (12%) newborns with known data had a low birth weight <2500 g of which 1 was born preterm at week 35 and 6 were born at term (≥37 weeks). Two of those children were exposed to IM in late pregnancy (treatment started at weeks 17 and 34), 1 was exposed both to IFN in 2nd trimester and IM started from week 30, 1 was exposed to HC since week 32 and 1 was exposed to PEG-IFN in 2nd and 3rd trimester, while 2 children were born from untreated women (Table 4). A calculation according to the week of gestation revealed those 7 newborns to be “small for gestational age” (SGA) as having a birth weight less than the 10th percentile including 4 newborns with a weight less than 3rd percentile.
Table 4.
Exposure to therapy during pregnancy and outcome in 7 cases with low birth weight (<2500 g) newborns.
| No of case | Therapy during pregnancy by trimester | Outcome | Week of gestation at birth | Gender | Birth weight, g | Percentilea | ||
|---|---|---|---|---|---|---|---|---|
| 1st trimester | 2nd trimester | 3rd trimester | ||||||
| 1 | – | – | – | Birth at term | 38 | Male | 2400 | <5 |
| 2 | – | – | – | Birth at term | 39 | Female | 2400 | <3 |
| 3 | – | PEG-IFN | PEG-IFN | Birth at term | 37 | Male | 2190 | <5 |
| 4 | – | IFN | Imatinib | Birth at term | 37 | Male | 2340 | <10 |
| 5 | – | Imatinib | Imatinib | Birth at term | 40 | Male | 2360 | <3 |
| 6 | – | Imatinib | Imatinib | Preterm birth | 35 | Female | 1700 | <3 |
| 7 | - | - | HC | Birth at term | 41 | female | 2400 | < 3 |
aCalculated according to INTERGROWTH-21st Project tables [32] http://intergrowth21.ndog.ox.ac.uk/.
All low birth weighted babies recovered normal weight and none needed medical intervention. The median follow-up was 36 months (range 8 months to 17 years) and has been uneventful to date.
Outcomes of subsequent pregnancies
Twenty-one of the 87 (24%) patients had 29 subsequent pregnancies. Fifteen patients who delivered live children had 19 pregnancies at a median of 3 years (range 1–7 years) from the 1st pregnancy; 4 pregnancies ended in abortion (2 spontaneous and 2 elective). Six patients who terminated their 1st pregnancy had 10 subsequent pregnancies at a median time of 5 years (range 3–9 years): 7 pregnancies resulted in live births, with 1 elective and 2 spontaneous abortions on one woman.
Discussion
We describe here the largest collection of the management and outcome of patients diagnosed with CML during pregnancy. They represent a sub-group of 400 patients with CML associated pregnancies reported to the ELN Pregnancy Registry with 87 (29%) cases of CML diagnosed during pregnancy. It is not possible to evaluate whether this rate reflects the real-life situation, although hematologists managing these patients may have been motivated to select and report those cases.
Notably, the proportion of the cases resulting in live births in years 2012–2022 increased to 83% compared to 57% in the previous decade (2001–2011), and this can be explained by a number of factors including overall improvement of prognosis in TKI era, publications relating to successful pregnancies and increasing personal experience of the physicians and awareness of the patients. Fortunately, all the reported patients had CP CML at the time of diagnosis and the majority (86%) were either low or intermediate Sokal score. It was not possible to calculate an EUTOS long-term survival (ELTS) score as this was introduced after the start of our collection period [26] and many primary data were missing.
The “watch and wait” approach without any treatment throughout pregnancy was used in 77% of women who carried their babies to term until 2011. However, after 2012, treatment during pregnancy was started in 75% of the reported cases, although it should be noted that all of the drugs were used off-label as the manufacturers warn against use in pregnancy. It is also important to note that we do not have full information concerning blood counts at diagnosis for all the cases and are unable to comment on the precise reasons for treating or not during pregnancy. We accept that there might be a reporting bias for successful pregnancies and some underreporting of miscarriages or abortions.
The potential teratogenic risk of IM during pregnancy was the main concern as there were early reports of congenital malformations predominantly associated with its use in the 1st trimester [7]. Subsequently the data of “safe” IM use in late pregnancy were gradually accumulating [10, 11, 17]. Possible explanations include limited placental crossing of IM ex vivo [12] and in vivo [13, 14, 27, 28] and use of IM after completion of major organogenesis. In our series IM was used exclusively in the 2nd and 3rd trimester, with a median start in the 18th week of gestation, i.e. at a time when major organogenesis was already completed and the placentation process would be established.
The incidence of congenital malformations was 3% in our patient cohort. This value is close to the estimates in the general population which range between 20–55 per 1000 live births (2–5.5%) [29]. One premature child had a patent foramen ovale, the most frequent congenital heart abnormality, which is present in approximately 25% of the adult population [30]. Another newborn in our case series (exposed to PEG-IFN and HC in utero) had a congenital cutaneous angioma which has an incidence of 17 per 10,000 newborns (or 0.17%) [31]. Thus, we did not identify an increased rate of congenital malformations, and moreover, there were no severe or life-threatening birth abnormalities or complications.
Seven (12%) newborns were SGA as they had a fetal size less than the 10th percentile. According to large multinational population-based studies, the rate of low birth weight in the general population ranges from 6 to 11% [32] while the SGA rate is around 10% [33]. Both are rather similar to our data. The possibility of low birth weight in newborns of women who received IM during pregnancy has been reported previously [34]. We were unable to confirm this as there was no consistent association of low birth weight and therapy, but acknowledge our numbers are limited. Interpretation of our results is further confounded by recognized differences in infant sizes in different national populations [35, 36]. SGA only partially overlaps with fetal growth restriction (FGR), a pathologic condition characterized by hypoxia and lack of nutrition [33, 37, 38], which occurs in 3–10% on newborns in the general population and up to 25% of pregnancies in low- and middle-income countries. The level of detail required to assess FGR was not collected in our study. Again, we would like to underline that growth and development of all children were reported to be normal.
More patients received IFN rather than IM, most probably because of the greater confidence in its safety. The variability of IFN dosage reflects the efficacy in controlling blood counts, tolerability and the personal experience of the treating physicians. Unsurprisingly, IM used during pregnancy was more effective than IFN in inducing a CHR. All but one patient (treated for less than one month) achieved CHR on IM compared to only half of the patients on IFN, with a comparable treatment duration. However, it is well known that IFN has a slow kinetics for reducing tumor burden, thus more time is needed compared to IM or HC to normalize counts [39]. Normality of blood counts at the time of delivery may be an important benefit in terms of reducing the risk of perinatal complications such as bleeding or impaired placental blood flow. Twelve of 28 (42%) women who were treated with IFN received its pegylated form; PEG-IFN is better tolerated compared to regular IFN and, with the withdrawal of regular IFN by the manufacturers in many countries, it may be the only formulation available in the future.
Seven women received HC during their pregnancy (Fig. 2). The choice of HC by physicians and combinations of HC and IFN possibly represented the attempts to normalize the extremely high blood counts (hyperleucocytosis, hyperthrombocytosis) in the absence of other treatment options as HC is usually considered hazardous because it crosses the placenta, can interfere with DNA synthesis and cause teratogenic effects [20, 21]. As reported, one child in our series (exposed to HC in 3 trimester and to PEG-IFN in 2nd-3rd trimester) was born with an abdominal cutaneous angioma, however the connection with the IFN and/or HC use in this case is debatable, considering the relative common frequency of this abnormality in general population.
Providing information about the tolerability of treatments during pregnancy was optional in the study and thus could be underreported. However, no unexpected treatment-related AEs were observed. Any interpretation regarding causality is challenging because some complications associated with TKI are also common during pregnancy (e.g. nausea, fluid retention). With the more frequent use of CML treatment options during pregnancy we consider it important to pay more attention to the maternal AEs evaluation in future in order to choose the treatment with the best tolerability.
Follow-up of the patients after pregnancy was performed according to national guidelines The 20-year period of case collection and different access to treatment and monitoring options (i.e. molecular analyses) in different countries, precluded an accurate evaluation of treatment responses. In general, the proportion of patients who underwent a change of their 1st line TKI (28%) seems to be comparable to the expected in a non-pregnant CML population. As has been described in many trials the rates of primary treatment change was from 26.5% to 37.5% for IM and approximately 40% for TKI 2G used in 1st line [40–43]. We acknowledge that we are describing a special cohort with median age 27 years (range 18–41) akin to “adolescents and young adults” (AYA), most frequently defined as between 15–16 and 39 years [44, 45]. Younger patients are known to present with more ‘aggressive’ features, (i.e. larger spleen size, lower rate of early molecular response) [45, 46] but there is no evidence that this adversely affects responses to TKI and/or survival. More recently guidelines have suggested that younger patients may be more suitable for initial therapy with 2GTKI and early switching for suboptimal responses in order to identify candidates for the extremes of referral for stem cell transplantation or consideration of TFR [46].
There were no cases of CML progression during pregnancy although 3 women unfortunately progressed and died during a long-term period of follow-up. Two of those cases represented primary resistance of disease and one patient developed secondary resistance after non-compliance. It is unlikely that any delay to effective treatment because of pregnancy led to clonal evolution and disease progression: one patient received serially IFN and IM during pregnancy and achieved a CHR before the delivery, the other one underwent an elective termination and commenced IM immediately afterwards.
Almost a quarter of women had subsequent pregnancies which confirms the ability to parent children also in established CML, and underlines the advances in our approach to this disease, where quality of life has improved alongside duration of life following the introduction of TKI.
So, what is the best approach: to treat or not to treat in CML diagnosed during pregnancy? There are some disturbing reports of blast crisis developing shortly after delivery when TKI were not given [11, 47] highlighting the importance of a timely treatment start. In contrast, even during the last decade 25% of cases of CML presenting in pregnancy were untreated and none experienced disease progression. In our small series no difference was found between the rates of resistance/suboptimal response to 1st line therapy in patients with and without therapy before delivery, with median treatment delay of 6.5 month. Possibly, certain number of pregnancies diagnosed with CML in 3rd trimester and having no signs of a rapid blood count increase may be observed without any treatment intervention. There are no data to suggest a safe value of leukocytes (a threshold of 100–150 × 109/L was described in cases treated with leucapheresis) or platelets (while a threshold over 1000 × 109 /L was suggested as extreme thrombocytosis in pregnant patients with MPN [48]) indicating immediate treatment intervention. The logical conclusion is that the nature and timing of therapy during pregnancy should be judged individually. Leucapheresis was an acceptable option in previous years, but is not available widely.
Nowadays with the highly effective treatment options for CML and accumulating evidence of the safety of particular TKIs in late pregnancy (i.e. IM and emerging data for nilotinib [13, 15]) and having IFN as an alternative, there is more basis and greater confidence for giving treatment during pregnancy. In a recent publication from an international group, the authors suggested that it was reasonable to adjust therapy according to the competing risks to mother and child [4].
Before the advent of TKIs, IFN was the best available treatment for CML. IFN is able to inhibit cell proliferation by its effect on protein synthesis, RNA degradation and by immune system modulation [49], while it does not inhibits DNA synthesis. Besides, the large molecular weight of IFN (19,000 Dalton) prevents placental crossing, decrease any risk to the fetus [50].
Therapy with IFN, in patients with CP CML, demonstrated a CHR rate of 70% and a cytogenetic response rate of 40% [51]. The limits for IFN use are the relatively slow response and the poor tolerability. However, it is a manageable drug with the possibility to modulate dosage, and newer pegylated formulations require only weekly or biweekly administration with considerably fewer side effects [52]. Furthermore, the use of IFN during pregnancy, including early stages, is an accepted practice not only in hematology but also in other diseases [53]. The dose can be chosen individually considering the optimal disease control and tolerance, starting at 3–9 MU daily for regular IFN, or 45–180 µg weekly of the PEG formulations currently available.
IM is possibly safe in 2nd–3rd trimester considering the completion of organogenesis and its limited placental crossing and there are a number of reports of normal childbirth with IM administered late in pregnancy [5, 10, 11]. Nilotinib, although not used in this case series also has limited placental transfer and there are reports of normal childbirth with nilotinib exposure in 2nd–3rd trimester [4, 13, 15, 17]. The data of the other TKIs use during pregnancy are very limited, and dasatinib use even at late pregnancy has shown serious fetal AEs, including hydrops fetalis and fetal death [8, 18].
The use of HC is not a reasonable option as it has few advantages compared to IM and passes freely through the placenta. In exceptional cases it might be implemented for urgent cytoreduction if leucapheresis is unavailable.
Conclusions and preliminary recommendations
Taking into consideration the accumulated experience we suggest that CML onset in CP during pregnancy in the era of TKIs is a manageable situation with a significant chance of a normal delivery.
According to the data in the ELN CML pregnancy registry, the particular experience of a certain physician/clinic and the access to different drugs have a significant impact on a decision of a treatment start or delay during pregnancy and a treatment choice. An expected time to delivery is an important issue. Obviously, patients with CML onset in 1st trimester may require treatment during pregnancy to benefit both mother and a child. The safest treatment in the 1st trimester is IFN, including PEG-IFN formulations. However, IFN has a reduced chance of achieving a CHR by the time of delivery while IM used in the 2nd–3rd trimester (after 15 gestational weeks) is more effective in inducing CHR while the risk of congenital abnormalities seems to be similar to the general population [4, 10, 13, 27]. Nilotinib, a drug without preclinical teratogenic effects but with less clinical experience, may also be a possibility in late pregnancy [13, 15] in selected cases, particularly when a TKI 2 G is preferable (e.g. in patients with high risk score).
This approach incorporating the strategy of the safe treatment options balanced in accordance with a gestation period takes a step forward to have normal childbirth in women with newly diagnosed CML and further development of the evidence-based recommendations.
Supplementary information
Acknowledgements
The authors express their gratitude to the colleagues who treated and consulted patients within the multidisciplinary team. Our warm gratitude to the patients and their children. On behalf of ELN Pregnancy Registry Committee we express our special gratitude for the contribution to collecting cases for the ELN Pregnancy Registry to Dr. Dong-Wook Kim from Catholic Hematology Hospital, Seoul St. Mary’s Hospital, Seoul, S. Korea, Dr. Konstantin Kotlyarchuk from SI ‘Institute of Blood Pathology and Transfusion Medicine UAMS, Lviv, Ukraine and Dr. Penka Ganeva from Specilaized Hospital for Active Treatment of Hematological Diseases, Sofia, Bulgaria. We are grateful to Sandro Pittori for management of the REDCap database in the international registry. We thank Mr. Christophe Bouvier, datamanager, for data collection in Lyon, France. We thank Yulia Chabaeva and Sergey Kulikov from National Medical Hematology Research Center, Moscow, for the data management and express our special gratitude to «National Hematological Society» for promoting the development of hematology, transfusiology and bone marrow transplantation in Russian Federation. JFA acknowledges the support of the Imperial College NIHR Biomedical Research Centre Our special thanks to Professor Rudiger Hehlmann for many years of inspiring support for ELN international cooperation.
Author contributions
EC and EA were responsible for designing the study protocol, collecting and extracting the data and interpreting results. EC, JA, AT and EA were responsible for preparing the initial report. All the co-authors contributed into data collection and were involved into manuscript preparation.
Funding
The study was supported by European LeukemiaNet.
Data availability
The data supporting the findings of this study are not openly available to preserve individuals privacy under the European General Data Protection Regulation. However, they can be obtained from the corresponding author upon reasonable request and permission.
Competing interests
EC – speaker: Novartis, Pfizer, R-Pharm, consultancy: Ascentage Pharma, JA – speaker: Novartis, Bristol Myers Squibb, Incyte, honoraria: Novartis, Bristol Myers Squibb, Incyte, Research Funding: Pfizer, Incyte, AT – speaker: Novartis, Pfizer, R-Pharm, Amgen, consultancy/advisory board: Novartis, DR – Novartis, Incyte, Pfizer, Terns, FEN – consultancy: Sun Pharma Ltd, Novartis, speaker: Incyte Biosciences, board entity: Incyte Biosciences, Pfizer, Novartis, BMS-Celgene, research fundings: Incyte Biosciences, Novartis, ASA – consultancy: Novartis, JB – honoraria: Novartis, Incyte, Pfizer, MC – advisory board: Insight, Novartis, Italfarmaco, speaker: Servier, Abbvie, Janssen, PF – consultancy: Jazz, Jannsen, Abbvie, MMT – consultancy: Novartis, Takeda, EA – consultancy: BMS, Novaris, Incyte, Pfizer, advisory: BMS, Novaris, Incyte, Pfizer, SAK, MAY, DB, KhK, SS, HFR, RS and EP – nothing to disclose.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/17/2024
A Correction to this paper has been published: 10.1038/s41375-024-02412-6
Supplementary information
The online version contains supplementary material available at 10.1038/s41375-024-02183-0.
References
- 1.Chronic granulocytic leukaemia: comparison of radiotherapy and busulphan therapy. Report of the Medical Research Council’s working party for therapeutic trials in leukaemia. Br Med J. 1968;1:201–8 [DOI] [PMC free article] [PubMed]
- 2.Law AD, Kim DDH, Lipton JH. Pregnancy: part of life in chronic myelogenous leukemia. Leuk Lymphoma. 2017;58:280–7. [DOI] [PubMed] [Google Scholar]
- 3.Robertson HF, Apperley JF. Treatment of CML in pregnancy. Hematol Am Soc Hematol Educ Program. 2022;2022:123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abruzzese E, Mauro M, Apperley J, Chelysheva E. Tyrosine kinase inhibitors and pregnancy in chronic myeloid leukemia: opinion, evidence, and recommendations. Ther Adv Hematol. 2020;11:2040620720966120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chelysheva E, Turkina A. Risks and challenges of CML management during pregnancy: looking for a balanced decision. Eur J Hematol. 2019;102:378–9. [DOI] [PubMed] [Google Scholar]
- 6.Berdman E. Family planning and pregnancy in patients with chronic myeloid leukemia. Curr Hematol Malignancy Rep. 2023;18:1–7. [DOI] [PubMed] [Google Scholar]
- 7.Pye SM, Cortes J, Ault P, Hatfield A, Kantarjian H, Pilot R, et al. The effects of imatinib on pregnancy outcome. Blood. 2008;111:5505–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortes JE, Abruzzese E, Chelysheva E, Guha M, Wallis N, Apperley JF. The impact of dasatinib on pregnancy outcomes. Am J Hematol. 2015;90:1111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abruzzese E, Trawinska MM, de Fabritiis P, Baccarani M. Management of pregnant chronic myeloid leukemia patients. Expert Rev Hematol. 2016;9:781–91. [DOI] [PubMed] [Google Scholar]
- 10.Cole S, Kantarjian H, Ault P, Cortés JE. Successful completion of pregnancy in a patient with chronic myeloid leukemia without active intervention: a case report and review of the literature. Clin Lymphoma Myeloma. 2009;9:324–7. [DOI] [PubMed] [Google Scholar]
- 11.Iqbal J, Ali Z, Khan AU, Aziz Z. Pregnancy outcomes in patients with chronic myeloid leukemia treated with imatinib mesylate: short report from a developing country. Leuk Lymphoma. 2014;55:2109–13. [DOI] [PubMed] [Google Scholar]
- 12.Jovelet C, Seck A, Mir O, Simasotchi C, Broutin S, Goffinet F, et al. Variation in transplacental transfer of tyrosine kinase inhibitors in the human perfused cotyledon model. Ann Oncol. 2015;26:1500–4. [DOI] [PubMed] [Google Scholar]
- 13.Chelysheva E, Turkina A, Polushkina E, Shmakov R, Zeifman A, Aleshin S, et al. Placental transfer of tyrosine kinase inhibitors used for chronic myeloid leukemia treatment. Leuk Lymphoma. 2018;59:733–8. [DOI] [PubMed] [Google Scholar]
- 14.Russell MA, Carpenter MW, Akhtar MS, Lagattuta TF, Egorin MJ. Imatinib mesylate and metabolite concentrations in maternal blood, umbilical cord blood, placenta and breast milk. J Perinatol. 2007;27:241–3. [DOI] [PubMed] [Google Scholar]
- 15.Abruzzese E, Aureli S, Bondanini F, Ciccarone M, Cortis E, Di Paolo A, et al. Chronic myeloid leukemia and pregnancy: when dreams meet reality. State of the art, management and outcome of 41 cases, nilotinib placental transfer. J Clin Med. 2022;11:1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cortes JE, Gambacorti-Passerini C, Deininger M, Abruzzese E, De Annuntis L, Brümmendorf TH. Pregnancy outcomes in patients treated with bosutinib. Int J Hematol Oncol. 2020;9:IJH26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alizadeh H, Jaafar H, Rajnics P, Khan MI, Kajtár B. Outcome of pregnancy in chronic myeloid leukaemia patients treated with tyrosine kinase inhibitors: short report from a single centre. Leuk Res. 2015;39:47–51. [DOI] [PubMed] [Google Scholar]
- 18.Berveiller P, Andreoli A, Mir O, Anselem O, Delezoide AL, Sauvageon H, et al. A dramatic fetal outcome following transplacental transfer of dasatinib. Anticancer Drugs. 2012;23:754–7. [DOI] [PubMed] [Google Scholar]
- 19.Lasica M, Willcox A, Burbury K, Ross DM, Branford S, Butler J, et al. The effect of tyrosine kinase inhibitor interruption and interferon use on pregnancy outcomes and long-term disease control in chronic myeloid leukemia. Leuk Lymphoma. 2019;60:1796–802. [DOI] [PubMed] [Google Scholar]
- 20.Celiloglu M, Altunyurt S, Undar B. Hydroxyurea treatment for chronic myeloid leukemia during pregnancy. Acta Obstet Gynecol Scand. 2000;79:803–4. [PubMed] [Google Scholar]
- 21.Jain D, Atmapoojya P, Colah R, Lodha P. Sickle cell disease and pregnancy. Mediterr J Hematol Infect Dis. 2019;11:e2019040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rohilla M, Rai R, Yanamandra U, Chaudhary N, Malhotra P, Varma N, et al. Obstetric complications and management in chronic myeloid leukemia. Indian J Hematol Blood Transfus. 2016;32:62–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Assi R, Kantarjian H, Keating M, Pemmaraju N, Verstovsek S, Garcia-Manero G, et al. Management of chronic myeloid leukemia during pregnancy among patients treated with a tyrosine kinase inhibitor: a single-Center experience. Leuk Lymphoma. 2021;62:909–17. [DOI] [PubMed] [Google Scholar]
- 25.Claudiani S, Gatenby A, Szydlo R, Nesr G, Abulafia AS, Palanicawandar R, et al. MR4 sustained for 12 months is associated with stable deep molecular responses in chronic myeloid leukemia. Haematologica. 2019;104:2206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfirrmann M, Baccarani M, Saussele S, Guilhot J, Cervantes F, Ossenkoppele G, et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia. 2016;30:48–56. [DOI] [PubMed] [Google Scholar]
- 27.Ali R, Ozkalemkas F, Kimya Y, Koksal N, Ozkocaman V, Gulten T, et al. Imatinib use during pregnancy and breast feeding: a case report and review of the literature. Arch Gynecol Obstet. 2009;280:169–75. [DOI] [PubMed] [Google Scholar]
- 28.Burwick RM, Kuo K, Brewer D, Druker BJ. Maternal, fetal, and neonatal imatinib levels with treatment of chronic myeloid leukemia in pregnancy. Obstet Gynecol. 2017;129:831–4. [DOI] [PubMed] [Google Scholar]
- 29.Egbe A, Uppu S, Lee S, Stroustrup A, Ho D, Srivastava S. Congenital malformations in the newborn population: a population study and analysis of the effect of sex and prematurity. Pediatr Neonatol. 2015;56:25–30. [DOI] [PubMed] [Google Scholar]
- 30.Homma S, Messé SR, Rundek T, Sun YP, Franke J, Davidson K, et al. Patent foramen ovale. Nat Rev Dis Prim. 2016;2:15086. [DOI] [PubMed] [Google Scholar]
- 31.Tripathi R, Mazmudar RS, Knusel KD, Ezaldein HH, Belazarian LT, Bordeaux JS, et al. Impact of congenital cutaneous hemangiomas on newborn care in the United States. Arch Dermatol Res. 2021;313:641–51. [DOI] [PubMed] [Google Scholar]
- 32.Stirnemann J, Villar J, Salomon LJ, Ohuma E, Ruyan P, Altman DG, et al. International estimated fetal weight standards of the INTERGROWTH-21st Project. Ultrasound Obstet Gynecol. 2017;49:478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Damhuis SE, Ganzevoort W, Gordijn SJ. Abnormal fetal growth: small for gestational age, fetal growth restriction, large for gestational age: definitions and epidemiology. Obstet Gynecol Clin N Am. 2021;48:267–79. [DOI] [PubMed] [Google Scholar]
- 34.Al Kindi S, Dennison D, Pathare A. Imatinib in pregnancy. Eur J Haematol. 2005;74:535–7. [DOI] [PubMed] [Google Scholar]
- 35.Barron SL. Birthweight and ethnicity. Br J Obstet Gynaecol. 1983;90:289–90. [DOI] [PubMed] [Google Scholar]
- 36.Urquia ML. Variability in birthweight, birthweight charts, and adverse outcomes: Is the “right size” the right question? Paediatr Perinat Epidemiol. 2019;33:433–5. [DOI] [PubMed] [Google Scholar]
- 37.Nardozza LM, Caetano AC, Zamarian AC, Mazzola JB, Silva CP, Marçal VM, et al. Fetal growth restriction: current knowledge. Arch Gynecol Obstet. 2017;295:1061–77. [DOI] [PubMed] [Google Scholar]
- 38.Romo A, Carceller R, Tobajas J. Intrauterine growth retardation (IUGR): epidemiology and etiology. Pediatr Endocrinol Rev. 2009;6:332–6. [PubMed] [Google Scholar]
- 39.Hehlmann R, Heimpel H, Hasford J, Kolb HJ, Pralle H, Hossfeld DK, et al. Randomized comparison of interferon-alpha with busulfan and hydroxyurea in chronic myelogenous leukemia. The German CML Study Group. Blood. 1994;84:4064–77. [PubMed] [Google Scholar]
- 40.Hehlmann R, Lauseker M, Saussele S, Pfirrmann M, Krause S, Kolb HJ, et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia. 2017;31:2398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim DW, Issaragrisil S, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boque C, et al. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naive chronic myeloid leukemia patients trial. J Clin Oncol. 2016;34:2333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. EuropeanLeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wood WA, Lee SJ. Malignant hematologic diseases in adolescents and young adults. Blood. 2011;117:5803–15. [DOI] [PubMed] [Google Scholar]
- 45.Pemmaraju N, Cortes J. Chronic myeloid leukemia in adolescents and young adults: patient characteristics, outcomes and review of the literature. Acta Haematol. 2014;132:298–306. [DOI] [PubMed] [Google Scholar]
- 46.Kalmanti L, Saussele S, Lauseker M, Proetel U, Müller MC, Hanfstein B, et al. Younger patients with chronic myeloid leukemia do well in spite of poor prognostic indicators: results from the randomized CML study IV. Ann Hematol. 2014;93:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Staley EM, Simmons SC, Feldman AZ, Lorenz RG, Marques MB, Williams LA 3rd, et al. Management of chronic myeloid leukemia in the setting of pregnancy: when is leukocytapheresis appropriate? A case report and review of the literature. Transfusion. 2018;58:456–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gangat N, Tefferi A. Myeloproliferative neoplasms and pregnancy: Overview and practice recommendations. Am J Hematol. 2021;96:354–66. [DOI] [PubMed] [Google Scholar]
- 49.Talpaz M, Mercer J, Hehlmann R. The interferon-alpha revival in CML. Ann Hematol. 2015;94:S195–207. [DOI] [PubMed] [Google Scholar]
- 50.Mubarak AA, Kakil IR, Awidi A, Al-Homsi U, Fawzi Z, Kelta M, et al. Normal outcome of pregnancy in chronic myeloid leukemia treated with interferon-alpha in 1st trimester: report of 3 cases and review of the literature. Am J Hematol. 2002;69:115–8. [DOI] [PubMed] [Google Scholar]
- 51.Kantarjian HM, Deisseroth A, Kurzrock R, Estrov Z, Talpaz M. Chronic myelogenous leukemia: a concise update. Blood. 1993;82:691–703. [PubMed] [Google Scholar]
- 52.Quinta´s-Cardama A, Kantarjian HM, Giles F, Verstovsek S. Pegylated interferon therapy for patients with Philadelphia chromosome-negative myeloproliferative disorders. Thromb Hemost. 2006;32:409–16. [DOI] [PubMed] [Google Scholar]
- 53.Varytė G, Arlauskienė A, Ramašauskaitė D. Pregnancy and multiple sclerosis: an update. Curr Opin Obstet Gynecol. 2021;33:378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are not openly available to preserve individuals privacy under the European General Data Protection Regulation. However, they can be obtained from the corresponding author upon reasonable request and permission.