Abstract
The phenomenon of cuckoos’ brood parasitism is well known and can be investigated using applied mathematical techniques. Among adaptive features of this phenomenon are certain egg parameters that ensure their shortened incubation period (I) and thus the successful survival of their offspring. In particular, the volume of a cuckoo egg is not less than, or exceeds, that of the host species, which should, in theory, increase I. Also, cuckoo eggs have thicker shell than that of nest hosts. Here, we analyzed the available geometric dimensions of eggs in 447 species and found an inverse correlation (−0.585, p < 0.05) between I and the shell thickness-to-egg surface area ratio (T/S). A mathematical relationship was derived to calculate I depending on T/S. This premise was confirmed by comparative calculations using egg images of two parasitic species, common (Cuculus canorus) and plaintive cuckoo (Cacomantis merulinus) and their hosts: great reed warbler (Acrocephalus arundinaceus), European robin (Erithacus rubecula), rufescent prinia (Prinia rufescens), and common tailorbird (Orthotomus sutorius). An average calculated I value for cuckoo eggs was one day less than that for host eggs. Our findings unravel additional details of how cuckoos adapt to brood parasitism and specific host-parasite relationships.
Keywords: Avian eggs, Cuckoo's brood parasitism, Egg incubation period, Shell thickness, Egg surface area and volume, Metabolic rate, Host-parasite relationships
Graphical abstract
Highlights
-
•
We assumed that cuckoo eggs have shorter incubation time (I) due to thicker shells.
-
•
To test this, we analyzed published data for 454 eggs from 447 bird species.
-
•
We found that I can be determined by shell thickness-to surface area ratio (T/S).
-
•
Mother cuckoo seems to be adapted to increasing the T/S ratio by increasing T.
-
•
We derived empirical formulae for calculating I based on S/V and T/S ratios.
1. Introduction
The brood parasitism of cuckoos is a well-described concept present beyond the peer-reviewed zoological literature (e.g., Wang et al., 2016; Pradeep et al., 2016). “Cuckoo in the nest” is a commonly used idiom in popular culture, universally taken to mean an unwanted intruder in any given situation or location. By disguising their eggs as those of other birds, cuckoos devolve the efforts of parental care, i.e. hatching, feeding and raising of their offspring, to parents of other species. Moreover, hatched cuckoo chicks either eject eggs and/or chicks of the real (natural) progenies of their adoptive parents from the nest, or else make them starve by aggressively competing for food resources (Honza et al., 2007; Anderson et al., 2009; Schulze-Hagen et al., 2009; Moksnes et al., 2013; Canestrari et al., 2014). In turn, host species often evolve to recognize abandoned cuckoo eggs and thus can neutralize the worst of the parasitic effects (Davies and Brooke, 1988; Grim, 2008; Antonov et al., 2008; Feeney et al., 2014). Indeed, some host species are so successful in such identification that they eject almost 100% of parasitic cuckoo eggs (e.g., Yang et al., 2022). As part of the evolutionary “arms race”, the mother cuckoo does her best to make her eggs as similar as possible to those of the host species (Brooke and Davies, 1988; Stoddard and Stevens, 2010, 2011; see also Fig. 1).
Fig. 1.
A mimicking egg of the common cuckoo nominate subspecies (Cuculus canorus canorus; left) in a clutch of Marmora's warbler (Curruca sarda; right). Image source: https://commons.wikimedia.org/wiki/File:Cuculus_canorus_canorus_MHNT.ZOO.2010.11.150.36.jpg; collection of Jacques Perrin de Brichambaut, Muséum de Toulouse, France; by Ercé, CC-BY-SA-4.0.
It is nonetheless evolutionarily advantageous for the cuckoo egg to be slightly larger in size than that of its host (Fig. 1), instantly providing a weight advantage for the cuckoo hatchling (Alvarez, 1994, 2000; Moksnes and Røskaft, 1995; Krüger and Davies, 2004). At the same time, it is also evolutionarily advantageous to be incubated faster than its egg neighbors in the nest. Given that there is a direct relationship between the incubation time and the egg weight (W) or size (Rahn and Ar, 1974; Ar and Rahn, 1978; Deeming et al., 2006) and thus a larger egg should spend more time till the hatch, a paradox exists. That is, it is both evolutionarily advantageous and disadvantageous for cuckoos to have larger eggs than that of their hosts. In nature cuckoo chicks do indeed hatch ahead of their nestmates (Gill, 1980; Briskie and Sealy, 1990; Strausberger, 1998; Birkhead et al., 2011; Igic et al., 2015; Cao et al., 2018). Many studies have been devoted to uncovering the causes for this phenomenon, with its most studied and popular prerequisite being accelerated cuckoo chick embryo development in an already formed but not yet laid egg, i.e. located in the mother cuckoo's body (Liversidge, 1961; Perrins, 1967; Birkhead et al., 2011). Applied mathematical modelling approaches have also been used to explore the brood parasitism phenomenon (e.g., Wang et al., 2016; Pradeep et al., 2016).
Such an adaptive feature of brood parasitism in cuckoos, i.e., the egg incubation beginning while still in the mother's body, is crucial for reducing I in the host nest; however, it is not always feasible in practice. Indeed, the cuckoo has to adapt to the egg's adoptive parents, synchronizing the laying of its eggs with those already in the nest (e.g., Moskàt et al., 2006). Such a synchronization requires the cuckoo to make a prompt decision to lay an egg in an “emergency”, which does not always include a sufficiently long incubation of the egg inside the mother's body.
In this respect, other (alternative) prerequisites should be considered to explain the possible reasons for the reduction in I for eggs of brood parasite species. One possibility in this regard involves adaptive changes in the structure of the yolk, as it is the main source of vital substances, including energy reserves (Török et al., 2004; Igic et al., 2015; Cao et al., 2018). Geltsch et al. (2016) provided evidence that the explanation may be relatively simple in that the majority of cuckoo eggs are laid before host incubation begins; however, this may only be a contributory factor, among several others.
In studies to assess the differences between the eggs of brood parasites and their hosts, many researchers have noted that the shell thickness (T) of the former is somewhat greater than that of the latter (Spaw and Rohwer, 1987; Brooker and Brooker, 1991; Antonov et al., 2006; Pujol and Mermoz, 2011; Igic et al., 2011, 2017; Holleley et al., 2022). Moreover, such an excess in T is observed even when the eggs of both species are of the same size. It would seem, given the fierce struggle for the survival of parasitic species, the most likely hypothesis of a thicker shell of parasite eggs is protection from damage to such an egg by the hosts trying to break, or at least puncture, it (Spaw and Rohwer, 1987; López et al., 2023) leading to proposition of the ‘puncture resistance hypothesis’ (e.g., Holleley et al., 2022). A number of authors have, however, suggested that, to a greater extent, the thicker shells of brood parasites are associated not with protection from damage to the eggs by the beak of the nest owners, but in order to reduce risk of damage to the eggs “when eggs are dropped into nests” (Holleley et al., 2022), or “to protect the parasite's egg from damage if the nest is multiply parasitized” (Brooker and Brooker, 1991). Igic et al. (2017) suggested that a thicker shell enables developing embryos to consume more calcium and other minerals contained in its structure. However, as a result of the research, this version was rejected due to the fact that the decalcification of the shell in cuckoo eggs was no different from the eggs of the hosts.
Another hypothesis was put forward by Ian Wyllie (1981) suggesting that, either before or during incubation, a cuckoo's thick eggshell may help to prevent heat loss, which could hasten the development of the embryo. Yang et al. (2018) agreed with Wyllie's assumption by stating that “the unusually thick-shelled eggs laid by parasitic cuckoos retain more heat for the developing embryo and thus facilitate early hatching.” To test this supposition, Yang et al. (2018) measured shell temperature during incubation of host and cuckoos' eggs. As a result, the authors confirmed that the shell temperature of cuckoo eggs was higher than that of host eggs.
Confirmation of this hypothesis was also found in studies conducted on poultry eggs. For example, Lourens et al. (2007) demonstrated that higher shell temperatures in chicken eggs decreased hatch time. In a study conducted by Yamak et al. (2016) when incubating eggs of chukar partridge (Alectoris chukar), the authors, although not finding significant differences in I, noted, however, that “thin-shelled eggs had a relatively longer hatching period than medium- and thick-shelled eggs." Undoubtedly, this premise requires a more thorough analysis by involving in research as many eggs of different species as possible.
In a series of our previous studies (Narushin et al., 2024a,b,c), we demonstrated that I of eggs in various bird species is associated not only with their W or volume (V), but also with the ratio of different geometric parameters. For example, this can be the egg surface area-to-volume ratio (S/V), the value of which can conditionally characterize the metabolism of the developing embryo. It is unlikely that the S/V value can be used in relation to the shortened I of cuckoo eggs. Most often, these eggs either correspond to, or exceed, the S and V values of the host eggs. According to our results (Narushin et al., 2024b), the larger the egg size, the lower the S/V value and, therefore, the longer I. It is possible that other parameters, especially the relationships between these indicators, also influence the period of incubation development.
Considering the promising direction of research into the possible effect of T on I, a more thorough study of this relationship, taking into account other egg characteristics, could be of special interest. In particular, this can include the ratio of T with other egg parameters. Many works have shown sufficient effectiveness of this relationship. For instance, the ratios of T2/W (Juang et al., 2017) or T/R (where R is the egg curvature radius) or some mathematically transformed set of geometric dimensions (Macleod et al., 2006; Ma et al., 2008; Zhang et al., 2017) can characterize the shell strength traits.
The objective of this study, therefore, was to assess the relationship between the duration of incubation of bird eggs depending on the ratio of their morphological parameters. This was followed by substantiation of their possible effect on the shortened development time of the cuckoo embryo and/or other parasitic species.
2. Material and methods
The experimental work was carried out in two stages. Initially, we assessed the possibility of predicting the value of I depending on the geometric and/or physical egg parameters of wild bird species. Methodologically, work on measuring parameters such as shell thickness (T) and its weight (Ws) requires destructive approaches, which is unacceptable in view of the existing wildlife protection regulations. In this regard, we decided to use published data, with the most extensive database of oomorphological information being contained in the reference book by Schönwetter et al. (1960–1992). Particularly important is the fact that Schönwetter et al. (1960–1992), in addition to oomorphological parameters, also placed many images of bird eggs. This enabled to carry out the necessary geometric measurements of the required parameters as follows: the egg's length (L), maximum breadth (B), diameter (Dp) at the point where the pointed end is L/4 away from the egg's center, and the distance w that the B axis is moved from the egg's center to the point where the egg is L/2 away (Narushin et al., 2021, 2023). The measured values allowed us to calculate the volume (V) and surface area (S) of the eggs using the formulae from Narushin et al. (2024d):
| (1) |
| (2) |
The procedure for measuring images of bird eggs was described in detail by us in the results of our previous studies (Narushin et al., 2024a,b). Briefly, the egg image was measured in pixels using an electronic ruler in Microsoft Office Picture Manager. The pixel measurements were then converted to cm according to the metric egg length data given in the tables of Schönwetter et al. (1960–1992).
In addition to geometric dimensions, data from Schönwetter et al. (1960–1992) on egg weight (W), shell thickness (T) and shell weight (Ws) were used in the present analysis.
Information on I values was gathered from publicly accessible ornithological websites located online (e.g., Avibase 2003; Celebrate Urban Birds 2016; Animal Diversity Web 2020; Project FeederWatch 2021; eBird, 2023; Macaulay Library 2023; Bird Academy 2024; Birds of the World 2024; Great Backyard Bird Count 2024; NestWatch 2024).
Schönwetter included pictures of 434 eggs from 433 bird species in his oological reference book (1960–1992). The relatively narrow egg weight (W) range of eggs with available images—from 1 to 100 g—was a limitation of Schönwetter's investigation, despite the vast diversity of data he was able to gather. We were missing data on birds laying eggs with larger W values, which would have allowed for a more thorough examination. In these cases, we relied on photos of these eggs that we retrieved from other sources, such as the digitized collection of images of bird eggs from the Natural History Collections of the Museum Wiesbaden (Wikimedia Commons, 2014), while using the numerical values of these eggs from the reference book by Schönwetter et al. (1960–1992). This resulted in 454 eggs altogether, representing 447 bird species, 95 families, and 13 orders.
Correlation analysis made it possible to evaluate the most significant relationships between the I value depending on combinations of T and other parameters of avian eggs. The data that showed the closest correlation were approximated by formulae for calculating I.
The task of the second research stage was to practically test the equations for calculating I obtained in the first stage. For these purposes, in the available scientific publications, we selected photographs of cuckoo eggs along with host eggs, allowing us to measure their geometric parameters. To conduct a comparative analysis, we used images of eggs of the following parasitic species and their hosts from the respective sources.
-
1.
Common cuckoo (Cuculus canorus) eggs were compared with great reed warbler (Acrocephalus arundinaceus) eggs depicted by Moskàt et al. (2009, 2012) and Bán et al. (2011).
-
2.
Common cuckoo (Cuculus canorus) eggs were compared with European robin (Erithacus rubecula) eggs as reported by Bán et al. (2011).
-
3.
Plaintive cuckoo (Cacomantis merulinus) eggs were compared with rufescent prinia (Prinia rufescens) eggs presented by Liang et al. (2017) and Yang et al. (2021).
-
4.
Plaintive cuckoo (Cacomantis merulinus) eggs were compared with those of the common tailorbird (Orthotomus sutorius) reported by Yang et al. (2021).
To convert pixels into cm, we used the reference of the host egg to its real size, presented either by the authors of the respective publication, or, in the absence of such data, in the handbook by Schönwetter et al. (1960–1992) or in another source. Information about T of both types of eggs was taken from the same sources. This approach made it possible to maintain the proportions between specific eggs of the cuckoo and the hosts when converting them into metric measurement systems.
A number of statistical and mathematical procedures, which can be found in the STATISTICA 5.5 program (StatSoft, Inc./TIBCO, Palo Alto, CA, USA) and applications for the Microsoft Excel program, were utilized to process the data. Here, the Pearson correlation coefficient (R) and regression models employing the coefficient of determination (R2) were used to evaluate the validity of the found associations, with significance being confirmed at the p < 0.05 level.
3. Results and discussion
3.1. Effects of T on I of bird eggs
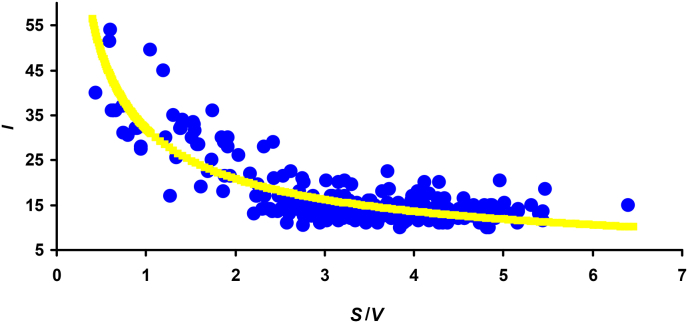
When performing correlation analysis of various egg traits, our main attention was focused on the relationship between egg parameters that indirectly characterize certain physiological, biological and/or physical processes that influence embryonic development. As expected, the strongest relationship was observed between the incubation period (I) and the S/V ratio, which indirectly characterizes the metabolism level of the developing embryo (Narushin et al. 2024a,b). Despite the fact that the present research used a different database of egg images from the reference book by Schönwetter et al. (1960–1992) than that from Museum Wiesbaden images (Wikimedia Commons, 2014) in the previous study (Narushin et al., 2024b), the nature of the relationship between I and S/V (Fig. 2) and the resultant calculation formula echoed the outcome produced by Narushin et al. (2024b).
Fig. 2.
Visualization of data approximation of the relationship between the incubation period value (I) and the egg surface area-to-volume ratio (S/V).
In particular, according to Narushin et al. (2024b) who used the Museum Wiesbaden images (Wikimedia Commons, 2014):
| (3) |
with R2 = 0.725 (p < 0.05),
where I is measured in days, S in cm2, and V in cm3.
The current investigation based on the egg images from the reference book by Schönwetter et al. (1960–1992) resulted in the following similar mathematical dependence (shown as a yellow line in Fig. 2):
| (4) |
with R2 = 0.726 (p < 0.05).
To create a single mathematical calculation algorithm, we decided to combine both above equations (Eqns (3), (4))). As a result, a universal dependence was obtained, the practical use of which did not affect the decrease in the accuracy of the calculations, both current and previous (Narushin et al., 2024b) data:
| (5) |
with R2 = 0.726 (p < 0.05).
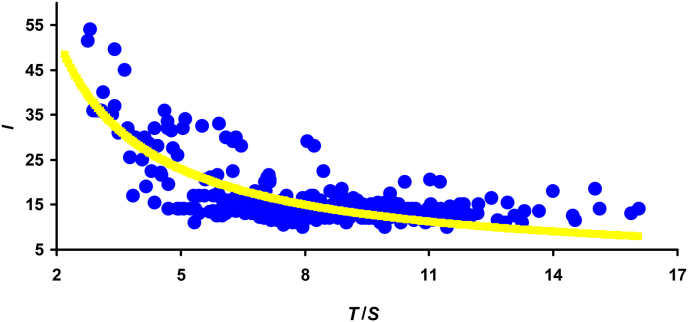
Furthermore, we paid the closest attention to the analysis of ratios containing T and revealed their inverse relationship with the value of I, i.e., reducing I when increasing T. Among these, we selected the ratios of T/S (R = −0.585, p < 0.05) and T/V (R = −0.565, p < 0.05) and the geometric mean between the main dimensional characteristics of the egg, i.e., T/(SV)0.5 (R = −0.579, p < 0.05). The highest correlation was noted between I and T/S (Fig. 3), based on of which the following calculation formula was derived:
| (6) |
Fig. 3.
Visualization of data approximation of the relationship between the incubation period value (I) and the shell thickness-to-egg surface area ratio (T/S) according to Eqn (6).
with R2 = 0.602 (p < 0.05),
where I is measured in days, S in cm2, and T in μm.
Considering the hypothesis suggested by Yang et al. (2018) to explain the reasons for thicker shells in eggs of cuckoos and/or other parasitic species, i.e., as a way to keep more heat inside the egg, it can be assumed that the T/S index characterizes the physical feature of the egg in retaining heat along its outer surface.
3.2. Comparative analysis of the parameters of cuckoo eggs and their hosts
Using the T values taken from Schönwetter et al. (1960–1992) and after averaging the results presented there, this parameter equaled 0.098 and 0.08 mm for the shells of two parasitic species, common cuckoo (Cuculus canorus) plaintive cuckoo (Cacomantis merulinus), respectively. Among four host species, it was equal to 0.082 mm in the great reed warbler (Acrocephalus arundinaceus), being almost completely consistent with the values given by Picman and Honza (2020); 0.08 mm in the European robin (Erithacus rubecula); 0.063 mm in the rufescent prinia (Prinia rufescens); and 0.0563 mm in the common tailorbird (Orthotomus sutorius). The results of the calculation of the averaged main parameters pertaining to the compared species, their eggs and incubation time are given in Table 1.
Table 1.
Values of the main egg parameters and their incubation periods for four pairwise cuckoo–host species comparisons.
| Parameters | Cuckoo | Host |
|---|---|---|
| Comparison 1 | Common cuckoo (Cuculus canorus) n = 21 | Great reed warbler (Acrocephalus arundinaceus) n = 21 |
| Egg volume, V (cm3) | 3.3 | 3.0 |
| Egg surface area, S (cm2) | 10.9 | 10.3 |
| S/V (cm2/cm3) | 3.3a | 3.5a |
| T/S (μm/cm2) | 8.9a | 8.0a |
| Estimated incubation period according to Eqn (6), I (days) | 13.6a | 15.0a |
| Standard average incubation period for host eggs according to Planet of Birds (2011) |
14 |
|
| Comparison 2 | Common cuckoo (Cuculus canorus) n = 1 | European robin (Erithacus rubecula) n = 1 |
| Egg volume, V (cm3) | 2.4 | 2.3 |
| Egg surface area, S (cm2) | 8.8 | 8.5 |
| S/V (cm2/cm3) | 3.7 | 3.7 |
| T/S (μm/cm2) | 10.2 | 9.4 |
| Estimated incubation period according to Eqn (6), I (days) | 12.0 | 12.9 |
| Standard average incubation period for host eggs according to Bouglouan (2024) |
13 |
|
| Comparison 3 | Plaintive cuckoo (Cacomantis merulinus) n = 4 | Rufescent prinia (Prinia rufescens) n = 8 |
| Egg volume, V (cm3) | 1.7a | 1.3a |
| Egg surface area, S (cm2) | 7.0a | 5.8a |
| S/V (cm2/cm3) | 4.1a | 4.6a |
| T/S (μm/cm2) | 11.4 | 10.9 |
| Estimated incubation period according to Eqn (6), I (days) | 10.8 | 11.3 |
| Standard average incubation period for host eggs according to Krishnan (2021) |
12 |
|
| Comparison 4 | Plaintive cuckoo (Cacomantis merulinus) n = 2 | Common tailorbird (Orthotomus sutorius) n = 2 |
| Egg volume, V (cm3) | 1.6 | 1.1 |
| Egg surface area, S (cm2) | 6.7 | 5.3 |
| S/V (cm2/cm3) | 4.3 | 4.8 |
| T/S (μm/cm2) | 12.0 | 10.7 |
| Estimated incubation period according to Eqn (6), I (days) | 10.3 | 11.5 |
| Standard average incubation period for host eggs according to Chan (2012) | 12 |
n is the quantity of egg images taken for the analysis.
Significance of pairwise parameter comparisons (p < 0.05); the values without superscript index are insignificant.
The limited sampling of available images of cuckoo eggs and hosts that would allow for a full comparative analysis prevented us from unambiguously judging the significance of the differences between a number of parameters and their relationships. However, the following general trends can be observed for all parasite–host pairs of the species considered.
-
1.
The average value of V, although in some cases not by much, still exceeded that of the hosts.
-
2.
The S/V ratio in cuckoo eggs was lower than that in host eggs, which, according to our previous studies (Narushin et al., 2024b), should lead to an increase in incubation time in comparison with host eggs.
-
3.
The T/S ratio of cuckoo eggs was greater than that of hosts, despite the fact that the S value was greater than that of hosts. That is, this effect occurred due to the thicker shell of cuckoo eggs.
-
4.
The calculated value of I for cuckoo eggs was approximately 0.5–1.5 days less than that for host eggs.
Thus, there seems to be a certain fine line in the mother cuckoo's ability to form the “correct” egg, from the viewpoint of nest parasitism. A skew in the egg's characteristics, either in one direction or the other, is undesirable, as it calls into question the survival of her offspring. It is inconceivable that the cuckoo subjects its actions to complex mathematical calculations and analyses that help her form an egg with clearly defined parameters suitable for a specific host nest. However, we do believe that most likely her reproductive behavior is instinctively “guided” by the evolutionarily fixed experience of many past generations adapted to nest parasitism. Since we do not have such an ability to judge this directly, herein are our efforts to follow a similar analytical path using strict mathematical logic.
3.3. Evolutionary adaptation or a clear mathematical calculation?
In addition to achieving similarity in the shell pigmentation with the eggs of the owners of the nest, the mother cuckoo faces another dilemma: how to shape the egg in such a way that it is the same size or slightly larger in size than that of the hosts. This thereby provides the cuckoo with an evolutionary advantage in nestling weight after hatching, and, at the same time, reduces the period of its incubation. Considering this problem from a mathematical point of view, we have the value of I, i.e., the standard incubation time of eggs, depending on their size, or rather, on the S/V ratio, which indirectly characterizes embryonic metabolism (Narushin et al., 2024b). The calculation of the standard value of I can be done according to formula (5). For the convenience of further analysis, we will slightly transform Eqn (5), expressing the value of S via V. Undoubtedly, the accuracy of the calculation will be somewhat reduced, however, this fact will not affect the reliability of consequent mathematical logic.
In our previous work (Narushin et al., 2024d), we derived a universal relationship between S and V that is characteristic of an egg of any shape found in nature:
| (7) |
where B is the egg's breadth, L is its length, w is the distance that the B axis is moved away from the egg's center to the point L/2, and Dp is diameter at the point where the pointed end is L/4 away.
Simplifying Eqn (7) and substituting the following average values of the respective coefficients: B/L = 0.736; w/L = 0.05; Dp/B = 0.794, obtained as a result of our measurements of bird egg images. Then, Eqn (7) will take the following form:
| (8) |
Taking into account the resultant formula (8), Eqn (5) is transformed into the following:
| (9) |
where I is measured in days, and V in cm3.
In a similar way, we transform another dependence to predict the I value, according to formula (6):
| (10) |
where T is measured in μm.
Conventionally, Eqns (9), (10)) reflect the standard dependence of I relevant to the size (V) and T of a specific egg, in particular, within the framework of our conditions, the host egg.
Now considering the following “endeavor” of the mother cuckoo.
-
(i)
Assume that she wants to reduce the value of I by at least 1 day.
-
(ii)
In this case, the size (volume) of her egg should be greater than the volume of the host egg (V). Let us express this condition in such a way that the volume of a cuckoo egg is equal to KVV, where KV is a certain coefficient whose value is greater than 1.
-
(iii)
The only way for the cuckoo to achieve the above conditions is to increase the shell thickness in comparison with the shell thickness (T) of the host eggs. Again, this condition can be written mathematically as the product of T by a certain coefficient KT, the value of which is also greater than 1.
Mathematically, the cuckoo's “endeavor” to shorten I can be expressed by the following relationship based on Eqn (10):
| (11) |
The difference and some mathematical transformations of formulae (10) and (11) allows us to obtain the relationship between the coefficients KT and KV:
| (12) |
Again, for simplicity of analyzing formula (12), we express the T value in terms of V using the calculation data for egg images from the oological reference book by Schönwetter et al. (1960–1992):
| (13) |
with R2 = 0.970 (p < 0.05),
where T is measured in μm, and V in cm3.
Substituting Eqn (13) into formula (12), we obtain:
| (14) |
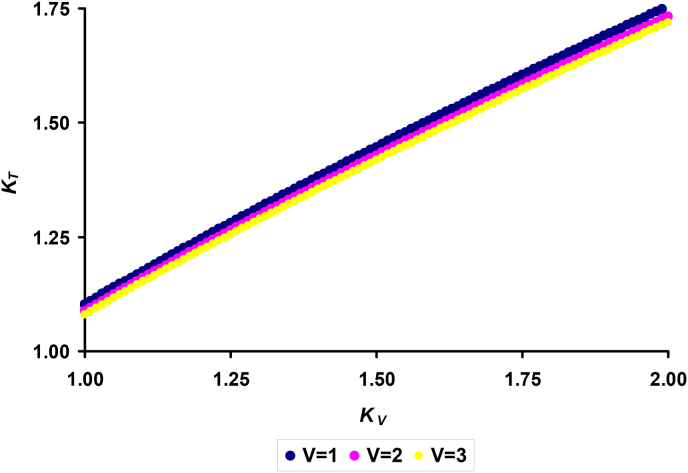
Then, dependence (14) can be presented graphically as shown in Fig. 4 where, for example, we chose three options for V: 1, 2, and 3 cm3.
Fig. 4.
Visualization of the mathematical relationship between the coefficients KT and KV depending on the volume of host eggs: V = 1, 2 and 3 cm3.
Despite some possible errors in the dependence of the coefficients KT and KV caused by the assumptions we made in the prediction calculations of the values S (Eqn (8)), I (Eqn (10)) and T (Eqn (13)), it can be unequivocally stated that in order to shorten the incubation time I, the cuckoo needs to lay eggs with thicker shells. In our example, a reduction in the I value by 1 day, even when laying an egg with the same V value as that of the hosts of the nest, requires an increase in T by 8–10% depending on the size of the egg (V). At the same time, the analysis of Eqn (14) and Fig. 4 suggests that changing V by 2- or even 3-fold did not significantly change the numerical values of KT relative to the values of KV,. All three lines of functional relationship practically coincide (Fig. 4). In this case, the coefficient of T increase (KT) is approximately proportional to the coefficient of V increase (KV) to the power of 2/3. This relationship warrants that the cuckoo nestling hatching 1 day earlier than the chick (or chicks) of the nest owners.
Considering that our comparative calculations of the I value for cuckoo eggs and their hosts (section “Comparative analysis of the parameters of cuckoo eggs and their hosts”) demonstrated an average 1 day difference in I, we can test the adequacy of the derived dependence (14) for the coefficients KT on KV. Taking, for example, the data on the calculation for eggs of the common cuckoo (Cuculus canorus) and its host, the great reed warbler (Acrocephalus arundinaceus), due to the greater representativeness of this sample, numbering 21 eggs in each species. The average V value of great reed warbler eggs, according to our measurements, was 3.0 cm3, and that of the common cuckoo was 3.3 cm3, or 1.1 times more, i.e., the value of KV = 1.1. The T value for the great reed warbler was taken to be 82 μm, and that for the common cuckoo 96 μm (Schönwetter et al., 1960–1992). Thus, the KT value was 1.17. Recalculation of the theoretical value of KT according to Eqn (14) gave a similar result KT = 1.15. Such ratios of parameters led to the fact that the estimated incubation time of common cuckoo eggs was 13.6 days, while that of great reed warbler was 15 days.
3.4. Calculation of I for avian eggs
Thus, based on both previous (Narushin et al., 2024a,b) and current studies, it can be argued that the duration of incubation of bird eggs depends on two indices expressed by the ratio S/V and T/S. Our natural instinct was to combine the results of current and previous calculations, proposing a single, most adequate dependence that enables to predict the I value most accurately. As a result of approximation of the obtained measurements and/or calculations of the values of V, S and T, we derived the following relationship:
| (15) |
with R2 = 0.727 (p < 0.05),
in which I is measured in days, S in cm2, V in cm3, and T in μm.
The results of the present research and subsequent theoretical analysis suggested that T can have a significant impact on the duration of incubation. To a greater extent, the value of I is determined not even by T, but by the T/S ratio. The higher the value of this ratio, the less time the bird spends incubating future chicks. The T/S index seems to have multiple effects on the bird's egg. In addition to the effect on I, T/S indirectly expresses the strength properties of the shell, whereas many researchers prefer to use in this ratio a complex of geometric dimensions of the egg instead of just the S value (Macleod et al., 2006; Ma et al., 2008; Zhang et al., 2017). The mother cuckoo seems to have adapted to skillfully use the T/S indicator with maximum efficiency, increasing its value in her eggs. A stronger shell prevents mechanical damage to the egg when laid in another nest (Holleley et al., 2022), or in case of possible aggression from the hosts (Spaw and Rohwer, 1987; López et al., 2023). At the same time, early hatching makes it possible for the cuckoo nestling to eliminate competition from the host chicks by force. Namely, in view of the use of force by the cuckoo nestling to neutralize competitors, the mother cuckoo is possibly inclined to increase the T/S ratio not at the expense of reducing S, which affects the size of the egg, but by increasing T. After all, to use a force ejection action, the cuckoo chick should be no smaller than, and, if possible, somewhat larger in size than other host nestlings.
As part of our measurements and further calculations (Table 1), we discovered that I for eggs of two cuckoo species, common cuckoo (Cuculus canorus) and plaintive cuckoo (Cacomantis merulinus), was 0.5–1.5 days less than that for eggs of four hosts: great reed warbler (Acrocephalus arundinaceus), European robin (Erithacus rubecula), rufescent prinia (Prinia rufescens), and common tailorbird (Orthotomus sutorius).
4. Conclusions
Collectively, the following suggestions can be drawn from the results of our research. Firstly, the T/S index, reflecting the ratio of eggshell thickness to its surface area, is an indirect indicator of the duration of incubation of bird eggs. At the same time, an increase in this indicator leads to a decrease in hatching time. Secondly, based on geometric measurements of egg images from 447 species and information on the value of T from the oological reference book by Schönwetter et al. (1960–1992), we derived an empirical relationship that enabled to calculate the value of I (Eqn (6)). Thirdly, through a comparative analysis of cuckoo eggs and their hosts, we confirmed the hypothesis that I of cuckoo eggs is reduced due to the greater T/S value. Most likely, the adaptive ability of cuckoos somehow to adjust this indicator is only one on the list of “tricks” used by cuckoos to hatch their offspring earlier. Fourthly, considering the presence of a few indicators based on the parameters of a bird's egg to predict the I value, we proposed an empirical calculated dependence of I on the ratios S/V and T/S (Eqn (15)). Our findings provide more insight into the ways in which cuckoos adapt to specific brood parasitism and host-parasite relationships.
Ethical aspects
Importantly, due to the currently existing protection and research ethics restrictions aimed to prevent human impact on wildlife, we did not directly examine the nests and eggs of wild avian species in the natural conditions. Instead, we used the previously published data available in the ornithological literature and web resources.
Funding
The authors declare that there is no funding associated with the work featured in this article.
Data and materials availability
All data are available in the main text.
CRediT authorship contribution statement
Valeriy G. Narushin: Writing – review & editing, Writing – original draft, Visualization, Project administration, Methodology, Investigation, Conceptualization. Michael N. Romanov: Writing – review & editing, Writing – original draft, Visualization, Project administration. Nili Avni-Magen: Writing – review & editing. Darren K. Griffin: Writing – review & editing, Visualization, Validation, Supervision.
Declaration of competing interest
Authors declare that they have no competing interests.
Acknowledgments
The skilled technical assistance of Olga M. Romanova in preparing images for the Graphical Abstract is kindly appreciated.
Contributor Information
Valeriy G. Narushin, Email: val@vitamarket.com.ua.
Michael N. Romanov, Email: m.romanov@kent.ac.uk.
Nili Avni-Magen, Email: niliavnimagen@gmail.com.
Darren K. Griffin, Email: D.K.Griffin@kent.ac.uk.
References
- Alvarez F. A gens of cuckoo Cuculus canorus parasitizing rufous bush chat Cercotrichas galactotes. J. Avian Biol. 1994;25:239–243. doi: 10.2307/3677081. [DOI] [Google Scholar]
- Alvarez F. Response to common cuckoo Cuculus canorus model egg size by a parasitized population of rufous bush chat Cercotrichas galactotes. Ibis. 2000;142:683–686. doi: 10.1111/j.1474-919X.2000.tb04470.x. [DOI] [Google Scholar]
- Anderson M.G., et al. Egg eviction imposes a recoverable cost of virulence in chicks of a brood parasite. PLoS One. 2009;4 doi: 10.1371/journal.pone.0007725. pmid: 19907639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Animal Diversity Web . Regents of the University of Michigan; Ann Arbor, MI, USA: 2020. https://animaldiversity.org/ [Google Scholar]
- Antonov A., et al. Eggshell strength of an obligate brood parasite: a test of the puncture resistance hypothesis. Behav. Ecol. Sociobiol. 2006;60:11–18. doi: 10.1007/s00265-005-0132-6. [DOI] [Google Scholar]
- Antonov A., Stokke B.G., Moksnes A., Røskaft E. Getting rid of the cuckoo Cuculus canorus egg: why do hosts delay rejection? Behav. Ecol. 2008;19:100–107. doi: 10.1093/beheco/arm102. [DOI] [Google Scholar]
- Ar A., Rahn H. In: Part of Proceedings in Life Sciences. Piiper J., editor. Springer; 1978. “Interdependence of gas conductance, incubation length, and weight of the avian egg” in Respiratory Function in Birds, Adult and Embryonic; pp. 227–236. [DOI] [Google Scholar]
- Avibase Denis Lepage. ON; Canada: 2003. Data Science and Technology, Birds Canada, Port Rowan.https://avibase.bsc-eoc.org/ [Google Scholar]
- Bán M., et al. The analysis of common cuckoo's egg shape in relation to its hosts' in two geographically distant areas. J. Zool. 2011;284:77–83. doi: 10.1111/j.1469-7998.2011.00795.x. [DOI] [Google Scholar]
- Bird Academy . Cornell University; Ithaca, NY, USA: 2024. The Cornell Lab of Ornithology.https://academy.allaboutbirds.org/ [Google Scholar]
- Birds of the World . Cornell University; Ithaca, NY, USA: 2024. The Cornell Lab of Ornithology.http://birdsoftheworld.org/ [Google Scholar]
- Birkhead T.R., et al. Internal incubation and early hatching in brood parasitic birds. Proc. Biol. Sci. 2011;278:1019–1024.. doi: 10.1098/rspb.2010.1504. pmid: 20880882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouglouan N. Oiseaux-Birds; 2024. European Robin Erithacus rubecula.https://www.oiseaux-birds.com/card-european-robin.html [Google Scholar]
- Briskie J.V., Sealy S.G. Evolution of short incubation periods in the parasitic Cowbirds, Molothrus spp. Auk. 1990;107:789–794. doi: 10.2307/4088016. [DOI] [Google Scholar]
- Brooke M. de L., Davies N.B. Egg mimicry by cuckoos Cuculus canorus in relation to discrimination by hosts. Nature. 1988;335:630–632. doi: 10.1038/335630a0. [DOI] [Google Scholar]
- Brooker M.G., Brooker L.C. Eggshell strength in cuckoos and cowbirds. Ibis. 1991;133:406–413. doi: 10.1111/j.1474-919X.1991.tb04589.x. [DOI] [Google Scholar]
- Canestrari D., et al. From parasitism to mutualism: unexpected interactions between a cuckoo and its host. Science. 2014;343:1350–1352.. doi: 10.1126/science.1249008. pmid: 24653032. [DOI] [PubMed] [Google Scholar]
- Cao P., Sun B.-J., Wang L.-W., Liang W., Du W.-G. Proximate mechanisms of earlier hatching in parasitic cuckoos: yolk energy and embryonic metabolism. Biol. J. Linn. Soc. 2018;123:63–71. doi: 10.1093/biolinnean/blx136. [DOI] [Google Scholar]
- Celebrate Urban Birds . Cornell University; Ithaca, NY, USA: 2016. The Cornell Lab of Ornithology.https://celebrateurbanbirds.org/ [Google Scholar]
- Chan S. Tailors at work. NParks Buzz. 2012;1(12) https://www.nparks.gov.sg/nparksbuzz/issue-12-vol-1-2012/conservation/tailors-at-work [Google Scholar]
- Davies N.B., Brooke M. de L. Cuckoos versus reed warblers: adaptations and counteradaptations. Anim. Behav. 1988;6:262–284. doi: 10.1016/S0003-3472(88)80269-0. [DOI] [Google Scholar]
- Deeming D.C., Birchard G.F., Crafer R., Eady P.E. Egg mass and incubation period allometry in birds and reptiles: effects of phylogeny. J. Zool. 2006;270:209–218. doi: 10.1111/j.1469-7998.2006.00131.x. [DOI] [Google Scholar]
- eBird . The Cornell Lab of Ornithology. Cornell University; Ithaca, NY, USA: 2023. https://ebird.org/ [Google Scholar]
- Feeney W.E., Welbergen J.A., Langmore N.E. Advances in the study of coevolution between avian brood parasites and their hosts. Annu. Rev. Ecol. Evol. Syst. 2014;45:227–246. doi: 10.1146/annurev-ecolsys-120213-091603. [DOI] [Google Scholar]
- Geltsch N., Bán M., Hauber M.E., Moskát C. When should Common Cuckoos Cuculus canorus lay their eggs in host nests? Hous. Theor. Soc. 2016;63:46–51. doi: 10.1080/00063657.2015.1125851. [DOI] [Google Scholar]
- Gill B.J. PhD Thesis. University of Canterbury; Christchurch, New Zealand.: 1980. Breeding of the Grey Warbler with Special Reference to Brood-Parasitism by the Shining Cuckoo.https://core.ac.uk/download/pdf/35467437.pdf [Google Scholar]
- Great Backyard Bird Count . Cornell University; Ithaca, NY, USA: 2024. The Cornell Lab of Ornithology.https://www.birdcount.org/ [Google Scholar]
- Grim T. The evolution of nestling discrimination by hosts of parasitic birds: why is rejection so rare? Evol. Ecol. Res. 2008;8:785–802. https://www.evolutionary-ecology.com/abstracts/v08/2034.html [Google Scholar]
- Holleley C.E., Grieve A.C., Grealy A., Medina I., Langmore N.E. Thicker eggshells are not predicted by host egg ejection behaviour in four species of Australian cuckoo. Sci. Rep. 2022;12:6320.. doi: 10.1038/s41598-022-09872-9. pmid: 35428801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honza M., Voslajerová K., Moskát C. Eviction behaviour of the common cuckoo Cuculus canorus chicks. J. Avian Biol. 2007;38:385–389. doi: 10.1111/j.2007.0908-8857.03901.x. [DOI] [Google Scholar]
- Igic B., et al. Alternative mechanisms of increased eggshell hardness of avian brood parasites relative to host species. J. R. Soc. Interface. 2011;8:1654–1664.. doi: 10.1098/rsif.2011.0207. pmid: 21561966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igic B., et al. A comparison of egg yolk lipid constituents between parasitic Common Cuckoos and their hosts. Auk. 2015;132:817–825. doi: 10.1642/AUK-15-14.1. [DOI] [Google Scholar]
- Igic B., et al. Brood parasite and host eggshells undergo similar levels of decalcification during embryonic development. J. Zool. 2017;301:165–173. doi: 10.1111/jzo.12408. [DOI] [Google Scholar]
- Juang J.Y., Chen P.Y., Yang D.C., Wu S.-P., Yen A. The avian egg exhibits general allometric invariances in mechanical design. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-14552-0. pmid: 29079743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A. Prinias of Karnataka. JLR Explore. 2021 https://jlrexplore.com/explore/focus/prinias-of-karnataka [Google Scholar]
- Krüger O., Davies N.B. The evolution of egg size in the brood parasitic cuckoos. Behav. Ecol. 2004;15:210–218. doi: 10.1093/beheco/arg104. [DOI] [Google Scholar]
- Liang W., Yang C., Takasu F. How can distinct egg polymorphism be maintained in the rufescent prinia (Prinia rufescens)–plaintive cuckoo (Cacomantis merulinus) interaction—a modeling approach. Ecol. Evol. 2017;7:5613–5620.. doi: 10.1002/ece3.3090. pmid: 28808541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Library Macaulay. Cornell University; Ithaca, NY, USA: 2023. The Cornell Lab of Ornithology.https://www.macaulaylibrary.org/ [Google Scholar]
- Liversidge R. Pre-incubation development of Clamator jacobinus. Ibis. 1961;103:624. doi: 10.1111/j.1474-919X.1961.tb02466.x. [DOI] [Google Scholar]
- López A.V., et al. Avian obligate brood parasitic lineages evolved variable complex polycrystalline structures to build tougher eggshells. iScience. 2023;26 doi: 10.1016/j.isci.2023.108552. pmid: 38144448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourens A., van den Brand H., Heetkamp M.J., Meijerhof R., Kemp B. Effects of eggshell temperature and oxygen concentration on embryo growth and metabolism during incubation. Poultry Sci. 2007;86:2194–2199.. doi: 10.1093/ps/86.10.2194. pmid: 17878449. [DOI] [PubMed] [Google Scholar]
- Ma Y.Q., Wang C.M., Ang K.K., Xiang Y. Buckling of super ellipsoidal shells under uniform pressure. IES J. A Civ. Struct. Eng. 2008;1:218–225. doi: 10.1080/19373260801928150. [DOI] [Google Scholar]
- Macleod N., Bain M.M., Hancock J.W. The mechanics and mechanisms of failure of hens' eggs. Int. J. Fract. 2006;142:29–41. doi: 10.1007/s10704-006-9018-5. [DOI] [Google Scholar]
- Moksnes A., Røskaft E. Egg-morphs and host preference in the common cuckoo (Cuculus canorus): an analysis of cuckoo and host eggs from European museum collections. J. Zool. 1995;236:625–648. doi: 10.1111/j.1469-7998.1995.tb02736.x. [DOI] [Google Scholar]
- Moksnes A., Fossøy F., Røskaft E., Stokke B.G. Reviewing 30 years of studies on the Common Cuckoo: accumulated knowledge and future perspectives. Avian Res. 2013;(4):3–14. doi: 10.5122/cbirds.2013.0001. [DOI] [Google Scholar]
- Moskàt C., et al. Increased host tolerance of multiple cuckoo eggs leads to higher fledging success of the brood parasite. Anim. Behav. 2009;77:1281–1290. doi: 10.1016/j.anbehav.2009.01.030. [DOI] [Google Scholar]
- Moskàt C., et al. Cuckoo parasitism on two closely-related Acrocephalus warblers in distant areas: a case of parallel coevolution? Avian Res. 2012;3:320–329. doi: 10.5122/cbirds.2012.0038. [DOI] [Google Scholar]
- Moskàt C., Barta Z., Hauber M.E., Honza M. High synchrony of egg laying in common cuckoos (Cuculus canorus) and their great reed warbler (Acrocephalus arundinaceus) hosts. Ethol. Ecol. Evol. 2006;18:159–167. doi: 10.1080/08927014.2006.9522720. [DOI] [Google Scholar]
- Narushin V.G., Romanov M.N., Griffin D.K. Egg and math: introducing a universal formula for egg shape. Ann. N. Y. Acad. Sci. 2021;1505:169–177.. doi: 10.1111/nyas.14680. pmid: 34426991. [DOI] [PubMed] [Google Scholar]
- Narushin V.G., Orszulik S.T., Romanov M.N., Griffin D.K. A novel approach to egg and math: improved geometrical standardization of any avian egg profile. Ann. N. Y. Acad. Sci. 2023;1529:61–71.. doi: 10.1111/nyas.15059. pmid: 37642389. [DOI] [PubMed] [Google Scholar]
- Narushin V.G., Romanov M.N., Griffin D.K. Pear-shaped eggs evolved to maximize surface area-to-volume ratio, increase metabolism and shorten incubation time in birds. Integr. Zool. 2024 submitted. [Google Scholar]
- Narushin V.G., Romanov M.N., Avni-Magen N., Griffin D.K. Avian egg incubation period: revisiting existing allometric relationships. Sci. Rep. 2024 submitted for publication. [Google Scholar]
- Narushin V.G., Romanov M.N., Avni-Magen N., Griffin D.K. Accurate calculation of the content volume, density and original weight of museum curated eggs. Sci. Rep. 2024 submitted for publication. [Google Scholar]
- Narushin V.G., et al. Reimagining Archimedes: An innovative and accurate calculation of volumes and asserting another standard method for defining the surface area of quail and any avian eggs. Food Bioprod. Process. 2024;147:327–334. doi: 10.1016/j.fbp.2024.07.013. [DOI] [Google Scholar]
- NestWatch . Cornell University; Ithaca, NY, USA: 2024. The Cornell Lab of Ornithology.https://nestwatch.org/ [Google Scholar]
- Perrins C.M. The short apparent incubation period of the cuckoo. Br. Birds. 1967;60:51–52. [Google Scholar]
- Picman J., Honza M. How strong are eggs of the common cuckoo Cuculus canorus? J. Vertebr. Biol. 2020;70:20109. doi: 10.25225/jvb.20109. 1. [DOI] [Google Scholar]
- Planet of Birds Great reed-warbler (Acrocephalus arundinaceus) 2011. https://planetofbirds.com/passeriformes-acrocephalidae-great-reed-warbler-acrocephalus-arundinaceus
- Pradeep B.S.A., Ma W., Jiang Z. Mathematical analysis of the effect of cuckoo bird's incubation period in population dynamics. Appl. Math. Model. 2016;40:10167–10180. doi: 10.1016/j.apm.2016.06.048. [DOI] [Google Scholar]
- Project FeederWatch . Cornell University; Ithaca, NY, USA: 2021. The Cornell Lab of Ornithology.https://feederwatch.org/ [Google Scholar]
- Pujol E.M., Mermoz M.E. Do life-history traits in the ancestor of Cowbirds (Molothrus spp.) predispose them to become brood parasites? Ornitol. Neotropica (LA Plata) 2011;22:553–568. https://bibliotecadigital.exactas.uba.ar/download/paper/paper_10754377_v22_n4_p553_ManuelaPujol.pdf [Google Scholar]
- Rahn H., Ar A. The avian egg: incubation time and water loss. Condor. 1974;76:147–152. doi: 10.2307/1366724. [DOI] [Google Scholar]
- Schönwetter M., Handbuch der Oologie, Meise W. (Eds.) Vol. 1–4. Akademie Verlag; 1960. https://core.ac.uk/download/pdf/9319774.pdf [Google Scholar]
- Schulze-Hagen K., Stokke B.G., Birkhead T.R. Reproductive biology of the European Cuckoo Cuculus canorus: early insights, persistent errors and the acquisition of knowledge. J. Ornithol. 2009;150:1–16. doi: 10.1007/s10336-008-0340-8. [DOI] [Google Scholar]
- Spaw C.D., Rohwer S. A comparative study of eggshell thickness in cowbirds and other passerines. Condor. 1987;89:307–318. doi: 10.2307/1368483. [DOI] [Google Scholar]
- Stoddard M.C., Stevens M. Pattern mimicry of host eggs by the common cuckoo, as seen through a bird’s eye. Proc. R. Soc. B Biol. Sci. 2010;277:1387–1393.. doi: 10.1098/rspb.2009.2018. pmid: 20053650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard M.C., Stevens M. Avian vision and the evolution of egg color mimicry in the common cuckoo. Evolution. 2011;65:2004–2013.. doi: 10.1111/j.1558-5646.2011.01262.x. pmid: 21729055. [DOI] [PubMed] [Google Scholar]
- Strausberger B.M. Temperature, egg mass, and incubation time: a comparison of Brown-headed Cowbirds and Red-winged Blackbirds. Auk. 1998;115:843–850. doi: 10.2307/4089503. [DOI] [Google Scholar]
- Török J., Moskát C., Michl G., Péczely P. Common cuckoos (Cuculus canorus) lay eggs with larger yolk but not more testosterone than their great reed warbler (Acrocephalus arundinaceus) hosts. Ethol. Ecol. Evol. 2004;16:271–277. doi: 10.1080/08927014.2004.9522638. [DOI] [Google Scholar]
- Wang Y. Mutualisms in a parasitism–predation system consisting of crows, cuckoos and cats. Appl. Math. Model. 2016;40:9656–9674. doi: 10.1016/j.apm.2016.03.032. [DOI] [Google Scholar]
- Wikimedia Commons . Category: Eggs of the Natural History Collections of the Museum Wiesbaden. 2014. https://commons.wikimedia.org/wiki/Category:Eggs_of_the_Natural_History_Collections_of_the_Museum_Wiesbaden [Google Scholar]
- Wyllie I. Batsford); 1981. The Cuckoo (B. T.https://archive.org/embed/cuckoo0000wyll [Google Scholar]
- Yamak U.S., Sarica M., Boz M.A., Ucar A. The effect of eggshell thickness on hatching traits of partridges. Braz. J. Poult. Sci. 2016;18(spe):13–18. doi: 10.1590/1806-9061-2015-0039. [DOI] [Google Scholar]
- Yang C., et al. Keeping eggs warm: thermal and developmental advantages for parasitic cuckoos of laying unusually thick-shelled eggs. Sci. Nat. 2018;105:10.. doi: 10.1007/s00114-017-1532-y. pmid: 29294204. [DOI] [PubMed] [Google Scholar]
- Yang C., Wang L., Møller A.P., Liang W. Egg polymorphism and highly sensitive egg recognition of cross-phenotypes in rufescent prinias Prinia rufescens as effective defenses against brood parasitism. Integr. Zool. 2021;16:280–285.. doi: 10.1111/1749-4877.12474. pmid: 32644219. [DOI] [PubMed] [Google Scholar]
- Yang C., Chen X., Wang L., Liang W. Defensive adaptations to cuckoo parasitism in the black-browed reed warbler (Acrocephalus bistrigiceps): recognition and mechanism. Anim. Cognit. 2022;25:1299–1306.. doi: 10.1007/s10071-022-01613-9. pmid: 35320446. [DOI] [PubMed] [Google Scholar]
- Zhang J., Wang M., Wang W., Tang W. Buckling of egg-shaped shells subjected to external pressure. Thin-Walled Struct. 2017;113:122–128. doi: 10.1016/j.tws.2017.01.017. [DOI] [Google Scholar]