Abstract
DNA polymerase α-primase (pol-prim), a complex consisting of four subunits, is the major species-specific factor for mouse polyomavirus (PyV) and simian virus 40 (SV40) DNA replication. Although p48 is the most conserved subunit of pol-prim, it is required for in vitro PyV DNA replication but can inhibit cell-free SV40 DNA replication. Production of chimeric human-mouse p48 revealed that different regions of p48 are involved in supporting PyV DNA replication and inhibiting SV40 DNA replication. The N and C-terminal parts of p48 do not have species-specific functions in cell-free PyV DNA replication, but the central part (amino acids [aa] 129 to 320) controls PyV DNA replication in vitro. However, PyV T antigen physically binds to mouse, human, and chimeric pol-prim complexes independently, whether they support PyV DNA replication or not. In contrast to the PyV system, the inhibitory effects of mouse p48 on SV40 DNA replication are mediated by N- and C-terminal regions of p48. Thus, a chimeric p48 containing human aa 1 to 128, mouse aa 129 to 320, and human aa 321 to 418 is active in both PyV and SV40 DNA replication in vitro.
Papovaviruses are small DNA tumor viruses (47). For their DNA replication these viruses contribute a viral origin of replication (ori) and the large tumor antigens (Tag) of mouse polyomavirus (PyV) and simian virus 40 (SV40) or the E1 and E2 proteins of papillomaviruses; all other replication factors are supplied by the host (6, 42, 47, 49). Therefore, papovaviral DNA replication in vivo and in vitro has served as a model system to study virus-host interactions and the mechanisms of DNA replication in mammalian cells (37, 47, 49).
These studies allowed the development of a model for eukaryotic DNA replication which relies on unwinding of double-stranded (ds) DNA and stepwise assembly of multiprotein complexes mediated by the viral initiator of DNA synthesis, large Tag. After Tag has recognized and formed a double hexamer at the replication origin, specific distortions of the dsDNA occur (10, 25). The following steps of DNA replication require the specific recruitment of host replication proteins, such as DNA polymerase α-primase (pol-prim), eukaryotic single-stranded (ss) DNA-binding protein, replication protein A (RPA), and topoisomerase I (topo I), to the origin of replication (7, 11, 12, 14, 26, 27, 39, 40, 41). The interaction of Tag with pol-prim stimulates ori binding by Tag (29). Melting and unwinding of ori dsDNA by Tag's helicase activity requires stabilization of the ssDNA by RPA. The melting of ori DNA by Tag also needs the relaxation of supercoiled DNA by topo I. The first RNA primer is then synthesized by the primase activity of pol-prim. The preinitiation and initiation steps of viral DNA synthesis at the origin of DNA replication require, in addition to the DNA-binding and enzymatic activities of the replication factors, specific physical contacts between these protein complexes (11, 48, 50, 51). The newly synthesized RNA is elongated by the DNA polymerase activity of pol-prim. After a replication factor C(RF-C)-catalyzed DNA polymerase switch to a leading strand synthesis complex consisting of proliferating cell nuclear antigen and DNA polymerase δ, this enzyme complex carries out processive DNA synthesis (5, 19, 49). On the lagging strand initiation of the Okazaki fragment, DNA synthesis is also performed by pol-prim in cooperation with Tag and RPA (11, 26, 30, 50, 51). These RNA-DNA primers are then elongated by DNA polymerase δ or ɛ (5, 19, 49). This model, which originated from work with cell-free systems of polyomaviruses and papillomaviruses, most likely also illustrates basic mechanisms of chromosomal DNA replication (19).
Despite their similarities, PyV and SV40 show a significant difference in their DNA replication: PyV propagates only in mouse cells, whereas SV40 multiplies only in primate cells. Mouse pol-prim is the major species-specific factor for PyV DNA replication, whereas human pol-prim mediates host-specific replication of SV40 DNA (4, 13, 28, 31, 44). Each of the four pol-prim subunits directly interacts with Tag (8, 12, 50), and these interactions play key roles in the permissiveness of the host cells: PyV Tag requires mouse p48 for its function (4, 13, 28), whereas SV40 Tag depends on the primate p180 subunit (21, 22, 31, 44).
Of the pol-prim subunits, the p48 polypeptide is the most highly conserved one between human and mouse (91% amino acid identity [43]), yet this polypeptide is the major species-specific factor for PyV DNA replication. Therefore, sequences of human and mouse p48 cDNA were exchanged, and chimeric human-mouse p48 cDNAs were expressed using baculovirus vectors. These polypeptides were coexpressed with the p180, p68, and p58 subunits of pol-prim and purified as enzyme complexes. The chimeric pol-prim complexes were used to determine the parts of the mouse p48 subunit responsible for species-specific functions in PyV DNA replication.
MATERIALS AND METHODS
Production of the mutant p48 proteins.
To obtain the chimeric human-mouse p48 subunits (see Fig. 1A), cDNAs were produced using the conserved restriction sites for BanII and NdeI to exchange the 5′ and 3′ ends, respectively. The constructs h(ml-320) and h(ml29-418) were transferred into the vectors pVL1392 and pVL1393, respectively. The cDNA containing the central part from mouse but human 5′ and 3′ ends was obtained by digesting pVL1392-h(m129-418) and pVL1393-h(m1-320) with PflMI and with BglII or BamHI. These DNAs were ligated to construct pVL1392-h(m129-320). After in vivo recombination baculoviruses expressing recombinant proteins were created using Baculo-Gold DNA and standard procedures (Pharmingen, Hamburg, Germany) (35).
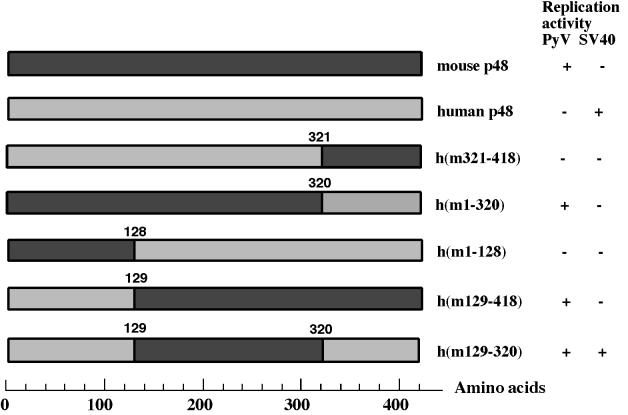
FIG. 1.
Construction of chimeric human-murine p48 subunits. Chimeric p48s containing human and murine sequences were named according to their murine amino acids. The remaining amino acids are from human origin. The ability of these hybrid p48 polypeptides to support PyV and SV40 DNA replication is summarized on the right.
Proteins.
PyV Tag, SV40 Tag, and the pol-prim complex (p180-p68-p58-p48) were purified from baculovirus-infected insect cells as described previously (4, 43, 44). RPA was bacterially expressed and purified as outlined before (18, 34, 45). Human topo I expressed in yeast and purified as described by Lisby et al. (24) was a generous gift of M. Lisby, University of Århus, Denmark. Monoclonal antibodies SJK237-71, SJK287-38 (46), F5 (36), and PAb101 (15, 16) specific for PyV and SV40 Tag were purified by affinity chromatography (17).
Protein manipulations.
Protein concentration was determined according to the method of Bradford (3) using a commercial reagent with bovine immunoglobulin G as a standard (Bio-Rad, Munich, Germany). Sodium dodecyl sulfate (SDS) gel electrophoresis was carried out as described previously (20), with 10-kDa ladders (Life Technologies) as molecular mass markers. After polyacrylamide gel electrophoresis, proteins were detected by silver staining according to the method of Nasheuer and Grosse (32).
DNA synthesis on ΦX174 ssDNA.
The DNA replication of ΦX174 ssDNA was carried out in a reaction containing 66 ng of ΦX174 ssDNA (New England Biolabs) (33), 20 mM Tris-acetate (pH 7.3), 5 mM magnesium acetate, 20 mM potassium acetate, 1 mM dithiothreitol, 0.1 mg of bovine serum albumin (BSA) per ml, 1 mM ATP, 0.1 mM each of CTP, GTP, UTP, dATP, dCTP, dGTP, TTP, and 0.1 mM [α-32P]dCTP (100 cpm/pmol) (Amersham Pharmacia Biotech, Freiburg, Germany). Comparisons between different pol-prim preparations were made by adding 0.2 U of primase per assay. The incorporation of dCMP was determined by acid precipitation of DNA and scintillation counting.
Preparation of S100 extracts and replication of PyV in vitro.
S100 extracts were prepared from logarithmically growing human 293S or mouse FM3A cells as previously described (43, 44). Cells were harvested by centrifugation and then washed twice with phosphate-buffered saline (PBS) and once with hypotonic buffer. The cells were resuspended in hypotonic buffer, incubated for 10 min on ice, and broken by 12 strokes in a Dounce homogenizer. The extracts were centrifuged at 4°C and 11,000 × g. The supernatant was then adjusted to 100 mM NaCl and clarified by a second centrifugation at 100,000 × g (S100 extract). Depletion of pol-prim from S100 extracts was performed essentially as previously described (43, 44).
The replication of PyV DNA in vitro was performed as previously described (4, 44). Briefly, the assay contained 1.2 μg of PyV Tag, 250 ng of pUC-Py1 DNA (PyV origin DNA [39]), and 200 μg of S100 or depleted S100 extract in 30 mM HEPES-NaOH (pH 7.8), 1 mM dithiothreitol, 7 mM magnesium acetate, 1 mM EGTA (pH 7.8), 4 mM ATP, 0.3 mM each of CTP, GTP, and UTP, 0.1 mM each of dATP and dGTP, 0.05 mM each of dCTP and dTTP, 40 mM creatine phosphate, 80 μg of creatine kinase per ml, and 5 μCi each of [α32P]dCTP and [α32P]dTTP (3,000Ci/mmol) (Amersham Pharmacia Biotech). The cell-free SV40 DNA replication contained 0.6 μg of SV40 Tag and 250 ng of pUC-HS (39). Pol-prim was added as indicated. The incorporation of radioactive deoxynucleoside monophosphates (dNMPs) was measured by acid precipitation of DNA and scintillation counting. The total radioactivity was measured after spotting 5 μl of a 200-fold dilution of the replication assay onto GF52 filters (Schleicher & Schüll, Dassel, Germany).
Initiation of replication on PyV and SV40 DNA.
Initiation reactions were performed essentially as previously described (4, 39, 44). Briefly, the PyV initiation assay (40 μl) was assembled on ice and contained 0.25 μg of pUC-Pyl DNA (PyV origin DNA), 1.6 μg of PyV Tag, and 1 μg of RPA in 30 mM HEPES-KOH (pH 7.8), 7 mM magnesium acetate, 1 mM EGTA, 1 mM dithiothreitol, 0.2 mM UTP, 0.2 mM GTP, 0.01 mM CTP, 4 mM ATP, 40 mM creatine phosphate, 1 μg of creatine kinase, 0.3 μg of topo I, 0.25 mg of heat-treated BSA per ml, and 20 μCi of [α-32P]CTP (3,000 Ci/mmol) (Amersham Pharmacia Biotech). Recombinant pol-prim was added as indicated in the figure legends. SV40 initiation reactions (40 μl) were carried out as described above but contained 0.25 μg of pUC-HS DNA, 0.6 μg of SV40 Tag, and 0.5 μg of RPA. After incubation for 1 h at 37°C, one-eighth of the reaction mixture was used to estimate the amount of incorporated nucleotides by spotting it onto DE81 paper (38). The reaction products were precipitated with 0.8 M LiCl, 10 mM MgCl2, 10 μg of sonicated salmon sperm DNA (Sigma), and 120 μl of ethanol for 1 h on dry ice, washed twice with 75% ethanol-water, dried, redissolved in 45% formamide–5 mM EDTA–0.05% xylene cyanol FF–0.05% bromphenol blue at 65°C for 30 min, heated for 3 min at 95°C, and electrophoresed in denaturing 20% polyacrylamide gels for 3 to 4 h at 600 V as described previously (4, 39). The reaction products were visualized by autoradiography.
ELISA.
Modified enzyme-linked immunosorbent assays (ELISAs) were carried out as described previously (39). Briefly, pol-prim complexes or BSA (1 μg in 50 μl of PBS) was immobilized for 1 h at room temperature (RT). After washing three times with PBS, blocking solution (5% BSA [Fraction V; Sigma] in PBS) was incubated for 1 h at RT. The blocking solution was removed by washing three times with PBS. Then PyV Tag (1 μg in 50 μl of PBS) was incubated for 1 h at RT in binding buffer (PBS supplemented with 8 mM MgCl2 and 4 mM adenylylimidodiphosphate [AMP-PNP]) and removed by washing three times with PBS. Bound Tag was recognized by monoclonal antibody F5 (5 μg in 50 μl of PBS for 1 h at RT) and a secondary horseradish peroxidase-conjugated antibody (Dianova, Hamburg, Germany). The ELISA was developed with the horseradish peroxidase substrate kit according to the supplier's instructions (Bio-Rad).
RESULTS
Production of pol-prim containing chimeric human-murine p48 primase.
The murine p48 subunit controls the replication of PyV DNA in vitro. Human p180 determines the species specificity of SV40 DNA replication in vitro, whereas mouse p48 poisons the initiation of DNA replication of this primate virus in vitro (4, 44). These different activities raise the question of whether the same amino acids and activities of mouse p48 are involved in the regulation of DNA replication of both polyomaviruses. To address this question, regions of murine and human p48 were exchanged and chimeric proteins were produced using baculovirus vectors (Fig. 1). The chimeric p48 subunits of pol-prim are named according to their murine amino acids. Thus, h(ml-128) contains the murine amino acids (aa) 1 to 128, with the remaining amino acids being encoded by the human cDNA, whereas h(m321-418) consists of the C-terminal aa 321 to 418 from mouse and the human aa 1 to 320. These proteins were then coexpressed with human or mouse p180, p68, and p58 subunits (HHH and MMM, respectively, in complex designations). The recombinant proteins assembled into protein complexes which contained all four subunits and could be purified to near homogeneity by phosphocellulose and immunoaffinity chromatography (Fig. 2). All purified enzyme complexes had DNA polymerase and primase activity. The specific DNA polymerase and primase activities of the protein complexes varied from 1,300 to 15,600 U/mg and from 170 to 2,280 U/mg, respectively (Table 1).
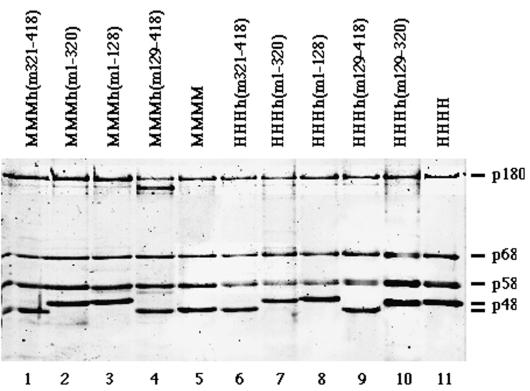
FIG. 2.
Production of pol-prim complexes containing a chimeric p48 subunit. One microgram of each purified pol-prim complex (pol-prim with murine p180, p68, p58, and chimeric p48 [lanes 1 to 4], murine pol-prim [lane 5], pol-prim with human p180, p68, p58, and chimeric p48 [lanes 6 to 10], and human pol-prim [lane 11]) was analyzed by SDS gel electrophoresis and stained with silver.
TABLE 1.
Enzyme activities of recombinant pol-prim complexes
| Enzyme complex | Protein concn (mg/ml) | Sp act of DNA polymerase (U/mg) | Sp act of primase (U/mg) |
|---|---|---|---|
| MMMM | 0.23 | 3,560 | 260 |
| MMMh(m321-418) | 2.8 | 1,270 | 305 |
| MMMh(ml-320) | 1.3 | 2,340 | 492 |
| MMMh(ml-128) | 0.4 | 6,900 | 1,900 |
| MMMh(ml29-418) | 0.89 | 3,370 | 820 |
| HHHh(m321-418) | 0.23 | 10,800 | 1,080 |
| HHHh(m1-128) | 0.52 | 5,000 | 750 |
| HHHh(m1-320) | 0.14 | 15,600 | 2,280 |
| HHHh(m129-418) | 0.34 | 7,350 | 764 |
| HHHh(m129-320) | 0.45 | 3,130 | 170 |
| HHHH | 0.52 | 4,923 | 960 |
SDS gel electrophoresis of these immunoaffinity-purified chimeric complexes revealed that the pol-prim complex MMMh(m129-418) (MMMx refers to hybrid pol-prims containing chimeric p48 plus murine p180, p68, and p58) had a p180 subunit which was significantly more degraded than those of the other purified complexes (Fig. 2, lane 4). This proteolysis of p180 could be due to a high protease activity during purification. The SDS gel electrophoresis also showed that the p48 polypeptides containing the human aa 321 to 418 had a significantly higher apparent molecular mass than those containing the murine C terminus (Fig. 2). The shifts of these polypeptides cannot be explained by an increase of molecular mass, since the predicted molecular masses of murine and human p48 have a difference of 0.6 kDa. Therefore, structural features that cannot be resolved by SDS gel electrophoresis are most likely the cause of these shifts.
DNA synthesis by pol-prim with a chimeric human-murine p48 primase.
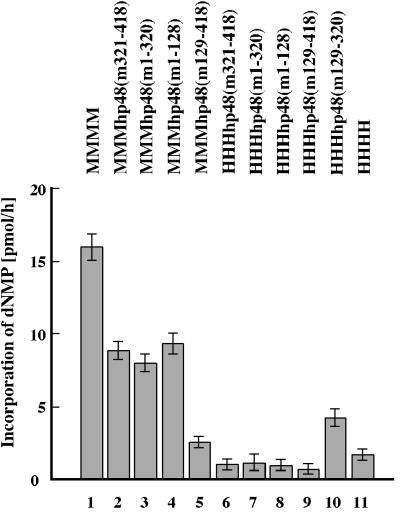
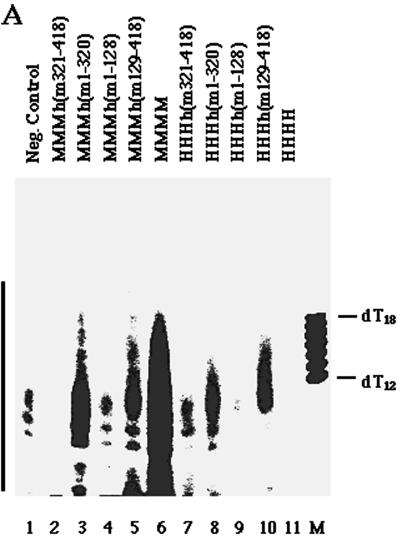
To determine whether the subunits of chimeric complexes cooperate, their ability to synthesize DNA on natural ssDNA templates was studied. In the presence of the four deoxy- and ribonucleotides and Mg2+, all pol-prim complexes synthesized DNA (Fig. 3).The mouse pol-prim and the chimeric protein complexes containing the three large murine subunits were in general more effective at DNA synthesis on ssDNA than the other enzyme complexes. However, the enzyme complexes containing chimeric p48 synthesized DNA at least with an activity similar to that of the four-subunit human pol-prim (Fig. 3, compare columns 6 to 11).
FIG. 3.
DNA synthesis on ssDNA by chimeric pol-prim complexes. The purified pol-prim complexes (0.3 U of primase) (murine pol-prim [column 1], pol-prim with murine p180, p68, p58, and chimeric p48 [columns 2 to 5], pol-prim with human p180, p68, p58, and chimeric p48 [columns 6 to 10], and human pol-prim [column 11]) were used to synthesize nucleic acids on ssDNA in the presence of nucleoside triphosphates, deoxynucleoside triphosphates, and Mg2+. The assay was repeated twice, and the means and standard deviations are presented.
The N and C termini of mouse p48 are not required for species-specific replication of PyV DNA in vitro.
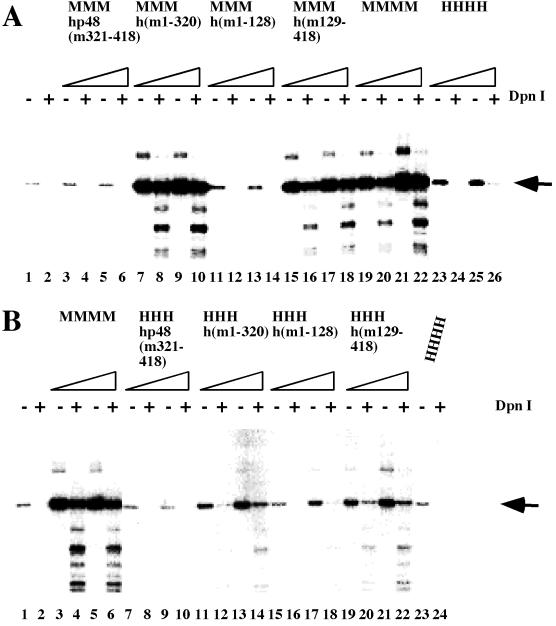
The mouse p48 subunit controls PyV DNA replication in vitro during the initiation of leading strand DNA synthesis through an unknown mechanism. The chimeric p48 subunits were tested in the replication of PyV DNA. To compensate for enzyme instabilities of recombinant proteins we used the same level of enzyme activity in each assay as indicated in the figures. As expected, mouse pol-prim supported in vitro PyV DNA replication, whereas the human enzyme did not (Fig. 4). Low levels of active protein complexes which contained aa 1 to 320 or 129 to 418 of mouse p48 supported PyV DNA replication. The observed DNA synthesis products were resistant to DpnI, which degrades both the unmethylated input DNA from Escherichia coli and partially synthesized products (e.g., Fig. 4A, lanes 2 and 4), indicating that they were the result of semiconservative DNA replication (Fig. 4A, lanes 7 to 10 and 15 to 22, and B, lanes 3 to 6, 13 to 16, and 19 to 22). However, hybrid pol-prim with p48 subunits containing only the N-terminal aa 1 to 128 or the C-terminal aa 321 to 418 of mouse p48 did not support cell-free PyV DNA replication. There were no qualitative differences between complexes with the three mouse subunits p180, p68, and p58 and those with the three human polypeptides (Fig. 4, compare panels A and B). In summary, all complexes with a p48 containing the central region of mouse p48 (aa 129 to 320) synthesized DNA that was resistant to DpnI digestion (Fig. 4A and B; summarized in Fig. 1), whereas complexes lacking this region of mouse p48 did not produce DpnI-resistant synthesis products (Fig. 4A and B).
FIG. 4.
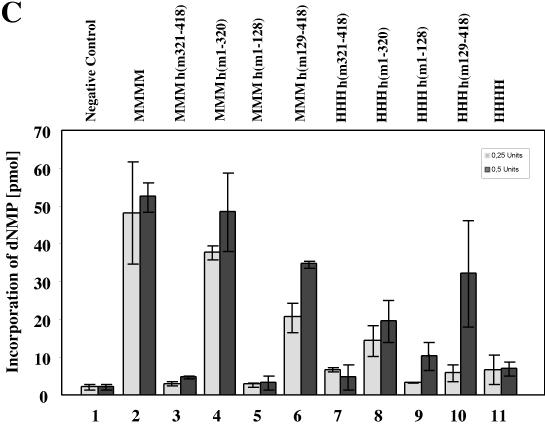
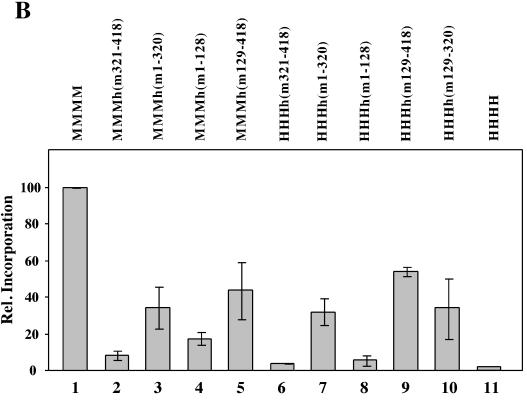
Replication of PyV DNA by chimeric pol-prim complexes. (A) Increasing amounts (0.25 and 0.5 U of DNA polymerase) of recombinant pol-prim containing murine p180, p68, p58, and chimeric p48 (lanes 3 to 18), four mouse subunits (lanes 19 to 22), or four human subunits (lanes 23 to 26) were added to depleted human 293S cell extracts (lanes 1 and 2) supplemented with PyV Tag and a PyV origin-containing plasmid. (B) The replication products of these extracts were also determined with recombinant enzymes containing three human subunits and hybrid p48. The assays were carried out in the absence of recombinant pol-prim (lanes 1 and 2) or in the presence of recombinant mouse (lanes 3 to 6), human (lanes 23 and 24), and hybrid pol-prim containing the three human subunits p180, p68, and p58, together with a chimeric subunit p48 (lanes 7 to 22). DNA synthesis products for panels A and B were analyzed for complete DNA replication by digestion with 10 U each of EcoRI and DpnI (even-numbered lanes). In parallel, the products were linearized with 10 U of EcoRI (odd-numbered lanes). The DNA synthesis products were visualized by autoradiography. The arrow at the right side of the figure indicates the linearized DNA synthesis products. (C) The incorporation of dNMPs into PyV DNA was determined by acid precipitation of DNA and scintillation counting (light and dark columns, showing means and standard deviations of a minimum of three replication assays with 0.25 and 0.5 primase units, respectively, of the indicated pol-prim). Columns 1, negative control of depleted extracts; columns 2, mouse pol-prim; columns 3 to 10, hybrid pol-prim as indicated; columns 11, human pol-prim.
Quantification of the cell-free PyV DNA replication revealed that pol-prim with three mouse proteins [MMMh(m1-320) and MMMh(m129-418)] supported DNA replication more efficiently than those with three human subunits (Fig. 4C, compare columns 4, 6, 8, and 10). These data suggest that the mouse p48 mediates species-specific replication of PyV DNA, whereas the other subunits have stimulatory effects on the replication reactions.
Amino acids within the central region of mouse p48 control species specificity of cell-free PyV DNA replication.
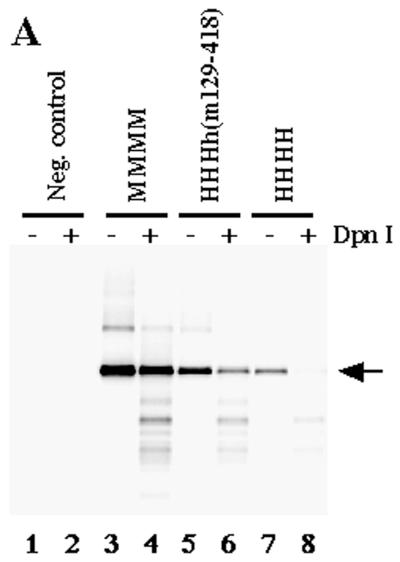
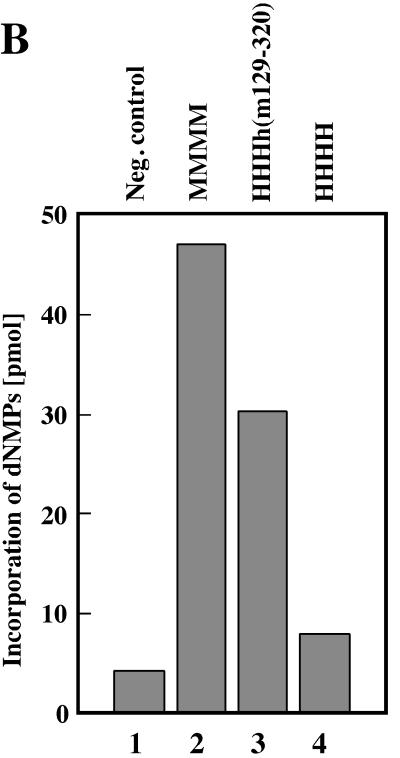
Since all proteins containing the central part of mouse p48 were active in PyV DNA replication these findings suggest that aa 129 to 320 control PyV DNA replication. Therefore, a chimeric p48 containing this region from mouse, together with human N- and C-terminal amino acids, was produced. The enzyme complex HHHh(m129-320) was highly active in PyV DNA replication in vitro, and DpnI-resistant DNA was synthesized (Fig. 5A, lane 6). Quantification of the reaction showed that the incorporation of radioactively labeled dNMPs by HHHh(m129-320) was about 65% of that by mouse pol-prim (Fig. 5B, compare columns 2 and 3).
FIG. 5.
aa 129 to 320 of mouse p48 control species specificity of cell-free PyV DNA replication. The ability of the chimeric pol-prim complex (0.5 DNA polymerase units) containing human p180, p68, p58, and chimeric h(m129-320) to support PyV DNA replication in vitro was determined by using depleted extracts from human 293 cells (A, lanes 5 and 6). This activity was compared with the replication activity of murine (A, lanes 3 and 4) and human (A, lanes 7 and 8) (0.5 DNA polymerase units) pol-prim. DNA synthesis products for panel A were analyzed for complete DNA replication by digestion with 10 U each of EcoRI and DpnI (even-numbered lanes). In parallel, the products were linearized with 10 U of EcoRI (odd-numbered lanes). For lanes 1 and 2, the synthesis products of depleted 293S extracts are presented. To compare the replication activities in a quantitative way the incorporated dNMPs were precipitated with trichloroacetic acid and measured by scintillation counting (B). Columns 1 to 4, depleted 293S extracts and mouse, chimeric HHHh(m129-320), and human pol-prim, respectively.
Initiation of PyV DNA replication with hybrid pol-prim in the presence of purified proteins.
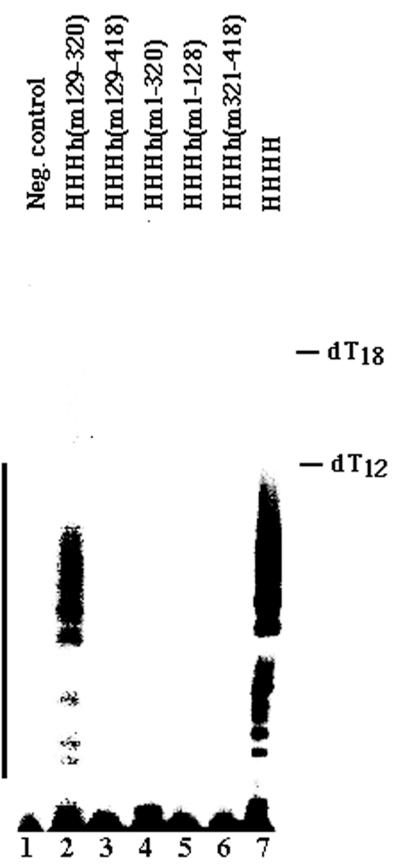
The initiation of PyV DNA replication is the species-specific step. Therefore, we tested whether we could reproduce the above results by using the initiation reaction of PyV DNA replication exclusively with purified proteins. Various pol-prim complexes (0.5 primase units) were used to initiate PyV DNA replication. These low amounts of mouse enzyme complex were sufficient to support the reaction, whereas the same amounts of human pol-prim were not (Fig. 6).The enzyme complexes containing the central part (aa 129 to 320) of mouse p48 were active, whereas those missing this region from mouse were inactive (Fig. 1 and 6).The other pol-prim subunits did not interfere with the initiation activity of the chimeric enzyme complexes, and the active chimeric complexes reached 35 to 50% of the activity of mouse pol-prim (Fig. 6B, compare columns 1, 3, 5, 7, 9, and 10). Thus, the initiation assay, which exclusively depends on purified proteins, underlines the fact that the central part of mouse p48 mediates the species specificity of PyV DNA replication.
FIG. 6.
Initiation of PyV DNA replication by recombinant pol-prim. The PyV initiation activity of the chimeric pol-prim complexes (0.5 primase units) containing murine or human p180, p68, and p58, together with a chimeric p48, compared with recombinant mouse and human pol-prim. (A) Lane 1, negative control without pol-prim; lanes 2 to 5, complexes with three murine subunits and a chimeric subunit; lane 6, mouse pol-prim; lanes 7 to 8, complexes with three human subunits and a chimeric subunit; lane 11, human pol-prim. Lane M shows 5′ end-labeled oligo(dT12-18) markers as indicated at the right. The bar at the left marks the initiation products. (B) The radioactive incorporation of at least three experiments was determined, and means and standard deviations of these experiments are presented. For each assay, the incorporation of mouse pol-prim was set to 100, and the relative incorporation activity of the other enzyme complexes was calculated. Column 1, mouse pol-prim; columns 2 to 10, hybrid enzyme complexes with murine and human p180, p68, and p58, together with a chimeric p48 as indicated; column 11, human pol-prim.
PyV, Tag physically interacts with mouse, human, and chimeric pol-prim complexes.
These results raised the question of whether the interaction of PyV Tag with pol-prim is species specific. Equal amounts of each enzyme complex were immobilized and then incubated with PyV Tag. PyV Tag physically bound to mouse and human pol-prim, as well as to the chimeric complexes (Fig. 7). The results suggest that the physical interaction of PyV Tag with the pol-prim complex is not the species-specific step, since Tag binds with a similar efficiency to both pol-prim complexes that are active and those which are inactive in cell-free PyV DNA replication (Fig. 4 to 7). This finding is consistent with data published earlier which show that SV40 Tag binds to human and mouse pol-prim (39).
FIG. 7.
Interaction of PyV Tag with recombinant pol-prim. BSA (1 μg) (column 1) or purified, recombinant pol-prim complexes (1 μg) (columns 2 to 8, as indicated) were immobilized. After incubation with 1 μg of PyV Tag for 1 h at RT, Tag–pol-prim complexes were detected with Tag-specific antibody F5. The means of three experiments are presented. OD, optical density.
Amino acids within the N and C termini of p48 inhibit SV40 DNA replication in vitro.
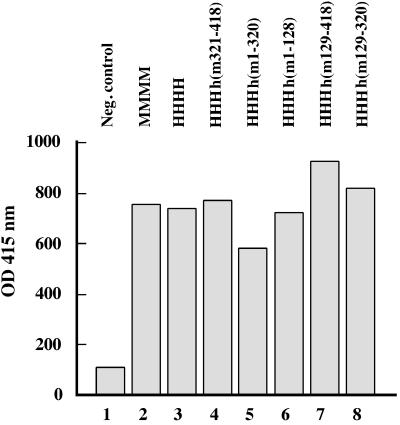
The mouse p48 subunit is required for PyV DNA replication, but in a complex with the three human subunits mouse p48 poisons SV40 DNA replication. This phenotype can be rescued by mouse p58 (4, 44). This behavior raised the question of whether the same region of mouse p48 is involved in the negative regulation of SV40 DNA replication and in the positive control of PyV DNA replication (44). The four chimeric complexes containing the N or C terminus of mouse p48 did not support initiation of SV40 DNA replication (Fig. 8, lanes 3 to 6; summarized in Fig. 1), although these enzyme complexes initiated DNA synthesis and elongated these primers on natural ssDNA templates (Fig. 3). Interestingly, the pol-prim complex HHHh(m129-320) containing the central part from mouse p48 synthesized primers in the cell-free SV40 system (Fig. 8, lane 2). Its efficiency was about 53% of that of human pol-prim (Fig. 8, compare lanes 2 and 7). In addition, HHHh(m129-320) supported SV40 DNA replication, and the incorporation of dNMPs with HHHh(m129-320) reached about 62% of that with human pol-prim (data not shown). These results indicate that amino acids within the N or C terminus of mouse p48 can suppress initiation of SV40 DNA replication in vitro.
FIG. 8.
Initiation of SV40 DNA replication by recombinant pol-prim. Pol-prims (0.8 U of primase activity) were tested in the SV40 initiation reaction. Lane 1, assay without pol-prim; lanes 2 to 6, pol-prim containing three human subunits and chimeric p48; lane 7, human pol-prim. Labeled oligo (dT12-18) marker is indicated at the right. The bar at the left marks the initiation products.
DISCUSSION
The cell-free polyomavirus DNA replication systems of PyV and SV40 have provided detailed insights into the mechanisms of eukaryotic DNA replication (for review, see reference 49 and references therein). The comparison of these DNA replication assays allowed the discrimination of separate essential functions of pol-prim in the control of viral DNA replication and explained the host specificity of these two viruses at least in part (reviewed in reference 42).
Control of PyV DNA replication by mouse p48.
The p48 subunit of pol-prim has essential functions in the initiation of leading and lagging strand DNA synthesis and in the control of PyV and SV40 DNA replication (4, 5, 13, 49, 50, 51). The initiation and DNA replication of PyV in vitro is supported by mouse p48 together with three human subunits of heterotetrameric pol-prim (4). We show here that the three large mouse subunits of the enzyme complex influence functions of mouse p48 and chimeric p48 sequences during PyV DNA replication and stimulate the replication efficiency of cell-free DNA replication (Fig. 4). MMMh(m1-320) and MMMh(m129-418) supported DNA replication more efficiently in the cell-free PyV system than the complexes HHHh(m1-320) and HHHh(m129-418) (Fig. 4). This increased activity seems to be dependent on a higher DNA synthesis rate of MMMh(m1-320) and MMMh(m129-418), since their initiation activity in the PyV system does not significantly differ from that of the other two chimeric enzyme complexes (Fig. 6).This interpretation is supported by the finding that MMMh(m1-320) and MMMh(m129-418) synthesized DNA more efficiently on a natural Φ×174 ssDNA template than did the other two enzyme complexes (Fig. 3).
The replication of dsDNA containing a PyV ori revealed that mouse p48 controls species specificity of PyV DNA replication even in the presence of three other murine subunits. Especially the central residues of mouse p48 carry essential functions to support PyV DNA replication in vitro. The shift in apparent molecular mass determined by SDS gel electrophoresis is not a parameter that affects the host specificity of PyV DNA replication, since this shift is most likely caused by amino acids in the region aa 321 to 421 of human p48 which do not interfere with PyV DNA replication in human extracts (Fig. 2 and 4 to 6). However, the species specificity of PyV DNA replication only becomes manifest within the initiation complex composed of PyV Tag, pol-prim, and RPA at an origin of DNA replication. Although PyV Tag physically binds to pol-prim, the direct physical contacts do not show species-specific effects (Fig. 7) (4). Therefore, the primase subunit p48 of the nonpermissive host might differ in its preferential initiation sequence at the viral ori. Alternatively, conformational restrictions within the initiation complex consisting of PyV Tag, RPA, pol-prim, topo I, and the PyV ori may hinder recognition of the DNA template by the human primase. These restrictions may occur at any stage of the initiation process, as our assay does not distinguish between them (2, 5, 6, 19, 23, 49). Especially, Tag serves as a mediator protein (1) to support RNA synthesis by pol-prim on an RPA-bound template by primase. PyV Tag is most likely able to carry out these activities at the PyV ori with mouse p48 but not with the human p48.
Activity of mouse p48 in SV40 DNA replication.
In combination with human p180, p68, and p58, the mouse p48 has been found to poison initiation of SV40 DNA replication (4, 44). The results presented here indicate that the inhibitory effect of mouse p48 on SV40 DNA replication and its supporting function(s) in PyV DNA replication depend on different mechanisms and can be separated. In the presence of either the murine N or C terminus the initiation of SV40 DNA replication is inhibited, whereas the central part of mouse p48 is required for the initiation of PyV DNA replication (Fig. 1, 6, and 8). However, the chimeric protein h(m129-320) supports the cell-free DNA replication of both PyV and SV40 (Fig. 1, 4, 5, 6, and 8). The mechanism for the inhibitory effect of mouse p48, and more precisely its N- and C-terminal amino acids, on SV40 DNA replication is still unclear, but this inhibition is relieved by substituting the human primase subunit p58 with the mouse polypeptide (4). These findings suggest that the N and C termini of p48 cooperate with p58 during the initiation of SV40 DNA replication. The human p58 might fail to functionally interact with these regions of mouse p48, whereas mouse p58 does interact (4). This interpretation is supported by earlier findings that the N and C termini of p48 are involved in its physical binding to p58 (9). However, this cooperation of the two mammalian subunits is not disturbed in the initiation of DNA synthesis on ssDNA, the initiation of leading strand DNA synthesis in the PyV origin, and the initiation of Okazaki fragments on the lagging strand (Fig. 3 to 6)(39, 44).
ACKNOWLEDGMENTS
We thank F. Grosse and R. Smith for critical reading of the manuscript and A. Willitzer for synthesizing oligonucleotides.
This work was financially supported by the Deutsche Forschungsgemeinschaft (Na 190/8, Na 190/10, and Na 190/12) and the EC (CT970125). The IMB is a Gottfried-Wilhelm-Leibniz Institut and is financially supported by the federal government and the Land Thüringen.
REFERENCES
- 1.Beernink H T, Morrical S W. RMPs: recombination/replication mediator proteins. Trends Biochem Sci. 1999;24:385–389. doi: 10.1016/s0968-0004(99)01451-6. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharyya S, Lorimer H, Prives C. Murine polyomavirus and simian virus 40 large T antigens produce different structural alterations in viral origin DNA. J Virol. 1995;69:7579–7585. doi: 10.1128/jvi.69.12.7579-7585.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 4.Brückner A, Stadlbauer F, Guarino L A, Brunahl A, Schneider C, Rehfuess C, Prives C, Fanning E, Nasheuer H-P. The mouse DNA polymerase α-primase subunit p48 mediates species-specific replication of polyomavirus DNA in vitro. Mol Cell Biol. 1995;15:1716–1724. doi: 10.1128/mcb.15.3.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgers P M. Eukaryotic DNA polymerases in DNA replication and DNA repair. Chromosoma. 1998;107:218–227. doi: 10.1007/s004120050300. [DOI] [PubMed] [Google Scholar]
- 6.Challberg M, Kelly T J. Animal virus DNA replication. Annu Rev Biochem. 1989;58:671–717. doi: 10.1146/annurev.bi.58.070189.003323. [DOI] [PubMed] [Google Scholar]
- 7.Collins K L, Kelly T J. The effects of T antigen and replication protein A on the initiation of DNA synthesis by DNA polymerase α-primase. Mol Cell Biol. 1991;11:2108–2115. doi: 10.1128/mcb.11.4.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins K L, Russo A A R, Tseng B Y, Kelly T J. The role of the 70kDa subunit of human DNA polymerase α in DNA replication. EMBO J. 1993;12:4555–4566. doi: 10.1002/j.1460-2075.1993.tb06144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Copeland W C, Tan X. Active site mapping of the catalytic mouse primase subunit by alanine scanning mutagenesis. J Biol Chem. 1995;270:3905–3913. doi: 10.1074/jbc.270.8.3905. [DOI] [PubMed] [Google Scholar]
- 10.Dean F B, Borowiec J A, Eki T, Hurwitz J. The simian virus 40 T antigen double hexamer assembles around the DNA at the replication origin. J Biol Chem. 1992;267:14129–14137. [PubMed] [Google Scholar]
- 11.Dornreiter I, Erdile L F, Gilbert I U, von Winkler D, Kelly T J, Fanning E. Interaction of DNA polymerase α-primase with cellular replication protein A and SV40 T antigen. EMBO J. 1992;11:769–776. doi: 10.1002/j.1460-2075.1992.tb05110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dornreiter I, Höss A, Arthur A K, Fanning E. SV40 T antigen binds directly to the catalytic subunit of DNA polymerase alpha. EMBO J. 1990;9:3329–3336. doi: 10.1002/j.1460-2075.1990.tb07533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eki T, Enomoto T, Masutani C, Miyajima A, Takada R, Murakami Y, Ohno T, Hanaoka F, Ui M. Mouse DNA primase plays the principal role in determination of permissiveness for polyomavirus DNA replication. J Virol. 1991;65:4874–4881. doi: 10.1128/jvi.65.9.4874-4881.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gannon J V, Lane D P. Interactions between SV40 T antigen and DNA polymerase α. New Biol. 1990;2:84–92. [PubMed] [Google Scholar]
- 15.Gurney E G, Harrison R O, Fenno J. Monoclonal antibodies against simian virus 40 T antigens: evidence for distinct subclasses of large T antigen and for similarities among nonviral T antigens. J Virol. 1980;34:752–763. doi: 10.1128/jvi.34.3.752-763.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurney E G, Tamowski S, Deppert W. Antigenic binding sites of monoclonal antibodies specific for simian virus 40 large T antigen. J Virol. 1986;57:1168–1172. doi: 10.1128/jvi.57.3.1168-1172.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harlow E, Lane D P. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 18.Henricksen L A, Umbricht C B, Wold M S. Recombinant replication protein A: expression, complex formation, and functional characterization. J Biol Chem. 1994;269:11121–11132. [PubMed] [Google Scholar]
- 19.Hübscher U, Nasheuer H-P, Syväjoja J. Eukaryotic DNA polymerases, a growing family. Trends Biochem Sci. 2000;25:143–147. doi: 10.1016/s0968-0004(99)01523-6. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Li J J, Kelly T J. Simian virus 40 DNA replication in vitro. Proc Natl Acad Sci USA. 1984;81:6973–6977. doi: 10.1073/pnas.81.22.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J J, Kelly T J. Simian virus 40 DNA replication in vitro: specificity of initiation and evidence for bidirectional replication. Mol Cell Biol. 1985;5:1238–1246. doi: 10.1128/mcb.5.6.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L, Li B L, Hock M, Wang E, Folk W R. Sequences flanking the pentanucleotide T-antigen binding sites in the polyomavirus core origin help determine selectivity of DNA replication. J Virol. 1995;69:7570–7578. doi: 10.1128/jvi.69.12.7570-7578.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lisby M, Krogh B O, Boege F, Westergaard O, Knudsen B R. Camptothecins inhibit the utilization of hydrogen peroxide in the ligation step of topoisomerase I catalysis. Biochemistry. 1998;37:10815–10827. doi: 10.1021/bi980757r. [DOI] [PubMed] [Google Scholar]
- 25.Mastrangelo I A, Hough P V C, Wall J S, Dodson M, Dean F B, Hurwitz J. ATP-dependent assembly of double hexamers of SV40 T antigen at the viral origin of DNA replication. Nature. 1989;338:658–662. doi: 10.1038/338658a0. [DOI] [PubMed] [Google Scholar]
- 26.Melendy T, Stillman B. An interaction between replication protein A and SV40 T antigen appears essential for primosome assembly during SV40 DNA replication. J Biol Chem. 1993;268:3389–3395. [PubMed] [Google Scholar]
- 27.Moses K, Prives C. A unique subpopulation of murine DNA polymerase α-primase specifically interacts with polyoma virus T antigen and stimulates DNA replication. Mol Cell Biol. 1994;14:2767–2776. doi: 10.1128/mcb.14.4.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murakami Y, Eki T, Yamada M, Prives C, Hurwitz J. Species-specific in vitro synthesis of DNA containing the polyoma virus origin of replication. Proc Natl Acad Sci USA. 1986;83:6347–6351. doi: 10.1073/pnas.83.17.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murakami Y, Hurwitz J. Functional interactions between SV40 T antigen and other replication proteins at the replication fork. J Biol Chem. 1993;268:11008–11017. [PubMed] [Google Scholar]
- 30.Murakami Y, Hurwitz J. DNA polymerase α stimulates the ATP-dependent binding of simian virus tumor T antigen to the SV40 origin of replication. J Biol Chem. 1993;268:11018–11027. [PubMed] [Google Scholar]
- 31.Murakami Y, Wobbe C R, Weissbach L, Dean F B, Hurwitz J. Role of DNA polymerase α and DNA primase in simian virus 40 DNA replication in vitro. Proc Natl Acad Sci USA. 1986;83:2869–2873. doi: 10.1073/pnas.83.9.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nasheuer H-P, Grosse F. DNA polymerase α-primase from calf thymus. Determination of the polypeptide responsible for primase activity. J Biol Chem. 1988;263:8981–8988. [PubMed] [Google Scholar]
- 33.Nasheuer H-P, Grosse F. Immunoaffinity-purified DNA polymerase α displays novel properties. Biochemistry. 1987;26:8458–8466. doi: 10.1021/bi00399a064. [DOI] [PubMed] [Google Scholar]
- 34.Nasheuer H-P, von Winkler D, Schneider C, Dornreiter I, Gilbert I, Fanning E. Purification and functional characterization of bovine RP-A in an in vitro SV40 DNA replication system. Chromosoma. 1992;102:S52–S59. doi: 10.1007/BF02451786. [DOI] [PubMed] [Google Scholar]
- 35.O'Reilly D R, Miller L K, Luckow V A. Baculovirus expression vectors—a laboratory manual. W. H. New York, N.Y: Freeman and Co.; 1992. [Google Scholar]
- 36.Pallas D C, Schley C, Mahoney M, Harlow E, Schaffhausen B S, Roberts T M. Polyomavirus small T antigen: overproduction in bacteria, purification, and utilization for monoclonal and polyclonal antibody production. J Virol. 1986;60:1075–1084. doi: 10.1128/jvi.60.3.1075-1084.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reynisdottir I, Bhattacharyya S, Zhang D, Prives C. The retinoblastoma protein alters the phosphorylation state of polyomavirus large T antigen in murine cell extracts and inhibits polyomavirus origin DNA replication. J Virol. 1999;73:3004–3013. doi: 10.1128/jvi.73.4.3004-3013.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 39.Schneider C, Weisshart K, Guarino L A, Dornreiter I, Fanning E. Species-specific functional interactions of DNA polymerase α-primase with SV40 T antigen require SV40 origin DNA. Mol Cell Biol. 1994;14:3176–3185. doi: 10.1128/mcb.14.5.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simmons D T, Melendy T, Usher D, Stillman B. Simian virus 40 large T antigen binds to topoisomerase I. Virology. 1996;222:365–374. doi: 10.1006/viro.1996.0433. [DOI] [PubMed] [Google Scholar]
- 41.Smale S T, Tjian R. T antigen-DNA polymerase α complex implicated in simian virus 40 DNA replication. Mol Cell Biol. 1986;6:4077–4087. doi: 10.1128/mcb.6.11.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith R W P, Nasheuer H-P. Pandalai (ed.), Recent research developments in virology. Vol. 2. Trivandrum, India: Transworld Research Network; 2000. Control of papovaviral DNA replication; pp. 67–92. [Google Scholar]
- 43.Stadlbauer F, Brueckner A, Rehfuess C, Eckerskorn C, Lottspeich F, Förster V, Tseng B Y, Nasheuer H-P. DNA replication in vitro by recombinant DNA-polymerase-α-primase. Eur J Biochem. 1994;222:781–793. doi: 10.1111/j.1432-1033.1994.tb18925.x. [DOI] [PubMed] [Google Scholar]
- 44.Stadlbauer F, Voitenleitner C, Brückner A, Fanning E, Nasheuer H-P. Species-specific replication of simian virus 40 DNA in vitro requires the p180 subunit of human DNA polymerase α-primase. Mol Cell Biol. 1996;16:94–104. doi: 10.1128/mcb.16.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stigger E, Dean F B, Hurwitz J, Lee S H. Reconstitution of functional human single-stranded DNA-binding protein from individual subunits expressed by recombinant baculoviruses. Proc Natl Acad Sci USA. 1994;91:579–583. doi: 10.1073/pnas.91.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka S, Hu S-Z, Wang T S-F, Korn D. Preparation and preliminary characterization of monoclonal antibodies against human DNA polymerase α. J Biol Chem. 1982;257:8386–8390. [PubMed] [Google Scholar]
- 47.Tooze J. Molecular biology of tumor virus, part 2. DNA tumor viruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1981. [Google Scholar]
- 48.Trowbridge P W, Roy R, Simmons D T. Human topoisomerase I promotes initiation of simian virus 40 DNA replication in vitro. Mol Cell Biol. 1999;19:1686–1694. doi: 10.1128/mcb.19.3.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waga S, Stillman B. The DNA replication fork in eukaryotic cells. Annu Rev Biochem. 1998;67:721–751. doi: 10.1146/annurev.biochem.67.1.721. [DOI] [PubMed] [Google Scholar]
- 50.Weisshart K, Förster H, Kremmer E, Schlott B, Grosse F, Nasheuer H-P. Protein-protein interactions of the primase subunits p58 and p48 with simian virus 40 T antigen are required for efficient primer synthesis in a cell-free system. J Biol Chem. 2000;275:17328–17337. doi: 10.1074/jbc.M000717200. [DOI] [PubMed] [Google Scholar]
- 51.Weisshart K, Taneja P, Fanning E. The replication protein A binding site in simian virus 40 (SV40) T antigen and its role in the initial steps of SV40 DNA replication. J Virol. 1998;72:9771–9781. doi: 10.1128/jvi.72.12.9771-9781.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]