Abstract
Following bowel surgery, infectious complications, including anastomotic leak (AL), remain major sources of morbidity and mortality. Bowel preparation is often administered with the assumption that gut decontamination reduces post-surgical complications. In this study, we tested this hypothesis using a murine model of colon surgery. The mice were fed either regular chow or a high-fat, high-sugar Western diet. The day before surgery, the mice received one of four interventions: water (control), mechanical bowel preparation (MBP), oral antibiotics (OA), or both MBP and OA. We found no differences in the rates of AL among the experimental groups, and diet did not appear to affect the outcomes. Exploratory analyses showed changes in the gut microbiome consistent with the different treatments, but investigations of fecal short-chain fatty acids and RNA sequencing of colonic tissue did not reveal specific effects of the treatments or the presence of AL. However, we did identify bacterial genera that may be causally associated with AL and developed a predictive index from stool samples as a marker for the presence of AL. Future research is needed to identify and validate a microbial predictive tool and to uncover the microbial-driven mechanisms that lead to AL.
Keywords: Microbiome, Anastomotic leak, Colorectal surgery, Mouse model, Western diet
Subject terms: Clinical microbiology, Gastrointestinal diseases, Preclinical research
Introduction
Colorectal disease is a major health burden and significant contributor to healthcare costs with increasing prevalence related to Western-type diet and an expanding aging population1. Surgical resection and anastomosis (healthy bowel connection) are often necessary for successful treatment of these diseases; accordingly, more than 600,000 colectomies are performed annually in the United States2–4. There are more than 2.7 million outpatient visits and 200,000 inpatient admissions for diverticulitis per year5, and nearly 15% of patients diagnosed eventually require surgery2,3. An estimated 147,950 adults in the United States in 2020 were diagnosed with colorectal cancer, of which the mainstay treatment is surgery6. Up to 20% of all patients with ulcerative colitis, and up to 80% of patients with Crohn’s disease will undergo bowel resection. Microbiota-driven complications, such as anastomotic leak (AL) and surgical site infections (SSI), remain major problems and sources of patient morbidity, mortality, and economic burden. Despite major advances in the technical aspects of surgery and post-surgical clinical care of patients, avoiding these severe complications remains a major challenge.
Surgical bowel preparation (SBP) was introduced by Nichols and Condon in the 1970s to decrease the bacterial and fecal load of the colon prior to surgery, and has remained a controversial topic7. The field of colorectal surgery has vacillated between the use of mechanical bowel preparation (MBP) and/or oral antibiotics (OA). Currently, the most implemented bowel regimen includes polyethylene glycol (PEG) for MBP and combination neomycin plus metronidazole for OA8. The theoretical role of MBP is to mechanically cleanse the intestine to allow for anastomosis (stapled or hand-sewn) without the presence of formed stool, and reduce risk of subsequent fecal impaction at the anastomosis, thereby reducing tension and local ischemia. The purpose of OA is to facilitate the function of the host immune defense system by decreasing bacterial growth at the surgical site9. Many clinical outcomes studies have been performed to evaluate the efficacy of each component and serve as the foundation upon which the enhanced recovery after surgery (ERAS) protocols were published10. Despite its clinical support, the mechanisms behind the efficacy of combination MBP and OA remains unknown. The fact remains that SBP is a non-specific intervention that also leads to the eradication of pathogenic and beneficial microbes, or ‘probiont’ species, that enhance mucosal repair of wounds11. Furthermore, AL and SSI continue to occur after SBP, highlighting room for growth through a more patient-centered approach to pre-operative microbial optimization and a need for basic science investigations to provide mechanistic context to clinical observations.
The intestinal microbiota plays a causative role in AL by antagonizing wound healing through collagenase production12. It has also been demonstrated in murine models that the injured mucosa is markedly different from intact mucosa and is characterized by a microenvironment consisting of cellular migration, proliferation, and tissue remodeling13. Distinct subpopulations of the microbiota preferentially colonize sites of damaged mucosa in response to local environmental cues and drive epithelial wound healing11. The role of bacteria in wound healing is exemplified in germ-free mice, which display impaired intestinal epithelial cell migration that is essential for appropriate healing14. We have previously observed significant shifts in the composition of the intestinal microbiome following SBP15. However, a biological understanding of the direct role that the resultant post-SBP microbiota composition has on wound healing remains unclear.
The aim of this study was to investigate each component of SBP individually and in combination to determine subsequent changes in the microbiota composition that are associated with the development of anastomotic dehiscence. We used a wild-type murine model of colon surgery in the context of regular mouse chow or murine Western diet (WD) to test the relationship between diet-induced dysbiosis and bowel preparation (Fig. 1). Based on clinical observations, we predicted that mice administered SBP would have a significant reduction in AL and SSI. Furthermore, we predicted that this benefit would be increased in WD-fed mice with resultant gut dysbiosis.
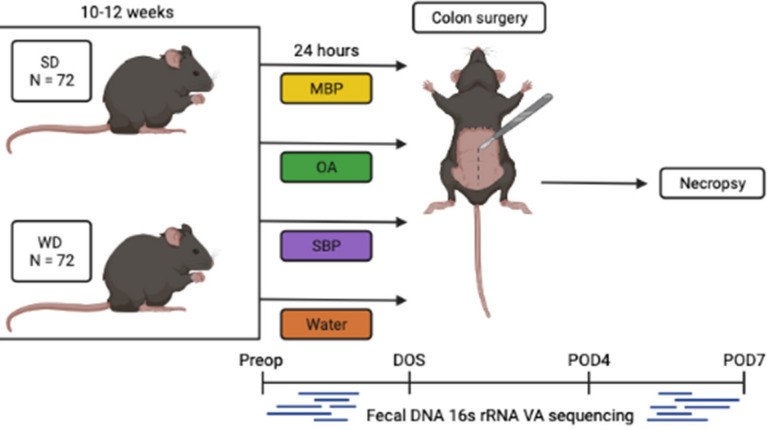
Fig. 1.
Experimental design. N = 144 mice were placed on SD or WD for 10–12 weeks. They received either MBP, OA, SBP, or water ab lib for 24 h prior to colon anastomosis. Necropsy was performed on POD7 to assess anastomotic healing. Fecal pellets were collects 24 h preoperatively (preop), on the morning of surgery (DOS), on POD4, and POD7. This image was created using Biorender.com.
Results
Effect of bowel preparation on clinical endpoints
Of 144 mice, 140 (97%) survived until POD7. All surviving mice demonstrated full clinical recovery with tolerance of post-operative diet, return of bowel function, and lack of signs of infection. Among mice that did not survive until POD7, one mouse was found to have frank anastomotic dehiscence with gross contamination on POD5 and another had colonic obstruction at the site of anastomosis without AL on POD6. The remaining 2 died of unknown reasons in the early perioperative period and were excluded from analysis. Both of their anastomoses were intact. No significant differences in survival were found between dietary and bowel preparation groups (Fisher’s exact P = 1.00; Supplementary Table 1).
Anastomotic leak
All anastomotic leaks were contained with peri-anastomotic abscess, except for the one above mentioned mouse that had frank peritonitis and was excluded. Thus, anastomoses were categorized as either having presence or absence of leak. Leak rates among SD and WD mice were not significantly different: 31/71 (44%) and 21/71 (30%), respectively (Chi-square P = 0.08; Table 1). Similarly, there were no significant differences in AL rate among bowel preparation groups: MBP 17/35 (49%); OA 8/35 (23%); SBP 16/36 (44%); and water 11/36 (31%) (Chi-square P = 0.09; Table 1). Similarly, when the effect of bowel preparation on AL was analyzed separately by diet, no differences were observed (Chi-square P = 0.27 and 0.39 for SD and WD, respectively; Table 1). There was a trend toward fewer ALs in mice treated with OA alone compared to the other treatment groups, with 5/18 (28%) in the SD group and 3/17 (18%) in the WD group experiencing AL (P = 0.07).
Table 1.
AL contingency table: no significant difference in AL observed between dietary groups, bowel preparation treatment groups, or between treatment groups within diets.
| No AL (n) | AL (n) | P value | |
|---|---|---|---|
| SD | 40 | 31 | 0.08 |
| WD | 50 | 21 | |
| MBP | 18 | 17 | 0.09 |
| OA | 27 | 8 | |
| SBP | 20 | 16 | |
| Water | 25 | 11 | |
| SD MBP | 7 | 10 | 0.27 |
| SD OA | 13 | 5 | |
| SD SBP | 9 | 9 | |
| SD Water | 11 | 7 | |
| WD MBP | 11 | 7 | 0.39 |
| WD OA | 14 | 3 | |
| WD SBP | 11 | 7 | |
| WD Water | 14 | 4 |
*Values are for Chi-squared test. Fisher’s exact test used for cells with counts < 5.
Skin closure breakdown
Superficial wound healing was assessed on the day of necropsy and the abdominal skin closure was categorized as either healed or not healed if any amount of incisional breakdown was present. SD mice demonstrated significantly fewer non-healed wounds 33/71 (46%) compared to 58/71 (82%) mice in the WD group (Chi-square P < 0.0001, Table 2). There were no differences in superficial wound healing between bowel preparation groups (Chi-square P = 0.63, Table 2), or when bowel preparation was analyzed separately by diet (Chi-square P = 0.84 and 0.71 for SD and WD, respectively, Table 2).
Table 2.
Superficial wound healing contingency table: significant difference in rate of abdominal skin closure breakdown between diets, but not between bowel preparation groups.
| Healed (n) | Skin breakdown (n) | P value* | |
|---|---|---|---|
| SD | 38 | 33 | < 0.0001 |
| WD | 13 | 58 | |
| MBP | 11 | 24 | 0.63 |
| OA | 11 | 24 | |
| SBP | 16 | 20 | |
| Water | 13 | 23 | |
| SD MBP | 8 | 9 | 0.84 |
| SD OA | 9 | 9 | |
| SD SBP | 11 | 7 | |
| SD Water | 10 | 8 | |
| WD MBP | 3 | 15 | 0.71 |
| WD OA | 2 | 15 | |
| WD SBP | 5 | 13 | |
| WD Water | 3 | 15 |
*Values are for Chi-squared test. Fisher’s exact test used for cells with counts < 5.
Body weight
After the preoperative feeding period, mean body weight on the day of surgery (DOS) was higher in the WD compared to SD mice: 32.1 ± 3.0 g and 30.2 ± 2.7 g for WD- and SD-fed mice, respectively (ANOVA F = 15.9, P = 0.0001).
Community diversity
Following normalization to 5,000 and 9,350 sequence reads for fecal and serum/tissue samples, respectively, the estimated Good’s coverage for all samples was ≥ 98% (Supplementary Table 2). Only four serum samples had sufficient reads following quality control to be included in the dataset. Microbial communities characterized from tissue samples were more diverse than those from fecal samples, with serum samples showing significantly lower richness despite having a statistically greater Shannon index than other tissue types, potentially due to a greater rarefaction depth (Supplementary Table 2). Among fecal samples, regardless of treatment group, mice fed a SD had significantly greater Shannon and Chao1 indices (3.21, [3.07–3.31] and 300, [259–353], respectively) than did mice fed WD (2.98, [2.79–3.16] and 239, [204–287]; Kruskal–Wallis P < 0.0001). Specifically, at the pre-op timepoint, SD-fed mice had significantly greater Shannon and Chao1 indices (3.21, [3.08–3.31] and 284, [249–328], respectively) than WD-fed mice (2.99, [2.78–3.17] and 227, [192–265]; Kruskal–Wallis P < 0.0001). Similarly, on DOS, mice fed a SD had significantly greater Shannon and Chao1 indices (3.20, [3.10–3.27] and 307, [275–369], respectively) than WD mice (2.88, [2.71–3.12] and 235, [201–289]; Kruskal–Wallis P < 0.0001). Moreover, there were significant differences in beta diversity between SD- and WD-fed mice at pre-op and DOS timepoints (ANOSIM R = 0.389, P < 0.001 and R = 0.340, P < 0.001, respectively). Due to significant differences by diet, SD and WD groups were evaluated separately in subsequent analysis.
Among SD mice, temporal variation was only observed in the group receiving oral antibiotics (OA), where the pre-op group had significantly greater Shannon indices than communities characterized at POD7 (Dunn’s post-hoc P = 0.005; Table 3). Moreover, the OA group at POD7 had significantly lower Shannon indices than the SBP group at the same time point (P = 0.007). Among WD mice, both the OA and SBP groups saw significant temporal changes in alpha diversity. In WD OA mice, diversity on DOS was significantly lower than at POD4 (P = 0.004). Similarly, in WD SBP mice, diversity at DOS was significantly lower than at pre-op (P = 0.004). No significant differences were observed among treatment groups at any single time point. These relationships were maintained when evaluated using the Chao1 index, but fewer comparisons achieved statistical significance (data not shown).
Table 3.
Shannon indices (media [IQR]) summarized by diet, treatment group, and time point.
| Diet | Treatment | Pre-op | DOS | POD4 | POD7 | P value |
|---|---|---|---|---|---|---|
| SD | MBP | 3.13, [3.08–3.26] | 3.17, [3.09–3.26] | 3.26, [3.06–3.35] | 3.15, [3.13–3.27] XY | 0.541 |
| OA | 3.24, [3.14–3.35] A | 3.20, [3.09–3.32] AB | 3.18, [3.05–3.26] AB | 2.93, [2.72–3.08] B;Y | 0.021 | |
| SBP | 3.25, [3.05–3.32] | 3.15, [3.09–3.24] | 3.21, [3.08–3.31] | 3.22, [3.10–3.31] X | 0.824 | |
| Water | 3.20, [3.11–3.28] | 3.23, [3.12–3.31] | 3.27, [3.23–3.35] | 3.22, [3.10–3.31] XY | 0.244 | |
| P-value | 0.560 | 0.459 | 0.233 | 0.020 | ||
| WD | MBP | 3.18, [2.89–3.31] | 3.11, [2.89–3.26] | 3.08, [2.90–3.22] | 2.97, [2.87–3.19] | 0.779 |
| OA | 2.86, [2.74–2.99] AB | 2.79, [2.58–2.87] B | 3.01, [2.87–3.22] A | 3.01, [2.88–3.12] AB | 0.018 | |
| SBP | 3.00, [2.90–3.09] A | 2.74, [2.66–2.88] B | 2.97, [2.75–3.09] AB | 2.93, [2.84–2.97] AB | 0.029 | |
| Water | 2.99, [2.72–3.15] | 3.12, [2.84–3.19] | 3.02, [2.81–3.30] | 3.02, [2.81–3.12] | 0.754 | |
| P-value | 0.207 | 0.754 | 0.578 | 0.579 | ||
ABCells sharing the same letter across a row did not differ significantly by Dunn’s post-hoc test with Bonferroni correction for multiple comparisons (adjusted α = 0.0083).
XYCells sharing the same letter down a column, within a dietary group, did not differ significantly by Dunn’s post-hoc test with Bonferroni correction for multiple comparisons (adjusted α = 0.0083).
Kruskal–Wallis analyses were done on each row and each diet, separately for standard chow diet (SD) and western diet (WD).
Microbiota signature for AL
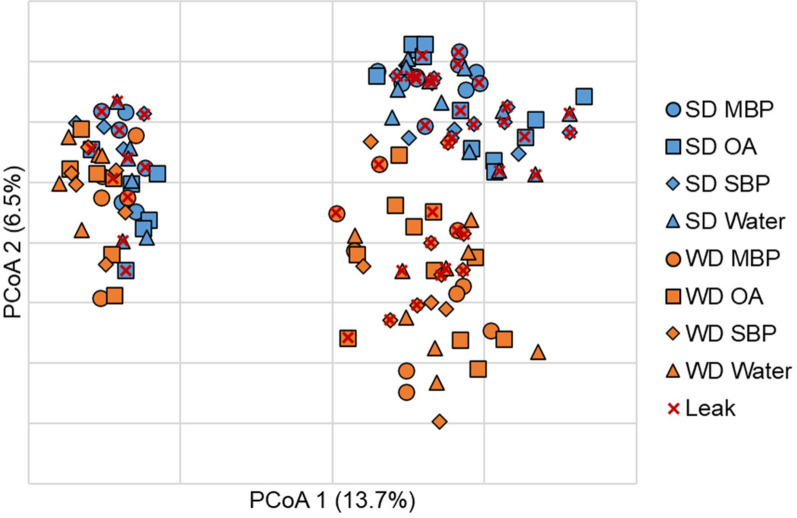
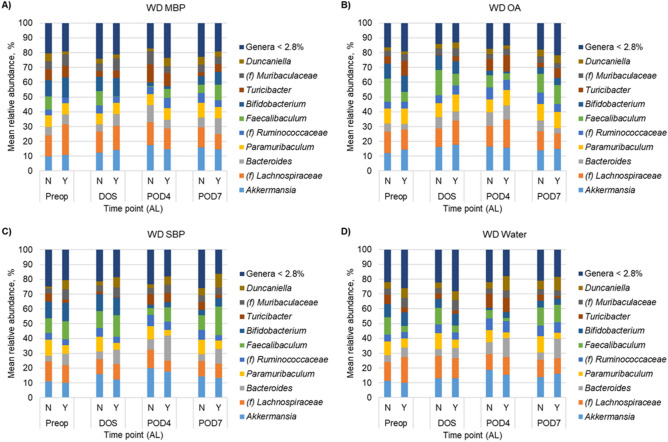
Similar to alpha diversity, beta diversity analysis revealed significant separation of SD- and WD-fed mice who did not have AL (ANOSIM R = 0.215, P < 0.001). However, among mice with AL, differences due to diet were not significant (R = 0.114, P = 0.048, at Bonferroni-corrected α = 0.008). Unexpectedly, no clustering was observed among treatment groups on either diet (POD4 shown in Fig. 2). Few differences in genera were observed in mice that experienced AL when treatment groups were compared individually at DOS and POD4. Therefore, SD- and WD-fed mice were combined by diet, without respect to preparation treatment group, to determine genera associated with AL. Among SD groups, no significant differences in genera were observed at DOS or POD4 between mice with or without AL (Supplementary Fig. 1; Kruskal–Wallis P ≥ 0.066). However, among WD-fed mice, Bacteroides and further unclassified members of family Muribaculaceae had significantly greater relative abundances in mice with AL on DOS (K = 4.51 and 12.92; P = 0.034 and < 0.001, respectively; Fig. 3). These relationships were maintained at POD4 (K = 8.41 and 4.87; P = 0.022 and 0.027). In addition, at POD4, relative abundances of Paramuribaculum and further unclassified members of Ruminococcaceae were greater in WD-mice that did not have AL (K = 5.28 and 4.48; P = 0.022 and 0.034).
Fig. 2.
Principal coordinate analysis of Bray–Curtis dissimilarities (r2 = 0.57) at POD4. SD-fed mice are shown in blue and WD-fed mice are shown in orange. Treatment groups are indicated by symbols which are conserved across both diets. Samples overlayed with × indicated mice that had AL across all time points.
Fig. 3.
Distribution of predominant genera among fecal samples for WD-fed mice. For clarity, the top 10 predominant genera are shown and the less abundant genera were consolidated. Each genus consolidated had a mean relative abundance < 2.8% of normalized sequence reads. (f) Indicates the taxon could not be classified to greater resolution than family.
We have previously developed an index to predict AL at POD4 based on abundances of putative protective genera (sum of abundances of Paramuribaculum, Clostridium senso stricto, and Alistipes) as the numerator and putative drivers of AL (sum of abundances of Bacteroides, Akkermansia, and Dubosiella) as the denominator16. In our experience, mice with low values for this index (< 0.45) are more likely to have AL. Using our previous index, applied to the current data, it had an overall accuracy of 50% with sensitivity of 79% and specificity of 34% to predict AL16. When taxa that were found to have significant differential abundances related to leak in the current dataset were incorporated into a similar regression tree analysis, model accuracy was 74%, with sensitivity of 45% and specificity of 91%. These results indicate that our original index, while less accurate overall, has approximately 30% greater chance to identify AL versus an index based on the current data, despite improved specificity using the latter. Comparison of our original AL index across diet and treatment groups revealed insignificant differences among all groups (ANOVA P = 0.510).
Changes in fecal short-chain fatty acids (SCFA) and immunoglobulin A (IgA)
To assess changes in microbial metabolites, fecal SCFA were quantified. In the SD-fed mice, there were significant differences in the average concentration of butyrate on DOS versus POD7 in the water group (P = 0.039), propionate DOS versus POD7 in the water and SBP groups (P = 0.0003 and 0.006, respectively), and in acetate DOS versus POD7 in the water and SBP groups (P = 0.002 and 0.028, respectively) (Supplementary Fig. 2A-C).There were no significant differences in the average concentration of butyrate, propionate, and acetate in WD-fed mice across timepoints (preop, DOS, and POD7) or between bowel preparation treatment groups (Supplementary Fig. 2D-F). However, there were significant differences in the average concentration of acetate, propionate, butyrate, and valerate across diets at pre-op, DOS, and POD7 timepoints (Supplementary Fig. 3A,D-F).
To characterize immune-associated changes, IgA was also quantified (Supplementary Fig. 4). WD was associated with significantly higher levels of fecal IgA (measured on POD7) with mean concentrations of 325 ng IgA/mg stool in the no AL group and 469 ng IgA/mg stool in the AL group compared to SD, which had a mean of 132 ng IgA/mg stool in the no AL group and 177 ng IgA/mg stool in the AL group. Mean IgA was significantly higher in WD mice with AL versus no AL (469 ng IgA/mg stool vs. 325 ng IgA/mg stool, P = 0.007). SD mice with leak had greater mean IgA, however this was not significant (177 ng IgA/mg stool for leak vs. 132 ng IgA/mg stool for no leak, P = 0.38).
Serum and tissue communities associated with AL
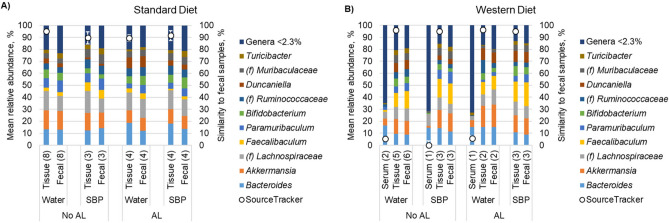
To evaluate potential translocation of the fecal microbiota to either the AL lesions or the serum, the microbiota was characterized from matched fecal, colonic tissue adjacent to the anastomosis, and serum samples at POD7 (Fig. 4). Among samples from mice fed SD, no serum samples achieved sufficient sequence reads to be included in the analysis, while four were included among samples from mice fed WD, with only one from a mouse who had AL (Supplementary Fig. 5). We defined fecal communities as “source” communities using the Bayesian algorithm SourceTracker5, and evaluated the percent of tissue and sera communities that were attributable. Both taxonomically and as determined by SourceTracker, communities from tissue samples were highly similar to fecal communities with > 85% of the community attributable to fecal samples. In contrast, communities from serum samples showed much lower (≤ 10.7%) similarity. Fecal signatures in serum samples consisted of: (i) Bacteroides (3.4%), Paramuribaculum (5.1%), and Faecalibacterium (2.3%); (ii) Turicibacter (0.1%) and Lactobacillus (0.1%); or (iii) Bacteroides (3.4%) and Turicibacter (2.3%), and this third community was observed in the mouse that had AL. The final serum sample had no attributable taxa to fecal communities.
Fig. 4.
Distribution of predominant genera among matched fecal, serum, and tissue samples collected at POD7. Numbers in parentheses reflect sample sizes used for data analysis. For clarity, the top 10 predominant genera are shown and the less abundant genera were consolidated. Each genus consolidated had a mean relative abundance < 2.3% of normalized sequence reads. (f) Indicates the taxon could not be classified to greater resolution than family. SourceTracker indicates the percent of the community in tissue and serum samples that could be attributed to the fecal communities.
Few changes in murine gene expression associated with AL
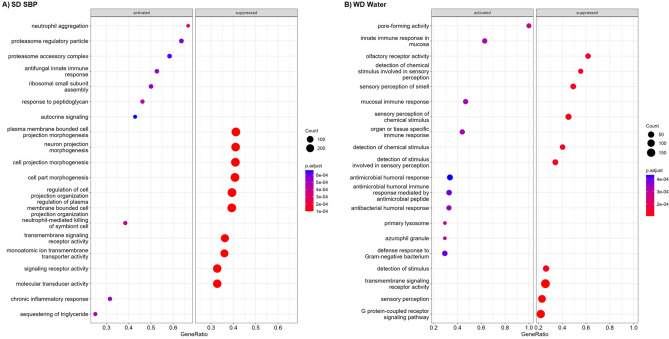
RNAseq of host colonic tissue was done for mice in the SBP (n = 8) and water (n = 12) treatment groups for both SD and WD (n = 40, total). No significant differences in host transcript abundances for individual genes were detected in either treatment between diets (adjusted P > 0.3 for SD and > 0.9 for WD). However, significant differences in transcript abundances were observed for genes in the SBP group fed SD and in the water group fed WD (Fig. 5, Supplementary Fig. 6A-D). To determine relationships between gene transcripts and the fecal microbiome, canonical correlation analysis was performed (Supplementary Fig. 6E). By this analysis, members of family Lachnospiraceae were correlated with C–C motif chemokine ligand 25 (Ccl25), a protein associated with LIN7 2 (Pals2), paired-like homeodomain transcription factor 2 (Pitx2), and ST3 beta-galactoside alpha-2,3-sialyltransferase 5 (St3gal5) genes. The strongest association was observed for Pitx2 which is a transcription factor that establishes the asymmetry of the left and right sides of the tissues that give rise to the vertebrate gut17. Class Coriobacteriia was associated with anoctamin 1 (Ano1), cytochrome P450: family 2, subfamily c, polypeptides 68 and 69 (Cyp2c68 and Cyp2c69 respectively), grainyhead like transcription factor 3 (GRHL3), paired box 5 (Pax5), transglutaminase 3 (TGM3), and synaptic Ras GTPase activating protein 1 (Srgap1). The strongest association was observed with the GRHL3 gene which is essential for formation of the skin barrier during embryonic development18.
Fig. 5.
Dot plots showing pathways that were significantly activated or suppressed in mice with AL. The y-axis shows pathways with differential transcript abundances. Adjusted p-values in the legend and gene ratios (axis title in x-axis) for each significantly enriched pathway are represented as a function of color and size of dots, respectively. GeneRatio represents count/setSize wherein 'count' is the number of genes that belong to a given gene-set (here, KEGG), while 'setSize' is the total number of genes in the gene-set (here, KEGG).
Discussion
While perioperative protocols have resulted in major improvements in patient recovery, current approaches designed to modify gut microbial composition for optimized wound healing remain poorly understood and suboptimized. Here, we tested the impact of perioperative bowel preparation on colonic anastomotic and surgical site wound healing across two different diets and assessed microbiota-associated physiologic impacts. We predicted that SBP would have the most significant impact in reducing diet and surgically induced dysbiosis resulting in a reduction in AL and enhanced wound healing. Conversely, we observed no differences in AL and wound healing rates resulting from exposure to bowel preparation in mice with and without diet-induced dysbiosis.
SBP is a widely implemented intervention, as high as 79% among colorectal surgeons in the United States, to prepare patients for gastrointestinal surgery19. AL complicates up to 14% of all colorectal surgical operations20, while SSI complicate up to 27% of all operations20,21. Thus, identifying and refining tools to reduce these complications remain critical needs. While many clinical outcomes studies have been performed evaluating the efficacy of each component, most trials have not included OA which has added confusion regarding the optimal protocol. A Cochrane review of 18 randomized trials with 5,805 patients did not detect significant differences in rates of AL with the use of MBP following colorectal operations22. Indeed, these trials and data served as the foundation upon which the enhanced recovery after surgery (ERAS) protocols recommended against the routine use of MBP10. However, evaluating each individual component may not accurately reflect an increased efficacy when MBP and OA are used in combination. Accordingly, multiple studies have shown a reduction in AL with combined MBP and OA prior to undergoing colonic surgery23–26. Notably, a recent randomized controlled trial, the MOBILE2 trial, showed significant reductions in AL and SSI with use of MBP and OA relative to MBP and placebo27. Even with our robust number of operations, neither AL nor superficial wound healing was impacted by any of the bowel preparation interventions, despite impacts on the microbial composition. As expected, mice receiving oral antibiotics had the greatest changes in microbial composition. Given the deleterious impact of WD on metabolism and healing28,29, it was also expected that WD fed mice would exhibit poorer healing capacity. While this was observed by the three-fold higher rate of wound breakdown in WD fed mice relative to SD fed mice, we did not observe a difference in overall AL rates between the two diet cohorts. Differences among diet with respect to AL may have been mitigated due to the uniform implementation of DietGel post-operatively. This was done purposefully to evaluate the clinical impact of the preoperative diets and interventions. However, even with the same postoperative diet, there was still major separation in the microbiota composition driven by preoperative diet.
A tool identifying the presence of AL would be highly valuable in the clinical setting, thus our application of our previously developed index16. The intestinal microbiota has been proposed as a primary vehicle through which genetics and environment interact to directly affect gut homeostasis and healing. Distinct subpopulations of the microbiota preferentially colonize sites of damaged mucosa in response to local environmental cues and drive epithelial wound healing11. It is then logical to expect that a predictive tool may be created that reflects dysbiosis, which may predict anastomotic breakdown thus allowing for interventions early in the postoperative period. It is noteworthy that no differences were observed in the composition of mice that had AL due to diet, suggesting that AL may be driven by stochastic microbiota processes unrelated to overall community composition. Utilizing this tool in applying it to each intervention and diet, we observed no differences in the scores produced from each intervention further supporting the conclusion that any effect on clinical outcomes is unlikely to occur directly through changes produced in the microbiota16. This is further supported by our human studies in which we have observed greater abundances of Enterococcus, Lactobacillus, and Streptococcus in AL15. Enterococcus has been mechanistically linked to the development of AL via the production of matrix metalloproteinase 9, which degrades collagen and results in tissue breakdown12. Enterococcus and Streptococcus are also potent bacteriocin and lactic acid producing bacteria30,31.
We measured fecal levels of SCFA to explore changes in microbial metabolites, critical in the regulation of colonic inflammation and health. Dysbiotic microbiota with depleted SCFA have been shown to dysregulate immune function, propagate intestinal and systemic inflammation, and impede wound healing11. SCFA levels were not significantly different among the cohorts, thus suggesting that they may not mediate clinical benefits related to bowel preparation. In our human investigations, we have observed significant reductions in butyrate shortly following surgery, in line with our observations that SCFA are unlikely to mediate any benefit of bowel preparation in surgery15. Interestingly, we did observe a difference in fecal levels of IgA. As expected, mice fed WD had significantly increased levels of fecal IgA. This is consistent with others who have demonstrated increased IgA driven by the presence of increased pro-inflammatory microbiota32–34. We and others have previously shown that fecal IgA levels inversely correlate with alpha diversity15,35. Given the inflammation driven by a robust process such as AL, we expected fecal IgA to discriminate between the presence or absence of AL regardless of baseline diet. While this was true among WD fed mice, this was not so for SD fed mice. While diet had a strong role in influencing baseline levels of IgA, it is likely that the immune response elicited by surgery and the development of AL are also markedly different within the background of the different diets. This difference may suggest that each additional inciting event promotes a more robust inflammatory response, as demonstrated by markedly increased levels in WD fed mice with AL. Future studies are required to delineate whether IgA may serve as a clinical marker for the detection of AL.
To evaluate direct host effects of bowel preparation, RNAseq was performed on the colonic tissue adjacent to the anastomosis due to the potential confounding effects of significant inflammation should there be the presence of AL. Interestingly, we observed significant differences in leak versus no leak cohorts in both the SD SBP and WD water cohorts, and no differences among the SD water and WD SBP cohorts, regardless of leak status. These observations may suggest that mice fed SD have a more resilient microbial community that was only disturbed enough to impact host immunity in the SBP group. In contrast, WD-fed mice may have much less resilient communities such that any intervention perturbed or improved the community to reduce immunogenicity. Altogether, no clear mechanistic pattern recognition was seen by SBP that may account for enhanced surgical recovery and healing.
Murine models have shown that the injured mucosa is markedly different from intact mucosa and is characterized by a microenvironment consisting of cellular migration, proliferation, and tissue remodeling13. It has also been shown that mucous-adherent microbiota at the anastomosis may have a different composition when compared to expelled stool36. To evaluate this in our model, we compared the microbiome composition of expelled stool to that at the anastomosis, and furthermore in the serum to evaluate for bacterial translocation. We identified that expelled stool was highly similar in composition to that at the anastomosis, and an accurate reflection of its composition. Interestingly, no serum samples from mice on regular chow showed detectable levels of microbiota, whereas four samples from WD fed mice achieved sufficient sequence reads. Although compositionally different from stool, this is consistent with bacterial translocation that is known to occur in the setting of WD-induced gut inflammation37,38.
There are important limitations to this study. This is a murine preclinical model which attempts to mimic the clinical situation. Each anastomosis was tested to ensure patency without leak, and if a leak was identified, additional sutures were placed to create a complete seal leading to differences in number of sutures used but with the result that no leak was present upon completion of surgery. While we attempted to maintain translational accuracy, further studies are required in patients to verify our observations. Intravenous antibiotics are routinely given at the time of surgery to reduce wound infections. We purposefully did not administer perioperative antibiotics as this would have significantly impacted the microbiota and confounded the outcomes of this study39. While our study involved a robust number of murine operations (n = 144), we may have been underpowered to identify significant differences in AL resulting from bowel preparation using our murine model of surgery as compared to a model of anastomotic leak or poor healing. Power calculations based on our observed leak rates suggest that ~ 200 mice/arm would be necessary to allow for detection of significant differences in leak rates. We attempted in-depth investigations to overcome this potential, without clear underlying mechanisms to potentially explain a clinical benefit induced by bowel preparation. All mice were placed on DietGel following surgery, which we expect to potentially have some influence on the microbiome and potentially SCFA production. While DietGel may influence the composition of the microbiota, we have successfully utilized it previously in our murine anastomotic model16 as well as in a murine model of the sleeve gastrectomy investigating role of the post-surgical microbiota in regulating the metabolic benefits of surgery39,40. Regarding IgA levels, we observed no differences in SD fed mice with or without AL raising the possibility that other markers of inflammation may be more sensitive requiring additional investigation in the future. Another important limitation is that only male C57BL/6 J mice were used as female mice are more resistant to the obesogenic effects of WD, with different responses in weight gain, food consumption, energy expenditure and glucose tolerance. However, identifying mechanisms of sex disparity and differences in AL rates may have important implications for identifying high risk patients in the future41,42.
In conclusion, our study did not identify significant clinical impacts on AL or wound healing from the administration of any component of bowel preparation, despite changes observed in the gut microbiota. We reaffirm the use of an AL tool based on changes in the microbiota that may be applied to identify AL independent of major differences in preoperative composition driven by differing baseline diet. This remains an important potential clinical application as stool-based tests for the detection of AL do not currently exist. Future studies are required to identify therapeutic interventions to mitigate pathogenic microbiota shifts in a more targeted approach to reduce the occurrence of postoperative complications.
Methods
Mice, dietary intervention, and bowel preparation treatments
C57BL/6J male mice (n = 144) purchased from Jackson Laboratories at 2 weeks of age were conventionally housed (see Fig. 1 for overview of experimental design). Mice were randomized to a 10–12 week feeding period of either standard diet (SD; n = 72; Envigo, 2018 Teklad Global 18% Protein Rodent Diet) or Western diet (WD; n = 72; Envigo, TD.88137 Adjusted calories diet [42% from fat]; see Supplementary Table 1 for detailed nutritional information). Water and chow were available ad lib. until the perioperative period. Twenty-four hours prior to surgery, mice were given sipper bottles of either (1) mechanical bowel preparation (MBP; Suprep, Sebela Pharmaceuticals Inc., Roswell, GA), (2) oral antibiotics (OA; neomycin and metronidazole, both at concentrations of 1 mg/mL), (3) surgical bowel preparation (SBP; oral antibiotics combined with mechanical bowel preparation), or 4) regular drinking water, all available ad lib. In developing this model, perioperative soft diet (DietGel, ClearH2O, Westbrook, ME) was found to reduce the incidence of colonic obstruction that occurred if mice were continuously fed pelleted food. Thus, all mice were transitioned to DietGel 5 days prior to surgery and were maintained on it until necropsy. Compliance with established guidelines for humane use and care of laboratory animals was carried out as approved by the University of Minnesota Institutional Animal Care and Use Committee. The study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org).
Surgery, anastomotic leak, and superficial wound healing
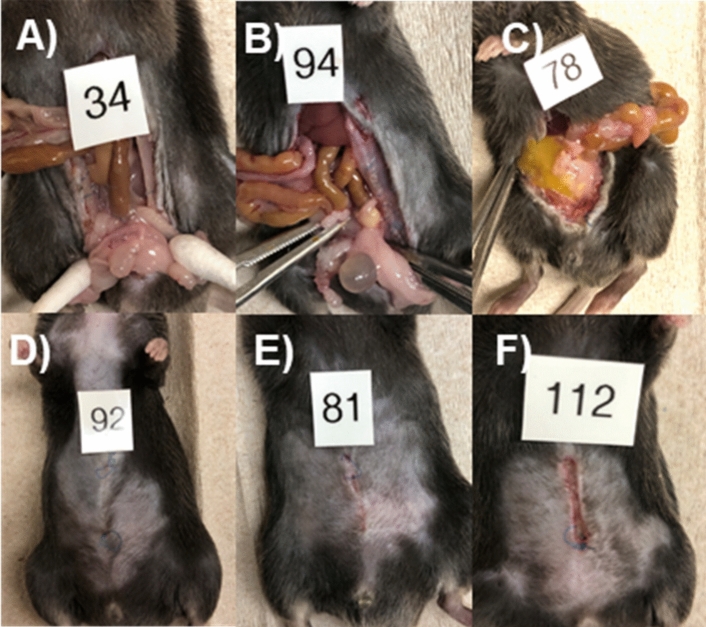
Mice were cohoused before surgery, and then were all individually housed after surgery. They were fasted for 4–6 h prior to surgery, then placed under general anesthesia, shaved, and prepped in sterile fashion. All mice received sustained-release Buprenorphine (Zoopharm, Fort Collins, CO) prior to surgery for pain control and received isoflurane for anesthesia. All operations were performed by a single surgeon approaching the anastomosis at the same level and technique each operation, as previously described16. A 2-cm low-midline incision was made to enter the abdomen. The colon was identified and transected at a point 2–3 cm from the anus. An end-to-end anastomosis was performed with interrupted 8–0 prolene sutures and anastomotic leak test was performed via saline enema to ensure that all anastomoses were sealed. An additional one to two sutures were placed until the saline leak test was negative ensuring that all anastomoses were sealed at the time of completion of the operation. Fascia and skin were each closed with running 6–0 prolene sutures. Mice were advanced to DietGel 4 h after surgery and were maintained on this until euthanasia by CO2 on post-operative day 7 (POD7), or sooner if they displayed clinical deterioration. Necropsies were performed upon euthanasia and anastomotic healing was evaluated. The anastomosis was classified as (1) intact with or without adjacent adhesions, (2) contained anastomotic leak with perianastomotic abscess, or (3) gross abdominal contamination with frank dehiscence (Fig. 6A–C). The skin incision of each mouse was assessed at necropsy for wound healing and classified as either healed or not healed if any amount of incisional breakdown was present (Fig. 6D–F). Post-operative monitoring of mice is described in Supplementary Methods.
Fig. 6.
Anastomotic healing classification: (A) no AL, (B) contained AL (abscess), (C) gross peritonitis. (D–F) Superficial wound healing classification: (D) healed, (E, F) incisional breakdown present.
DNA extraction and sequencing
Microbiota was characterized from fecal pellets collected at 4 timepoints: (1) just prior to starting bowel preparation treatment (Preop), (2) day of surgery (DOS), (3) post-operative day 4 (POD4), and (4) POD7. Pellets were stored at − 80 °C prior to DNA extraction. Sequence data were processed and analyzed as described previously and in supplementary material (see Supplementary Methods)43.
Metabolite and fecal IgA characterization
SCFA were quantified by gas chromatography-mass spectrometry. Samples were extracted in 0.5% phosphoric acid and 50% ethyl acetate. Concentrations of acetate, propionate, and butyrate, and their isomers, were determined using standard curves with an internal standard of 4-methyl valeric acid. Fecal IgA levels were quantified by murine IgA ELISA (Abcam; ab157717). Samples were run in duplicates with a negative control. Absorbance was read using BioTek synergy 2 multi-detection microplate reader.
Statistical analysis
Clinical data (body weight, AL, and survival) were evaluated by analysis of variance (ANOVA) with Duncan’s post-hoc test for multiple comparisons, or Fisher’s exact test for categorical variables where appropriate, using XLSTAT (version 2015.01.0; Addinsoft, Belmont, MA). Shannon indices (calculated in mothur) were used to determine alpha diversity of microbial communities and were compared using ANOVA. Kruskal–Wallis was performed to assess differences in abundance of genera between experimental groups. Bray–Curtis dissimilarity matrices were used to calculate beta diversity and visualized by ordination using principal coordinate analysis (PCoA)44,45. These matrices were also used to assess differences in beta diversity by analysis of similarity (ANOSIM) with Bonferroni correction46. Relative abundances of genera were correlated to axis position using Spearman’s correlations, and taxa significantly correlated to group clustering were overlaid on the PCoA plot using the corr.axes command in mothur. All statistics were evaluated at α = 0.05.
Supplementary Information
Acknowledgements
Sequence data were generated by the University of Minnesota Genomics Center and processed and analyzed using resources of the Minnesota Supercomputing Institute.
Author contributions
S.B.: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing—Original draft preparation, Writing—Reviewing and Editing, Visualization. M.H.K.: Methodology, Software, Formal analysis, Data curation, Visualization. N.G.: Investigation. H.N.-B.: Methodology, Validation. J.K.: Writing—Reviewing and Editing. A.T.: Writing—Reviewing and Editing. Z.Z.: Methodology, Formal analysis, Data curation, Visualization, Writing—Reviewing and Editing. R.M.: Writing—Reviewing and Editing. W.G.: Writing—Reviewing and Editing. C.S.: Conceptualization, Methodology, Software, Formal analysis, Resources, Data curation, Writing—Original draft preparation, Writing—Reviewing and Editing, Visualization, Supervision, Project administration. C.J.: Conceptualization, Methodology, Validation, Writing—Original draft preparation, Writing—Reviewing and Editing, Visualization, Supervision, Project administration, Funding acquisition.
Funding
This work was supported Grant CDA-020 Career Development Award from the American Society of Colon & Rectal Surgeons.
Data availability
Sequence data were deposited in the NCBI SRA under accession number SRP408098 [https://www.ncbi.nlm.nih.gov/Traces/study/?acc=SRP408098&o=acc_s%3Aa].
Competing interests
WBG is a consultant and advisory board member for Intuitive Surgical, Coloplast, Steel Therapeutics, BD, and Applied Medical. The remaining authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Cyrus Jahansouz and Christopher Staley.
Contributor Information
Cyrus Jahansouz, Email: jahan023@umn.edu.
Christopher Staley, Email: cmstaley@umn.edu.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-72520-x.
References
- 1.Everhart, J. E. & Ruhl, C. E. Burden of digestive diseases in the United States part II: Lower gastrointestinal diseases. Gastroenterology136(3), 741–754. 10.1053/j.gastro.2009.01.015 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Morris, A. M., Regenbogen, S. E., Hardiman, K. M. & Hendren, S. Sigmoid diverticulitis: A systematic review. JAMA311(3), 287–297. 10.1001/jama.2013.282025 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Anaya, D. A. & Flum, D. R. Risk of emergency colectomy and colostomy in patients with diverticular disease. Archiv. Surg.140(7), 681–685. 10.1001/archsurg.140.7.681 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Surgeons SoGaE. Colon Resection Surgery Patient Information from SAGES. Accessed May 23, 2023. https://www.sages.org/publications/patient-information/patient-information-for-laparoscopic-colon-resection-from-sages/
- 5.Peery, A. F. K. T. et al. Distribution and characteristics of colonic diverticula in a United States screening population. Clin. Gastroenterol. Hepatol.14(7), 980–985. 10.1016/j.cgh.2016.01.020.Distribution (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics 2020. CA A Cancer J. Clin.70(3), 145–164. 10.3322/caac.21590 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Nichols, R. L., Broido, P., Condon, R. E., Gorbach, S. L. & Nyhus, L. M. Effect of preoperative neomycin erythromycin intestinal preparation on the incidence of infectious complications following colon surgery. Ann. Surg.178(4), 453–462. 10.1097/00000658-197310000-00008 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ioannidis, A., Zoikas, A. & Wexner, S. D. Current evidence of combination of oral antibiotics and mechanical bowel preparation in elective colorectal surgery and their impact on anastomotic leak. Surg. Innovation.27(1), 101–102. 10.1177/1553350619851672 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Poggio, J. L. Perioperative strategies to prevent surgical-site infection. Clin. Colon Rectal Surg.26(3), 168–173. 10.1055/s-0033-1351133 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustafsson, U. O. et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced recovery after surgery (ERAS®) society recommendations: 2018. World J. Surg.43(3), 659–695. 10.1007/s00268-018-4844-y (2019). [DOI] [PubMed] [Google Scholar]
- 11.Alam, A. et al. The microenvironment of injured murine gut elicits a local pro-restitutive microbiota. Nat. Microbiol.10.1038/nmicrobiol.2015.21 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shogan, B. D. et al. Collagen degradation and MMP9 activation by Enterococcus faecalis contribute to intestinal anastomotic leak. Sci. Transl. Med.7(286), 286ra68-286ra68. 10.1126/scitranslmed.3010658 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leoni, G., Neumann, P.-A., Sumagin, R., Denning, T. L. & Nusrat, A. Wound repair: Role of immune–epithelial interactions. Mucosal Immunol.8(5), 959–968. 10.1038/mi.2015.63 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pull, S. L., Doherty, J. M., Mills, J. C., Gordon, J. I. & Stappenbeck, T. S. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc. Natl. Acad. Sci. USA10.1073/pnas.0405979102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nalluri-Butz, H. et al. A pilot study demonstrating the impact of surgical bowel preparation on intestinal microbiota composition following colon and rectal surgery. Sci. Rep.12(1), 10559. 10.1038/s41598-022-14819-1 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boatman, S. et al. Diet-induced shifts in the gut microbiota influence anastomotic healing in a murine model of colonic surgery. Gut Microbes.15(2), 2283147. 10.1080/19490976.2023.2283147 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanketi, B. D. et al. Pitx2 patterns an accelerator-brake mechanical feedback through latent TGFbeta to rotate the gut. Science377(6613), eabl3921. 10.1126/science.abl3921 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng, Z., Cangkrama, M., Butt, T., Jane, S. M. & Carpinelli, M. R. Grainyhead-like transcription factors: guardians of the skin barrier. Vet Dermatol.32(6), 553-e152. 10.1111/vde.12956 (2021). [DOI] [PubMed] [Google Scholar]
- 19.McChesney, S. L. Z. M., Green, R. L. & Nichols, R. L. Current U.S. Pre-operatibe bowel preparation trends: A 2018 survey of the American society of colon and rectal surgeons members. Surg. Infect. (Larchmt)21(1), 1–8 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Young, H. et al. Surgical site infection after colon surgery: National healthcare safety network risk factors and modeled rates compared with published risk factors and rates. J. Am. College Surg.214(5), 852–859. 10.1016/j.jamcollsurg.2012.01.041 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Wick, E. C. et al. Implementation of a surgical comprehensive unit-based safety program to reduce surgical site infections. J. Am. Coll. Surg.215(2), 193–200. 10.1016/j.jamcollsurg.2012.03.017 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Guenaga, K. K. F. G., Matos, D. & Wille-Jørgensen, P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst. Rev.9, CD001544-CD01544. 10.1002/14651858.CD001544.pub3 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scarborough, J. E., Mantyh, C. R., Sun, Z. & Migaly, J. Combined mechanical and oral antibiotic bowel preparation reduces incisional surgical site infection and anastomotic leak rates after elective colorectal resection: An analysis of colectomy-targeted ACS NSQIP. Ann. Surg.262, 331–7. 10.1097/SLA.0000000000001041 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Moghadamyeghaneh, Z. et al. Nationwide analysis of outcomes of bowel preparation in colon surgery. J. Am. College Surg.220, 912–920. 10.1016/j.jamcollsurg.2015.02.008 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Midura, E. F. et al. Combination oral and mechanical bowel preparations decreases complications in both right and left colectomy. Surgery163, 528–534. 10.1016/j.surg.2017.10.023 (2018). [DOI] [PubMed] [Google Scholar]
- 26.McSorley, S. T., Steele, C. W. & McMahon, A. J. Meta-analysis of oral antibiotics, in combination with preoperative intravenous antibiotics and mechanical bowel preparation the day before surgery, compared with intravenous antibiotics and mechanical bowel preparation alone to reduce surgical-site infec. BJS Open2, 185–194. 10.1002/bjs5.68 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koskenvuo, L. et al. Morbidity after mechanical bowel preparation and oral antibiotics prior to rectal resection: The MOBILE2 randomized clinical trial. JAMA Surg159(6), 606–614. 10.1001/jamasurg.2024.0184 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hyoju, S. K. et al. Low-fat/high-fibre diet prehabilitation improves anastomotic healing via the microbiome: An experimental model. Br. J. Surg.107(6), 743–755. 10.1002/bjs.11388 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaines, S. et al. Western diet promotes intestinal colonization by collagenolytic microbes and promotes tumor formation after colorectal surgery. Gastroenterology158(4), 958-970.e2. 10.1053/j.gastro.2019.10.020 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kommineni, S. et al. Bacteriocin production augments niche competition by enterococci in teh mammalian GI tract. Nature526(7575), 719–722. 10.1038/nature15524.Bacteriocin (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rashidi, A. et al. Gut dysbiosis during antileukemia chemotherapy versus allogeneic hematopoietic cell transplantation. Cancer126(7), 1434–1447. 10.1002/cncr.32641 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Alam, A. & Neish, A. Role of gut microbiota in intestinal wound healing and barrier function. Tissue Barriers6(3), 1539595. 10.1080/21688370.2018.1539595 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pabst, O. & Slack, E. IgA and the intestinal microbiota: the importance of being specific. Mucosal Immunol.13(1), 12–21. 10.1038/s41385-019-0227-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palm, N. W. et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell158, 1000–1010. 10.1016/j.cell.2014.08.006 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janzon, A., Goodrich, J. K., Koren, O., Waters, J. L. & Ley, R. E. Interactions between the gut microbiome and mucosal immunoglobulins A, M, and G in the developing infant gut. mSystems4(6), 1–17. 10.1128/msystems.00612-19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shogan, B. D. et al. Intestinal anastomotic injury alters spatially defined microbiome composition and function. Microbiome2(1), 1–10. 10.1186/2049-2618-2-35 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monte, S. V. et al. Reduction in endotoxemia, oxidative and inflammatory stress, and insulin resistance after Roux-en-Y gastric bypass surgery in patients with morbid obesity and type 2 diabetes mellitus. Surgery151(4), 587–593. 10.1016/j.surg.2011.09.038 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Lassenius, M. I. et al. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care34(8), 1809–1815. 10.2337/dc10-2197 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jahansouz, C. et al. Antibiotic-induced disruption of intestinal microbiota contributes to failure of vertical sleeve gastrectomy. Ann. Surg.269(6), 1092–1100. 10.1097/SLA.0000000000002729 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Jahansouz, C. et al. Sleeve gastrectomy drives persistent shifts in the gut microbiome. Surg. Obes. Relat. Dis.13(6), 916–924. 10.1016/j.soard.2017.01.003 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Casimiro, I., Stull, N. D., Tersey, S. A. & Mirmira, R. G. Phenotypic sexual dimorphism in response to dietary fat manipulation in C57BL/6J mice. J. Diabetes Complicat.35(2), 107795. 10.1016/j.jdiacomp.2020.107795 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gelineau, R. R. et al. The behavioral and physiological effects of high-fat diet and alcohol consumption: Sex differences in C57BL6/J mice. Brain Behav.7(6), e00708. 10.1002/brb3.708 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Staley, C. et al. Predicting recurrence of Clostridium difficile infection following encapsulated fecal microbiota transplantation. Microbiome6(1), 166. 10.1186/s40168-018-0549-6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bray, J. & Curtis, J. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr.27(4), 325–349 (1957). [Google Scholar]
- 45.Anderson, M. J. & Willis, T. J. Canonical analysis of principal coordinates: A useful method of constrained ordination for ecology. Ecology.84(2), 511–525 (2003). [Google Scholar]
- 46.Clarke, K. R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol.18, 117–143 (1993). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data were deposited in the NCBI SRA under accession number SRP408098 [https://www.ncbi.nlm.nih.gov/Traces/study/?acc=SRP408098&o=acc_s%3Aa].