Abstract
Introduction
This study compared the efficacy of hydroxypropyl guar (HPG)/hyaluronic acid (HA) and carboxymethylcellulose (CMC)/HA lubricant eye drops for post-cataract surgery dry eye disease (DED).
Methods
This was a prospective, open-label, assessor-masked, parallel, randomized controlled study. Seventy patients with DED who underwent cataract surgery were randomized in a 1:1 ratio to receive 1–2 drops of HPG/HA or CMC/HA lubricant four times daily for 3 weeks. Efficacy assessments included changes from baseline in corneal fluorescein staining (CFS) score, Ocular Surface Disease Index score, Schirmer’s test score (without anesthesia), tear break-up time, and central corneal sensitivity at weeks 1 and 3.
Results
There were 35 patients in each group. The HPG/HA group demonstrated superior improvements in CFS scores (expressed as means and standard deviations) to the CMC/HA group at week 1 ( – 1.0 [1.7] vs. – 0.1 [1.7], p = 0.039) and demonstrated comparable results at week 3 ( – 1.6 [1.8] vs. – 1.3 [1.9], p = 0.552). No statistical differences were observed in other secondary outcomes between groups at weeks 1 and 3 (p > 0.05). Only one adverse event was reported in this study, which occurred in the HPG/HA group. The AE of ocular hypertension was mild, deemed unrelated to the study treatment, and resolved within a week.
Conclusions
The HPG/HA lubricant eye drops resulted in greater CFS scores at 1 week after treatment compared with CMC/HA drops. The HPG/HA and CMC/HA drops were safe and well tolerated.
ClinicalTrials.gov identifier
Keywords: Cataract surgery, Dry eye disease, Hyaluronic acid, Hydroxypropyl-guar, Carboxymethylcellulose
Key Summary Points
| Why carry out this study? |
| Cataracts are usually treated with surgery to replace the intraocular lens, which may lead to or worsen dry eye disease (DED) through various mechanisms. |
| The relative benefits of hydroxypropyl guar (HPG)/hyaluronic acid (HA) and carboxymethylcellulose (CMC)/HA lubricant eye drops have been demonstrated in treating post-cataract surgery DED. However, HPG/HA and CMC/HA have not been directly compared to determine which is more beneficial in treating post-cataract surgery DED. |
| The present study addressed this gap by comparing HPG/HA and CMC/HA lubricant eye drops in terms of subjective and objective outcomes in patients with DED following cataract surgery. |
| What was learned from the study? |
| The HPG/HA and CMC/HA groups demonstrated improvement in dry eye parameters after treatment, with the HPG/HA group achieving greater and faster improvements in corneal fluorescein staining scores than the CMC/HA group as early as 1 week after treatment. |
| This study’s findings offer clinicians insights into the effectiveness of HPG/HA and CMC/HA lubricant eye drops. |
Introduction
Cataracts are opacities in the crystalline ocular lens that are the leading cause of visual impairment worldwide [1–4]. Cataracts are typically treated with surgery to replace the lens [1], which may induce or exacerbate dry eye disease (DED) through several mechanisms, specifically corneal nerve transection, inflammatory factor elevation, goblet cell loss, and meibomian gland dysfunction [5, 6]. Symptoms of DED include foreign-body sensation and irritation of the affected eye lasting 1 month or longer post-operation [5, 7, 8].
Artificial tears are the primary therapy for DED and are available in numerous formulations [6, 9]. Viscosity-enhancing agents, such as hyaluronic acid (HA), hydroxypropyl guar (HPG), carboxymethylcellulose (CMC), and polyethylene glycol (PEG) are common components of artificial tears that relieve dry eye symptoms through various mechanisms [9, 10]. HA, in particular, acts as a lubricant promoting cell proliferation, anti-inflammation, and wound repair [11] and is widely used in tear supplements due to its proven ocular benefits [9]. The two products used in this study, the combination of HPG/HA or CMC/HA lubricant eye drops, are commercially available artificial tears containing HA. The HPG/HA lubricant eye drops contain the demulcents of PEG, propylene glycol (PG), HPG, and HA [12, 13]. These active ingredients bind to damaged epithelial cells to add volume to the tear film and restructure it, providing lasting protection [12]. By contrast, CMC/HA lubricant eye drops include the demulcents of CMC and HA, which lubricate and bind to the ocular surface for extended periods [13, 14]. Both types of lubricant eye drops have benefits for patients with post-cataract surgery DED. In a retrospective, multicenter cohort study, patients with post-cataract surgery DED treated with HPG/HA lubricant eye drops had significantly lower Standard Patient Evaluation of Eye Dryness scores (expressed as means and standard deviations [SD]) at 8 weeks compared with those who did not receive any perioperative artificial tears (1.1 [1.4] vs. 4.0 [3.4], p < 0.001) [15]. In another prospective, randomized, open-label study, CMC/HA lubricant eyedrops were effective in supplementing the standard of care (SOC) compared with SOC alone in patients with DED following cataract surgery [7]. At 5 weeks, the mean tear break-up time (TBUT) for patients treated with CMC/HA lubricant eye drops as supplements to SOC was significantly higher than that for patients treated with SOC alone (10.7 vs. 9.3 s, p = 0.003) [7].
The relative benefits of HPG/HA and CMC/HA lubricant eye over alternative formulations have been demonstrated. Nevertheless, HPG/HA and CMC/HA have not been directly compared to determine which is more beneficial in treating post-cataract surgery DED. Additionally, according to the Tear Film and Ocular Surface Society’s Dry Eye Workshop II, a head-to-head comparison study to determine the effectiveness of specific artificial tears has yet to be conducted [9]. Therefore, the present study addressed this gap by comparing HPG/HA and CMC/HA lubricant eye drops in terms of subjective and objective outcomes in patients with DED following cataract surgery.
Methods
Study Design
This study adopted a 3-week, prospective, open-label, assessor-masked, parallel, randomized controlled design and was conducted over a period between December 2021 and July 2023 at the Department of Ophthalmology, Keelung Chang Gung Memorial Hospital, Keelung, Taiwan (ClinicalTrials.gov identifier NCT06221345). This study was approved by the Institutional Review Board (IRB) of the Chang Gung Medical Foundation (IRB No.: 202002539A3) prior to any study procedures, and the research team members adhered to the tenets of the Declaration of Helsinki throughout the study. Informed consent was obtained from all participants involved in the study. No changes were made to the study design, procedures, or outcomes after the trial commenced.
Patients
Patients with DED in the first week after cataract surgery who met the inclusion criteria were enrolled after providing written informed consent. The inclusion criteria were being aged 20 years or older, having been scheduled for unilateral cataract surgery, and having a positive screening result for symptoms of DED after cataract surgery (Ocular Surface Disease Index [OSDI] > 14.8, positive corneal fluorescein staining [CFS], Schirmer’s test score ≤ 10 mm per 5 min, and TBUT ≤ 5 s). Patients who met the following criteria during screening were excluded: having an allergy to any of the study medications, having conjunctival allergy or infectious diseases; having a history of ocular chemical or thermal burns; having Stevens–Johnson syndrome or ocular pemphigoid; having glaucoma or ocular hypertension; having eyelid or lacrimal diseases; receiving an ocular operation within the previous 3 months; having graft-versus-host disease, having non-dry eye ocular inflammation, trauma, or uncontrolled systemic diseases; having worn corneal contact lenses before enrollment, history of severe systemic disease, having other conditions that precluded enrollment per the judgment of the investigator; experiencing complications during surgery; or having postsurgical ocular hypertension, endophthalmitis, or infectious keratitis.
Sample Size
The sample size required to determine an appreciable change in CFS scores was calculated based on a previous trial that compared PEG 400/PG-based lubricant eye drops to CMC-based drops [12]. Specifically, to detect a reduction in the mean (standard deviation) CFS scores of 1 (1.4) points and given an estimated type 1 error (α) of 0.05, a sample size of approximately 31 eyes per patient group was required for a positive difference between groups to be detected at a power of 80%. To provide a buffer of 10% for the drop-out rate, the total sample size required was 70 eyes (35 patients in each group).
Randomization and Blinding
Eligible patients were assigned in a 1:1 ratio to HPG/HA and CMC/HA groups. An independent statistical programmer generated random numbers for group assignment utilizing computerized block-randomization with block sizes of 2 and 4. Subsequently, independent personnel prepared sequentially numbered, opaque sealed envelopes. Upon enrollment, the study coordinator opened the envelope to reveal the patient’s allocated treatment. After being assigned to a treatment group, patients received HPG/HA or CMC/HA lubricant eye drops from a pharmacist.
This was an open-label, assessor-masked study, only the assessor who evaluated CFS score, Schirmer’s test score, TBUT, and central corneal sensitivity was masked to the patient group assignments.
Intervention
The same surgeon (Chi-Chin Sun) performed standard phacoemulsification (Infiniti from Alcon, Fort Worth, TX, USA) at the study hospital using posterior chamber intraocular lens implantation and made a 2.65-mm sutureless clear corneal incision at the superior corneal position in each eye. All patients received prednisolone acetate (Pred Forte Ophthalmic Suspension; Allergan, Irvine, CA, USA) and levofloxacin (Cravit Ophthalmic Solution; Santen Pharmaceutical, Osaka, Japan) as the SOC at a dosage of 1–2 drops four times daily for 4 weeks (i.e., from the day of cataract surgery until the fourth-week post-operation).
During weeks 1 to 3 (the first to fourth weeks post-operation), the HPG/HA group received HPG/HA (Systane HYDRATION Preservative-Free Lubricant Eye Drops; Alcon), whereas the CMC/HA group received CMC/HA (Optive Fusion Lubricant Eye Drops [Unit Dose]; Allergan). Both groups were prescribed 1–2 drops of artificial tears four times daily for 3 weeks.
Outcome Measures
A total of three visits were scheduled in this study: screening (the first-week post operation), week 1 (the second-week post operation), and week 3 (the fourth-week post-operation).
Efficacy and safety assessments were performed during all visits. Efficacy assessments included CFS score, OSDI score, Schirmer’s test score, TBUT, and central corneal sensitivity. Clinicians conducted safety assessments by recording adverse events (AEs) during the study period. All outcomes were evaluated by a masked assessor, except OSDI scores, which were patient-reported.
The five cornea areas (inferior, superior, central, nasal, and temporal) were stained with fluorescein paper and external eye photography was performed with a biomicroscopy image system. The CFS grade was scored according to the National Eye Institute scale [16], with a total score ranging from 0 to 15; a higher score indicates more severe epitheliopathy [17]. The 12-item patient-reported OSDI questionnaire has an overall score ranging from 0 to 100 [18, 19]. On the basis of the OSDI score, patients were determined to have normal (0–12), mild (13–22), moderate (23–32), or severe (33–100) ocular surface disease [19]. Schirmer’s test was performed without anesthesia [20]. A Schirmer’s test score of greater than 10 mm indicates normal tear production, whereas a test score of less than 5 mm indicates tear deficiency [21]. TBUT was assessed by recording the interval between the last blink and the first appearance of a dry spot. A TBUT less than 10 s suggests an abnormal tear film, a TBUT between 5 and 10 s suggests a marginal tear film and a TBUT less than 5 s indicates dry eye [22–24]. Corneal sensitivity was measured using a Cochet–Bonnet esthesiometer [25]. The longer the monofilament length, the greater the sensitivity of the patients’ corneas [26].
The primary outcome was a change between groups from the baseline CFS scores at weeks 1 and 3. The secondary outcomes were a change from baseline in OSDI scores, Schirmer’s test scores, TBUT, and central corneal sensitivity between groups at weeks 1 and 3. Safety outcomes were assessed as the incidence of AEs from weeks 1 to 3.
Statistical Analysis
All efficacy analyses were performed based on the full analysis set (FAS) and per protocol (PP) populations, whereas safety analyses were performed based on the FAS population only. The FAS population comprised all randomized patients who received artificial tears, regardless of protocol violations, whereas the PP population comprised only the patients in the FAS population who completed 3 weeks of treatment without any major protocol violations during this time.
Continuous variables are presented as means and standard deviations, and categorical variables are presented as frequencies and percentages. A two-sample t test and paired t test were used to analyze between-group and within-group comparisons of continuous variables, respectively, whereas a Chi-square test was performed for categorical variables. All analyses were conducted using SAS software version 9.4 (SAS Institute, Cary, NC, USA). A p value of < 0.05 indicated statistical significance.
Results
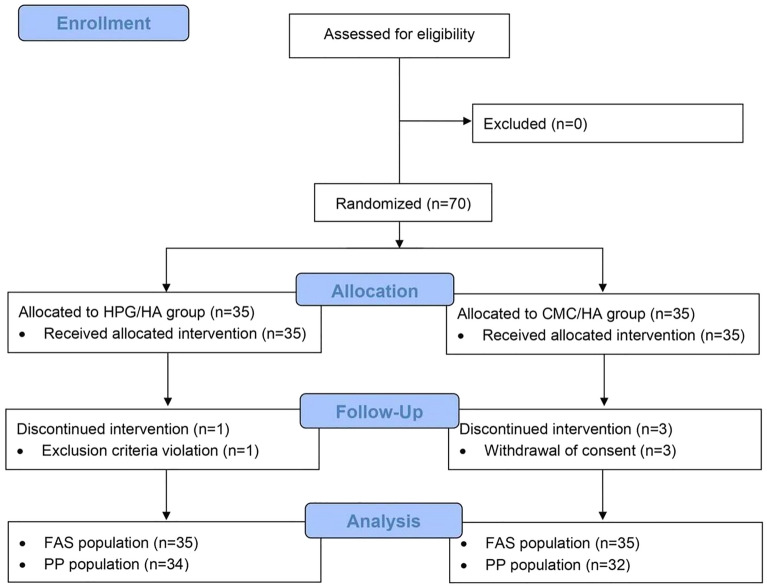
Of the 35 patients assigned in a 1:1 ratio to each study group, one (1, 2.9%) patient from the HPG/HA group withdrew early due to violating exclusion criteria, and 3 (8.6%) patients from the CMC/HA group withdrew their consent and discontinued study treatment before the study’s conclusion (Fig. 1). The FAS and PP populations thus comprised 70 and 66 patients, respectively. Table 1 presents the demographic characteristics of both groups. The patients in both groups were older individuals (> 65 years old), predominantly female, and Taiwanese. The patients’ approximate mean height and weight were 158 cm and 61 kg, respectively. Both groups exhibited well-balanced and comparable demographic and baseline characteristics (p > 0.05) (Table 1). The most common pre-existing medical conditions were hypertension (37, 52.9%), hyperlipidemia (35, 50.0%), and diabetes mellitus (DM; 17, 24.3%).
Fig. 1.
Patient disposition. FAS full analysis set, PP per protocol
Table 1.
Demographic and baseline characteristics
| Parameter | HPG/HA group (n = 35) | CMC/HA group (n = 35) | p value |
|---|---|---|---|
| Age, years | 67.5 (13.0) | 70.8 (8.8) | 0.231 |
| Sex | |||
| Male | 6 (17.1) | 11 (31.4) | 0.163 |
| Female | 29 (82.9) | 24 (68.6) | |
| Height, cm | 156.3 (7.4) | 159.0 (7.4) | 0.144 |
| Weight, kg | 58.8 (9.2) | 62.8 (12.8) | 0.143 |
| Race | |||
| Taiwanese | 35 (100.0) | 35 (100.0) | NA |
| CFS scores | 3.4 (1.9) | 3.9 (2.1) | 0.285 |
| OSDI scores | 19.1 (5.0) | 18.7 (7.1) | 0.797 |
| Schirmer’s test scores, mm/5 min | 8.2 (5.3) | 7.9 (2.4) | 0.795 |
| TBUT, s | 4.4 (1.6) | 4.3 (1.4) | 0.692 |
| Central corneal sensitivity, cm | 5.9 (0.2) | 5.9 (0.4) | 0.415 |
CFS corneal fluorescein staining, CMC carboxymethylcellulose, HA hyaluronic acid, HPG hydroxypropyl guar, NA not available, OSDI Ocular Surface Disease Index, TBUT tear break-up time
Values are presented as mean (SD) or counts (%)
Primary Endpoint
The HPG/HA group had a greater reduction in CFS scores at week 1 than did the CMC/HA group (− 1.0 [1.7] vs. − 0.1 [1.7], p = 0.039). The CFS scores of both groups continued to decline until week 3, with the HPG/HA group having a slightly larger mean change than the CMC/HA group (− 1.6 [1.8] vs. − 1.3 [1.9]); however, the difference was not statistically significant (p = 0.552; Fig. 2). The results for the PP population were consistent with those for the FAS population (data not shown).
Fig. 2.
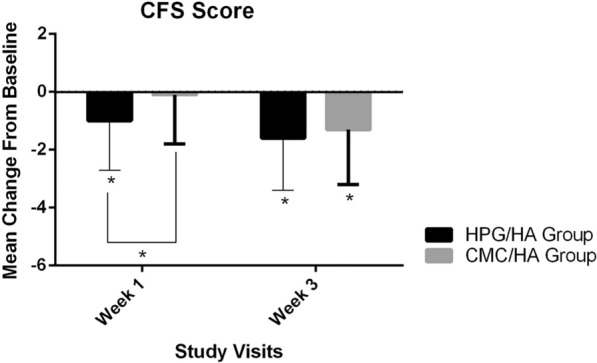
Change from baseline in CFS scores at weeks 1 and 3 (FAS population). The CFS scores (expressed as means and standard deviations) of HPG/HA group were greater than the CMC/HA group at week 1 ( – 1.0 [1.7] vs. – 0.1 [1.7], p = 0.039). At week 3, although the difference between groups was not statistically significant, the HPG/HA group had a slightly greater mean change than the CMC/HA group ( – 1.6 [1.8] vs. – 1.3 [1.9], p = 0.552). *p < 0.05. CMC carboxymethylcellulose, CFS corneal fluorescein staining, FAS full analysis set, HA hyaluronic acid, HPG hydroxypropyl guar
Secondary Endpoints
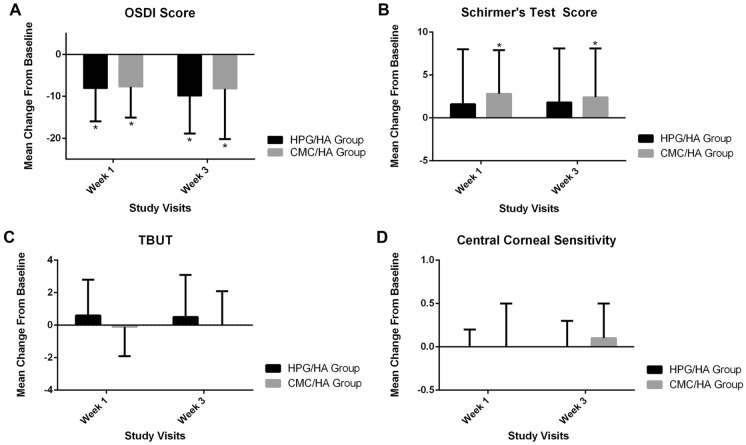
Figure 3 shows the change from baseline to weeks 1 and 3 for all secondary outcomes. Collectively, the p values of all secondary outcomes revealed no statistically significant differences between groups. The two treatments had comparable effects on OSDI, Schirmer’s test, TBUT, and central corneal sensitivity at weeks 1 and 3 (p > 0.05). These trends were observed in both the FAS and PP populations (data not shown).
Fig. 3.
Change from baseline in A OSDI scores, B Schirmer’s test scores, C TBUT, and D central corneal sensitivity at weeks 1 and 3 (FAS population). A OSDI scores (expressed as means and SDs) of the HPG/HA and CMC/HA groups were 8.1 (7.9) vs. – 7.7 (7.4), p = 0.799 at week 1 and – 9.9 (9.0) vs. – 8.2 (12.0), p = 0.506 at week 3. B Schirmer’s test scores (expressed as means and SDs) of HPG/HA and CMC/HA groups were 1.6 (6.4) vs. 2.8 (5.1) mm/ 5 min, p = 0.373 at week 1 and 1.8 (6.3) vs. 2.4 (5.7) mm/ 5 min, p = 0.688 at week 3. C TBUT (expressed as means and SDs) of HPG/HA and CMC/HA groups were 0.6 (2.2) vs. – 0.1 (1.8) s, p = 0.184 at week 1 and 0.5 (2.6) vs. 0.0 (2.1) s, p = 0.389 at week 3. D Central corneal sensitivity measurements (expressed as means and SDs) of HPG/HA and CMC/HA groups were 0.0 (0.2) vs. – 0.0 (0.5), p = 0.854 at week 1 and 0.0 (0.3) vs. 0.1 (0.4), p = 0.275 at week 3. *p < 0.05. CMC carboxymethylcellulose, FAS full analysis set, HA hyaluronic acid, HPG hydroxypropyl guar, OSDI Ocular Surface Disease Index, TBUT tear break-up time
The HPG/HA and CMC/HA groups exhibited continual reductions in OSDI scores until week 3 (week 1, − 8.1 [7.9] vs. − 7.7 [7.4], p = 0.799; week 3, − 9.9 [9.0] vs. − 8.2 [12.0], p = 0.506; Fig. 3, top left). Additionally, Schirmer’s test scores of the HPG/HA and CMC/HA groups changed from baseline (week 1, 1.6 [6.4] vs. 2.8 [5.1] mm/5 min, p = 0.373; week 3, 1.8 [6.3] vs. 2.4 [5.7] mm/ 5 min, p = 0.688; Fig. 3, top right). In terms of TBUT, only a minimal change from baseline was observed in the HPG/HA and CMC/HA groups (week 1, 0.6 [2.2] vs. − 0.1 [1.8] s, p = 0.184; week 3, 0.5 [2.6] vs. 0.0 [2.1] s, p = 0.389; Fig. 3, bottom left). The mean central corneal sensitivity of the HPG/HA and CMC/HA groups differed negligibly from the baseline (week 1, 0.0 [0.2] vs. − 0.0 [0.5], p = 0.854; week 3, 0.0 [0.3] vs. 0.1 [0.4], p = 0.275; Fig. 3, bottom right).
Safety Endpoints
Generally, both artificial tears were well tolerated. Only one AE occurred during the study. One patient (2.9%) in the HPG/HA group experienced a mild AE of ocular hypertension, which was determined to be unrelated to the HPG/HA lubricant eye drops. The AE completely resolved after the patient received 1 week of ocular medications. The CMC/HA group did not report any AEs throughout the course of the study.
Discussion
Cataract surgery is an effective treatment for improving visual acuity [3]. However, at least one study observed a clear association between cataract surgery and the development or exacerbation of DED [27]. Intraoperative factors causing DED include incisional nerve damage and ocular surface trauma. Additionally, surgical procedures encompass repeated drying and irrigation of the ocular surface and using antiseptic agents such as povidone-iodine or antibiotics may cause toxicity to the ocular surface. Other contributing factors to DED include the phototoxic effects of the operating microscope and surgical trauma caused by femtosecond-laser-assisted cataract surgery. Consequently, these factors may compromise the patients’ ocular surface by disrupting tear film homeostasis, causing DED [27]. Numerous topical artificial tears are widely available to treat DED [9]. However, direct comparisons between specific artificial tears are still necessary to determine which products are most suitable for which patients. The present study is the first head-to-head comparison study evaluating the efficacy of HPG/HA and CMC/HA lubricant eye drops in patients with DED after cataract surgery.
More than half of the 70 patients in this study had hypertension and hyperlipidemia, and approximately one-quarter of the patients had DM. Advanced age and female sex are risk factors for post-cataract surgery DED, and DM is a risk factor for developing DED even in the absence of cataracts [28, 29]. DM-related DED is likely caused by several mechanisms, such as antiglycemic medications, abnormal tear dynamics due to osmolarity changes, tear film instability resulting from dysfunction of the lacrimal functional unit or meibomian gland, altered enzyme metabolism, and decreased mucin secretion [29].
At weeks 1 and 3, the HPG/HA and CMC/HA groups experienced improvement in several outcomes related to DED. As for the primary endpoints, the HPG/HA group had the most improved CFS scores at 1-week post-operation. CFS scores and the National Eye Institute scale enable a numerical assessment of corneal tissue cell damage following treatment [7]. The improvements in CFS scores observed in this study may be due to the application of lubricant eye drops containing HA. HA binds to hyaladherins to activate various intracellular signaling pathways dependent on concentration, molecular weight, and modifications to the HA molecule, promoting wound healing [30]. Furthermore, the lubricating properties of HA can mitigate the effects of mechanical trauma and facilitate the re-epithelization of ocular cells [30]. The HPG and CMC in artificial tears containing HA promote a synergistic effect between the polymer and HA, increasing the effectiveness of the eye drops [13, 31]. In the HPG/HA lubricant eye drops, the surface-active HPG binds to the damaged portion of the epithelial cells and forms a mucin–guar demulcent network with the natural mucin layer. These processes provide additional lubricity and promote natural surface epithelial cell repair [32]. Furthermore, the bioadhesive properties of HPG promote the retention of HA on the ocular surface, promoting corneal wound healing through a dual-polymer synergistic action [31]. By contrast, in the CMC/HA lubricant eye drops, CMC has strong mucoadhesive and lubricating properties that enhance the retention of HA on the ocular surface and accelerate recovery [14, 33]. CMC and HA form a bridged matrix of polymers that maintain hydration and stabilize the tear film [7]. Although both HPG and CMC work with HA to promote corneal epithelization [14, 31], the results of this study indicate that HPG/HA lubricant eye drops enable faster corneal wound recovery than CMC/HA drops, as indicated by improvements in CFS score. This finding aligns with the results of a preclinical study that demonstrated a higher corneal re-epithelialization rate using HPG/HA lubricant eye drops compared with artificial tears containing HA, including CMC/HA lubricant eye drops [31]. One study comparing HPG and CMC lubricant eye drops without added HA indicated that HPG drops were more effective than CMC drops in improving CFS scores and managing other dry eye signs and symptoms [32]. The HPG/HA drops used in this study had a molecular weight as high as 1334 kDA and were HPG-predominant [13]. Because artificial tears with higher viscosity are preferable in treating ocular surface damage [13], the greater CFS score improvements observed in the HPG/HA group in this study may be due to the higher viscosity of HPG. Moreover, the pH-sensitive nature of HPG increases the viscosity of the drops exposed to the ocular surface [32], promoting faster corneal wound recovery.
Regarding the secondary endpoints, although improvements in dry eye parameters was observed in both groups, no statistically significant differences were observed between groups at weeks 1 and 3. The OSDI scores of both groups decreased to less than 12, indicating a change from mild dry eye to a normal healthy state as early as 1 week after treatment [19]. Moreover, this improvement was sustained until the end of the study. The OSDI scores of both groups decreased more than the minimal clinically important difference (MCID) of 4.5 to 7.3 for mild or moderate DED [19], with the HPG/HA group MCID decreasing by 8.1 and 9.9 and the CMC/HA group MCID decreasing by 7.7 and 8.2 at weeks 1 and 3, respectively. The clinical improvement in patient-reported outcomes may be explained by the synergistic effects of HPG/CMC and HA that lubricate the eyes for sustained periods [34, 35]. Additionally, due to the diverse mechanisms contributing to DED, the Schirmer test is only weakly associated with post-cataract surgery DED [28]. However, the test is still worth exploring owing to its utility in assessing the tear secretion and reflex [21, 36]. In this study, after 1 week of treatment, both groups exhibited a mean change in Schirmer’s test results comparable to the day 7 results observed in another study (mean change of 1.6) [37]. The minimal changes of 0.5 and 0.0 s in TBUT for the HPG/HA and CMC/HA groups at week 3 (the fourth-week post-operation) were consistent with the findings from other studies, which showed TBUT changes of less than 0.5 s at the first month compared with the first day after surgery [8, 38]. However, substantial increases in TBUT were only observed from the second month onward [8, 15, 39]. Hence, studies with longer follow-up time are warranted to investigate the long-term benefits of artificial tears on TBUT. Regarding central corneal sensitivity, one small-scale study on patients with Sjögren’s syndrome identified a negative correlation between CFS scores and corneal sensitivity [40]. Consequently, an increase in central corneal sensitivity was expected in the present study following a decrease in CFS scores over weeks. In the present study, measurements of the central corneal sensitivity of the patients revealed minimal changes from baseline. However, this negligible change was not entirely unexpected, as the patients had corneal sensitivity scores close to the maximum value of 6 cm at baseline.
Overall, both artificial tears examined in the present study were safe, with only one mild AE of ocular hypertension reported in the HPG/HA group. Ocular hypertension is a common postoperative complication, as another study indicated [41].
Artificial tears containing HA alleviate DED symptoms by promoting wound healing and protecting damaged surfaces during healing [13, 30]. HA binds to hyaladherins, activating intracellular signaling pathways to modulate inflammation, cellular migration, and angiogenesis. Several factors may affect the performance of artificial tears, including variations in HA concentration, variations in molecular weight, additions of polymers, variations along the polydispersion index, and variations in osmolarity across different formulations. The concentrations of HA in the HPG/HA and CMC/HA lubricant eye drops utilized in the present study were 0.15 and 0.1%, respectively [13]. Improvements in epithelial cell damage and tear film stability were observed in an animal model when the concentration of HA was increased from 0.1 to 0.3%; in human studies, HA concentrations between 0.1 and 0.2% provided superior objective outcomes, symptom relief, and patient comfort [30]. The addition of copolymers (HPG and CMC) to the artificial tears containing HA improved performance in treating the symptoms of DED [13, 42].
Treatment for DED present before cataract surgery is also crucial as it may affect postoperative outcomes and patient satisfaction following surgery [43]. Although cataract surgery may exacerbate pre-existing dry eye conditions [27], pre-existing DED may also affect preoperative anterior corneal power measurements, leading to inaccurate intraocular lens power predictions for surgery [43]. Therefore, following cataract surgery, patients must receive adequate treatment for both pre-existing and newly developed DED. Additionally, DED is significantly associated with decreased quality of life and increased economic burden [27, 44].
This study has several limitations. First, this was a single-center study and only included Taiwanese patients; therefore, the findings may not be generalizable to other populations. Second, caution must be exercised when interpreting the secondary outcomes because the sample size was calculated based on the primary outcome. Third, the self-reported OSDI scores may have been influenced by the experiences of the nonstudy eye. However, the influence of nonstudy eyes on the study should be minimal because masked assessors objectively evaluated the other outcome measurements. Fourth, this study only provides evidence of the effects of applying artificial tears containing HA over a 3-week treatment period for patients with post-cataract surgery DED. Some studies have indicated that TBUT measurements return to preoperative values from the second month onwards [8, 15, 39], which may indicate that the amelioration of TBUT requires a longer time frame than that used in the present study. Hence, studies with longer treatment duration may be necessary to assess the long-term efficacy of artificial tears containing HA.
Lubricant eye drops containing HPG, CMC, and HA have long been used to manage DED effectively [5, 30, 37, 45–47]. Several studies have compared HPG and CMC lubricant eye drops for managing DED [48, 49]. However, the study team was unaware of studies directly comparing HPG/HA and CMC/HA lubricant eye drops in patients with DED after cataract surgery and could not locate any relevant trials on PubMed at the time of manuscript preparation. Thus, the findings of this study may provide insights into the efficacy of HPG/HA and CMC/HA lubricant eye drops [50].
Conclusions
In summary, the HPG/HA and CMC/HA groups demonstrated improvement in dry eye parameters after treatment, with the HPG/HA group achieving greater and faster improvements in CFS scores than the CMC/HA group as early as 1 week after treatment.
Author Contribution
Chi-Chin Sun and Pei-Wei Huang devised the project and its main conceptual ideas. Yuan-Hsi Chan and Nan-Ni Chen contributed to implementing the research. Chi-Chin Sun contributed to data interpretation, took the lead in writing the manuscript, and supervised the project.
Funding
The study received support from a grant provided by Alcon Services AG, Taiwan Branch (Investigator-Initiated Trial # 60298913). The journal’s Rapid Service fee was also funded by Alcon Services AG, Taiwan Branch.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Conflict of Interest
Chi-Chin Sun, Yuan-Hsi Chan, Pei-Wei Huang, and Nan-Ni Chen confirm that they have no competing interests to declare.
Ethical Approval
This study was approved by the Institutional Review Board (IRB) of the Chang Gung Medical Foundation (IRB No.: 202002539A3) prior to any study procedures. The research team members adhered to the tenets of the Declaration of Helsinki throughout the study. Informed consent was obtained from all participants involved in the study.
References
- 1.Fogagnolo P, Favuzza E, Marchina D, et al. New therapeutic strategy and innovative lubricating ophthalmic solution in minimizing dry eye disease associated with cataract surgery: a randomized, prospective study. Adv Ther. 2020;37(4):1664–74. 10.1007/s12325-020-01288-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute for Health and Care Excellence: Guidelines. Cataracts in adults: management. National Institute for Health and Care Excellence (NICE). 2017. [PubMed]
- 3.Moshirfar M, Milner D, Patel BC. Cataract Surgery. StatPearls. StatPearls Publishing, StatPearls Publishing LLC.; 2023. [PubMed]
- 4.Fang R, Yu YF, Li EJ, et al. Global, regional, national burden and gender disparity of cataract: findings from the global burden of disease study 2019. BMC Public Health. 2022;22(1):2068. 10.1186/s12889-022-14491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mencucci R, Boccalini C, Caputo R, Favuzza E. Effect of a hyaluronic acid and carboxymethylcellulose ophthalmic solution on ocular comfort and tear-film instability after cataract surgery. J Cataract Refract Surg. 2015;41(8):1699–704. 10.1016/j.jcrs.2014.12.056. [DOI] [PubMed] [Google Scholar]
- 6.Sutu C, Fukuoka H, Afshari NA. Mechanisms and management of dry eye in cataract surgery patients. Curr Opin Ophthalmol. 2016;27(1):24–30. 10.1097/ICU.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 7.Mencucci R, Boccalini C, Caputo R, Favuzza E. Effect of a hyaluronic acid and carboxymethylcellulose ophthalmic solution on ocular comfort and tear-film instability after cataract surgery. J Cataract Refract Surg. 2015;41(8):1699–704. 10.1016/j.jcrs.2014.12.056. [DOI] [PubMed] [Google Scholar]
- 8.Cetinkaya S, Mestan E, Acir NO, Cetinkaya YF, Dadaci Z, Yener HI. The course of dry eye after phacoemulsification surgery. BMC Ophthalmol. 2015;15:68. 10.1186/s12886-015-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575–628. 10.1016/j.jtos.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal P, Craig JP, Rupenthal ID. Formulation considerations for the management of dry eye disease. Pharmaceutics. 2021. 10.3390/pharmaceutics13020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Labetoulle M, Schmickler S, Galarreta D, et al. Efficacy and safety of dual-polymer hydroxypropyl guar- and hyaluronic acid-containing lubricant eyedrops for the management of dry-eye disease: a randomized double-masked clinical study. Clin Ophthalmol. 2018;12:2499–508. 10.2147/opth.S177176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davitt WF, Bloomenstein M, Christensen M, Martin AE. Efficacy in patients with dry eye after treatment with a new lubricant eye drop formulation. J Ocul Pharmacol Ther. 2010;26(4):347–53. 10.1089/jop.2010.0025. [DOI] [PubMed] [Google Scholar]
- 13.Aragona P, Simmons PA, Wang H, Wang T. Physicochemical properties of hyaluronic acid-based lubricant eye drops. Transl Vis Sci Technol. 2019;8(6):2. 10.1167/tvst.8.6.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrett Q, Simmons PA, Xu S, et al. Carboxymethylcellulose binds to human corneal epithelial cells and is a modulator of corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2007;48(4):1559–67. 10.1167/iovs.06-0848. [DOI] [PubMed] [Google Scholar]
- 15.Favuzza E, Cennamo M, Vicchio L, Giansanti F, Mencucci R. Protecting the ocular surface in cataract surgery: the efficacy of the perioperative use of a hydroxypropyl guar and hyaluronic acid ophthalmic solution. Clin Ophthalmol. 2020;14:1769–75. 10.2147/opth.S259704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemp MA. Report of the National Eye Institute/industry workshop on clinical trials in dry eyes. Clao j. 1995;21(4):221–32. [PubMed] [Google Scholar]
- 17.Amparo F, Wang H, Yin J, Marmalidou A, Dana R. Evaluating corneal fluorescein staining using a novel automated method. Investig Ophthalmol Visual Sci. 2017;58(6):BIO168–73. 10.1167/iovs.17-21831. [DOI] [PubMed] [Google Scholar]
- 18.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118(5):615–21. 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 19.Miller KL, Walt JG, Mink DR, et al. Minimal clinically important difference for the ocular surface disease index. Arch Ophthalmol. 2010;128(1):94–101. 10.1001/archophthalmol.2009.356. [DOI] [PubMed] [Google Scholar]
- 20.Chan YH, Sun CC. Efficacy and safety of topical cyclosporine 0.1% in moderate-to-severe dry eye disease refractory to topical cyclosporine 0.05% regimen. Taiwan J Ophthalmol. 2023;13(1):68–74. 10.4103/tjo.TJO-D-22-00140 [DOI] [PMC free article] [PubMed]
- 21.Brott NR, Ronquillo Y. Schirmer Test. StatPearls. StatPearls Publishing [PubMed]
- 22.Copyright © 2023, StatPearls Publishing LLC.; 2023.
- 23.Sweeney DF, Millar TJ, Raju SR. Tear film stability: a review. Exp Eye Res. 2013;117:28–38. 10.1016/j.exer.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Vidas Pauk S, Petriček I, Jukić T, et al. Noninvasive tear film break-up time assessment using handheld lipid layer examination instrument. Acta Clin Croat. 2019;58(1):63–71. 10.20471/acc.2019.58.01.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pflugfelder SC, Tseng SC, Sanabria O, et al. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea. 1998;17(1):38–56. 10.1097/00003226-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Tai YC, Sun CC. Effects of flap diameter on dry eye parameters and corneal sensation after femtosecond laser-assisted LASIK. Taiwan J Ophthalmol Jul-Sep. 2019;9(3):166–72. 10.4103/tjo.tjo_59_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahman EZ, Lam PK, Chu CK, Moore Q, Pflugfelder SC. Corneal sensitivity in tear dysfunction and its correlation with clinical parameters and blink rate. Am J Ophthalmol. 2015;160(5):858-866.e5. 10.1016/j.ajo.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naderi K, Gormley J, O’Brart D. Cataract surgery and dry eye disease: a review. Eur J Ophthalmol. 2020;30(5):840–55. 10.1177/1120672120929958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miura M, Inomata T, Nakamura M, et al. Prevalence and characteristics of dry eye disease after cataract surgery: a systematic review and meta-analysis. Ophthalmol Ther. 2022;11(4):1309–32. 10.1007/s40123-022-00513-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan LY, Kuo YK, Chen TH, Sun CC. Dry eye disease in patients with type II diabetes mellitus: a retrospective, population-based cohort study in Taiwan. Front Med (Lausanne). 2022;9: 980714. 10.3389/fmed.2022.980714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hynnekleiv L, Magno M, Vernhardsdottir RR, et al. Hyaluronic acid in the treatment of dry eye disease. Acta Ophthalmol. 2022;100(8):844–60. 10.1111/aos.15159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carlson E, Kao WWY, Ogundele A. Impact of hyaluronic acid-containing artificial tear products on reepithelialization in an in vivo corneal wound model. J Ocul Pharmacol Ther. 2018;34(4):360–4. 10.1089/jop.2017.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christensen MT, Cohen S, Rinehart J, et al. Clinical evaluation of an HP-guar gellable lubricant eye drop for the relief of dryness of the eye. Curr Eye Res. 2004;28(1):55–62. 10.1076/ceyr.28.1.55.23495. [DOI] [PubMed] [Google Scholar]
- 34.Garrett Q, Simmons PA, Xu S, et al. Carboxymethylcellulose binds to human corneal epithelial cells and is a modulator of corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2007;48(4):1559–67. 10.1167/iovs.06-0848. [DOI] [PubMed] [Google Scholar]
- 35.Rangarajan R, Kraybill B, Ogundele A, Ketelson HA. Effects of a hyaluronic acid/hydroxypropyl guar artificial tear solution on protection, recovery, and lubricity in models of corneal epithelium. J Ocul Pharmacol Ther. 2015;31(8):491–7. 10.1089/jop.2014.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallerstein A, Jackson WB, Chambers J, Moezzi AM, Lin H, Simmons PA. Management of post-LASIK dry eye: a multicenter randomized comparison of a new multi-ingredient artificial tear to carboxymethylcellulose. Clin Ophthalmol. 2018;12:839–48. 10.2147/opth.S163744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocular Surface. 2017/07/01/ 2017;15(3):539–574. 10.1016/j.jtos.2017.05.001 [DOI] [PubMed]
- 38.Simmons PA, Liu H, Carlisle-Wilcox C, Vehige JG. Efficacy and safety of two new formulations of artificial tears in subjects with dry eye disease: a 3-month, multicenter, active-controlled, randomized trial. Clin Ophthalmol. 2015;9:665–75. 10.2147/opth.S78184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zamora MG, Caballero EF, Maldonado MJ. Short-term changes in ocular surface signs and symptoms after phacoemulsification. Eur J Ophthalmol. 2020;30(6):1301–7. 10.1177/1120672119896427. [DOI] [PubMed] [Google Scholar]
- 40.Xue W, Zhu MM, Zhu BJ, et al. Long-term impact of dry eye symptoms on vision-related quality of life after phacoemulsification surgery. Int Ophthalmol. 2019;39(2):419–29. 10.1007/s10792-018-0828-z. [DOI] [PubMed] [Google Scholar]
- 41.Adatia FA, Michaeli-Cohen A, Naor J, Caffery B, Bookman A, Slomovic A. Correlation between corneal sensitivity, subjective dry eye symptoms and corneal staining in Sjögren’s syndrome. Can J Ophthalmol. 2004;39(7):767–71. 10.1016/s0008-4182(04)80071-1. [DOI] [PubMed] [Google Scholar]
- 42.Jürgens I, Matheu A, Castilla M. Ocular hypertension after cataract surgery: a comparison of three surgical techniques and two viscoelastics. Ophthalmic Surg Lasers. 1997;28(1):30–6. [PubMed] [Google Scholar]
- 43.Srinivasan S, Garofalo R, Williams R. Safe and effective management of dry eye symptoms with hydroxypropyl guar and hyaluronic acid dual-polymer lubricating eye drops: a review of preclinical and clinical studies. Clin Ophthalmol. 2023;17:3883–98. 10.2147/opth.S428725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim J, Kim MK, Ha Y, Paik HJ, Kim DH. Improved accuracy of intraocular lens power calculation by preoperative management of dry eye disease. BMC Ophthalmol. 2021;21(1):364. 10.1186/s12886-021-02129-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dana R, Meunier J, Markowitz JT, Joseph C, Siffel C. Patient-reported burden of dry eye disease in the United States: results of an online cross-sectional survey. Am J Ophthalmol. 2020;216:7–17. 10.1016/j.ajo.2020.03.044. [DOI] [PubMed] [Google Scholar]
- 46.Springs CL. Novel hydroxypropyl-guar gellable lubricant eye drops for treatment of dry eye. Adv Ther. 2010;27(10):681–90. 10.1007/s12325-010-0052-3. [DOI] [PubMed] [Google Scholar]
- 47.Yang YJ, Lee WY, Kim YJ, Hong YP. A meta-analysis of the efficacy of hyaluronic acid eye drops for the treatment of dry eye syndrome. Int J Environ Res Public Health. 2021. 10.3390/ijerph18052383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Labetoulle M, Chiambaretta F, Shirlaw A, Leaback R, Baudouin C. Osmoprotectants, carboxymethylcellulose and hyaluronic acid multi-ingredient eye drop: a randomised controlled trial in moderate to severe dry eye. Eye (Lond). 2017;31(10):1409–16. 10.1038/eye.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Labetoulle M, Messmer EM, Pisella PJ, Ogundele A, Baudouin C. Safety and efficacy of a hydroxypropyl guar/polyethylene glycol/propylene glycol-based lubricant eye-drop in patients with dry eye. Br J Ophthalmol. 2017;101(4):487–92. 10.1136/bjophthalmol-2016-308608. [DOI] [PubMed] [Google Scholar]
- 50.Guillon M, Maissa CA, Wong S, Griffin JM, Christensen MT. Functional visual performance of Systane ultra after 4 weeks of use. Invest Ophthalmol Vis Sci. 2011;52(14):3834–3834. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.